Abstract
Depressive symptoms are common in multiple psychiatric disorders and are frequent sequelae of trauma. A dimensional conceptualization of depression suggests that symptoms should be associated with a continuum of deficits in specific neural circuits. However, most prior investigations of abnormalities in functional connectivity have typically focused on a single diagnostic category using hypothesis-driven seed-based analyses. Here, using a sample of 105 adult female participants from three diagnostic groups (healthy controls, n = 17; major depression, n = 38; and post-traumatic stress disorder, n = 50), we examine the dimensional relationship between resting-state functional dysconnectivity and severity of depressive symptoms across diagnostic categories using a data-driven analysis (multivariate distance-based matrix regression). This connectome-wide analysis identified foci of dysconnectivity associated with depression severity in the bilateral amygdala. Follow-up seed analyses using subject-specific amygdala segmentations revealed that depression severity was associated with amygdalo-frontal hypo-connectivity in a network of regions including bilateral dorsolateral prefrontal cortex, anterior cingulate and anterior insula. In contrast, anxiety was associated with elevated connectivity between the amygdala and the ventromedial prefrontal cortex. Taken together, these results emphasize the centrality of the amygdala in the pathophysiology of depressive symptoms, and suggest that dissociable patterns of amygdalo-frontal dysconnectivity are a critical neurobiological feature across clinical diagnostic categories.
INTRODUCTION
Multiple psychiatric disorders beyond major depressive disorder (MDD) are associated with symptoms of depression.1 This reality is recognized by the NIMH Research Domain Criteria (RDoC), which seeks to map dimensions of psychopathology that are present across clinical diagnostic criteria to abnormalities in specific brain circuitry.2 Two disorders characterized by mood disturbances, MDD and post-traumatic stress disorder (PTSD), are common disorders that cause significant morbidity and mortality. Both are enduring conditions with significant impairment in social and occupational functioning, high levels of recurrence and frequent suicide.3–6
Notably, PTSD and MDD are frequently comorbid, and common behavioral and neural abnormalities have been described.7–11 Prior research has shown that MDD occurs in 48–69% of individuals with PTSD.3,12–14 Likewise, PTSD in the context of MDD often goes unrecognized.13,15 The National Comorbidity Survey reported that PTSD preceded MDD 78% of the time in individuals with both PTSD and MDD, suggesting that PTSD may be a strong predictor of future MDD.3 In addition, recent research has shown that high rates of comorbidity occur even when overlapping symptoms were removed from the diagnoses.12,16 One conceptualization is a general ‘anxious-misery’ class of disorders (including PTSD and MDD), which fits within the negative valence construct defined within RDoC.15,17
Potentially, such comorbid presentation and shared phenomenology could be the result of dysfunction of specific neural circuits that are common to both MDD and PTSD. One powerful tool for the examination of circuit-level abnormalities is resting-state (intrinsic) functional connectivity.18 Prior studies of functional connectivity in both MDD and PTSD provide ample evidence for similar deficits.8,9,19–23 However, previous investigations of changes in functional connectivity have typically focused on comparisons between each diagnosis (MDD and PTSD) and controls, but have not examined common dimensional effects across both clinical groups. Studies of both disorders report abnormalities in a common set of brain regions that span both frontal regions involved in cognitive control as well as affective limbic regions. Specifically, the amygdala is one region that is among the most commonly implicated in both disorders.9,20,21,23–29 Similarly, hypo-connectivity of frontal control regions such as the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) with the amygdala and other brain regions has often been reported.8–10,21,22,27,30,31 Despite this, to our knowledge no studies have directly examined depression severity on a dimensional basis across both MDD and PTSD. Furthermore, most prior studies have been limited by analyses that have examined a specific subset of regions (using seed-based analyses) or within a restricted set of brain networks.
Accordingly, here we investigated common dimensional effects of depression on functional connectivity in a large sample of unmedicated patients with MDD or PTSD. To explore dysconnectivity beyond a specific set of brain regions, we conducted a connectome-wide association study using a recently introduced technique called multivariate distance-based matrix regression (MDMR32). Previously applied in analysis of large-scale ecological and genetic data sets, MDMR allows one to interrogate how the overall multivariate pattern of connectivity differs at each voxel in association with symptoms of depression while controlling for covariates. We hypothesized that this fully data-driven analysis would reveal common dimensional effects of depression severity across MDD, PTSD and matched controls. As described below, this approach yielded novel evidence of common, dimensional patterns of amygdalo-frontal dysconnectivity in association with depressive symptoms across disorders.
MATERIALS AND METHODS
Participants and clinical assessment
This study considered 105 unmedicated females from three groups, including 38 with major depression (MDD), 50 patients with PTSD, and 17 demographically matched healthy controls. Demographic details are provided in Table 1. All participants were female, right-handed, English-speaking and aged 18–55. This study focused on females because all PTSD patients were female victims of intimate partner violence. All participants provided written informed consent; the Human Subjects Committees of both Washington University and the University of Missouri-St Louis approved all study procedures. Inclusion diagnosis for MDD and PTSD was established according to DSM-IV-TR criteria using the SCID33 and the CAPS-IV.34 Participants were excluded from the study due to [1] comorbid neurological disorders; [2] current alcohol or substance use disorder; [3] history of psychotic disorder, bipolar disorder or obsessive-compulsive disorder; [4] and current suicide risk; [5] recent treatment with any psychotropic or central nervous system–active drug. Specifically, subjects were required to have not been treated with any psychotropic medication for at least 3 weeks (5 weeks for fluoxetine). Depression and anxiety symptom severity were assessed on the same day as imaging. Depression severity was assessed by a trained clinician using the Montgomery Asberg Depression Rating Scale.35 To evaluate depressive symptoms while controlling for comorbid symptoms of anxiety, anxiety symptoms were assessed using the state subscale of the State-Trait Anxiety Inventory.36 To be included, subjects were required to have complete imaging and clinical data including depression and anxiety rating scales. Of those eligible for inclusion, nine participants were excluded due to high motion any individual series with a mean relative displacement >0.25 mm;37 or poor image coverage.
Table 1.
Sample demographics and participant characteristics
| Healthy comparators | MDD | PTSD | P-value | |
|---|---|---|---|---|
| n | 17 | 38 | 50 | NA |
| % Female | 100 | 100 | 100 | NA |
| % Taking psychotropic medication | 0 | 0 | 0 | NA |
| Mean age (years; s.d.) | 31.7 (10.5) | 33.2 (8.4) | 30.1 (9.2) | 0.51 |
| Depression severity (total MADRS; s.d.) | 1.6 (2.0) | 28.1 (6.1) | 19 (8.3) | < 0.001 |
| Anxiety severity (total STAI-S; s.d.) | 27 (8.3) | 60.1 (9.6) | 49.8 (14.1) | < 0.001 |
| In-scanner motion (mean relative displacement; s.d.) | 0.09 (0.04) | 0.1 (0.04) | 0.09 (0.04) | 0.36 |
Abbreviations: MADRS, Montgomery Asberg Depression Rating Scale; MDD, major depressive disorder; NA, not applicable; PTSD, post-traumatic stress disorder; STAI-S, State-Trait Anxiety Inventory-S.
Image acquisition and processing
All subjects were imaged on the same scanner 3T Tim Trio (Siemens, Erlangen, Germany) using the same acquisition protocol. High-resolution structural images were acquired using a T1-weighted MPRAGE sequence: TR 2400 ms, TE 3.13 ms, TI 1000 ms, flip angle 8°, slice thickness/gap 1 mm/0 mm and effective voxel resolution 1.0 mm3. Resting-state gradient spin-echo functional images were acquired in two series of 210 volumes (7:42 duration each) using the following parameters: TR 2200 ms, TE 27 ms, flip angle 90°, slice thickness/gap 4 mm/0 mm and effective voxel resolution 4.0 mm3.
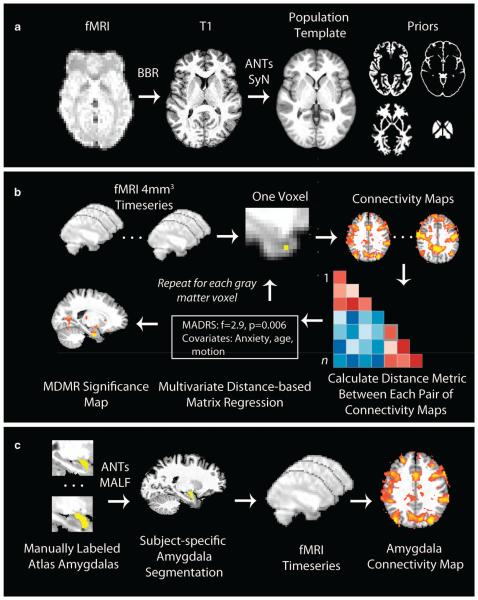
To avoid registration bias and maximize sensitivity to detect regional effects that can be impacted by registration error, a custom template was created with advanced normalization tools (ANTs);38 T1 images were normalized to this population-specific template space using the top-performing SyN diffeomorphic registration method implemented in ANTs (Figure 1a39). Structural images were then processed with ‘antsCorticalThickness’,40 which uses the custom template to guide brain extraction, N4 bias correction,41 and Atropos probabilistic tissue segmentation.42
Figure 1.
Image registration and analysis procedures. (a) Image registration. Functional images were registered to a population-specific template using advanced normalization tools (ANTs). T1 image skull stripping, bias correction and segmentation were guided using prior information provided by the template. Functional images were coregistered to the T1 using boundary-based registration (BBR). (b) Connectome-wide analysis using multivariate distance-based matrix regression (MDMR). Template-space functional time series were resampled at 4 mm3 for computational feasibility. For each gray matter voxel a connectivity map was created for each subject, which were compared with a pairwise manner to create a distance matrix. MDMR used these distance matrices to evaluate the complex multivariate pattern of connectivity associated with depression severity across subjects while controlling for clinical group, anxiety, age and in-scanner motion. This yielded a pseudo-F statistic; a P-value was determined using permutation testing. This procedure was repeated for each gray matter voxel, yielding a voxel-wise significance map. (c) Amygdala seed connectivity using multi-atlas label fusion (MALF). To obtain a maximally accurate estimate of amygdala connectivity, we used MALF to create subject-specific amygdala segmentations that were in turn used for seed-based connectivity analyses. Manually labeled amygdalas from 30 images were registered to each participant’s T1 image using ANTs, and MALF was used to derive a subject-specific amygdala segmentation. This was projected into native fMRI space and used to extract the average amygdala time course, which was in turn used to generate a seed connectivity map. These native-space maps were registered to the population template as in a before group-level analysis. Elements of b modified with permission from Shehzad et al.32
Resting-state time series data was processed using a validated confound regression procedure that has been optimized to reduce the influence of subject motion.43 After the first four volumes of the functional time series were removed to allow signal stabilization, functional images were realigned using MCFLIRT,44 and smoothed with a Gaussian filter at 6 mm full width at half maximum. Confound regression included nine confounding signals (six motion parameters+global/white matter/cerebral spinal fluid) as well as the temporal derivative, quadratic term and temporal derivative of the quadratic of each (36 regressors total43). Finally, time series were band-pass filtered to retain frequencies between 0.01 and 0.08 Hz; all motion parameters and confound time courses were band-pass filtered in an identical manner as the time series data itself to prevent frequency mismatch.45 Functional images were coregistered to the T1 image using boundary-based registration,46 and aligned to template space using the ANTs transform for the T1 image as above. Time-series images were resampled to 4 mm3 isotropic voxels in the template space before connectome-wide association study for computational feasibility;32 higher spatial resolution images (2 mm3) was used for follow-up seed analyses. Throughout, all transformations were concatenated so that only one interpolation was performed.
Multivariate distance-based matrix regression
As previously described,32 MDMR operates in three steps (Figure 1b). First, the template-space 4-mm voxel-wise participant time series data are used to conduct a seed-based connectivity analysis at each voxel within gray matter, by calculating the Pearson’s correlation coefficient between each voxel’s time series and the time series of every other gray matter voxel. Second, the overall multivariate pattern of connectivity for each voxel is compared among participants using a distance metric that is a function of Pearson’s correlation. Third, MDMR is used to test how well each phenotypic variable explains the distances between each participant’s connectivity patterns created in the second step. This provides a measure of how the overall pattern of connectivity is impacted by each group-level variable (that is, dimensional depression severity). Notably, as matrix regression evaluates the overall pattern of connectivity across the entire connectome, it is sensitive to robust, distributed patterns of dysconnectivity but not more focal deficits. In contrast to other multivariate methods, this allowed us to examine the dimensional association between depression severity and multivariate patterns of connectivity while controlling for potentially confounding variables. Here, we controlled for clinical group, subject age, anxiety and in-scanner motion (mean relative displacement averaged over both series37). For each voxel’s connectivity pattern, MDMR yields a pseudo-F statistic, the significance of which was assessed using 5000 iterations of a permutation test.47 This pseudo-F statistic is robust to differences in group sample size. The ultimate product of this procedure is a voxel-wise significance map showing where depression severity impacts the overall pattern of connectivity at each voxel. As in Shehzad et al.32 type I error was controlled using cluster-correction with a voxel height of z>1.64; corrected significance was set at P < 0.001 as determined by 10 000 Monte-Carlo simulations.48
Amygdala segmentation and connectivity analysis using multi-atlas label fusion
Although MDMR identifies clusters where a group difference in the overall multivariate pattern of connectivity is present, it does not describe what pattern of connectivity is driving the observed result. Accordingly, we conducted post-hoc seed-based analyses to understand this dysconnectivity associated with depression severity across categorical diagnoses. As described below, MDMR revealed that depression severity was associated with dysconnectivity of medial temporal lobe voxels consistent with the anatomical location of the amygdala. Notably, although the initial MDMR allows for a connectome-wide search for multivariate effects, it does so using relatively coarse resolution data (4 mm voxels). A substantial body of work has shown that for small subcortical structures such as the amygdala, region definition using individualized segmentation with a T1 image is far superior to template-level region definition.49
Accordingly, amygdala segmentation of each individual T1 image was performed using an advanced multi-atlas label fusion (Figure 1c) procedure implemented in ANTs. A set of 30 T1 magnetic resonance imaging images from the OASIS data set were manually labeled by Neuromorphometrics, Inc. (http://Neuromorphometrics.com/); the 30-labeled atlases were registered to each subject’s T1 image using ANTs and a final amygdala segmentation was synthesized by joint label fusion.49 To aid visualization of the variability between the subject-specific T1 segmentation and the group-level template, each individual amygdala was projected to the custom template and an overlap map was constructed (see Figure 2, below).
Figure 2.
Depression severity is associated with multivariate patterns of dysconnectivity in bilateral amygdala. The connectome-wide association study using multivariate distance-based matrix regression (MDMR) identified two clusters (yellow) where the multivariate pattern of dysconnectivity was related to dimensional depression severity (total Montgomery Asberg Depression Rating Scale (MADRS)) across clinical groups. Cluster threshold z>1.64, P < 0.001; model covariates included clinical diagnostic group, anxiety (State-Trait Anxiety Inventory-S (STAI-S)), participant age and in-scanner motion. Subject-specific amygdala segmentations provided by multi-atlas label fusion (MALF; Figure 1c) are shown in green over the population template, which underlines both the variability in amygdala definition across subjects and also the high degree of overlap with the MDMR results.
For amygdala connectivity analyses, the individually segmented structural amygdala for each subject was coregistered to the BOLD time series, and the mean time series was extracted. Next, a seed map was created for each subject by calculating the Pearson’s correlation between the subject-specific amygdala time series and every other voxel in the brain. Seed maps were Fischer z-transformed to approximate normality and projected back to the group-level custom template through the T1 image by concatenating the blood oxygen level dependent to T1 co-registration and the T1 to template deformation. These maps were finally evaluated with a group-level regression to determine the association between amygdala connectivity and depression severity (Montgomery Asberg Depression Rating Scale). Motion and age were included as covariates in all analyses; as described below, anxiety and clinical group were additionally evaluated in subsequent models that were used to examine specificity. For follow-up seed analyses, clusters were considered significant above a voxel height of z>2.3, and a corrected cluster probability of P < 0.001.48 Results were displayed with a cortical surface projection rendered using Caret.50 However, it should be emphasized that this follow-up seed-based analysis subsequent to MDMR does not constitute a unique hypothesis test, as the amygdala was identified based on the significance of the MDMR result. Rather, this follow-up seed analysis is a necessary post-hoc test to understand the MDMR result.
Supplementary analyses
Although the above analyses focused on the dimensional relationship with depression severity, we conducted several supplementary analyses to evaluate the specificity of the observed results within the context of other variables of interest including clinical group and anxiety. First, we re-evaluated clusters identified in the dimensional analysis of depression severity while including anxiety and/or group as covariates. As described below, this analysis suggested that amygdala-vmPFC connectivity was linked to anxiety. Accordingly, we reran the amygdala seed analysis while considering each variable (Montgomery Asberg Depression Rating Scale and State-Trait Anxiety Inventory-S) alone and also when modeled jointly. Second, to evaluate if dimensional results were similar within the patient groups alone, we re-evaluated significant clusters while excluding healthy control participants. Third, to explore effects of anxiety beyond the amygdala, we used MDMR to search the connectome for patterns of dysconnectivity associated with the State-Trait Anxiety Inventory-S while controlling for age, motion and depressive symptoms (Montgomery Asberg Depression Rating Scale). Finally, although the focus of this study was dimensional dysconnectivity associated with depression, we also evaluated categorical group differences in amygdala connectivity using a 3×1 group-level analysis of variance using ANTsR (http://stnava.github.io/ANTsR/). Pairwise post-hoc tests were conducted within each cluster using the least-squares means procedure, controlling for multiple comparisons using the Tukey method.
RESULTS
Connectome-wide analysis using MDMR identifies foci of dysconnectivity in bilateral medial temporal lobe
We evaluated multivariate patterns of dysconnectivity associated with depression severity across clinical diagnostic groups using MDMR. This connectome-wide analysis revealed that depression severity was associated with changes in connectivity in the bilateral medial temporal lobe, centered on the amygdala (Figure 2; left: 85 voxels (4 mm) with cluster center of gravity in Montreal Neurological Institute coordinates: − 27, − 9, − 22; right: 92 voxels at 31, − 11, − 21). No other significant clusters were detected. However, MDMR does not describe which specific connections form the basis for the observed multivariate results. Furthermore, the relatively coarse (4 mm) resolution of the analysis limited the ability to specifically isolate small subcortical structures. Accordingly, we next conducted a focused seed-based analysis of amygdala connectivity.
Depression severity is related to dimensional impairment in amygdalo-frontal connectivity
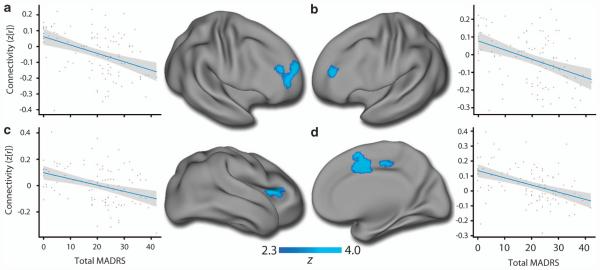
Given the small size of the amygdala and known inaccuracies in using template-space demarcations of small subcortical structures, we individually segmented each subject’s amygdala using a top-performing multi-atlas labeling procedure.49 Seed-based analyses from these customized amygdala segmentations revealed that depression severity across diagnostic categories is associated with diminished connectivity between the amygdala and a network of frontal regions (Supplementary Table 1A). Regions impacted included bilateral DLPFC, anterior cingulate and anterior insula (Figure 3). When these clusters were re-evaluated with anxiety and clinical group included as model covariates, or only among patients, effects remained consistent (Supplementary Table 2A).
Figure 3.
Depression severity is associated with amygdalo-frontal hypo-connectivity across clinical diagnostic groups. Subject-specific amygdala segmentations from multi-atlas label fusion (MALF) were used to conduct seed-based connectivity analyses. Depression severity (total Montgomery Asberg Depression Rating Scale (MADRS)) was correlated with diminished amygdala connectivity with a network of frontal regions including the bilateral dorsolateral prefrontal cortex (DLPFC; a and b), anterior insula (c) and anterior cingulate (d). All panels reflect right amygdala connectivity except for (d); for detailed results see Supplementary Table S2. Model covariates included age and in-scanner motion; inclusion of anxiety and clinical diagnostic group yielded similar results within these regions (Supplementary Table S3). Images thresholded at z>2.3, P < 0.001.
Amygdala hyper-connectivity with ventral medial prefrontal cortex (vmPFC) is related to anxiety
In contrast with such amygdalo-frontal hypo-connectivity, depression severity was also associated with hyper-connectivity between the right amygdala and the vmPFC and subgenual anterior cingulate cortex (sgACC) (Figure 4a, Supplementary Table 1B). However, the relationship between depression and amygdala-vmPFC/sgACC hyper-connectivity was substantially attenuated when anxiety was included as a covariate (Supplementary Table 2B). Accordingly, we conducted voxel-wise analyses where we examined each variable alone and also jointly. As displayed in Figure 4b, amygdala-vmPFC/sgACC hyper-connectivity was more robustly associated with anxiety than depression. When both variables were modeled together on a voxel-wise basis, only the relationship with anxiety remained significant (Figure 4c). Notably the pattern of amygdala hyper-connectivity with the sgACC/vmPFC and hypo-connectivity with DLPFC were significantly anti-correlated (t(204) = 3.0; P = 0.004 for right DLPFC).
Figure 4.
Amygdala hyper-connectivity with the vmPFC is more closely linked with anxiety than depression. Although depression (a; Montgomery Asberg Depression Rating Scale (MADRS)) was related to elevated right amygdala-vmPFC connectivity, the relationship with anxiety (State-Trait Anxiety Inventory-S (STAI-S)) was more robust (b). When both variables were modeled together (c), only the anxiety remained significant.
To explore effects of anxiety beyond the amydgala, we also conducted a MDMR analysis of anxiety (State-Trait Anxiety Inventory-S) while controlling for depression, age and motion. This revealed a significant cluster in the left parahippocampal gyrus, which was driven by enhanced connectivity with the anterior cingulate as well as somatosensory, auditory and visual cortex (Supplementary Figure 1).
Categorical analyses based on clinical diagnosis
Although this investigation was focused on mechanisms of dysconnectivity associated symptom severity across clinical diagnoses, we additionally conducted categorical analyses of amygdala connectivity based on diagnostic group. These analyses revealed disrupted amygdala connectivity with regions implicated in dimensional analyses in both patient groups (Supplementary Table 3). However, in general results were less robust than the dimensional findings reported above. Regions impacted included the anterior cingulate, anterior insula and DLPFC, where amygdala connectivity in control subjects was higher than both patient groups.
DISCUSSION
In this study, we conducted a connectome-wide study examining how patterns of dysconnectivity relate to dimensionally defined symptoms of depression in a large sample of medication-free MDD and PTSD patients. This data-driven approach revealed multivariate patterns of dysconnectivity centered on the bilateral amygdala. Follow-up seed-based analyses using subject-specific amygdala segmentations revealed that depression was associated with diminished amygdala connectivity with frontal regions including bilateral DLPFC and ACC. In contrast, elevated amygdala connectivity with the sgACC and vmPFC was more tightly linked with symptoms of anxiety rather than depression. Taken together, these findings provide novel evidence for amygdala dysconnectivity as a critical mechanism of psychopathology across negative valence disorders such as MDD and PTSD.
Connectome-wide search reveals common patterns of amygdala dysconnectivity
Most studies investigating abnormalities of functional connectivity in PTSD or MDD have employed either seed-based or network-based approaches with regions of interest (nodes) defined a priori. In contrast, we used MDMR32 to conduct a fully data-driven analysis of the entire functional connectome. Remarkably, this multivariate analysis identified abnormalities in the bilateral amygdala. As described below, this parallels prior findings in single-disorder case–control studies, where the amygdala is one of the regions most frequently implicated in the pathophysiology of both disorders.51–54
Amygdalo-frontal hypo-connectivity is associated with symptoms of depression
As MDMR produces a multivariate result but does not provide information on what features of connectivity are driving the finding, we conducted follow-up seed-based connectivity analyses. Seed analyses based on highly accurate subject-specific segmentations revealed that connectivity with a network of frontal regions including the dorsolateral prefrontal cortex, anterior insula and anterior cingulate was reduced in proportion to depressive symptoms across clinical groups.
These results parallel prior findings in case–control studies of MDD and PTSD, which have frequently reported amygdalo-frontal hypo-connectivity.8,9,20,21,23,28,29,31 For example, Matthews et al.21 presented evidence for diminished coupling between the amygdala and dorsal cingulate that scaled with depression severity. Similarly, Fonzo et al.20, reported diminished connectivity between the amygdala and cingulate as well as the insula in a sample of women with PTSD. Such results showing abnormal patterns of functional connectivity in depression and PTSD add to the extensive literature describing abnormalities of amygdala activation in both disorders.24,30,55–57 Importantly, the present dimensional findings suggest that amygdala hypo-connectivity with frontal cortex may be a common pathophysiological mechanism that scales with symptom severity across clinical groups. The regions impacted belong to cognitive control networks,58,59 and the observed abnormalities may be associated with a diminished ability of such networks to effectively regulate the amygdala in a top–down manner.22,30
Elevated amygdala-vmPFC/sgACC connectivity is more tightly linked to anxiety
In contrast to the observed hypo-connectivity between amygdala and dorsal frontal regions (DLPFC, ACC and anterior insula) in association with depression, anxiety was associated with hyper-connectivity between the amygdala and vmPFC/sgACC. Notably, the connectome-wide analysis using MDMR evaluated depressive symptoms after controlling for anxiety (as well as group, age and in-scanner motion). Similarly, specificity analyses examining clusters identified by the follow-up seed analysis revealed that amygdalo hypo-connectivity with the DLPFC, ACC and insula was more strongly linked to depression than anxiety. However, the converse was true of amygdala hyper-connectivity with the vmPFC/sgACC, which was in fact more closely associated with symptoms of anxiety across both MDD and PTSD.
Prior studies of both MDD and PTSD have implicated abnormalities of the vmPFC and sgACC.9,19,21,23,24,26,31 These regions are a hub of the default mode network;60 default mode network connectivity has been found to be abnormal in multiple studies of MDD.61–63 In addition, now-canonical work by Mayberg et al. identified hyperactivity in the sgACC as a predictor of treatment response to deep brain stimulation in treatment-resistant depression.64 Although studies in PTSD are somewhat more heterogeneous,65–67 several have described both hyper-activation and hyper-connectivity of the vmPFC.19,23,24 Hypo-activation of the sgACC/vmPFC has been reported in PTSD as well, but usually in the context of fear paradigms designed to provoke symptoms.10,54 Although further research is necessary to disentangle these effects, such findings are not mutually exclusive; it is possible that the sgACC/vmPFC has both elevated connectivity with the amygdala at rest but also diminished activation during fear-related paradigms.
These results suggest a double-dissociation in abnormalities of amygdala connectivity, whereby amygdala hypo-connectivity with dorsal frontal regions is more related to depression whereas amygdala hyper-connectivity with the vmPFC/sgACC is more closely tied to symptoms of anxiety. Such results accord with research from both lesion studies27 and human imaging10,68 suggesting that dorsal (cognitive control) and ventral (default mode) regions within frontal cortex have differential roles in regulating amygdala function. As the sgACC/vmPFC is known to send output to GABAergic intercalated cells in the amygdala that provide inhibitory input to the central nucleus of the amygdala,10 the present results may reflect an inefficient, compensatory effort to provide amygdala regulation by the vmPFC in association with anxiety in both MDD and PTSD. The hyper-connectivity seen between the amygdala and vmPFC stands in notable contrast to the amygdalar hypo-connectivity with DLPFC and anterior insula seen in association with depressive symptoms, and suggests that abnormalities in distinct regulatory circuits may be associated with different symptom dimensions within negative valence disorders. Interestingly, these two deficits were in fact inversely related to each other. Although speculative, this data suggests that brain-based connectivity phenotypes may have more individual specificity than clinical symptoms that are often highly correlated.
Limitations
Several limitations of the current study should be noted. First, cross-sectional results cannot disentangle effects of mood state from trait effects that may be present across mood states. Longitudinal studies that track patients across mood episodes are necessary to establish specific links between mood state and the patterns of dysconnectivity observed here. Second, both depression and anxiety are heterogeneous constructs that include symptoms across potentially separable psychological domains. Studies that include detailed symptom assessments of specific domains will help further parse the present results. Third, through our focus on patients who met full diagnostic criteria for PTSD or MDD, we did not evaluate individuals with other disorders (bipolar disorder, obsessive-compulsive disorder) or those who were distressed by symptoms but sub-syndromal at the time. Future studies that include a broader range of both disorders and symptom severity may provide enhanced power and increased generalizability. Finally, as the focus of this study was on common mechanisms of dysconnectivity that are present across both disorders, we did not comprehensively evaluate group differences on a connectome-wide level. Although the present data and other recent work69 support the importance of dimensional models of depression and anxiety, recent work by Oathes et al.8 emphasizes that clinical diagnosis provides valuable information as well.
Conclusions and future directions
These limitations notwithstanding, these results provide a critical link between studies in the usually separate literatures of MDD and PTSD.
The results buttress prior research using case–control designs in single disorders and importantly place such abnormalities on a common dimensional scale, suggesting that similar neural mechanisms of amygdala dysfunction may occur in both disorders. As such, these results accord with the negative valence construct embedded within new RDoC framework.2,70,71
The presence of such common mechanisms underscore the need for clinical trials which are focused on abnormalities of specific biological circuits, which may provide insight whether effective treatment could in fact normalize such deficits. Ultimately, such research may help develop precision medicine for mood disorders through use of neuroimaging-based biomarkers, which would allow for substantial improvements in treatment response by tailoring interventions to each individual patient.72,73
Supplementary Material
Footnotes
CONFLICT OF INTEREST
CC was previously on the speaker’s bureau for Bristol-Myers Squibb and Otsuka Pharmaceuticals and has received research funding from Bristol-Myers Squibb, Cyberonics, the Stanley Baer Foundation, the Brain and Behavior Research Foundation and the Taylor Foundation for Innovative Psychiatric Research. He serves as a non-paid consultant to Cyberonics. Remaining authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
REFERENCES
- 1.Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the united states. Annu Rev Public Health. 2008;29:115–129. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- 2.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (rdoc): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. J Clin Psychiatry. 2000;61:4–12. [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 6.Zlotnick C, Rodriguez BF, Weisberg RB, Bruce SE, Spencer MA, Culpepper L, et al. Chronicity in posttraumatic stress disorder and predictors of the course of post-traumatic stress disorder among primary care patients. J Nerv Ment Dis. 2004;192:153–159. doi: 10.1097/01.nmd.0000110287.16635.8e. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 8.Oathes DJ, Patenaude B, Schatzberg AF, Etkin A. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol Psychiatry. 2015;77:385–393. doi: 10.1016/j.biopsych.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-mri and treatment response in major depressive disorder. J Affect Disord. 2014;172C:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhai JD, Grubaugh AL, Kashdan TB, Frueh BC. Empirical examination of a proposed refinement to DSM-IV posttraumatic stress disorder symptom criteria using the national comorbidity survey replication data. J Clin Psychiatry. 2008;69:597–602. doi: 10.4088/jcp.v69n0411. [DOI] [PubMed] [Google Scholar]
- 13.Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- 14.Keane TM, Kaloupek DG. Comorbid psychiatric disorders in PTSD. Implications for research. Ann N Y Acad Sci. 1997;821:24–34. doi: 10.1111/j.1749-6632.1997.tb48266.x. [DOI] [PubMed] [Google Scholar]
- 15.Grant DM, Beck JG, Marques L, Palyo SA, Clapp JD. The structure of distress following trauma: posttraumatic stress disorder, major depressive disorder, and generalized anxiety disorder. J Abnorm Psychol. 2008;117:662–672. doi: 10.1037/a0012591. [DOI] [PubMed] [Google Scholar]
- 16.Ford JD, Elhai JD, Ruggiero KJ, Frueh BC. Refining posttraumatic stress disorder diagnosis: evaluation of symptom criteria with the national survey of adolescents. J Clin Psychiatry. 2009;70:748–755. doi: 10.4088/JCP.08m04692. [DOI] [PubMed] [Google Scholar]
- 17.Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clin Psychol Rev. 2009;30:839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 19.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Théberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 20.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown VM, LaBar KS, Haswell CC, Gold AL, McCarthy G, Morey RA. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39:351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fmri study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCabe C, Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage. 2011;57:1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veer IM, Oei NYL, Spinhoven P, van Buchem MA, Elzinga BM. Rombouts SARB. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57:1534–1541. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- 27.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, et al. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-mri studies in major depression. J Affect Disord. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Shehzad Z, Kelly C, Reiss PT, Cameron Craddock R, Emerson JW, McMahon K, et al. A multivariate distance-based analytic framework for connectome-wide association studies. Neuroimage. 2014;93:74–94. doi: 10.1016/j.neuroimage.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, patient edition (SCID-P), version 2. Biometrics Research; New York, New York State Psychiatric Institute: 1996. [Google Scholar]
- 34.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 36.Spielberger CD, Gorsuch RL, Lushene RE, editors. Manual for the state-trait anxiety inventoryManual for the State-trait Anxiety Inventory. 1970. [Google Scholar]
- 37.Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ants similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, et al. Large-scale evaluation of ants and freesurfer cortical thickness measurements. Neuroimage. 2014;99:166–179. doi: 10.1016/j.neuroimage.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 41.Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9:381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 45.Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fmri preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82C:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiss PT, Stevens MHH, Shehzad Z, Petkova E, Milham MP. On distance-based permutation tests for between-group comparisons. Biometrics. 2010;66:636–643. doi: 10.1111/j.1541-0420.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- 48.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Suh JW, Das SR, Pluta J, Craige C, Yushkevich PA. Multi-Atlas segmentation with joint label fusion. IEEE Trans Pattern Anal Mach Intell. 2013;35:611–623. doi: 10.1109/TPAMI.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 53.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 56.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fmri study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 57.Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, et al. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010;49:42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 61.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 65.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, et al. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Y, Wang Z, Qin L-D, Wan J-Q, Sun Y-W, Su S-S, et al. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PLoS One. 2012;7:e46833. doi: 10.1371/journal.pone.0046833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satterthwaite TD, Kable JW, Vandekar L, Katchmar N, Bassett DS, Baldassano CF, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression reward dysfunction in depression. Neuropsychopharmacology. 2015;40:2258–2268. doi: 10.1038/npp.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, et al. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- 71.Cuthbert BN. The rdoc framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou Z-W, Williams LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci USA. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.