Significance
Leaf traits are used to drive models of global carbon fluxes and understand plant evolution. Many syntheses have highlighted relationships between plant leaf nitrogen and photosynthesis as evidence of a strong evolutionary drive to “intercept light and capture CO2.” Different from previous studies, we compiled a global dataset constrained to sites and studies where nitrogen-fixing plants (N2FP) and nonfixing species [other plants (OP)] could be directly compared. We show that photosynthesis is not related to leaf nitrogen for N2FP, irrespective of climate or growth form. N2FP have clear advantages in water use efficiency over OP. These findings contribute to a more complete explanation of global distributions of N2FP and can help improve models of global carbon and nitrogen cycles.
Keywords: legume, actinorhizal species, nitrogen, photosynthesis, water use efficiency
Abstract
Using robust, pairwise comparisons and a global dataset, we show that nitrogen concentration per unit leaf mass for nitrogen-fixing plants (N2FP; mainly legumes plus some actinorhizal species) in nonagricultural ecosystems is universally greater (43–100%) than that for other plants (OP). This difference is maintained across Koppen climate zones and growth forms and strongest in the wet tropics and within deciduous angiosperms. N2FP mostly show a similar advantage over OP in nitrogen per leaf area (Narea), even in arid climates, despite diazotrophy being sensitive to drought. We also show that, for most N2FP, carbon fixation by photosynthesis (Asat) and stomatal conductance (gs) are not related to Narea—in distinct challenge to current theories that place the leaf nitrogen–Asat relationship at the center of explanations of plant fitness and competitive ability. Among N2FP, only forbs displayed an Narea–gs relationship similar to that for OP, whereas intrinsic water use efficiency (WUEi; Asat/gs) was positively related to Narea for woody N2FP. Enhanced foliar nitrogen (relative to OP) contributes strongly to other evolutionarily advantageous attributes of legumes, such as seed nitrogen and herbivore defense. These alternate explanations of clear differences in leaf N between N2FP and OP have significant implications (e.g., for global models of carbon fluxes based on relationships between leaf N and Asat). Combined, greater WUE and leaf nitrogen—in a variety of forms—enhance fitness and survival of genomes of N2FP, particularly in arid and semiarid climates.
Through symbioses with diazotrophic bacteria, legumes and other N2-fixing plants (N2FP) acquire atmospheric dinitrogen (N2) and are widely expected to maintain greater leaf nitrogen than nonfixing or other plants (OP) (1). N2FP can profoundly influence both ecosystem development and responses to changing climate by alleviating nitrogen shortages that limit capacity of ecosystems to fix and sequester CO2 (2–4). A central tenet of trait-based ecology (5, 6) is that carbon fixation and transpiration are directly related to leaf nitrogen; in turn, leaf nitrogen is used to drive global models of carbon (and water) exchanges between plants and the atmosphere (7).
The distribution, abundance, and activity of N2FP in terrestrial ecosystems have remained unexplained, even “paradoxical” (8, 9), especially in relation to local and global nitrogen cycles. For the northern hemisphere, one recent explanation of the distribution of N2FP (2) and their dominance in wet tropical forests relied on their greater ability to acquire phosphorus from old tropical soils and temperature maxima for N2 fixation of around 25 °C (i.e., similar to prevailing temperatures in the tropics). Menge et al. (8) subsequently noted that the diazotrophic symbioses are typically rhizobial and facultative toward the tropics but actinorhizal and obligate north of about 35° N. Facultative symbioses in the tropics make evolutionary sense inasmuch as soil nitrogen availability is typically greater there than at the poles and nitrogen fixation carries a carbon cost for the plant. In support, concurrent research suggested that rates of nitrogen fixation may be less in N-rich tropical forests than previously thought (10).
N2FP differ in their distribution in northern and southern hemispheres, albeit that N2FP are common in the tropics in both hemispheres. By comparison with the north, beyond 35° S, there is relatively little land at all. Bryophyte–cyanobacteria associations again contribute significant nitrogen (11), albeit to much smaller areas than in the northern hemisphere, and actinorhizal plants (e.g., Morella/Myrica spp. in Africa and South America and Casuarina spp. in Australia) are as likely found in the tropics as closer to the southern pole (12). A distinctive feature of all three major continents in the southern hemisphere is the large areas of arid, semiarid, and Mediterranean (summer drought) climates between the equator and 35° S. In divergence from the “view from the north” (13), the “southern paradox” of the distribution of N2FP is that woody legumes, notably of the genus Acacia (sensu lato) but also, from numerous other genera, dominate much of the large arid and semiarid areas, despite an abundance of other drought-tolerant woody species. For Australia, the paradox is exemplified by the dominance of Acacia aneura and Acacia harpophylla over large areas, whereas nominally drought-adapted species from the genus Eucalyptus are restricted to drainage lines or where groundwater is accessible.
Analysis of plant traits is now routinely used (14–18) to seek explanations for distributions of plant species and growth forms as well as their functional attributes. Leaf nitrogen is among the most significant and widely explored of plant traits. For example, it is frequently observed that leaf nitrogen is greater per unit mass or area for N2FP than for OP (1). Leaf nitrogen has been a focus for trait-based studies of plants owing in part to strong positive relationships between leaf nitrogen and photosynthetic rate (19) and the implications for stomatal conductance (gs) and transpiration (20, 21). Increased leaf nitrogen (especially increased abundance of the principal nitrogen-rich enzyme involved in carbon fixation; RubisCo) can increase consumption of intercellular CO2, such that gs is reduced (and rates of water loss are reduced), because a strengthened CO2 diffusion gradient helps maintain supply of CO2. A corollary is that maintaining rates of photosynthesis (Asat) with reduced leaf nitrogen may require increased gs and water loss. Recently, Prentice et al. (22) built on earlier analysis by Wright et al. (5) and proposed a new theoretical framework for plant ecology based on leaf traits, such as nitrogen per leaf area (Narea), Asat, gs, and the ratio of internal to external concentration of carbon dioxide (ci/ca). Prentice et al. (22) focused on the relative constancy of ci/ca over a wide range of conditions, tested their theory using sites in Australia, including Acacia spp. and other N2FP, and argued that Narea should increase with aridity and that high Narea is an adaptation to drought. Despite some recent studies (23), that theory lacks testing for N2FP across the globe.
To test “paradoxes” associated with the global distribution of N2FP, we formalized hypotheses in accordance with the literature. Leaf nitrogen should reflect rates of Asat (hypothesis A)—irrespective of whether the plant species can fix nitrogen. Increases in leaf N should, thus, result in reduced gs and loss of water (hypothesis B) and as a result of either or both, increase water use efficiency [WUE; as indicated by intrinsic water use efficiency (WUEi) or carbon isotope ratio of leaf tissue (δ13C); hypothesis C].
We tested our hypotheses using a climate-stratified dataset constrained to sites where both N2FP and OP (paired dataset) were measured for either (i) Narea, Asat, gs, and WUEi or (ii) Narea and δ13C (that is, sites where N2FP and OP were both growing and measured in situ). We complemented this parsimonious, albeit more limited dataset (81 sites) with a larger dataset, in which either N2FP or OP were studied (nonpaired dataset) for WUEi (including Asat and gs) and Narea (63 sites) or δ13C and nitrogen concentration per unit leaf mass (Nmass; 351 sites). We adopted the Koppen system—the most frequently used and robust method for climate classification and related analyses (24, 25).
Results
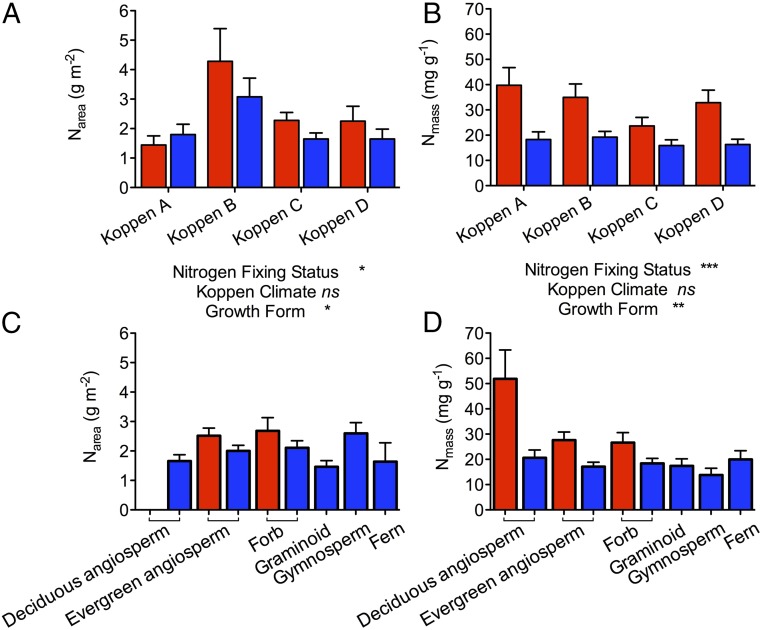
Based on our paired dataset (direct comparison of N2FP and OP) and with the exception of Koppen A climates, N2FP maintained a significant advantage over OP in Narea (Fig. 1A and Table S1). All plants in arid and semiarid Koppen B climates produce foliage distinctly enriched in N relative to other climate zones (Fig. 1A and Table S1), an advantage that was also revealed by the nonpaired dataset (Table S2). On average, foliage of N2FP in arid and semiarid regions (Koppen B) (Fig. 1A) has Narea around threefold that of N2FP in the tropics (Koppen A climate), whereas OP show a more modest N enrichment in Koppen B relative to Koppen A zones. Advantages of N2FP over OP in Narea were retained in nontropical climate zones (i.e., Koppen B–D climates), despite wide variation in lifeforms (Fig. 1C and Tables S1 and S2).
Fig. 1.
Leaf nitrogen (either mass- or area-based) for N2FP (red bars) and OP (blue bars) across Koppen climate classifications and growth forms. Koppen A is tropical, Koppen B is arid and semiarid, Koppen C is temperate, and Koppen D is continental. Linear mixed models were completed on log10-transformed data. Data shown are estimated marginal means and 1 SEs that were back-transformed from log10. Only main effects are shown; interaction terms are given in Table S1. ns, not significant. *P < 0.05; **P < 0.01; ***P < 0.001.
Table S1.
Paired dataset
| Climate or growth form and nitrogen-fixing status | Narea | Nmass | Asat | gs | WUEi | δ13C | ||||||
| Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | |
| All | ||||||||||||
| N2FP | 2.59 | (0.27) | 30.9 | (3.44) | 11.4 | (1.28) | 0.28 | (0.06) | 42.9 | (5.68) | −27.3 | (0.68) |
| OP | 1.93 | (0.17) | 17.9 | (1.87) | 9.7 | (0.88) | 0.22 | (0.04) | 46.7 | (5.56) | −28.0 | (0.66) |
| Koppen climate classification × nitrogen-fixing status | ||||||||||||
| Koppen A | ||||||||||||
| N2FP | 1.44 | (0.31) | 39.7 | (7.0) | 9.3 | (2.2) | 0.35 | (0.13) | 27.2 | (6.6) | −27.8 | (0.9) |
| OP | 1.80 | (0.35) | 18.3 | (3.0) | 9.6 | (1.9) | 0.41 | (0.13) | 23.9 | (5.1) | −28.6 | (0.9) |
| Koppen B | ||||||||||||
| N2FP | 4.28 | (1.11) | 34.9 | (5.3) | 8.8 | (2.6) | 0.22 | (0.10) | 46.4 | (13.5) | −25.3 | (0.8) |
| OP | 3.08 | (0.63) | 18.2 | (2.3) | 8.4 | (1.8) | 0.17 | (0.06) | 57.2 | (12.4) | −26.1 | (0.7) |
| Koppen C | ||||||||||||
| N2FP | 2.28 | (0.27) | 23.6 | (3.4) | 14.1 | (1.8) | 0.28 | (0.07) | 51.9 | (8.2) | −27.4 | (0.9) |
| OP | 1.65 | (0.20) | 15.9 | (2.3) | 10.1 | (1.3) | 0.18 | (0.04) | 57.6 | (9.2) | −27.4 | (0.9) |
| Koppen D | ||||||||||||
| N2FP | 2.25 | (0.51) | 32.8 | (5.0) | 15.2 | (3.6) | 0.37 | (0.14) | 39.5 | (9.9) | −29.3 | (0.8) |
| OP | 1.65 | (0.33) | 16.3 | (2.1) | 10.5 | (2.1) | 0.25 | (0.08) | 40.3 | (8.7) | −29.7 | (0.8) |
| Growth form × nitrogen-fixing status | ||||||||||||
| Deciduous angiosperm | ||||||||||||
| N2FP | 51.9 | (11.4) | −25.3 | (1.0) | ||||||||
| OP | 1.66 | (0.21) | 20.6 | (3.1) | 10.2 | (1.5) | 0.27 | (0.07) | 39.8 | (6.4) | −27.8 | (0.9) |
| Evergreen angiosperm | ||||||||||||
| N2FP | 2.52 | (0.26) | 27.6 | (3.2) | 10.3 | (1.1) | 0.23 | (0.05) | 48.8 | (6.5) | −27.6 | (0.7) |
| OP | 2.00 | (0.19) | 17.1 | (1.8) | 9.4 | (0.9) | 0.22 | (0.04) | 45.3 | (5.6) | −28.4 | (0.7) |
| Fern | ||||||||||||
| OP | 1.64 | (0.64) | 20.0 | (3.4) | 8.3 | (4.0) | 0.08 | (0.07) | 99.5 | (45.4) | −27.5 | (0.9) |
| Forb | ||||||||||||
| N2FP | 2.68 | (0.45) | 26.6 | (4.0) | 12.6 | (2.4) | 0.34 | (0.11) | 37.7 | (7.6) | −28.1 | (0.9) |
| OP | 2.11 | (0.24) | 18.4 | (2.0) | 10.9 | (1.3) | 0.25 | (0.05) | 45.6 | (6.4) | −28.4 | (0.7) |
| Graminoid | ||||||||||||
| OP | 1.46 | (0.21) | 17.4 | (2.8) | 14.7 | (2.3) | 0.29 | (0.08) | 49.6 | (8.6) | −28.1 | (0.9) |
| Gymnosperm | ||||||||||||
| OP | 2.60 | (0.36) | 13.8 | (2.7) | 6.7 | (1.0) | 0.15 | (0.04) | 45.8 | (7.8) | −27.2 | (0.9) |
| Koppen climate classification × growth form × nitrogen-fixing status | ||||||||||||
| Koppen A | ||||||||||||
| N2FP deciduous angiosperm | 45.0 | (10.4) | −27.0 | (1.1) | ||||||||
| N2FP evergreen angiosperm | 1.44 | (0.31) | 35.0 | (7.2) | 9.3 | (2.2) | 0.35 | (0.13) | 27.2 | (6.6) | −28.7 | (1.1) |
| OP deciduous angiosperm | 1.74 | (0.41) | 10.5 | (2.7) | 0.45 | (0.19) | 23.5 | (6.2) | ||||
| OP evergreen angiosperm | 1.86 | (0.35) | 18.3 | (3.0) | 8.7 | (1.7) | 0.37 | (0.11) | 24.3 | (5.0) | −28.6 | (0.9) |
| Koppen B | ||||||||||||
| N2FP deciduous angiosperm | 59.8 | (21.0) | −23.7 | (1.4) | ||||||||
| N2FP evergreen angiosperm | 4.08 | (0.86) | 24.5 | (2.85) | 10.9 | (2.4) | 0.19 | (0.06) | 65.5 | (14.5) | −26.0 | (0.7) |
| N2FP forb | 4.48 | (2.15) | 29.0 | (3.6) | 7.1 | (4.0) | 0.24 | (0.23) | 32.8 | (18.3) | −26.1 | (0.7) |
| OP deciduous angiosperm | 1.74 | (0.76) | 18.2 | (2.4) | 10.3 | (5.5) | 0.28 | (0.23) | 44.6 | (21.7) | −26.4 | (0.8) |
| OP evergreen angiosperm | 3.05 | (0.60) | 19.2 | (2.2) | 9.3 | (1.9) | 0.14 | (0.04) | 75.3 | (15.4) | −27.1 | (0.7) |
| OP fern | 26.1 | (6.5) | −25.3 | (1.1) | ||||||||
| OP forb | 3.82 | (1.00) | 18.7 | (2.2) | 11.1 | (3.2) | 0.27 | (0.12) | 48.3 | (14.2) | −26.4 | (0.7) |
| OP graminoid | 14.7 | (1.8) | −25.6 | (0.7) | ||||||||
| OP gymnosperm | 4.44 | (1.24) | 14.7 | (4.8) | 4.6 | (1.5) | 0.08 | (0.04) | 66.1 | (20.0) | −25.8 | (1.3) |
| Koppen C | ||||||||||||
| N2FP evergreen angiosperm | 2.72 | (0.32) | 18.3 | (2.6) | 10.8 | (1.3) | 0.17 | (0.04) | 65.4 | (10.1) | −27.5 | (0.9) |
| N2FP forb | 1.91 | (0.32) | 30.4 | (5.6) | 18.4 | (3.5) | 0.46 | (0.15) | 41.2 | (8.5) | −27.3 | (1.0) |
| OP deciduous angiosperm | 1.46 | (0.18) | 15.9 | (2.5) | 9.1 | (1.2) | 0.15 | (0.04) | 61.4 | (9.8) | −26.7 | (0.9) |
| OP evergreen angiosperm | 1.83 | (0.19) | 12.8 | (1.8) | 10.4 | (1.1) | 0.17 | (0.04) | 61.4 | (8.8) | −27.6 | (0.9) |
| OP fern | 1.64 | (0.64) | 8.3 | (4.0) | 0.08 | (0.07) | 99.5 | (45.4) | ||||
| OP forb | 1.64 | (0.19) | 18.2 | (3.0) | 11.5 | (1.4) | 0.23 | (0.05) | 50.5 | (7.8) | −27.9 | (0.9) |
| OP graminoid | 1.53 | (0.29) | 17.3 | (4.9) | 14.7 | (3.2) | 0.27 | (0.10) | 56.2 | (12.9) | −27.4 | (1.2) |
| OP gymnosperm | 1.81 | (0.39) | 8.0 | (2.0) | 0.23 | (0.09) | 34.3 | (8.7) | ||||
| Koppen D | ||||||||||||
| N2FP evergreen angiosperm | 36.8 | (7.9) | −28.3 | (1.0) | ||||||||
| N2FP forb | 2.25 | (0.51) | 29.3 | (4.1) | 15.2 | (3.6) | 0.37 | (0.14) | 39.5 | (9.9) | −30.3 | (0.8) |
| OP deciduous angiosperm | 1.72 | (0.39) | 19.4 | (2.5) | 11.1 | (2.6) | 0.27 | (0.10) | 39.0 | (9.4) | −29.2 | (0.8) |
| OP evergreen angiosperm | 1.53 | (0.41) | 18.8 | (2.6) | 9.5 | (2.7) | 0.24 | (0.11) | 37.5 | (10.7) | −30.5 | (0.8) |
| OP fern | 15.2 | (2.8) | −29.7 | (0.9) | ||||||||
| OP forb | 1.51 | (0.31) | 18.3 | (2.5) | 10.2 | (2.1) | 0.25 | (0.08) | 39.1 | (8.6) | −30.9 | (0.8) |
| OP graminoid | 1.39 | (0.32) | 14.4 | (2.0) | 14.6 | (3.6) | 0.32 | (0.13) | 43.8 | (11.3) | −29.3 | (0.8) |
| OP gymnosperm | 2.19 | (0.56) | 13.0 | (2.5) | 8.3 | (2.3) | 0.19 | (0.08) | 42.5 | (11.7) | −28.5 | (0.9) |
| P values | ||||||||||||
| NFS | 0.017 | 0.000 | 0.132 | 0.119 | 0.398 | 0.002 | ||||||
| GF | 0.038 | 0.001 | 0.002 | 0.041 | 0.045 | 0.000 | ||||||
| KCC | 0.050 | 0.670 | 0.513 | 0.169 | 0.009 | 0.000 | ||||||
| NFS × GF | 0.372 | 0.015 | 0.711 | 0.680 | 0.269 | 0.062 | ||||||
| NFS × KCC | 0.003 | 0.028 | 0.417 | 0.814 | 0.558 | 0.158 | ||||||
| GF × KCC | 0.206 | 0.008 | 0.131 | 0.094 | 0.221 | 0.007 | ||||||
| NFS × GF × KCC | 0.792 | 0.147 | 0.038 | 0.121 | 0.964 | 0.083 | ||||||
Leaf nitrogen (area- and mass-based) and physiological parameters (Asat, micromoles meter−2 second−1; gs, moles meter−2 second−1; WUEi, micromoles CO2 moles−1 H2O; δ13C, percentage) for N2FP and OP across Koppen climate classifications and growth forms. Koppen A, tropical; Koppen B, arid and semiarid; Koppen C, temperate; Koppen D, continental. Linear mixed models were completed on log10-transformed data. Data shown are estimated marginal means and 1 SEM that were back-transformed from log10. GF, growth form; KCC, Koppen climate classification; NFS, nitrogen-fixing status.
Table S2.
Unpaired dataset
| Climate or growth form and nitrogen-fixing status | Narea | Nmass | Asat | gs | WUEi | δ13C | ||||||
| Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | Estimated marginal means | 1 SEM | |
| All | ||||||||||||
| N2FP | 3.0 | (0.31) | 29.1 | (2.1) | 12.2 | (1.6) | 0.26 | (0.05) | 47 | (5.4) | −25.0 | (0.5) |
| OP | 2.0 | (0.19) | 17.1 | (1.1) | 9.4 | (1.0) | 0.20 | (0.03) | 46 | (4.3) | −26.2 | (0.4) |
| Koppen climate classification × nitrogen-fixing status | ||||||||||||
| Koppen A | 1.3 | (0.29) | 30.6 | (4.4) | 10.7 | (2.8) | 0.33 | (0.12) | 32 | (7.5) | −24.0 | (0.9) |
| N2FP | 1.4 | (0.28) | 16.9 | (2.2) | 8.1 | (2.2) | 0.22 | (0.07) | 37 | (8.2) | −26.8 | (0.7) |
| OP | 3.7 | (0.93) | 32.1 | (3.8) | 11.5 | (3.5) | 0.25 | (0.10) | 45 | (12.4) | −23.7 | (0.6) |
| Koppen B | 2.7 | (0.49) | 16.8 | (1.2) | 12.0 | (2.5) | 0.19 | (0.05) | 61 | (11.5) | −24.6 | (0.4) |
| N2FP | 3.7 | (0.55) | 25.9 | (2.3) | 12.6 | (2.3) | 0.23 | (0.06) | 56 | (9.4) | −26.6 | (0.5) |
| OP | 2.1 | (0.21) | 15.8 | (1.1) | 9.4 | (1.2) | 0.16 | (0.03) | 57 | (6.5) | −26.5 | (0.4) |
| Koppen C | 2.2 | (0.38) | 30.7 | (3.1) | 13.8 | (3.0) | 0.36 | (0.11) | 38 | (7.4) | −27.3 | (0.5) |
| N2FP | 1.6 | (0.19) | 15.6 | (1.0) | 9.4 | (1.4) | 0.25 | (0.05) | 36 | (4.7) | −27.2 | (0.4) |
| Growth form × nitrogen-fixing status | ||||||||||||
| Deciduous angiosperm | ||||||||||||
| N2FP | 3.6 | (1.35) | 45.1 | (8.5) | 17.8 | (8.2) | 0.42 | (0.29) | 43 | (18.5) | −23.8 | (0.8) |
| OP | ||||||||||||
| Evergreen angiosperm | 1.9 | (0.23) | 22.0 | (2.4) | 11.1 | (1.5) | 0.27 | (0.05) | 41 | (5.0) | −25.9 | (0.7) |
| N2FP | 2.5 | (0.26) | 26.2 | (1.8) | 11.5 | (1.5) | 0.23 | (0.04) | 50 | (5.6) | −26.3 | (0.4) |
| OP | 1.8 | (0.21) | 16.2 | (0.9) | 8.8 | (1.1) | 0.21 | (0.04) | 41 | (4.7) | −26.8 | (0.4) |
| Fern | ||||||||||||
| OP | 2.1 | (0.76) | 16.6 | (2.2) | 7.9 | (3.6) | 0.09 | (0.06) | 92 | (38.7) | −26.0 | (0.6) |
| Forb | ||||||||||||
| N2FP | 2.7 | (0.43) | 26.6 | (3.0) | 13.2 | (2.6) | 0.36 | (0.10) | 36 | (6.4) | −24.5 | (0.8) |
| OP | 1.9 | (0.26) | 18.1 | (1.2) | 9.4 | (1.6) | 0.23 | (0.05) | 41 | (6.0) | −26.9 | (0.4) |
| Graminoid | ||||||||||||
| OP | 1.8 | (0.26) | 17.2 | (2.3) | 14.8 | (2.7) | 0.26 | (0.07) | 56 | (9.3) | −26.0 | (0.8) |
| Gymnosperm | ||||||||||||
| OP | 2.8 | (0.38) | 11.5 | (0.9) | 6.0 | (0.9) | 0.12 | (0.02) | 48 | (6.6) | −25.4 | (0.5) |
| Koppen climate classification × growth form × nitrogen-fixing status | ||||||||||||
| Koppen A | ||||||||||||
| N2FP deciduous angiosperm | 39.0 | (7.4) | −25.5 | (0.8) | ||||||||
| N2FP evergreen angiosperm | 1.3 | (0.29) | 30.7 | (4.3) | 10.7 | (2.8) | 0.33 | (0.12) | 32 | (7.5) | −27.2 | (0.7) |
| OP deciduous angiosperm | 24.0 | (9.0) | −19.2 | (2.5) | ||||||||
| OP evergreen angiosperm | 1.7 | (0.37) | 26.3 | (6.2) | 12.6 | (3.2) | 0.41 | (0.14) | 30 | (7.1) | −26.0 | (1.0) |
| Koppen B | 1.6 | (0.28) | 16.1 | (1.6) | 10.2 | (1.9) | 0.34 | (0.09) | 29 | (5.0) | −27.1 | (0.6) |
| N2FP deciduous angiosperm | 12.0 | (3.5) | −26.4 | (1.3) | ||||||||
| N2FP evergreen angiosperm | 1.0 | (0.57) | 16.2 | (2.9) | 4.2 | (3.7) | 0.07 | (0.08) | 58 | (37.2) | −27.7 | (0.8) |
| N2FP forb | ||||||||||||
| OP deciduous angiosperm | 52.3 | (17.2) | −22.2 | (1.3) | ||||||||
| OP evergreen angiosperm | 3.5 | (0.69) | 22.6 | (1.5) | 14.0 | (2.9) | 0.21 | (0.06) | 64 | (12.8) | −24.5 | (0.4) |
| OP fern | 3.9 | (1.80) | 28.1 | (2.3) | 9.6 | (5.5) | 0.29 | (0.25) | 32 | (16.8) | −24.5 | (0.5) |
| OP forb | 2.1 | (0.56) | 17.0 | (1.5) | 11.8 | (3.7) | 0.19 | (0.08) | 62 | (17.9) | −25.0 | (0.5) |
| OP graminoid | 2.6 | (0.47) | 16.8 | (1.1) | 11.9 | (2.3) | 0.16 | (0.04) | 73 | (13.1) | −25.5 | (0.4) |
| OP gymnosperm | 24.8 | (5.6) | −23.8 | (0.9) | ||||||||
| Koppen C | 3.0 | (0.56) | 18.2 | (1.2) | 15.5 | (3.5) | 0.33 | (0.10) | 46 | (9.2) | −24.9 | (0.4) |
| N2FP evergreen angiosperm | 2.1 | (0.89) | 15.2 | (1.0) | 19.4 | (10.1) | 0.31 | (0.24) | 62 | (29.9) | −24.2 | (0.4) |
| N2FP forb | 4.0 | (1.06) | 11.3 | (1.8) | 5.8 | (1.7) | 0.09 | (0.04) | 66 | (18.6) | −24.4 | (0.7) |
| OP deciduous angiosperm | ||||||||||||
| OP evergreen angiosperm | 3.6 | (1.35) | 17.8 | (8.2) | 0.42 | (0.29) | 43 | (18.5) | ||||
| OP fern | 3.3 | (0.35) | 20.4 | (1.7) | 10.2 | (1.4) | 0.17 | (0.03) | 61 | (7.2) | −26.6 | (0.5) |
| OP forb | 2.4 | (0.38) | 32.8 | (4.5) | 17.5 | (3.4) | 0.46 | (0.12) | 39 | (6.8) | −26.5 | (0.7) |
| OP graminoid | 1.9 | (0.18) | 18.1 | (1.4) | 9.1 | (1.1) | 0.17 | (0.03) | 56 | (5.9) | −26.1 | (0.5) |
| OP gymnosperm | 2.2 | (0.20) | 14.9 | (1.0) | 10.0 | (1.2) | 0.18 | (0.03) | 56 | (5.5) | −26.6 | (0.4) |
| Koppen D | 2.1 | (0.76) | 7.9 | (3.6) | 0.09 | (0.06) | 92 | (38.7) | ||||
| N2FP evergreen angiosperm | 2.1 | (0.20) | 19.9 | (2.2) | 11.1 | (1.4) | 0.24 | (0.04) | 46 | (5.1) | −26.9 | (0.6) |
| N2FP forb | 1.9 | (0.28) | 15.9 | (2.9) | 12.3 | (2.2) | 0.20 | (0.05) | 61 | (9.8) | −26.9 | (0.9) |
| OP deciduous angiosperm | 2.3 | (0.33) | 11.5 | (1.6) | 6.8 | (1.2) | 0.15 | (0.04) | 45 | (7.3) | −25.8 | (0.8) |
| OP evergreen angiosperm | ||||||||||||
| OP fern | 33.5 | (5.0) | −26.8 | (0.7) | ||||||||
| OP forb | 2.2 | (0.38) | 28.2 | (2.6) | 13.8 | (3.0) | 0.36 | (0.11) | 38 | (7.4) | −27.7 | (0.5) |
| OP graminoid | 1.7 | (0.21) | 18.5 | (1.3) | 10.4 | (1.7) | 0.28 | (0.06) | 36 | (5.1) | −26.8 | (0.4) |
| OP gymnosperm | 1.5 | (0.28) | 16.9 | (1.4) | 8.1 | (1.8) | 0.27 | (0.08) | 30 | (6.1) | −28.1 | (0.5) |
| Koppen climate classification × growth form × nitrogen-fixing status | 15.4 | (2.1) | −27.7 | (0.6) | ||||||||
| Koppen A | 1.5 | (0.20) | 18.2 | (1.5) | 9.6 | (1.7) | 0.26 | (0.06) | 37 | (5.6) | −28.2 | (0.5) |
| N2FP deciduous angiosperm | 1.4 | (0.24) | 13.8 | (1.1) | 13.6 | (3.0) | 0.29 | (0.09) | 46 | (9.1) | −26.2 | (0.5) |
| N2FP evergreen angiosperm | 1.9 | (0.30) | 11.7 | (1.0) | 6.6 | (1.3) | 0.19 | (0.05) | 33 | (5.7) | −26.1 | (0.5) |
| P value | ||||||||||||
| NFS | 0.003 | 0.000 | 0.050 | 0.047 | 0.340 | 0.000 | ||||||
| GF | 0.001 | 0.000 | 0.000 | 0.000 | 0.060 | 0.000 | ||||||
| KCC | 0.014 | 0.797 | 0.777 | 0.840 | 0.175 | 0.000 | ||||||
| NFS × GF | 0.480 | 0.328 | 0.484 | 0.464 | 0.455 | 0.016 | ||||||
| NFS × KCC | 0.003 | 0.445 | 0.357 | 0.827 | 0.585 | 0.020 | ||||||
| GF × KCC | 0.198 | 0.010 | 0.068 | 0.019 | 0.127 | 0.007 | ||||||
| NFS × GF × KCC | 0.585 | 0.008 | 0.023 | 0.089 | 0.948 | 0.004 | ||||||
Leaf nitrogen and physiological parameters (as for Table S1) for N2FP and OP across Koppen climate classifications and growth forms. Koppen A, tropical; Koppen B, arid and semiarid; Koppen C, temperate; Koppen D, continental. Linear mixed models were completed on log10-transformed data. Data shown are estimated marginal means and 1 SEM that were back-transformed from log10. GF, growth form; KCC, Koppen climate classification; NFS, nitrogen-fixing status.
Differences in Nmass and Narea between Koppen A and Koppen B zones reflect differences in specific leaf area. Consequently and as expected, Nmass was consistently greater in N2FP than OP growing on the same site (Fig. 1B and Table S1) across all climate zones. In the Koppen A zone, foliage of N2FP was, on average, twice as rich in N as that of OP, and the advantage in terms of leaf N was never less than 40% across climate zones. Effects of N-fixing status on Nmass were strongest at low and relatively high latitudes and in deciduous angiosperms (Fig. 1D). This pattern was replicated when we included indirect comparisons of N2FP and OP (nonpaired dataset) (Table S2).
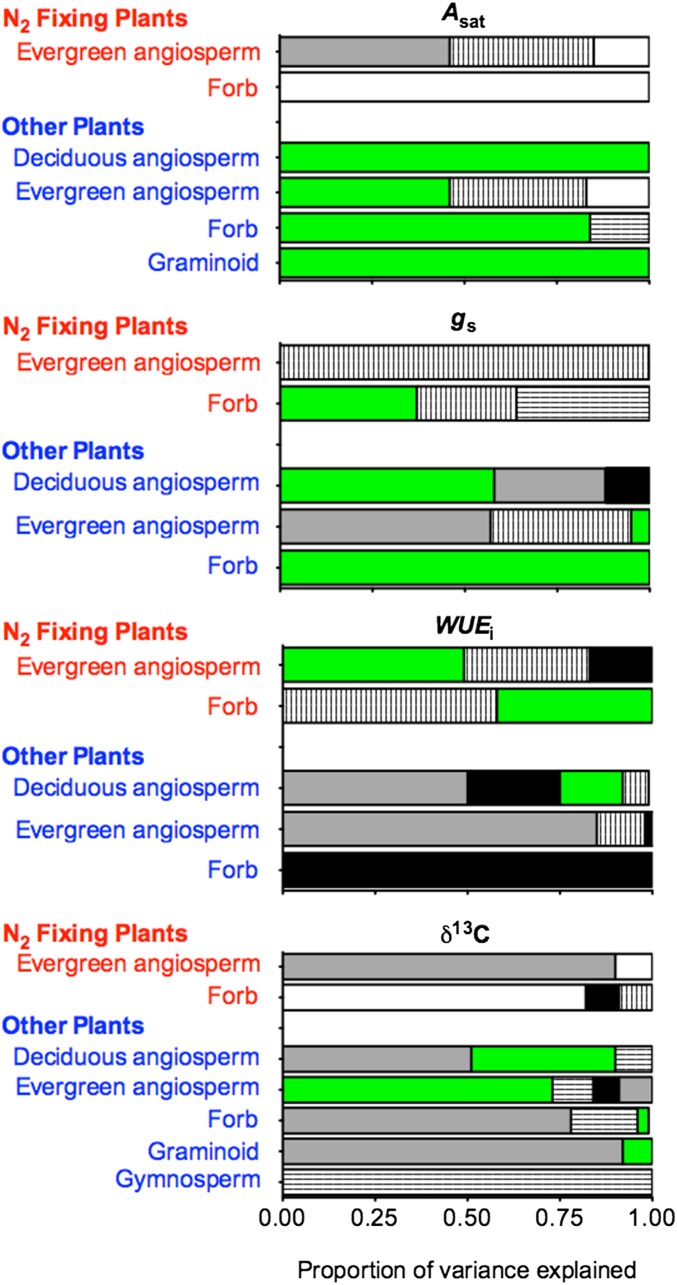
Multivariate analysis showed that Narea dominated predictions of Asat (model of best fit) for OP of all growth forms (Table 1). This pattern can be readily seen (Fig. 2) in the large proportion of variance in Asat that was attributed to Narea (accept hypothesis A for OP). In contrast, Narea had no influence on predicted Asat for N2FP (Fig. 2 and Table 1) (reject hypothesis A for N2FP). Narea contributed to the model of best fit for predicting gs in N2FP forbs but played no role for N2FP evergreen, woody angiosperms (Table 1) (reject hypothesis B). For OP, Narea was again a key driver of gs (Table 1). It is noteworthy that Narea had a positive relationship with gs for all OP and forbs within N2FP (reject hypothesis B).
Table 1.
Stepwise multiple regressions between Asat, gs, WUEi, and δ 13C and predictive variables: Narea, latitude, mean annual precipitation, mean annual temperature, dryness index, and elevation
| Growth form | Equation | R2 | P value |
| Log10 Asat | |||
| N2FP evergreen angiosperm | Log10Asat = 1.253 − 0.0002MAP + 0.003Lat − 0.024DI | 0.52 | 0.000 |
| N2FP forb | Log10Asat = 1.330 − 0.24DI | 0.34 | 0.015 |
| OP deciduous angiosperm | Log10Asat = 0.902 + 0.602log10Narea | 0.39 | 0.000 |
| OP evergreen angiosperm | Log10Asat = 0.909 + 0.419log10Narea + 0.002Lat − 0.018DI | 0.26 | 0.000 |
| OP forb | Log10Asat = 1.015 + 0.568log10Narea − 0.0001Elev | 0.25 | 0.001 |
| OP graminoid | Log10Asat = 1.116 + 0.720log10Narea | 0.35 | 0.035 |
| Log10 gs | |||
| N2FP evergreen angiosperm | Log10gs = −0.694 + 0.006Lat | 0.35 | 0.000 |
| N2FP forb | Log10gs = −0.40 + 1.186log10Narea − 0.0004Elev − 0.006Lat | 0.70 | 0.001 |
| OP deciduous angiosperm | Log10gs = −0.833 + 1.067log10Narea + 0.0003MAP − 0.020MAT | 0.64 | 0.000 |
| OP evergreen angiosperm | Log10gs = −1.034 + 0.0002MAP + 0.005Lat + 0.293log10Narea | 0.46 | 0.000 |
| OP forb | Log10gs = −0.597 + 0.401log10Narea | 0.08 | 0.014 |
| Log10 WUEi | |||
| N2FP evergreen angiosperm | Log10WUEi = 1.816 + 0.394log10Narea − 0.003Lat − 0.014MAT | 0.47 | 0.000 |
| N2FP forb | Log10WUEi = 1.642 − 0.722log10Narea + 0.005Lat | 0.67 | 0.000 |
| OP deciduous angiosperm | Log10WUEi = 0.891 − 0.002MAP + 0.036MAT − 0.452log10Narea + 0.14Lat | 0.74 | 0.000 |
| OP evergreen angiosperm | Log10WUEi = 2.103 − 0.002MAP − 0.003Lat − 0.008MAT − 0.011DI | 0.70 | 0.000 |
| OP forb | Log10WUEi = 1.426 + 0.016MAT | 0.12 | 0.002 |
| δ13C | |||
| N2FP evergreen angiosperm | δ13C = −25.537 − 0.003MAP + 0.233DI | 0.52 | 0.000 |
| N2FP forb | δ13C = −31.809 + 5.328DI − 0.229MAT − 0.063Lat | 0.72 | 0.000 |
| OP deciduous angiosperm | δ13C = −27.020 − 0.003MAP + 3.809log10Narea − 0.001Elev | 0.43 | 0.000 |
| OP evergreen angiosperm | δ13C = −29.883 + 2.003log10Narea + 0.002Elev + 0.125MAT − 0.002MAP | 0.60 | 0.000 |
| OP forb | δ13C = −25.746 − 0.008MAP + 0.001Elev + 2.739log10Narea | 0.83 | 0.000 |
| OP graminoid | δ13C = −22.809 − 0.009MAP + 2.352log10Narea | 0.66 | 0.000 |
| OP gymnosperm | δ13C = −24.547 − 0.012Elev | 0.87 | 0.021 |
Equations were developed for growth forms within N2FP and OP using log10-transformed data for Asat, gs, WUEi, and Narea and untransformed data for other variables. Absence of an equation for a specific combination of growth form and nitrogen-fixing status signifies either insufficient data or a statistically insignificant regression. Predictive variables were Narea, latitude (Lat), mean annual precipitation (MAP), mean annual temperature (MAT), dryness index (DI), and elevation (Elev).
Fig. 2.
Proportional contributions to explain variance in multivariate relationships describing physiological parameters (shown in Table 1). Contributions are shown for Narea (green bars), latitude (vertical line bars), precipitation (gray bars), temperature (black bars), dryness index (white bars), and elevation (horizontal line bars).
Patterns for WUEi and δ13C were very different to those for Asat and gs. Narea was particularly important to predicting WUEi (Fig. 2 and Table 1) for all growth forms of N2FP and of much lesser significance for OP; δ13C was best predicted using a variety of combinations of precipitation, latitude, temperature, elevation, and dryness index.
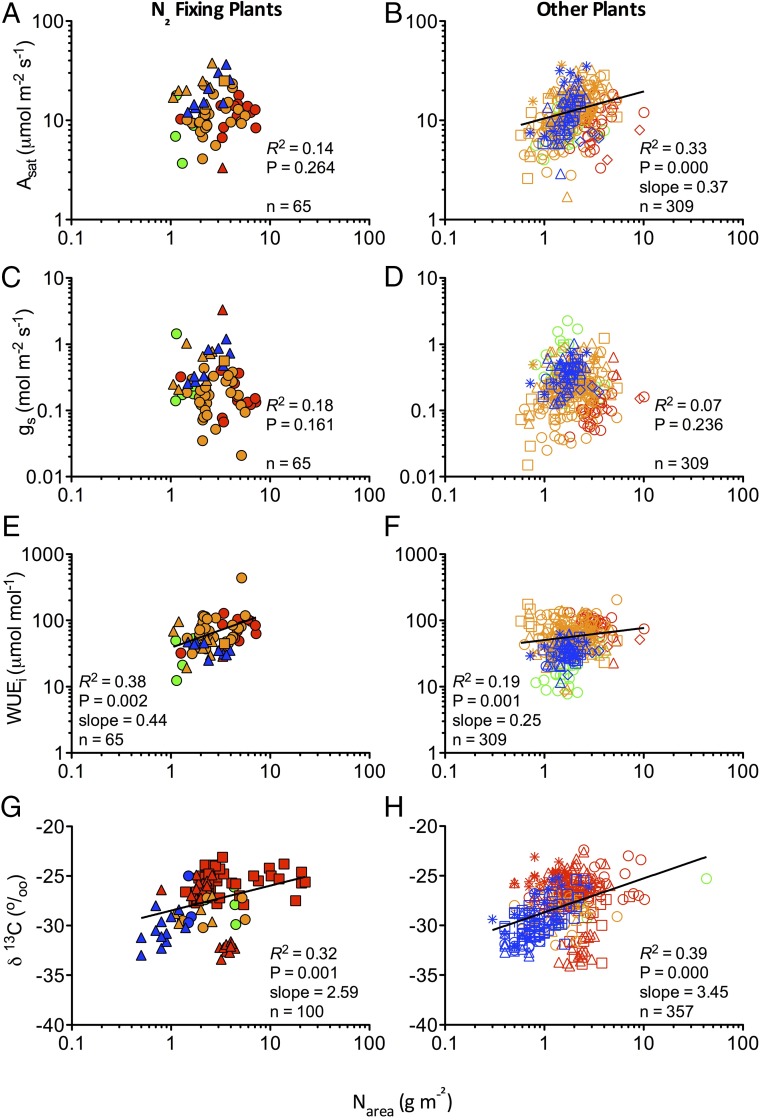
Bivariate analyses of the data mostly lend support to multivariate analyses showing Narea of N2FP unrelated to Asat (reject hypothesis A) (Fig. 3A and Table 2) or gs (reject hypothesis B) (Fig. 3C). For OP, Narea was significantly related to Asat (accept hypothesis A) (Fig. 3B) but not gs (Fig. 3D). Instantaneous WUE was related to Narea for both N2FP and OP but more significantly and tightly so for the former (accept hypothesis C) (Fig. 3 E and F). Relative to OP, N2FP showed marginally faster rates of both photosynthetic carbon fixation and gs in Koppen zones B–D, irrespective of whether data were constrained to sites where direct comparisons could be made (Table S1) or not so constrained (Table S2). Both OP and N2FP show clearly significant relationships between δ13C and Narea (Fig. 3 G and H). Additional bivariate analysis (Table 2) helped elucidate specific non-N influences on physiological properties. For both N2FP and OP, latitude was a surprisingly strong predictor of Asat, gs, and WUEi; δ13C, however, was much better predicted by precipitation (Table 2) and was not significantly related to latitude. Our larger, nonpaired dataset produced similar results, albeit that the relationships were generally weaker than those of the paired data (Table S3).
Fig. 3.
Relationships between Narea (grams meter−2) and light-saturated Asat (micromoles meter−2 second−1), light-saturated rate of gs (moles meter−2 second−1), WUEi (micromoles CO2 moles−1 H2O), and δ13C (percentage) for (A, C, E, and G) N2FP and (B, D, F, and H) OP. Symbol shape corresponds to growth form: evergreen angiosperm (circle), deciduous angiosperm (square), forb (triangle), fern (dash), gymnosperm (diamond), and graminoid (asterisk). Symbol color denotes Koppen climate classification: A (green; tropical), B (red; arid and semiarid), C (orange; temperate), and D (blue; continental). Pearson correlations completed on log10-transformed data for all variables. Slopes are shown for significant relationships only.
Table 2.
Bivariate relationships among Asat, gs, WUEi, δ13C, and climate-related variables for N2FP and OP
| Independent variable and nitrogen-fixing status | Log10Asat | Log10gs | Log10WUEi | δ13C | ||||||||
| R2 | P value | Slope | R2 | P value | Slope | R2 | P value | Slope | R2 | P value | Slope | |
| Latitude | ||||||||||||
| N2FP | 0.55 | 0.000 | 0.003 | 0.64 | 0.000 | 0.006 | 0.48 | 0.000 | −0.003 | 0.10 | 0.276 | |
| OP | 0.29 | 0.000 | 0.002 | 0.43 | 0.000 | 0.004 | 0.31 | 0.000 | −0.002 | 0.08 | 0.105 | |
| MAP (mm) | ||||||||||||
| N2FP | 0.40 | 0.001 | 0.0002 | 0.03 | 0.822 | 0.30 | 0.016 | −0.0001 | 0.70 | 0.000 | −0.005 | |
| OP | 0.22 | 0.000 | 0.0006 | 0.30 | 0.000 | 0.0001 | 0.57 | 0.000 | −0.0002 | 0.57 | 0.000 | −0.004 |
| MAT (°C) | ||||||||||||
| N2FP | 0.41 | 0.001 | −0.015 | 0.19 | 0.123 | 0.06 | 0.647 | 0.07 | 0.464 | |||
| OP | 0.09 | 0.127 | 0.01 | 0.849 | 0.09 | 0.128 | 0.05 | 0.333 | ||||
| Dryness index | ||||||||||||
| N2FP | 0.15 | 0.235 | 0.01 | 0.963 | 0.12 | 0.351 | 0.49 | 0.000 | 0.685 | |||
| OP | 0.02 | 0.758 | 0.08 | 0.183 | 0.08 | 0.144 | 0.39 | 0.000 | 0.547 | |||
| Elevation (meters above sea level) | ||||||||||||
| N2FP | 0.19 | 0.112 | 0.19 | 0.143 | 0.11 | 0.390 | 0.06 | 0.486 | ||||
| OP | 0.17 | 0.003 | −0.004 | 0.14 | 0.012 | −0.0006 | 0.04 | 0.464 | 0.03 | 0.584 | ||
Pearson correlations were completed on log-transformed data for all variables, with the exception of δ13C. Slopes are shown for significant relationships only. MAP, mean annual precipitation; MAT, mean annual temperature.
Table S3.
Unpaired dataset
| Independent variable and nitrogen-fixing status | Log10Asat | Log10gs | Log10WUEi | δ13C | ||||||||
| R2 | P value | Slope | R2 | P value | Slope | R2 | P value | Slope | R2 | P value | Slope | |
| Log10Narea | ||||||||||||
| N2FP | 0.12 | 0.316 | 0.19 | 0.121 | 0.39 | 0.001 | 0.44 | 0.33 | 0.000 | 2.6 | ||
| OP | 0.32 | 0.000 | 0.395 | 0.10 | 0.020 | −0.184 | 0.16 | 0.000 | 0.21 | 0.40 | 0.000 | 3.493 |
| Latitude | ||||||||||||
| N2FP | 0.55 | 0.000 | 0.003 | 0.64 | 0.000 | 0.006 | 0.48 | 0.000 | −0.003 | 0.04 | 0.600 | |
| OP | 0.09 | 0.037 | 0.001 | 0.28 | 0.000 | 0.003 | 0.29 | 0.000 | −0.002 | 0.02 | 0.470 | |
| Mean annual precipitation (mm) | ||||||||||||
| N2FP | 0.37 | 0.002 | −0.0002 | 0.03 | 0.828 | 0.28 | 0.023 | −0.0001 | 0.13 | 0.040 | 0.001 | |
| OP | 0.26 | 0.000 | −0.0001 | 0.10 | 0.034 | 0.00005 | 0.37 | 0.000 | −0.0001 | 0.37 | 0.000 | −0.002 |
| Mean annual temperature (°C) | ||||||||||||
| N2FP | 0.39 | 0.001 | −0.016 | 0.22 | 0.077 | 0.01 | 0.918 | 0.24 | 0.000 | 0.097 | ||
| OP | 0.07 | 0.123 | 0.05 | 0.465 | 0.00 | 0.981 | 0.12 | 0.000 | −0.042 | |||
| Dryness index | ||||||||||||
| N2FP | 0.26 | 0.101 | 0.14 | 0.364 | 0.02 | 0.915 | −0.001 | 0.25 | 0.000 | 0.453 | ||
| OP | 0.11 | 0.023 | 0.012 | 0.02 | 0.760 | 0.13 | 0.008 | 0.015 | 0.25 | 0.000 | 0.395 | |
| Elevation (meters above sea level) | ||||||||||||
| N2FP | 0.16 | 0.196 | 0.16 | 0.200 | 0.10 | 0.421 | 0.02 | 0.740 | ||||
| OP | 0.19 | 0.000 | −0.0001 | 0.04 | 0.330 | 0.12 | 0.008 | −0.00004 | 0.11 | 0.000 | 0.0004 | |
Bivariate relationships among Asat, gs, WUEi, δ13C, and leaf nitrogen (area-based) and climate-related variables for N2FP and OP. Pearson correlations were completed on log-transformed data for all variables with the exception of δ13C. Slopes are shown for significant relationships only.
Discussion
Positive relationships between leaf N and Asat have been widely reported at scales ranging from individual plant species to the globe. For example, our independent analysis for OP (Fig. 3B) is qualitatively similar to those in the works by Evans (19) and Wright et al. (5). However, our analysis also shows that this is not the case for N2FP in nonagricultural ecosystems (Fig. 3A), and the literature shows that it is not true for agricultural systems (26). Our results also challenge the prevailing theory that additional leaf N will increase Asat or reduce gs (20). We found that additional leaf N was only ever a positive influence on both Asat and gs.
Osnas et al. (6) and many others draw on the broad observation that leaves have evolved primarily to intercept light and capture CO2 to propose that photosynthetic capabilities are mostly proportional to leaf area. There are, however, other evolutionary forces at work. Given the lack of support among N2FP for either greater carbon gain (hypothesis A) or reduced leaf water loss (hypothesis B) but good evidence for enhanced WUE (hypothesis C), can these other forces help explain leaf N and the dominance of many arid and semiarid zones by woody legumes?
Rates of leaf and plant growth are only part of evolutionary success and must be considered alongside a plant’s ability to survive and reproduce. Relative to photosynthetic needs, overinvestment of nitrogen in leaves in harsh semiarid to arid regions has remained unexplained (22). In these areas, there is little selection pressure for light, to create a large canopy, or to grow quickly. A potent selective force is the ability to survive (as either plant or seed) periods of drought that might last weeks to months or even a decade or more.
For annual agricultural legumes, Hardwick (27) noted that canopy Asat varies according to the rate of growth of the seed—not the other way around. There is also abundant evidence that remobilization of nitrogen from foliage and other plant tissues may account for 70–90% of seed nitrogen in annual agricultural legumes (28). Prolific flowering and generation of seedpods and seeds are features of many N2FP (Fig. S1). Although it is not known how much nitrogen is remobilized from leaves to seeds for the thousands of species of N2FP in nonagricultural ecosystems, current knowledge suggests that leaf N is an investment in the ability of N2FP to produce seed and the “survival of the genome” (27). Furthermore, the competitive ability of N2FP is enhanced by their ability to take up other forms of N available in the soil (29) or when diazotrophy is restricted by water availability (30, 31). N2FP also make efficient use of N temporarily stored in foliage. For example, in the forms of amines, polyamines, alkaloids, cyanogenic glucosides, and many others, N-rich molecules help N2FP cope with drought (by osmotic adjustment) as well as freezing conditions (32) and also, help deter herbivores in both tropical and nontropical forests (33, 34).
Fig. S1.
Flowering woody legumes from Africa and Australia. (Upper Left) Ormocarpum tricocarpum in Kruger National Park, South Africa. (Upper Right) Acacia boormanii in southeast Australia. (Lower Left) Acacia macradenia in northern Australia. (Lower Left) Swainsonia Formosa in northwestern Australia.
Despite relatively recent evolution (∼60 MyBP) (35, 36), possibly from a “single cryptic evolutionary innovation” (36), symbioses with diazotrophic bacteria ensure access of N2FP to nitrogen—one of the most limiting resources for plant growth, survival, and reproduction. That insurance and other nitrogen-related advantages have facilitated the spread of N2FP throughout the globe and their contributions to global N cycles (37, 38). The facultative nature of the symbiosis with respect to soil nitrogen (4, 8–10) is augmented by its flexibility in relation to soil water—N2FP seldom fix nitrogen under drought conditions (29–31), although their ability to nodulate may be unimpeded (39) and help restore fixation after drought is relieved. These features facilitate the dominant role played by N2FP in both wet and dry tropics as well as large areas of temperate and Mediterranean climates. WUE contributes further to the evolutionary advantages enjoyed by legumes and other N2FP. In their recent synthesis of the now large body of work that informs our understanding of δ-values in plants, Cernusak et al. (21) noted that, for C3 plants, the range in δ-values (Cernusak used Δ in place of δ) was constrained by coordination of gs and Asat. A more sophisticated and complex relationship between δ and WUE than what was once recognized does not detract from the evidence presented here that the latter contributes to our knowledge of the benefits enjoyed by legumes and why they are different from OP (40).
If trait-based models of regional and global carbon cycles (7) are to achieve promised predictive capabilities, they will need to incorporate WUE as well as traits, such as the ability of N2FP to store and use N in leaves for other survival-related functions. Increasingly dry conditions in many areas of the globe reinforce this point. In similar fashion, the absence of significant predictive power of leaf nitrogen for rates of carbon fixation by N2FP will pose ongoing challenges given their dominance of so many wet tropical forests that collectively are critical to global C cycles.
Methods
Data Acquisition.
We developed a database from a global meta-analysis of published literature (Table S4). Our database was targeted to our hypotheses; studies included from natural systems had to contain a measure of leaf nitrogen content and a measure of leaf WUE for N2FP and OP. We identified relevant literature by screening the Web of Science and Google Scholar search engines for keywords: carbon isotope discrimination, 13C, WUE, water use efficiency, leaf nitrogen, legume*, n-fix*, and nodulation; it also included relevant citations documented in these literature. We included targeted searches for each of the major actinorhizal genera.
Table S4.
List of references from which data were drawn
| Dataset | Author(s) | Reference | |||
| Paired WUEi | Ackerly D | (2004) | Ecological Monographs | 74 (1) | 25–44 |
| Unpaired WUEi, unpaired 13C | Albert KR, Kongstad J, Schmidt IK, Ro-Poulsen H, Mikkelsen TN, Michelsen A, van der Linden, Beier C | (2012) | Acta Oecologia | 45 | 79–87 |
| Unpaired 13C | Alstad KP, Welker JM, Williams SA, Trlica MJ | (1999) | Oecologia | 120 | 375–385 |
| Unpaired WUEi, unpaired 13C | Brendel O, Le Thiec D, Scotti-Santagne C, Bodenes C, Kremer A, Guehl J-M | (2008) | Tree Genetics & Genomes | 4 | 263–278 |
| Unpaired WUEi, unpaired 13C | Cano FJ, Sanchez-Gomez D, Rodrigues-Calcerrada J, Warren CR, Gil L, Aranda I | (2013) | Plant, Cell and Environment | 36 | 1961–1980 |
| Unpaired 13C | Carey EV, Callaway RM, DeLucia E | (1998) | Ecology | 79 | 2281–2291 |
| Unpaired 13C | Case AL, Barrett SCH | (2001) | Ecology | 82 (9) | 2601–2616 |
| Unpaired WUEi | Chaturvedi RK, Prasad S, Rana S, Obaidullah SM, Pandey V, Singh H | (2013) | Env Monit Assess | 185 | 383–391 |
| Unpaired 13C | Chen S, Bai Y, Ahang L, Han X | (2005) | Environmental and Experimental Botany | 53 | 65–75 |
| Unpaired WUEi | Clearwater MJ, Meinzer FC | (2001) | Tree Physiology | 21 | 683–690 |
| Unpaired 13C | Cordell S, Goldstein G, Meinzer FC, Handley LL | (1998) | Functional Ecology | 13 | 811–818 |
| Unpaired WUEi | Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM | (1998) | Oecologia | 113 | 188–196 |
| Unpaired 13C | del Mar Alguacil M, Roldan A, Salinas-Garcia J R, Querejeta JI | (2010) | J Sci Food Agric | 91 | 268–272 |
| Unpaired 13C | Del Pozo A, Matus I, Serret MD, Araus JL | (2014) | Environmental and Experimental Botany | 103 | 180–189 |
| Unpaired WUEi | Diaz-Espejo A, Nocolas E, Fernandez JE | (2007) | Plant, Cell and Environment | 30 | 922–933 |
| Unpaired 13C | Donovan L, Dudley SA, Rosenthal DM Ludwig F | (2007) | Oecologia | 152 | 13–25 |
| Unpaired WUEi, unpaired 13C | Donovan L, West JB, McLeod KW | (1999) | Tree Physiology | 20 | 929–936 |
| Unpaired WUEi | Donovan LA, Pappert RA | (1998) | Journal of the Torrey Botanical Society | 125 (1) | 3–10 |
| Unpaired 13C | Dorodnikov M, Kuzy akov Y, Fanmeier A, Wiesenberg GLB | (2011) | Soil Biology and Biochemistry | 43 | 579–589 |
| Unpaired 13C | Ehleringer JR, Cook CS Tieszen LL | (1986) | Oecologia | 68 | 279–284 |
| Paired WUEi | Ewe | (2003) | Forest Ecology and Management | 179 | 27–36 |
| Unpaired 13C | Falxa-Raymond N, Patterson AE, Schuster WSF, Griffin KL | (2012) | Tree Physiology | 32 | 1092–1101 |
| Unpaired WUEi | Field C, Merino J, Mooney HA | (1984) | Oecologia | 60 | 384–389 |
| Paired 13C | Flanagan LB, Cook CS, Ehleringer JR | (1997) | Oecologia | 111 | 481–489 |
| Paired WUEi | Forrester DI, Lancaster K, Collopy JJ, Warren CR, Tausz M | (2012) | Trees | 26 | 1203–1213 |
| Unpaired WUEi, unpaired 13C | Forseth IN, Wait DA, Casper BB | (2001) | Journal of Ecology | 89 | 670–680 |
| Paired 13C | Foster TE, Brooks JR | (2005) | Oecologia | 144 | 337–352 |
| Unpaired WUEi | Fredeen AL, Gamon JA, Field CB | (1991) | Plant, Cell and Environment | 14 | 963–970 |
| Unpaired WUEi | Friend AD, Woodward FI Switsur VR | (1989) | Functional Ecology | 3 (1) | 117–122 |
| Unpaired WUEi | Funk J, Jones CG, Lerdau MT | (2007) | Tree Physiology | 27 | 1731–1739 |
| Unpaired 13C | Geßler A, Duarte HM, Franco AC, Luutge U, de Mattos EA, Nahm M, Rodrigues PJFP, Scarano FR, Rennenberg H | (2005) | Trees | 19 | 523–530 |
| Unpaired 13C | Gong XU, Chen Q, Lin S, Brueck H, Dittert K, Taube F, Schnyder H | (2011) | Plant Soil | 340 | 227–238 |
| Unpaired WUEi, unpaired 13C | Gornall JL | (2007) | Canadian Journal of Botany | 85 (12) | 1202–1213 |
| Unpaired 13C | Gubsch M, Buchmann N, Schmid B, E-D Schulze, Lipowsky A, Roscher C | (2011) | Annals of Botany | 107 | 157–169 |
| Paired WUEi | Gulías J, Flexas J, Mus M, Cifre J, Lefi E, Medrano H | (2003) | Annals of Botany | 92 | 215–222 |
| Unpaired WUEi, unpaired 13C | Han Q | (2011) | Tree Physiology | 31 | 976–984 |
| Unpaired 13C | Hanba YT, Noma N, Umeki K | (2000) | Ecological Research | 15 | 393–403 |
| Unpaired 13C | Harrington RA, Fownes JH, Meinzer F, Scowcroft PG | (1995) | Oecologia | 102 | 277–284 |
| Unpaired 13C | Holscher D | (2003) | Basic and Applied Ecology | 5 | 163–172 |
| Unpaired 13C | Hubbard RM, Bond BJ, Ryan MG | (1999) | Tree Physiology | 19 | 165–172 |
| Unpaired 13C | Hultine KR, Marshall JD | (2000) | Oecologia | 123 | 32–40 |
| Unpaired WUEi | Huxman TE, Barron-Gafford G, Gerst KL, Angert AL, Tyler AP, Venable DL | (2008) | Ecology | 89 (6) | 1554–1563 |
| Unpaired 13C | Ibell PT, Xu Z, Blumfield TJ | (2013) | Plant Soil | 369 | 199–217 |
| Unpaired 13C | Ignace DD, Huxman TE | (2009) | Journal of Arid Environments | 73 | 626–633 |
| Unpaired 13C | Inagaki Y, Miyamoto K, Okuda S, Noguchi M, Itou T, Noguchi K | (2011) | Soil Sci and Plant Nutrition | 57 | 710–718 |
| Unpaired WUEi | Jensen CR, Mogensen VO, Mortensen G, Andersen MN, Schjoerring JK, Thage JH, Koribitis J | (1996) | Australian Journal of Plant Physiology | 23 | 631–644 |
| Unpaired 13C | Juhrbandt J, Leuschner C, Holscher D | (2004) | Forest Ecology and Management | 202 | 245–256 |
| Paired 13C | Jumpponen A, Mulder CPH, Huss-Danell K, Högberg P | (2005) | Journal of Ecology | 93 (6) | 1136–1147 |
| Unpaired WUEi | Kenzo T | (2012) | Japan Agricultural Research Quarterly | 46 (2) | 167–180 |
| Unpaired 13C | Kittelson P, Maron J, Marler M | (2008) | Ecology | 89 | 1344–1351 |
| Unpaired 13C | Knight JD, Thies JE, Singleton PW, van Kessel C | (1995) | Plant and Soil | 177 | 101–109 |
| Unpaired WUEi, unpaired 13C | Koerber GR, Seekamp JV, Anderson PA, Whalen MA, Tyerman SD | (2012) | Australian Journal of Botany | 60 | 358–367 |
| Unpaired 13C | Kong G, Luo T, Liu X, Zhang L, Liang E | (2012) | Plant Ecology | 213 | 1843–1855 |
| Unpaired 13C | Lajtha K, Getz J | (1993) | Oecologia | 94 | 95–101 |
| Paired WUEi | Lee TD, Tjoelker MG, Ellsworth DS, Reich PB | (2001) | New Phytologist | 150 | 405–418 |
| Unpaired 13C | Letts MG | (2009) | Ecoscience | 16 | 125–137 |
| Unpaired WUEi, unpaired 13C | Letts MG, Nakonechny KN, Van Gaalen KEV, Smith CM | (2009) | Canadian Journal of Forest Research | 39 | 629–641 |
| Unpaired WUEi, unpaired 13C | Letts MG, Phelan CA, Johnson DRE, Rood SB | (2008) | Tree Physiology | 28 | 1037–1048 |
| Paired 13C | Li C, Xu G, Aang R, Korpelainen H, Berninger F | (2007) | Tree Phys | 27 | 399–406 |
| Paired 13C | Li C, Zhang X, Liu X, Luukkanen O, Berninger F | (2006) | Silva Fennica | 40 (1) | 5–13 |
| Unpaired WUEi, unpaired 13C | Li Z, Zhang S, Hu H, Li Z | (2009) | Journal of Plant Research | 121 | 559–569 |
| Paired 13C | Liu X, Ahao LJ, Gasaw M, Gao D, Qin DH, Ren JW | (2007) | Chinese Science Bulletin | 52 (9) | 1265–1273 |
| Unpaired 13C | Livingston NJ, Guy RD, Sun ZJ, Ethier GJ | 1999 | Plant, Cell and Environment | 22 | 281–289 |
| Unpaired WUEi, unpaired 13C | Llorens L, Peneulas J, Filella I | (2003) | Physiologia Plantarum | 118 | 84–95 |
| Unpaired 13C | Ludwig F, Rosenthal DM, Johnston JA, Kane N, Gross BL, Lexer C, Dudley SA, Rieseberg LH, Donovan LA | (2004) | Evolution | 58 | 2682–2692 |
| Unpaired 13C | Luo J, Zang R, Li C | (2006) | Forest Ecology and Management | 221 | 285–290 |
| Unpaired 13C | Luo T, Li M, Luo J | (2011) | Ecology Research | 26 | 253–263 |
| Unpaired 13C | MacFarlane C, Adams MA, White DA | (2004) | Plant, Cell and Environment | 27 | 1515–1524 |
| Unpaired WUEi, unpaired 13C | Marchin RM, Sage E, Ward J | (2008) | Tree Physiology | 28 | 151–159 |
| Unpaired 13C | Maricle BR, Zwenger SR, Lee RW | (2011) | Environmental and Experimental Botany | 71 | 1–9 |
| Unpaired 13C | Marshall JD, Linder S | (2013) | Tree Physiology | 33 | 1132–1144 |
| Unpaired WUEi, unpaired 13C | Martin KC, Bruhn D, Lovelock CE, Feller IC, Evans JR, Ball MC | (2010) | Plant, Cell and Environment | 33 | 344–357 |
| Paired 13C | Martínez-Mena M, Garcia-Franco N, Almagro M, Ruiz-Navarro A, Albaledjo J, Melgares de Aguilar J, Gonzales D, Querejeta JI | (2013) | European J Agronomy | 49 | 149–157 |
| Unpaired WUEi | Massonet C, Costes E, Rambal S, Dreyer E, Regnard JL | (2007) | Annals of Botany | 100 | 1347–1356 |
| Unpaired 13C | McDowell SCL, Turner DP | (2002) | Oecologia | 133 | 102–111 |
| Unpaired WUEi | Medhurst JL, Pinkard EA, Beadle CL, Worledge D | (2006) | Forest Ecology and Management | 233 | 250–259 |
| Unpaired 13C | Meinzer FC, Rundell PW, Goldstein G, Sharifi MR | (1992) | Oecologia | 91 | 305–311 |
| Unpaired 13C | Meinzer FC, Woodruff DR, Shaw DC | (2004) | Plant, Cell and Environment | 27 | 937–946 |
| Paired 13C | Midgely GF, Ara ibar JN, Mantlana KB, Macko S | (2004) | Global Change Biology | 10 | 309–317 |
| Unpaired 13C | Mohale KC, Belane AK, Dakora FD | (2012) | Biol Fertil Soils | 50 | 307–319 |
| Unpaired 13C | Monclus R, Villar M, Barbaroux C, Bastein C, Fichot R, Delmotte FM, Delay D, Petit J-M, Brechet C, Dreyer E, Brignolas F | (2009) | Tree Physiology | 29 | 1329–1339 |
| Unpaired 13C | Morecroft MD, Woodward FI, Marris RH | (1992) | Functional Ecology | 6 | 730–740 |
| Unpaired WUEi | Mulkey SS, Smith AP, Wright SJ | (1991) | Oecologia | 88 | 263–273 |
| Unpaired WUEi | Nabeshima E, Hiura T | (2004) | Tree Physiology | 24 | 745–752 |
| Unpaired 13C | Ninou E, Tsialtas JT, Dordas CA, Papakosta DK | (2013) | Agricultural Water Management | 116 | 235–241 |
| Unpaired WUEi | Oleksyn J, Karolewski P, Giertych MJ, Zytkowiak R, Reich, PB, Tjoelker | (1998) | New Phytologist | 140 | 239–249 |
| Unpaired WUEi | Ono K, Maruyama A, Kuwagata T, Mano M, Takimoto T, Hayashi K, Hasegwa T, Miyata A | (2013) | Global Change Biology | 19 | 2209–2220 |
| Unpaired WUEi | Paquin R, Margolis HA, Doucet R, Coyea MR | (2000) | Tree Physiology | 20 | 229–237 |
| Unpaired 13C | Pascual M, Lordan J, Villar JM, Fonseca F, Rufat J | (2013) | Scientia Horticulturae | 157 | 99–107 |
| Unpaired 13C | Peng G | (2010) | Polish Journal of Ecology | 60 (2) | 311–321 |
| Paired WUEi, paired 13C | Prentice C, Dong N, Gleason SM, Maire V, Wright IJ | (2014) | Ecology Letters | 17 | 82–91 |
| Paired 13C | Prentice C, Meng T, Wang H, Harrison SP, Ni J, Wang G | (2011) | New Phytolologist | 190 | 169–180 |
| Paired WUEi | Quilici A, Medina E | (1998) | Photosynthetica | 35 (4) | 525–534 |
| Unpaired 13C | Ramirez-Valiente JA, Lorenzo Z, Soto A, Valladares F, Gil L, Aranda I | (2009) | Molecular Ecology | 18 | 3803–3815 |
| Unpaired 13C | Ramirez-Valiente JA, Sanchez-Gomes D, Aranda I, Valladares F | (2010) | Tree Physiology | 30 | 618–627 |
| Unpaired 13C | Ramirez-Valiente JA, Valladares F, Delgado Huertas A, Granados S, Aranda I | (2010) | Tree Genetics & Genomes | 7 | 285–295 |
| Unpaired WUEi | Ran F, Zhang X, Zhang Y, Korpelainen H, Li C | (2013) | Trees | 27 | 1405–1416 |
| Unpaired WUEi | Reich A, Holbrook NM, Ewel JJ | (2004) | American Journal of Botany | 91 (4) | 582–589 |
| Paired WUEi | Reich Pb, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD | (1999) | Ecology | 80 (6) | 1955–1969 |
| Unpaired WUEi, unpaired 13C | Renninger HJ, Meinzer FC, Gartner BL | (2007) | Tree Physiology | 27 | 33–42 |
| Unpaired 13C | Rodriguez-Calcerrada J, Nanos N, Aranda I | (2011) | Trees | 25 | 873–884 |
| Unpaired 13C | Roscher C, Schmid B, Buchmann N, Weigelt A, E-D Schulze | (2011) | Oecologia | 165 | 437–452 |
| Unpaired 13C | Sala A, Peters GD, McIntyre LR, Harrington MG | (2005) | Tree Physiology | 25 | 339–348 |
| Unpaired 13C | Sales-Come R, Holscher D | (2007) | Forest Ecology and Management | 260 | 846–855 |
| Unpaired 13C | Scarano FR, Duarte HM, Franco AC, Gessler A, de Mattos EA, Nahm M, Rennenberg H, Zaluar HL, Luttge U | (2005) | Trees | 19 | 497–509 |
| Unpaired 13C | Schulze E-D, Turner NC, Nicolle D, Schumacher J | (2006) | Physiologia Plantarum | 127 | 434–444 |
| Paired 13C | Schulze E-D, Gebauer G, Ziegler H, Lange OL | (1991) | Oecologia | 88 | 451–455 |
| Unpaired 13C | Schulze E-D, Lange OL, Ziegler H, Gebauer G | (1991) | Oecologia | 88 | 457–462 |
| Paired 13C | Schulze E-D, Williams RJ, Farquhar GD, Schulze W, Langridge J, Miller JM, Walker BH | (1998) | Australian Journal of Plant Physiology | 25 | 413–425 |
| Unpaired WUEi | Sellin A, Tullus A, Niglas A, Ounapuu E, Karasion A, Lohmus K | (2013) | Ecology Research | 28 | 525–535 |
| Unpaired 13C | Sharma S, Williams DG | (2009) | Biogeosciences | 6 | 25–31 |
| Paired 13C | Sharp ED, Sullivan PF, Steltzer H, Csank AZ, Welker JM | (2013) | Global Change Biology | 19 | 1780–1792 |
| Unpaired 13C | Shimoda S | (2012) | Photosynthetica | 50 (3) | 387–396 |
| Paired WUEi | Sobrado MA | (1991) | Functional Ecology | 5 (5) | 608–616 |
| Paired 13C | Sobrado MA | (2010) | Journal of Tropical Ecology | 26 (2) | 215–226 |
| Paired 13C | Song L-L, Fan J-W, Harris W, Wu S-H, Zhong H-P, Zhou Y-C, Wang N, Zhu X-D | (2012) | Plant Ecology | 213 | 89–101 |
| Unpaired 13C | Sparks JP, Ehleringer JR | (1997) | Oecologia | 109 | 362–367 |
| Unpaired 13C | Stockel M, Meyer C, Gebauer G | (2011) | New Phytologist | 189 | 790–796 |
| Paired WUEi, paired 13C | Sumbele S, Fotelli MN, Nikolopoulos D, Tooulakou G, Liakoura V, Liakopoulos G, Bresta P, Dotsika E, Adams MA, Karabourniotis G | (2012) | AOB plants | 25 | 1–10 |
| Unpaired 13C | Takahashi K, Miyajima Y | (2008) | Botany | 86 | 1233–1241 |
| Paired 13C | Tanaka-Oda A, Kenzo T, Koretsune S, Sasaki H, Fukuda | 2010 | Forest Ecology and Management | 259 | 953–957 |
| Unpaired WUEi | Terashima I, Masuzawa T, Ohba H | (1993) | Oecologia | 95 (2) | 194–201 |
| Paired WUEi | Tjoelker MG, Craine JM, Wedin D, Reich PB and Tilman D | (2005) | New Phytologist | 167 (2) | 493–508 |
| Unpaired 13C | Toillon J, Fichot R, Dalle E, Berthelot A, Brignolas F, Marron N | (2013) | Forest Ecology and Management | 304 | 345–354 |
| Paired 13C | Tsialtas JT, Handley LL, Kassioumi MT, Veresoglou DS, Gagianas AA | 2001 | Functional Ecology | 15 | 605–614 |
| Unpaired WUEi, unpaired 13C | Turnbull MH, Whitehead D, Tissue DT, Schuster WSF, Brown KJ, Engel VC, Griffin KL | (2002) | Oecologia | 130 | 515–524 |
| Unpaired WUEi | Turnbull TL, Adams MA, Warren CR | (2007) | Tree Physiology | 27 | 1481–1492 |
| Unpaired 13C | Turner NC, E-D Schulze, Nicolle D, Kuhlmann I | (2010) | Tree Physiology | 30 | 741–474 |
| Unpaired 13C | Uemura A, Harayama H, Koike N, Ishida A | (2006) | Tree Physiology | 26 | 633–641 |
| Unpaired WUEi, unpaired 13C | Voltas J, Serrano L, Hernandez M, Peman J | (2006) | New Forests | 31 | 435–451 |
| Paired WUEi, paired 13C | von Caemmerer S, Ghannoum O, Conroy JP, Clark H, Newton PCD | (2001) | Australian Journal of Plant Physiology | 28 | 439–450 |
| Unpaired WUEi, unpaired 13C | Wallin K F, Kolb TE, Skov KR, Wagner MR | (2003) | Restoration Ecology | 12 (2) | 239–247 |
| Unpaired 13C | Walters MB, Gerlach JP | (2013) | Tree Physiology | 33 | 297–310 |
| Unpaired WUEi | Wan C, Sosebee RE | (1990) | Botanical Gazette | 151 (1) | 14–20 |
| Paired WUEi, paired 13C | Wand SJE, Esler KJ, Rundel PW, Sherwin HW | (1999) | Plant Ecology | 142 | 149–160 |
| Unpaired 13C | Warren CR, Adams MA | (2000) | Oecologia | 124 | 487–494 |
| Unpaired WUEi, unpaired 13C | Warren CR, Tausz M, Adams MA | (2005) | Tree Physiology | 25 | 1369–1378 |
| Unpaired 13C | Watkins JE, Rundel PW, Cardelus CL | (2007) | Oecologia | 153 | 225–232 |
| Unpaired 13C | Welker JM, Wookey PA, Parsons AN, Press MC, Callaghan TV, Lee JA | (1993) | Oecologia | 95 | 463–469 |
| Unpaired 13C | White JW, Castillo JA, Ehleringer JR, Garcia JA, Singh SP | (1994) | Journal of Agricultural Science | 122 | 275–284 |
| Unpaired 13C | Williams DG, Ehleringer JR | (2000) | Western N American Naturalist | 60 | 121–129 |
| Paired 13C | Wittmer M, Auerswald K, Tungalag R, Bai YF, Schäufele R, Bai CH, Schnyder H | (2008) | Biogeosciences discussions | 5 | 903–935 |
| Unpaired 13C | Woodrow IE, Sclocum DJ, Gleadow RM | (2002) | Functional Plant Biology | 29 | 103–110 |
| Unpaired 13C | Yan C, Han S, Zhou Y, Zheng X, Yu D, Zheng J, Dai G, Li M-H | (2013) | Trees | 27 | 389–399 |
| Unpaired 13C | Yang SJ, Sun M, Zhang Y, Chochard H, Cao K | (2014) | Plant Ecology | 215 | 97–109 |
| Unpaired 13C | Zausen GL, Kolb TE, Bailey JD, Wagner MR | (2005) | Forest Ecology and Management | 218 | 291–305 |
| Unpaired 13C | Zhao C, Chen L, Ma F, Yao B, Liu J | (2008) | Tree Physiology | 28 | 133–141 |
| Paired WUEi | Zhu J-T, Li X-Y, Zhang X-M, Yu Q, Lin l-S | (2012) | Australian Journal of Botany | 60 | 61–67 |
| Unpaired 13C | Zianis D, Mencucci M | (2005) | Tree Phys | 25 | 713–722 |
Also identified are paired or unpaired dataset and subset. Subsets were either δ13C (contained data for Nmass and δ13C) or WUEi (contained data for Narea, WUEi, or both light-saturated Asat and gs to water vapor).
We constructed two datasets: one based on studies with concurrent data that were collected from the same site for both N2FP and OP (paired dataset) and one that included studies with data for either N2FP or OP presented (nonpaired dataset). For each of the paired and nonpaired datasets, we had two subsets: one comprised of data of Nmass (milligrams gram−1) and δ13C (percentage) recorded concurrently and one comprised of data for studies of Narea (grams meter−2) reported concurrently with WUEi (micromoles CO2 moles−1 H2O) or both Asat (micromoles CO2 meter−2 second−1) and gs to water vapor (moles meter−2 second−1), such that we could calculate WUEi. The paired dataset includes 22 sites across the globe for studies that presented data in a form from which we could record or calculate Narea together with WUEi and 81 sites containing data in a form from which we could record or calculate Nmass and δ13C, with 57 of those sites also presenting data for specific leaf area (meters2 kilogram−1) or leaf mass per unit area (grams centimeter−2), which enabled calculation of Narea. The nonpaired dataset contains 63 sites across the globe for Narea and WUEi and 351 sites for Nmass and δ13C. For studies where a treatment was applied, only data from the control were used. Species were identified as N2FP (including actinorhizal and nodulating plants) or OP and classified by their growth form: fern, forb, graminoid, gymnosperm, woody evergreen angiosperm, or woody deciduous angiosperm. In total, 11 actinorhizal species were included, the majority of which are from the families Rosaceae or Casuarinaceae (Fig. S2). Digital latitude and longitude of each site were recorded and used to identify site mean annual temperature (degrees Celsius), mean annual precipitation (millimeters), dryness index (mean annual precipitation/potential evaporation), and elevation (meters a.s.l.). We also identified sites according to their Koppen classification (A, tropical/megathermal; B, dry/arid/semiarid; C, temperate/mesothermal; and D, continental/microthermal).
Fig. S2.
Growth forms represented in each Koppen climate classification [A (tropical), B (dry, arid, and semiarid), C (temperate) and D (continental)] for N2FP (red bars) and OP (blue bars) across (A) the paired δ13C dataset and (B) the WUEi dataset. Numbers above the bars represent numbers of observations, with the numbers in parentheses representing actinorhizal species.
Data Analysis.
Shapiro–Wilk tests showed that data for all variables were significantly nonnormal (skewed to the right). Log10 transformations improved normality distributions of data for all variables except δ13C, which had distribution that did not improve with either log10 or square root transformation; hence, all analyses were performed on nontransformed δ13C data.
We used multivariate analyses (linear mixed models and maximum likelihood) to quantify the combined influence of N-fixing status, climate variables, and growth form on leaf nitrogen. Site and author were treated as random factors for all analyses to counter nonindependence. We used bivariate analyses (Pearson correlations) to assess simple relationships between measures of WUE and leaf nitrogen content or measures of WUE and climate-related variables. Multivariate stepwise multiple regressions better explained relationships in toto among leaf nitrogen, climate, and leaf WUE. The large range in data for bivariate analyses was conserved between N2FP and OP groups. All analyses were performed with SPSS. Unless denoted otherwise, data and analyses refer to the paired dataset.
Acknowledgments
We thank Alexandra Barlow for helping us screen the literature. We also thank the numerous authors who provided additional data on request and the two reviewers for their suggestions that significantly improved this article. We thank the Australian Research Council for support. ETH Zurich is thanked for its support to M.A.A. as a visiting professor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523936113/-/DCSupplemental.
References
- 1.Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB. Global resorption efficiencies and concentrations or carbon and nutrients in leaves of terrestrial plants. Ecol Monogr. 2012;82(2):205–220. [Google Scholar]
- 2.Houlton BZ, Wang Y-P, Vitousek PM, Field CB. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature. 2008;454(7202):327–330. doi: 10.1038/nature07028. [DOI] [PubMed] [Google Scholar]
- 3.Batterman SA, et al. Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature. 2013;502(7470):224–227. doi: 10.1038/nature12525. [DOI] [PubMed] [Google Scholar]
- 4.Menge DNL, Levin SA, Hedin LO. Evolutionary tradeoffs can select against nitrogen fixation and thereby maintain nitrogen limitation. Proc Natl Acad Sci USA. 2008;105(5):1573–1578. doi: 10.1073/pnas.0711411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 6.Osnas JLD, Lichstein JW, Reich PB, Pacala SW. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science. 2013;340(6133):741–744. doi: 10.1126/science.1231574. [DOI] [PubMed] [Google Scholar]
- 7.Krinner G, et al. A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Global Biogeochem Cycles. 2005;19:GB1015. [Google Scholar]
- 8.Menge DNL, Lichstein JW, Angeles-Pérez G. Nitrogen fixation strategies can explain the latitudinal shift in nitrogen-fixing tree abundance. Ecology. 2014;95(8):2236–2245. doi: 10.1890/13-2124.1. [DOI] [PubMed] [Google Scholar]
- 9.Hedin LO, Brookshire ENJ, Menge DNL, Barron AR. The nitrogen paradox in tropical forest ecosystems. Annu Rev Ecol Evol Syst. 2009;40:613–635. [Google Scholar]
- 10.Sullivan BW, et al. Spatially robust estimates of biological nitrogen (N) fixation imply substantial human alteration of the tropical N cycle. Proc Natl Acad Sci USA. 2014;111(22):8101–8106. doi: 10.1073/pnas.1320646111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arróniz-Crespo M, et al. Bryophyte-cyanobacteria associations during primary succession in recently Deglaciated areas of Tierra del Fuego (Chile) PLoS One. 2014;9(5):e96081. doi: 10.1371/journal.pone.0096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gtari M, Dawson JO. An overview of actinorhizal plants in Africa. Funct Plant Biol. 2011;38(9):653–661. doi: 10.1071/FP11009. [DOI] [PubMed] [Google Scholar]
- 13.Adams MA, Simon J, Pfautsch S. Woody legumes: A (re)view from the South. Tree Physiol. 2010;30(9):1072–1082. doi: 10.1093/treephys/tpq061. [DOI] [PubMed] [Google Scholar]
- 14.Thuiller W, Lavorel S, Midgley G, Lavergne S, Rebelo T. Relating plant traits and species distributions along bioclimatic gradients for 88 Leucadendron taxa. Ecology. 2004;85(6):1688–1699. [Google Scholar]
- 15.Ordonez JC, et al. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob Ecol Biogeogr. 2009;18(2):137–149. [Google Scholar]
- 16.Pollock LJ, Morris WK, Vesk PA. The role of functional traits in species distributions revealed through a hierarchical model. Ecography (Cop.) 2012;35(8):716–725. [Google Scholar]
- 17.Stahl U, Reu B, Wirth C. Predicting species’ range limits from functional traits for the tree flora of North America. Proc Natl Acad Sci USA. 2014;111(38):13739–13744. doi: 10.1073/pnas.1300673111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Violle C, Reich PB, Pacala SW, Enquist BJ, Kattge J. The emergence and promise of functional biogeography. Proc Natl Acad Sci USA. 2014;111(38):13690–13696. doi: 10.1073/pnas.1415442111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JR. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989;78(1):9–19. doi: 10.1007/BF00377192. [DOI] [PubMed] [Google Scholar]
- 20.Farquhar GD, Buckley TN, Miller JM. Optimal stomatal control in relation to leaf area and nitrogen content. Silva Fenn. 2002;36(3):625–637. [Google Scholar]
- 21.Cernusak LA, et al. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 2013;200(4):950–965. doi: 10.1111/nph.12423. [DOI] [PubMed] [Google Scholar]
- 22.Prentice IC, Dong N, Gleason SM, Maire V, Wright IJ. Balancing the costs of carbon gain and water transport: Testing a new theoretical framework for plant functional ecology. Ecol Lett. 2014;17(1):82–91. doi: 10.1111/ele.12211. [DOI] [PubMed] [Google Scholar]
- 23.Song M, Djagbletey G, Nkrumah EE, Huang M. Patterns in leaf traits of leguminous and non-leguminous dominant trees along a rainfall gradient in Ghana. J Plant Ecol. 2016;9(1):69–76. [Google Scholar]
- 24.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorol Z. 2006;15(3):259–263. [Google Scholar]
- 25.Mahlstein I, Daniel JS, Solomon S. Pace of shifts in climate regions increases with global temperature. Nat Clim Chang. 2013;3(8):739–743. [Google Scholar]
- 26.Del Pozo A, Garnier E, Aronson J. Contrasted nitrogen utilization in annual C3 grass and legume crops: Physiological explorations and ecological considerations. Acta Oecol. 2000;21(1):79–89. [Google Scholar]
- 27.Hardwick RC. Critical physiological traits in pulse crops. In: Summerfield RJ, editor. World Crops: Cool Season Food Legumes. Kluwer; Dordrecht, The Netherlands: 1988. pp. 885–896. [Google Scholar]
- 28.Schiltz S, Munier-Jolain N, Jeudy C, Burstin J, Salon C. Dynamics of exogenous nitrogen partitioning and nitrogen remobilization from vegetative organs in pea revealed by 15N in vivo labeling throughout seed filling. Plant Physiol. 2005;137(4):1463–1473. doi: 10.1104/pp.104.056713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erskine PD, et al. Water availability—a physiological constraint on nitrate utilization in plants of Australian semi-arid mulga woodlands. Plant Cell Environ. 1996;19(10):1149–1159. [Google Scholar]
- 30.Serraj R, Sinclair TR, Purcell LT. Symbiotic N2 fixation response to drought. J Exp Bot. 1999;50(331):143–155. [Google Scholar]
- 31.Valentine AJ, Benedito VA, Kang Y. Abiotic stress in legume N2 fixation: From physiology to genomics and beyond. In: Foyer C, Zhao M, editors. Annual Plant Reviews Volume 42: Nitrogen Metabolism in Plants in the Post-Genomic Era. Wiley-Blackwell; Oxford: 2010. pp. 207–248. [Google Scholar]
- 32.Bohnert HJ, Nelson DE, Jensen RG. Adaptations to environmental stresses. Plant Cell. 1995;7(7):1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wink M. Evolution of secondary metabolites in legumes (Fabaceae) S Afr J Bot. 2013;89:164–175. [Google Scholar]
- 34.Kursar TA, et al. The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proc Natl Acad Sci USA. 2009;106(43):18073–18078. doi: 10.1073/pnas.0904786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavin M, Herendeen PS, Wojciechowski MF. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Syst Biol. 2005;54(4):575–594. doi: 10.1080/10635150590947131. [DOI] [PubMed] [Google Scholar]
- 36.Werner GDA, Cornwell WK, Sprent JI, Kattge J, Kiers ET. A single evolutionary innovation drives the deep evolution of symbiotic N2-fixation in angiosperms. Nat Commun. 2014;5:4087. doi: 10.1038/ncomms5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vitousek PM, Menge DNL, Reed SC, Cleveland CC. Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philos Trans R Soc Lond B Biol Sci. 2013;368(1621):20130119. doi: 10.1098/rstb.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powers JS, Tiffin P. Plant functional type classifications in tropical dry forests in Costa Rica: Leaf habit versus taxonomic approaches. Funct Ecol. 2010;24(4):927–936. [Google Scholar]
- 39.Wurzburger N, Miniat CF. Drought enhances symbiotic dinitrogen fixation and competitive ability of a temperate forest tree. Oecologia. 2014;174(4):1117–1126. doi: 10.1007/s00442-013-2851-0. [DOI] [PubMed] [Google Scholar]
- 40.McKey D. Legumes and nitrogen: The evolutionary ecology of a nitrogen-demanding lifestyle. In: Sprent JJ, McKey D, editors. Advances in Legume Systematics, Vol 5: The Nitrogen Factor. Royal Botanic Gardens; Burlington, ON, Canada: 1994. pp. 211–228. [Google Scholar]