Significance
The two-pore-domain K+ (K2P) family of potassium channels provides basal inhibitory tone to regulate resting membrane potential and excitability of neurons. Although physiological and biochemical studies have indicated that distinct members of the family can heterodimerize, the tendency to do so, rather than homodimerize, and the functional effects of heterodimerization are minimally understood. We find that Twik-related K+ channel 1 (TREK1) dimerizes as readily with TREK2 as with itself and also with Twik-related arachidonic-acid stimulated K+ channel (TRAAK), although less efficiently. The heterodimers combine functional properties of their constituents in terms of three key modes of regulation: by external pH, internal pH, and phospholipase D2. The unique regulatory properties of TREK1-TREK2 and TREK1-TRAAK heterodimers provide insight into the extracellular and intracellular gating mechanisms of K2P channels.
Keywords: potassium channels, single-molecule fluorescence, leak current, combinatorial diversity, heteromerization
Abstract
Twik-related K+ channel 1 (TREK1), TREK2, and Twik-related arachidonic-acid stimulated K+ channel (TRAAK) form the TREK subfamily of two-pore-domain K+ (K2P) channels. Despite sharing up to 78% sequence homology and overlapping expression profiles in the nervous system, these channels show major differences in their regulation by physiological stimuli. For instance, TREK1 is inhibited by external acidification, whereas TREK2 is activated. Here, we investigated the ability of the members of the TREK subfamily to assemble to form functional heteromeric channels with novel properties. Using single-molecule pull-down (SiMPull) from HEK cell lysate and subunit counting in the plasma membrane of living cells, we show that TREK1, TREK2, and TRAAK readily coassemble. TREK1 and TREK2 can each heterodimerize with TRAAK, but do so less efficiently than with each other. We functionally characterized the heterodimers and found that all combinations form outwardly rectifying potassium-selective channels but with variable voltage sensitivity and pH regulation. TREK1-TREK2 heterodimers show low levels of activity at physiological external pH but, unlike their corresponding homodimers, are activated by both acidic and alkaline conditions. Modeling based on recent crystal structures, along with mutational analysis, suggests that each subunit within a TREK1-TREK2 channel is regulated independently via titratable His. Finally, TREK1/TRAAK heterodimers differ in function from TRAAK homodimers in two critical ways: they are activated by both intracellular acidification and alkalinization and are regulated by the enzyme phospholipase D2. Thus, heterodimerization provides a means for diversifying functionality through an expansion of the channel types within the K2P channels.
Twik-related K+ channel 1 (TREK1), TREK2, and Twik-related arachidonic-acid stimulated K+ channel (TRAAK) are two-pore-domain K+ (K2P) ion channels that belong to the TREK channel subfamily and assemble as dimers to produce an inhibitory, outwardly rectifying potassium current. They are not very active under basal conditions but can be dynamically stimulated by a wide range of stimuli, including mechanical stretch (1), heat (2, 3), phospholipids (4–6), and polyunsaturated fatty acids (1, 7). pH is an especially prominent regulator of these channels. Intracellular acidification stimulates both TREK1 and TREK2 (8, 9), whereas TRAAK is stimulated by intracellular alkalinization (10). In contrast, extracellular acidification is able to inhibit TREK1 and TRAAK, but activates TREK2 (11). Members of the TREK channel subfamily are also regulated extensively by intracellular scaffolding and signaling proteins (10). We recently characterized the regulation of these channels by the enzyme phospholipase D2 (PLD2) and found that PLD2 associates with TREK1 and TREK2, but not TRAAK, which localizes these channels to a microdomain rich in phosphatidic acid (PA) that tonically activates the channels (5).
TREK1 and TREK2 share ∼65% sequence identity, whereas TREK1 and TRAAK share ∼40% (12–14). All three subunits are highly expressed throughout the nervous system (12, 14), where they display distinct but overlapping distributions (15). Due to their ubiquitous expression throughout the brain and based on gene KO studies, these channels are thought to play crucial roles in neuroprotection and anesthesia (16), depression (17), and pain perception (10).
Heteromultimerization is a mechanism commonly used to increase the functional diversity of protein complexes. Heteromerization within the K2P channel family was first described in 2002, with the identification of the TASK1–TASK3 complex (18). In this case, the existence of specific sensitivity to ruthenium red allowed the authors to prove the existence of this heteromer functionally. Recently, it has been found that THIK2 requires heteromerization with THIK1 to be active (19). TREK1 has been reported to heteromerize with TWIK-1 in astrocytes (20), but in another study, TREK1 was shown not to heteromerize with TWIK-1 (21). Physiologically, heteromerization of K2P channels can be extremely functionally relevant in neurons. For example, the TASK1-TASK3 heteromer has been shown to contribute to the standing, outward potassium current in cerebellar granule cells (22), which plays a crucial role in apoptosis (20). Despite the increasing interest in heteromerization of K2P channels, it is unknown whether members of the TREK channel subfamily are able to complex with each other and what the functional properties are of these potential heteromers.
Here, we show, using single-molecule fluorescence techniques, that TREK1, TREK2, and TRAAK heterodimerize and that the heterodimers reach the plasma membrane. TREK1-TREK2 heterodimers are functional and produce a current with intermediate amplitude and increased outward rectification compared with TREK1 and TREK2 homodimers. Most interestingly, external pH regulation of the TREK1-TREK2 heterodimer is different from TREK1 and TREK2 homodimers. This heterodimer shows minimal activity at pH 7.4 but is activated by both acidic and alkaline conditions. Mutational and structural analysis of the heterodimer indicate that each subunit has a His pH sensor that operates through intrasubunit electrostatic interactions. We also characterized the TREK1-TRAAK heterodimer and found an intermediate current amplitude and regulation by external pH. Surprisingly, TREK1-TRAAK showed biphasic activation in response to either intracellular acidification or alkalinization. Finally, we addressed the ability of the intracellular partner protein PLD2 to regulate the TREK1-TRAAK heterodimer and found that only one TREK1 subunit is sufficient to permit current potentiation.
Results
Single-Molecule Analysis of Coassembly of TREK1, TREK2, and TRAAK Channels in Mammalian Cells.
To determine the ability of TREK channels to heteromerize, we used the recently developed single-molecule pull-down (SiMPull) assay (23). SiMPull enables direct visualization of Ab-immobilized individual protein complexes on PEG-passivated coverslips to determine the composition and stoichiometry within individual protein complexes by observing fluorophore bleaching steps.
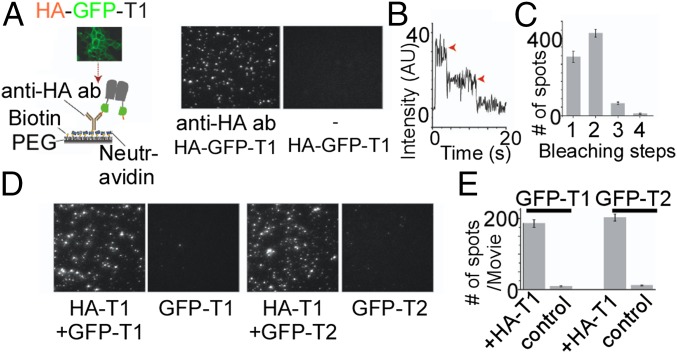
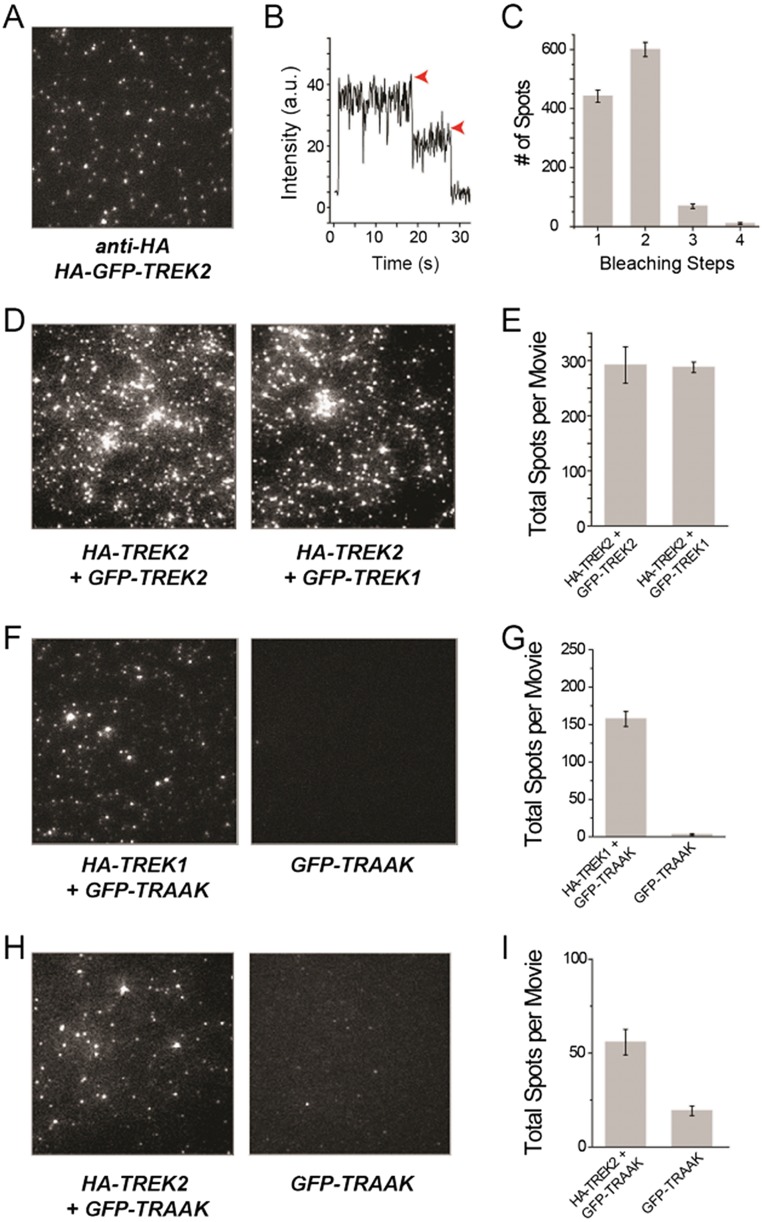
We first confirmed that TREK channels may be immobilized as dimers in SiMPull by expressing a construct containing an N-terminal HA tag followed by a GFP and then TREK1 (HA-GFP-TREK1) in HEK 293T cells. After 24 to 48 h of expression, cells were lysed and single HA-GFP-TREK1 complexes were pulled down using an anti-HA Ab and imaged with total internal reflection fluorescence (TIRF) microscopy (Fig. 1A). In the absence of the HA Ab, there were no fluorescent spots, confirming the specificity of the Ab and the passivation of the coverslip (Fig. 1A). The majority of fluorescence intensity trajectories (Fig. 1B) showed two-step bleaching (∼53%), with the remaining spots bleaching in one step (∼38%) or, occasionally, three or more steps (<10%) (Fig. 1C). This distribution agrees with the binomial distribution for a strict dimer based on an estimated GFP maturation probability of ∼75% (24). SiMPull experiments with HA-GFP-TREK2 confirmed that these channels also form strict dimers in mammalian cells (Fig. S1 A–C).
Fig. 1.
SiMPull of TREK1 from HEK 293T cells reveals TREK1 homodimers and TREK1-TREK2 heteromers. (A, Left) Schematic of SiMPull of TREK1. HEK 293T cells expressing HA-GFP-TREK1 (HA-GFP-T1) are lysed and then immobilized on a passivated coverslip conjugated to a biotinylated anti-HA Ab. (A, Right) TIRF images of single molecules showing that HA-GFP-TREK1 immobilization is dependent on the anti-HA Ab. (B) Representative trace showing two-step photobleaching (red arrows) of HA-GFP-TREK1. AU, arbitrary units. (C) Summary of photobleaching step distribution for HA-GFP-TREK1. (D) Representative images showing that HA-TREK1 (HA-T1) can pull down GFP-TREK1 (GFP-T1) or GFP-TREK2 (GFP-T2) with comparable efficiency. Controls without HA-TREK1 confirm specificity. (E) Summary of HA-TREK1 pull-down of GFP-TREK1 and GFP-TREK2.
Fig. S1.
SiMPull from HEK 293T cells reveals TREK2 homodimers and TREK1/TRAAK and TREK2/TRAAK heteromers. (A) TIRF images of immobilized single molecules of HA-GFP-TREK2. (B) Representative trace showing two-step photobleaching (red arrows) of TREK2. a.u., arbitrary unit. (C) Summary of photobleaching step distribution for HA-GFP-TREK2. (D) Representative images showing that HA-TREK2 is able to pull down GFP-TREK1 or GFP-TREK2 with comparable efficiency. (E) Summary of HA-TREK2 pull-down of GFP-TREK1 and GFP-TREK2. Representative images and summary graphs showing that HA-TREK1 (F and G) or HA-TREK2 (H and I) is able to pull down GFP-TRAAK above background levels, as measured by GFP-TRAAK alone.
We next tested the ability of TREK1 to coassemble with its most closely related homolog, TREK2. We coexpressed HA-TREK1 with GFP-TREK1 or GFP-TREK2 and assessed the ability of HA-TREK1 to coimmunoprecipate the other channels via the anti-HA Ab. In both cases, HA-TREK1 was able to pull down many fluorescent spots via interactions with the GFP-tagged constructs (Fig. 1D). Importantly, when HA-TREK1 was not coexpressed, neither of the GFP-tagged channels was isolated (Fig. 1 D and E). HA-TREK1 pulled down both GFP-TREK1 and GFP-TREK2 with comparable efficiency (Fig. 1E), indicating that TREK1 can coassemble with TREK2 without any clear preference for itself. We also found that HA-TREK2 was able to pull down GFP-TREK2 or GFP-TREK1 with comparable efficiency (Fig. S1 D and E).
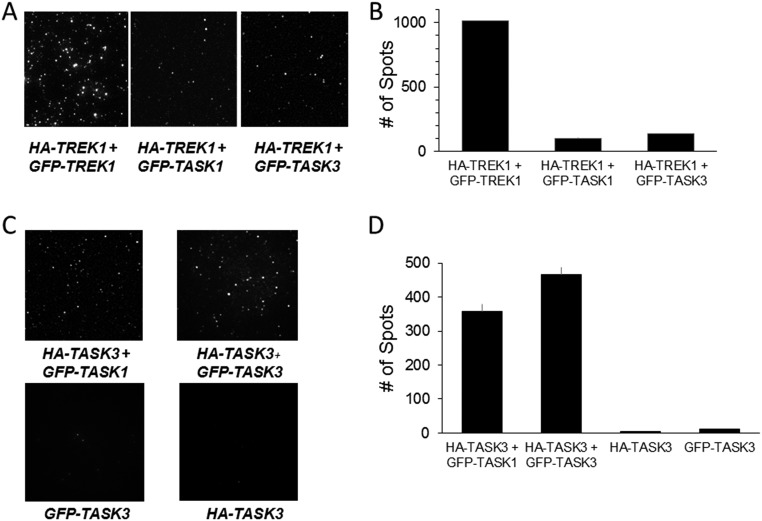
We also extended this study to TRAAK. Strikingly, HA-TREK1 or HA-TREK2 was able to pull down GFP-TRAAK despite only ∼40% sequence identity (Fig. S1 F and G). The extent of pull-down of GFP-TRAAK with HA-TREK1 or HA-TREK2 showed weaker efficiency compared with GFP-TREK1 (Fig. S1 F–I). We next wondered if TREK1 can coassemble with more distant K2P channels from another subfamily, such as TASK1 or TASK3, which share ∼30% sequence identity with TREK1. The number of detected spots for both GFP-TASK1 and GFP-TASK3 was ∼10-fold less than the number of spots detected for GFP-TREK1 (Fig. S2 A and B). Consistent with previous studies (18, 25), we found that HA-TASK3 pulled down GFP-TASK3 or GFP-TASK1 with similar efficiency (Fig. S2C).
Fig. S2.
SiMPull reveals a lack of heteromerization between TREK1 and TASK1 or TASK3, but clear heteromerization between TASK1 and TASK3. (A) TIRF images of immobilized single molecules of HA-TREK1–mediated pull-down of GFP-TREK1 (Left), GFP-TASK1 (Middle), or GFP-TASK3 (Right). (B) Summary of HA-TREK1 pull-down showing much more efficient pull-down of TREK1 compared with TASK1 or TASK3. Dilution factors describe how much lysate was diluted before application onto a coverslip. (C) Representative images showing that HA-TASK3 is able to pull down GFP-TASK1 or GFP-TASK3 with comparable efficiency. (D) Summary of HA-TASK1 pull-down of GFP-TASK1 and GFP-TASK3 compared with controls with GFP-TASK1 or HA-TASK1 alone.
Because SiMPull was used to isolate complexes at high levels of expression, we wanted to determine whether the same interactions would occur at low protein density exclusively on the plasma membrane.
TREK1 and TREK2 Coassemble and Are Targeted to the Plasma Membrane.
To determine the ability of the identified TREK1-TREK2 heterodimer to form on the plasma membrane of living cells, we used single-molecule subunit counting of membrane-bound proteins in Xenopus oocytes (24). The assay is performed at low levels of expression, minimizing nonspecific interactions.
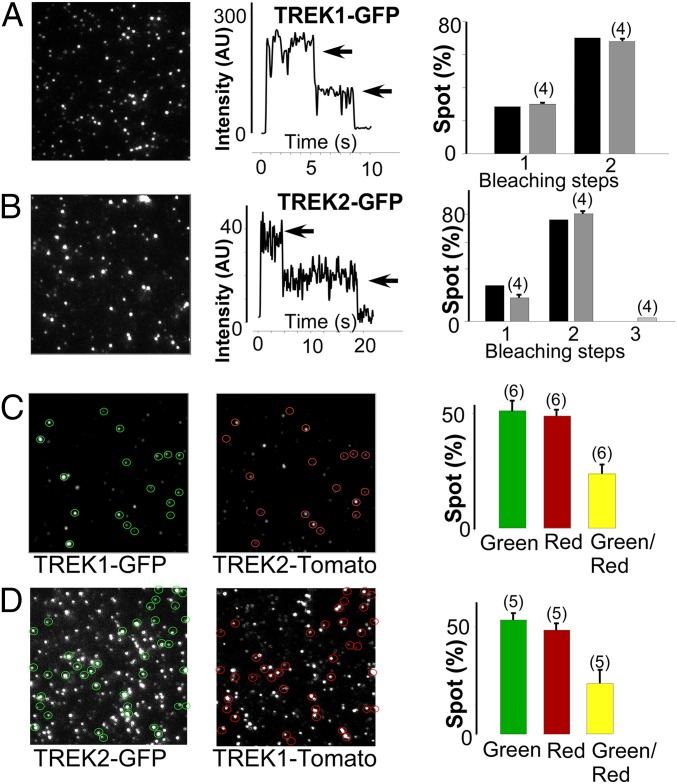
We first validated the technique by expressing either a C-terminally GFP-tagged TREK1 (TREK1-GFP) or TREK2 (TREK2-GFP) alone and counting GFP bleaching steps to confirm the dimerization of TREK1 and TREK2. For both TREK1-GFP and TREK2-GFP, ∼30% of spots bleached in one step, whereas ∼70% bleached in two steps (Fig. 2 A and B). This observation agrees well with the binomial distribution expected for a dimer with GFP maturation of ∼75% and is consistent with results from SiMPull (Fig. 1).
Fig. 2.
TREK1 and TREK2 form heterodimers on the plasma membrane of Xenopus oocytes. Single-molecule subunit counting of TREK1 (A) and TREK2 (B) confirms strict dimerization. (Left) Images showing the first frame of a movie obtained for TREK1-GFP or TREK2-GFP. (Middle) Representative examples showing the time course of fluorescence photobleaching (gray arrows) from a single spot. (Right) Summary of photobleaching step distribution (gray bars) compared with the predicted distribution for a dimer based on 80% GFP maturation (black bars). (C) Representative TIRF images in the GFP (green) and tdTomato (red) channels for oocytes expressing similar levels of TREK1-GFP and TREK2-tdTomato. A bar graph summarizes the total number of red, green, and colocalized spots (yellow). (D) Similar data to C with TREK2-GFP and TREK1-tdTomato. The numbers of cells tested are indicated in parentheses.
We next applied a two-color, colocalization-based version of subunit counting to test for coassembly between TREK1 and TREK2. When both TREK1-GFP and a C-terminally tdTomato-tagged TREK2 (TREK2-tdTomato) subunits were coexpressed, some spots were only green, some were only red, and the rest were colocalized spots containing both green and red fluorescence (Fig. 2C). Although background colocalization rates for noninteracting membrane proteins have been estimated at <5% (24), ∼25% of the total spots were colocalized in this experiment (114 of 476 spots colocalized). Coexpression of TREK2-GFP and TREK1-tdTomato showed a similar spot distribution with considerable colocalization between the two subunits (105 of 348 spots colocalized). These results confirm that when TREK1 and TREK2 are coexpressed at low densities on the plasma membrane, a mix of TREK1 and TREK2 homodimers and TREK1-TREK2 heterodimers exists. We next tested the hypothesis that TREK1-TREK2 heterodimers would have unique functional properties.
Functional Characterization of TREK1-TREK2 and TREK1-TRAAK Heterodimers.
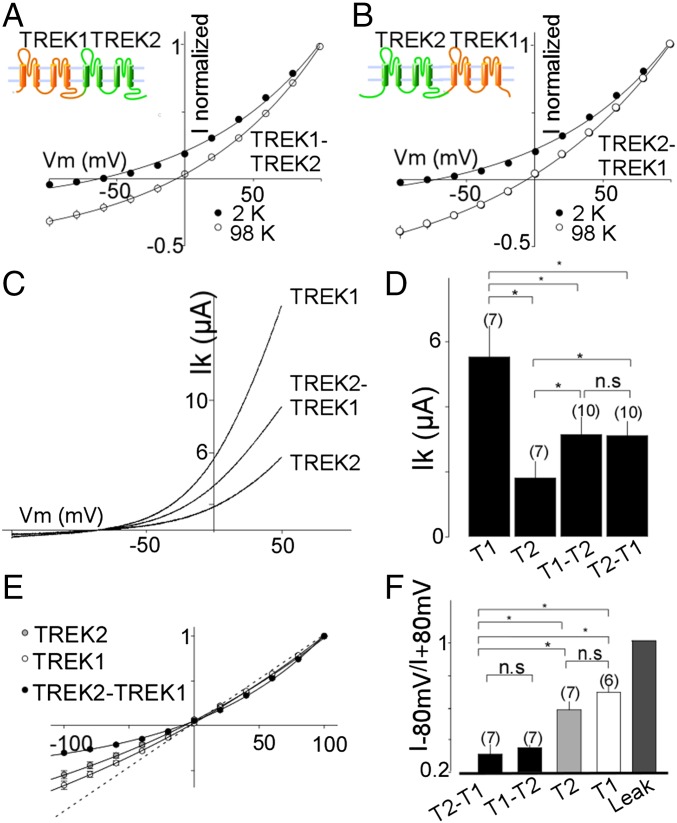
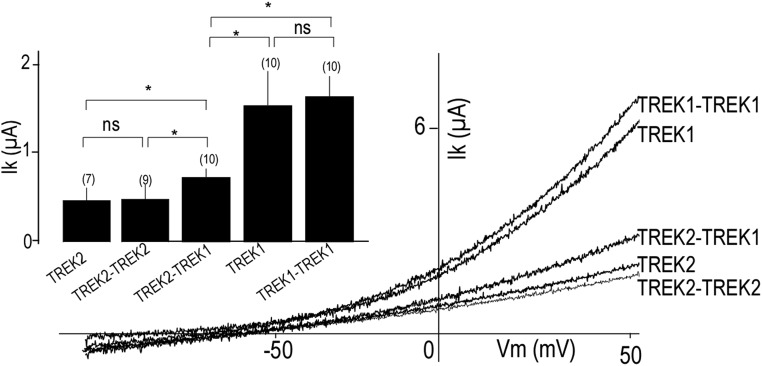
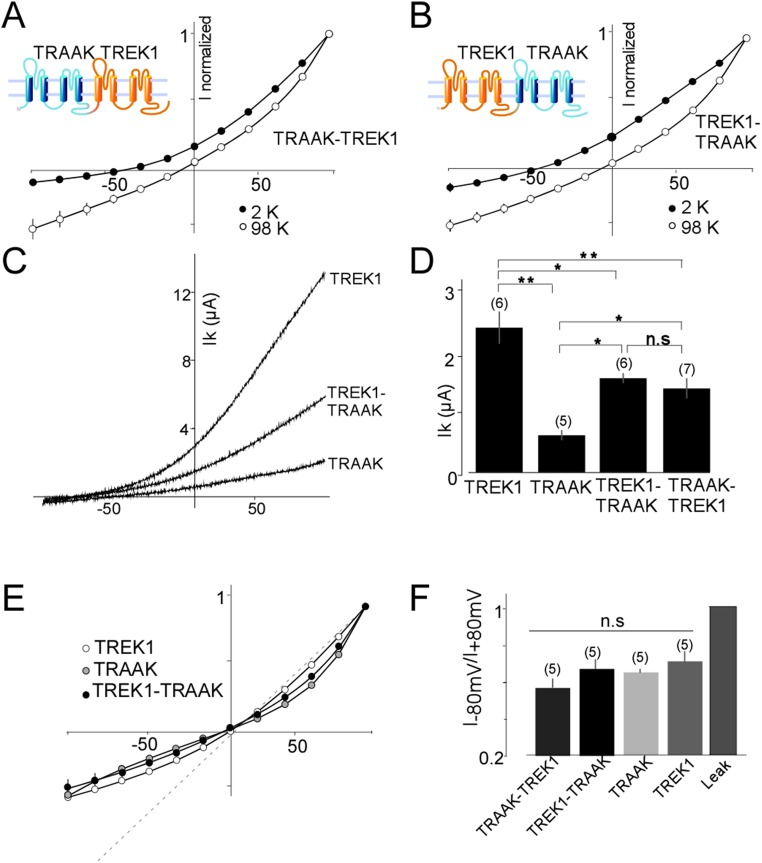
To obtain a homogeneous population of heterodimers, we fused TREK1 and TREK2 subunits to produce either a TREK1-TREK2 or TREK2-TREK1 tandem (Fig. 3 A and B). Both tandems formed functional potassium-selective channels (Fig. 3 A and B) with similar basal current amplitudes (3.13 ± 0.5 μA for TREK1-TREK2 and 3.10 ± 0.4 μA for TREK2-TREK1) that were intermediate between the basal current amplitudes of TREK1 (5.5 ± 1 μA) and TREK2 (1.8 ± 0.5 μA) homodimers (Fig. 3 C and D). TREK1-TREK2 and TREK2-TREK1 had the same channel properties (Fig. 3), indicating that the order of the subunits within the tandem is not important. Importantly, TREK1-TREK1 and TREK2-TREK2 tandem homodimers showed the same amplitude as WT-TREK1 (P > 0.4) or WT-TREK2 (P > 0.2), respectively, indicating that the tandem construct does not alter basal expression or functional properties (Fig. S3). We also fused TRAAK to TREK1 to produce TREK1-TRAAK and TRAAK-TREK1 tandems that also formed potassium-selective channels with current amplitudes (1.5 ± 0.1 μA TREK1-TRAAK and 1.4 ± 0.2 μA TRAAK-TREK1) that were intermediate between the amplitudes observed for homodimers of TRAAK (0.6 ± 0.1 μA) and TREK1 (2.4 ± 0.3 μA) (Fig. S4). We next examined the functional and regulatory properties of the heterodimeric channels.
Fig. 3.
Functional characterization of TREK1-TREK2 heterodimers. Normalized current–voltage (I–V) curves for TREK1-TREK2 (T1-T2) (A) or TREK2-TREK1 (T2-T1) (B) tandem dimers in the presence of two concentrations of external potassium (2 mM and 98 mM). (C) Representative traces showing TREK1 (T1), TREK2 (T2), and TREK2-TREK1 currents elicited by voltage ramps (from −100 to 50 mV, 1-s duration). (D) Summary of average current amplitudes. (E) Normalized I–V curves for TREK1, TREK2, and TREK2-TREK1 obtained in symmetrical potassium conditions (98 mM). (F) Bar graph representing the ratio (absolute values) of mean current recorded at −80 and +80 mV. The numbers of cells tested are indicated in parentheses. Student’s t test (*P < 0.05). Ik, potassium current; n.s., not significant; Vm, membrane potential.
Fig. S3.
TREK1-TREK1 and TREK2-TREK2 tandem homodimers show the same amplitude as WT TREK1 and TREK2, respectively. Representative traces showing TREK1, TREK1-TREK1, TREK2, TREK2-TREK2, and TREK2-TREK1 currents. Currents were elicited by voltage-ramps (from −100 to 50 mV, 1 s in duration). (Inset) Bar graph showing average TREK1, TREK1-TREK1, TREK2, TREK2-TREK2, and TREK2-TREK1. The numbers of cells tested are indicated in parentheses. Student’s t test (*P < 0.05). Ik, potassium current; n.s., not significant; Vm, membrane potential.
Fig. S4.
Basic characterization of TREK1-TASK tandem heterodimers. Normalized current–voltage (I–V) curves for TRAAK-TREK1 (A) or TREK1-TRAAK (B) tandem dimers obtained in the presence of two concentrations of external potassium (2 mM and 98 mM). (C) Representative traces showing TREK1, TRAAK, and TREK1-TRAAK currents. Currents were elicited by voltage ramps (from −100 to 50 mV, 1 s in duration). (D) Bar graph showing average TREK1, TREK2, TREK1-TRAAK, and TRAAK-TREK1 current amplitudes. (E) Normalized I–V curves for TREK1, TRAAK, and TREK1-TRAAK obtained in symmetrical potassium conditions (98 mM). (F) Bar graph representing the ratio (absolute values) of mean current recorded at −80 and +80 mV. The numbers of cells tested are indicated in parentheses. Student’s t test (*P < 0.05; **P < 0.01).
TREK1-TREK2 Heterodimers Show Modified Current Rectification.
In symmetrical K+ conditions, TREK-1 and TREK-2 produce outwardly rectifying currents that can be characterized by the ratio I−80 mV/I+80 mV, which are 0.68 ± 0.05 and 0.58 ± 0.02 for TREK1 and TREK2, respectively. Interestingly, the rectification was stronger for TREK1-TREK2 and TREK2-TREK1 tandem dimers compared with TREK1 and TREK2 homodimers (0.31 ± 0.05 for TREK1-TREK2 and 0.35 ± 0.02 for TREK2-TREK1). In contrast, the TREK1-TRAAK and TRAAK-TREK1 tandem dimers showed the same rectification as TREK1 and TRAAK homodimers (Fig. S4 E and F).
pH Regulation of TREK1-TREK2 and TREK1-TRAAK Heterodimers.
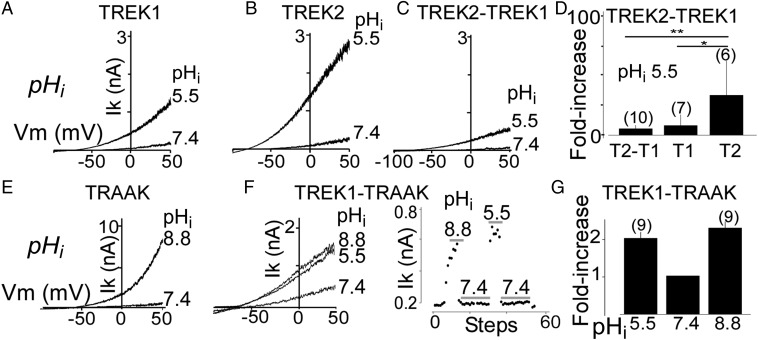
For both TREK1 and TREK2, intracellular acidification increases the potassium current by protonating Glu residues on the post-M4 C-terminal domain (Ctd), which controls channel gating through interaction with the plasma membrane (6, 26). Consistent with previous studies, acidifying the intracellular solution from internal pH (pHi) 7.4 to pHi 5.5 increased TREK2 current (ITREK2) by 27 ± 5-fold, whereas ITREK1 showed only an 8 ± 3-fold increase (P < 0.01) (Fig. 4 A and B). We tested the TREK2-TREK1 under the same conditions and observed a 6 ± 0.8-fold current increase, similar to the increase seen in TREK1 (P > 0.8) but significantly less than what was observed for TREK2 (P < 0.01) (Fig. 4 C and D). Unlike TREK1 and TREK2, TRAAK is activated by intracellular alkalinization (Fig. 4E). Using TREK1-TRAAK tandem dimers, we found that these heterodimers are weakly activated by both increases and decreases in pHi (Fig. 4 F and G).
Fig. 4.
Regulation of TREK family heterodimers by intracellular pH (pHi). Representative traces showing the effect of intracellular acidification on TREK1 (T1) (A), TREK2 (T2) (B), and TREK2-TREK1 tandem heterodimers (T2-T1) (C). (D) Bar graph summarizing the current fold increases induced by a pHi shift from 7.4 to 5.5. Representative traces showing the effect of changes in pHi on TRAAK homodimers (E) and TREK1-TRAAK tandem heterodimers (F). An example of the response to dynamic changes in pHi for TREK1-TRAAK is shown in F. Currents were elicited by voltage ramps (from −100 to 50 mV, 1-s duration, 1 step per 5 s). (G) Bar graph summarizing the increase in current induced by pHi shift from 7.4 to either 5.5 or 8.8 for TREK1-TRAAK. The numbers of cells tested are indicated in parentheses. Student’s t test (*P < 0.05; **P < 0.01).
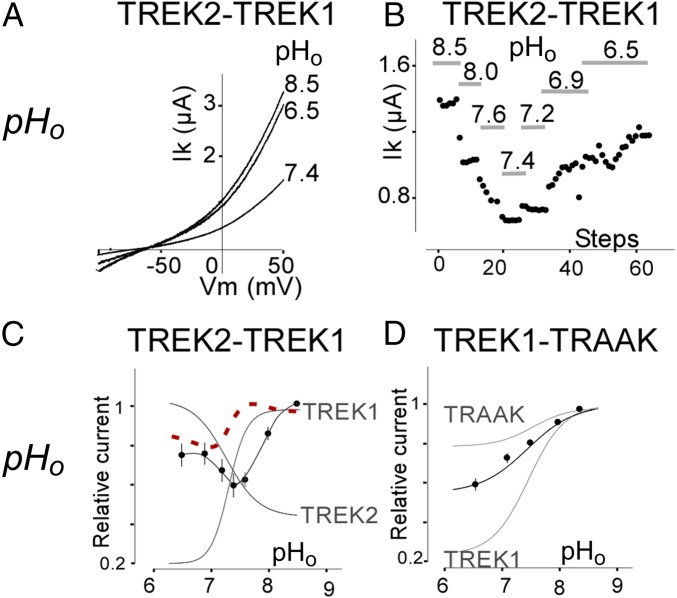
We next investigated the regulation of TREK heterodimers by external pH (pHo). Despite the high degree of sequence identity (∼70%), TREK1 and TREK2 display an opposite regulation by pHo (11): TREK1 is inhibited by acidification and activated by alkalinization, whereas TREK2 is activated by acidification and inhibited by alkalinization. TREK1-TREK2 showed unique regulation by pHo. Acidification from pHo 8.5–7.4 induced a rapid and large decrease in TREK1-TREK2 current amplitude (51 ± 6%) that was similar to TREK1 (71 ± 6%) (Fig. 5A). Further acidification from 7.4 to 6.5 induced a 34 ± 9% increase in TREK1-TREK2 current, similar to what was observed for TREK2 (49 ± 11%) (Fig. 5A). We titrated the pH and found that the heterodimer produces its lowest activity at physiological pH 7.4 and that either acidification or alkalinization leads to an increase in current (Fig. 5 B and C). In contrast to TREK2, TRAAK is inhibited by external acidification, but more weakly than TREK1 (11). As expected, TREK1-TRAAK was inhibited by external acidification but the observed inhibition was intermediate in amplitude between what is seen in TREK1 (highly sensitive) and TRAAK (poorly sensitive) (Fig. 5D).
Fig. 5.
Regulation of TREK family heterodimers by extracellular pH. (A) Representative traces showing the effect of changes in extracellular pH (pHo) on TREK2-TREK1 tandem heterodimers. Currents were elicited by voltage ramps (from −10 to 50 mV, 1-s in duration). (B) Representative example of dynamic regulation of TREK2-TREK1 tandem heterodimers by changes in pHo. (C) pHo dependence of TREK2-TREK1 tandem (black) compared with TREK2 and TREK1 (gray) homodimers. The linear sum of the pHo dependence of the TREK1 and TREK2 homodimers is shown as a dotted red line. (D) pHo dependence of TREK1-TRAAK tandem homodimers (black) compared with TREK1 and TRAAK homodimers (gray).
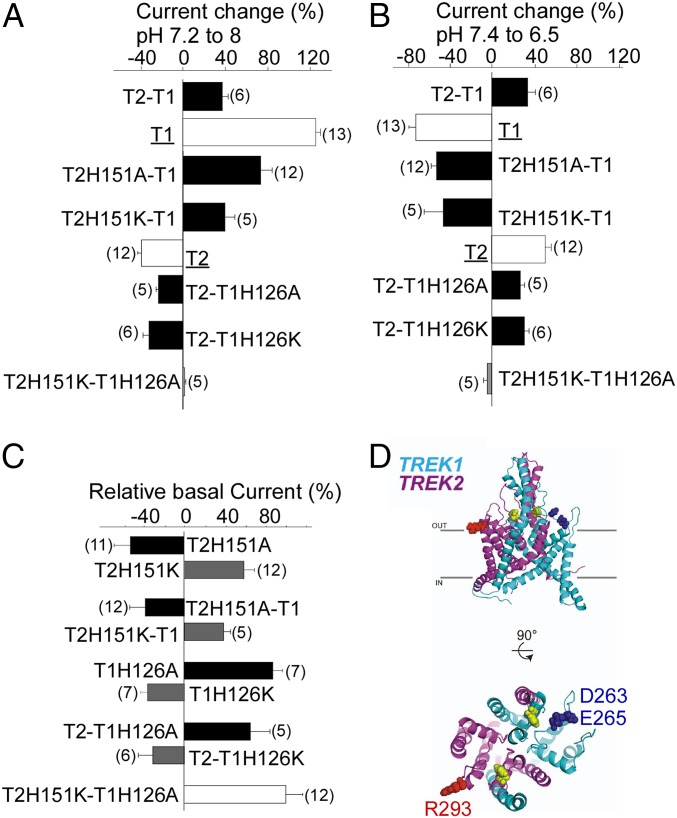
We next probed the atypical regulation of the TREK1-TREK2 heterodimer to determine the mechanism of pH-sensing. TREK1 and TREK2 channels have a homologous pH sensor: a conserved His located in the first extracellular loop [H126 in TREK1 and H151 in TREK2 (11)]. Mutation of both His to either Ala or Lys within the tandem TREK2-TREK1 heterodimer abolished pH sensitivity (Fig. 6 A and B), confirming their roles in pH sensing. Within a heterodimer, mutation of only the pH sensor (H151) of the TREK2 subunit did not fully abolish pH sensitivity but, instead, converted the channel into a TREK1-like “mode”: The tandem heterodimer was potentiated by 68 ± 13% or 40 ± 9% by alkalinization from pH 7.2–8 and inhibited by 53.5 ± 5% and 48 ± 16% by acidification from pH 7.4–6.5 for H151A and H151K, respectively (Fig. 6 A and B). In addition, H151K, which mimics the protonated state, increased the basal current of the heterodimer by 39 ± 7%, whereas H151A, which mimics the deprotonated state, induced a basal current decrease of 38.5 ± 15% (Fig. 6C). This measurement is in agreement with the effects of the H151K mutation (58 ± 12% increase in basal current) and the H151A mutation (30 ± 14% decrease in basal current) in TREK2 homodimers (Fig. 6C). Together, these data indicate that the His pH sensors within a heterodimer control basal current and the pHo response in a subunit-autonomous manner. In agreement with this interpretation, mutation of the pH sensor of the TREK1 subunit within the TREK1-TREK2 heterodimer (H126A or H126K) resulted in TREK2-like pH-sensing behavior (Fig. 6 A and B). Consistent with what was seen for TREK1 homodimers, heterodimers with TREK1-H126A showed increased basal current by 68 ± 15%, whereas tandem heterodimers with TREK1-H126K showed decreased basal current by 31.8 ± 5% (Fig. 6C).
Fig. 6.
Mechanism of Pho sensitivity of TREK2-TREK1 heterodimers. (A) Bar graph showing the percentage of current at pH 8 relative to pH 7.2 for TREK1 (T1), TREK2 (T2), TREK2-TREK1 (T2-T1), and TREK2-TREK1 (T2-T1) mutants. (B) Bar graph showing the percentage of current at pH 7.4 relative to pH 6.5. (C) Bar graph showing the current amplitude of TREK2 (T2) mutants relative to TREK2, TREK1 (T1) mutants relative to TREK1, and TREK2-TREK1 (T2-T1) mutants relative to TREK2-TREK1. (D) Structural model of TREK1-TREK2 heterodimers showing His pH sensors (yellow). The proposed negatively charged interacting residues of TREK1 (cyan) are shown in blue, and the proposed positively charged interacting residues of TREK2 (magenta) are shown in red.
To visualize how extracellular His within the first loop of TREK channels may control the sensitivity to pHo, we used crystal structures of TREK2 (27) and TREK1 to build a model of the TREK1-TREK2 heterodimer. The model shows that charged residues within the second extracellular loop (negatively charged E265 and D263 in TREK1 and positively charged R293 in TREK2), which were previously identified as potential electrostatic interaction partners (11), are indeed positioned to interact with the His, and that these interactions occur within a subunit (Fig. 6D). This proximity likely explains why pH sensing can operate independently in each subunit.
Regulation of TREK1-TRAAK Heterodimers by PLD2.
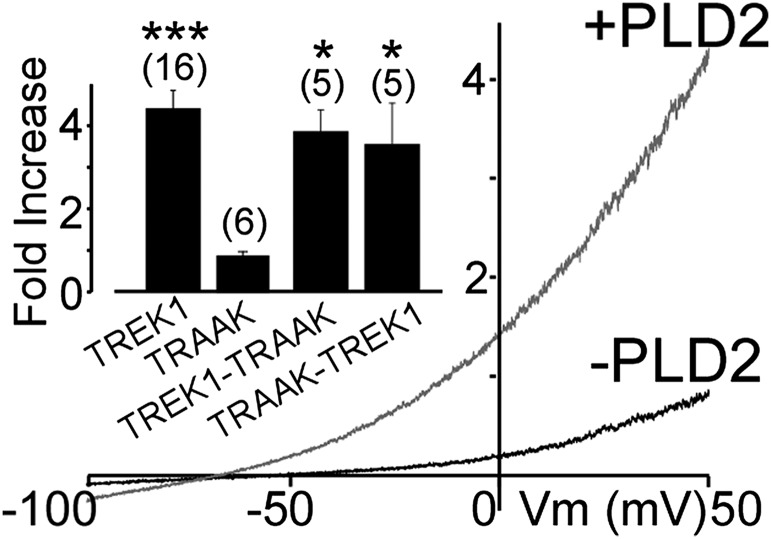
We recently described how, in both TREK1 and TREK2, interaction of the intracellular Ctd with the PA-producing enzyme PLD2 results in PA-dependent tonic activation (5). Despite its sensitivity to PA, TRAAK is not regulated by PLD2 because it does not form a channel–enzyme complex. We asked if the TREK1-TRAAK heterodimer, which presumably contains only one PLD2 binding site in the Ctd of the TREK1 subunit, can be regulated by PLD2 coexpression. TRAAK-TREK1 and TREK1-TRAAK tandems were potentiated by coexpression of PLD2 to a similar level as seen for TREK1 (Fig. 7), suggesting that only one Ctd is sufficient to bind PLD2 and produce enough PA for tonic activation.
Fig. 7.
Potentiation of TREK1-TRAAK heterodimers by PLD2. Representative traces showing effects of PLD2 coexpression on TREK1-TRAAK. (Inset) Summary of current potentiation by coexpression of PLD2. The numbers of cells tested are indicated in parentheses. Student’s t test (*P < 0.05; ***P < 0.001).
Discussion
In this study, we analyzed the ability of members of the TREK channel subfamily of K2P channels to coassemble and found that TREK1, TREK2, and TRAAK are able to heteromerize: TREK1 coassembles with TREK2 as efficiently as it does with itself and also assembles with TRAAK, although less efficiently. In contrast, TREK1 coassembles poorly with members of other K2P subfamilies, such as TASK channels. The overlapping expression patterns in the mouse brain (7, 28) suggest that heterodimers may form in native tissue. We found that the functional properties of heterodimers are distinct from the functional properties of the homodimers, thereby providing an added mechanism for functional diversity.
Extracellular acidification has previously been shown to inhibit homodimers of TREK1 and activate homodimers of TREK2 (11). We found that TREK1-TREK2 heterodimers display a unique blend of pH sensitivity, with activation by both extracellular acidification and alkalinization, which results in an unusual property of minimal activity at physiological pHo. Mutations in the pH sensor of the TREK1 subunit of the heterodimer converted the TREK1-TREK2 heterodimeric channel into TREK2-like pH-sensing, whereas mutations in the pH sensor of the TREK2 subunit converted the heterodimeric channel into TREK1-like pH-sensing. Together, these findings reveal that the pH sensors operate individually within each subunit.
Interestingly, extracellular acidification or mutation of the pH sensor His in the first extracellular loop to Lys boosts current in TREK1 and inhibits current in TREK2, whereas mutation to Ala has the opposite effects (11). Moreover, the opposite effect in TREK1 and TREK2 can be accounted for by the opposite charges of the interacting charged residues in the second extracellular loop: two acidic residues in TREK1 vs. a basic residue in TREK2. These characteristics are consistent with the model that repulsion between the external loops stabilizes the open state of the channel, whereas attraction between the external loops stabilizes the closed state of the channel (11). How could such interactions between the first and second external loops be communicated to the internal gate of the channel? An answer is suggested by the location of the charged residues in the second external loop just “above” the fourth transmembrane helix, which has been shown to serve as an intracellular gate in K2P channels (29–31), implying an activation rearrangement that propagates through the helix to the intracellular gate.
It is interesting to consider how the proposed pH sensor to gate linkages in the two subunits combines their influences on the gate. Given the opposite effect of titration of the sensor His in TREK1 and TREK2, one would have expected that the effect of pH would have been blunted in the TREK1-TREK2 heterodimer and perhaps that the effect on one of the subunits (e.g., TREK1, which is potentiated at alkaline pH by more than TREK2 is inhibited) would have dominated, leaving a reduced and monotonic effect of pH in the TREK1-TREK2 heterodimer. However, what we observed instead was that the TREK1-TREK2 heterodimer was activated at both low and high pH. This nonlinear influence on gating suggests a more complex process that may involve intersubunit interactions.
TREK subfamily channels are also modulated by pHi. Whereas TREK1 and TREK2 homodimers are activated by intracellular acidification (32), TRAAK is activated by intracellular alkalinization (27). We find that TREK1-TRAAK heterodimers are activated by both increases and decreases in pHi. This regulation indicates that, as seen with the extracellular His pHo sensors, the C termini of TREK and TRAAK individually sense pH and influence gating.
The unique sensitivity of TREK1-TREK2 and TREK1-TRAAK heterodimers to changes in extracellular or intracellular pH, respectively, may have important physiological consequences. Deviation from resting pH is associated with heavy activity or metabolic load, as well as with pathological states. Neurons coexpressing TREK1 and TREK2 or TREK1 and TRAAK may form heterodimers that are activated by both acidification and alkalinization. This coassembly may endow TREK channels with a neuroprotective function, whereby pH deviation from physiological values increases the hyperpolarizing outward potassium current to prevent potentially damaging excitation from spreading throughout the network.
In contrast to the complex subunit regulation by pH, we found that another class of regulation, by PLD2, operated in a simple manner. PLD2 has been shown recently to regulate TREK1 and TREK2, but not TRAAK, with specificity defined by the ability of PLD2 to bind to the C termini of TREK1 and TREK2, but not TRAAK (5). We find that TREK1-TRAAK heterodimers are regulated by PLD2 with a similar potency to TREK1, indicating that a channel needs only one PLD2-compatible C-terminal domain to bind PLD2. Thus, whereas the two C termini respond individually to changes in pHi and the two pHo sensors respond individually to changes in external pH, a single PLD2 binding site in one C-terminal domain is sufficient to anchor the enzyme, which then can create a PA-rich domain.
Methods
Standard molecular biological, biochemical, and electrophysiological techniques were used as described previously (5) and (SI Methods). Electrophysiology was performed 24–72 h after transfection for HEK 293T cells and 24–48 h after injection for Xenopus oocytes. For SiMPull experiments, coverslips were prepared as described (23). Single molecules were imaged using a 488-nm argon laser on a TIRF microscope with a 60× objective (Olympus). For subunit counting with Xenopus laevis oocytes, oocytes were enzymatically treated with hyaluronidase (1 mg/mL; Sigma) and neuraminidase (1 unit/mL; Sigma) for 15 min at 4 °C and manually devitellinized to enable close contact of the oocyte’s plasma membrane to the coverslip.
SI Methods
Molecular Biology, Cell Culture, and Gene Expression.
All channel DNA was used in the pIRES2eGFP and pEXO vectors. Capped cRNAs were synthesized in vitro from the linearized plasmid by using T7 RNA polymerase (Ambion). Tandems between TREK1 and TREK2 or TREK1 and TRAAK were generated by PCR.
HEK 293 or 293T cells were maintained in DMEM with 5% FBS on poly-l-lysine–coated glass coverslips in 12-well plates. Cells were were transiently cotransfected using Lipofectamine 2000 (Invitrogen) with a total of 1–1.6 μg of DNA total per 18-mm-diameter coverslip.
Defolliculated Xenopus oocytes were injected with cRNA (5 ng) encoding mouse TREK1, TREK2, and TRAAK or TREK1-TREK2, TREK1-TREK2, TRAAK-TREK1, and TREK1-TRAAK tandems. They were used for electrophysiological studies 2–4 d after injection. For single-molecule experiments, the amount of injected cRNA required to produce resolvable single channels on the cell’s surface was determined empirically.
Electrophysiology.
HEK 293 cell electrophysiology was performed 24–72 h after transfection in solution containing 145 mM NaCl, 4 mM KCl, 1 mM MgCl2, 2 mM CaCl2, and 10 mM Hepes. Glass pipettes of resistance between 3 MΩ and 6 MΩ were filled with intracellular solution containing 140 mM KCl, 10 mM Hepes, 5 mM EGTA, and 3 mM MgCl2 (pH 7.4). Cells were patch-clamped using an Axopatch 200A (Molecular Devices) amplifier in the whole-cell mode. Currents were elicited by voltage ramps (from −100 to 50 mV, 1 s in duration), and the current density was calculated at 0 mV. For acidic intracellular (5.5 pHi) solutions, Hepes was substituted with MES, and for basic (8.0 pHi) solution, Hepes was substituted with Tris.
Oocyte two-electrode voltage clamp electrophysiology was performed in a 0.3-mL perfusion chamber; a single oocyte was impaled with two standard microelectrodes (1–2.5 MΩ resistance) filled with 3 M KCl, and maintained under voltage clamp using a Dagan TEV 200 amplifier in standard ND96 solution [96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 2 mM MgCl2, 5 mM Hepes (pH 7.4 with NaOH]. For the high K solution, the NaCl was replaced by KCl. Stimulation of the preparation, data acquisition, and analysis were performed using pClamp software (Molecular Devices). Changes in extracellular pH were induced by a microperfusion system that allowed local and rapid changes of solutions. Hepes was replaced by MES (5 mM) for buffer solutions with a pH between 6.5 and 5.0.
SiMPull.
For SiMPull experiments, HEK 239T cells were harvested from coverslips by incubating with Ca2+-free PBS buffer for 20–30 min, followed by gentle pipetting. Cells were pelleted and lysed in buffer containing 150 mM NaCl, 1 mM EDTA, protease inhibitor mixture (Thermo Scientific), and 1.5% (vol/vol) IGEPAL (Sigma). After 30–60 min incubation at 4 °C, lysate was centrifuged for 20 min at 16,000 × g and the supernatant was collected. Coverslips passivated with PEG (∼99%)/biotin-PEG (∼1%) and treated with Neutravidin (Thermo Scientific) were prepared as described (23). Biotinylated anti-HA Ab (15 nM, no. ab26228; Abcam) was applied for 20 min and then washed out. Ab dilutions and washes were done in T50 buffer containing 50 mM NaCl and 10 mM Tris (pH 7.5). Lysate, diluted in standard patch-clamp electrophysiology extracellular recording solutions (SI Methods, Electrophysiology) was then applied to the chamber and washed away following brief incubation (∼2 min). Single molecules were imaged using a 488-nm argon laser on a TIRF microscope with a 60× objective (Olympus). We recorded the emission light after an additional 3× magnification and passage through a double-dichroic mirror and an emission filter (525/50 for GFP) with a back-illuminated EM CCD camera (iXon DV-897 BV; Andor). Movies of 500–800 frames were acquired at frame rates of 10–30 Hz. The imaged area was 13 × 13 μm2. At least five movies were recorded for each condition, and data were analyzed using custom software as previously described (24). Multiple independent experiments were performed for each condition. Representative datasets are presented to compare conditions tested on the same day quantitatively. For photobleaching experiments, error bars were calculated using counting statistics as described by Reiner et al. (33).
Subunit Counting with X. laevis Oocytes.
Twenty-four hours after RNA injection and expression at 18 °C, X. laevis oocytes were enyzmatically treated with hyaluronidase (1 mg/mL; Sigma) and neuraminidase (1 unit/mL; Sigma) for 15 min at 4 °C and manually devitellinized to enable close contact of the oocyte’s plasma membrane to the coverslip. The coverslips’ refractive index (n = 1.78) matched the refractive index of the microscope objective front lens (100/NA1.65; Olympus) and the immersion oil (Cargille). GFP was excited with a 488-nm argon laser, and ttTomato was excited with a 532-nm diode-pumped solid-state (DPSS) laser. We recorded the emission light after an additional 3× magnification and passage through a double-dichroic mirror and an emission filter (525/50 for GFP, 592.5/50 for ttTomato) with a back-illuminated EM CCD camera (iXon DV-897 BV). Movies of 500 frames were acquired at frame rates of 10–30 Hz. The imaged area was 13 × 13 μm2. For two-color experiments, fluorescence from ttTomato was measured first, and after almost complete bleaching of ttTomato to prevent Förster/fluorescence resonance energy transfer from green to red chromophores, GFP fluorescence was measured. Occasionally, a few ttTomato molecules did not bleach completely, but even unbleached ttTomato did not affect fluorescence from colocalized GFP significantly. Laser powers were between 0.5 mW and 2 mW, and the diameter of the beam at the sample was 20 μm. Switching the illumination on and off and changing emission filters were done using electromechanical shutters and a motorized filter wheel. The typical time for switching off the 532-nm illumination, changing emission filters, and switching on the 488-nm illumination was 1–2 s.
Structural Modeling.
The model of the TREK1-TREK2 heterodimer was constructed based on the crystal structures of TREK1 [Protein Data Bank (PDB) ID code 4TWK] and TREK2 (PDB ID code 4BW5) (30). The program Modeler (34) was used to fill in the unresolved regions of each structure separately. The resultant full-length model of TREK2 chain B was then aligned to chain B of the TREK1 dimer structure using Visual Molecular Dynamics (VMD) (35). Finally, the full-length model of TREK1 chain A, the aligned full-length model of TREK2 chain B, and the crystallographic waters and potassium ions from the TREK1 structure were written to a new PDB file with a patch applied to form the disulfide bridge between Cys69 in TREK1 and Cys58 in TREK2 using a custom tcl script run in VMD. Figures were then generated using PyMOL.
Acknowledgments
We thank S. Bharill, M. Ulbrich, and O. Soriani for technical assistance and helpful discussion. The work was supported by a grant (to G.S.) by the Action Thématique et Incitative sur Programme-AVENIR (ATIP-AVENIR) funds and the French National Center for Scientific Research, as well as by grants (to G.S.) from the Fondation NRJ–Institut de France, Agence Nationale de la Recherche (ANR) (Laboratory of Excellence “Ion Channel Science and Therapeutics,” Grant ANR-11-LABX-0015-01), and ANR Dynaselect (Grant ANR-14-CE13-0010); by grants (to E.Y.I.) from the National Science Foundation (Grant IOS-1451027) and NIH (Grant R01NS35549); and by a Chateaubriand fellowship (to J.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522459113/-/DCSupplemental.
References
- 1.Patel AJ, et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17(15):4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maingret F, et al. TREK-1 is a heat-activated background K(+) channel. EMBO J. 2000;19(11):2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564(Pt 1):103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Lysophospholipids open the two-pore domain mechano-gated K(+) channels TREK-1 and TRAAK. J Biol Chem. 2000;275(14):10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 5.Comoglio Y, et al. Phospholipase D2 specifically regulates TREK potassium channels via direct interaction and local production of phosphatidic acid. Proc Natl Acad Sci USA. 2014;111(37):13547–13552. doi: 10.1073/pnas.1407160111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandoz G, Bell SC, Isacoff EY. Optical probing of a dynamic membrane interaction that regulates the TREK1 channel. Proc Natl Acad Sci USA. 2011;108(6):2605–2610. doi: 10.1073/pnas.1015788108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink M, et al. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17(12):3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274(38):26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Gnatenco C, Bang H, Kim D. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflugers Arch. 2001;442(6):952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- 10.Noël J, Sandoz G, Lesage F. Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin) 2011;5(5):402–409. doi: 10.4161/chan.5.5.16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci USA. 2009;106(34):14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275(37):28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- 13.Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275(23):17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- 14.Fink M, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15(24):6854–6862. [PMC free article] [PubMed] [Google Scholar]
- 15.Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21(19):7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heurteaux C, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23(13):2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heurteaux C, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9(9):1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- 18.Czirják G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002;277(7):5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- 19.Blin S, et al. Tandem pore domain halothane-inhibited K+ channel subunits THIK1 and THIK2 assemble and form active channels. J Biol Chem. 2014;289(41):28202–28212. doi: 10.1074/jbc.M114.600437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo DH, et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151(1):25–40. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Plant LD, et al. One SUMO is sufficient to silence the dimeric potassium channel K2P1. Proc Natl Acad Sci USA. 2010;107(23):10743–10748. doi: 10.1073/pnas.1004712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauritzen I, et al. K+-dependent cerebellar granule neuron apoptosis. Role of task leak K+ channels. J Biol Chem. 2003;278(34):32068–32076. doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- 23.Jain A, et al. Probing cellular protein complexes using single-molecule pull-down. Nature. 2011;473(7348):484–488. doi: 10.1038/nature10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulbrich MH, Isacoff EY. Subunit counting in membrane-bound proteins. Nat Methods. 2007;4(4):319–321. doi: 10.1038/NMETH1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauritzen I, et al. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6(7):642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honoré E. The neuronal background K2P channels: Focus on TREK1. Nat Rev Neurosci. 2007;8(4):251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Bang H, Gnatenco C, Kim D. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflugers Arch. 2001;442(1):64–72. doi: 10.1007/s004240000496. [DOI] [PubMed] [Google Scholar]
- 28.Sandoz G, et al. Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J Neurosci. 2008;28(34):8545–8552. doi: 10.1523/JNEUROSCI.1962-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brohawn SG, del Mármol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335(6067):436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong YY, et al. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science. 2015;347(6227):1256–1259. doi: 10.1126/science.1261512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagriantsev SN, Peyronnet R, Clark KA, Honoré E, Minor DL., Jr Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 2011;30(17):3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honoré E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K(+) channel TREK-1. EMBO J. 2002;21(12):2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiner A, Arant RJ, Isacoff EY. Assembly stoichiometry of the GluK2/GluK5 kainate receptor complex. Cell Reports. 2012;1(3):234–240. doi: 10.1016/j.celrep.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 35.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graph. 1996;14(1):33–38, 27–28. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]