Abstract
The therapeutic effect in non–small cell lung cancer (NSCLC) patients is limited because of intrinsic and acquired resistance. Thus, an unmet need exists for the development of new drugs to improve the therapeutic efficacy in NSCLC patients. In this study, the novel small molecule indolizino[6,7-b]indole derivative BO-1978 was selected to evaluate its therapeutic effects on NSCLC and its preclinical toxicity in animal models. An in vitro cytotoxicity assay revealed that BO-1978 significantly suppressed the growth of various NSCLC cell lines with or without mutations in epidermal growth factor receptor (EGFR). Mechanistically, we demonstrated that BO-1978 exhibited multiple modes of action, including inhibition of topoisomerase I/II and induction of DNA cross-linking. Treatment of NSCLC cells with BO-1978 caused DNA damage, disturbed cell cycle progression, and triggered apoptotic cell death. Furthermore, BO-1978 significantly suppressed the growth of EGFR wild-type and mutant NSCLC tumors in xenograft tumor and orthotopic lung tumor models with negligible body weight loss. The combination of BO-1978 with gefitinib further suppressed EGFR mutant NSCLC cell growth in xenograft tumor and orthotopic lung tumor models. Preclinical toxicity studies showed that BO-1978 administration did not cause apparent toxicity in mice. Based on its significant therapeutic efficacy and low drug toxicity, BO-1978 is a potential therapeutic agent for treatment of NSCLC.
Abbreviations: EGFR, epidermal growth factor receptor; ICLs, interstrand DNA cross-links; MDR, multiple drug resistance; NSCLC, Non-small cell lung cancer; TKIs, tyrosine kinase inhibitors; Topo, topoisomerase
Introduction
Lung cancer causes one third of cancer deaths worldwide because of its high incidence and high mortality [1]. Non–small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases and has a 16% 5-year survival rate [2]. Curative NSCLC treatments include surgery, radiation therapy, chemotherapy, and targeted therapy. Cisplatin, carboplatin, mitomycin C, paclitaxel, ifosfamide, doxorubicin, irinotecan, and vinorelbine are frequently used as therapeutic agents for treatment of patients with NSCLC, either alone or in combination [3], [4]. Unfortunately, the responsiveness of NSCLC to chemotherapeutic agents is limited [5].
Because of technological advances during the past decade, epidermal growth factor receptor (EGFR) mutations have been identified in approximately 40% of patients with NSCLC in East Asia and 15% of Caucasians and African Americans [6], [7]. Thus, tyrosine kinase inhibitors (TKIs), such as bevacizumab, cetuximab, erlotinib, gefitinib, and afatinib, are clinically used as targeted therapeutics to treat NSCLC patients harboring mutations in EGFR [3], [8], [9]. Although the clinical outcome is impressive during initial treatment, the survival rate remains to be improved because of the emergence of drug resistance within 9 to 12 months [10], which mainly occurs because of a T790M secondary mutation, MET (hepatocyte growth factor receptor) amplification, or histologic transformation to small cell lung cancer [11], [12], [13]. Currently, numerous new-generation TKIs are under development [14]. In addition, a variety of combinations of chemotherapy and targeted agents are undergoing clinical trials to improve maintenance therapy [15].
In addition to EGFR, numerous oncogene mutations, either targetable or nontargetable, were identified in NSCLC patients, such as human epidermal growth factor receptor 2, MET, fibroblast growth factor receptor 1 and 2, anaplastic lymphoma kinase, ROS1 receptor tyrosine kinase, neuregulin 1, neurotrophic tyrosine kinase receptor type 1, RET receptor tyrosine kinase, and others [16]. For NSCLC patients who do not harbor targetable genetic abnormalities, platinum-based doublet chemotherapy remains the first-line treatment [17]. Unfortunately, the outcomes are generally frustrating. Therefore, the need exists to discover novel agents with improved efficacy and safety profiles for the treatment of NSCLC, including patients with wild-type EGFR, mutant EGFR, and acquired mutations that are resistant to TKIs.
Hybrid molecules, which integrate two drug pharmacophores into a single molecule, hold the potential to generate new compounds with dual mechanisms of action [18]. Based on this concept, we recently designed and synthesized a series of indolizino[6,7-b]indoles that consist of a β-carboline group, which acts as a topoisomerase (topo) I/II inhibitory moiety [19], [20], and a bis(hydroxymethyl)pyrrole fragment, which acts as a DNA cross-linking moiety [18], [21], [22]. Our previous report showed that the indolizino[6,7-b]indole derivatives were potent anticancer agents that significantly suppressed the growth of human breast carcinoma MX-1, lung adenocarcinoma A549, and colon cancer HT-29 xenograft tumor models [23]. Based on the solubility and animal tolerance, we recently found that compound [3-ethyl-6-methyl-6,11-dihydro-5H-indolizino[6,7-b]indole-1,2-diyl]dimethanol (Figure 1A), named BO-1978, exhibited significant cytotoxicity against the cell growth of various NSCLC cells in vitro. This observation drew our attention to further explore the antitumor activity of BO-1978 against NSCLC.
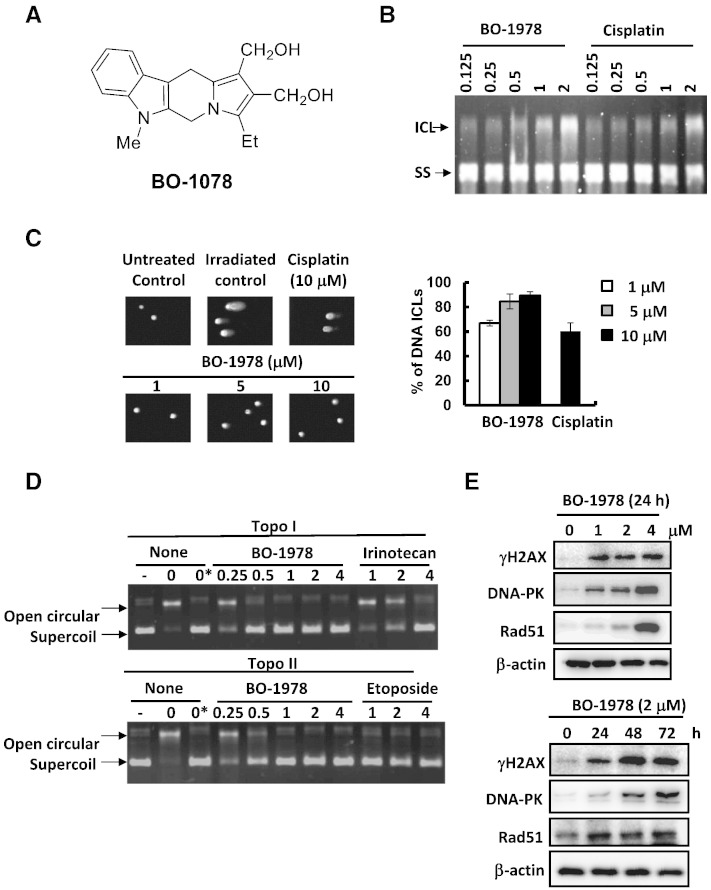
Figure 1.
DNA damage induced by BO-1978. (A) Chemical structure of BO-1978. (B) Formation of DNA cross-linking by BO-1978. Plasmid DNA was treated with various concentrations (0.125 to 2 μM) of BO-1978 or cisplatin for 2 hours at 37°C. The ICL and single-strand DNA was electrophoretically separated under alkaline conditions, as described in the Materials and Methods section. (C) Induction of DNA ICLs in H460 cells by BO-1978. H460 cells were treated with various concentrations (1, 5, and 10 μM) of BO-1978 or 10 μM cisplatin for 2 hours. DNA ICLs formed in H460 cells were identified using a modified comet assay. (D) Inhibition of intracellular topo I (upper panel) and II (lower panel) activities in H460 cells. The nuclear extracts of BO-1978–treated H460 cells (0.25 to 4 μM for 72 hours) were prepared and incubated with pEGFP-N1 plasmid DNA and specific topo I or topo II buffer for 30 minutes at 37°C as described in the Materials and Methods section. The relaxation of plasmid DNA was analyzed by electrophoresis. Irinotecan and etoposide served as positive controls for topo I and topo II, respectively. (E) The DNA damage response of H460 cells to BO-1978. H460 cells were treated with BO-1978 at the concentrations of 1 to 4 μM for 24 hours or 2 μM BO-1978 for various time periods (24 to 72 hours). γH2AX (Ser139), DNA-PK, and Rad51 protein expression levels were determined by western blotting.
In this study, we evaluated the cytotoxicity of BO-1978 in a batch of EGFR wild-type and mutant NSCLC cell lines in vitro and performed biological assays to confirm the compound’s biochemical activities in inducing interstrand DNA cross-links (ICLs) and inhibiting topo I/II. The anti-NSCLC activities of BO-1978 were investigated with xenograft and orthotopic lung models in nude mice. In addition, we also conducted a preclinical toxicity study of BO-1978 in animal models. Our results demonstrate that BO-1978 is a prospective compound for the treatment of patients with NSCLC.
Materials and Methods
Chemicals and Reagents
Compound BO-1978 (Figure 1A), a derivative of indolizino[6,7-b]indoles, was synthesized as previously described [23]. Gefitinib was purchased from Cayman Chemical Company. Other chemicals and reagents were purchased from Sigma-Aldrich except those as indicated elsewhere.
Cell Lines and Cell Culture
The NSCLC lines used in this study were listed in Table 1. Among them, H460, H1299, and A549 cells were obtained from the American Type Culture Collection, and H1650 and H1975 were provided by Dr. Tsu-An Hsu (National Health Research Institutes, Miaoli, Taiwan). CL141, CL1-5, CL100, CL97, PC9, PC9/gef B4 [24], and CL1-5/GFP-Luciferase [25] lines were kindly provided by Dr. Pan-Chyr Yang (National Taiwan University, Taipei, Taiwan). CL141T was derived from CL141 xenograft from a NOD-SCID mouse in our laboratory [26]. The luciferase overexpressed H1650 cells, designated as H1650-Luc cells, were established by transfection of 2.5 μg of pGL4.10[Luc2] plasmid DNA (Promega) into H460 cells using 5 μl of Lipofectamine 2000 Reagent (Invitrogen) for 4 hours and cultured with 1 mg/ml of geneticin (Gibco). In general, these cell lines were cultured in the RPMI 1640 medium (Gibco) containing 10% fetal bovine serum (HyClone), 100 U/ml of penicillin, 100 U/ml of streptomycin, 29.2 mg/ml of L-glutamine (Gibco), and 10 mM HEPES (pH 7.4) at 37°C in a humidified atmosphere with 5% CO2.
Table 1.
Characteristics of the NSCLC Lines Used in This Study.
| Cell Line | EGFR | TP53 | Kras | TKI Susceptibility |
|---|---|---|---|---|
| H460 | Wild-type | Wild-type | Q61H | Intrinsic resistance |
| A549 | Wild-type | Wild-type | G12S | Intrinsic resistance |
| H1299 | Wild-type | Deficient | Wild-type | Intrinsic resistance |
| CL141T | Wild-type | R248W | Wild-type | Intrinsic resistance |
| CL100 | Exon 19 deletion | ND | Wild-type | Sensitive |
| H1650 | Exon 19 deletion | Wild-type | Wild-type | Resistance (Pten loss) |
| H1975 | L858R/T790M | Wild-type | Wild-type | Acquired resistance |
| CL97 | G719A/T790M | R273H | Wild-type | Acquired resistance |
| CL1-5 | Wild-type | R248W | Wild-type | Intrinsic resistance |
| PC9 | Exon 19 deletion | Wild-type | Wild-type | Sensitive |
| PC9/gef B4 | Exon 19 deletion | Wild-type | Wild-type | Acquired resistance (Slug overexpression) |
ND, not determined.
In Vitro Cytotoxicity Assays
The cytotoxicity was assayed using alamarBlue reagent (AbD Serotec) as previously described [23]. In brief, the logarithmically growing cells were treated with BO-1978 at serial-diluted concentrations or in combination with gefitinib for 72 hours at 37°C. An aliquot of alamarBlue reagent was added. The cultures were incubated for 4 to 6 hours, and the absorbance at 570 nm and 600 nm was read with a plate reader. The proliferation rate was calculated according to the manufacturer’s instruction. The values of 50% inhibition concentration (IC50) and combination index for join treatment were determined from dose-effect relationship using the CompuSyn software (CompuSyn Inc., Paramus, NJ) [27].
Alkaline Gel Shift Assay
Formation of DNA cross-links was analyzed by alkaline agarose gel electrophoresis as previously described [26]. Briefly, purified pEGFP-N1 plasmid DNA (1500 ng) was mixed with various concentrations (0.125 to 2 μM) of BO-1978 or cisplatin in 40 μl of binding buffer (3 mM sodium chloride, 1 mM sodium phosphate, pH 7.4, and 1 mM EDTA). The reaction mixture was incubated at 37°C for 2 hours. At the end of incubation, the plasmid DNA was linearized by BamHI digestion at 37°C for 2 hours and then precipitated with 70% ice-cold ethanol at − 80°C for 16 hours. The DNA pellets were dissolved and denatured in an alkaline buffer (0.5 N NaOH and 10 mM EDTA). An aliquot of 20 μl of DNA solution (approximately 1500 ng) was mixed with 4 μl of six-fold DNA loading dye and electrophoretically resolved on a 0.8% alkaline agarose gel with a NaOH-EDTA buffer using 18 V at 4°C for 22 hours. After staining the gels with ethidium bromide solution, the DNA was visualized under UV light.
Modified Comet Assay
As previously described [28], H460 cells were treated with various concentrations of BO-1978 or cisplatin for 2 hours. Afterward, the cells were irradiated with X-rays at the dose of 20 Gy. An aliquot of 80 μl of cell suspension were mixed with 400 μl of 1.2% low–melting point agarose and plated on Fisherfinest microscope slide (Thermo Scientific), which was previously layered with 120 μl of 1% agarose gel. Cells were then lysed in the lysis buffer, and fragmented DNA was electrophoretically drawn out. The gels were neutralized before staining with 60 μl of 5 μM YOYO-1 iodide (491/509) (Invitrogen). The tail moment of 100 cells of each treatment was analyzed with Comet assay III software (Perceptive Instruments). The degree of DNA ICLs present in a drug-treated sample was determined by comparing the tail moments of the irradiated control. The formula is percentage of DNA with ICLs = [1 – (TMdi − TMcu/TMci − TMcu)] × 100%. TMdi means tail moment of drug-treated irradiated sample, TMcu indicates tail moment of untreated nonirradiated control, and TMci is tail moment of untreated irradiated control.
Topo I and II Activity Assays
The assays of topo I and II activity were performed following the established protocols [29]. In brief, H460 cells were treated with various concentrations of BO-1978 or reference drugs (irinotecan or etoposide) for 72 hours. The nucleus extracts (NE) were prepared using NE-PER Nuclear Protein Extraction Kit (Pierce). For topo I activity assay, the reaction was carried out by incubation of 2.5 μg of NE and 200 ng of plasmid pEGFP-N1 DNA in 20 μl of topo I reaction buffer (250 mM Tris, pH 7.5, 50 mM EDTA, 5 mM DTT, 0.5 M KCl, 25 mg/ml of BSA) at 37°C for 30 minutes. The reaction was terminated by the addition of 2 μl of 10% SDS. The samples were then loaded onto a 1% agarose gel and electrophoresed at 50 V for 90 minutes. The DNA was visualized by staining with ethidium bromide. For topo II assay, 10 μg of NE and 200 ng of plasmid pEGFP-N1 DNA were mixed in 20 μl of topo II reaction buffer (100 mM Tris, pH 7.5, 50 mM MgCl2, 5 mM ATP, 5 mM EDTA, 5 mM DTT, 0.75 M KCl, 150 mg/ml of BSA). The reaction, electrophoresis, and signal visualization were performed as described above.
Western Blot Analysis
The response of DNA damage biomarker (γH2AX) and proteins involved in repair of DNA double-strand breaks (DNA-dependent protein kinase [DNA-PK] and Rad51) and apoptosis (caspase 3 and 7 and poly-ADP ribose polymerase [PARP]) to BO-1978 was determined by Western blotting [26]. Briefly, H460 cells were treated with various concentrations of BO-1978 for 72 hours or 2 μM for different time periods. The whole cell extracts were then electrophoretically separated on an SDS polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (Amersham Biosciences). After blocking, the membrane was incubated with primary antibodies and then with horseradish peroxidase–conjugated antirabbit or antimouse secondary antibodies. Western blot signals were visualized by chemiluminescence using SuperSignal West Pico chemiluminescence reagent (Pierce). Antibodies against Rad51 and β-actin were obtained from Genetex; caspases 3 and 7, PARP, DNA-PK, and secondary antibodies from Cell Signaling; and γH2AX from EMD Millipore.
Cell Cycle Analysis
H460 cells (2.5 × 105 cells) were treated with various concentrations of BO-1978 for 24, 48, or 72 hours before being harvested and fixed with ice-cold 70% ethanol. The cells were stained with 4 μg/ml of propidium iodide in PBS containing 1% Triton X-100 and 0.1 mg/ml of RNase A and then subjected to flow cytometric analysis (FACScan flow cytometer) as previously described [23]. The distributions of cell cycle phases were determined using ModFit LT 3.0 software (Verity Software House).
Annexin V–Fluorescein Isothiocyanate (FITC) Apoptosis Analysis
The Annexin V–FITC apoptosis detection kit (eBioscience) was used to study cell apoptosis induced by BO-1978 according to the instructions. Cells were exposed to compounds with indicated concentrations before harvested by trypsinization, then suspended in Annexin V–FITC and propidium iodide contained binding buffer and subjected to flow cytometric analysis (FACScan flow cytometer).
Anticancer Activity in Xenograft Mouse Models
The animals used in this study exactly followed the guidelines of the Academia Sinica Institutional Animal Care and Utilization Committee. Male athymic nude mice 5 weeks of age were obtained from the National Laboratory Animal Center (Taipei, Taiwan) and housed for 1 week before performing experiments [28]. For implantations of xenograft tumors, an aliquot of H460 (3 × 106), PC9 (5 × 106), PC9/gef B4 (5 × 106), or H1650 (5 × 106) cells suspended in 100 μl of phosphate buffered saline (PBS, pH 7.4) was subcutaneously inoculated into the hind limb of mice. Tested compounds were dissolved in DMSO/Tween 80/saline (10:10:80; v/v/v) and intravenously injected into mice through tail vein. BO-1978 (40 mg/kg) or gefitinib (10 mg/kg) [30] was delivered for 5 consecutive days. Cisplatin (6 mg/kg) was given every 4 days for three times [31]. To monitor tumor growth, the longest and shortest diameters of the tumors were measured using calipers. Tumor volume (mm3) was calculated according to the following formula: tumor volume = (length × width2)/2. Mouse body weight was also measured as an indicator of the systemic toxicity of the treatments.
Anticancer Activity in Orthotopic Lung Tumor Models
The orthotopic lung tumor models were performed using intrathoracic implantation system [25], [32]. In brief, CL1-5/GFP-Luciferase (5 × 106) or H1650-Luc (2 × 106) cells were suspended in 50 μl of ice-cold Matrigel/PBS (1:1; v/v) and inoculated into the upper margin of the sixth intercostal rib on the left anterior axillary line of mice which were anesthetized via intraperitoneal (i.p.) injection with 2.5% Avertin (250 mg/kg). The injection depth was about 5 mm. The drug deliveries were performed as described above. The growths of cancer cells were monitored with In Vivo Imaging Systems (IVIS Spectrum System; Xenogen Corporation) after i.p. injection of 150 mg/kg of D-Luciferin (PerkinElmer). Mouse body weights were also monitored as described above.
Pharmacokinetics and Tissue Distribution in Sprague-Dawley (SD) Rats
The pharmacokinetic study of BO-1978 was performed using SD rats. In brief, BO-1978 was prepared at the concentration of 10 mg/ml in 20% ethanol and 80% PEG400. A single dose of BO-1978 was administrated into healthy male SD rats at the dose of 10 mg/kg via an indwelling catheter in jugular vein. Serial blood samples were collected from tail veins at 0, 1, 5, 15, 30, 60, and 120 minutes postdose from all animals. Concentrations of BO-1978 in blood were determined by HPLC using Agilent HC-C18 column (4.6 × 100 mm) and UV detector (228 nm). The solution of mobile phase was 35% acetonitrile and 65% 10 mM NaH2PO4 (pH 3.0). For tissue distribution, liver, heart, spleen, lung, kidney, and brain were sampled at 30 minutes after BO-1978 administration. The stability of BO-1978 was determined by incubation of this compound in rat plasma or cultural medium at room temperature for various time periods. The residual amount of BO-1978 was analyzed by HPLC.
Preclinical Toxicity in Institute for Cancer Research (ICR) Mice
The preclinical toxicological studies were conducted according to the values of LD50 with 6-week-male ICR mice (obtained from the National Laboratory Animal Center, Taipei, Taiwan). Two dosages below the values of LD50 were given to ICR mice by intravenous injection. After being monitored for 48 hours (namely, acute toxicity) and 14 days (namely, subacute toxicity) post drug administrations, the mice were anesthesia via i.p. injection with 2.5% Avertin (250 mg/kg) before hemospasia from hearts and harvesting heart, lung, liver, spleen and kidney samples. The whole blood and serum samples were subjected to complete blood count and blood chemistry (BC) test, and the main organs were fixed by formalin and embedded in paraffin block to make tissue sections. BC test was carried out with the blood chemistry analysis, including aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine (CRE), and glucose. The tissue sections were stained with hematoxylin and eosin, and the morphology was examined by a microscope.
Results
Cytotoxicity of BO-1978 in NSCLC Cells
We have previously demonstrated that indolizino[6,7-b]indoles have broad spectra of antitumor activity in both culture and xenograft tumor models [23]. Because lung cancer requires laborious clinical treatment, we therefore evaluated the anti-NSCLC potential of BO-1978 (Figure 1A). As summarized in Table 2, BO-1978 is a potent agent against a batch of 11 NSCLC cell lines, including five EGFR wild-type and six EGFR mutant cell lines. The IC50 values of BO-1978 against these NSCLC cells ranged from 1.06 to 3.50 μM, which were generally lower than those of previously tested therapeutic agents, such as cisplatin, irinotecan, and etoposide. Although TKIs are promising drugs against EGFR mutant NSCLC [33], the generation of resistance to TKIs is a major reason that treatments fail. Among the cell lines used, except PC9 cells, others were relatively resistant to gefitinib (Table 2). Intriguingly, BO-1978 also effectively killed the cells that acquired gefitinib resistance, PC9/gef B4 cells. These results imply that BO-1978 and its derivatives are potential chemotherapeutic agents against NSCLC with wild-type or mutant EGFR. Furthermore, we found that BO-1978 showed no cross-resistance to multiple drug–resistant and cisplatin-resistant cells (Table 3).
Table 2.
Comparative Cytotoxicity of BO-1978 with Therapeutic Agents in NSCLC Cells (IC50, μM).⁎
| Cell Line | BO-1978 | Cisplatin | Irinotecan | Etoposide | Gefitinib |
|---|---|---|---|---|---|
| H460 | 1.06 ± 0.11 | 9.94 ± 0.47 | 4.18 ± 1.29 | 7.31 ± 2.56 | 28.41 ± 7.44 |
| A549 | 3.15 ± 1.20 | 31.10 ± 3.03 | 9.29 ± 3.31 | 22.40 ± 14.39 | 17.65 ± 1.55 |
| H1299 | 3.02 ± 0.65 | 16.53 ± 0.90 | 24.46 ± 1.85 | 15.61 ± 7.45 | 27.87 ± 2.34 |
| CL1-5 | 1.47 ± 0.53 | 2.82 ± 0.28 | 10.53 ± 2.54 | 12.64 ± 3.93 | ND |
| CL141T | 1.32 ± 0.47 | 2.80 ± 0.36 | 44.99 ± 15.72 | 23.32 ± 3.34 | 20.56 ± 3.34 |
| H1650 | 3.50 ± 0.10 | 36.50 ± 8.14 | 17.05 ± 4.20 | 32.48 ± 17.56 | 32.81 ± 1.38 |
| H1975 | 2.34 ± 0.18 | 16.09 ± 4.03 | 36.83 ± 14.43 | 14.12 ± 2.90 | 50.72 ± 7.58 |
| CL100 | 1.51 ± 0.30 | 6.21 ± 3.64 | 0.66 ± 0.65 | 4.30 ± 3.87 | 27.60 ± 4.70 |
| CL97 | 1.25 ± 0.18 | 14.70 ± 0.34 | 9.99 ± 3.10 | 24.40 ± 17.39 | 8.91 ± 1.65 |
| PC9 | 1.41 ± 0.37 | 16.35 ± 3.29 | 11.23 ± 1.97 | 19.33 ± 6.28 | 0.29 ± 0.05 |
| PC9/gef B4 | 1.58 ± 0.33 [1.12]† | 34.34 ± 1.62 [2.10] | 31.58 ± 4.70 [2.81] | 36.66 ± 20.54 [1.90] | 25.68 ± 4.68 [88.55] |
IC50, the concentration of drug required to inhibit cell growth by 50% (mean ± S.D. of three independent experiments).
Numbers in brackets are the resistance factors of PC9/gef B4 to PC9 cells.
Table 3.
IC50 Values (μM) of BO-1978 in Multiple Drug–Resistant and Cisplatin-Resistant Cells.⁎
| Cell Line | BO-1978 | Vincristine (nM) | Doxorubicin | Cisplatin |
|---|---|---|---|---|
| CCRF-CEM | 0.46 ± 0.12 | 2.23 ± 0.19 | ND | ND |
| CCRF/VBL | 0.41 ± 0.03 [0.78x]† | 2128 ± 248.86 [954.3x] | ND | ND |
| KB | 1.96 ± 0.39 | 2.16 ± 0.25 | ND | ND |
| KB/vin10 | 1.56 ± 0.51 [0.79x] | 510 ± 170 [236.1x] | ND | ND |
| MES-SA | 0.77 ± 0.08 | ND | 0.038 ± 0.003 | 1.82 ± 0.66 |
| MES-SA/dx5 | 0.47 ± 0.12 [0.61x] | ND | 2.30 ± 0.98 [60.52x] | 2.27 ± 0.73 [1.25x] |
| NTUB1 | 1.14 ± 0.32 | ND | ND | 2.97 ± 1.43 |
| NTUB1/P | 1.92 ± 0.39 [1.68x] | ND | ND | 62.09 ± 15.30 [20.9x] |
IC50, the concentration of drug required to inhibit cell growth by 50% (mean ± S.D. of three independent experiments).
Numbers in brackets are resistance factors.
Induction of DNA Damage, Cell Cycle Disturbance, and Apoptosis by BO-1978
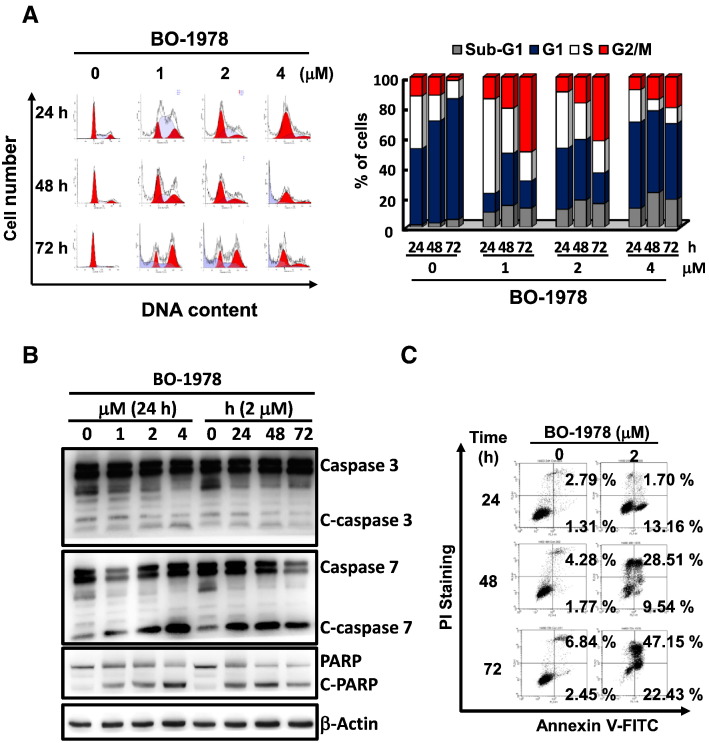
The hybrid indolizino[6,7-b]indoles consist of a bis(hydroxymethyl)pyrrole pharmacophore and a β-carboline moiety, indicating that they could form DNA ICL and inhibit topo I and II activities [23]. We first confirmed that incubation of plasmid DNA with BO-1978 formed DNA ICLs, as shown by an alkaline gel electrophoretic assay (Figure 1B). Using a modified comet assay, we further demonstrated that BO-1978 dose-dependently induced shortened tail moments in H460 cells irradiated with X-rays, indicating the formation of DNA ICLs in H460 cells by BO-1978 (Figure 1C). We also observed that topo I and II activities in H460 cells were significantly inhibited by BO-1978 at concentrations greater than 0.5 μM for 72 hours (Figure 1B), whereas irinotecan and etoposide were used as positive controls for the topo I and II assays, respectively. We further observed that BO-1978 dose-dependently increased the expression levels of proteins that participate in DNA repair, such as γH2AX, DNA-PK, and Rad51, in H460 cells (Figure 1E), supporting the suggestion that BO-1978 significantly induces DNA damage. These results confirmed our previous finding showing that indolizino[6,7-b]indoles could cause DNA damage via induction of DNA ICLs and inhibition of topo I and II activities [23]. One striking feature of DNA-damaging agents or topo I and II inhibitors is cell cycle interference [34]. As shown in Figure 2A, treatment of H460 cells with BO-1978 (1 to 4 μM) first resulted in S phase delay at 24 hours and then G2 arrest at 72 hours, whereas similar results were also observed in BO-1978–treated PC9 and PC9/gef B4 cells (Supplementary Figure S1). Because we observed that BO-1978 treatment resulted in increased cell populations at the sub-G1 phase, an indicator of apoptosis, we examined whether BO-1978–induced damage could trigger apoptosis by analyzing the activation of apoptotic executor proteins. As shown in Figure 2B, BO-1978 dose- and time-dependently induced cleavage of caspases 3 and 7. Using Annexin V staining and flow cytometric analysis, we confirmed a time-dependent increase in apoptotic cell numbers upon treatment of H460 cells with BO-1978 at 2 μM (Figure 2C). Taken together, these results indicate that BO-1978 inhibits topo I and II and induces DNA ICLs to cause DNA damage and thus to interfere with cell cycle progression, ultimately triggering apoptosis.
Figure 2.
Interference with cell cycle progression and induction of apoptosis by BO-1978 in H460 cells. (A) Cell cycle interference by BO-1978. H460 cells were treated with various concentrations of BO-1978 (1 to 4 μM) for various time periods (24 to 72 hours). The distribution of cell cycle phases was analyzed using a flow cytometer and ModFit LT 3.0 software (Verity Software House, Topsham, ME). (B) Activation of the apoptotic pathway by BO-1978. The appearance of cleaved caspase-3 and -7 and PARP in BO-1978–treated H460 cells was determined by Western blotting. (C) Induction of apoptosis by BO-1978. The apoptotic cells induced by BO-1978 were determined using an Annexin V–FITC apoptosis detection kit, as described in the Materials and Methods section.
Effective Suppression of EGFR Wild-Type NSCLC Cells by BO-1978 in Xenograft and Orthotopic Lung Tumor Models
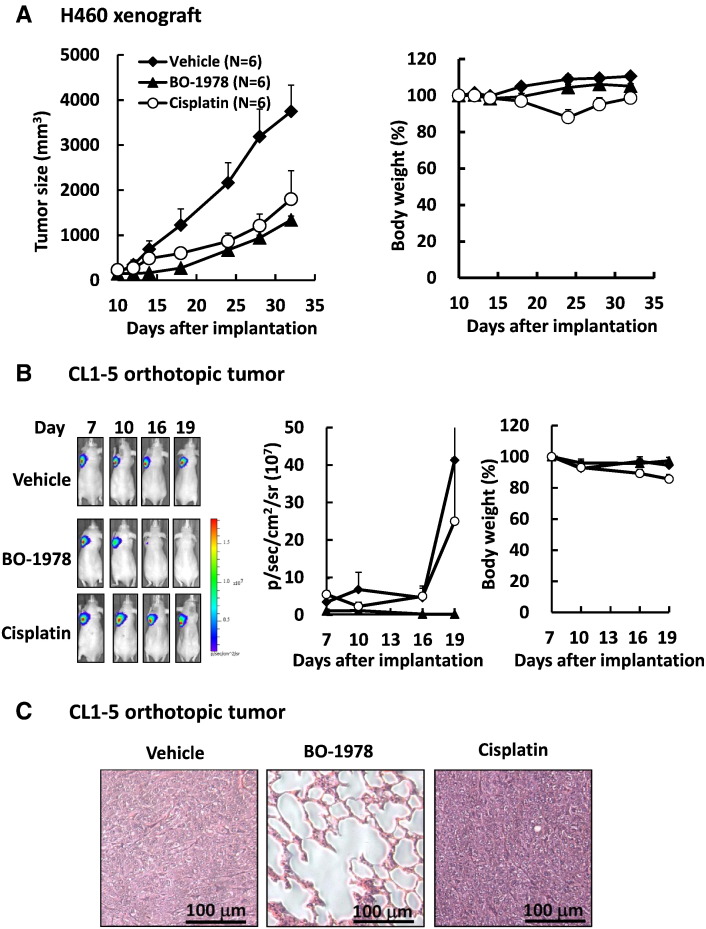
The antitumor activity of BO-1978 against NSCLC cells with wild-type EGFR was first evaluated using H460 xenografts in nude mice. To examine the therapeutic efficacy, the maximal tolerant dose of BO-1978 (40 mg/kg) was given for 5 consecutive days (QDx5). As shown in Figure 3A, BO-1978 significantly suppressed the growth of the H460 xenograft to approximately 65% in tumor size, whereas cisplatin reduced the tumor size to 52%. At the dose used, the effect of BO-1978 on mouse body weight was negligible, but cisplatin caused a body weight loss of approximately 12%. To further confirm the therapeutic efficacy of BO-1978 in EGFR wild-type NSCLC cells, we implanted CL1-5/GFP-Luciferase cells into the left lobes of lungs and traced the tumor growth using an IVIS. As shown in Figure 3B, treatment of tumor-bearing mice with BO-1978 dramatically abolished the growth of CL1-5/GFP-Luciferase cells in the lung, whereas cisplatin failed to suppress the growth of CL1-5/GFP-Luciferase in the lung. Histopathological examination further confirmed the absence of tumors in the lungs of mice treated with BO-1978 (Figure 3C). These results revealed that BO-1978 is a potent therapeutic agent against EGFR wild-type NSCLC cells in the lung.
Figure 3.
The therapeutic efficacy of BO-1978 against EGFR wild-type NSCLC cells. (A) Suppression of H460 xenografts by BO-1978. H460 cells (3 × 106) were subcutaneously implanted in nude mice. When the tumor size reached approximately 100 mm3, the tumor-bearing mice were intravenously treated with vehicle, BO-1978 (40 mg/kg, daily for 5 consecutive days), or cisplatin (6 mg/kg, three times every 4 days). The tumor size and body weight were measured at the times indicated. (B) Suppression of orthotopically implanted CL1-5/GFP-Luciferase cells by BO-1978. CL1-5/GFP-Luciferase cells (5 × 106) were orthotopically implanted in the lungs of nude mice. The tumor-bearing mice were treated with vehicle, BO-1978, or cisplatin, as described above. (Left) The representative images of mice implanted with CL1-5/GFP-Luciferase cells and treated with drugs. (Middle and right) The quantitative signals of luciferase (p/s per cm2 per sr) and the relative body weights of mice treated with vehicle, BO-1978, or cisplatin, respectively. p, photon; sr, steradian. (C) No tumor formation in the lungs of orthotopically implanted mice treated with BO-1978. On day 19, the lungs were harvested from mice treated with vehicle, BO-1978, or cisplatin; histopathologically sectioned; and stained with hematoxylin and eosin.
Synergistically Increased Cytotoxicity to EGFR Mutant NSCLC Cells by Combination Treatment with BO-1978 and Gefitinib
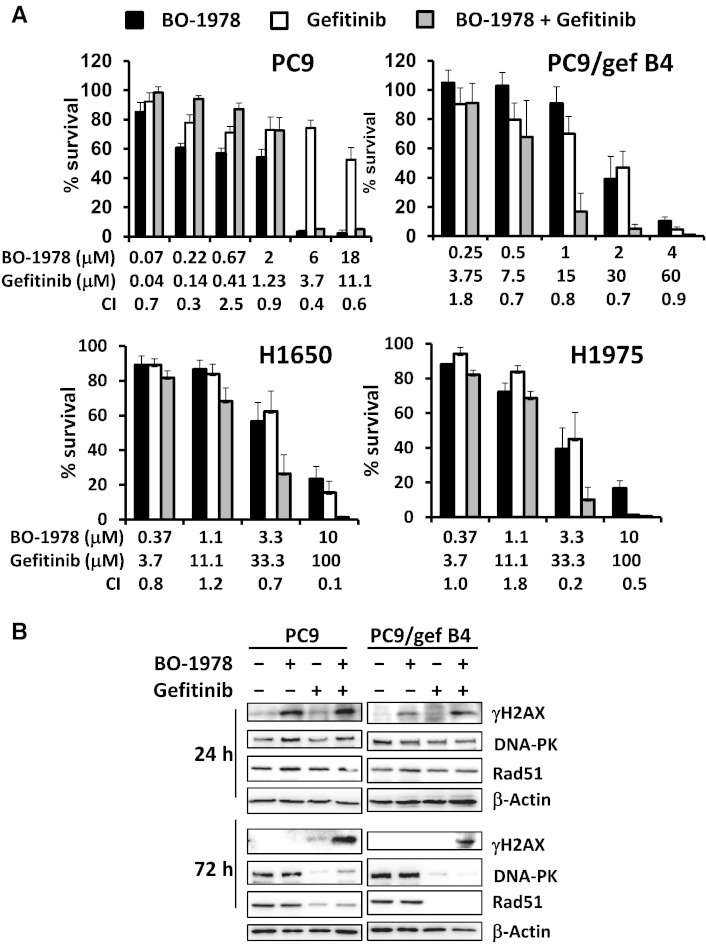
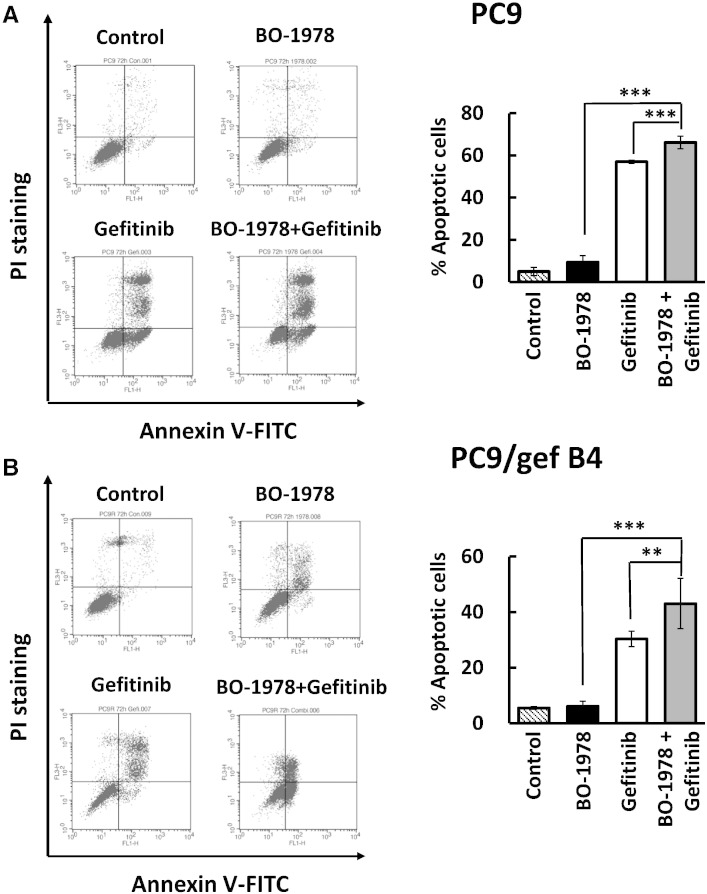
Although we found that BO-1978 significantly killed mutant EGFR NSCLC cells in vitro (Table 1), we further investigated the efficacy of this compound and its combination with gefitinib to suppress growth of NSCLC cells with mutant EGFR. We first performed an alamarBlue assay to demonstrate enhanced cytotoxicity by co-treatment with BO-1978 and gefitinib in PC9, PC9/gef B4, H1650, and H1975 cells in the toxic dose range (Figure 4A). The effective dose ratios of gefitinib to BO-1978 used were relatively associated with the resistance of cells to gefitinib. The ratio was 0.6 to 1 in gefitinib-sensitive PC9 cells, whereas the ratios were 15 to 1 in gefitinib-resistant PC9/gef B4 cells and 10 to 1 in H1650 and H1975 cells. Furthermore, we observed that treatment of cells with BO-1978 (2 μM) alone resulted in increased expression of γH2AX, a DNA damage marker, at 24 hours that then declined at 72 hours, implying that BO-1978–induced DNA damage was gradually repaired in PC9 and PC9/gef B4 cells. Treatment of cells with gefitinib (4 μM) alone significantly reduced the protein expression levels of DNA-PK and Rad51, which are essentially involved in DNA repair (Figure 4B). Intriguingly, upon co-treatment of PC9 and PC9/gef B4 cells with BO-1978 and gefitinib, the protein expression levels of DNA-PK and Rad51 were suppressed, whereas γH2AX remained and accumulated in the cells (Figure 4B). These results indicate that gefitinib likely suppresses repair of BO-1978–induced DNA damage. Consistently, combination treatment of PC9 and PC9/gef B4 cells with BO-1978 and gefitinib also resulted in increased apoptotic cells (Figure 5, A and B).
Figure 4.
Enhancement of BO-1978–induced toxic effects in EGFR mutant NSCLC cells upon gefitinib treatment. (A) Synergistic suppression of cell growth by combination treatment of EGFR mutant NSCLC with BO-1978 and gefitinib. Logarithmically growing PC9, PC9/gef B4, H1650, and H1975 cells were treated with BO-1978, gefitinib, or the combination for 72 hours. The cell growth was determined using an alamarBlue assay, as described in the Materials and Methods section. (B) Increased DNA damage marker (γH2AX) expression and suppression of DNA repair proteins (DNA-PK and Rad51) by gefitinib. PC9 and PC9/gef B4 cells were treated with BO-1978, gefitinib, or the combination for 24 and 72 hours. At the end of treatment, the cells were harvested, and γH2AX, DNA-PK, and Rad51 expression levels were analyzed by Western blotting.
Figure 5.
Increasing BO-1978–induced apoptotic cells with gefitinib treatment in PC9 (A) and PC9/gef B4 cells (B). Logarithmically growing PC9 and PC9/gef B4 cells were treated with BO-1978 (2 μM), gefitinib (0.4 μM for PC9 cells and 30 μM for PC9/gef B4 cells), or the combination for 72 hours. At the end of treatment, the cells were harvested and subjected to analysis of apoptotic cells using an Annexin V–FITC apoptosis detection kit. The data on the right are the means and SD of three independent experiments. ** and ***, P < .01 and .001 according to Student’s t test.
Effective Suppression of EGFR Mutant NSCLC Cells by Combination Treatment with BO-1978 and Gefitinib in Xenograft and Orthotopic Lung Tumor Models
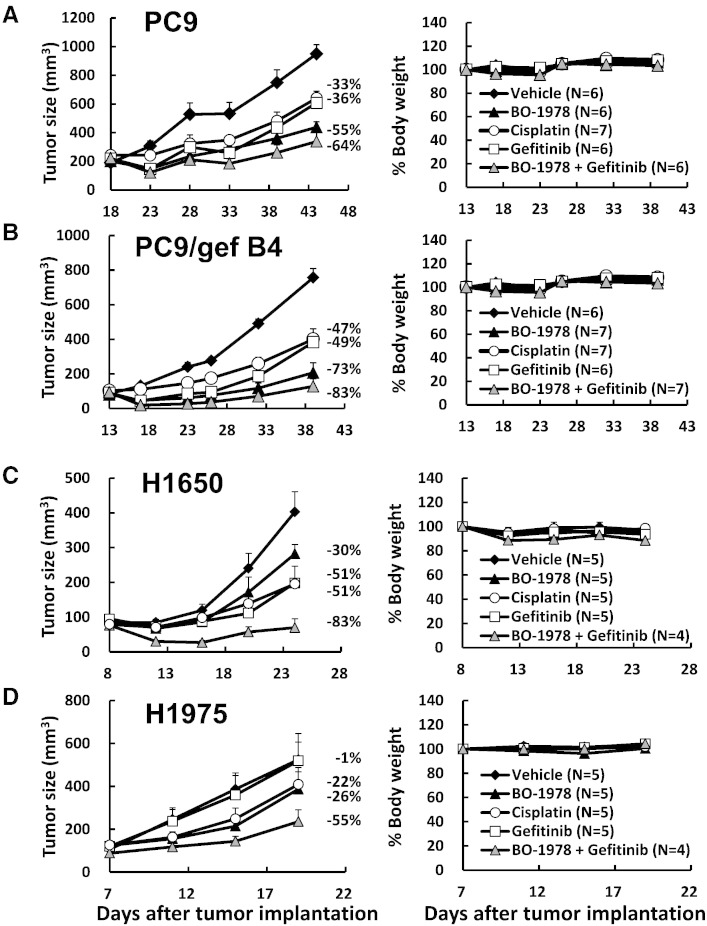
The xenograft models of PC9, PC9/gef B4, H1650, and H1975 cells were adopted to examine the therapeutic efficacy of BO-1978 alone or in combination with gefitinib against EGFR mutant NSCLC cells. As shown in Figure 6, daily treatment with BO-1978 at the dose of 40 mg/kg for 5 consecutive days significantly suppressed the growth of PC9, PC9/gef B4, H1650, and H1975 xenograft tumors by 55%, 73%, 30%, and 26%, respectively, without significant reductions in body weight during the treatments. The suppression effects on xenograft growth by gefitinib administered at the dose of 10 mg/kg for 5 consecutive days in PC9, PC9/gef B4, H1650, and H1975 xenograft mouse models were approximately 36%, 49%, 51%, and 1%, respectively. The efficacy of cisplatin was included for comparison. BO-1978 was more potent than cisplatin and gefitinib against PC9, PC9/gef B4, and H1975 cells but not H1650 cells. However, the combination treatment of BO-1978 and gefitinib promisingly suppressed the growth of xenografts by 64%, 83%, 83%, and 55% in PC9, PC9/gef B4, H1650, and H1975 cells, respectively. These results indicate that the combination of BO-1978 with gefitinib could be an effective regimen for treatment of EGFR mutant NSCLC cells.
Figure 6.
Antitumor activity of BO-1978 in EGFR mutant NSCLC xenografts. Aliquots of 5 × 106 PC9 (A), PC9/gef B4 (B), H1650 (C), and H1975 (D) cells were subcutaneously implanted in nude mice. When tumor sizes reached approximately 100 mm3, the mice were treated with vehicle, BO-1978 (40 mg/kg, daily for 5 consecutive days), gefitinib (10 mg/kg, daily for 5 consecutive days), BO-1978 + gefitinib, or cisplatin (6 mg/kg, three times every 4 days). The tumor volumes and body weights were monitored at the indicated times.
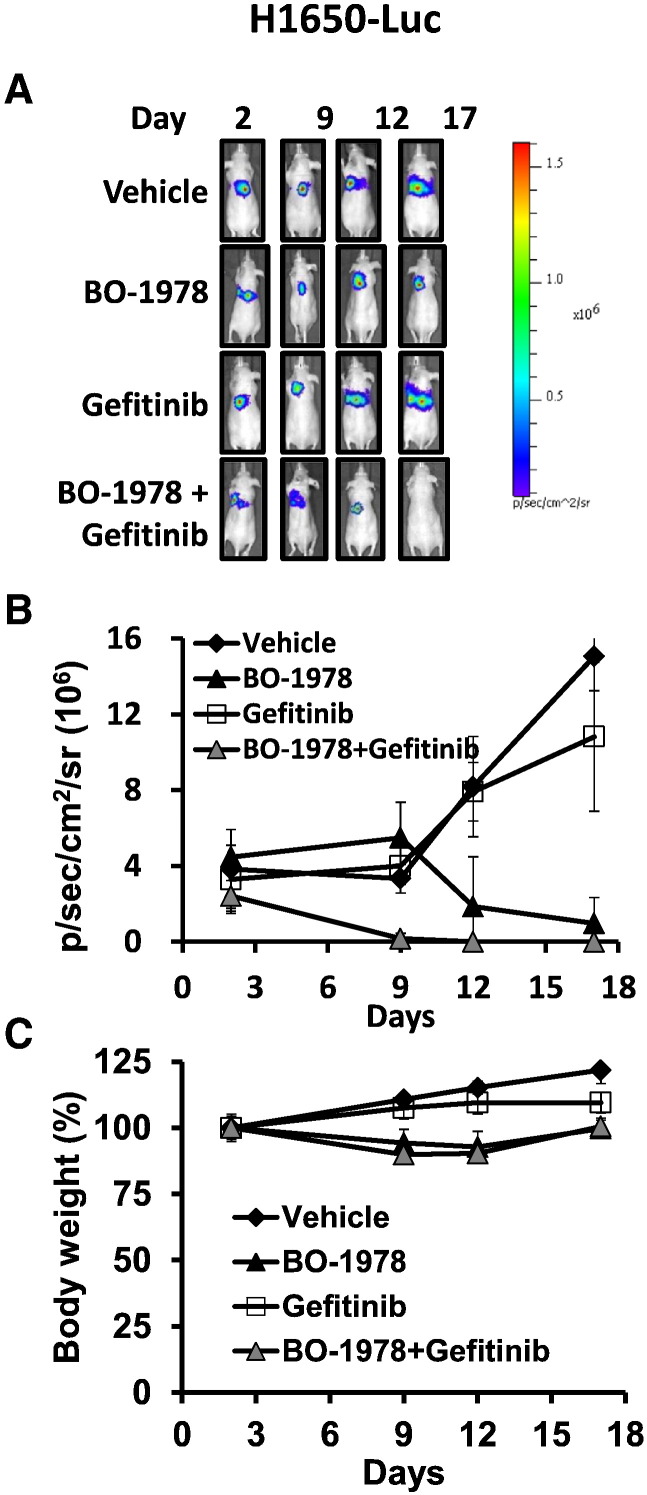
To further confirm the therapeutic efficacy of BO-1978 against EGFR mutant NSCLC cells, we established H1650-Luc cells by transfection of luciferase-expressing plasmids into cells and then intrathoracically implanted them into the left lobes. Two days after implantation, the mice were randomly grouped and received treatment. As shown in Figure 7, we found that H1650-Luc cells were resistant to gefitinib treatment alone but were effectively suppressed by BO-1978 treatment alone. Furthermore, the combination of BO-1978 with gefitinib completely suppressed tumor cell signaling in the orthotropic model (Figure 7). Consistently, the treatment did not lead to severe damage in terms of body weight changes. These results confirmed the antitumor activity of BO-1978 alone and in combination in the lungs.
Figure 7.
Suppression of orthotopic growth of H1650-Luc cells by BO-1978 alone or in combination with gefitinib. H1650-Luc cells (2 × 106) were intrathoracically implanted in the left lobes of nude mice. On day 2, the mice were treated with BO-1978 alone or in combination with gefitinib, as described in Figure 6. Tumor growth was monitored by IVIS. (A) Representative images of tumor growth; (B) averaged photon intensity; and (C) body weight.
Pharmacokinetics of BO-1978 in SD Rats
By incubation of BO-1978 in rat plasma or cultural medium at room temperature, we found thast BO-1978 was relatively stable. The half-lives of BO-1978 in rat plasma and culture medium were 89.41 ± 5.62 (n = 3) and 36.83 ± 2.19 hours (n = 3), respectively. The whole-blood pharmacokinetics of BO-1978 was evaluated in healthy male SD rats following a single intravenous administration of BO-1978 at a dose of 10 mg/kg. BO-1978 concentrations in plasma following drug administration were determined by HPLC and are shown in Supplementary Figure S2. Accordingly, the initial concentration or C0 (drug administration at time 0 minute) and the half-life (t1/2) of BO-1978 were 8.63 ± 2.18 μg/ml (i.e., 27.8 ± 7.0 μM) and 23.50 ± 7.09 minutes, respectively. The area under the curve (AUC0 -last) was estimated to be 188.10 ± 45.99 min μg/ml. The systemic clearance of BO-1978 was estimated to be 56.24 ± 14.90 ml/min per kg, which is approximately 19% of the cardiac output of rats (approximately 296 ml/min per kg), indicating that BO-1978 in rats has a medium clearance rate. The apparent volume of distribution at steady state was 1.46 ± 0.29 l/kg, which was more than two-fold the total body water of rats (0.668 l/kg), suggesting that BO-1978 may be moderately distributed into tissues. The mean residence time was 27.34 ± 7.97 minutes. The tissue distribution of BO-1978 in rats is summarized in Table 4. At 30 mintes after BO-1978 administration, the drug was widely distributed in various tissues, including liver, lung, and brain. However, how BO-1978 is metabolized in animals warrants our further investigation.
Table 4.
Tissue Distribution of BO-1978 in Rats.⁎
| Tissue | BO-1978 (μg/ml or μg/g) |
|---|---|
| Blood | 1.59 ± 0.48 |
| Liver | 10.71 ± 1.96 |
| Heart | 1.79 ± 0.72 |
| Spleen | 0.48 ± 0.08 |
| Lung | 3.13 ± 1.44 |
| Kidney | 2.19 ± 0.12 |
| Brain | 3.97 ± 0.96 |
Tissue samples were harvested 30 minutes after drug administration. The data are expressed as the mean ± SD (n = 6).
Limited Toxicity of BO-1978 in ICR Mice
Toxicity/safety is one of the main issues for new drug development. In our animal studies, we did not observe severe body weight changes in mice treated with either BO-1978 alone or BO-1978 plus gefitinib. To further investigate the toxic effect of BO-1978, we performed an acute toxicity study using ICR mice. First, we found that the LD50 value (50% lethal concentration) for BO-1978 was 93.7 mg/kg in ICR mice. We then studied the toxicity/safety of BO-1978 at the effective dose. The ICR mice were intravenously administered 40 mg/kg of BO-1978, and the blood samples and major organs were harvested on days 2 and 14 for analysis of their toxic effects. The results of the complete blood count and BC examinations are summarized in Table 5. No evidence of anemia, white cell abnormality, or hematopoietic deficiency was noted in mice administered with BO-1978. Although we observed obvious increments of AST and ALT post 48 hours in the BC test, the liver damage caused by BO-1978 recovered to normal range on day 14. However, we found that the hepatocytes and the hepatoportal area were intact, and no anoxic signs were noted around the central vein in the liver sections (Supplementary Figure S3). These results were also consistent with the findings showing that more BO-1978 was distributed to the liver than other organs (Table 5). The changes in BUN, CRE, and glucose were negligible, suggesting that renal metabolic functions were not excessively affected. In addition to the liver, histopathological examinations revealed no apparent alterations in the heart, lung, spleen and kidney. As shown in Supplementary Figure S2, BO-1978–treated mice had intact structures in the myocardium, septa, and the four- and three-chamber sections of the heart, indicating no cardiac injury, ventricular dilation, or ventricular hypertrophy. The lung sections revealed that BO-1978–treated mice had clear alveoli without obstruction of the airway. We did not find any lymphocyte depletion or enhancement within the spleens of BO-1978–treated mice. For the kidneys, the structure of the renal glomerulus was intact, and no filtrate in Bowman’s capsules or detached renal tubular debris was detected in the kidneys of BO-1978–treated mice. In summary, BO-1978 at a dose of 40 mg/kg did not cause apparent toxic effects in the major organs except for slight and recoverable liver damage.
Table 5.
Blood Cell and Blood Chemistry Tests following BO-1978 Administration after 48 Hours and 14 Days in ICR Mice.
| Items | Ctrl (n = 12) | BO-1978 |
|
|---|---|---|---|
| 48 Hours (n = 8) | 14 Days (n = 6) | ||
| WBC (109/l) | 3.92 ± 1.40 | 2.43 ± 1.19 | 4.00 ± 0.97 |
| NEU (109/l) | 0.67 ± 0.24 | 0.45 ± 0.21 | 0.82 ± 0.47 |
| LYM (109/l) | 2.95 ± 1.16 | 1.83 ± 1.17 | 2.86 ± 0.88 |
| MONO (109/l) | 0.12 ± 0.06 | 0.05 ± 0.04 | 0.11 ± 0.09 |
| EOS (109/l) | 0.10 ± 0.04 | 0.05 ± 0.04 | 0.12 ± 0.13 |
| BASO (109/l) | 0.09 ± 0.05 | 0.06 ± 0.03 | 0.09 ± 0.03 |
| RBC (1012/l) | 7.73 ± 0.51 | 7.14 ± 0.38 | 7.81 ± 0.33 |
| HGB (g/dl) | 12.99 ± 0.42 | 11.96 ± 0.53 | 12.78 ± 0.42 |
| HCT (%) | 44.69 ± 2.59 | 41.13 ± 1.95 | 44.02 ± 1.18 |
| MCV (fL) | 57.83 ± 1.49 | 57.61 ± 1.27 | 56.40 ± 1.69 |
| MCH (pg) | 16.86 ± 0.89 | 16.75 ± 0.45 | 16.38 ± 0.52 |
| MCHC (g/dl) | 29.13 ± 1.30 | 29.07 ± 0.40 | 29.05 ± 0.46 |
| PLT (109/l) | 915.42 ± 147.50 | 786.10 ± 90.60 | 958.00 ± 178.31 |
| AST (U/l) | 90.09 ± 38.80 | 220.27 ± 153.45 | 86.14 ± 25.25 |
| ALT (U/l) | 39.75 ± 12.46 | 156.25 ± 236.88 | 44.87 ± 19.72 |
| ALP (U/l) | 244.14 ± 49.91 | 297.50 ± 72.11 | 233.50 ± 36.74 |
| BUN (mg/dl) | 26.07 ± 4.64 | 19.48 ± 2.03 | 23.95 ± 1.71 |
| CRE (mg/dl) | 0.21 ± 0.11 | 0.18 ± 0.15 | 0.17 ± 0.05 |
BASO, basophil; EOS, eosinophil; HCT, hematocrit; HGB, Hemoglobin; LYM, lymphocyte; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MONO, monocyte; NEU, neutrophil; PLT, platelet; RBC, red blood cell; WBC, white blood cell.
Discussion
The present study was conducted to understand the anti-NSCLC activity of indolizino[6,7-b]indoles. Accordingly, we have demonstrated that the selected derivative of indolizino[6,7-b]indoles, BO-1978, is a potent agent against a variety of NSCLC cells in vitro and in vivo. Because BO-1978 at the doses used did not cause obvious toxicity in mice, we may consider that BO-1978 has high potential to be developed into a candidate drug for NSCLC treatment.
BO-1978 is a chemical with multimodal activities, including inducing DNA ICLs and inhibiting topo I and II activity. Because the lesions of DNA ICLs are generally highly toxic to cells, a variety of DNA ICL compounds have been widely used as chemotherapeutic agents against different types of cancer [35], [36]. Our results showed that BO-1978 induces DNA ICLs in vitro and in vivo and, hence, disturbs cell cycle progression and triggers apoptosis. The pharmacophoric group of BO-1978 responsible for attacking DNA to form ICLs is bis(hydroxymethyl)pyrrole [23], which displays potent antitumor activity [37], [38]. Numerous anticancer chemotherapeutics target topo I and II by either catalytic inhibition or poisoning [39], [40]. β-Carboline alkaloids are an important class of natural and synthetic medicinal molecules exerting their anticancer activities through diverse mechanisms [41], such as topo I/II inhibition [19], [20]. Recently, a variety of β-carboline hybrids have been synthesized and evaluated against different diseases, including cancer and neurological disorders [42], [43], [44]. We revealed that BO-1978, also a β-carboline hybrid [23], could inhibit topo I/II. Our results support that the conjugation of DNA ICL activity and topoisomerase inhibitory activity into a single molecule may exert a unique mode to kill NSCLC cells.
Clinicians still endeavor to find effective regimens for the treatment of NSCLC patients who do not harbor targetable genetic abnormalities [17], [45], [46]. In the cytotoxicity study, BO-1978 exhibited strong antitumor activity in vitro in NSCLC lines containing Kras mutations (H460 and A549 cells), p53 mutations (H1299, CL1-5, and CL141T cells), TKI-sensitive mutant EGFRs (PC9 cells), and TKI-resistant mutant EGFRs (PC9/gef B4, H1650, H1975, CL97, and CL100 cells). Unfortunately, a major portion of NSCLC patients harboring wild-type EGFR cannot control the disease using current treatments, including cisplatin-based adjuvant chemotherapy or TKIs [47]. These results show that BO-1978 effectively suppresses subcutaneously transplanted EGFR wild-type NSCLC cells in nude mouse models. Furthermore, orthotopically implanted CL1-5 cells, an EGFR wild-type NSCLC cell line, were completely eradicated by BO-1978 treatment, whereas cisplatin failed to suppress their growth in the lung. Intriguingly, our results also showed not only that BO-1978 was more cytotoxic to multiple drug–resistant cells than their parental cells but also that the compound displayed no cross-resistance in cisplatin-resistant cells. Therefore, the potential of BO-1978 as an anti-NSCLC agent without targetable alterations warrants our further investigation.
Because of the rapid development of resistance results in treatment failure [48], there is an unmet clinical need to search for effective third- or fourth-line therapeutic agents against TKI-resistant NSCLC [48], [49]. Several targeted therapies, including inhibitors of EGFR, anaplastic lymphoma kinase or MET, and others, are currently either clinically available or under development [16]. Herein, we demonstrated that BO-1978 could also overcome TKI resistance in several EGFR mutant NSCLC cells. In addition, combination treatment of PC9 or PC9/gef B4 cells with BO-1978 and gefitinib resulted in accumulation of γH2AX, a marker for monitoring DNA damage [50], and decreased Rad51 and DNA-PK, core components of DNA double-strand break repair [49], [51], indicating that EGFR inhibition by gefitinib resulted in suppression of DNA repair and potentiated BO-1978 toxicity. Combination therapy using TKIs and chemotherapeutic agents has been well reported in the literature [52]. Although BO-1978 treatment moderately suppressed the growth of H1650 xenograft, the signals of orthotopically implanted H1650 cells in the lungs were almost completely eliminated by combination treatment of BO-1978 and gefitinib. These results implicate that the therapeutic efficacy of DNA ICL agents could be further improved by rational combination therapy with DNA repair inhibitors or targeted therapeutic agents. However, the reason why BO-1978 actively eradicates tumor cells growing in the lung is unclear. As tumor microenvironment [16] and drug distribution [53] are apparently different between xenograft and orthotopic lung tumor models, the distribution of BO-1978 in various organs warrants further investigation.
Our single-dose pharmacokinetic study showed that the initial t1/2 of BO-1978 was 23.50 ± 7.09 minutes. Because the drug metabolic rate in animals is generally higher than that in humans, the elimination t1/2 of BO-1978 is close to that of bendamustine (approximately 40 minutes) [54], a unique alkylating agent used for treatment of chronic lymphocytic leukemia by the United States Food and Drug Administration in 2008 [55]. The medium clearance rate of BO-1978 suggests that we could administer BO-1978 daily or every other day. The value of volume of distribution at steady state was more than two-fold the total body water of rats, indicating the moderate distribution of BO-1978 in tissues. Slightly higher levels of BO-1978 distributed in the liver may cause slight but recoverable liver injury.
Drug development usually fails because of high toxicity [56]. However, for cancer treatment, current chemotherapeutic agents lead to severe drug toxicity, such as thrombocytopenia, leukocytopenia, and interstitial pneumonitis resulting from treatment with mitomycin C; nephrotoxicity, ototoxicity, and neurotoxicity from cisplatin and carboplatin; and cardiac toxicity from doxorubicin [57], [58], [59]. With regard to drug toxicity, BO-1978 has limited toxicity at the effective dose in mice. Furthermore, there is no significant body weight loss in mice treated with BO-1978 for 5 consecutive days in our animal studies, indicating that these compounds did not cause severe systematic toxicity.
In conclusion, our present study demonstrates that BO-1978 is a potent agent against EGFR wild-type and mutant NSCLCs in an orthotopic mouse model and has limited toxicity in mice. These results suggest that BO-1978, a derivative of indolizino[6,7-b]indoles with topo I/II inhibition and DNA ICL induction activities, is a potential candidate for future development as a therapeutic agent against NSCLC.
Acknowledgements
The authors thank the Taiwan Mouse Clinic (MOST 103-2325-B-001-015), which is funded by the National Research Program for Biopharmaceuticals at the Ministry of Science and Technology of Taiwan, for technical support with blood cells and blood chemistry in the preclinical toxicological experiments.
Footnotes
This article refers to supplementary materials, which are designated by Supplementary Figures S1 to S3.
This work was supported by Thematic Project Program Grant, Academia Sinica, Taiwan (AS-100-TP-B13 to T.C.L., T.L.S., and T.-H. Tsai); NRPB Grant, Ministry of Science and Technology, Taiwan (NSC 102-2325-B-001-002, MOST 103-2325-B-001-018, and 104-2325-B-001-001 to T.-C. Lee and T.-L. Su); and National Science and Technology Development Fund (MOST 103-3111-Y-001-031).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2016.02.005.
Appendix A. Supplementary data
Supplementary figures.
References
- 1.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA. Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Govindan R, Grannis FW., Jr. Non–small cell lung cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11:645–653. doi: 10.6004/jnccn.2013.0084. [quiz 653] [DOI] [PubMed] [Google Scholar]
- 3.Cataldo VD, Gibbons DL, Perez-Soler R, Quintas-Cardama A. Treatment of non–small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364:947–955. doi: 10.1056/NEJMct0807960. [DOI] [PubMed] [Google Scholar]
- 4.Clegg A, Scott DA, Hewitson P, Sidhu M, Waugh N. Clinical and cost effectiveness of paclitaxel, docetaxel, gemcitabine, and vinorelbine in non–small cell lung cancer: a systematic review. Thorax. 2002;57:20–28. doi: 10.1136/thorax.57.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lwin Z, Riess JW, Gandara D. The continuing role of chemotherapy for advanced non–small cell lung cancer in the targeted therapy era. J Thorac Dis. 2013;5:S556–S564. doi: 10.3978/j.issn.2072-1439.2013.08.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non–small-cell lung cancer. Nat Rev Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suda K, Mitsudomi T. Racial differences in lung cancer genetics. J Thorac Oncol. 2015;10:230–231. doi: 10.1097/JTO.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 8.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 9.Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 10.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 11.Alam N, Gustafson KS, Ladanyi M, Zakowski MF, Kapoor A, Truskinovsky AM, Dudek AZ. Small-cell carcinoma with an epidermal growth factor receptor mutation in a never-smoker with gefitinib-responsive adenocarcinoma of the lung. Clin Lung Cancer. 2010;11:E1–E4. doi: 10.3816/CLC.2010.n.046. [DOI] [PubMed] [Google Scholar]
- 12.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, Pao W, Ladanyi M, Miller VA. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17:1616–1622. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oxnard GR, Lo PC, Nishino M, Dahlberg SE, Lindeman NI, Butaney M, Jackman DM, Johnson BE, Janne PA. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8:179–184. doi: 10.1097/JTO.0b013e3182779d18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter AO, Sjin RT, Haringsma HJ, Ohashi K, Sun J, Lee K, Dubrovskiy A, Labenski M, Zhu Z, Wang Z. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 2013;3:1404–1415. doi: 10.1158/2159-8290.CD-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berge EM, Doebele RC. Re-examination of maintenance therapy in non–small cell lung cancer with the advent of new anti-cancer agents. Drugs. 2013;73:517–532. doi: 10.1007/s40265-013-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non–small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azzoli CG, Baker S, Jr., Temin S, Pao W, Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D. American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non–small-cell lung cancer. J Clin Oncol. 2009;27:6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su TL, Lee TC, Kakadiya R. The development of bis(hydroxymethyl)pyrrole analogs as bifunctional DNA cross-linking agents and their chemotherapeutic potential. Eur J Med Chem. 2013;69:609–621. doi: 10.1016/j.ejmech.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Castro AC, Dang LC, Soucy F, Grenier L, Mazdiyasni H, Hottelet M, Parent L, Pien C, Palombella V, Adams J. Novel IKK inhibitors: beta-carbolines. Bioorg Med Chem Lett. 2003;13:2419–2422. doi: 10.1016/s0960-894x(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 20.Guan H, Chen H, Peng W, Ma Y, Cao R, Liu X, Xu A. Design of beta-carboline derivatives as DNA-targeting antitumor agents. Eur J Med Chem. 2006;41:1167–1179. doi: 10.1016/j.ejmech.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Anderson WK. Activity of bis-carbamoyloxymethyl derivatives of pyrroles and pyrrolizines against human tumor xenografts in nude mice. Cancer Res. 1982;42:2168–2170. [PubMed] [Google Scholar]
- 22.Anderson WK, McPherson HL, Jr., New JS, Rick AC. Synthesis and murine antineoplastic activity of bis[(carbamoyloxy)methyl] derivatives of pyrrolo[2,1-a]isoquinoline. J Med Chem. 1984;27:1321–1325. doi: 10.1021/jm00376a017. [DOI] [PubMed] [Google Scholar]
- 23.Chaniyara R, Tala S, Chen CW, Zang X, Kakadiya R, Lin LF, Chen CH, Chien SI, Chou TC, Tsai TH. Novel antitumor indolizino[6,7-b]indoles with multiple modes of action: DNA cross-linking and topoisomerase I and II inhibition. J Med Chem. 2013;56:1544–1563. doi: 10.1021/jm301788a. [DOI] [PubMed] [Google Scholar]
- 24.Yeh CT, Wu AT, Chang PM, Chen KY, Yang CN, Yang SC, Ho CC, Chen CC, Kuo YL, Lee PY. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med. 2012;186:1180–1188. doi: 10.1164/rccm.201207-1180OC. [DOI] [PubMed] [Google Scholar]
- 25.Pan SH, Chao YC, Hung PF, Chen HY, Yang SC, Chang YL, Wu CT, Chang CC, Wang WL, Chan WK. The ability of LCRMP-1 to promote cancer invasion by enhancing filopodia formation is antagonized by CRMP-1. J Clin Invest. 2011;121:3189–3205. doi: 10.1172/JCI42975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaniyara R, Tala S, Chen CW, Lee PC, Kakadiya R, Dong H, Marvania B, Chen CH, Chou TC, Lee TC. Synthesis and antitumor evaluation of novel benzo[d]pyrrolo[2,1-b]thiazole derivatives. Eur J Med Chem. 2012;53:28–40. doi: 10.1016/j.ejmech.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 28.Lee PC, Kakadiya R, Su TL, Lee TC. Combination of bifunctional alkylating agent and arsenic trioxide synergistically suppresses the growth of drug-resistant tumor cells. Neoplasia. 2010;12:376–387. doi: 10.1593/neo.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitiss JL, Soans E, Rogojina A, Seth A, Mishina M. Topoisomerase assays. Curr Protoc Pharmacol. 2012;57:3.3.1–3.3.27. doi: 10.1002/0471141755.ph0303s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Guo P, Wang X, Zhou Q, Gallo JM. Preclinical pharmacokinetic/pharmacodynamic models of gefitinib and the design of equivalent dosing regimens in EGFR wild-type and mutant tumor models. Mol Cancer Ther. 2008;7:407–417. doi: 10.1158/1535-7163.MCT-07-2070. [DOI] [PubMed] [Google Scholar]
- 31.Sanjiv K, Su TL, Suman S, Kakadiya R, Lai TC, Wang HY, Hsiao M, Lee TC. The novel DNA alkylating agent BO-1090 suppresses the growth of human oral cavity cancer in xenografted and orthotopic mouse models. Int J Cancer. 2012;130:1440–1450. doi: 10.1002/ijc.26142. [DOI] [PubMed] [Google Scholar]
- 32.Onn A, Isobe T, Itasaka S, Wu W, O'Reilly MS, Ki Hong W, Fidler IJ, Herbst RS. Development of an orthotopic model to study the biology and therapy of primary human lung cancer in nude mice. Clin Cancer Res. 2003;9:5532–5539. [PubMed] [Google Scholar]
- 33.Zarogoulidis K, Zarogoulidis P, Darwiche K, Boutsikou E, Machairiotis N, Tsakiridis K, Katsikogiannis N, Kougioumtzi I, Karapantzos I, Huang H. Treatment of non–small cell lung cancer (NSCLC) J Thorac Dis. 2013;5(Suppl. 4):S389–S396. doi: 10.3978/j.issn.2072-1439.2013.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 35.Shukla P, Solanki A, Ghosh K, Vundinti BR. DNA interstrand cross-link repair: understanding role of Fanconi anemia pathway and therapeutic implications. Eur J Haematol. 2013;91:381–393. doi: 10.1111/ejh.12169. [DOI] [PubMed] [Google Scholar]
- 36.Brulikova L, Hlavac J, Hradil P. DNA interstrand cross-linking agents and their chemotherapeutic potential. Curr Med Chem. 2012;19:364–385. doi: 10.2174/092986712803414295. [DOI] [PubMed] [Google Scholar]
- 37.Anderson WK, Halat MJ. Antileukemic activity of derivatives of 1,2-dimethyl-3,4-bis(hydroxymethyl)-5-phenylpyrrole bis(N-methylcarbamate) J Med Chem. 1979;22:977–980. doi: 10.1021/jm00194a018. [DOI] [PubMed] [Google Scholar]
- 38.Kakadiya R, Dong H, Lee PC, Kapuriya N, Zhang X, Chou TC, Lee TC, Kapuriya K, Shah A, Su TL. Potent antitumor bifunctional DNA alkylating agents, synthesis and biological activities of 3a-aza-cyclopenta[a]indenes. Bioorg Med Chem. 2009;17:5614–5626. doi: 10.1016/j.bmc.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M, Sun D. Recent advances of natural and synthetic beta-carbolines as anticancer agents. Anticancer Agents Med Chem. 2015;15:537–547. doi: 10.2174/1871520614666141128121812. [DOI] [PubMed] [Google Scholar]
- 42.Shankaraiah N, Nekkanti S, Chudasama KJ, Senwar KR, Sharma P, Jeengar MK, Naidu VG, Srinivasulu V, Srinivasulu G, Kamal A. Design, synthesis and anticancer evaluation of tetrahydro-beta-carboline-hydantoin hybrids. Bioorg Med Chem Lett. 2014;24:5413–5417. doi: 10.1016/j.bmcl.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 43.Kamal A, Srinivasulu V, Nayak VL, Sathish M, Shankaraiah N, Bagul C, Reddy NV, Rangaraj N, Nagesh N. Design and synthesis of C3-pyrazole/chalcone-linked beta-carboline hybrids: antitopoisomerase I, DNA-interactive, and apoptosis-inducing anticancer agents. ChemMedChem. 2014;9:2084–2098. doi: 10.1002/cmdc.201300406. [DOI] [PubMed] [Google Scholar]
- 44.Lan JS, Xie SS, Li SY, Pan LF, Wang XB, Kong LY. Design, synthesis and evaluation of novel tacrine-(beta-carboline) hybrids as multifunctional agents for the treatment of Alzheimer's disease. Bioorg Med Chem. 2014;22:6089–6104. doi: 10.1016/j.bmc.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 45.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology G. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 46.Azzoli CG, Temin S, Giaccone G. 2011 Focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non–small-cell lung cancer. J Oncol Pract. 2012;8:63–66. doi: 10.1200/JOP.2011.000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laurie SA, Goss GD. Role of epidermal growth factor receptor inhibitors in epidermal growth factor receptor wild-type non–small-cell lung cancer. J Clin Oncol. 2013;31:1061–1069. doi: 10.1200/JCO.2012.43.4522. [DOI] [PubMed] [Google Scholar]
- 48.Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19:1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syrigos KN, Saif MW, Karapanagiotou EM, Oikonomopoulos G, De Marinis F. The need for third-line treatment in non–small cell lung cancer: an overview of new options. Anticancer Res. 2011;31:649–659. [PubMed] [Google Scholar]
- 50.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartley KO, Gell D, Smith GC, Zhang H, Divecha N, Connelly MA, Admon A, Lees-Miller SP, Anderson CW, Jackson SP. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- 52.Friedmann B, Caplin M, Hartley JA, Hochhauser D. Modulation of DNA repair in vitro after treatment with chemotherapeutic agents by the epidermal growth factor receptor inhibitor gefitinib (ZD1839) Clin Cancer Res. 2004;10:6476–6486. doi: 10.1158/1078-0432.CCR-04-0586. [DOI] [PubMed] [Google Scholar]
- 53.Fuso Nerini I, Morosi L, Zucchetti M, Ballerini A, Giavazzi R, D'Incalci M. Intratumor heterogeneity and its impact on drug distribution and sensitivity. Clin Pharmacol Ther. 2014;96:224–238. doi: 10.1038/clpt.2014.105. [DOI] [PubMed] [Google Scholar]
- 54.Owen JS, Melhem M, Passarell JA, D'Andrea D, Darwish M, Kahl B. Bendamustine pharmacokinetic profile and exposure-response relationships in patients with indolent non-Hodgkin's lymphoma. Cancer Chemother Pharmacol. 2010;66:1039–1049. doi: 10.1007/s00280-010-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Apostolopoulos C, Castellano L, Stebbing J, Giamas G. Bendamustine as a model for the activity of alkylating agents. Future Oncol. 2008;4:323–332. doi: 10.2217/14796694.4.3.323. [DOI] [PubMed] [Google Scholar]
- 56.Gewirtz DA, Bristol ML, Yalowich JC. Toxicity issues in cancer drug development. Curr Opin Investig Drugs. 2010;11:612–614. [PubMed] [Google Scholar]
- 57.van der Wall E, Beijnen JH, Rodenhuis S. High-dose chemotherapy regimens for solid tumors. Cancer Treat Rev. 1995;21:105–132. doi: 10.1016/0305-7372(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 58.Verweij J, Pinedo HM. Mitomycin C: mechanism of action, usefulness and limitations. Anticancer Drugs. 1990;1:5–13. [PubMed] [Google Scholar]
- 59.Chlebowski RT. Adriamycin (doxorubicin) cardiotoxicity: a review. West J Med. 1979;131:364–368. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.