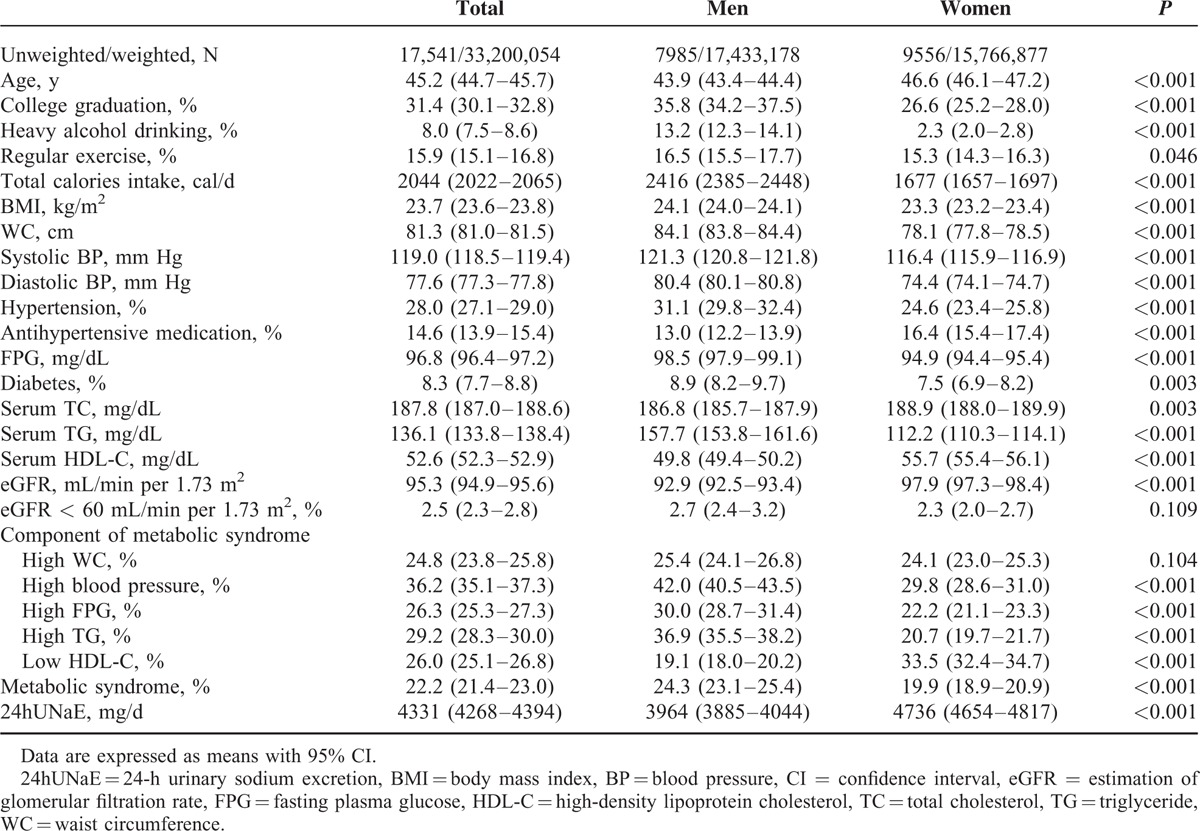
Abstract
High sodium intake is 1 of the modifiable risk factors for cardiovascular disease, but in Korea, daily sodium intake is estimated to be double the level recommended by World Health Organization. We investigated the association between the estimated 24-h urinary sodium excretion (24hUNaE) and metabolic syndrome using nationwide population data.
In total, 17,541 individuals (weighted n = 33,200,054; weighted men, 52.5% [95% confidence interval, CI = 51.8–53.3]; weighted age, 45.2 years [44.7–45.7]) who participated in the Korean Health and Nutrition Examination Survey 2009 to 2011 were investigated. NCEP-ATP III criteria for metabolic syndrome were used, and sodium intake was estimated by 24hUNaE using Tanaka equation with a spot urine sample.
The weighted mean 24hUNaE values were 3964 mg/d (95% CI = 3885–4044) in men and 4736 mg/d (4654–4817) in women. The weighted age-adjusted prevalence of metabolic syndrome was 22.2% (21.4–23.0), and it increased with 24hUNaE quartile in both men and women (mean ± standard error of the mean; men: 22.5 ± 1.0%, 23.0 ± 1.0%, 26.0 ± 1.2%, and 26.0 ± 1.2%; P = 0.026; women: 19.4 ± 0.8%, 17.7 ± 0.8%, 19.8 ± 1.0%, and 23.0 ± 1.1%; P = 0.002, for quartiles 1–4, respectively). Even after adjustment for age, daily calorie intake, heavy alcohol drinking, regular exercise, college graduation, and antihypertensive medication, the weighted prevalence of metabolic syndrome increased with the increase in 24hUNaE in men and women. The weighted 24hUNaE was positively associated with the number of metabolic syndrome components after adjustment for confounding factors in men and women. In subjects without antihypertensive medication, the odds ratio for metabolic syndrome in quartile 4 of 24hUNaE compared with quartile 1 was 1.56 (1.33–1.84, P < 0.001) in the total population, 1.66 (1.34–2.06, P < 0.001) in men, and 1.94 (1.49–2.53, P < 0.001) in women.
In this nationwide population study, we observed a significant independent association between high sodium intake, estimated by spot urine sodium excretion, and the presence of metabolic syndrome in men and women.
INTRODUCTION
Metabolic syndrome is associated with an increased risk of cardiovascular disease (CVD), diabetes, chronic kidney disease, and total mortality.1,2 The prevalence of metabolic syndrome is increasing in Korea. According to data from the Korean National Health and Nutrition Examination Surveys 1998 to 2007, the age-adjusted prevalence of metabolic syndrome increased from 24.9% to 31.3%.3 Thus, specific modifiable risk factors for metabolic syndrome should be defined and measures to modify them emphasized.
The mean sodium intake in Korea (5158 mg/d, 12 g of salt) is 2 to 3 times higher than that recommended by the World Health Organization (WHO) (<2000 mg/d).4,5 Excess sodium intake has a clinical impact on CVDs,6 and accumulating evidence suggests that even modest reductions in dietary sodium reduce CVD events and the substantial associated economic burden.7,8 Thus, many nations have developed campaigns to reduce salt intake to help prevent CVDs; such public health interventions would be even more useful in a population with a high average sodium intake.9
Although increasing evidence indicates an association between high sodium intake and CVDs,6 in Korea, there are limited nationwide survey data on the association of sodium intake and metabolic syndrome. Previously, we reported that high sodium intake was associated with an increased prevalence of albuminuria, a marker for the renal component of metabolic syndrome.10,11
Because high salt consumption is a modifiable risk factor associated with CVDs, it is important to investigate the population-based association between salt intake and metabolic syndrome in Korean subjects, who are known to have high salt intake. In this study, we investigated the association between salt intake, estimated using 24-h urinary sodium excretion (24hUNaE), and metabolic syndrome and its components, stratified by gender.
METHODS
Study Population
Details of the study population and methods have been published elsewhere.11,12 Briefly, from the 28,009 participants in KNHANES 2009 to 2011 (a cross-sectional, nationally representative, and stratified survey), we considered 21,119 individuals with age 19 years and older. Among this population, 17,541 individuals who participated in the laboratory examination were finally included in the analyses (Supplementary Figure 1). KNHANES is conducted regularly by the Korean Center for Disease Control using a representative population recruited using population-allocation systematic sampling with multistage stratification. Its operation and procedures have been reported before.12 Because KNHANES is a complex probability sample survey, the survey design should be explicitly used when producing statistical estimates or undertaking statistical analysis of the KNHANES data to present an unbiased cross-sectional estimates for the entire Korean population.13 After approval of the investigator's proposal by the Korean Center for Disease Control, a survey dataset was provided, including information about survey location, stratification by age, gender, and other factors, and sample weight for each participant. The sampling weight for each sample person created by Korean CDC reflect the reciprocal of the probabilities of selection (the primary sample units, household), an adjustment for nonresponse (household, person), and a poststratification factors such as age, sex, metropolitan area, or province category approximately equal to the total population of Korea (2005 Korean National Census Registry) to produce estimates representative of the noninstitutionalized Korean civilian population. KNHANES data include a standardized health interview using well-established questions to determine the demographic and socioeconomic characteristics of the subjects. In the present study, current smokers were defined as smokers. Heavy alcohol drinking was defined as those who drank at least 4 times/wk among the 6 answered items questioning the frequency of drinking days per a week or a month of the alcohol-related questionnaire in KNHANES V: never during recent 1 year, <1 time/mo, approximately 1 time/mo, 2 to 4 times/mo, 2 to 3 times/wk, and >4 times/wk. Physical activity was assessed in terms of intensity, duration, and frequency, and regular exercise was defined as exercising 5 or more times per week of moderate or 3 or more sessions per week of strenuous exercise, sustained for at least 30 min per session. We did not exclude the individual with depressive symptom because it was not evaluated by Diagnostic and Statistical Manual of Mental Disorders criteria. The study was approved by the Institutional Review Board of Ilsan-Paik Hospital (2015-09-003).
Definition of Metabolic Syndrome
Metabolic syndrome was defined according to the criteria of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATPIII) guidelines.14 The presence of any 3 of the following 5 factors is required for a diagnosis of metabolic syndrome: waist circumference (WC) ≥90 cm in men or ≥80 cm in women, hypertriglyceridemia (triglyceride, TG ≥ 150 mg/dL), low high-density lipoprotein cholesterol (HDL-C ≤40 mg/dL for men and ≤50 mg/dL for women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg and/or diastolic BP ≥ 85 mm Hg or current use of antihypertensive drugs), and impaired fasting glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL or use of glucose-lowering medicine).
Estimating the 24hUNaE and Glomerular Filtration Rate
The 24hUNaE was estimated from the sodium and creatinine of random urine samples using Tanaka equation15: 24-h urinary Na excretion (mmol/d) = 21.98 × UNa/UCr × [−2.04 × age + 14.89 × weight (kg) + 16.14 × height (cm) − 2244.45]0.392. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation: GFR = 141 × min (SCr/κ, 1)α × max (SCr/κ, 1)−1.209 × 0.993Age × 1.018 (if female) × 1.159 (if black), where SCr is the serum creatinine level in mg/dL, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of SCr/κ or 1, and max indicates the maximum of SCr/κ or 1.16
Statistical Analysis
All data are presented as weighted means and confidence intervals (CI) or standard error of the mean (SEM) unless otherwise specified. Weighted age-adjusted comparisons of the clinical characteristics according to the quartile of the estimated 24hUNaE were made using analysis of covariance (ANCOVA) stratified by gender (Tables 1 and 2). ANCOVA was used to explore the association between quartiles of 24hUNaE and the presence of metabolic syndrome or its components (Table 2). Weighted multivariate comparisons of 24hUNaE by number of metabolic syndrome components were made using ANCOVA (Table 3). Logistic regression analyses were performed to determine the odds ratios for each quartile of the estimated 24hUNaE, with quartile 1 as the reference (Table 4). These data were analyzed using the SPSS software (ver. 21.0 for Windows; SPSS, Chicago, IL), and the level of significance was P < 0.05.
TABLE 1.
Age-Adjusted Weighted Clinical Characteristics of Korean Adult Men by Estimated 24-h Urine Na Excretion
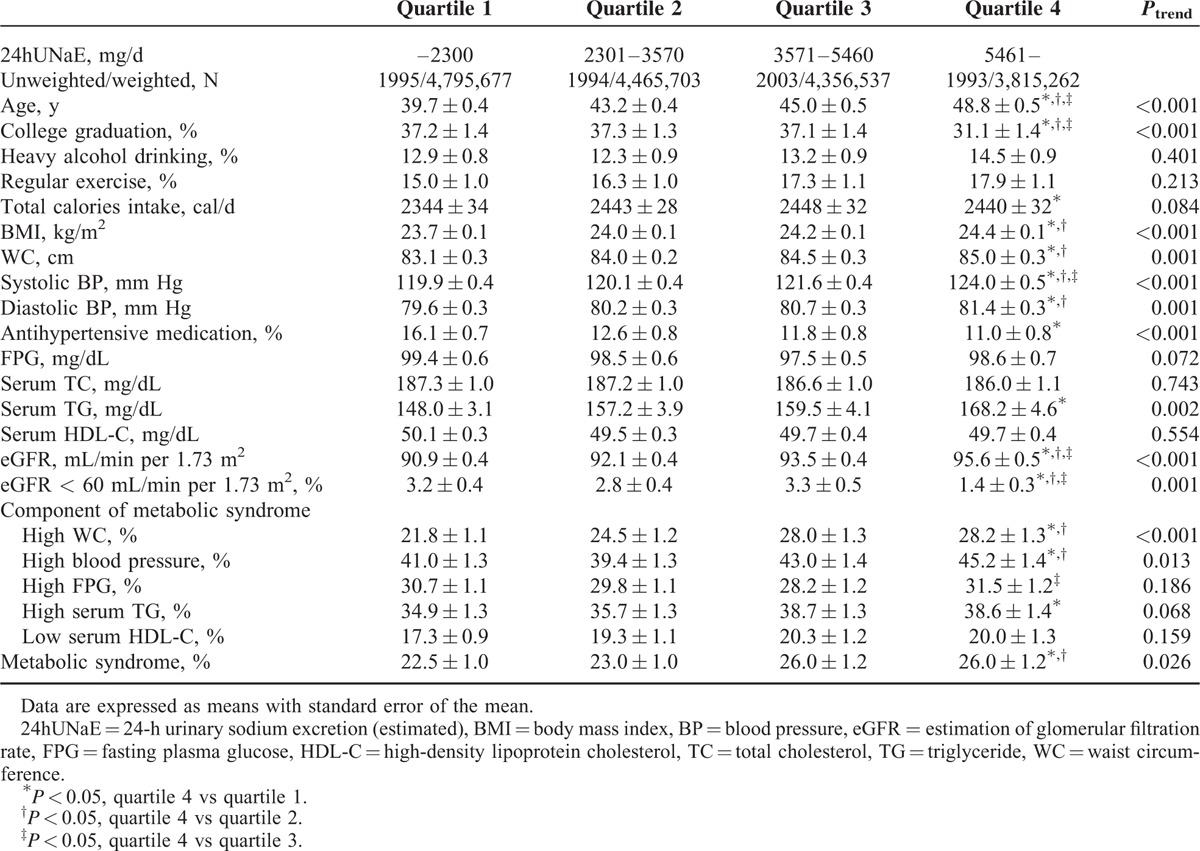
TABLE 2.
Age-Adjusted Weighted Clinical Characteristics of Korean Adult Women by Estimated 24-h Urine Na Excretion
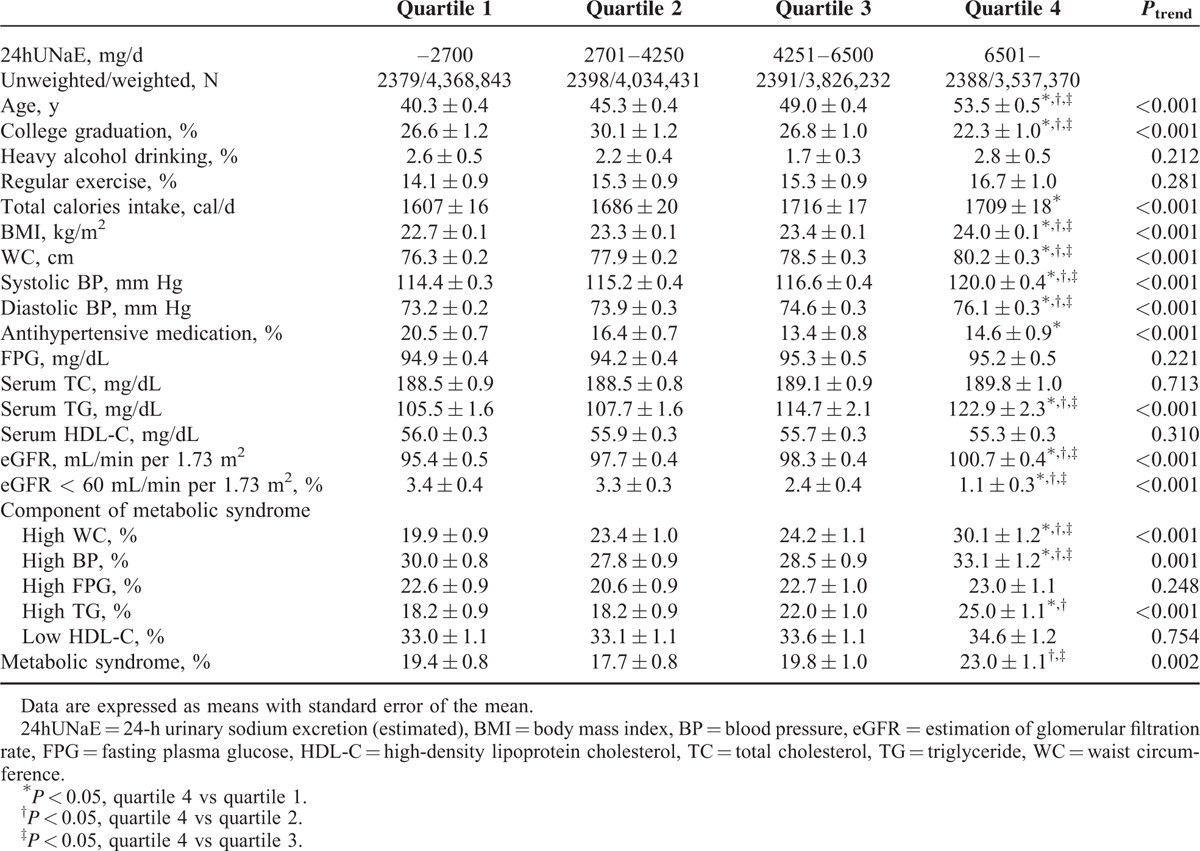
TABLE 3.
Sex-Stratified Weighted Estimated 24-h Urine Na Excretion of Korean Adult by Number of Metabolic Syndrome Components
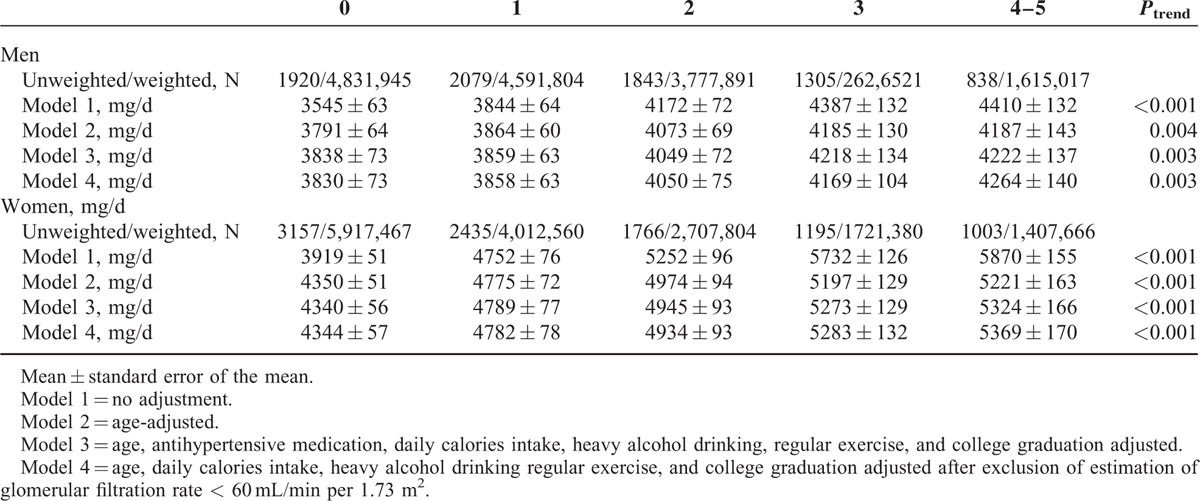
TABLE 4.
Sex-Stratified Logistic Regression Analyses for the Presence of Metabolic Syndrome by Quartiles of Estimated 24-h Urine Na Excretion
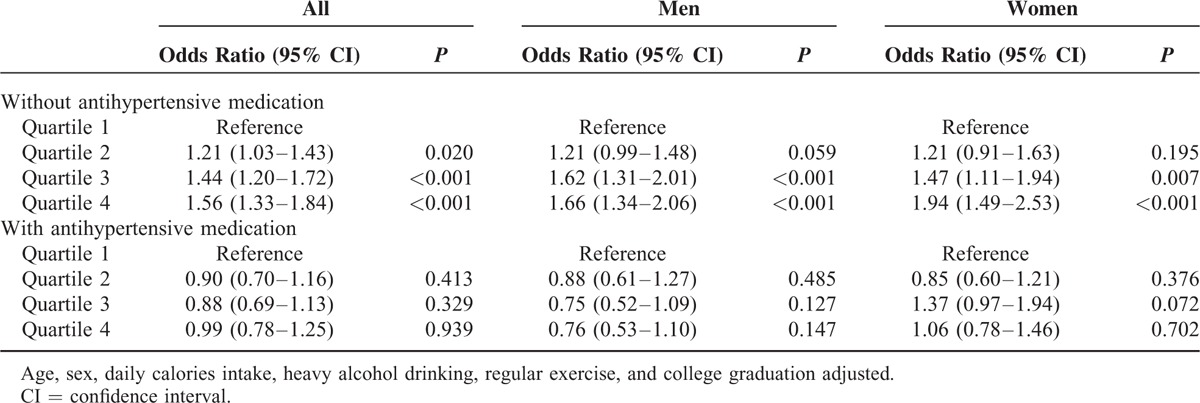
RESULTS
The weighted characteristics of study participants by gender are presented in Table 5. Men were more likely than women to report college graduation, current smoking, heavy alcohol drinking, and diabetes (all P < 0.05). The mean systolic and diastolic BP, body mass index (BMI), WC, TG, and FPG were significantly higher, whereas HDL-C was lower, in men than women. Overall, the weighted mean prevalence of metabolic syndrome was 22.2% (95% CI = 21.4–23.0). Metabolic syndrome was more prevalent in men than women (mean and 95% CI: 24.3% and 23.1–25.4 vs 19.9% and 18.9–20.9, respectively; P < 0.001). The mean 24hUNaE was 4331 mg/d in Korean adults overall, and those in men and women were 3964 mg/d (3885–4044) and 4736 mg/d (4654–4817), respectively. The weighted mean of 24hUNaE was higher in subjects with metabolic syndrome than in those without: 5812 mg/d and 5624 to 6000 versus 4469 mg/d and 4387 to 4552, respectively; P < 0.001.
TABLE 5.
Weighted Clinical Characteristics of Korean Adults
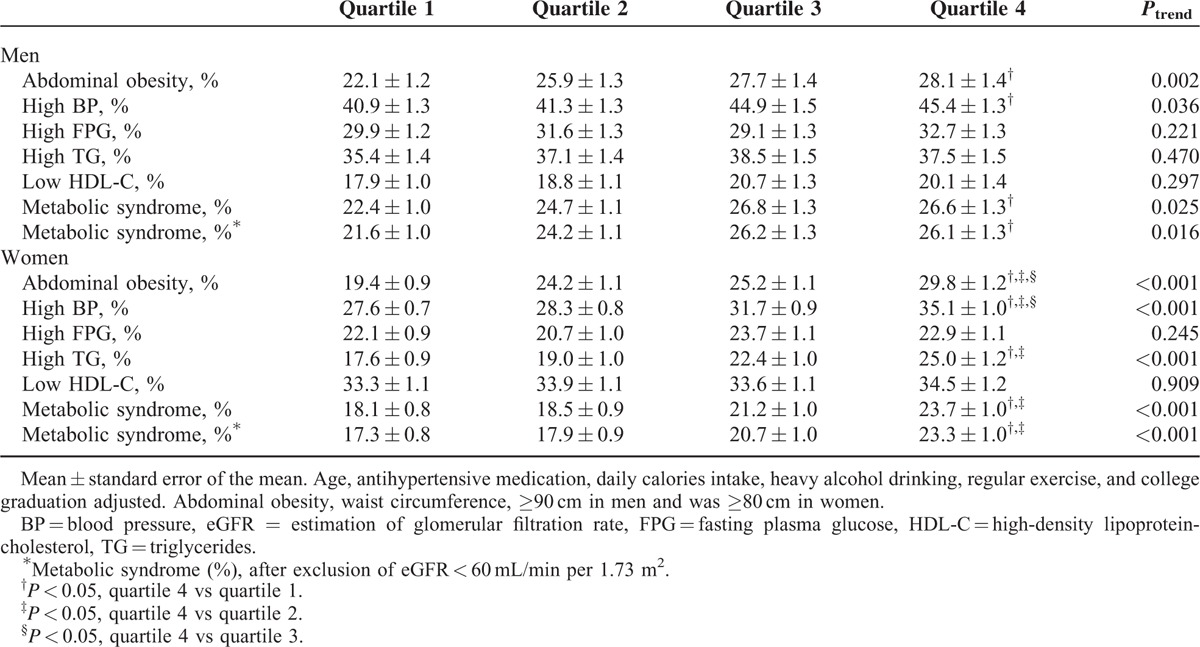
The weighted demographic and clinical characteristics of the 17,541 participants (weighted n = 33,200,054) according to quartiles of 24hUNaE stratified by gender are shown in Tables 1 and 2. To ensure the effect of population's age distribution, age-adjusted comparing the differences between population groups was measured. The weighted age-adjusted proportion of subjects with college graduation decreased by quartile in relation to 24hUNaE in both men and women. Whereas age, proportion of college graduation, BMI, WC, systolic and diastolic BP, serum TG, and eGFR increased with increasing 24hUNaE level across the quartiles in both men and women, the proportion of subjects with antihypertensive medication or decreased eGFR declined. The weighted age-adjusted prevalence of metabolic syndrome increased with 24hUNaE quartile in both men and women; the mean ± SEM values for quartiles 1 to 4 in men were 22.5 ± 1.0%, 23.0 ± 1.0%, 26.0 ± 1.2%, and 26.0 ± 1.2% (P = 0.026), and those in women were 19.4 ± 0.8%, 17.7 ± 0.8%, 19.8 ± 1.0%, and 23.0 ± 1.1% (P = 0.002), respectively. In weighted, age-adjusted comparisons for the presence of each component of metabolic syndrome, the proportion of abdominal obesity and hypertension increased significantly according to increases in 24hUNaE in men. In women, abdominal obesity, hypertension, and hypertriglyceridemia increased significantly with increasing 24hUNaE.
In the weighted age-adjusted comparison, for both men and women, the 24hUNaE of hypertensive subjects without antihypertensive medication was significantly higher than that in subjects without hypertension and in those with hypertension taking antihypertensive medication; in men, the mean ± SEM values were 4413 ± 91, 3896 ± 42, and 3707 ± 118 mg/d, respectively (all P < 0.001), and in women, 5564 ± 124, 4652 ± 42, and 4660 ± 125 mg/d, respectively (all P < 0.001).
In gender-stratified models adjusted for age, antihypertensive medication, daily calorie intake, heavy alcohol consumption, regular exercise, and college graduation, the 24hUNaE quartile was significantly associated with abdominal obesity, hypertension, and hence the presence of metabolic syndrome in both men and women (Table 6). Additionally, the proportion of subjects with hypertriglyceridemia increased with higher 24hUNaE quartiles in women. To minimize interference induced by decreased eGFR, we excluded subjects with eGFR < 60 mL/min per 1.73 m2. The positive association between increased 24hUNaE and the proportion of subjects with metabolic syndrome remained statistically significant after the exclusion of subjects with decreased eGFR (<60 mL/min per 1.73 m2). The weighted estimated 24hUNaE increased according to the number of metabolic syndrome components in both men and women, even after adjusting for those confounding variables (Table 3).
TABLE 6.
Adjusted Weighted Prevalence of Metabolic Syndrome Component of Korean Adult Men and Women by Estimated 24-h Urine Na Excretion
To further exclude any bias induced by antihypertensive medication, logistic regression analyses were performed to investigate the presence of metabolic syndrome by 24hUNaE quartile in gender-stratified subgroups according to antihypertensive medication status (Table 4). Here, in subjects without antihypertensive medication, odd ratios showed significant graded associations with the presence of metabolic syndrome from the second quartile of 24hUNaE compared with the lowest quartile in all subjects. Compared with subjects in the lowest 24hUNaE quartile, the odd ratios for metabolic syndrome among those in the highest quartile was 1.66 (95% CI = 1.34–2.06) in men and 1.94 (1.49–2.53) in women after adjustment for confounding factors. No significant association was observed in all subjects or in gender-stratified subgroups with antihypertensive medications.
DISCUSSION
In this nationwide survey, 22.2% of Korean adults had metabolic syndrome, and we observed that the mean 24hUNaE, estimated by spot urine sodium excretion, was higher in subjects with metabolic syndrome compared with those without, even though mean 24hUNaE was high (4331 mg/d) in Korean adults, which is more than double the recommended level. Even after adjusting for age, daily calorie intake, heavy alcohol drinking, regular exercise, college graduation, and antihypertensive medication, there was a significant independent association of high sodium intake with the presence of metabolic syndrome in men and women. In subjects not taking any antihypertensive medication, the weighted odds ratio for metabolic syndrome in the highest 24hUNaE quartile, with the lowest quartile as the reference, was 1.56 (95% CI = 1.33–1.84) in the total population, 1.66 (1.34–2.06) in men, and 1.94 (1.49–2.53) in women. Although the degree of renal function affects the 24hUNaE,17 restricting analyses to subjects without decreased eGFR (<60 mL/min per 1.73 m2) did not alter our findings.
To date, few studies have examined the association between sodium intake and metabolic syndrome. In a study of normotensive subjects in Brazil (<130/85 mm Hg, n = 781), there was no difference in 24hUNaE between subjects with and without metabolic syndrome (men: 96 ± 48 and 97 ± 49 mEq/12 h, P = 0.75; women: 83 ± 51 and 93 ± 45, P = 0.22, respectively), and weight-adjusted urinary sodium values were lower in subjects with than in those without metabolic syndrome (1.27 ± 0.64 and 1.42 ± 0.88 mEq/kg, respectively, P = 0.06).18 A previous Korean study using the KNHANES III database (2005) found that sodium intake, estimated from food composition tables, did not differ between subjects with and those without metabolic syndrome (5722.4 ± 3301.0 mg/d vs 5658.3 ± 3294.3 mg/d, respectively, P = 0.629).19 That study examined the association between dietary patterns, including sodium intake and BP, relying on 24-h dietary recall, which is not suitable for estimating population sodium intake in epidemiological studies because of changes in or under-reporting of energy or sodium intake and difficulty in quantifying the dietary sodium in dietary assessments.20 In contrast, whereas Hoffmann and Cubeddu reported that sodium intake was more closely associated with metabolic syndrome in Venezuelan men than in women (n = 251), Rhee et al reported that a significant association was only seen in Korean women (n = 272). These 2 studies used 24-h urine collection, which seems to be the most accurate method, but had small sample sizes and nonhomogenous study populations.21,22 These differences in outcomes may be due to differences in study populations, differences in mean levels of sodium intake or salt sensitivity between populations, cultural groups, or genders, diagnostic criteria for metabolic syndrome (e.g., WC criterion for abdominal obesity), the method of salt-intake measurement, differences in adjustment of confounding factors, or statistical methods.
A previous study by Huh et al, which included 3545 men and 3617 postmenopausal women ages 45 years older and used KNHANES 2008 to 2010 data, found that the highest 24hUNaE tertile was significantly associated with WC, hypertriglyceridemia, and hypertension in postmenopausal women but with only WC in men.23 The prevalence of metabolic syndrome was higher in subjects in the third tertile compared with the first quartile in women but not in men (P < 0.001 vs 0.259, respectively). Compared with that study, which did not adjust for the effects of confounding factors including age, gender, daily calorie intakes, and socio-demographic factors, our study included an extensive population not limited with regard to menopausal status and age and represented a weighted comparison adjusted for confounding factors using population-based data from a representative population. Using this approach, we showed similar associations between 24hUNaE and the prevalence of metabolic syndrome in gender-stratified analyses.
In most epidemiologic data, sodium intake is higher in men than women, but this difference was reversed after adjustment for daily calorie intake because sodium intake is determined, to a large extent, by calorie intake.24,25 Further studies about factors associated with high sodium intake in the daily diet are needed to clarify this difference in Koreans.
The mechanism of the connection between increased 24hUNaE and insulin resistance, the core component of metabolic syndrome, is not fully defined. Although a direct mechanism between abdominal obesity and high salt intake is not conclusive, a high-calorie diet could be a possible explanation.26 In agreement with a previous report,27 high 24hUNaE was associated with daily total calorie intake in the present study. However, after adjustment for daily calorie intake, the association between abdominal obesity and 24hUNaE remained statistically significance. Albuminuria is a common feature of insulin resistance and metabolic syndrome, and a marker of CVDs. We previously reported that the prevalence of albuminuria increased with 24hUNaE quartile, and the highest 24hUNaE quartile was significantly associated with the presence of albuminuria in the multiple logistic regression analysis adjusted for age, gender, diabetes, obesity, and hypertension.10 High sodium intake has various effects on each component of metabolic syndrome and thus on metabolic syndrome overall. Recently, it was suggested that insulin increases salt sensitivity; furthermore, high salt intake was associated with increased glucocorticoid production, and it may induce insulin resistance and metabolic syndrome.28,29 In addition, high salt intake increased intracellular Ca2+ leak which increased vascular tone in vascular smooth muscle cells and caused defective glucose homeostasis in β cells, via some mechanisms including the type 2 ryanodine receptor.30,31 Further studies will be needed to clarify the underlying mechanism of high-salt diet-induced insulin resistance and metabolic syndrome.
There is solid evidence that high sodium intake increases BP, and interventions to reduce the sodium intake have been shown to reduce BP.32,33 Our study showed that high BP could play a role in the association between sodium intake and metabolic syndrome. In this study, subjects taking antihypertensive medication showed lower 24hUNaE than did those without medication in both men and women; indeed, the results for the former group were comparable to results for those without hypertension. This finding raises the question of whether subjects receiving treatment for high BP restrict their sodium intake or whether the antihypertensive medications decrease sodium excretion. However, the latter possibility is inconsistent with the finding that sympathetic activation in subjects with hypertension increases renal sodium retention via stimulation of α1b-adrenoreceptors and activation of juxtaglomerular cells by β1-adrenoreceptors; additionally, antihypertensive medications, diuretics, and aldosterone blockers increase sodium excretion.34,35 A more reasonable explanation for this finding is that individuals taking antihypertensive medications, where more extensive lifestyle modifications are often applied, restrict their sodium intake as recommended. Thus, this result is similar to findings that hypertensive patients taking medications showed lower daily salt intake compared to those without mediations under ambulatory conditions.36 Thus, varying degrees of therapeutic lifestyle modification may be a confounding factor for the relationship between sodium intake and high BP in subjects with metabolic syndrome. Future studies may explain the observed differential association of decreased sodium intake with high BP by antihypertensive medication status.
There are several limitations to our study. We were not able to evaluate causality due to the cross-sectional design. Additionally, data on specific drug histories or other dietary components that might affect urinary sodium excretion were not available. Low dietary intake of potassium potentiates the effects of increased salt intake on BP.6 Another limitation was that urinary sodium excretion was not determined by 24-h collection, but by estimation from spot urine specimens. Single measurements of spot urinary sodium excretion do not allow precise quantification of an individual's sodium intake or validation of the variability of an individual's day-to-day dietary patterns. Although 24-h urine collection is used as the gold standard in validation studies, collecting 24-h urine was not feasible in this large-scale survey because it is labor intensive, and low response rates and under-collection create the potential for bias. In this study, a low-cost method with easy sampling and a large sample size was used to provide a valid population estimate of the level of sodium intake using spot urine sampling. In this study, low cost with readily sampling and a large sample size could account for valid estimate of population level of sodium intake using spot urine sampling. Lastly, the genetic variations with sensitivity of BP to dietary salt were not considered in this study. However, knowing specific genetic variants associated with salt sensitivity or hypertension, like the G-protein-coupled receptor kinases,37 calcium/calmodulin-dependent kinase IV,38 or platelet antigen 1,39 could understand susceptibility for hypertension and improve cardiovascular health in subjects with high sodium intake and metabolic syndrome. Despite these limitations, with the strength of a nationwide representative sample size, we found a clear association of the estimated 24hUNaE with the presence of metabolic syndrome. Thus, we suggest that sodium intake is involved in the development of metabolic syndrome.
In conclusion, subjects with metabolic syndrome had higher estimated 24hUNaE compared with those without it. 24hUNaE levels were increasing along the number of metabolic syndrome component, known as CVD risk factors. The relationship remained significant after adjustment for age, daily calorie intake, heavy alcohol drinking, regular exercise, college graduation, and antihypertensive medication, as well as excluding subjects with decreased renal function in men and women. This nationwide study of the relationship between 24hUNaE and metabolic syndrome may help to develop a comprehensive public health strategy to lower the prevalence of metabolic syndrome in Koreans.
Supplementary Material
Footnotes
Abbreviations: 24hUNaE = 24-h urinary sodium excretion, ANCOVA = analysis of covariance, BMI = body mass index, BP = blood pressure, CI = confidence interval, CVD = cardiovascular disease, DSM = Diagnostic and Statistical Manual of Mental Disorders, eGFR = estimation of glomerular filtration rate, FPG = fasting plasma glucose, HDL-C = high-density lipoprotein-cholesterol, KNHANES = Korea National Health and Nutrition Examination Survey, NCEP-ATPIII = the National Cholesterol Education Program Adult Treatment Panel, OR = odds ratio, SCr = serum creatinine, SD = standard deviation, SEM = standard error of the mean, TG = triglyceride, WC = waist circumference, WHO = World Health Organization.
The Priority Research Centers Program through the National Education, Science, and Technology (2010-0020224) supported this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lakka H-M, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama 2002; 288:2709–2716. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med 2004; 140:167–174. [DOI] [PubMed] [Google Scholar]
- 3.Lim S, Shin H, Song JH, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabet Care 2011; 34:1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yon M, Lee Y, Kim D, et al. Major sources of sodium intake of the Korean population at prepared dish level-based on the KNHANES 2008 & 2009. Korean J Community Nutr 2011; 16:473–487. [Google Scholar]
- 5.World Health Organization. Guideline: Sodium Intake for Adults and Children. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 6.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease—a delicate balance. N Engl J Med 2013; 368:1229–1237. [DOI] [PubMed] [Google Scholar]
- 7.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). Bmj 2007; 334:885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010; 362:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaglehole R, Bonita R, Horton R, et al. Priority actions for the non-communicable disease crisis. Lancet 2011; 377:1438–1447. [DOI] [PubMed] [Google Scholar]
- 10.Palaniappan L, Carnethon M, Fortmann SP. Association between microalbuminuria and the metabolic syndrome: NHANES III∗. Am J Hypertens 2003; 16:952–958. [DOI] [PubMed] [Google Scholar]
- 11.Han SY, Hong JW, Noh JH, et al. Association of the estimated 24-h urinary sodium excretion with albuminuria in adult Koreans: the 2011 Korea National Health and Nutrition Examination Survey. PLoS ONE 2014; 9:e109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Won JC, Lee YJ, Kim JM, et al. Prevalence of and factors associated with albuminuria in the Korean adult population: the 2011 Korea National Health and Nutrition Examination Survey. PLoS ONE 2013; 8:e83273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kweon S, Kim Y, Jang M-J, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014; 43:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005; 365:1415–1428. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens 2002; 16:97–103. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 1996; 27:481–490. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues SL, Baldo MP, de Sá Cunha R, et al. Salt excretion in normotensive individuals with metabolic syndrome: a population-based study. Hypertens Res 2009; 32:906–910. [DOI] [PubMed] [Google Scholar]
- 19.Kim BK, Lim YH, Kim SG, et al. Relationship between sodium intake and blood pressure according to metabolic syndrome status in the Korean National Health and Nutrition Examination Survey. Blood Press Monit 2012; 17:120–127. [DOI] [PubMed] [Google Scholar]
- 20.Leiba A, Vald A, Peleg E, et al. Does dietary recall adequately assess sodium, potassium, and calcium intake in hypertensive patients? Nutrition 2005; 21:462–466. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann IS, Cubeddu LX. Salt and the metabolic syndrome. Nutr Metabol Cardiovasc Dis 2009; 19:123–128. [DOI] [PubMed] [Google Scholar]
- 22.Rhee M-Y, Kim J-H, Kim Y-S, et al. High sodium intake in women with metabolic syndrome. Korean Circ J 2014; 44:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh JH, Lim JS, Lee MY, et al. Gender-specific association between urinary sodium excretion and body composition: analysis of the 2008–2010 Korean National Health and Nutrition Examination Surveys. Metabolism 2015; 64:837–844. [DOI] [PubMed] [Google Scholar]
- 24.Brown IJ, Tzoulaki I, Candeias V, et al. Salt intakes around the world: implications for public health. Int J Epidemiol 2009; 38:791–813. [DOI] [PubMed] [Google Scholar]
- 25.Anderson CA, Appel LJ, Okuda N, et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J Am Diet Assoc 2010; 110:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaw K-T, Bingham S, Welch A, et al. Blood pressure and urinary sodium in men and women: the Norfolk Cohort of the European Prospective Investigation into Cancer (EPIC-Norfolk). Am J Clin Nutr 2004; 80:1397–1403. [DOI] [PubMed] [Google Scholar]
- 27.Larsen SC, Ängquist L, Sørensen T, et al. 24 h urinary sodium excretion and subsequent change in weight, waist circumference and body composition. PLoS ONE 2013; 8:e69689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baudrand R, Campino C, Carvajal C, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol 2014; 80:677–684. [DOI] [PubMed] [Google Scholar]
- 29.Fujita T. Insulin resistance and salt-sensitive hypertension in metabolic syndrome. Nephrol Dial Transplant 2007; 22:3102–3107. [DOI] [PubMed] [Google Scholar]
- 30.Santulli G, Pagano G, Sardu C, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 2015; 125:4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexiewicz JM, Gaciong Z, Parise M, et al. Effect of dietary sodium intake on intracellular calcium in lymphocytes of salt-sensitive hypertensive patients. Am J Hypertens 1992; 5:536–541. [DOI] [PubMed] [Google Scholar]
- 32.Group ICR. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Bmj 1988; 297:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacGregor G, Sagnella G, Markandu N, et al. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet 1989; 334:1244–1247. [DOI] [PubMed] [Google Scholar]
- 34.Bakris GL, Townsend RR, Liu M, et al. Impact of renal denervation on 24-hour ambulatory blood pressure: results from SYMPLICITY HTN-3. J Am Coll Cardiol 2014; 64:1071–1078. [DOI] [PubMed] [Google Scholar]
- 35.DiBona GF. Physiology in perspective: the wisdom of the body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol 2005; 289:R633–R641. [DOI] [PubMed] [Google Scholar]
- 36.Kawamura M, Kusano Y, Takahashi T, et al. Effectiveness of a spot urine method in evaluating daily salt intake in hypertensive patients taking oral antihypertensive drugs. Hypertens Res 2006; 29:397–402. [DOI] [PubMed] [Google Scholar]
- 37.Santulli G, Trimarco B, Iaccarino G. G-protein-coupled receptor kinase 2 and hypertension: molecular insights and pathophysiological mechanisms. High Blood Press Cardiovasc Prev 2013; 20:5–12. [DOI] [PubMed] [Google Scholar]
- 38.Santulli G, Cipolletta E, Sorriento D, et al. CaMK4 gene deletion induces hypertension. J Am Heart Assoc 2012; 1:e001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galasso G, Santulli G, Piscione F, et al. The GPIIIA PlA2 polymorphism is associated with an increased risk of cardiovascular adverse events. BMC Cardiovasc Disord 2010; 10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


