Abstract
Objectives
This study aimed to clarify whether pretreatment human equilibrative nucleoside transporter (hENT1) expressions in endoscopic ultrasonography-guided fine-needle aspiration biopsy (EUS-FNAB) specimens obtained from resectable, borderline resectable, and locally advanced unresectable pancreatic ductal adenocarcinoma (PDAC) are concordant with those in the resected specimen after gemcitabine-based chemoradiotherapy (Gem-CRT) and to validate the utility of hENT1 expression using EUS-FNAB samples as a prognostic marker.
Methods
We evaluated the relationship between hENT1 expressions assessed by immunohistochemical staining and clinical outcomes in 51 of 76 patients with PDAC who were diagnosed by EUS-FNAB and received preoperative Gem-CRT.
Results
The concordance rate of hENT1 expressions was 89.2% (K = 0.681). Median survival time (month) in the 51 whole patients and 37 patients with resection was significantly longer in hENT1 positive than in hENT1 negative: 25.0 and 30.0 versus 9.0 and 9.0, respectively. A multivariate analysis confirmed that hENT1 expression was an independent prognostic factor in both whole patients and those with resection. Regardless of T3 and T4, hENT1-positive patients with resection had significantly better prognosis than hENT1-negative patients, whose prognosis was similar to those without resection.
Conclusions
The assessment of hENT1 expression using EUS-FNAB samples before Gem-CRT provides important information on patients with PDAC who can benefit from curative-intent resection.
Key Words: EUS-FNAB, hENT1, chemoradiotherapy, gemcitabine, pancreatic ductal adenocarcinoma
Gemcitabine (Gem) therapy has been the standard treatment for pancreatic ductal adenocarcinoma (PDAC) since Burris et al1 reported that Gem offered better overall survival (OS) than fluorouracil. However, its efficacy is limited; only 15% of patients with recurrent and metastatic PDAC2 and up to 30% in general3 can be expected to respond to treatment. Because Gem is strongly hydrophilic, passive diffusion through hydrophobic cellular membranes is slow. Efficient permeation of Gem into cells requires specialized integral membrane transporter proteins to cross plasma membranes.4 Among these transporters, the major mediators of Gem uptake into human cells are the human equilibrative nucleoside transporter 1 (hENT1) and, to a lesser degree, the human concentrative nucleoside transporter 3.5–7
The hENT1 has been reported as an important predictive marker of Gem-based therapy.8 In vitro studies indicated that hENT1 gene expression was positively associated with Gem-chemosensitivity.9 High hENT1 expression in resected specimen was also reported to be associated with increased OS in patients with PDAC who received postoperative Gem-based chemotherapy.8,10–16 These studies indicate that hENT1 expression is important in predicting the survival of patients with PDAC in the adjuvant setting. However, there have been a few reports describing the impact of hENT1 expression on the outcome after preoperative Gem-based chemoradiotherapy (Gem-CRT) in patients with PDAC. Our previous study showed that hENT1 expression was an independent predictor of OS after neoadjuvant Gem-CRT in the patients with Union Internationale Contrele Cancer (UICC) T3 to T4.17 We also reported that positive expression of hENT1 in the resected specimen was the significant prognostic factor especially for the treatment of locally unresectable (LUR) PDAC defined by the National Comprehensive Cancer Network (NCCN) guidelines (2010).18,19
Based on these results, pretreatment/preoperative evaluation of hENT1 expression in PDAC tissue can be beneficial in predicting the efficacy of Gem-based therapy before initial treatment. The specimens obtained by endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB) might be suitable for evaluating hENT1 expression; however, the immunohistochemical (IHC) analysis of hENT1 expression in the pancreatic tumor tissue taken by EUS-FNAB has not been established. There have been several studies that examined gene expression including hENT1 in pretreated tissue biopsy samples obtained by EUS-FNAB in patients with unresectable PDAC.20–22 Based on genetic analysis of EUS-FNAB tissue samples, it is suggested that hENT1 messenger RNA expression levels might be biomarkers for predicting and monitoring Gem sensitivity in patients with unresectable PDAC.22 The examination of total RNA isolated from EUS-FNAB tissue samples without microdissection has a risk of contaminating cells, which could lead to false results. In contrast, IHC analysis using EUS-FNAB samples can examine cancer-specific expression of hENT1. However, there have been no previous reports performing IHC analysis of hENT1 expression in the pretreatment tissue taken by EUS-FNAB and comparing with posttreatment resected specimens of PDAC. One of the reasons why such studies were rare is the difficulty in obtaining sufficient quantity of cancer cells for IHC analysis because the materials aspirated for analysis are often bloody and contain contamination from gastrointestinal tract epithelium.23–26 Recently, Yamao et al23 have revealed that EUS-FNAB with rapid on-site evaluation (ROSE) provides more accurate diagnosis than EUS-FNAB without it because a cytopathologist ensures that the samples taken by EUS-FNAB are adequate for assessment. Because the sampling rate of PDAC tissues in our institute has been high owing to the introduction of ROSE, we could retrospectively evaluate the stored cell block specimens for the IHC analysis of hENT1 expression.
The aims of our study were to clarify whether pretreatment hENT1 expressions in the EUS-FNAB specimens are concordant with those in the resected specimen after Gem-CRT and to validate the utility of hENT1 expression using EUS-FNAB samples as a prognostic marker in patients with locally advanced PDAC who underwent Gem-CRT.
MATERIALS AND METHODS
Between February 2005 and November 2011, we had enrolled 117 patients for our Gem-CRT protocol reported previously,17,18 who were cytologically or histologically diagnosed as having PDAC and having UICC T3 and T4 tumors determined by using 64-slice multidetector computed tomography (MDCT). Computed tomography was performed according to a defined pancreas protocol as 4-phasic contrast-enhanced MDCT with thin slices at intervals of 1 mm. Patients were excluded when they showed evident distant metastatic lesions at the time of enrollment. They all gave their written informed consent for inclusion in the study. These patients were also retrospectively reclassified into the 3 resectability groups: resectable (R), borderline resectable (BR), or LUR, according to the NCCN guidelines (2010).19
Of the 117 patients, 76 were diagnosed with PDAC by cytology and/or histology using EUS-FNAB specimen (Fig. 1). Among 76 cases, 94.7% (n = 72) were diagnosed by cytology, 81.6% (n = 62) were diagnosed by histology, 100% (n = 76) were diagnosed by either of the 2 methods. We retrospectively reviewed the formalin-embedded specimens obtained by EUS-FNAB for these 76 patients, and the adequate amount of histological specimens required for the examination of hENT1 expression could be found in 52 patients (68.4%), all of which could have IHC staining successfully performed. Among these 52 patients, hENT1 positive was found in 34 (65.4%), of whom 29 (85.3%) could receive resection and 5 (14.7%) could not, whereas hENT1 negative was found in 18 (34.6%), of whom 1 was excluded because of refusal of treatment, 8 (47.0%) could receive resection, and 9 (53.0%) could not.
FIGURE 1.
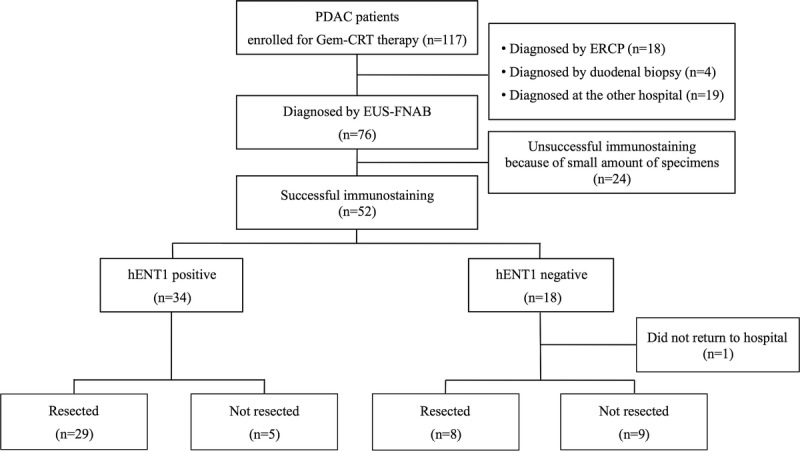
Flow diagram of the study participants. ERCP, endoscopic retrograde cholangiopancreatography.
We evaluated the relation between hENT1 expressions and clinical courses in these 51 patients. The study measured intratumoral hENT1 expression, concordance rates of hENT1 expressions of EUS-FNAB specimens with those of resected tumors, and survival analysis based on hENT1 expression of EUS-FNAB specimen.
EUS-FNAB Procedure
Endoscopic ultrasonography was performed using a linear array endoscope (GF-UCP240; Olympus Medical Systems Co, Ltd, Tokyo, Japan), connected to a processor with a color Doppler function (SSD-α10; Hitachi-Aloka Medical, Ltd, Tokyo, Japan). After the tumor was identified using B-mode imaging, we confirmed the absence of vessels in the target area with the color Doppler mode. After we punctured an aspiration needle into the tumor under ultrasonographic guidance, the stylet was pulled out, and the specimen was aspirated with a 20-mL syringe, and then, the needle moved back and forth several times within the tumor. Negative pressure was released before the needle was removed from the tumor. A cytologist immediately examined the specimen with ROSE using rapid stain (Diff-Quik stain; International Reagents, Kobe, Japan) to verify that sufficient sample was obtained. When a tentative diagnosis of malignancy could be made by the on-site evaluation, we finished the EUS-FNAB procedure. If not, we performed an additional 1 to 2 punctures to obtain the diagnosis. The specimen from each EUS-FNAB pass was fixed in alcohol and then stained using the Papanicolaou multichromatic procedure. The remaining material was fixed in 10% formalin and then embedded in paraffin for the cell block analysis to obtain histological diagnosis (hematoxylin and eosin [H&E]).
Immunohistochemical Analysis and Evaluation of hENT1 Expression
After cytological and/or histological diagnosis of PDAC had been confirmed, we retrospectively evaluated 76 stored cell block specimens for the IHC analysis of hENT1 expression: IHC staining was able to be performed successfully on 52 specimens, whereas the remaining 24 failed. The causes of failure were as follows: blood clot alone in 4, normal pancreatic tissue in 9, and an insufficient quantity of malignant cells in 11. For the hENT1 IHC analysis, we used only cell block samples, neither core biopsy samples nor cytological smear.
The cell blocks were sliced into 2-μm paraffin sections. The 2-μm sections were used for the assessment of intratumoral hENT1 expressions with immunohistochemistry as well as being stained with H&E. Immunostaining procedure was performed using the labeled streptavidin-biotin peroxidase complex method with the Benchmark XT auto-immunostaining system (Ventana Japan, Tokyo, Japan). The antigen retrieval step was performed at 90°C, 30 minutes, and then, the sections were incubated in rabbit-derived anti-hENT1 polyclonal antibody (Medical and Biological Laboratories Co, Ltd, Nagoya, Japan). The sections were labeled with an automated immunostaining system with I-View detection kit. Immunostained sections were lightly counterstained with Mayer's hematoxylin.
The resected specimens were fixed in a formalin solution, sliced into 5-mm sections, and embedded in paraffin blocks. A 3-μm section was obtained from each block and stained with H&E. The sections were routinely examined for pathological differentiation and resection margin status. The histological response of Gem-CRT was evaluated according to histopathological criteria of Evans et al.27 According to the result of H&E staining, the most appropriate one section that contained tumor cells rich enough for immunostaining was stained to assess intratumoral hENT1 expression in the same manner as the EUS-FNAB samples.
Two pathologists (T.S., K.U.) who were blinded to the clinical characteristics of the patients assessed EUS-FNAB samples and resected specimens. Scoring for hENT1 immunostaining was performed on the basis of the relative intensities of staining of the cancer cells, with reference to the normally strong hENT1 staining of cytoplasm within the lymphocytes in the EUS-FNAB samples and of cell membranes within the islets of Langerhans cells in the resected specimen as internal controls, respectively. The degree of hENT1 expression in the resected specimen was determined by the intensity as well as extent of positive staining according to our previous study.17 A revised scoring system expressing the degree of hENT1 expression in the EUS-FNAB samples was devised based on our previous study; the scoring system is represented as follows: a score ranging from 0 to 3 was assigned based on the intensity of staining, where 0 = no staining, 1 = weakly positive, 2 = moderately positive (same intensity as internal control), and 3 = strongly positive. The degree of hENT1 expression was defined as high (neoplastic cells with score 3 accounting for >50% of the total tumor cells), low (neoplastic cells with score 0 or 1 accounting for >50% of the total tumor cells), and intermediate (all other neoplastic cells). We defined high and intermediate staining as hENT1 positive and low staining as hENT1 negative in both EUS-FNAB samples and resected specimens (Fig. 2).
FIGURE 2.
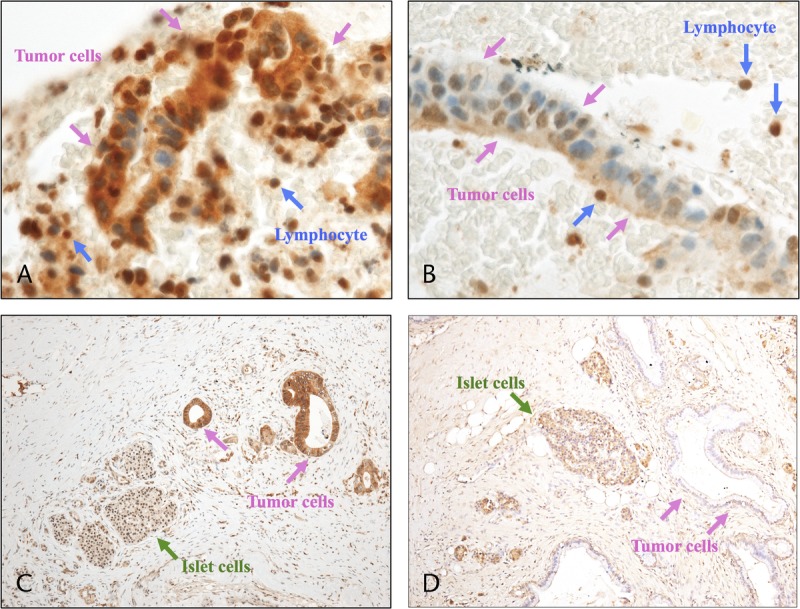
Immunohistochemical staining of PDAC for hENT1. A, EUS-FNAB sample showing high hENT1 expression relative to internal control (lymphocyte), “hENT1 positive.” B, EUS-FNAB sample showing low hENT1 expression, “hENT1 negative.” C, Resected specimen showing high hENT1 expression relative to internal control (islet cells), “hENT1 positive.” D, Resected specimen showing low hENT1 expression, “hENT1 negative.”
Treatment Protocol
The treatment protocol of Gem-CRT was described by our previous reports.17,18 Briefly, the total radiation dose was 45 Gy, delivered in 25 fractions (5 fractions per week), and the patients were administered an infusion of Gem at a dose of 800 mg/m2 on days 1, 8, 22, and 29 for 1 cycle. The patients underwent reassessment at 4 to 6 weeks after the completion of Gem-CRT; when we determined that curative-intent resection was possible, they were scheduled to undergo pancreatectomy. At the time of reassessment, especially in the case of LUR patients, we determined that curative-intent resection was possible when the following findings on MDCT were observed: no stenosis or change of shape in the celiac trunk and superior mesenteric artery as well as the absence of metastatic lesions in other distant organs. Even after we decided that the tumor was inoperable, we continued chemotherapy mainly using Gem. Pancreaticoduodenectomy (PD) or distal pancreatectomy was performed as previously described.17,18 From 6 weeks after resection, we planned to start the postoperative chemotherapy regimen, consisting of Gem at a dose of 800 mg/m2 biweekly for at least 6 months. After pancreatectomy, all patients were evaluated as follows: physical examination every month; laboratory tests including CEA serum levels and carbohydrate antigen (CA) 19-9 (CA19-9) levels every 2 or 3 months; and MDCT every 3 months within 2 years and thereafter every 6 months.17,18
Analysis of Factors Contributing to Survival
We analyzed various clinicopathological factors in the whole patients and those with resection to clarify the significant prognostic factors, including (1) pretreatment factors such as tumor location, tumor size before Gem-CRT, UICC-T classification, resectability according to NCCN guideline 2010, and hENT1 expression of EUS-FNAB samples and (2) posttreatment clinical factors, such as response to Gem-CRT evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST),28 reduction rate in serum CA19-9 level as previously described,18 presence of distant metastasis after Gem-CRT, and hENT1 expression of resected specimen.
Statistical Analyses
The results for continuous variables were expressed as mean or median. For the clinicopathological features of the patients, P values were calculated by χ2 test or Fisher exact test, as appropriate. In the whole patients, the date of the initial treatment was chosen as the starting point for the measurement of survival time. The day of final follow-up was December 31, 2013, and there was no loss of follow-up. Survival time was calculated using the Kaplan-Meier method and was compared between the groups using the Wilcoxon test. The factors affecting survival time were analyzed using the multivariate Cox proportional hazard model. Individual variables with a significance of P < 0.05 in the univariate Cox proportional hazard model were selected for inclusion into the multivariate analysis. In the multivariate analysis, variables with a significance of P < 0.05 were selected. For all statistical tests, a P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 20 software (IBM Inc., Chicago, Ill).
RESULTS
Immunostaining and Patient Background
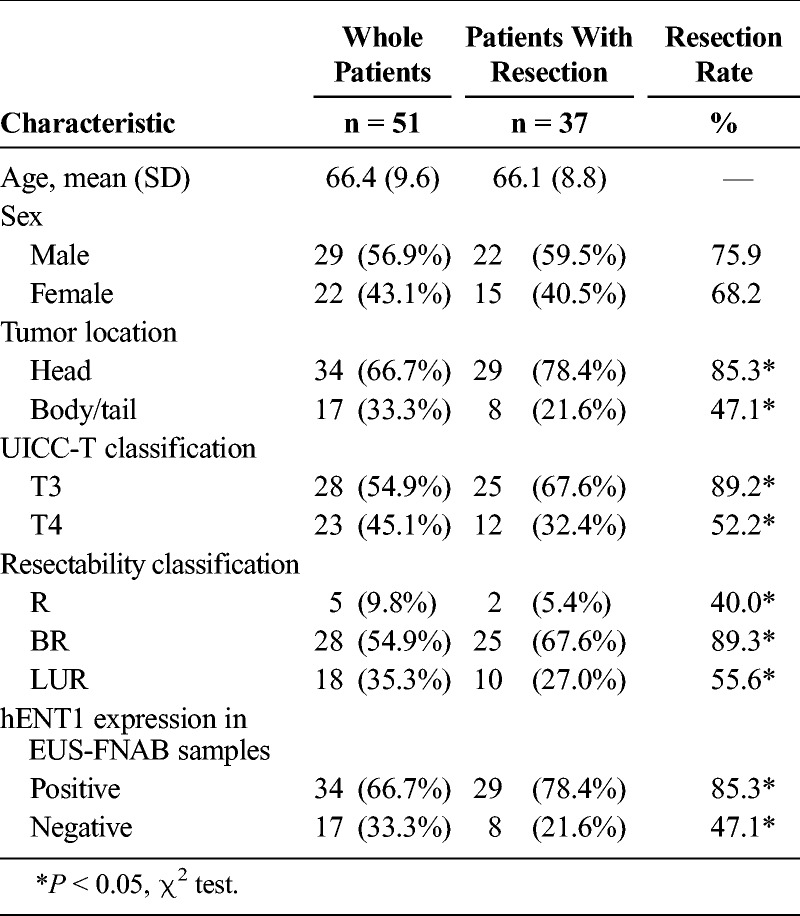
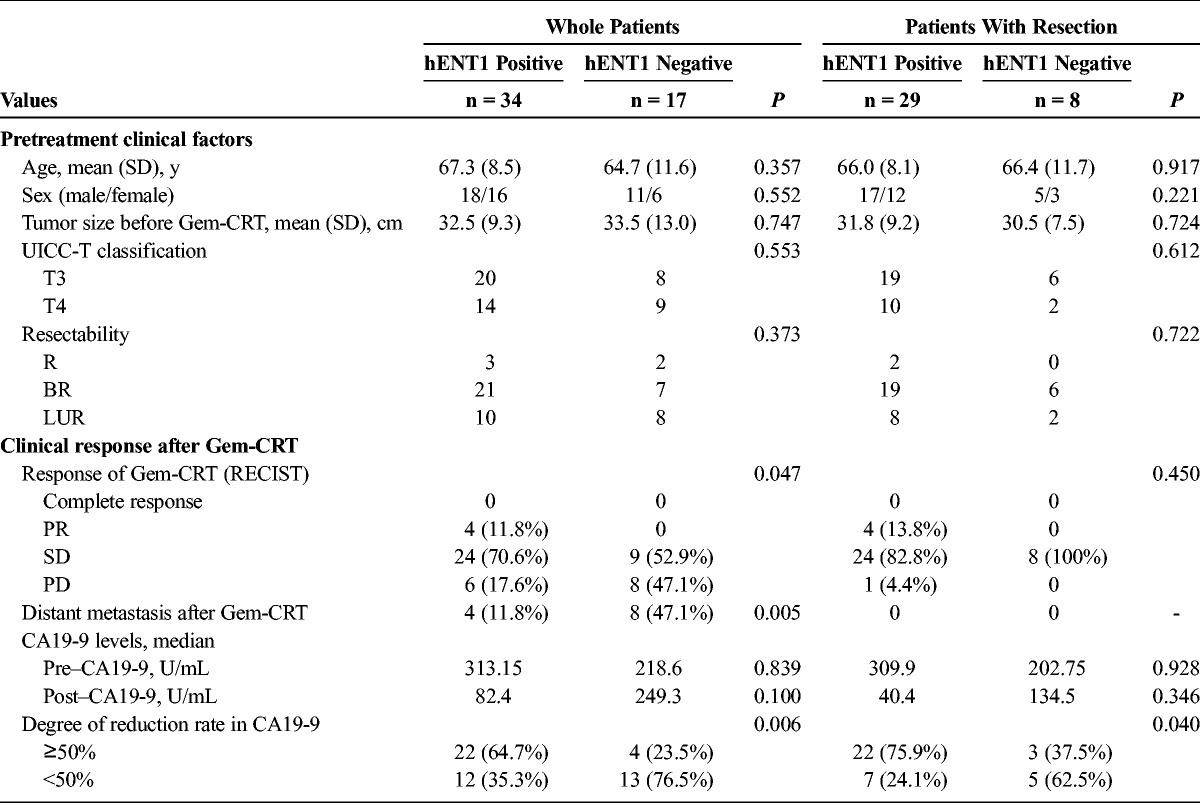
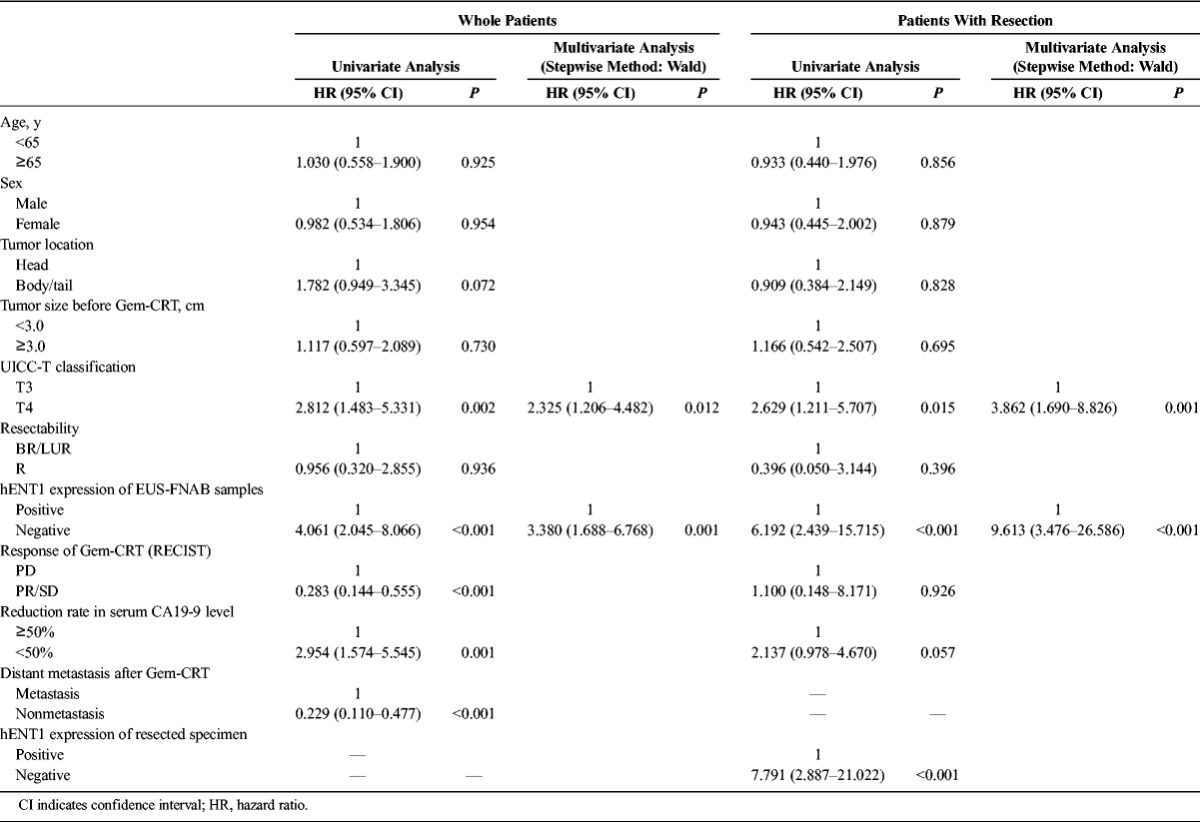
Patient characteristics are summarized in Table 1. Comparing with the whole patients and those with resection, the resection rate according to tumor location, UICC-T classification, resectability classification, and hENT1 expression in EUS-FNAB samples differed significantly: head versus body/tail (85.3% vs 47.1%, P = 0.004), T3 versus T4 (89.2% vs 52.2%, P = 0.003), resectable versus borderline resectable versus LUR (40.0% vs 89.3% vs 55.6%, P = 0.01), and hENT1 positive versus hENT1 negative (85.3% vs 47.1%, P = 0.004). The positive rate of hENT1 expression in EUS-FNAB samples was 66.7% in the whole 51 patients and 78.4% in the 37 patients with resection.
TABLE 1.
Background Characteristics of the Patients
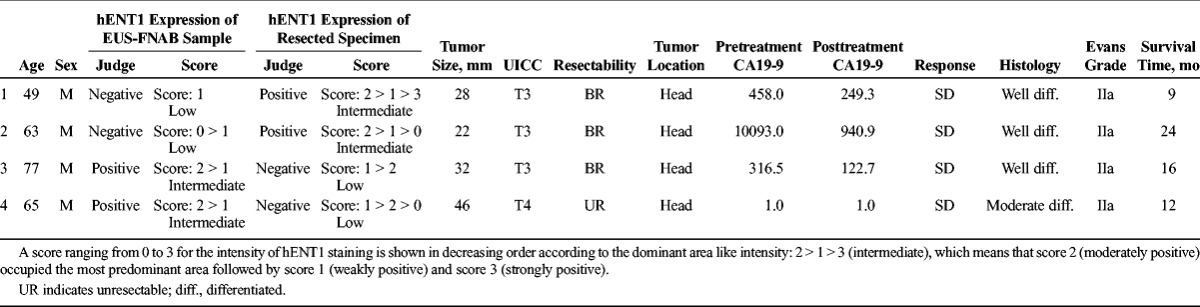
We examined the homology of hENT1 expression between pretreatment samples obtained by EUS-FNAB and resected specimens after Gem-CRT in 37 resected specimens (Table 2). As the status of hENT1 expression in the resected specimens was determined as control, sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of EUS-FNAB samples were 93.1%, 75.0%, 93.1%, 75.0%, and 89.2%, respectively. Therefore, the rate of concordance between EUS-FNAB samples and resected specimens was 89.2% (K = 0.681). We examined the characteristics of the 4 patients in whom hENT1 expression differed between EUS-FNAB samples and resected specimen (Table 3). In cases 1 and 2, hENT1 expression was found to be negative (low) in the EUS-FNAB samples, whereas positive (intermediate) in the resected specimen. On the other hand, in cases 3 and 4, it was positive (intermediate) in the EUS-FNAB sample, whereas negative (low) in the resected specimen. When we compared the intensity scores of hENT1 staining between the EUS-FNAB samples and the resected specimen (control) as shown in Table 3, the intensity scores in the resected specimen in all of 4 cases contained more than 2 kinds of intensity with various dominant area, and those in the EUS-FNAB samples contained one or more scores of the resected specimen with dominant area, which was different from the resected specimen. These findings suggested that the discrepancy between EUS-FNAB samples and resected specimen occurred because the intensity of staining and its area in the resected specimen varied widely in these 4 cases.
TABLE 2.
Homology of hENT1 Expression Between Pretreatment Samples Obtained by EUS-FNAB and Resected Specimens After Gem-CRT (Κ = 0.681)
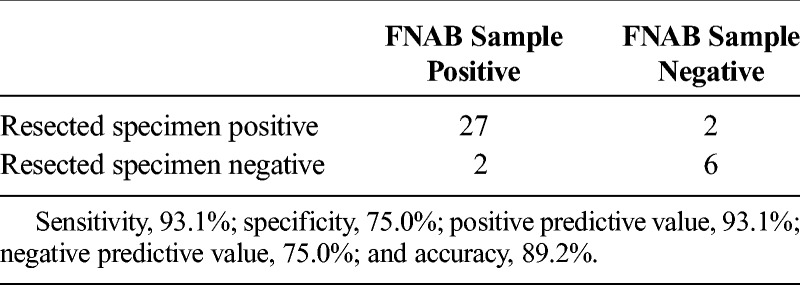
TABLE 3.
The Characteristics of the 4 Patients in Whom hENT1 Expression Differed Between EUS-FNAB Samples and Resected Specimen
Patient Characteristics and Effect of Gem-CRT According to hENT1 Expression
Pretreatment clinical factors and the clinical response after Gem-CRT in the whole patients and those with resection are summarized in Table 4. Pretreatment clinical factors in the whole patients and in those with resection did not differ between hENT1-positive and hENT1-negative expression. As for RECIST after Gem-CRT in the whole patients, the percentage of the patients with partial response (PR) and stable disease (SD) was significantly higher in hENT1 positive than in negative: 82.4% versus 52.9% (P = 0.047). Distant metastasis after Gem-CRT occurred significantly less frequently in hENT1 positive than in negative: 11.8% versus 47.1% (P = 0.005). The incidence of the patients with CA19-9 reduction rate of 50% or more was significantly higher in hENT1 positive than in negative: 64.7% versus 24.5% (P = 0.006). In the patients with resection, the incidence of patients with CA19-9 reduction rate of 50% or more was significantly higher in hENT1 positive than in negative: 75.9% versus 37.5% (P = 0.04), whereas other factors did not differ between the 2 groups.
TABLE 4.
Patient Characteristics and Effect of Gem-CRT According to hENT1 Expression
Univariable and Multivariable Analyses for Prognostic Factors
In the whole patients, UICC-T classification (P = 0.002), hENT1 expression of EUS-FNAB samples (P < 0.001), response of Gem-CRT (P < 0.001), CA19-9 reduction rate (P = 0.001), and distant metastasis after Gem-CRT (P < 0.001) were found to be significant in the univariate model; however, in the multivariate model, only hENT1 expression and UICC-T classification were found to be significant independent prognosis factors (Table 5). In the patients who underwent resection, UICC-T classification (P = 0.015), hENT1 expression of EUS-FNAB samples (P < 0.001), and hENT1 expression of resected specimen (P < 0.001) were found to be statistically significant in the univariable analyses; however, again, in the multivariate model, only hENT1 expression and UICC-T classification were found to be significant (Table 5).
TABLE 5.
Univariate and Multivariate Analyses of Factors Affecting Cox Proportional Hazard Model
In the 51 whole patients and 37 with resection, survival rates were significantly higher in hENT1 positive than in hENT1 negative as shown in Figure 3. Furthermore, we compared survival curves according to hENT1 expression in T3 (Fig. 4A, B) and T4 patients (Fig. 5A, B). In T3 patients, the survival rates were significantly higher in hENT1 positive than in hENT1 negative in the whole patients and in those with resection. In T4 patients, the survival rates did not significantly differ between hENT1 positive and negative in the whole patients, whereas in the patients with resection, the survival rates were significantly higher in hENT1 positive than in negative. Interestingly, survival curves in the patients without resection (14 patients in Fig. 3B, 3 in Fig. 4B, and 11 in Fig. 5B) were very similar to those of hENT1 negative with resection (8 in Fig. 3B, 6 in Fig. 4B, 2 in Fig. 5B).
FIGURE 3.
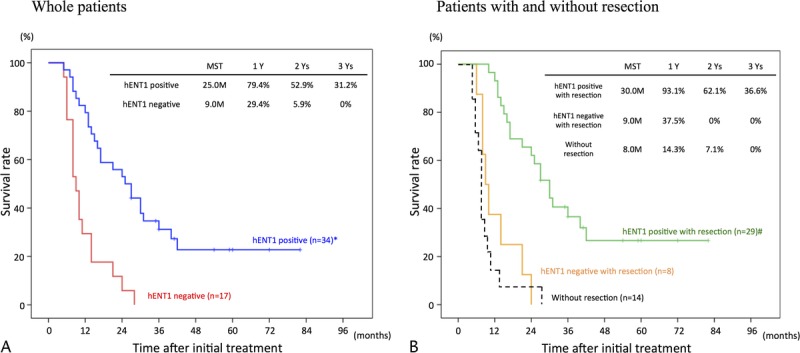
Cumulative survival curves according to hENT1 expression. A, Whole patients comparing hENT1 positive (n = 34) and negative (n = 17). B, Patients with resection comparing hENT1 positive (n = 29) and negative (n = 8), and those without resection (n = 14). *P < 0.001 versus hENT1 negative. #P < 0.001 hENT1 negative with resection.
FIGURE 4.
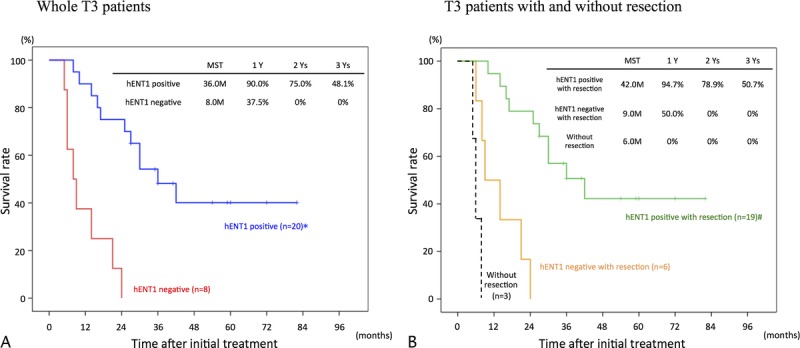
Cumulative survival curves in T3 patients according to hENT1 expression. A, Whole T3 patients comparing hENT1 positive (n = 20) and negative (n = 8). B, T3 patients with resection comparing hENT1 positive (n = 19) and negative (n = 6), and those without resection (n = 3). *P < 0.001 versus hENT1 negative. #P < 0.001 hENT1 negative with resection.
FIGURE 5.
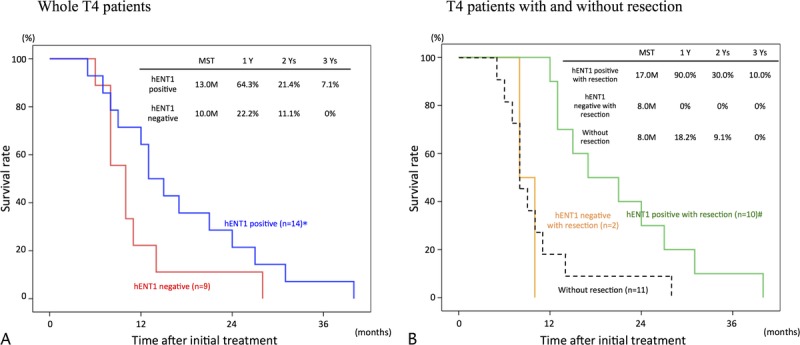
Cumulative survival curves in T4 patients according to hENT1 expression. A, Whole T4 patients comparing hENT1 positive (n = 14) and negative (n = 9). B, T4 patients with resection comparing hENT1 positive (n = 10) and negative (n = 2), and those without resection (n = 11). *P = 0.126 versus hENT1 negative. #P < 0.001 hENT1 negative with resection.
DISCUSSION
The hENT1 expression assessed immunohistochemically in the resected specimen has been proven to be a significant prognostic marker of patients with PDAC undergoing Gem-based adjuvant therapy,8,10–17 although the assessment method for grading of expression and reference cells (Langerhans cells or lymphocytes) differed among the studies. In our previous study on the 55 patients using Langerhans cells as a reference,17 staining intensity and extension of stained tumor cells (I-E) were graded as high (n = 14, 25.5%), intermediate (n = 25, 45.5%), and low (n = 16, 29.0%). High and intermediate were defined as positive (71.0%) and low was defined as negative (29.0%), and survival rate was significantly higher in the hENT1-positive group than in the hENT1-negative group. Using lymphocytes as a reference, Farrell et al10 reported that I-E was categorized as high (n = 34, 37.4%), low (n = 39, 42.8%), and no staining (n = 18, 19.8%), in which greater than 50% of cells showed no staining and that survival rate was significantly higher in the hENT1 high/low than in the no staining. Using Langerhans cells as a reference, Nakagawa et al15 also reported that I-E was graded high (n = 78, 71.6%) and low (n = 31, 28.4%) and that survival rate was significantly higher in the hENT1 high than in the low. Therefore, the proportion of hENT1 expression was similar among these previous 3 studies, although the assessment method based on I-E for grading of expression slightly differed. In contrast, Kawada et al29 revealed that hENT1 expression in the resected specimens was not associated with prognosis in the patients who underwent resection after preoperative Gem-CRT and immediately received postoperative liver perfusion chemotherapy using continuous infusion of 5-fluorouracil (for 28 days) into the hepatic artery and portal vein through a catheter inserted during the surgical procedure. They suggested that 5-FU liver perfusion had a negative impact on the role of hENT1 expression in prognosis.
If pretreatment evaluation of hENT1 expression in PDAC specimen obtained by EUS-FNAB becomes possible without difficulty, it is very useful to predict the efficacy of Gem-based therapy. The IHC analysis of hENT1 expression in the EUS-FNAB specimens has not been established, and thus, we first examined whether pretreatment hENT1 expressions in the EUS-FNAB specimens were concordant with those in the resected specimen after Gem-CRT. As a result, the rate of concordance between them was 89.2%, which is higher than the previous 2 reports concerning the other IHC studies: 86.5% in the study on SMAD4 protein and 73.9% in the study on ZIP4.30,31 The reason why the concordance rate in the 3 studies including ours did not reach 100% is unclear.
However, we could identify the features of the 4 patients in whom hENT1 expression differed between EUS-FNAB samples and resected specimen by comparing the intensity scores and its area of hENT1 staining between the EUS-FNAB samples and the resected specimen: the intensity of staining and its area in the resected specimen varied widely, indicating the existence of tumor heterogeneity in these 4 cases. A recent study on the evaluation of Ki-67 index in pancreatic neuroendocrine tumors also demonstrated intratumoral heterogeneity by comparing its index in EUS-FNAB specimens and resected specimens as the criterion standard: concordance rate remained 74.0% using the mean Ki-67 index.32 It is however interesting to note that Gem-CRT did not seem to change the preoperative/postoperative correlation of hENT1 staining.
With the use of EUS-FNAB specimens, our hENT1 IHC analysis could be successfully performed in 68.4% (52/76) among the cases diagnosed cytologically and/or histologically as PDAC, under the situations that the remaining materials followed by cytological/histological diagnosis were used and that some of adequate samples might be already consumed before the IHC analysis. These results suggested that EUS-FNAB specimens obtained from PDAC were appropriate for IHC analysis of hENT1. In the method similar to ours, which used the remaining samples after diagnosis to evaluate SMAD4 protein, only 44.4% (52/117) could be analyzed.30 It is therefore considered that the success rate of IHC analysis using EUS-FNAB samples obtained from PDAC specimens remains not so high. Concerning the reason why the success rate remains low, Navina et al33 recently evaluated the adequacy of EUS-FNAB samples of pancreatic masses for theranostic studies by assessing the cellularity of the cytology material. They retrospectively evaluated 169 EUS-FNAB specimens with positive diagnoses of solid epithelial pancreatic neoplasms (adenocarcinoma, 88%) for smear and cell block cellularity. Cellularity of cell blocks was scored on a scale of 1 to 4 (score 1 for <50 lesional cells; score 2 for 50–100, score 3 for 100–200, and score 4 for >200), and scores of 3 or 4 were deemed adequate for ancillary studies such as IHC analysis. As a result, only 12.4% of the positive cases had a cell block cellularity score that was adequate for theranostic studies. This score was not associated with ROSE, needle gauge, or number of passes. Tumor size and fibrosis score of resected tumors correlated with cellularity, but only larger size in pancreatic neuroendocrine tumors was significantly associated with adequacy. Furthermore, 75 PDAC cases were prospectively evaluated for cellularity score: score 0 in 39%, score 1 to 2 in 49%, and score 3 in 12%. When taking this cellularity score 1 to 3 of 61% and our result of yield 68.4% for hENT1 IHC analysis together, the cellularity score 1 or greater might be enough for hENT1 IHC analysis. Consequently, to enhance the clinical utility of pretreatment/preoperative IHC hENT1 examination, we have to develop a novel method to improve tumor cell yield, including modified cytological techniques and new needle designs.
Serum levels of CA19-9 have been accepted as a measure of pancreatic cancer burden, and the role of CA19-9 has been recently underscored for the evaluation of patients with pretreatment/preoperative therapy before planned surgical resection. Our previous 2 studies, which evaluated the clinical response after Gem-CRT for PDAC according to the hENT1 expression in the resected specimen, revealed that the hENT1-positive group had significantly higher reduction rate of CA19-9 than the hENT1-negative group, although RECIST did not differ between the 2 groups.17,18 In our present study using pretreatment/preoperative EUS-FNAB samples in the whole patients, the incidence of the patients with CA19-9 reduction rate of 50% or more was significantly higher in the hENT1-positive group than in the hENT1-negative group, and percentage of the patients with PR and SD in RECIST after Gem-CRT was significantly higher in the hENT1-positive than in the hENT1-negative group. In the patients with resection, however, RECIST did not differ between the 2 groups. Concerning the reason why RECIST results differed between the whole patients and those with resection, 47.1% (8/17) of hENT1 negative showed PD after Gem-CRT, and all of them could not receive pancreatectomy, whereas only 18.6% (6/34) of hENT1 positive showed PD, and one of them could receive pancreatectomy. It was therefore considered that hENT1 expression was not associated with RECIST in the patients with resection. Other than our studies comparing the clinical response between hENT1 positive and negative, Poplin et al34 evaluated clinical response using RECIST and survival in patients with metastatic PDAC, and hENT1 status had no influence on RECIST and survival (median survival time): the percentage of PR/complete response was 15.5% (9/58), and median survival time was 5.2 months in hENT1 high, whereas 26.3% (30/118) and 6.1 months, respectively, in hENT1 low. They considered that the role of hENT1 was less important in metastatic disease than after surgery with a presumed micrometastatic state. In contrast, our study included the patients with locally advanced (T3/T4) PDAC without distant metastasis at the time of enrollment, and at the time of reassessment (approximately 2–3 months after enrollment), distant metastasis became apparent in 23.5% (12/51) of the patients: hENT1 positive (n = 4) and negative (n = 8). These 12 patients died within 12 months regardless of hENT1 expression.
As for Gem-based pretreatment studies on hENT1 expression in PDAC, to the best of our knowledge, there have been 4 studies including our study: the clinical outcomes of patients undergoing Gem-CRT could be predicted by IHC analysis of hENT1 in EUS-FNAB samples obtained from T3/T4 (R/BR/LUR) PDAC. The one study, which evaluated messenger RNA expression levels of hENT1 using EUS-FNAB specimens obtained from patients with stage III/IV inoperable (LUR and metastatic) PDAC, did not show that its expression levels influenced survival.22 The remaining 2 studies on IHC hENT1 evaluation in PDAC, of which one used biopsy specimens of metastatic lesions33 and the other used biopsy specimens from the primary and metastatic lesions in stage III/IV inoperable (LUR and metastatic) patients,35 did not demonstrate any significant differences in prognosis between the high- and low-hENT1 subgroups either. The reason for the conflicting results between our study and the other 3 probably is that the other 3 studies included only inoperable patients who had basically poor prognosis in itself, whereas ours included the patients with locally advanced (T3/T4) PDAC without distant metastasis. It is considered that tumor progression influences the role of hENT1 expression in clinical response as well as prognosis in patients with PDAC, and we therefore compared survival curves according to hENT1 expression in T3 (R/BR) and T4 (BR/LUR) patients. In T3, prognosis was significantly better in hENT1 positive in the whole patients and in those with resection. In T4, it did not significantly differ between hENT1 positive and negative in the whole patients, whereas it was significantly better in hENT1 positive in those with resection. These results indicate that the role of hENT1 expression in Gem-based treatment becomes less important as tumor progresses.
Our treatment protocol of Gem-CRT for patients with locally advanced (T3/T4) PDAC was conducted for aiming to achieve curative-intent resection after reassessment, even though it was determined initially LUR. Therefore, we have to clarify the significance of preoperative/pretreatment assessment of hENT1 expression using EUS-FNAB specimens based on our results: its assessment identifies patients with PDAC who can benefit from curative-intent resection followed by Gem-based adjuvant therapy. Regardless of T3 and T4 tumors, hENT1-positive patients who underwent curative-intent resection had significantly better prognosis compared with hENT1-negative patients with resection, whose prognosis was similar to those without resection. To improve the prognosis in hENT1-negative patients, a novel regimen other than Gem-based treatment needs to be further investigated.
In conclusion, pretreatment hENT1 expressions in the EUS-FNAB specimens are concordant with those in the resected specimen after Gem-CRT, and its assessment before Gem-CRT provides us the important information on patients with PDAC who can benefit from curative-intent resection followed by Gem-based adjuvant therapy.
Footnotes
This study was supported by in part by Grants-in-Aid for scientific research from Japan Society for the Promotion of Science (25461033).
The authors declare no conflict of interest.
REFERENCES
- 1. Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15: 2403– 2413. [DOI] [PubMed] [Google Scholar]
- 2. Hashimoto K, Ueno H, Ikeda M, et al. Do recurrent and metastatic pancreatic cancer patients have the same outcomes with gemcitabine treatment? Oncology. 2009; 77: 217– 223. [DOI] [PubMed] [Google Scholar]
- 3. Andriulli A, Festa V, Botteri E, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012; 19: 1644– 1662. [DOI] [PubMed] [Google Scholar]
- 4. Mackey JR, Baldwin SA, Young JD, et al. Nucleoside transport and its significance for anticancer drug resistance. Drug Resist Updat. 1998; 1: 310– 324. [DOI] [PubMed] [Google Scholar]
- 5. Mackey JR, Yao SY, Smith KM, et al. Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J Natl Cancer Inst. 1999; 91: 1876– 1881. [DOI] [PubMed] [Google Scholar]
- 6. Ritzel MW, Ng AM, Yao SY, et al. Recent molecular advances in studies of the concentrative Na+-dependent nucleoside transporter (CNT) family: identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). Mol Membr Biol. 2001; 18: 65– 72. [DOI] [PubMed] [Google Scholar]
- 7. Mackey JR, Mani RS, Selner M, et al. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998; 58: 4349– 4357. [PubMed] [Google Scholar]
- 8. Maréchal R, Bachet JB, Mackey JR, et al. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology. 2012; 143: 664– 674. [DOI] [PubMed] [Google Scholar]
- 9. Mori R, Ishikawa T, Ichikawa Y, et al. Human equilibrative nucleoside transporter 1 is associated with the chemosensitivity of gemcitabine in human pancreatic adenocarcinoma and biliary tract carcinoma cells. Oncol Rep. 2007; 17: 1201– 1205. [PubMed] [Google Scholar]
- 10. Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009; 136: 187– 195. [DOI] [PubMed] [Google Scholar]
- 11. Maréchal R, Mackey JR, Lai R, et al. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin Cancer Res. 2009; 15: 2913– 2919. [DOI] [PubMed] [Google Scholar]
- 12. Kim R, Tan A, Lai KK, et al. Prognostic roles of human equilibrative transporter 1 (hENT-1) and ribonucleoside reductase subunit M1 (RRM1) in resected pancreatic cancer. Cancer. 2011; 117: 3126– 3134. [DOI] [PubMed] [Google Scholar]
- 13. Morinaga S, Nakamura Y, Watanabe T, et al. Immunohistochemical analysis of human equilibrative nucleoside transporter-1 (hENT1) predicts survival in resected pancreatic cancer patients treated with adjuvant gemcitabine monotherapy. Ann Surg Oncol. 2012; 19: 558– 564. [DOI] [PubMed] [Google Scholar]
- 14. Kondo N, Murakami Y, Uemura K, et al. Combined analysis of dihydropyrimidine dehydrogenase and human equilibrative nucleoside transporter 1 expression predicts survival of pancreatic carcinoma patients treated with adjuvant gemcitabine plus S-1 chemotherapy after surgical resection. Ann Surg Oncol. 2012; 19: 646– 655. [DOI] [PubMed] [Google Scholar]
- 15. Nakagawa N, Murakami Y, Uemura K, et al. Combined analysis of intratumoral human equilibrative nucleoside transporter 1 (hENT1) and ribonucleotide reductase regulatory subunit M1 (RRM1) expression is a powerful predictor of survival in patients with pancreatic carcinoma treated with adjuvant gemcitabine-based chemotherapy after operative resection. Surgery. 2013; 153: 565– 575. [DOI] [PubMed] [Google Scholar]
- 16. Greenhalf W, Ghaneh P, Neoptolemos JP, et al. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer Inst. 2014; 106: djt347. [DOI] [PubMed] [Google Scholar]
- 17. Murata Y, Hamada T, Kishiwada M, et al. Human equilibrative nucleoside transporter 1 expression is a strong independent prognostic factor in UICC T3-T4 pancreatic cancer patients treated with preoperative gemcitabine-based chemoradiotherapy. J Hepatobiliary Pancreat Sci. 2012; 19: 413– 425. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi M, Mizuno S, Murata Y, et al. Gemcitabine-based chemoradiotherapy followed by surgery for borderline resectable and locally unresectable pancreatic ductal adenocarcinoma: significance of the CA19-9 reduction rate and intratumoral human equilibrative nucleoside transporter 1 expression. Pancreas. 2014; 43: 350– 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network (NCCN) practice guidelines for pancreatic cancer. 2010. Available at: http://www.ncc- n.org. Accessed April 1, 2012.
- 20. Ashida R, Nakata B, Shigekawa M, et al. Gemcitabine sensitivity-related mRNA expression in endoscopic ultrasound-guided fine-needle aspiration biopsy of unresectable pancreatic cancer. J Exp Clin Cancer Res. 2009; 28: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujita H, Ohuchida K, Mizumoto K, et al. Gene expression levels as predictive markers of outcome in pancreatic cancer after gemcitabine-based adjuvant chemotherapy. Neoplasia. 2010; 12: 807– 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eto K, Kawakami H, Kuwatani M, et al. Human equilibrative nucleoside transporter 1 and Notch3 can predict gemcitabine effects in patients with unresectable pancreatic cancer. Br J Cancer. 2013; 108: 1488– 1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamao K, Sawaki A, Mizuno N, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol. 2005; 40: 1013– 1023. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz MR. Endoscopic ultrasound-guided fine-needle aspiration. Cancer. 2004; 102: 203– 206. [DOI] [PubMed] [Google Scholar]
- 25. Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012; 75: 319– 331. [DOI] [PubMed] [Google Scholar]
- 26. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013; 139: 300– 308. [DOI] [PubMed] [Google Scholar]
- 27. Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992; 127: 1335– 1339. [DOI] [PubMed] [Google Scholar]
- 28. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228– 247. [DOI] [PubMed] [Google Scholar]
- 29. Kawada N, Uehara H, Katayama K, et al. Human equilibrative nucleoside transporter 1 level does not predict prognosis in pancreatic cancer patients treated with neoadjuvant chemoradiation including gemcitabine. J Hepatobiliary Pancreat Sci. 2012; 19: 717– 722. [DOI] [PubMed] [Google Scholar]
- 30. Boone BA, Sabbaghian S, Zenati M, et al. Loss of SMAD4 staining in pre-operative cell blocks is associated with distant metastases following pancreaticoduodenectomy with venous resection for pancreatic cancer. J Surg Oncol. 2014; 110: 171– 175. [DOI] [PubMed] [Google Scholar]
- 31. Xu C, Wallace MB, Yang J, et al. ZIP4 is a novel diagnostic and prognostic marker in human pancreatic cancer: a systemic comparison between EUS-FNA and surgical specimens. Curr Mol Med. 2014; 14: 309– 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014; 46: 32– 38. [DOI] [PubMed] [Google Scholar]
- 33. Navina S, McGrath K, Chennat J, et al. Adequacy assessment of endoscopic ultrasound-guided, fine-needle aspirations of pancreatic masses for theranostic studies: optimization of current practices is warranted. Arch Pathol Lab Med. 2014; 138: 923– 928. [DOI] [PubMed] [Google Scholar]
- 34. Poplin E, Wasan H, Rolfe L, et al. Randomized, multicenter, phase II study of CO-101 versus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma: including a prospective evaluation of the role of hENT1 in gemcitabine or CO-101 sensitivity. J Clin Oncol. 2013; 31: 4453– 4461. [DOI] [PubMed] [Google Scholar]
- 35. Ormanns S, Heinemann V, Raponi M, et al. Human equilibrative nucleoside transporter 1 is not predictive for gemcitabine efficacy in advanced pancreatic cancer: translational results from the AIO-PK0104 phase III study with the clone SP120 rabbit antibody. Eur J Cancer. 2014; 50: 1891– 1899. [DOI] [PubMed] [Google Scholar]


