Abstract
The common marmoset (Callithrix jacchus) has garnered interest recently as a powerful model for the future of neuroscience research. Much of this excitement has centered on the species’ reproductive biology and compatibility with gene editing techniques, which together have provided a path for transgenic marmosets to contribute to the study of disease as well as basic brain mechanisms. In step with technical advances is the need to establish experimental paradigms that optimally tap into the marmosets’ behavioral and cognitive capacities. While conditioned task performance of a marmoset can compare unfavorably with rhesus monkey performance on conventional testing paradigms, marmosets’ social cognition and communication are more similar to that of humans. For example, marmosets are amongst only a handful of primates that, like humans, routinely pair bond and care cooperatively for their young. They are also notably pro-social and exhibit social cognitive abilities, such as imitation, that are rare outside of the Apes. In this review, we describe key facets of marmoset natural social behavior and demonstrate that emerging behavioral paradigms are well suited to isolate components of marmoset cognition that are highly relevant to humans. These approaches generally embrace natural behavior and communication, which has been rare in conventional primate testing, and thus allow for a new consideration of neural mechanisms underlying primate social cognition and communication. We anticipate that through parallel technical and paradigmatic advances, marmosets will become an essential model of human social behavior, including its dysfunction in nearly all neuropsychiatric disorders.
I. Introduction
There has been considerable interest recently in the common marmoset (Callithrix jacchus) as a neuroscientific model organism. This renewed focus on an already established animal model species (Bendor and Wang, 2005; Chaplin et al., 2013; Fritsches and Rosa, 1996; Roberts et al., 1994; Roberts and Wallis, 2000; Wang et al., 2005) has been driven, at least in part, by the prospect of developing primate transgenic lines (Belmonte et al., 2015; Kaiser and Feng, 2015). There is some debate as to the suitability of marmosets, compared to more widely used animal models, especially the rhesus monkey for tapping into higher aspects of human cognition and rodents for functional dissection of neural circuitry. On the one hand, marmosets share with humans core features of brain architecture and function (Bendor and Wang, 2005; Chaplin et al., 2013; Mitchell and Leopold, 2015) and the complex social and cognitive behaviors typical of the primate Order (Digby, 1995; Digby and Barreto, 1993; Huber and Voelkl, 2009; Voelkl and Huber, 2007). On the other hand, marmosets are a unique species with their own distinct evolutionary history and behavioral repertoire. Most notably, they are unusually small primates, and in that respect nearly opposite to humans. While this difference can be a hindrance in studying some aspects of brain organization and function, the small size offers a number of distinct experimental advantages. In weighing these factors against those of more widely used experimental models, many researchers have concluded that the marmoset model is likely to play a prominent role in the next chapter of neuroscience (Belmonte et al., 2015). Here we argue that this species is particularly promising as a model for studying neural circuits of social behaviors and their dysfunctions in neuropsychiatric disorders.
Primates are perhaps best distinguished from other animals by their sophisticated societies and the manner in which individuals understand and navigate these complexly structured and dynamic social systems. Like other social mammals (Barnett, 1958; Blumstein, 2013; Crook et al., 1976; Hofer and East, 1993; Mann et al., 2000; Wittemeyer and Getz, 2007), primate social hierarchies are organized along familial lines (Mitani et al., 2012), but since social knowledge is not limited to ego-centric relationships (Barton and Dunbar, 1997; Cheney and Seyfarth, 1990, 2007; Seyfarth and Cheney, 2015; Seyfarth et al., 2005), the capacity for communication may be much larger and the complexity of the rules governing social interaction may be much higher. Social rule learning is a critical component of primate cognitive development (Byrne and Bates, 2010; Cheney et al., 1995; Seyfarth and Cheney, 2012; Tomasello and Call, 1997), for which primates appear to have specialized mechanisms (Flombaum and Santos, 2005; Hare et al., 2001; van de Waal et al., 2013; Voelkl and Huber, 2007). Nonhuman primates can exhibit social tactics familiar to humans, such as deception and strategic alliances (Byrne and Whiten, 1989; le Roux et al., 2012; Palombit et al., 1997). Thus the social landscape of primates is fraught with unique challenges and opportunities (Toarmino et al., In Press).
Circuits for interpreting social landscapes and making decisions must be adaptive and accommodate learning over both short and long time scales to understand the context and consequences of behavior. Primates excel in modeling and predicting the actions of other group members based on knowledge of their respective relationships gained during prior interactions (Byrne and Bates, 2010; Cheney and Seyfarth, 1990, 2007; Toarmino et al., In Press). An individual's social participation and knowledge encompasses visual observation, active communication, the testing of social rules, and strategic thinking – skills that together have shaped the evolution of the primate brain (Adolphs, 2009; Frith and Frith, 1999; Ghazanfar and Santos, 2004). These core human behaviors are notably affected in a range of human mental disorders, where it is the interaction with other members of society that is most obviously affected (Kennedy and Adolphs, 2012). However, our understanding of the brain circuits that steer real-world human social interaction is limited, in large part due to the constraints of measuring brain activity in humans and the limits of existing behavioral paradigms in relevant models.
A major challenge for studying active social behavior in primates has been the limitations of existing paradigms. Marmosets may be better suited for developing and implementing such paradigms than other species, particularly as a model for human social interaction. Like humans, marmoset are amongst the few primates that pair-bond (Digby and Barreto, 1993) and engage in cooperative care of their young (French, 1997; Solomon and French, 1997). Cooperative breeding in humans and marmosets is thought to have coevolved with a unique set of social cognitive behaviors, including prosociality (Burkart et al., 2009a; Burkart and van Schaik, 2010). A case in point of this prosociality is food sharing. Marmosets allow group members to remain in close proximity during feeding (Day et al., 2003) and share foods with unrelated conspecifics even without an opportunity for reciprocity (Burkart et al., 2007). Most of the cognitive elements underlying prosocial behavior are likely to be a shared feature of the primate brain; however, the cooperative behaviors shared by marmosets and humans are uncommon among primates, including those great apes most similar to humans (Hare et al., 2007; Wobber et al., 2010). It is only through the interaction with species-specific societies that these and other types of cooperative behaviors are expressed in some primate societies. Marmosets also exhibit sophisticated forms of observational social learning (Burkart et al., 2009b) and are one of the only non-ape species to demonstrate imitation (Bugnyar and Huber, 1997; Voelkl and Huber, 2000). Because marmosets are small in body size (~300-400g), they can be housed in the laboratory in their species-typical family units that naturally give rise to rich interaction and social communication in laboratory conditions. The marmoset brain, though lissencephalic (smooth), possesses the shared neural architecture of primates (Chaplin et al., 2013; de la Mothe et al., 2006, 2012; Mitchell and Leopold, 2015; Solomon and Rosa, 2014), including potential homologous cortical substrates underlying complex perceptual processes, such as pitch discrimination (Bendor and Wang, 2005; Song et al., 2016; Wang and Walker, 2012). Modern technological methods make it feasible to track the activity of neural circuits under such conditions (Eliades and Wang, 2008a, b; Eliades and Wang, 2013; Miller et al., 2015; Roy and Wang, 2012). Thus, just as active exploration of natural landscapes by rats led to the discovery of place cells and grid cells, the core building blocks of circuits for spatial cognition (O'Keefe and Dostrovsky, 1971), studies of marmosets exploring their social landscape may lead to similarly fundamental insights into social brain function. While it is possible that some of the resemblance is only superficial, such as cooperative parenting (Saito, 2015), the extent to which shared social behaviors are supported by homologous neural mechanisms remains an empirical question.
The primary aim of this review is to highlight recent experimental, technological and conceptual advances that provide a foundation for developing the marmoset into a model for the neuroscientific study of social behaviors as are found in humans and their psychiatric disorders. First, we briefly review the merits and limitations of current animal models of human social circuits. We then discuss how active visual and acoustic signaling in marmosets could provide a road map for developing models of natural human social interaction. We conclude with a brief discussion of the prospects for gene editing methods in the marmoset and how these could be combined with natural social experimental paradigms to understand the basis for human neuropsychiatric disease.
II. Rodent and Monkey Models of Human Social Behavior
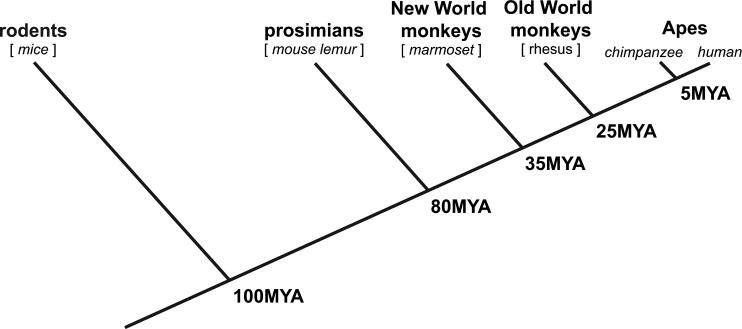
Establishing animal models of human social behavior necessitates evidence of similarities in sociality, as well as its underlying neurobiological mechanisms. Certain core features of social behavior are shared among mammals, as are the corresponding features of circuits in the brain. Much of what we understand about the neurobiology of social interaction comes from research in rats, mice, and voles (Beery and Kaufer, 2015; Insel and Young, 2001; Young, 2002). This is despite peripheral adaptations that are very different from humans, most notably the importance of olfaction for social perception (Barnett, 1958; Grant, 1963; Wolff and Sherman, 2007). Rodents engage in intricate parental care (Insel and Young, 2001), demonstrate reciprocal cooperation in some situations (Viana et al., 2010), and aid distressed cage mates even at a cost to themselves (Bartal et al., 2011). Social interactions amongst rodent species are typically mitigated by the issuance of specific signals, such as vocalizations (Arriaga and Jarvis, 2013; Portfors, 2007) and odor cues (Arakawa et al., 2008; Lin et al., 2005), which can be perceived by conspecifics at a distance. Nearby interactions are also facilitated by tactile behavior (Bobrov et al., 2014; Brecht, 2007; Grant, 1963). These cues convey information ranging from social recognition to the presence of a predators or other threats (Johnston, 2003; Shelley and Blumstein, 2005; Wesson, 2013), and facilitate forms of social learning, such as that for food preference (Galef and Kennett, 1987; Laland and Plotkin, 1993). These social tendencies have often been used to support the use of rodents as models of human social behavior. Indeed, humans exhibit analogous social behaviors, as do many other gregarious mammals (Bradbury and Vehrencamp, 2011; Gariepy et al., 2014; Hecht et al., 2012; Heinrich, 2011; Madden et al., 2009, 2011; Mann et al., 2000; Rendell and Whitehead, 2001; Wittemeyer and Getz, 2007). Rather than serve as a model of human-specific sociality, these aspects of rodent sociality likely reflect more general mammalian characteristics that evolved prior to our last common ancestor and the divergence of the species (Emes et al., 2003; Li et al., 1990; Murphy et al., 2001) (Figure 1).
Figure 1.
Cladogram showing the evolutionary divergence between humans, rodents and each of the main primate taxonomic groups, and estimated time points of divergence. [MYA-Millions of Years Ago].
The neurobiology of rodent social interaction has led to several important discoveries on underlying neural circuits and neurochemicals relevant to many social vertebrates, including humans. Studies of oxytocin in rodents, for example, have shown that this neuropeptide is critical to a broad range of social behaviors, such as maternal care, attachment and affiliation (Insel and Young, 2001). Oxytocin knock-out mice (OTKO) develop normal olfactory bulbs but have significantly impoverished social recognition (Ferguson et al., 2000). Notably, oxytocin plays a similarly fundamental role in social attachment for humans (Feldman, 2012; Feldman et al., 2015; Kosfeld et al., 2005). Likewise, social aggression and recognition in mice are mediated by the vasopressin 1b receptor in hippocampal CA2 pyramidal neurons (Caldwell et al., 2008; Pagani et al., 2015; Wersinger et al., 2002). Mice with lesioned (Stevenson and Caldwell, 2014) or optogenetically inactivated (Hitti and Siegelbaum, 2014) CA2 showed a significant reduction in social recognition (Dudek et al., 2016). Although direct evidence of CA2 function in humans is limited, some patients with social neuropsychiatric disorders (e.g. schizophrenia, autism, etc) have exhibited abnormalities in CA2 (Meyer-Lindberg et al., 2011). These similarities in the neurobiology of social behavior in rodents and humans likely reflect a common mammalian heritage and have maintained their adaptive value and function in most, if not all, mammals because the social contexts in which they function have remained critical in all mammalian societies (e.g. parental care, recognition, affiliation, etc).
However, because individual mammalian taxa have evolved distinctive, idiosyncratic social behaviors, rodent studies must be complemented by studies in primates. Many of these idiosyncratic behaviors relate directly to aspects of social interaction. Each species faces a unique set of challenges in its social landscape and must employ distinct strategies for social interactions. As sociality varies immensely among mammals, individual species’ brains are marked by very different social adaptations. In primates, social behavior relies strongly upon visual and acoustic communication and, in contrast to most mammals, depends minimally on chemical and olfactory signaling. In the visual domain, the interpretation of faces and bodily postures is particularly important, with primates’ high visual acuity permitting the analysis of these visual cues from a distance of several meters or more. Most experimental work to date has focused on faces, which are particularly important for human social communication. Faces are inherently salient, are controlled with a high degree of precision by specialized muscles and neural mechanisms, and serve as the basis for conveying a spectrum of meaningful social signals (Fridlund, 1994; Leopold and Rhodes, 2010). At each moment, a person's face carries a wealth of information for a perceiver, including individual identity, gender and emotional state (Micheletta et al., 2015; Parr and Heintz, 2009; Preuschoft, 1995, 2000). The importance of facial communication is reflected in the fine differentiation of facial musculature in primates and the putative specialization for face processing in high-level visual cortex and subcortical structures (Desimone et al., 1984; Gothard et al., 2007; Hoffman et al., 2007; Maior et al., 2010; Perrett et al., 1985; Sugase et al., 1999; Tsao et al., 2006; Vick et al., 2007; Weiner and Grill-Spector, 2015). Brain imaging and electrophysiological experiments have shown that multiple ‘face patches’ are evident in the temporal lobe of humans, rhesus monkeys, and marmosets (Hung et al., 2015; Kanwisher et al., 1997; Tsao et al., 2008), suggesting that such a network might be a core feature of the primate social brain. Current studies are investigating specialization among such regions for extracting different types of information from faces, such as head orientation, direction of gaze, individual identity, body context, and facial motion (Fisher and Freiwald, 2015a, b; Freiwald and Tsao, 2010; Hoffman and Haxby, 2000; Leopold et al., 2006; Polosecki et al., 2013).
While much has been learned regarding layout and basic response properties of face-specialized areas, our knowledge vis-à-vis social interaction runs up against the limitations imposed by conventional paradigms in which animals are restrained. For example, neurophysiological investigations of face selectivity have primarily relied on contrasting responses to the brief presentation of stimuli from different visual categories. A recent study found that responses collected under such circumstances were minimally predictive of the role of individual cells under more naturalistic viewing conditions (McMahon et al., 2015). Face-selective neurons recorded during free viewing of natural social videos responded to features other than faces, such as the perceived distance to an individual or group. Furthermore, neighboring neurons within a few hundred microns of one another responded at very different times during the video. Importantly, individual neurons were highly deterministic in their responses when the same video clip was repeated, thus the difference among neurons reflected a genuine specialization to different features of the video. Free viewing of video stimuli revealed a fascinating new dimension of social perception; neurons in the amygdala were sometimes highly sensitive to where an animal was looking. Specifically, a population of neurons responded the most when the subject looked at the eye of an animal in the video, while a subclass of neurons responding only during “eye contact”, when the animal in the video was also looking at the camera (Mosher et al., 2014). Given the importance of eye contact and the rules governing it for primate social communication, these neural responses may be critical for understanding high-level aspects of primate social signaling, such as communication, attachment, threat, and gaze-following. Together, these early studies using free viewing of social videos illustrate that even a relatively moderate relaxation of conventional testing paradigms can lead to deep new insights into the neurobiological basis of primate social perception.
Other behavioral and neurophysiological experiments in rhesus monkeys have used paradigms that emphasize active decision making, especially during direct social interaction between animals. As a precursor, an early experiment studying the inclination of subjects to select which photo they preferred to look at demonstrated distinct preferences for certain socially relevant stimuli (e.g. female perinea) over others (e.g. low ranking males) (Deaner et al., 2005), with these choices reflected in the activity of neurons in parietal cortex (Klein et al., 2008). Such choice behavior depends not only on the stimulus but also on the impact a choice has on another animal observer (Chang et al., 2011). For example, in one experiment, the choice of one animal (the Actor) determined whether the Actor, the Observer, both, or neither animal received a reward. Distinct areas of frontal cortex and anterior cingulate cortex played complementary roles in subjects’ choices based on outcome (Chang et al., 2013). In perhaps the most compelling of these results, neurons in the dorsal anterior cingulate were monitored while two monkeys interacted during a Prisoner's Dilemma game, where the monkeys could choose to cooperate for a larger reward outcome in the long term (Haroush and Williams, 2015). Individual cingulate neurons were sensitive to distinct aspects of these interactions, including predictions about the other monkey's ensuing choice. These and related studies (Azzi et al., 2012; Lee, 2010) illustrate that social information is weighed when primates make decisions, and that neurons associated with decision-making are impacted by the nuances of the context and related social perception. More importantly, they suggest that certain neurobiological processes may only be evident during active social interactions. Decisions are not solely based on the value of a particular stimulus, but on the relative choices of another conspecific in the interaction. Given that much of the sophistication evident in primate social cognition relates to the acquisition of social knowledge and its implementation for strategies to succeed in the primate social landscape, further study of active social interactions and signaling is likely to be particularly significant in future discoveries about the neurobiology of primate social behavior.
To unravel the neural basis of human social behavior, animal models have been and will continue to be critical. The strength of rodent models is the precision modern technologies afford for dissecting neural circuits (Ashton-Jones and Deisseroth, 2013; Deisseroth, 2011; Oh et al., 2014), along with the relative ease in setting up direct social interaction (Gunaydin et al., 2014; Peca et al., 2011). The strengths of primate models are shared perceptual domains and immediate relevance to humans. In the next section, we argue that marmosets provide a path to apply the advantages of circuit dissection to the primate brain. The marmoset model supporting this effort is rooted in important aspects of its natural behavior, as well as recent technical and conceptual advances that afford the opportunity to study the neurobiology of active social interactions in the primate brain.
III. Paradigms to Investigate the Neurobiology of Marmoset Social Behavior
Marmosets are prolific explorers of their social landscape. In addition to the sophisticated facets of social behavior discussed above, marmosets possess an expansive system for social signaling for navigating the complex nuances of their societies. Communication in this species includes social grooming (Lazaro-Perea et al., 2004) and scent marking behavior (Abbott, 1984; Abbott et al., 1997; Lazaro-Perea et al., 1999; Smith, 2006; Smith et al., 2001), but as is typical amongst primates, there is a clear dominance of visual (de Boer et al., 2013; Kemp and Kaplan, 2013) and vocal signaling (Bezerra and Souto, 2008; Chen et al., 2009; Miller et al., 2010b; Miller and Wang, 2006; Norcross and Newman, 1993; Pistorio et al., 2006). Traditional head-restrained preparations constrain neurobiological studies of social signaling in marmosets, as in other primates. Where this small New World primate differs, however, is that neural studies of their social behavior are not limited to this paradigm. Because marmosets can live in large extended family groups in captivity and engage in normal social interactions, it is possible to study the neural basis of natural interactions in an experimentally controlled fashion. In Old World primates, such as the rhesus monkey, such studies are difficult due to the high costs associated with building suitably large enriched habitats as well as the more aggressive disposition of the species. Techniques developed for recording single-unit activity in freely-moving marmosets significantly expands the range of behaviors that can be studied with electrophysiology (Eliades and Wang, 2008a; Roy and Wang, 2012). Neurophysiology is but one of the many methods used to study neural function. Several other techniques could be modified from existing technology employed in rodents for use in these small-bodied primates, including calcium imaging (Flusberg et al., 2008; Helmchen et al., 2001; Sadakane et al., 2015) and optogenetics (MacDougall et al., 2016; Watakabe et al., 2015). Ultimately, the greatest value of marmosets may emerge when methods to record and manipulate neural activity are applied to the study of active social behavior and signaling.
Social signaling in primates presents something of a paradox. Despite many examples of high social aptitude within the rules of their society (Hare et al., 2001, 2006; Rosati et al., 2010), the repertoire of social signals (e.g. facial expressions, vocalizations, piloerection, etc) is notably limited. One might expect that the demonstrable complexity in social interaction might be matched by a broad “vocabulary” for social communication. Yet, one of the lessons from primate ethology is that communication does not amount to a catalogue of social signals, but must instead be seen within the broader context of an existing social scene of interacting individuals, where environment, social dominance, and recent history strongly influence how the limited number of signals is used and interpreted (Engh et al., 2006; Seyfarth and Cheney, 2014). Even in the case of human language, which is much richer in its content, the meaning behind a word or sentence is often highly contextual (Dunbar, 2003; Fitch et al., 2010). Future studies of active social signaling, likely in the marmoset, may shed greater light on the roles of social monitoring, memory and other cognitive operations in language comprehension. Importantly, it also provides a path to study neural mechanisms relevant to language, which need not be limited to vocal signals (McGurk and MacDonald, 1976). Thus, isolated aspects of social communication can be studied in other taxa (e.g. signal processing, vocal-learning, etc). But the idiosyncratic nuances of how social signals weave into the fabric of the primate social landscape, and the distinct neural processes that may emerge as a result of coordinating interactions in these scenes (Hasson et al., 2012), may necessitate a primate model.
Marmosets provide unique opportunities for laboratory studies of the neural basis of active communication. We discuss two components of marmoset social signaling - gaze following and antiphonal calling - as representative case studies of how this approach can be applied in the species.
Gaze-Following
More than other mammals, primates rely on vision to monitor their social landscape and coordinate social interactions (Allman, 1977). Key to this process is the face and the direction it is pointed. The direction of a conspecific's gaze also provides conspecifics with critical information about relevant information in the environment, such as food, predators and pertinent social interactions. Primates use each other's gaze direction as a spatial cue to direct their attention towards common points of interest, a group of behaviors termed ‘joint attention’(Tomasello, 1995). The significance of this visual behavior in primates is evidenced by its rapid development and role in more sophisticated aspects of social cognition. In human infants, for example, recognition of whether a face is making direct eye contact or not emerges by 1-4 months, while the ability to follow the direction of an averted gaze is evident soon after (4-6 months) (Farroni et al., 2002; Farroni et al., 2003; Gomez, 2005). By comparison, other aspects of face processing, such as recognition of emotional expression (Herba and Phillps, 2004) and individual identity (Carey and Diamond, 1994; McKone et al., 2012) appear later in childhood. Joint attention provides a key channel to communicate the location of important items, for example, in predator avoidance or food foraging. The basic features of joint attention are found across primates, and where data are available, also appear early in development (Tomasello et al., 2005), although at differing levels of sophistication across species (Rosati and Hare, 2009).
Marmosets, like other primates, are keenly interested in social stimuli such as faces (Hung et al., 2015; Mitchell et al., 2014). Evidence for the reading of faces and bodily postures comes from studies of observational learning of adult foraging skills by juvenile observers (Schiel and Huber, 2006). While marmosets routinely direct their gaze to faces and bodies (Mitchell et al., 2014), the kind of information that they extract and utilize is an open area of research. Close inspection of Figure 2 (panel A) reveals that gaze is primarily directed to internal facial features. This observation suggests that detailed information related to identity or facial behavior is gathered rather than more general species-related information, which might be gathered from external features like the ear tufts.
Figure 2.
Scan patterns of a marmoset attending to social stimuli (Mitchell et al., 2014). Note the repeated saccades to the face in each image. In panel B, the first saccade away from the face was in the direction of the gaze.
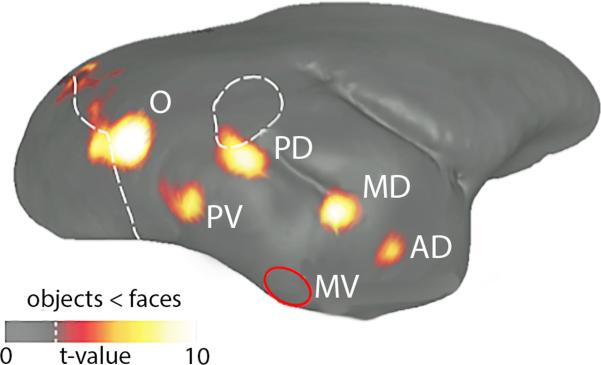
In contrast, there already is clear evidence that marmosets use gaze information as a cue for shared attention in both experimental and natural contexts. Although Old World primates use the eyes to determine direction of gaze in addition to head, Calltrichids appear to rely predominantly on head-direction cues (Santos and Hauser, 1999). This difference likely reflects the fact that larger monkeys and apes often move their eyes only to shift gaze, while marmosets tend to rotate their head rather than their eyes when shifting their gaze (Heiney and Biazquez, 2011; McCrea and Gdowki, 2003). Marmosets have been shown to perform geometrical gaze following, using gaze cues of other individuals to orient towards objects beyond their line of sight (Burkart and Heschl, 2006), although their understanding of what is seen by the other individual (i.e, visual access) is less clear (Burkart et al., 2007; Rosati and Hare, 2009). Determining the extent to which a primate can infer the mental state or knowledge of a conspecific based on where they are looking is an inherently challenging experimental question (Flombaum and Santos, 2005; Hare et al., 2001, 2006) which has not been explicitly studied in marmosets. In natural contexts, several behaviors that coordinate activity between group members are likely to rely on the shared attention signals mediated by gaze following. The “mobbing” behavior exhibited by groups when a terrestrial predator intrudes on their territory provides one example (Epple, 1975; Ferrari and Ferrari, 1990; Passamani, 1995). When a predator is detected, a group of marmosets engage in an elaborate and coordinated response (Barros et al., 2002; Clara et al., 2008). A marmoset detecting a threat will orient itself towards its location, maintain gaze on it, and repeatedly emit a sharp “mobbing” (tsik) call. The extent to which this orientation cue is used by other group members to detect the predator has not been manipulated experimentally. Marmosets are also willing to follow the gaze of human experimenters, for example, in searching for particular food locations (Burkart and Heschl, 2006). These behaviors could be leveraged under experimental contexts to provide a platform for studying gaze and social interactions. With paradigms suitable to track gaze-following in less restrained contexts, such as family groups living in a home cage, it would be possible to study its emergence in ontogeny (Teufel et al., 2010; Tomasello et al., 2001) and developmental neurobiology. Marmosets possess a system of face-selective regions across temporal cortex (Hung et al., 2015) similar to that in rhesus monkeys and humans (Tsao et al., 2006; Tsao et al., 2008) (i.e. ‘face-patches; Figure 3). Focused neurophysiological recordings in these and neighboring locations (Marciniak et al., 2014) as marmosets engage in gaze-following and other communication behaviors involving faces and facial expressions is likely to be particularly significant for determining the functional role that this circuit plays in active social signaling.
Figure 3.
Evidence of the ‘Face-Patch’ system in the extrastriate cortex of marmosets (Hung et al., 2015). fMRI functional map contrasting faces and objects reveals five discrete areas that significantly more responsive to face stimuli in awake marmosets. A sixth face patch, indicated by a red circle and labeled area MV, was detected with ECoG but not with fMRI due to signal dropout. Color bar below represents the t value scale.
Antiphonal Calling
Marmosets are highly vocal primates. Their proclivity to produce vocal signals at high rates contrasts with many Old World primates, including rhesus monkeys, and is thought to be an adaptation to the dense vegetation in the forest habitats marmosets have lived throughout their evolutionary history (Morrill et al., 2013; Rylands et al., 2009). Limits imposed on visual signaling from vegetation may have necessitated the use of vocal signals for maintaining social contact with conspecifics. Using its rich vocal repertoire, the species engages in near tonic level of vocal signaling to communicate with conspecific group members and maintain social interactions in their forest habitat (Agamaite et al., 2015; Bezerra and Souto, 2008; Epple, 1968). The significance of vocal signals for mitigating social interactions in marmosets presents an opportunity to investigate the dynamic relationship between social behavior, cognition and communication in this primate model.
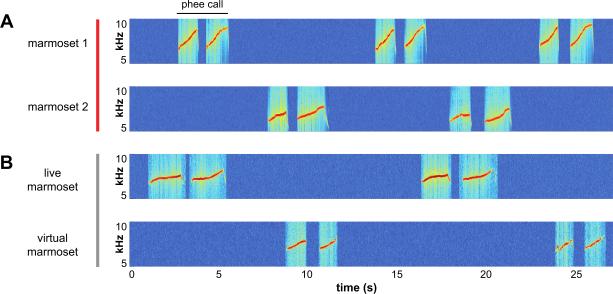
When marmosets are visually occluded from each other, they commonly engage in a conversational exchange known as antiphonal calling (Miller et al., 2010b; Miller and Wang, 2006; Norcross and Newman, 1993) (Figure 4A). Adult marmosets rarely interrupt each other during these conversations, but rather take turns alternating successive calls in bouts of vocal exchanges (Miller and Wang, 2006; Takahashi et al., 2013). The rhythm of these exchanges is governed by rules reflecting social relationships (e.g. cagemate, non-group mate, sex, age etc) (Chen et al., 2009; Chow et al., 2015; Miller et al., 2010b; Miller and Wang, 2006; Norcross and Newman, 1997). Marmosets will cease to interact with callers who deviate from the correct temporal pattern (Miller et al., 2009a). Callers exert control over several aspects of signal production during this behavior, ranging from a motor plan about the structure of the vocalization to avoiding sources of acoustic interference (Miller et al., 2009b; Roy et al., 2011). During an experiment in which noise was broadcast with different periodicities, for example, pairs of marmosets would coordinate the timing of their behavior to both avoid the interference and maintain turn-taking within the constraints of the environment (Roy et al., 2011). The dynamics of these vocal exchanges are also learned during ontogeny (Chow et al., 2015). Young marmosets must learn to take turns, use the correct call type and follow the appropriate periodicity of the exchange based on the social context. Evidence also suggests that parents may play a role in guiding the development of this behavior (Chow et al., 2015). Several characteristics of this behavior parallel conversations in humans (Bruner, 1975; Snow, 1977; Stivers et al., 2009), suggesting that antiphonal calling may be a valuable model for understanding the social and developmental influences on language.
Figure 4.
Antiphonal calling conversations in marmosets. (A) Plots spectrograms of two visually occluded marmosets engaged in an antiphonal conversational exchange. The vocalizations of each individual monkey is shown in the spectrogram shown on each row. A 2-pulse Phee call is indicated at the top. (B) Shows an antiphonal conversation between a live marmoset (above) Virtual Marmoset (VM; below). Note that marmosets directly engage VMs similarly to live marmosets. This behavioral paradigm affords the unique opportunity to actively participate in primate social signaling while also maintaining experimental control for explicit tests of social recognition and decision-making.
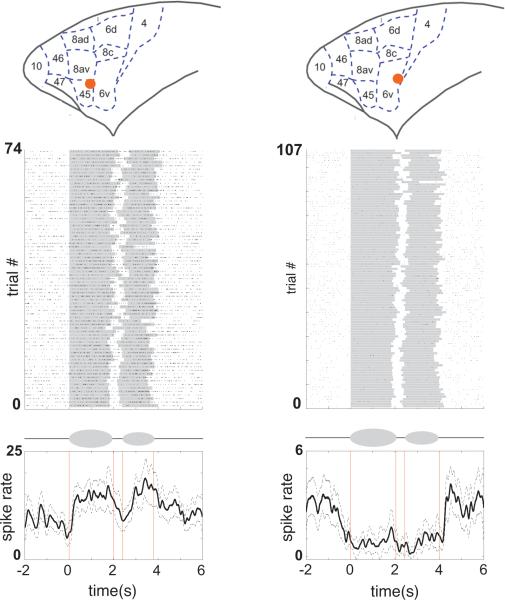
Antiphonal calling is an ideal behavior in which to apply recent technical advances to neurobiological studies of primate social behavior and active social signaling. A series of experiments recording the activity of auditory cortex neurons as marmosets naturally produced vocalizations were the first to apply the freely-moving neurophysiology method (Eliades and Wang, 2012; Eliades and Wang, 2008b; Eliades and Wang, 2013). These experiments showed that, despite being generally suppressed during vocal production, individual auditory neurons were more sensitive to deviations in subtle changes to auditory feedback during vocal production than simply hearing the same vocalization played back passively (Eliades and Wang, 2008b). To more explicitly test active social signaling, however, data from animals directly engaged in vocal interactions are necessary. A novel experimental paradigm was developed that involved generating ‘Virtual Marmosets’ (VMs), via a computer-controlled playback system, who directly engage live marmosets in their natural antiphonal calling interaction (Figure 4B). One of the key benefits of this method is that each VMs vocal behavior and vocal signals can be systematically manipulated in order to not only directly engage marmosets in their natural vocal interactions but also experimentally test the significance of various social and acoustic cues (Miller et al., 2009a; Miller and Thomas, 2012; Miller and Wang, 2006). This behavioral paradigm was recently used in a neurophysiological study of marmoset prefrontal and premotor cortex (Miller et al., 2015). Previous studies showed vocalization-induced immediate early gene expression across these areas of marmoset frontal cortex (Miller et al., 2010a; Simoes et al., 2010). Neurophysiological recordings during natural antiphonal calling interactions showed that marmoset frontal cortex neurons exhibited motor related changes in activity both prior to and during vocal production, including populations across ventrolateral premotor and prefrontal regions (Figure 5), such as Area 44 (i.e. a putative Broca's area homolog). These data contrast with recent studies of rhesus monkeys trained to vocalize in response to a visual stimulus (Coude et al., 2011; Hage and Nieder, 2013). In each of these studies, not only was the neural response primarily 500-1000ms prior to vocal onset but was also limited to only vocalizations produced in response to the stimulus. Naturally produced vocalizations did not elicit vocal-motor related neural responses. This is suggestive that vocal production during active communication in marmosets may reveal different dimensions of neural function. This was the first neurophysiological study of active signaling in a primate and serves as a foundation for more sophisticated interactive paradigms (Miller and Thomas, 2012). Neurophysiological recordings using interactive paradigms could determine perceptual boundaries for social and acoustic categories. More broadly, such paradigms can be applied to examine a myriad of sophisticated cognitive mechanisms at play during social signaling (King, 2015; Miller and Bee, 2012) and the role of these behaviors in the broader context of navigating the intricacies of primate societies. This approach offers new avenues of research for marmosets as a model of language. While songbirds have often been cited as a model of language (Doupe and Kuhl, 1999), most of the important insights from that work have centered on sensory-motor learning and motor control (Brainard and Doupe, 2000; Dave and Margoliash, 2000; Long and Fee, 2008; Roberts et al., 2012). The marmoset model offers a complementary approach to study language-related aspects of social communication that are unique to primates and, given the close phylogenetic mechanisms, likely to be supported by common brain mechanisms.
Figure 5.
Vocal-motor activity in marmoset frontal cortex neurons during natural vocal production (Miller et al., 2015). Two representative examples of neurons recorded during natural vocal production are shown. A schematic drawing at top shows the anatomical location of each cell in marmoset frontal cortex. Below plots the response during vocal production (Raster and PSTH). Grey bars on each line of the Raster plot the onset and offset of each 2-pulse phee, while vertical black lines indicate an action potential. The red vertical lines in the PSTH indicate the average onset and offset of each pulse of the phee.
Caveats and Considerations of Natural Behavior
The use of natural social behaviors in neuroscientific studies has many merits, but they are not without their limitations. One of the great strengths of traditional primate neurophysiological approaches is the ability to rigorously control and study neural function in awake, behaving animals. Subjects are highly trained to perform a regimented task in which many of the factors that may modulate neural activity are controlled. This approach allows for the precise measurement of repeatable stimulus classes. This degree of control is not possible in freely-moving, naturally behaving animals. Exactly how the inherent variability in social behaviors affects the various endogenous and exogenous factors that modulate neural activity in primate neocortex is not known. One likely factor in the differences reported in ventrolateral prefrontal cortex responses to vocalizations in freely-moving marmosets (Miller et al., 2015) and restrained rhesus monkeys (Gifford et al., 2005; Romanski et al., 2005), in which neurons are driven by the acoustic stimuli, relates to variability in baseline neural activity during stimulus presentations in the former. While clearly the brain is able to function without the need to control for these factors, the sources of variance also confound our ability to test and parse their relative contributions to neural function. Ultimately, progress on this front necessitates a balance of these issues. Our selection of gaze-following and antiphonal calling as examples of how to pursue these issues was to highlight the opportunity to examine facets of natural primate social behaviors, while also maintaining some degree of experimental control.
IV. Transgenic Marmosets: Implications for Social Neuropsychiatry
Neuroscience is in the midst of an empirical revolution. The advent of gene-editing techniques has afforded the opportunity to map, manipulate and record facets of neural function that were not possible only a decade ago (Ashton-Jones and Deisseroth, 2013; Tye and Deisseroth, 2012). Many of the most exciting advances from the application of these techniques have come from rodent models, while progress in primates has been more modest (Geritis and Vanduffel, 2013). The recent development of transgenic lines with germline transmission in marmosets (Sasaki et al., 2009) has pushed this species to the forefront of neuroscience as it highlights the prospect of a broader genomic tool kit (Belmonte et al., 2015) and the potential for primate models of human neuropsychiatric and neurodegenerative disease (Okano et al., 2012). While transgenic techniques have been implemented in other primate species, including recent work applying the CRISPR/Cas9 system and Talen mediated gene editing in rhesus monkeys (Kang et al., 2015; Niu et al., 2014; Wan et al., 2015), it is characteristics of marmoset reproductive biology that make the species particularly attractive for developing a broad range of transgenic disease models. Because of the short short gestation (~140days) and natural propensity to produce multiple offspring at each birth (Tardif et al., 2003) in marmosets, for example, a large population of a particular transgenic line could be generated relatively quickly. Though it should be noted that genetic chimerism of paternal twins may present certain challenges (Ross et al., 2007). The lissencephalic (i.e. smooth) cortical surface of the marmoset brain provides a different advantage for transgenic lines expressing a genetically-encoded calcium indicator (GECI) molecules (Broussard et al., 2014), such as GCaMP, or opsin molecules (Emiliani et al., 2015), that allow cell-specific control of neural activity. Because nearly all areas of the brain are accessible directly under the skull, optical imaging and activation methods can be implemented more effectively in marmosets than in many other primates. There is untold potential for transgenic marmosets in many areas, but as outlined above, their behavioral biology lends itself particularly well to experiments in the social domain. The types of behavioral paradigms discussed in the previous section would provide unique avenues to phenotype these primates following gene insertions and deletions in disease models, while modern optical imaging and activation methods could be aptly applied to studies of social circuits in the marmoset brain.
Primates are social animals with many attributes that are specific to our Order. Neuropsychiatric disorders that affect our ability to function in the social domain are immensely damaging. Autism Spectrum Disorder (ASD), for example, is a neurodevelopmental disorder that can manifest in different ways but individuals typically exhibit a range of deficits in social interaction and communication (Frith and Happe, 2005). Many humans on the ASD exhibit clear deficits in social signaling, such as avoiding looking at faces and eyes (Grice et al., 2005; Jones and Klin, 2013) and language impairments(Bartak et al., 1975; Charman et al., 2003). Because of ASD's prevalence and debilitating effects, extensive efforts have been made to understand the underlying causes. Recent advances in genomics have begun to identify the numerous genes that may underlie ASD (Abrahams and Geschwind, 2008), such as Shank3 (Durand et al., 2006; Moessner et al., 2007). Deletions of the Shank3 gene in mice results in anomalous social behavior (Duffney et al., 2015; Peca et al., 2011). Notably, the changes in mouse social behavior are typically characterized by the avoidance of, or reduction in time interacting with, conspecifics and atypical patterns of social interactions. Although these broad behavioral changes are often symptomatic of ASD, they certainly do not capture the breadth of social deficits evident in humans as discussed above. While mice and human do exhibit some similarities as a result of their shared mammalian heritage, much has obviously changed in the evolution of each taxa's brain and behavior since the taxa diverged (Shultz et al., 2011). Deletion of Shank3 in a primate may also impact primate social behaviors, but in ways that are notably distinct from other mammals. A primate model is critical to further explicating the genetic basis of ASD. Due to the broad experimental techniques available to study the neurobiology and behavior of marmoset social interactions, a Shank3 marmoset model would be able to diagnose not only general changes to social behavior in these animals but also key deviations in primate specific social signaling and behavior (e.g. eye gaze, coordinated vocal interactions, etc) using some of the behavioral paradigms discussed above. Furthermore, because the functional neuroanatomy of the primate brain has been conserved, changes in the behavioral phenotype following genetic manipulations would more likely have a similar impact on the underlying neural circuits in human and nonhuman primates than may be expected with a rodent model (Kaiser and Feng, 2015). The ability to identify the interaction between genes, neurons and behavior represents a powerful approach to explicating the mechanisms underlying neuropsychiatric disorders in the primate brain.
V. Conclusions
Here we argued that the greatest value of marmosets in neuroscience is as a model of the neural circuitry underlying social behavior and its disorders. Certainly, the marmoset model is more broadly applicable to several other areas of neuroscience research (Fox et al., 2010; Mitchell and Leopold, 2015; Philippens et al., 2010; Roberts and Wallis, 2000). However, facets of the species behavioral biology, such as small size, rich social signaling systems and cooperative society, as well as the broader array of neural recording techniques possible in this species, make them particularly amenable to questions about sociality.
As with any research model organism, the marmoset is not without its limitations, some examples of which have been addressed previously (Mitchell and Leopold, 2015). Two often voiced shortcoming of the marmoset are that, first, the phylogenetic separation from humans is too great, and second, that the lissencephalic brain is simply too different to allow for comparison with humans. These concerns have some degree of validity, though they are often overstated. Humans’ common ancestor with New World monkeys lived about ten million years earlier than its common ancestor with rhesus monkeys (Figure 1). Extensive comparative studies in primate species have shown that this phylogenetic separation alone is unlikely to account for large differences in the brain, since anthropoid primates (i.e. monkeys and apes) have a very similar constellation of brain areas (Kaas, 2013). Whether there are subtler differences in the brain that endowed the clade of Old World monkeys with cognitive abilities absent in New World monkeys remains to be demonstrated. At the same time, it must be said that the marmoset, in being one of the smallest anthropoid primates, also has one of the smallest brains. It is the absolute brain size, rather than the lissencephaly per se, that may shape aspects of marmoset cognition and behavior in a direction opposite to that of humans, who possess the largest primate brain (Mitchell and Leopold, 2015).
Most knowledge about the primate brain derives from the rhesus monkey, with findings from this work shaping our conception of the human brain, as well as our interpretation of direct measurement of human brain activity via fMRI (Alexander et al., 1986; Connor, 2007; Desimone et al., 1984; Felleman and Van Essen, 1991; Gold and Shadlen, 2007; Middleton and Strick, 1994; Romo and de Lafuente, 2013; Romo et al., 2004; Salzman et al., 1990; Tsao et al., 2006; Tsao and Livingstone, 2008; Tsao et al., 2008; Tsunada et al., 2016; Ungerleider and Mishkin, 1982). That the marmoset differs in some ways from the rhesus should be taken as an advantage, rather than a disadvantage. The two species offer complementary perspectives on our primate brain, which can be well directed to studying some of the most personal and fascinating aspects of human cognition.
The types of active social signaling paradigms highlighted in this manuscript represent a potentially powerful approach for which the marmoset is particularly well-suited, but they are by no means the only ones that are likely to be fruitful. Since marmosets display a similarly flexible pattern of sociosexual behavior as humans (Buss and Schmitt, 1993; Cavanaugh et al., 2015; Cavanaugh et al., 2014), continued research on the neurochemical basis of social attachment and recognition in marmosets (Cavanaugh et al., 2015; Cavanaugh et al., 2014; Mustoe et al., 2015; Smith et al., 2010), for example, provides direct comparisons to many analogous studies in rodents discussed earlier on this topic and offers the opportunity to identify shared and derived features of these core mammalian social behavioral systems in primates. Furthermore, marmoset active social signaling paradigms represent potential models for understanding the neural mechanisms underlying facets of language, in particular the intricate interplay between social communication and social cognition. This would include, but not be limited to, modeling social monitoring, recognition, and decision-making. Like transgenic marmoset models, the application of modern molecular techniques using viruses (Watakabe et al., 2015), such as two-photon calcium imaging (Sadakane et al., 2015) and optogenetics (MacDougall et al., 2016), to studies of the marmoset brain are able to build on the richness of marmoset social behaviors and existing experimental paradigms to explicate the underlying functional neural circuitry. While much work remains, we anticipate that a marmoset model of human social behavior is uniquely suited as a powerful approach to expand the frontiers of Neuroscience.
Acknowledgements
We thank Ed Connor, Nicho Hatsopoulos, Eric Knudsen and Jitendra Sharma for helpful comments on previous versions of this manuscript. CTM was supported by grants from the NIH (R01 DC012087 & R21 MH104756) and NSF (IDBR 1254309) . VAF was supported by The Simons Collaboration on the Global Brain, NSF (INSPIRE Track 2 DBI-1343174) and the New York Stem Cell Foundation. DAL was supported (in part) by the Intramural Research Program of the NIMH under grants ZIA MH002898 and ZIA MH002838. JFM was supported by grants from the NIH (R21 MH104756, U01 NS094330). ACS was supported by the Intramural Research Program of the NIH, NINDS. XW was supported by grants from the NIH (R01 DC03180 & R01 DC005808).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abbott DH. Behavioral and physiological suppression of fertility in subordinate marmoset monkeys. American Journal of Primatology. 1984;6:169–186. doi: 10.1002/ajp.1350060305. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Saltzman W, Schutz-Darken NJ, Smith TE. Specific neuroendocrine mechanisms not involving generalized stress mediate social regulation of fremale reproduction in cooperative breeding marmoset monkeys. In: Carver CS, Kirkpatrick B, Lederhendler LL, editors. The Integrative Neurobiology of Affiliation. Annals of the New York Academy of Sciences; NY: 1997. pp. 219–238. [DOI] [PubMed] [Google Scholar]
- Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. The social brain: Neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agamaite JA, Chang CJ, Osmanski MS, Wang X. Quantitative acoustic analysis of the vocal repertoire of the common marmoset (Callithrix jacchus). Journal of the Acoustic Society of America. 2015;138:2906–2928. doi: 10.1121/1.4934268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allman JM. Evolution of the visual system in early primates. In: Sprague JM, Epstein AM, editors. Progress in Psychobiology and Physiological Psychology. Academic Press; New York: 1977. pp. 1–53. [Google Scholar]
- Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odoran communication in mice. Neuroscience & Biobehavioral Reviews. 2008;32:1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriaga G, Jarvis ED. Mouse vocal communication system: Are ultrasounds learned or innate? Brain and Language. 2013;124:96–116. doi: 10.1016/j.bandl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton-Jones G, Deisseroth K. Recent advances in optogenetics and pharmacogenetics. Brain Research. 2013;1511:1–5. doi: 10.1016/j.brainres.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi JCB, Sirigu A, Duhamel JR. Modulation of value representation by social context in the primate orbitofrontal cortex. Proceedings of the National Academy of Sciences. 2012;109:2126–2131. doi: 10.1073/pnas.1111715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. An analysis of social behaviour in wild rats. Proceedings of the Zoolological Society, London. 1958;130:107–152. [Google Scholar]
- Barros M, Boere V, Mello EL, Tomaz C. Reactions to potential predators in captive-born marmosets (Callitrhix penicillata). International Journal of Primatology. 2002;23:443–454. [Google Scholar]
- Bartak L, Rutter M, Cox A. A comparative study of infantile autism and specific developmental receptive language disorder. British Journal of Psychiatry. 1975;126:127–145. doi: 10.1192/bjp.126.2.127. [DOI] [PubMed] [Google Scholar]
- Bartal IB, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton RA, Dunbar RIM. Evolution of the social brain. In: Whiten A, Byrne R, editors. Machiavellian intelligence 2. Cambridge University Press; Cambridge, UK: 1997. [Google Scholar]
- Beery AK, Kaufer D. Stress, social behavior and resilience: Insights from rodents. Neurobiology of Stress. 2015;1:116–127. doi: 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte J, Callaway EM, Caddick SJ, Churchland P, Feng G, Homatics GE, Lee K, Leopold DA, Miller CT, Mitchell JF, et al. Brains, Genes and Primates. Neuron. 2015;86:617–631. doi: 10.1016/j.neuron.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor DA, Wang X. The neuronal representation of pitch in primate auditory cortex. Nature. 2005;436:1161–1165. doi: 10.1038/nature03867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra BM, Souto A. Structure and usage of the vocal repertoire of Callithrix jacchus. International Journal of Primatology. 2008;29:671–701. [Google Scholar]
- Blumstein DT. Yellow-bellied marmosets: Insights from an emergent view of sociality. Philosophical Transactions of the Royal Society, B. 2013;368:20120349. doi: 10.1098/rstb.2012.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrov E, Wolfe J, Rao RP, Brecht M. The representation of social facial touch in rat barrel cortex. Current Biology. 2014;24:109–115. doi: 10.1016/j.cub.2013.11.049. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of Animal Communication. 2 edn Sinauer Associates; Sunderland, MA: 2011. [Google Scholar]
- Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nature Reviews Neuroscience. 2000;1:31–40. doi: 10.1038/35036205. [DOI] [PubMed] [Google Scholar]
- Brecht M. Barrel cortex and whisker-mediated behaviors. Current Opinion in Neurobiology. 2007;17:408–416. doi: 10.1016/j.conb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Broussard GJ, Liang R, Tian L. Monitoring activity in neural circuits with genetically encoded indicators. Front Mol Neurosci. 2014;7:97. doi: 10.3389/fnmol.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner JS. The ontogenesis of speech acts. Journal of Child Language. 1975;2:1–19. [Google Scholar]
- Bugnyar T, Huber L. Push or pull: An experimental study of imitation in marmosets. Animal Behaviour. 1997;54:817–831. doi: 10.1006/anbe.1996.0497. [DOI] [PubMed] [Google Scholar]
- Burkart JM, Fehr E, Efferson C, van Schaik CP. Other-regarding preferences in a nonhuman primate: Common marmosets provision food altruisically. Proceedings of the National Academy of Sciences. 2007;104:19762–19766. doi: 10.1073/pnas.0710310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart JM, Heschl A. Geometrical gaze following in marmosets (Callithrix jacchus). Journal of Comparative Psychology. 2006;120:120–130. doi: 10.1037/0735-7036.120.2.120. [DOI] [PubMed] [Google Scholar]
- Burkart JM, Hrdy SB, van Schaik CP. Cooperative breeding and human cognitive evolution. Evolutionary Anthropology. 2009a;18:175–186. [Google Scholar]
- Burkart JM, Strasser A, Foglia M. Trade-offs between social learning and individual innovativeness in common marmosets, Callithrix jacchus. Animal Behaviour. 2009b;77:1291–1301. [Google Scholar]
- Burkart JM, van Schaik CP. Cognitive consequences of cooperative breeding in primates? Animal Cognition. 2010;13:1–19. doi: 10.1007/s10071-009-0263-7. [DOI] [PubMed] [Google Scholar]
- Buss DM, Schmitt DP. Sexual strategies theory: An evolutionary perspective on human mating. Psychological Review. 1993;100:204–232. doi: 10.1037/0033-295x.100.2.204. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Bates LA. Primate social cognition: uniquely primate, uniquely social or just unique? Neuron. 2010;65:815–830. doi: 10.1016/j.neuron.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Whiten A. Machiavellian Intelligence: Social Expertise and the Evolution of Intllect in Monkeys, Apes and Humans. Oxford University Press; Oxford, UK: 1989. [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS. Vasopressin: Behavioral roles o an ‘original’ neuropeptide. Progress in Neurobiology. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey S, Diamond R. Are faces perceived as configurations more by adults than by children? Visual Cognition. 1994;1:253–274. [Google Scholar]
- Cavanaugh J, Huffman MC, Harnisch AM, French JA. Marmosets treated with oxytocin are more socially attractive to their long-term mate. Frontiers in Behavioral Neuroscience. 2015;9:251. doi: 10.3389/fnbeh.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, French JA. Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology. 2014;49:1–10. doi: 10.1016/j.psyneuen.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Gariepy JF, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nature Neuroscience. 2013;16:243–250. doi: 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (Macaca mulatta). Frontiers in Neuroscience. 2011;5:27. doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin T, Yu H, Soares J, Gattass R, Rosa MGP. A conserved pattern of differential expansion of cortical areaas in simian primates. Journal of Neuroscience. 2013;18:15120–15125. doi: 10.1523/JNEUROSCI.2909-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Drew A, Cox A. Predicting language outcome in infants with autism and pervasiive developmental disorder. International Journal of Language & Communication Disorders. 2003;38:265–285. doi: 10.1080/136820310000104830. [DOI] [PubMed] [Google Scholar]
- Chen HC, Kaplan G, Rogers LJ. Contact calls of common marmosets (Callithrix jacchus): influence of age of caller on antiphonal calling and other vocal responses. American Journal of Primatology. 2009;71:165–170. doi: 10.1002/ajp.20636. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. How Monkeys See the World: Inside the mind of Another Species. Chicago University Press; Chicago: 1990. [Google Scholar]
- Cheney DL, Seyfarth RM. Baboon metaphysics: the evolution of a social mind. University of Chicago Press; Chicago: 2007. [Google Scholar]
- Cheney DL, Seyfarth RM, Silk J. The responses of female baboons (Papio cynocephalus ursinus) to anomalous social interactions: Evidence for causal reasoning? Journal of Comparative Psychology. 1995;109:134–141. doi: 10.1037/0735-7036.109.2.134. [DOI] [PubMed] [Google Scholar]
- Chow C, Mitchell J, Miller CT. Vocal turn-taking in a nonhuman primate is learned during ontogeny. Proceedings of the Royal Society, B. 2015;282:210150069. doi: 10.1098/rspb.2015.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clara E, Tommasi L, Rogers LJ. Social mobbing calls in common marmosets (Callitrhix jacchus): Effects of experience nad associated cortisol levels. Animal Cognition. 2008;11:349–358. doi: 10.1007/s10071-007-0125-0. [DOI] [PubMed] [Google Scholar]
- Connor CE. Transformation of shape information in the ventral pathway. Current Opinion in Neurobiology. 2007;17:140–147. doi: 10.1016/j.conb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Coude G, Ferrari PF, Roda F, Marensi M, Borelli E, Veroni V, Monti F, Rozzi S, Fogassi L. Neurons controlling voluntary vocalization in the macaque ventral premotor cortex. PLoS ONE. 2011;6:e26822. doi: 10.1371/journal.pone.0026822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JH, Ellis JE, Goss-Custard JD. Mammalian Social Systems: Structure and Function. Animal Behaviour. 1976;24:261–274. [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Day RL, Coe RL, Kendal JR, Laland KN. Neophilia, innovation and social learning: A study of intergeneric differences in callitrichid monkeys. Animal Behaviour. 2003;65:559–571. [Google Scholar]
- de Boer RA, Overduin-de Vries AM, Louwerse AL, Sterck EHM. The behavioral context of visual displays in common marmosets. American Journal of Primatology. 2013;75 doi: 10.1002/ajp.22167. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Cortical connections of the auditory cortex in marmoset monkeys: core and medial belt regions. Journal of Comparative Neurology. 2006;496:27–71. doi: 10.1002/cne.20923. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Cortical connections of the auditory cortex in marmoset monkeys: Lateral belt and parabelt regions. The Anatomical Record. 2012;295:800–821. doi: 10.1002/ar.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Current Biology. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nature Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. Stimulus selective properties of inferior temporal neurons in the macaque. The Journal of Neuroscience. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby LJ. Social organization in a wild population of Callithrix jacchus. Part 2: Intragroup social behavior. Primates. 1995;36:361–375. [Google Scholar]
- Digby LJ, Barreto CE. Social organization in a wild population of Callithrix jacchus: Part 1: Group composition and dynamics. Folia primatologica. 1993;61:123–134. doi: 10.1159/000156739. [DOI] [PubMed] [Google Scholar]
- Doupe A, Kuhl P. Birdsong and human speech: Common themes and mechanisms. Annual Review of Neuroscience. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Alexander GM, Farris S. Rediscovering area CA2: Unique properties and functions. Nature Reviews Neuroscience. 2016;17:89–102. doi: 10.1038/nrn.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffney LJ, Zhong P, Wei J, Matas E, Cheng J, Qin L, Ma K, Dietz DM, Kajiwara Y, Buxbaum JD, Yan Z. Autism-like deficits in SHANK3-deficient mice are rescued by targeting Actin regulators. Cell reports. 2015;11:1400–1413. doi: 10.1016/j.celrep.2015.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM. The social brain: Mind, language and society in evolutionary perspective. Annual Review of Anthropology. 2003;32:163–181. [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Brockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gilberg IC, Anckarsater H, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorder. Nature Genetics. 2006;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliades S, Wang X. Neural correlates of the Lombard effect in primate auditory cortex. Journal of Neuroscience. 2012;32:10737–10748. doi: 10.1523/JNEUROSCI.3448-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Chronic multi-electrode neural recording in free-roaming monkeys. Journal of Neuroscience Methods. 2008a;172:201–214. doi: 10.1016/j.jneumeth.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008b;453:1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Comparison of auditory-vocal interactions across multiple types of vocalizations in marmoset cortex. Journal of Neurophysiology. 2013;109:1638–1657. doi: 10.1152/jn.00698.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes RD, Goodstadt L, Winter EE, Ponting CP. Comparison of the genomes of human and mouse lays the foundation of genome zoology. Human Molecular Genetics. 2003;12:701–709. doi: 10.1093/hmg/ddg078. [DOI] [PubMed] [Google Scholar]
- Emiliani V, Cohen AE, Deisseroth K, Hausser M. All-Optical Interrogation of Neural Circuits. J Neurosci. 2015;35:13917–13926. doi: 10.1523/JNEUROSCI.2916-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh AL, Hoffeier RR, Cheney DL, Seyfarth RM. Who, me? Can baboons infer the target of vocalizations? Animal Behaviour. 2006;71:381–387. [Google Scholar]
- Epple G. Comparative studies on vocalizations in marmoset monkeys. Folia primatologica. 1968;8:1–40. doi: 10.1159/000155129. [DOI] [PubMed] [Google Scholar]
- Epple G. The behavior of marmoset monkeys (Callithricidae). Primate Behavior: Developments in field and laboratory research. 1975;4:195–239. [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni T, Mansfield EM, Lai C, Johnson MH. Infants perceiving and acting on the eyes: Tests of an evolutionary hypothesis. Journal of Experimental Child Psychology. 2003;85:199–212. doi: 10.1016/s0022-0965(03)00022-5. [DOI] [PubMed] [Google Scholar]
- Feldman R. Ocytocin and social affiliation in humans. Hormones and Behavior. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. Ocytocin pathway genes: Evolutionary ancest system impacting on human affiliation, sociality and psychopathology. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.008. In Press. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed Hierarchical Processing in the Primate Cerebral Cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winsow JT. Social amenesia in mice lacking the oxytocin gene. Nature Genetics. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferrari SF, Ferrari MAL. Predator avoidance behaviour in the buffy-headed marmoset. Callithrix flaviceps. Primates. 1990;31:323–338. [Google Scholar]
- Fisher C, Freiwald WA. Contrasting specializations for facial motion within the macaque face-processing system. Current Biology. 2015a;25:261–266. doi: 10.1016/j.cub.2014.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C, Freiwald WA. Whole-agent selectivity within the macaque face processing system. Proceedings of the National Academy of Sciences. 2015b;112:14717–14722. doi: 10.1073/pnas.1512378112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WT, Huber L, Bugnyar T. Social Cognition and the Evolution of Language: Constructing Cognitive Phylogenies. Neuron. 2010;65:795–814. doi: 10.1016/j.neuron.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flombaum J, Santos LR. Rhesus monkeys attribute perceptions to others. Current Biology. 2005;15:447–452. doi: 10.1016/j.cub.2004.12.076. [DOI] [PubMed] [Google Scholar]
- Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RPJ, Ko TH, Burns LD, Jung JC, Schnitzer MJ. High-speed, miniaturized fluorescence microscopy in freely-moving mice. Nature Methods. 2008;5:935–938. doi: 10.1038/nmeth.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM. Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson's disease. Canadian Journal of Neurological Sciences. 2010;37:86–95. doi: 10.1017/s0317167100009707. [DOI] [PubMed] [Google Scholar]
- Freiwald WA, Tsao DY. Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science. 2010;330:845–851. doi: 10.1126/science.1194908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA. Proximate regulation of singular breeeding in callitrichid primates. In: Solomon N, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; Cambridge, UK: 1997. pp. 34–75. [Google Scholar]
- Fridlund A. Human Facial Expressions: An Evolutionary Perspective. Academic Press; New York: 1994. [Google Scholar]
- Frith CD, Frith U. Interacting minds - A biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Happe F. Autism spectrum disorder. Current Biology. 2005;15:R786–R790. doi: 10.1016/j.cub.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Fritsches KA, Rosa MGP. Visual organization of striate cortex in the marmoset monkey (Callitrhix jacchus). Journal of Comparative Neurology. 1996;372:264–282. doi: 10.1002/(SICI)1096-9861(19960819)372:2<264::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Galef BGJ, Kennett DJ. Different mechanisms for social transmission of diet preference in rat pups of different ages. Developmental Psychobiology. 1987;20:209–215. doi: 10.1002/dev.420200209. [DOI] [PubMed] [Google Scholar]
- Gariepy JF, Watson KK, Du E, Xie DL, Erb J, Amasino D, Platt ML. Social learning in humans and other animals. Frontiers in Neuroscience. 2014;8:1–13. doi: 10.3389/fnins.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geritis A, Vanduffel W. Optogenetics in primates: A shining future? Trends in Genetics. 2013;29:403–411. doi: 10.1016/j.tig.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Santos LR. Primate brains in the wild: the sensory basis for social interactions. Nature Reviews Neuroscience. 2004;5:603–616. doi: 10.1038/nrn1473. [DOI] [PubMed] [Google Scholar]
- Gifford GW, MacLean KA, Hauser MD, Cohen YE. The neurophysiology of functionally meaningful categories: macaque ventrolateral prefrontal cortex plays a critical role in spontaneous categorization of species-specific vocalizations. Journal of Cognitive Neuroscience. 2005;17:1471–1482. doi: 10.1162/0898929054985464. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annual Review of Neuroscience. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Gomez JC. Species comparative studies and cognitive development. Trends in Cognitive Science. 2005;9:118–125. doi: 10.1016/j.tics.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. Journal of Neurophysiology. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Grant EC. An analysis of the social behaviour of the male laboratory rat. Behaviour. 1963;21:260–281. [Google Scholar]
- Grice SJ, Halit H, Farroni T, Baron-Cohen S, Bolton P, Johnson MH. Neural correlates of eye-gaze detection in young children with autism. Cortex. 2005;41:342–353. doi: 10.1016/s0010-9452(08)70271-5. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Gosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage SR, Nieder A. Singl neurons in monkey prefrontal cortex encode volitional initiation of vocalizations. Nature Communications. 2013;4:2409. doi: 10.1038/ncomms3409. DOI: 10.1038/ncomms3409. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Do chimpanzees know what conspecifics know. Animal Behaviour. 2001;61:139–151. doi: 10.1006/anbe.2000.1518. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Chimpanzees deceive a human competitor by hiding. Cognition. 2006;101:495–514. doi: 10.1016/j.cognition.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Hare B, Melis AP, Woods V, Hastings S, Wrangham RW. Tolerance allows Bonobos to outperform Chimpanzees on a Cooperative task. Current Biology. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Haroush K, Williams ZM. Neuronal prediction of opponent's behavior during cooperative social interchange in primates. Cell. 2015;160:1–13. doi: 10.1016/j.cell.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Ghazanfar AA, Galantucci B, Garrod S, Keysers C. Brain-to-brain coupling: A mechanism for creating and sharing a social world. Trends in Cognitive Science. 2012;16:114–121. doi: 10.1016/j.tics.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht EE, Patterson R, Barbey AK. What can other animals tell us about human social cognition? An evolutionary perspetive on reflective and reflexive processing. Frontiers in Human Neuroscience. 2012;6:1–13. doi: 10.3389/fnhum.2012.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiney SA, Biazquez PM. Behavioral responses of trained squirrel and rhesus monkeys during occulomotor tasks. Experimental Brain Research. 2011;212:409–416. doi: 10.1007/s00221-011-2746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich B. Conflict, Cooperation and Cognition in the Common Raven. Advances in the Study of Behavior. 2011;43:189–238. [Google Scholar]
- Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope: High resolution brain imaging in freely-moving animals. Neuron. 2001;31:903–912. doi: 10.1016/s0896-6273(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Herba C, Phillps M. Development of facial expression recognition from childhood adolescence: Behavioural and neurological perspectives. Journal of Child Psychology and Psychiatry. 2004;45:1185–1198. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippcampal CA2 regio is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H, East ML. The commuting system of Serengeti spotted hyaenas: How a predator copes with migratory prey. I. Social Organization. Animal Behaviour. 1993;46:547–557. [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Current Biology. 2007;17:766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Huber L, Voelkl B. Social and physical cognition in marmsoets and tamarins. In: Ford SM, Porter LM, Davis LC, editors. The Smallest Anthropoids: The Marmoset/Callimico Radiation. Springer Verlag; New York, N.Y.: 2009. pp. 183–201. [Google Scholar]
- Hung C, Yen CC, Cluchta JL, Papoti D, Bock NA, Leopold DA, Silva AC. Functional mapping of Face-Selective Regions in the extrastriate visual cortex of the monkey. Journal of Neuroscience. 2015;35:1160–1172. doi: 10.1523/JNEUROSCI.2659-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Chemical communication in rodents: From pheromones to individual recognition. Journal of Mammology. 2003;84:1141–1162. [Google Scholar]
- Jones W, Klin A. Attention to eyes is present by in decline in 2-6month old infants later diagnosed with autism. Nature. 2013;504:427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. The evolution of brains from early mammals to humans. Wiley Interdisciplinary Reviews in Cognitive Science. 2013;4:33–45. doi: 10.1002/wcs.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser T, Feng G. Modeling psychiatric disorders for developing effective treatments. Nature Medicine. 2015;21:979–988. doi: 10.1038/nm.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Zheng B, Shen B, Chen Y, Wang L, Wang J, Niu Y, Cui Y, Zhou J, Wang H, et al. CRISPR/Cas9-mediated Dax1 knockout in the monkey recapitualtes human AHC-HH. Human Molecular Genetics. 2015;24:7255–7264. doi: 10.1093/hmg/ddv425. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C, Kaplan G. Facial expressions in common marmosets (Callithrix jacchus) and their use by conspecifics. Animal Cognition. 2013;16:773–788. doi: 10.1007/s10071-013-0611-5. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends in Cognitive Science. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SL. You talkin’ to me? Interactive playback is a powerful yet underused tool in animal communication research. Biology Letters. 2015;11:20150403. doi: 10.1098/rsbl.2015.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JT, Deaner RO, Platt ML. Neural correlates of social target value in Macaque Parietal Cortex. Current Biology. 2008;18:1–6. doi: 10.1016/j.cub.2008.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Henrichs M, Zak PJ, Fischbacher U, Fehr E. Ocytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Laland KN, Plotkin HC. Social transmission of food preferences among Norway rats by marking of food sites and by bustatory contact. Animal Learning and Behavior. 1993;21:35–41. [Google Scholar]
- Lazaro-Perea C, Arruda M, Snowdon CT. Grooming as reward? Social function of grooming between females in cooperatively breeding marmosets. Animal Behaviour. 2004;67:627–636. doi: 10.1016/j.anbehav.2003.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro-Perea C, Snowdon CT, de Fatima Arruda M. Scent-marking behavior in wild groups of common marmosets. Behavioral Ecology and Sociobiology. 1999;46:313–324. [Google Scholar]
- le Roux A, Snyder-Mackler N, Roberts EK, Beehner JC, Bergman TJ. Evidence of tactical concealment in a wild primate. Nature Communications. 2012;4:1–6. doi: 10.1038/ncomms2468. [DOI] [PubMed] [Google Scholar]
- Lee D. Neuroethology of Decision Making. In: Platt M, Ghazanfar AA, editors. Primate Neuroethology. Oxford University Press; New York, NY: 2010. pp. 550–569. [Google Scholar]
- Leopold DA, Bondar IV, Giese MA. Norm-based face encoding by single neurons in the monkey inferotemporal cortex. Nature. 2006;442:572–575. doi: 10.1038/nature04951. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Rhodes G. A comparative view of face perception. Journal of Comparative Psychology. 2010;124:233–251. doi: 10.1037/a0019460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WH, Gouy M, Sharp PM, O'hUigin C, Yang YW. Molecular phylogeny of Rodentia, Labomorpha, Primates, Artiodactyla, and Carnivora and molecular clocks. Proceedings of the National Academy of Sciences. 1990;87:6703–6707. doi: 10.1073/pnas.87.17.6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M, Nummela SU, Coop S, Mitchell JF, Miller CT. Optogenetic photostimulation of neural circuits in awake marmosets. 2016 doi: 10.1152/jn.00197.2016. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden JR, Drewe JA, Pearce GP, Clutton-Brock TH. The social network structure of a wild meerkat population: 2. Intragroup interactions. Behavioral Ecology and Sociobiology. 2009;64:81–95. [Google Scholar]
- Madden JR, Drewe JA, Pearce GP, Clutton-Brock TH. The social network structure of a wild meerkat population: 3. Position of individuals within networks. Behavioral Ecology and Sociobiology. 2011;65:1857–1871. [Google Scholar]
- Maior RS, Hori E, Tomaz C, Ono T, Nishijo H. The monkey pulvinar neurons differentially respond to emotional expressions of human faces. Behavioral and Brain Research. 2010;215:129–135. doi: 10.1016/j.bbr.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Mann J, Connor RC, Tyack PL, Whitehead H. Cetacean Societies: Field studies of Dolphins and Whales. University of Chicago Press; Chicago, Il: 2000. [Google Scholar]
- Marciniak K, Atabaki A, Dicke PW, Thier P. Disparate substrates for head gaze following and face perception in the monkey superior temporal sulcus. eLife. 2014;3:e03222. doi: 10.7554/eLife.03222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Gdowki GT. Firing behaviour of squirrel monkey eye movement-related vestibular nucleus neurons during gaze saccades. Journal of Physiology. 2003;546:207–224. doi: 10.1113/jphysiol.2002.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264:746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- McKone E, Crookes K, Jeffery L, Dilks DD. A critical review of the development of face recognition: Experience is less important than previously believed. Cognitive Neuropsychology. 2012;29:174–212. doi: 10.1080/02643294.2012.660138. [DOI] [PubMed] [Google Scholar]
- McMahon DB, Russ BE, Elnaiem HD, Kurnikova AI, Leopold DA. Single-Unit Activity during Natural Vision: Diversity, Consistency and Spatial Sensitivity among AF Face Patch Neurons. Journal of Neuroscience. 2015;35:5537–5548. doi: 10.1523/JNEUROSCI.3825-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindberg A, Domes G, Kirsch P, Heinrichs M. Ocytocin and vasopressin in the human brain: Social neuropeptices for translational medicine. Nature Reviews Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Micheletta J, Whitehouse J, Parr LA, Waller BM. Facial expression recognition in ctested macaques (Macaca nigra). Animal Cognition. 2015;18:985–990. doi: 10.1007/s10071-015-0867-z. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Miller CT, Beck K, Meade B, Wang X. Antiphonal call timing in marmosets is behaviorally significant: Interactive playback experiments. Journal of Comparative Physiology A. 2009a;195:783–789. doi: 10.1007/s00359-009-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Bee MA. Receiver psychology turns 20: Is it time for a broader approach? Animal Behaviour. 2012;83:331–343. doi: 10.1016/j.anbehav.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Dimauro A, Pistorio A, Hendry S, Wang X. Vocalization induced cFos expression in marmoset cortex. Frontiers in Integrative Neuroscience. 2010a;4(128):121–115. doi: 10.3389/fnint.2010.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Eliades SJ, Wang X. Motor-planning for vocal production in common marmosets. Animal Behaviour. 2009b;78:1195–1203. doi: 10.1016/j.anbehav.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Mandel K, Wang X. The communicative content of the common marmoset phee call during antiphonal calling. American Journal of Primatology. 2010b;72:974–980. doi: 10.1002/ajp.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Thomas AW. Individual recognition during bouts of antiphonal calling in common marmosets. Journal of Comparative Physiology A. 2012;198:337–346. doi: 10.1007/s00359-012-0712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Thomas AW, Nummela S, de la Mothe LA. Responses of primate frontal cortex neurons during natural vocal communication. Journal of Neurophysiology. 2015;114:1158–1171. doi: 10.1152/jn.01003.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Wang X. Sensory-motor interactions modulate a primate vocal behavior: antiphonal calling in common marmosets. Journal of Comparative Physiology A. 2006;192:27–38. doi: 10.1007/s00359-005-0043-z. [DOI] [PubMed] [Google Scholar]
- Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The evolution of primate societies. University of Chicago; Chicago, IL: 2012. [Google Scholar]
- Mitchell J, Reynolds J, Miller CT. Active vision in marmosets: A model for visual neuroscience. Journal of Neuroscience. 2014;34:1183–1194. doi: 10.1523/JNEUROSCI.3899-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Leopold DA. The marmoset monkey as a model for visual neuroscience. Neuroscience Research. 2015;93:20–46. doi: 10.1016/j.neures.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, Scherer SW. Contribution of SHANK3 mutations to autistm spectrum disorder. American Journal of Human Genetics. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill R, Thomas AW, Schiel N, Souto A, Miller CT. The effect of habitat acousics on common marmoset vocal signal transmission. American Journal of Primatology. 2013:904–916. doi: 10.1002/ajp.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, Gothard KM. Neurons in monkey amygdala detect eye contact during naturalistic social interactions. Current Biology. 2014;24:2459–2464. doi: 10.1016/j.cub.2014.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy WJ, Eizirik E, Johnson WE, Zhang YP, Ryder OA, O'Brien SJ. Molecular phylogenetics and the origins of placental mammals. Nature. 2001;409:614–618. doi: 10.1038/35054550. [DOI] [PubMed] [Google Scholar]
- Mustoe AC, Cavanaugh J, Harnisch AM, Thompson BE, French JA. Do marmosets care to share? Oxtocin treatment reduces prosocial behavior towards strangers. Horm Behav. 2015;71:83–90. doi: 10.1016/j.yhbeh.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, Li W, et al. Generation of gene modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Context and gender specific differences in the acoustic structure of common marmoset (Callithrix jacchus) phee calls. American Journal of Primatology. 1993;30:37–54. doi: 10.1002/ajp.1350300104. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Social context affects phee call production by nonreproductive common marmosets (Callithrix jacchus). American Journal of Primatology. 1997;43:135–146. doi: 10.1002/(SICI)1098-2345(1997)43:2<135::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely moving rat. Brain Research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Oh SW, Harris L, NG B, Winslow N, Cain S, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H, Hikishima K, Iriki A, Sasaki E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin Fetal Neonatal Med. 2012;17:336–340. doi: 10.1016/j.siny.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Pagani JH, Zhao M, Williams Avram SK, Caruana DA, Dudek SM, Young WS. Role of vasopresssin 1b receptor in rodent aggressive behavior and synaptic platicity in hippcampal area CA2. Molecular Psychiatry. 2015;20:490–499. doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombit R, Seyfarth RM, Cheney DL. The adaptive value of ‘friendships’ to female baboons: experimental and observational evidence. Animal Behaviour. 1997;54:599–614. doi: 10.1006/anbe.1996.0457. [DOI] [PubMed] [Google Scholar]
- Parr LA, Heintz M. Facial expression recognition in rhesus monkeys, Macaca mulatta. Animal Behaviour. 2009;77:1507–1513. doi: 10.1016/j.anbehav.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamani M. Field observation of a group of Geoffroy's marmosets mobbing a margay cat. Folia Primatologica. 1995;65:121–137. doi: 10.1159/000156848. [DOI] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatram TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, Smith PAJ, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proceedings of the Royal Society, London. 1985;223:293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Philippens I, Hart BA, Torres G. The MPTP marmoset model of Parkinsonism: A multi-purpose non-human primate model for neurodegenerative diseases. Drug Discovery Today. 2010;15:985–990. doi: 10.1016/j.drudis.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Pistorio A, Vintch B, Wang X. Acoustic analyses of vocal development in a New World primate, the common marmoset (Callithrix jacchus). Journal of the Acoustical Society of America. 2006;120:1655–1670. doi: 10.1121/1.2225899. [DOI] [PubMed] [Google Scholar]
- Polosecki P, Moeller S, Schweers N, Romanski LM, Tsao DY, Freiwald WA. Faces in motion: Selectivity of macaque and human face processing areas for dynamic stimuli. Journal of Neuroscience. 2013;33:11768–11773. doi: 10.1523/JNEUROSCI.5402-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of the American Association for Laboratory Animal Science. 2007;46:28–34. [PubMed] [Google Scholar]
- Preuschoft S. ‘Laughter’ and ‘smiling’ in macaques - an evolutionary approach. University of Utrecht Press; Utrecht: 1995. [Google Scholar]
- Preuschoft S. Primate faces and facial expressions. Social Research. 2000;67:245–271. [Google Scholar]
- Rendell LE, Whitehead H. Culture in whales and dolphins. Behavioral and Brain Sciences. 2001;24:309–324. doi: 10.1017/s0140525x0100396x. [DOI] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Colins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. Journal of Neuroscience. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Wallis JD. Inhibitory control and affective processing in the prefrontal cortex: Neuropsychological studies in the marmoset. Cerebral Cortex. 2000;10:252–262. doi: 10.1093/cercor/10.3.252. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Gobes SMH, Murugan M, Olveczky BP, Mooney R. Motor circuits are required to encode a sensory model for imitative learning. Nature Neuroscience. 2012;15:1454–1459. doi: 10.1038/nn.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. Journal of Neurophysiology. 2005;93:734–747. doi: 10.1152/jn.00675.2004. [DOI] [PubMed] [Google Scholar]
- Romo R, de Lafuente V. Conversion of sensory signals into perceptual decisions. Progress in Neurobiology. 2013;103:41–75. doi: 10.1016/j.pneurobio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor. Neuron. 2004;41:165–173. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
- Rosati A, Santos L, Hare B. Primate social cognition: Thirty years after Premack and Woodruff. In: Platt M, Ghazanfar AA, editors. Primate Neuroethology. Oxford University Press; Oxford, UK: 2010. pp. 117–142. [Google Scholar]
- Rosati AG, Hare B. Looking past the model species: diversity in gaze-following skills across primates. Current Opinion in Neurobiology. 2009;19:45–51. doi: 10.1016/j.conb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Ross CN, French JA, Orti G. Germ-line chimerism and paternal care in marmosets. Proceedings of the National Academy of Sciences. 2007;104:6278–6282. doi: 10.1073/pnas.0607426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Miller CT, Gottsch D, Wang X. Vocal control by the common marmoset in the presence of interfering noise. Journal of Experimental Biology. 2011;214:3619–3629. doi: 10.1242/jeb.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Wang X. Wireless multi-channel single unit recording in freely moving and vocalizing primates. Journal of Neuroscience Methods. 2012;203:28–40. doi: 10.1016/j.jneumeth.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylands AB, Coimbra-Filho AF, Mittermeier RA. The systematics and distributions of the marmosets (Callithrix, Callibela, Cebuella, and Mico) and Callimico (Callimico)(Callitrichidae, Primates). In: Ford SM, Porter LM, Davis LC, editors. The Smallest Anthropoids: The Marmoset/Callimico Radiation. Springer; New York: 2009. pp. 25–62. [Google Scholar]
- Sadakane O, Masamizu Y, Watakabe A, Terada S, Ohtsuka M, Takaji M, Mizukami H, Ozawa K, Kawasaki H, Matsuzaki M, Yamamori T. Long-term Two-Photon calcium imaging of neuronal populations with subcellular resolution in adult non-human primates. Cell reports. 2015;13:1–11. doi: 10.1016/j.celrep.2015.10.050. [DOI] [PubMed] [Google Scholar]
- Saito A. The marmoset as a model for the study of primate parental behavior. Neuroscience Research. 2015;93:99–109. doi: 10.1016/j.neures.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Britten KH, Newsome WT. Cortical microstimulation influences perceptual judgements of motion direction. Nature. 1990;346:174–177. doi: 10.1038/346174a0. [DOI] [PubMed] [Google Scholar]
- Santos LR, Hauser MD. How monkeys see the eyes: cotton-top tamarins’ reaction to changes in visual attention and action. Animal Cognition. 1999;2:131–139. [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto R, et al. Generation of transgenic non-human priamtes with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Schiel N, Huber L. Social influences on the development of foraging beahvior in free-living common marmosets (Callithrix jacchus). American Journal of Primatology. 2006;68:1–11. doi: 10.1002/ajp.20284. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Knowledge of Social Relations. In: Mitani J, Call J, Kappeler PM, Palombit R, Silk JB, editors. The Evolution of Primate Societies. University of Chicago Press; Chicago, IL: 2012. pp. 629–642. [Google Scholar]
- Seyfarth RM, Cheney DL. Evolution of language from social cognition. Current Opinion in Neurobiology. 2014;28:5–9. doi: 10.1016/j.conb.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Social cognition. Animal Behaviour. 2015;103:191–202. [Google Scholar]
- Seyfarth RM, Cheney DL, Bergman TJ. Primate social cognition and the origins of language. Trends in Cognitive Science. 2005;9:264–266. doi: 10.1016/j.tics.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Shelley EL, Blumstein DT. The evolution of vocal alarm communication in rodents. Behavioral Ecology. 2005;16:169–177. [Google Scholar]
- Shultz S, Opie C, Atkinson QD. Stepwise evolution of stable sociality in primates. Nature. 2011;479:219–222. doi: 10.1038/nature10601. [DOI] [PubMed] [Google Scholar]
- Simoes CS, Vianney PVR, Marcondes de Moura M, Freire MAM, Mello LE, Sameshima K, Araujo JF, Nicolelis MAL, Mello CV, Ribeiro S. Activation of Frontal Neocortical Areas by Vocal Production in Marmosets. Frontiers in Integrative Neuroscience. 2010;4:1–12. doi: 10.3389/fnint.2010.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE. Individual olfactory signatures in common marmosets (Callithrix jacchus). American Journal of Primatology. 2006;68:585–604. doi: 10.1002/ajp.20254. [DOI] [PubMed] [Google Scholar]
- Smith TE, Tomlinson AJ, Mlotkiewcz JZ, Abbott DH. Female marmoset monkeys (Callithrix jacchus) can be identified from chemical composition of their scent marks. Chemical Senses. 2001;26:449–458. doi: 10.1093/chemse/26.5.449. [DOI] [PubMed] [Google Scholar]
- Snow C. The development of conversation between mothers and babies. Journal of Child Language. 1977;4:1–22. [Google Scholar]
- Solomon N, French JA. The study of mammalian cooperative breeding. In: Solomon N, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; Cambridge, UK: 1997. pp. 1–10. [Google Scholar]
- Solomon SG, Rosa MGP. A simpler primate brain: The visual system of the marmoset monkey. Frontiers in Neural Circuits. 2014;8:1–24. doi: 10.3389/fncir.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Osmanski MS, Guo Y, Wang X. Complex pitch perception mechanisms are shared by humans and a New World monkey. Proceedings of the National Academy of Sciences. 2016;113:781–786. doi: 10.1073/pnas.1516120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. Lesions to the CA2 region of the hippcampus impair social memory in mice. European Journal of Neuroscience. 2014;40:3294–3301. doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stivers T, Enfield N, Brown PF, Englert C, Hayashi M, Heinemann T, Hoymann G, Rossano F, de Ruiter J, Yoon K, Levinson S. Universals and cultural variation in turn-taking in coversation. Proceedings of the National Academy of Sciences. 2009;106:10587–10592. doi: 10.1073/pnas.0903616106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugase Y, Shigeru Y, Ueno S, Kawano K. Global and fine information coded by single neurons in teh temporal visual cortex. Nature. 1999;400:869–873. doi: 10.1038/23703. [DOI] [PubMed] [Google Scholar]
- Takahashi D, Narayanan D, Ghazanfar AA. Coupled oscillator dynamics of vocal turn-taking in monkeys. Current Biology. 2013;23:2162–2168. doi: 10.1016/j.cub.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus). Comparative Medicine. 2003;53:364–368. [PubMed] [Google Scholar]
- Teufel C, Gutmann A, Pirow R, Fisher JW. Facial expressions modulate the ontogenetic trajectory of gaze-following among monkeys. Developmental Science. 2010;13:913–922. doi: 10.1111/j.1467-7687.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- Toarmino CR, Jovanovic V, Miller CT. Decisions to communicate in the primate ecological and social landscapes. In: Bee MA, Miller CT, editors. Perception and Cognition in Animal Communication. Springer Verlag; In Press. [Google Scholar]
- Tomasello M. Joint attention as social cognition. In: Moore C, Dunham P, editors. Joint Attention: Its Origins and Role in Development. Psychology Press; New York: 1995. pp. 103–130. [Google Scholar]
- Tomasello M, Call J. Primate Cognition. Oxford University Press; Oxford: 1997. [Google Scholar]
- Tomasello M, Carpenter M, Hobson RP. The emergence of social cognition in three young chimpanzees. Monographs of the Society for Research in Child Development. 2005;70:1–152. doi: 10.1111/j.1540-5834.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Fogleman T. The ontogeny of gaze following in chimpanzees, Pan troglodytes, and rhesus macaques, Macaca mulatta. Animal Behaviour. 2001;61:335–343. [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Livingstone MS. Neural mechanisms for face perception. Annual Review of Neuroscience. 2008;31:411–438. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald W. Comparing face patch systems in macaques and humans. Proceedings of the National Academy of Sciences. 2008;49:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunada J, Liu ASK, Gold JI, Cohen YE. Causal contribution of primate auditory cortex to auditory perceptual decision-making. Nature Neuroscience. 2016;19:135–142. doi: 10.1038/nn.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation fo neural circuits underlying brain disease in animal models. Nature Reviews Neuroscience. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DG, Goodale MA, Mansfield RJQ, editors. Analysis of visual behavior. MIT Press; Cambridge: 1982. pp. 549–586. [Google Scholar]
- van de Waal E, Borgeaud C, Whiten A. Potent social learning and conformity shape a wild primate's foraging decisions. Science. 2013;340:483–485. doi: 10.1126/science.1232769. [DOI] [PubMed] [Google Scholar]
- Viana DS, Gordo I, Sucena E, Moita MAP. Cognitive and motivational requiremens for the emegence of cooperation in a rat social game. PLOS One. 2010;5:e8483. doi: 10.1371/journal.pone.0008483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick SJ, Waller BM, Parr LA, Smith-Pasqualini MC, Bard KA. A cross-species comparison of facial morphology and movement in humans and chimpanzees using the facial action coding system (FACS). Journal of Nonverbal Behavior. 2007;31:1–20. doi: 10.1007/s10919-006-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkl B, Huber L. True imitation in marmosets. Animal Behaviour. 2000;60:195–202. doi: 10.1006/anbe.2000.1457. [DOI] [PubMed] [Google Scholar]
- Voelkl B, Huber L. Imitation as faithful copying of a novel technique in marmoset monkeys. PLoS ONE. 2007;2(7):e611. doi: 10.1371/journal.pone.0000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Feng C, Teng F, Yang S, Hu B, Niu Y, Xiang AP, Fang W, Ji W, Li W, et al. One-step generation of p53 gene biallelic mutant Cynomolgus monkey via the CRISPR/Cas system. Cell Research. 2015;25:258–261. doi: 10.1038/cr.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature. 2005;435:341–346. doi: 10.1038/nature03565. [DOI] [PubMed] [Google Scholar]
- Wang X, Walker KMM. Neural mechanisms for the abstraction of pitch information in auditory cortex. Journal of Neuroscience. 2012;32:13339–13342. doi: 10.1523/JNEUROSCI.3814-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Takaji M, Isa K, Mizukami H, Ozawa K, Isa T, Yamamori T. Comparative analyses of adeno-associated viral vector serotypes 1,2,5,8 and 9 in marmoset, mouse and macaque cerebral cortex. Neuroscience Research. 2015;93:144–157. doi: 10.1016/j.neures.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Grill-Spector K. The evolution of face processing networks. Trends in Cognitive Science. 2015;19:240–241. doi: 10.1016/j.tics.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O'Carroll AM, Lolait SJ, Young WS. Vasopressin V1b receptor konockout reduces aggressive behavior in male mice. Molecular Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wesson DW. Sniffing communicates social hierarchy. Current Biology. 2013;23:575–580. doi: 10.1016/j.cub.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Wittemeyer G, Getz WM. Hierarchical dominance structure and social organization in African elephants, Loxodonta africana. Animal Behaviour. 2007;73:671–681. [Google Scholar]
- Wobber V, Wrangham RW, Hare B. Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Current Biology. 2010;20:226–230. doi: 10.1016/j.cub.2009.11.070. [DOI] [PubMed] [Google Scholar]
- Wolff JO, Sherman PW. Rodent Societies: An Ecological and Evolutionary Perspective. University of Chicago Press; Chicago, IL: 2007. [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach and avoidance. Biological Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]