Abstract
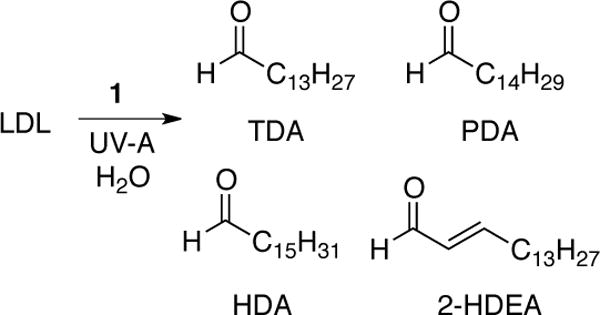
The oxidation of lipids by endogenous or environmental reactive oxygen species (ROS) generates a myriad of different lipid oxidation products that have important roles in disease pathology. The lipid oxidation products obtained in these reactions is dependent upon the identity of the reacting ROS. The photoinduced deoxygenation of various aromatic heterocyclic oxides has been suggested to generate ground state atomic oxygen (O(3P)) as an oxidant; however, very little is known about reactions between lipids and O(3P). To identify lipid oxidation products arising from the reaction of lipids with O(3P), photoactivatable precursors of O(3P) were irradiated in the presence of lysoplasmenylcholine, low-density lipoprotein, and RAW 264.7 cells under aerobic and anaerobic conditions. Four different aldehyde products consistent with the oxidation of plasmalogens were observed. The four aldehydes were: tetradecanal, pentadecanal, 2-hexadecenal, and hexadecanal. Depending upon the conditions, either pentadecanal or 2-hexadecenal was the major product. Increased amounts of the aldehyde products were observed in aerobic conditions.
INTRODUCTION
Oxidative damage caused by reactive oxygen species (ROS) has long been implicated to have an important role in human disease and aging (1). Despite their toxicity, increasing evidence indicates nature has evolved to use ROS as important secondary messengers in cell signaling (2). Additionally, the reactive nature of ROS has been exploited to probe the structure and function of biomolecules in techniques such as chromophore-assisted ligand inactivation and hydroxyl radical footprinting (3–5). The diverse roles played by ROS highlights the importance of understanding their fundamental reactivity with biomolecules.
Ground-state atomic oxygen (O(3P)) is an important species in atmospheric chemistry and has been postulated to form during the photodeoxygenation of aromatic heterocyclic oxides (6–10). The oxidative species generated during photodeoxygenation of aromatic heterocyclic oxides undergoes reactions consistent with what would be expected for O(3P). For example, the putative O(3P) reacts with O2 to form O3, selectively oxidizes tertiary hydrogen of alkanes, displays diffusion-limited rate constants of a magnitude that would be expected for a very small oxidant, and yields similar product ratios compared to O(3P) produced by microwave discharge (9, 11–13). However, due to the difficulties in directly detecting O(3P) in solution, definitive evidence for freely diffusing O(3P) in these photochemical reactions has not been achieved.
Regardless of the exact nature of the oxidant, the reactivity profile of the putative O(3P) is distinct from other ROS. For example, comparison of reaction rate constants for a series of compounds for reactions between the putative O(3P) and hydroxyl radical found O(3P) was considerably more selective than hydroxyl radical. In this previous study, reaction rate constants ranged over three orders of magnitude for O(3P), whereas for hydroxyl radical, the reaction rate constants were nearly identical for the same series of compounds (11). More recently, the photodeoxygenation of 2,8-hydroxymethyl-dibenzothiophene S-oxide (1) was shown to more easily oxidize thiols than other reactive functional groups in aqueous solutions (13). This suggested O(3P) could be used to selectively oxidize cysteine residues over other amino acids within a protein. The photodeoxygenation of 1 resulted in the near quantitative oxidation of a critical cysteine residue of Arabidopsis thaliana adenosine-5′-phophosulfate kinase (14). This observation was consistent with the expected selectivity of O(3P) since the use of most other ROS would be expected to result in more promiscuous oxidation of other amino acid residues.
While the irradiation of O(3P)-precursors has been shown to selectively oxidize cysteine residues in proteins and cause single strand-scission in DNA (14–16), very little is known about the oxidation of lipids by O(3P). The oxidation of lipids by ROS results in a variety of lipid oxidation products that have been implicated as mediators of human disease. The alkene bonds of polyunsaturated fatty acids and sterol ring of cholesterol are targets for ROS. These oxidized lipid species are produced during inflammation in vascular walls and thus participate in atherosclerosis, during infiltration of immune cells into ischemic and ischemic reperfused tissues as well as into tumors. Furthermore, lipids in the lung are exposed to environmental oxidants and ROS produced by subsequent inflammatory responses to environmental-elicited lung injury.
Plasmalogens are class of lipids that are particularly sensitive to oxidation by ROS due their vinyl ether linkage to the glycerol backbone. The vinyl ether bond has been shown to have a higher reactivity with a variety of ROS including ozone, peroxyl radicals, superoxide, and HOBr/HOCl (17–19). Gas phase reaction between O(3P) and alkenes are known to occur rapidly, and the reaction between styrene and O(3P) in acetonitrile yields styrene oxide and phenylacetaldehyde as products (12). Given the sensitivity of plasmalogens to oxidation, it was hypothesized that plasmalogens would be sensitive to oxidation by O(3P) and yield distinct oxidation products. By pursuing this hypothesis, it was sought to determine if O(3P) could be used to generate unique lipid oxidation products in a biological sample.
MATERIALS AND METHODS
Materials
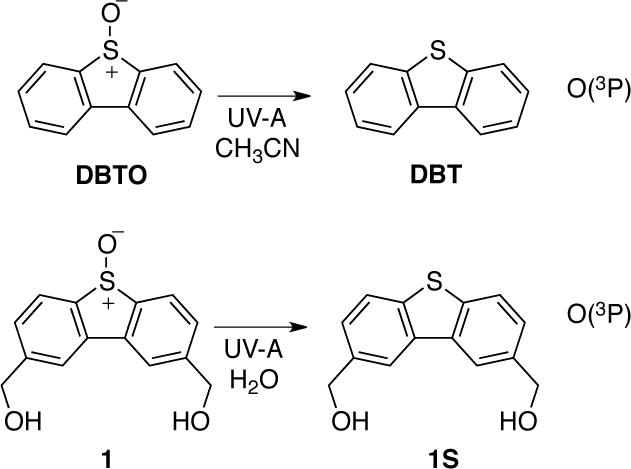
Dibenzothiophene S-oxide (DBTO) was prepared by the oxidation of dibenzothiophene (DBT) with mCPBA by a slight modification to a previously reported procedure (see Supporting Information) (20). 2,8-hydroxymethyldibenzothiophene S-oxide (1) was prepared from DBT by previously described procedures (13). Authentic tetradecanal (TDA), pentadecanal (PDA), and hexadecanal (HDA), were all purchased from TCI America and used as received. Authentic 2-hexadecenal (2-HDEA) was purchased from Toronto Research Chemicals and 2-[15,15,16,16,16-d5]-hexadecenal (2-[d5]-hexadecenal) was prepared as previously described (21).
Preparation of Lysoplasmenylcholine
The lysoplasmenylcholine molecular species, 1-O-hexadec-1′-enyl-sn-glycero-3-phosphocholine (pLPC), was prepared from bovine heart lecithin and purified as previously described (22). Following base methanolysis of bovine heart lecithin, the reaction product, lysoplasmenylcholine, was purified by HPLC, and the lysoplasmenylcholine molecular species, pLPC, was subsequently purified by reversed-phase HPLC. Prepared pLPC was greater than 95% pure as determined by thin-layer chromatography, straight phase HPLC, reversed-phase HPLC, and capillary gas chromatography. pLPC was subjected to acid methanolysis in the presence of arachidic acid (20:0 fatty acid) as an internal standard and quantified by capillary gas chromatography by comparisons of the integrated peak area of the dimethyl acetal of palmitaldehyde (derived from the sn-1 aliphatic chain of pLPC) to that of the methyl ester of arachidic acid (derived from the internal standard).
LDL isolation from peripheral blood
Human blood was collected for the preparation of LDL as authorized by Saint Louis University Institutional Review Board Protocol 10014. LDL from peripheral blood was isolated as described previously (23). In brief, whole blood (50 ml) was taken from healthy human volunteers and anticoagulated with EDTA (final concentration, 5.4 mM) before centrifugation at 500 × gmax for 20 min at 24 °C. The resulting supernatant was collected and adjusted to a density of 1.019 g/ml by the addition of KBr and then ultracentrifuged at 388,000 × gmax for 8 h at 4 °C without braking. After removing the top VLDL layer, the density of the remaining sample was adjusted to 1.063 g/ml by the addition of KBr, and then it was ultracentrifuged again under the conditions specified previously. The yellow lipemic layer floating in the top half of the tube was collected and desalted using PD10 columns. LDL protein concentration was assayed by a Markwell-modified Lowry assay (24).
Irradiation of DBTO and pLPC
A 50 μL aliquot from a solution containing pLPC dissolved in chloroform (9.41 mg/mL) was added to a fused-silica quartz test tube. The chloroform was then removed by a gentle stream of nitrogen. The pLPC was then reconstituted with 1.5 mL of acetonitrile (HPLC grade) containing 1.0 mM of DBTO and 1.0 mM of dodecane as an internal standard. The test tubes were then sealed with a rubber septa, and for anaerobic samples, the samples were sparged with argon for 30 minutes. The samples were then loaded onto a rotating merry-go-round apparatus, and irradiated in a Luzchem LZC-4C photoreactor using eight broadly emitting fluorescent bulbs centered at 350 nm for five hours.
Ozonolysis of pLPC and photolysis mixtures
Samples were exposed to ozone by bubbling through a stream of ozone generated by an Ozotech Poseidon ozone generator (Yreka, CA) being supplied with pure oxygen for 10 minutes. For control experiments, a stream of oxygen was passed through the samples for 10 minutes.
Quantification of aldehyde products from pLPC oxidation
Samples were directly analyzed by capillary column gas chromatography-electron impact mass spectrometry (EI-GC-MS). The GC-MS analysis was carried out using a Shimadzu 30 m (0.25 mm ID × 0.25 μm film thickness) SHRXI-5MS column attached to Shimadzu GCMS-QP2010S mass spectrometer. Helium was used as the carrier gas at a flow rate of 1.0 mL min−1, and the column temperature was initially set at 100 °C for 3 min, then increased by 10 °C min−1 until 250 °C, and then held at 250 °C for 10 min. The ion source temperature was set to 200 °C and the electron energy to 70 eV. Standard curves were made using the authentic samples of the detected aldehydes using dodecane as an internal standard.
Irradiation of LDL and 2,8-hydroxymethyldibenzothiophene S-oxide (1)
A 100 μL aliquot from the solution containing 0.63 mg mL−1 of LDL was added to 1.5 mL of water containing 1 mM of 1. Four drops of 1-octanol was added to samples to decrease foaming while samples were degassed. Additions of more than four drops of 1-octanol resulted in the formation of precipitate during sparging, and thus, careful addition of the 1-octanol was required. The samples were then degassed by sparging with argon for 30 minutes. The samples were then loaded onto a rotating merry-go-round apparatus, and irradiated in a Luzchem LZC-4C photoreactor using eight broadly emitting fluorescent bulbs centered at 350 nm for four hours.
RAW 264.7 cell studies
RAW 264.7 cells were seeded on six well 34.6 mm diameter cell culture plates in high glucose DMEM supplemented with 10% FBS. Prior to experimental conditions, cell culture media was removed and then 1 mL of a 2 mM solution of 1 in PBS or PBS without 1 was added to the cells. The plates were then incubated for 25 min. The plates were then irradiated with a Luzchem LZC-4C with 14 Hitachi FL8BL-B broadly emitting fluorescent bulbs centered at 350 nm bulbs for 2 hours.
Lipid Extraction for LDL and RAW-264.7 cells
The media was spun down to remove any cell fragments and collected for media analysis. Cells were scraped twice with 0.75 mL 154 mM saline solution. The cells and media, as well as LDL samples, were extracted using the Bligh and Dyer lipid extraction technique in the presence of 5 pmol of 2-[d5]-hexadecenal (25). Extraction was conducted three times per sample. After lipid extraction, samples underwent silica extraction on LC-Si (Supelco) columns using chloroform solvent to separate plasmalogens from free fatty aldehydes (26). The samples were then prepared for GC-MS analysis by derivatization with pentafluorobenzyl (PFB) hydroxylamine. Derivatization with PFB hydroxylamine was performed by resuspending the reaction products in 300 μL of 6 mg mL−1 PFB hydroxylamine in water. The ethanol-water mixture was vortexed for 5 min at room temperature with further incubation at room temperature for 25 min. The reaction products were extracted with 4:1 v:v cyclohexane/diethyl ether followed by resuspension in petroleum ether prior to GC-MS analysis.
Aldehyde quantification for LDL and RAW-264.7 cells
After derivatization with PFB hydroxylamine, the samples were analyzed by capillary gas chromatography-mass spectrometry (GC-MS). GC-MS analysis was performed in the negative ion chemical ionization mode (NICI) with methane as the reagent gas using a DB-1 column (12 m, 0.2 mm inner diameter, 0.33 mm methyl silicone film coating: P.J. Cobert), and a 6890 gas chromatograph-5973 mass spectrometer (Agilent). The source temperature was set at 150 ° C. The electron energy was 193.3 eV and the emission current was 49.4 A. The injector and transfer lines were maintained at 250 °C and 280 °C, respectively. The GC oven was maintained at 150 °C for 3.5 min, increased at the rate of 25 °C min−1 to 310 °C and held at 310 °C for another 5 min. Quantification of 2-hexadecenal was performed utilizing selected ion monitoring (SIM) by comparing the integrated area corresponding to m/z = 413 to that produced from the deuterated internal standard 2-[d5]-hexadecenal at m/z = 418. Similarly hexadecanal and pentadecanal were monitored by SIM at m/z = 415 and m/z = 401, respectively. Standard curves were made using the authentic samples of the detected aldehydes using 2-[d5]-hexadecenal as an internal standard.
RESULTS AND DISCUSSION
The photodeoxygenation of dibenzothiophene S-oxide (DBTO) has been suggested as a clean method of generating O(3P) (8, 27). Since side reaction are common with other possible precursors of O(3P), DBTO was selected as the photoactivatable source of O(3P). However, the aqueous solubility of DBTO is low, and thus, other aqueous soluble precursors of O(3P), such as 2,8-hydroxymethyl-dibenzothiophene S-oxide (1), have been recently developed (13). As shown in Scheme 1, DBTO was used as the photoactivatable O(3P)-precursor in acetonitrile, and 1 was used for generating O(3P) in aqueous solutions.
SCHEME 1.
The toxicity of lipid oxidation products has been thoroughly demonstrated and reviewed over the past few decades (28–30). For example, 4-hydroxynonenal has been shown to demonstrate cytotoxic and carcinogenic effects at doses of less than 1 μM (31–32). Lipid oxidation products are often the result of the oxidation of unsaturated fatty acids. Likewise, unsaturated fatty acids were the expected targets for oxidation by O(3P) since O(3P) reacts with alkenes with rate constants on the order of 1 × 109 M s−1 in acetonitrile (11, 12). To examine the reaction of O(3P) with a variety of different lipids quickly, low-density lipoprotein was irradiated in the presence of 2,8-hydroxymethyl-dibenzothiophene S-oxide (1).
Irradiation of LDL and 2,8-hydroxymethyl-dibenzothiophene S-oxide (1) yields aldehydes consistent with the oxidation of plasmalogens
Low-density lipoprotein (LDL) from human blood was chosen as a model system. The predominant protein in LDL is apolipoprotein B. Also, LDL is comprised of phospholipids on the surface of the lipoprotein protocol and a central lipid core containing triglycerides and cholesteryl esters. Furthermore approximately 40% of the esterified fatty acid in LDL is polyunsaturated (33).32 Aqueous solutions containing 0.063 mg mL−1 and 1 mM of 1 were irradiated with UV-A light (350 nm). After irradiation, the sample were derivatized with PFB hydroxylamine and then analyzed by GC-MS. As shown in Scheme 1, the PFB derivatives of four aldehydes were observed tetradecanal (TDA), pentadecanal (PDA), 2-hexadecenal (2-HDEA), and hexadecanal (HDA), which were identified by comparison to the PFB derivatives of authentic aldehydes.
The detected aldehyde products were suspected of arising from the oxidation of plasmalogens within the LDL particles. The amount of plasmalogens present in the fatty acid pools is variable in different tissues and has been estimated to represent as much as 12% of all phospholipids (34). Plasmalogens are particularly sensitive to oxidation due their vinyl ether linkage to the glycerol backbone, which has been shown to have a higher reactivity with a variety of ROS (17–19). The products obtained from the oxidation of plasmalogens is dependent upon the ROS. For example, long-chain aldehydes were observed after plasmalogens in Chinese hamster ovary cells were exposed to singlet oxygen. The oxidative cleavage of the vinyl ether π-bond was proposed to proceed by Hock cleavage of a dioxetane intermediate (35). Conversely, exposure of low and high-density lipoprotein to hydroxyl radical resulted in the formation of α-hydroxy aldehydes (36, 37). Also, Wynalda and Murphy have shown that the ozonolysis of plasmalogen yields long-chain, saturated aldehydes, and plasmalogens were considerably more sensitive to ozonolysis than other unsaturated fatty acids (19).
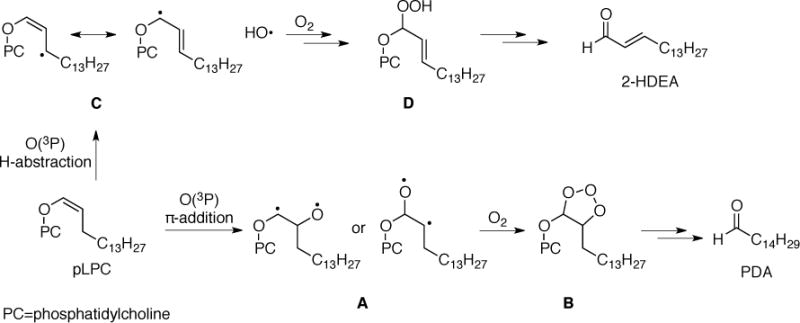
Like other ROS, direct oxidation of the vinyl ether linkage by O(3P) was expected to yield long-chain aldehydes. As mentioned above, plasmalogens are sensitive to ozonylsis, and thus, in aerobic conditions, the formation of O3 from the reaction of O(3P) and O2 could potentially account for some observed aldehydes such as TDA, PDA, and HDA. Oxidation of other monounsaturated or polyunsaturated fatty acids by O(3P) or O3 would be expected to result in different aldehydes. For example, common monounsaturated fatty acids such as palmitoleic acid (16:1 n-7) or oleic (18:1 n-9) would be expected to yield heptanal or nonanal, respectively, upon oxidative cleavage. Also, the ozonolysis of common monounsaturated and polyunsaturated fatty acids are not known to yield 2-HDEA.
The reaction between O2 and O(3P) yields O3. To examine the role of O3 in aerobic conditions, LDL and DBTO were irradiated with UV-A light with and without prior sparging with argon. Controls experiments of samples without 1 or without exposure to light were also performed, and it should be noted that measurable amounts of the observed products were observed in these controls arising from sample handling during PFB derivatization. The results of these experiments are shown in Figure 1. As shown in Figure 1A, 2-HDEA and PDA were the dominant products in aerobic conditions where 106 and 78 pmol per 0.1 mg of LDL was observed, respectively. However, a significant change, compared to controls, was also observed for TDA and HDA as well. If O3 was being generated in appreciable amounts, it was expected that 2-HDEA would react to form TDA. Thus, it was postulated that 2-HDEA was the result of a direct reaction between O(3P) and the lipids, possibly a plasmalogen or another unsaturated fatty acid, within the LDL.
Figure 1.
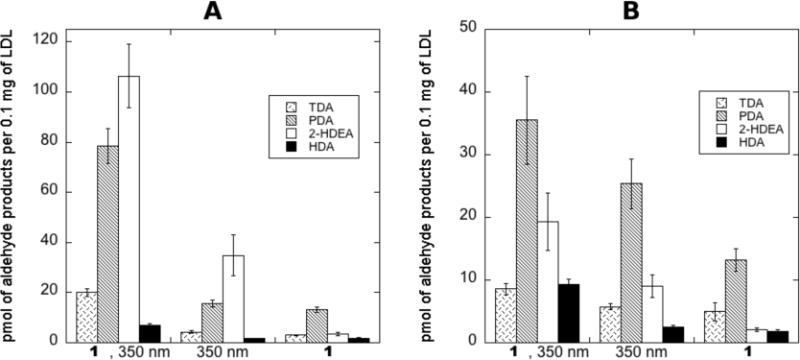
Amount of aldehyde products observed upon irradiation of LDL and 1 with broadly emitting 350 nm bulbs (UV-A) in anaerobic and aerobic conditions. A: aerobic conditions, B: anaerobic conditions. Error bars represent the standard error from three or more experiments. A Q-test with 99% confidence was performed to remove outlying data due to gross errors.
To examine how the removal of O2 from the reaction would influence the observed products, samples were sparged with argon prior to irradiation. To achieve anaerobic conditions, extreme care was required since foaming was observed when a gentle stream of argon gas was passed through the samples. Foaming was greatly reduced by the addition of 1-octanol; however, except at very low concentrations of 1-octanol, the combination of sparging and 1-octanol resulted in the formation of a precipitate that could not be dissolved even with sonication. These difficulties resulted in a greater amount of error associated with the data in anaerobic conditions. As shown in Figure 1B, decreased levels of all the aldehyde products was observed in anaerobic conditions compared to aerobic conditions. Also, the increase observed between the sample (1, 350 nm) and the controls was considerably smaller. Compared to the irradiated control (350 nm), an increase of only 2.8, 10.0, 10.3, and 6.8 pmol per 0.1 mg of LDL for TDA, PDA, 2-HDEA, and HDA, respectively, was observed. Additionally, the increase in PDA was not significant from the photo-control when the standard error for the measurements was considered. When taking account of the uncertainity represented by the standard error, an increase of only 1.3, 4.0, and 5.5 pmol per 0.1 mg of LDL for TDA, 2-HDEA, and HDA, respectively, could be claimed. This was somewhat unexpected since 2-HDEA was expected to increase in the absence of O3. In attempt to provide greater clarity for these unexpected results, the oxidation of an isolated plasmalogen was pursued.
Aldehyde products from the oxidation of pLPC by O(3P)
To examine the reaction of O(3P) with plasmalogens, pLPC was selected as a model plasmalogen. As shown in Scheme 3, irradiation of a solution of DBTO in acetonitrile with UV-A light in the presence of pLPC resulted in the formation of tetradecanal (TDA), pentadecanal (PDA), hexadecanal (HDA), and 2-hexadecenal (2-HDEA). These were the only four aldehydes that were detectable by GC-MS analysis. As described below, the identity of the aldehyde products was determined by the comparison to authentic samples of TDA, PDA, HDA, and 2-HDEA. Comparison of the GC-MS results for authentic 2-HDEA and the putative 2-HDEA from the photolysis mixture is shown in Figure 2. The peak retention times for authentic 2-HDEA and the unknown aldehyde were 14.88 and 14.89 minutes, respectively. Inspection of the authentic 2-HDEA and unknown mass spectra revealed similar fragmentation patterns. Thus, the unknown aldehyde was tentatively assigned to 2-HDEA. Identification of the remaining TDA, PDA, and HDA was performed in a similar fashion (see Supporting Information).
SCHEME 3.
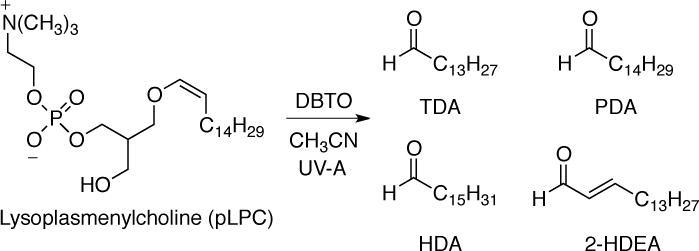
Figure 2.
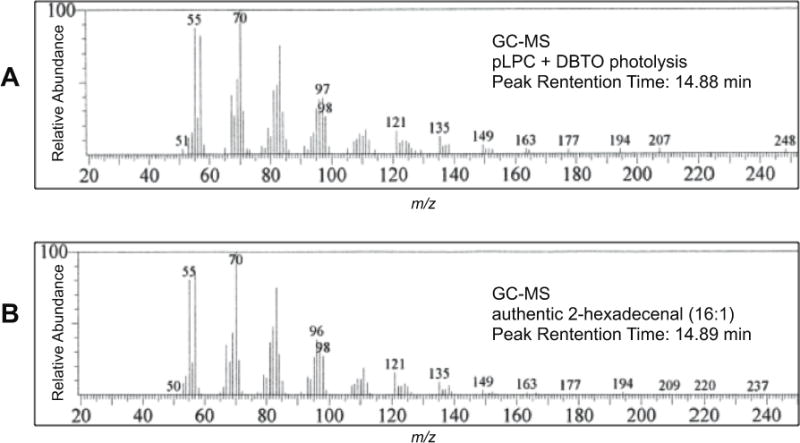
Comparison GC-MS results of unknown product from pLPC oxidation by UV irradiation of DBTO to authentic sample of 2-hexadecenal. A: GC-MS of unknown aldehyde. B: GC-MS data for an authentic sample of 2-hexadecenal.
Additional evidence for 2-hexadecenal
While GC-MS comparison to authentic 2-HDEA supported the assignment of the unknown product as 2-HDEA, more evidence was desired to rule out other possible potential isomers. To confirm the position of the carbon-carbon double bond, solutions of pLPC (700 μM) and DBTO (1 mM) were irradiated with UV-A light for 4 hours, which as expected resulted in the formation of the four different aldehyde products. The irradiated samples were then exposed to ozone for ten minutes. After ozonolysis, the aldehyde tentatively assigned to 2-HDEA was completely consumed, and a 200 μM increase of both TDA and PDA was observed, which were the expected ozonolysis products of 2-HDEA and unreacted pLPC, respectively. No other new aldehyde products were observed. To confirm TDA as the expected ozonolysis product of 2-HDEA, a sample of 100 μM of authentic 2-HDEA in acetonitrile was exposed to ozone in a similar fashion. Following ozonolysis, no 2-HDEA was detectable by GC-MS, and the only aldehyde observed was 80 ± 30 μM of TDA. The absence of shorter alkyl aldehydes after ozonolysis and nearly identical GC-MS compared to authentic 2-HDEA provides strong evidence that the assignment of the olefin containing aldehyde product as 2-HDEA was correct.
Quantitation of aldehyde products in aerobic and anaerobic conditions
The reaction between molecular oxygen and O(3P) to form ozone has a measured rate constant of 4 × 109 M s−1 in water (38). Thus, in aerobic conditions, formation of O3 during the photodeoxygenation of DBTO and its analogs is possible. To investigate the effect of removing oxygen, sample of 710 μM of pLPC and 1 mM DBTO in acetonitrile were irradiated with and without sparging with argon prior to irradiation. Formation of the aldehyde products and deoxygenation product dibenzothiophene (DBT) are given in Table 1. When the samples were deoxygenated, irradiation of the solution resulted in the formation of 220 ± 90 μM of PDA and 28 ± 8 μM of 2-HDEA and small increases in TDA and HDA (entry 1). In the absence of DBTO (entry 2), a much smaller increase (~30 μM) of PDA was observed, and the other aldehydes were either not detected or showed no change. For both aerobic and anaerobic conditions, samples that were not irradiated showed no formation of the aldehyde products (entry 3 and 6). It should be noted that in all samples, trace amounts of HDA were always detected during GC-MS analysis. The other aldehydes products were not detectable prior to irradiation. Therefore, for HDA, the change in HDA concentrations prior and after the experiments (entries 1–8) are reported in Table 1.
Table 1.
Amounts of pLPC aldehyde products produced during UV irradiation of DBTO and control experiments.a
| Entry | Cond. | O2b | [DBT]c | Total [CHO]c | [TDA]c | [PDA]c | [2-HDEA]c | [HDA]c |
|---|---|---|---|---|---|---|---|---|
| 1 | DBTOd, hνe | N | 470 ± 170 | 250 ± 100 | 6.7 ± 2.3 | 220 ± 90 | 28 ± 8 | 2.8 ± 1.7 |
| 2 | hν | N | – | 26 ± 11 | n.d.h | 29 ± 9 | n.d. | −2 ± 7 |
| 3 | DBTO | N | 6.9 ± 7.7 | 1.1 ± 2.0 | n.d. | n.d | n.d | 1.1 ± 2.0 |
| 4 | DBTO, hν | Y | 360 ± 100 | 730 ± 240 | 13 ± 4 | 630 ± 230 | 80 ± 30 | 4.5 ± 1.7 |
| 5 | hν | Y | – | 71 ± 18 | 2 ± 3 | 52 ± 17 | 13 ± 7 | 3.7 ± 2.4 |
| 6 | DBTO | Y | −2 ± 4 | 0.0 ± 2.0 | n.d. | n.d. | n.d. | 0.0 ± 2.0 |
| 7 | Ozonef | – | – | 730 ± 260 | 15 ± 5 | 700 ± 190 | n.d. | 14 ± 4 |
| 8 | Airg | – | – | 0.6 ± 1.2 | n.d. | n.d. | n.d. | 0.6 ± 1.2 |
Samples of 653 μM of pLPC in 6 mL acetonitrile contained within fused-silica test tubes were used for all experiments.
(N) Oxygen removed from samples by sparging with argon for 30 minutes. (Y) samples irradiated without sparging.
Concentrations in μM determined by GC-MS reported as mean ± 95% confidence interval for four or more experiments. A Q-test with 99% confidence was performed to remove outlying data due to gross errors.
Samples Contains 1.0 mM of DBTO.
sample irradiated with eight broadly emitting fluorescent bulbs centered at 350 nm for five hours.
Ozone gas gently passed through sample for 10 minutes.
Air gently passed through sample for 10 minutes.
not detected.
In the aerobic conditions, significantly more of PDA and 2-HDEA, 630 ± 230 μM and 80 ± 30 μM respectively, and about double the amount of TDA and HDA, 13 ± 4 μM and 4.5 ± 1.7 μM respectively, were produced upon irradiation of the sample (entry 4). Unlike in anaerobic conditions, small amounts of TDA and 2-HDEA were also detected in the irradiated control (entry 5). The increase observed in aerobic conditions was statistically significant in the total amount of aldehyde being produced in aerobic conditions. The similar amount of DBT being produced in anaerobic and aerobic conditions (entries 1 and 4) indicates the increase in amount of aldehyde being produced in aerobic conditions is not due to simply more oxidant being produced. It is tempting to suggest that in aerobic conditions that O3 results in greater oxidation of the vinyl ether linkage which has been shown to be very sensitive to ozonolysis (19). However, the relative ratio of the two major aldehyde products PDA and 2-HDEA remained the same at 7.8:1 of PDA to 2-HDEA, respectively. Since O3 reacts with pLPC to largely form PDA (entry 7), the similar ratio of PDA to 2-HDEA in anaerobic and aerobic conditions was counter to appreciable amounts of O3 being formed. If some of the O(3P) was being converted to O3, an increase in the amount of TDA and PDA at the expense of 2-HDEA would have been expected. This result does not rule out the formation of O3 in these samples, but suggested that O3 was not a significant fraction of the active oxygen species.
In the oxidation of LDL by 1, the observed aldehydes were suggested to arise from the oxidation of plasmalogens by O(3P). The significant formation of TDA, PDA and 2-HDEA during the oxidation of pLPC by DBTO suggests this was plausible. The decrease in the amount of observed aldehyde products in deoxygenated samples was also consistent with the results obtained for the oxidation of LDL. Therefore, the presence O2 uniformly increased the amount of aldehyde products being observed. However, as discussed above, the results were inconsistent with the involvement of O3, and no evidence for the formation of singlet oxygen during the irradiation of 1 in aerobic conditions has been previously reported (13). An alternative explanation for the increase in oxidation products in aerobic conditions is that O2 reacts with intermediates formed after the reaction between O(3P) and the plasmalogen. The epoxidation of styrene by O(3P) was suggested previously to arise from diradical (12). Consistent with this previously suggested mechanism, formation of ozonide B after the reaction between the diradical A and O2 would be expected to yield PDA as shown in Scheme 4 (π-addition pathway). Formation of 2-HDEA could potentially arise from an allylic H-abstraction leading to the allylic hydroperoxide D in the presence of oxygen, which has been proposed to convert to 2-HDEA (35). While these proposed mechanisms are consistent with previous suggested mechanisms, the true mechanism and the role of O2 in the oxidation of these lipids during UV-A irradiation of 1 and DBTO was not resolved by the experiments reported here.
SCHEME 4.
Exposure of RAW 264.7 cells to O(3P) does not result in the formation aldehyde products
The predominant ether linked phospholipid in RAW 264.7 cells is plasmenylethanolamine, which comprises about 36% of the total ethanolamine glycerolipid pool (39). These cells were irradiated with 350 nm light in 1 enriched media to test whether or not plasmalogen oxidation would be observed in a more complex sample. After extraction and PFB derivatization similar to the LDL procedures, only trace amounts of the previously observed aldehydes could be detected, and no statistically relevant change compared to control samples that either were not irradiated or contained no 1 was observed. Simply not generating enough oxidant was a potential explanation for this observation. However, the 2 mM starting concentration of 1 was expected to generate approximately 800 μM of O(3P) under these conditions (14).14 From previous work, 4-vinylbenzoic acid was not easily oxidized by O(3P) in water, where as 3-mercaptobezonic acid was quantitatively converted to the corresponding disulfide. Glutathione (GSH) concentrations in cells can reach millimolar concentrations. Thus, an alternative explanation was another antioxidant, such as GSH, was more competitive for O(3P) than the plasmalogens present in the cell samples. However, further experiments are needed to confirm this speculation.
Supplementary Material
SCHEME 2.
Acknowledgments
Portions of this material is based upon work supported by the National Science Foundation under CHE-1255270. The authors thank the National Science Foundation for support under grant CHE-0963363 for renovations to the research laboratories in Monsanto Hall. This work was additionally supported by donors to the Herman Frasch Foundation and the Presidential Research Fund at Saint Louis University.
Footnotes
SUPPLEMENTARY MATERIALS
A description of the preparation of DBTO and Figures S1–S3 can be found at DOI: 10.1562/2006-xxxxxx.s1.
References
- 1.Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson K, Rajfur Z, Vitriol E, Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008;18:443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deperalta G, Alvarez M, Bechtel C, Dong K, McDonald R, Ling V. Structural analysis of a therapeutic monoclonal antibody dimer by hydroxyl radical footprinting. MAbs. 2013;5:86–101. doi: 10.4161/mabs.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenowitz M, Chance MR, Dhavan G, Takamoto K. Probing the structural dynamics of nucleic acids by quantitative time-resolved and equilibrium hydroxyl radical “footprinting”. Curr Opin Struct Biol. 2002;12:648–653. doi: 10.1016/s0959-440x(02)00366-4. [DOI] [PubMed] [Google Scholar]
- 6.Leonori F, Occhiogrosso A, Balucani N, Bucci A, Petrucci R, Casavecchia P. Crossed Molecular Beam Dynamics Studies of the O(3P) Allene Reaction: Primary Products, Branching Ratios, and Dominant Role of Intersystem Crossing. J Phys Chem Lett. 2012;3:75–80. [Google Scholar]
- 7.Albini A, Alpegiani M. The photochemistry of the N-oxide function. Chem Rev. 1984;84:43–71. [Google Scholar]
- 8.Wan S, Jenks WS. Oxenoid reactivity observed on the photolysis of ceratin aromatic sulfoxides. J Am Chem Soc. 1995;117:2667–2668. [Google Scholar]
- 9.Lucien E, Greer A. Electrophilic oxidant produced in the photodeoxygenation of 1,2-benzodiphenylene sulfoxide. J Org Chem. 2001;66:4576–4579. doi: 10.1021/jo010009z. [DOI] [PubMed] [Google Scholar]
- 10.McCulla RD, Jenks WS. Deoxygenation and other photochemical reactions of aromatic selenoxides. J Am Chem Soc. 2004;126:16058–16065. doi: 10.1021/ja045935k. [DOI] [PubMed] [Google Scholar]
- 11.Bucher G, Scaiano J. Laser flash photolysis of pyridine N-oxide: kinetic studies of atomic oxygen [O(3P)] in solution. J Phys Chem. 1994;98:12471–12473. [Google Scholar]
- 12.Thomas KB, Greer A. Gauging the significance of atomic oxygen [O(3P)] in sulfoxide photochemistry. A method for hydrocarbon oxidation. J Org Chem. 2003;68:1886–1891. doi: 10.1021/jo0266487. [DOI] [PubMed] [Google Scholar]
- 13.Korang J, Grither WR, McCulla RD. Photodeoxygenation of dibenzothiophene S-oxide derivatives in aqueous media. J Am Chem Soc. 2010;132:4466–4476. doi: 10.1021/ja100147b. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Ravilious GE, Hicks LM, Jez JM, McCulla RD. Redox Switching of Adenosine-5′-phosphosulfate Kinase with Photoactivatable Atomic Oxygen Precursors. J Am Chem Soc. 2012;134:16979–16982. doi: 10.1021/ja3078522. [DOI] [PubMed] [Google Scholar]
- 15.Wauchope OR, Shakya S, Sawwan N, Liebman JF, Greer A. Photocleavage of plasmid DNA by dibenzothiophene S-oxide under anaerobic conditions. J Sulfur Chem. 2007;28:11–16. [Google Scholar]
- 16.Korang J, Emahi I, Grither WR, Baumann SM, Baum DA, McCulla RD. Photoinduced DNA cleavage by atomic oxygen precursors in aqueous solutions. RSC Adv. 2013;3:12390–12397. [Google Scholar]
- 17.Stadelmann-Ingrand S, Pontcharraud R, Fauconneau B. Evidence for the reactivity of fatty aldehydes released from oxidized plasmalogens with phosphatidylethanolamine to form Schiff base adducts in rat brain homogenates. Chem Phys Lipids. 2004;131:93–105. doi: 10.1016/j.chemphyslip.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Murphy RC. Free-radical-induced oxidation of arachidonoyl plasmalogen phospholipids: antioxidant mechanism and precursor pathway for bioactive eicosanoids. Chem Res Toxicol. 2001;14:463–472. doi: 10.1021/tx000250t. [DOI] [PubMed] [Google Scholar]
- 19.Wynalda KM, Murphy RC. Low-concentration ozone reacts with plasmalogen glycerophosphoethanolamine lipids in lung surfactant. Chem Res Toxicol. 2010;23:108–117. doi: 10.1021/tx900306p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelsen SF, Luo Y, Weaver MN, Lockard JV, Zink JI. Optical spectra of protected diamine 10-bond-bridged intervalence radical cations related to N,N,N,N-tetraalkylbenzidine. J Org Chem. 2006;71:4286–4295. doi: 10.1021/jo060466l. [DOI] [PubMed] [Google Scholar]
- 21.Brahmbhatt VV, Hsu F-F, Kao JL-F, Frank EC, Ford DA. Novel carbonyl and nitrile products from reactive chlorinating species attack of lysosphingolipid. Chem Phys Lipids. 2007;145:72–84. doi: 10.1016/j.chemphyslip.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Han XL, Zupan LA, Hazen SL, Gross RW. Semisynthesis and purification of homogeneous plasmenylcholine molecular species. Anal Biochem. 1992;200:119–124. doi: 10.1016/0003-2697(92)90286-g. [DOI] [PubMed] [Google Scholar]
- 23.Hazen SL, Hsu FF, Gaut JP, Crowley JR, Heinecke JW. Modification of proteins and lipids by myeloperoxidase. Meth Enzymol. 1999;300:88–105. doi: 10.1016/s0076-6879(99)00117-2. [DOI] [PubMed] [Google Scholar]
- 24.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 25.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Brahmbhatt VV, Nold C, Albert CJ, Ford DA. Quantification of pentafluorobenzyl oxime derivatives of long chain aldehydes by GC-MS analysis. Lipids. 2008;43:275–280. doi: 10.1007/s11745-008-3153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregory GD, Wan ZH, Jenks WS. Photodeoxygenation of dibenzothiophene sulfoxide: evidence for a unimolecular S-O cleavage mechanism. J Am Chem Soc. 1997;119:94–102. [Google Scholar]
- 28.Reddy JK. Lipid metabolism and liver inflammation. II. fatty liver disease and fatty acid oxidation. AJP-GI. 2005;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 29.Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J Photoch Photobio B. 2001;63:103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 30.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr. 1993;57:779S–785S. doi: 10.1093/ajcn/57.5.779S. [DOI] [PubMed] [Google Scholar]
- 31.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 32.Emerit I, Khan SH, Esterbauer H. Hydroxynonenal, a component of clastogenic factors? Free Radic Biol Med. 1991;10:371–377. doi: 10.1016/0891-5849(91)90045-5. [DOI] [PubMed] [Google Scholar]
- 33.Viens L, Athias A, Lizard G, Simard G, Gueldry S, Braschi S, Gambert P, Lallemant C, Lagrost L. Effect of lipid transfer activity and lipolysis on low density lipoprotein (LDL) oxidizability: evidence for lipolysis-generated non-esterified fatty acids as inhibitors of LDL oxidation. J Lipid Res. 1996;37:2179–2192. [PubMed] [Google Scholar]
- 34.Diagne A, Fauvel J, Record M, Chap H, Douste-Blazy L. Studies on ether phospholipids. II. Comparative composition of various tissues from human, rat and guinea pig. Biochim Biophys Acta. 1984;793:221–231. doi: 10.1016/0005-2760(84)90324-2. [DOI] [PubMed] [Google Scholar]
- 35.Morand OH, Zoeller RA, Raetz CRH. Disappearance of plasmalogens from membranes of animal cells subjected to photosensitized oxidation. J Bio Chem. 1988;263:11597–11606. [PubMed] [Google Scholar]
- 36.Jira W, Spiteller G. Plasmalogens and their oxidative degradation products in low and high density lipoprotein. Chem Phys Lipids. 1996;79:95–100. doi: 10.1016/0009-3084(95)02511-1. [DOI] [PubMed] [Google Scholar]
- 37.Felde R, Spiteller G. Plasmalogen oxidation in human serum lipoproteins. Chem Phys Lipids. 1995;76:259–267. doi: 10.1016/0009-3084(94)02448-e. [DOI] [PubMed] [Google Scholar]
- 38.Kläning U, Sehested K, Wolff T. Ozone formation in laser flash photolysis of oxoacids and oxoanions of chlorine and bromine. J Chem Soc, Faraday Trans. 1984;80:2969–2979. [Google Scholar]
- 39.Zoeller RA, Rangaswamy S, Herscovitz H, Rizzo WB, Hajra AK, Das AK, Moser HW, Moser A, Lazarow PB, Santos MJ. Mutants in a macrophage-like cell line are defective in plasmalogen biosynthesis, but contain functional peroxisomes. J Biol Chem. 1992;267:8299–8306. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


