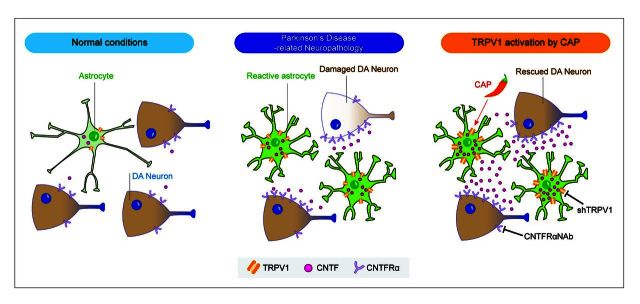
Ciliary neurotrophic factor (CNTF) has a neuroprotective effect on dopaminergic neurons. Nam et al. report that the capsaicin receptor TRPV1 expressed on astrocytes mediates the production of endogenous CNTF to inhibit degeneration of dopaminergic neurons in two rodent models of Parkinson's disease.
Keywords: Parkinson’s disease, TRPV1, astrocyte, ciliary neurotrophic factor (CNTF), dopamine neurons
Ciliary neurotrophic factor (CNTF) has a neuroprotective effect on dopaminergic neurons. Nam et al. report that the capsaicin receptor TRPV1 expressed on astrocytes mediates the production of endogenous CNTF to inhibit degeneration of dopaminergic neurons in two rodent models of Parkinson's disease.
Abstract
Currently there is no neuroprotective or neurorestorative therapy for Parkinson’s disease. Here we report that transient receptor potential vanilloid 1 (TRPV1) on astrocytes mediates endogenous production of ciliary neurotrophic factor (CNTF), which prevents the active degeneration of dopamine neurons and leads to behavioural recovery through CNTF receptor alpha (CNTFRα) on nigral dopamine neurons in both the MPP+-lesioned or adeno-associated virus α-synuclein rat models of Parkinson’s disease. Western blot and immunohistochemical analysis of human post-mortem substantia nigra from Parkinson’s disease suggests that this endogenous neuroprotective system (TRPV1 and CNTF on astrocytes, and CNTFRα on dopamine neurons) might have relevance to human Parkinson’s disease. Our results suggest that activation of astrocytic TRPV1 activates endogenous neuroprotective machinery in vivo and that it is a novel therapeutic target for the treatment of Parkinson’s disease.
Introduction
Parkinson’s disease is a common neurodegenerative disorder characterized by the loss of dopamine neurons in the substantia nigra pars compacta (SNpc) and the appearance of fibrillary aggregates of insoluble α-synuclein (encoded by SNCA) called Lewy bodies (Dauer and Przedborski, 2003; Braak et al., 2004). Various Parkinson’s disease animal models generated by administration of toxins including 1-methyl-4-phenylpyridinium (MPP+) (Park et al., 2012), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Chung et al., 2010), or 6-hydroxydopamine (6-OHDA) (Drinkut et al., 2012) or α-synuclein over-expression through viral transduction in rats and genetic methods in mice (Kirik et al., 2002; Chesselet, 2008; Decressac et al., 2012) have been used to identify therapeutic targets and test disease-modifying therapies. Although little is known about the cause of Parkinson’s disease, major concerns include the loss of dopamine neurons and the subsequent depletion of striatal dopamine, which causes motor abnormalities such as resting tremor, bradykinesia and rigidity (Dauer and Przedborski, 2003; Savitt et al., 2006). A role for non-neuronal cells such as astrocytes in producing neuropathological or neuroprotective functions in Parkinson’s disease is becoming increasingly recognized (Block et al., 2007; Rappold and Tieu, 2010).
Astrocytes are the most abundant glial cells in the mammalian brain and they can play both beneficial and detrimental roles in Parkinson’s disease (Vila et al., 2001; Rappold and Tieu, 2010; Episcopo et al., 2013). Astrocytes confer neuroprotection by producing neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF) (Nakagawa and Schwartz, 2004; Aron and Klein, 2011), mesencephalic astrocyte-derived neurotrophic factor (MANF) (Shen et al., 2012) and ciliary neurotrophic factor (CNTF) (Fischer et al., 2008).
Transient receptor potential vanilloid 1 (TRPV1), the capsaicin receptor, is involved in pain perception and is highly expressed in sensory neurons (Gunthorpe and Szallasi, 2008). TRPV1 is also present in the brain where it may play a role in modulating neuronal function (Starowicz et al., 2008; Kauer and Gibson, 2009), controlling motor behaviour (Lee et al., 2006; Morgese et al., 2007; Gonzalez-Aparicio and Moratalla, 2014), and regulating neuroinflammation (Park et al., 2012). The activation of TRPV1 can be achieved by systemic administration of blood–brain barrier-permeable capsaicin (Guler et al., 2012). Here we show that capsaicin activation of TRPV1 on astrocytes produces endogenous CNTF in vivo, which prevents degeneration of dopamine neurons by acting through CNTF receptor alpha (CNTFRα) on dopamine neurons in animal models of Parkinson’s disease. This endogenous neuroprotective system (TRPV1 and CNTF on astrocytes, and CNTFRα on dopamine neurons) could be harnessed as a novel beneficial therapeutic target for the treatment of Parkinson’s disease.
Materials and methods
Animals
All experiments were done in accordance with the approved animal protocols and guidelines established by Kyung Hee University. Female Sprague–Dawley rats (10 weeks of age, 240–270 g) were housed under a 12:12 h light:dark cycle at an ambient temperature of 22°C. Water and rat chow were available ad libitum.
Stereotaxic surgery
Stereotaxic surgery under chloral hydrate was performed as described (Paxinos, 1998; Park et al., 2012). Using coordinates relative to the bregma, stereotaxic injections of MPP+ (right medial forebrain bundle; A/P −3.6, M/L −2.0, D/V −7.5; MPP+, 7.4 µg in 2 µl phosphate-buffered saline, Sigma) (Park et al., 2012), or α-synuclein [kindly gifted from Michael J. Fox Foundation]; right substantia nigra (SN); A/P −5.3, M/L −2.3, D/V −7.6; AAV2-eGFP, AAV2-α-synuclein; 0.2 μl/ min, total 2 μl; adeno-associated virus (AAV) virus used here is driven by the chicken β-actin promoter (Kirik et al., 2002), lentivirus (right SN; shTRPV1, shCtrl; 0.2 μl/ min, total 3 μl), CNTFRα neutralizing antibody (right SN; R&D, AF-303-NA; 0.01 μg/μl, 0.2 μl/ min, total 2 μl) and respective control were done according to the atlas of Paxinos and Watson (Paxinos, 1998).
Capsaicin injection
Capsaicin (1 mg/kg, intraperitoneally; a single injection/day for 7 days, Sigma) (Veldhuis et al., 2003; Park et al., 2012) was injected at 1 week and 1 day post MPP+, and at 7 weeks and 1 day post α-synuclein. Lentivirus was injected immediately after MPP+ injection.
Rotational behaviour test
D-Amphetamine (5 mg/kg, intraperitoneally) was used to monitor ipsilateral rotations in rats with unilaterally lesioned nigrostriatal dopamine neurons (Jin and Iacovitti, 1995). The ipsilateral rotations were counted for 1 h at 1, 2 or 6 weeks post MPP+, and 7 or 8 weeks post α-synuclein.
Stereological estimation
As previously described (West et al., 1991; Choi et al., 2003; Bartus et al., 2011; Park et al., 2012), the total number of TH+ neurons was counted in the various animal groups using the optical fractionator method performed on a brightfield microscope (Olympus Optical, BX51) using Stereo Investigator software (MBF Bioscience). This unbiased stereological method of cell counting is not affected by either the reference volume (SNpc) or the size of the counted elements (neurons).
Morphological analysis
Optical densities of the TH+ striatal fibres were measured using Science Lab 2001 Image Gauge (Fujifilm) (Nam et al., 2015).
ImageJ analysis
Imaging data were analysed in ImageJ (National Institutes of Health) as described recently (Lee et al., 2010). ImageJ with co-localization plugin was used to quantify immunofluorescence and with colour deconvolution plugin was used to quantify chromogenic signal intensity on image.
Western blot
The SN area was rapidly removed and western blot analysis was performed as previously described (Choi et al., 2003; Shi et al., 2012). The following primary antibodies and dilutions were used: rabbit TRPV1 (1:1000, Alomone labs), mouse anti-CNTF (1:1000, Millipore), mouse anti-GFAP (1:500, Sigma), mouse anti-tyrosine hydroxylase (TH, 1:1000, Millipore), rabbit anti-phosphorylation Ser31 tyrosine hydroxylase (1:1000, Millipore), and mouse anti-beta-actin (1:5000, Abcam). The following secondary antibodies and dilutions were used; horseradish peroxidase-conjugated anti-rabbit or mouse IgG (1:5000, Bethyl).
Western blot analysis of human brain tissues
Human brain tissues (Supplementary Tables 1 and 2) from SN and cortex were homogenized in lysis buffer [10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40, 10 mM Na-β-glycerophosphate, 1% sodium dodecyl sulphate, 0.5% sodium deoxycholate, Phosphatase Inhibitor Mixture I and II (Sigma), and Complete Protease Inhibitor Mixture (Roche)], using a Diax 900 homogenizer (Sigma). After homogenization, samples were rotated at 4°C for 30 min for complete lysis, the homogenate was centrifuged at 52 000 rpm for 20 min, and the resulting fractions were collected. Protein levels were quantified using the BCA Kit (Pierce) with bovine serum albumin (BSA) standards, separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and subjected to immunoblot analysis with indicated antibodies. Immunoblot signals were visualized with chemiluminescence.
Immunostaining
Human brain tissues [age matched control and Parkinson’s disease subjects obtained from the Victoria Brain Bank Network (VBBN), Supplementary Table 3] were deparaffinized in xylene and subjected to citrate antigen retrieval prior to immunohistochemistry (Martins et al., 1999). Tissues sections were washed in cold phosphate-buffered saline (PBS) for 15 min and block with universal blocking solution (0.3% Triton™ X-100, 1% BSA, 0.05% Tween 20, 0.1% cold fish gelatin and 0.05% sodium azide in PBS) for 1 h at room temperature. Primary antibodies of mouse anti-GFAP (1:500, Sigma), rabbit anti-TRPV1 (1:1000, Alomone labs), rabbit anti-CNTF (1:200, Santa-Cruz) and goat anti-CNTFRα (1:200, Santa-Cruz) were diluted in 1–5% BSA or normal goat serum and incubated according to manufacturer recommendations. For fluorescent microscopy, FITC-conjugated-anti-mouse or goat (1:400, Millipore), Cy3-conjugated-anti-rabbit (1:400, Millipore) and Texas Red-conjugated-anti-rabbit (1:400, Vector Laboratories) secondary antibodies with 4’,6-diamidino-2-phenylindole (DAPI) nuclear counterstain (Vector Laboratories). After washing with PBS, coverslips were mounted on glass slides using mounting media (Vector Laboratories), and analysed using a confocal microscope (LSM 700, Carl Zeiss). For light microscopy, brain tissues were labelled using alkaline phosphatase-conjugated secondary antibodies (Vector Laboratories). After incubation, immunostaining visualized with the peroxidase with Alkaline Phosphatase Substrate Kit III (blue) (Vector Laboratories). To examine the expression of the CNTFRα, brain tissues were used appropriate biotin secondary antibody, followed by avidin-biotin complex (Vector Laboratories) and visualized with 3,3’-diaminobenzidine (DAB) peroxidase substrate solution-Blue (0.05% DAB, 0.05% cobalt chloride, 0.05% nickel ammonium sulphate and 0.015% H2O2 in PBS, pH 7.2).
For rat brain, as described (Park et al., 2012), animals were transcardially perfused, fixed and brain tissues (40-μm thick) were processed for immunohistochemical staining. In brief, sections were rinsed in PBS then incubated with the following primary antibodies: mouse anti-α-synuclein (1:1000, Abcam), rabbit anti-TRPV1 (1:1000, Alomone labs), rabbit anti-CNTF (1:200, Santa-Cruz), mouse and rabbit anti-GFAP (1:500, mouse, Sigma; 1:5000, rabbit, Neuromics) for astrocytes, rabbit or mouse anti-tyrosine hydroxylase (TH, 1:2000, rabbit, Pel-Freez; 1:2000; mouse, Millipore) for dopamine neurons and mouse anti-OX42 (1:400, Serotec) and anti-Iba-1 (1:1000, Wako) for microglia. The next day, tissues were rinsed and incubated with FITC-conjugated-anti-rabbit, mouse or goat IgG (1:400, Millipore), Cy3-conjugated-anti-rabbit IgG (1:400, Millipore), Texas Red-conjugated-anti-mouse IgG (1:400, Vector Laboratories), CF405M-conjugated- anti- rabbit or mouse IgG (1:400, Biotium) and 405-conjugated-anti-goat IgG (1:200, Jackson Immunoresearch) 1 h. Stained tissues were viewed using a confocal microscopy (LSM700, Carl Zeiss) or were analysed under a bright-field micro-scope (Olympus).
TRPV1 shRNA and lentivirus production
For plasmid‐based short hairpin (sh)RNA expression, the following complementary oligonucleotides were annealed and inserted into the HindIII/BglII sites of pSUPER‐GFP vector: gcgcatcttctacttcaac (sense) TTAGCACTG (loop) gttgaagtagaagatgcgc (antisense), corresponding to nucleotide sequence of TRPV1 (Christoph et al., 2008). For lentivirus‐based shRNA expression, a lentiviral vector containing TRPV1 gene was constructed by inserting synthetic double‐strand oligonucleotides 5’‐CGCTGCAGTTGCCAACTTGTCAATGAATTCAAGAGATTCATTG ACAAGTTGGCAATTTTTGATATCTAGACA‐3’ into the HpaI–XhoI restriction enzyme sites of the pSicoR-mcherry lentiviral vector. A shLenti construct containing scrambled oligonucleotides: 5’‐CGCATAGCGTATGCCGTTTTCAAGAGAAACGGCATACGCTATGCGATTTTTTC‐3’ was used as a control.
Statistical analysis
All values are expressed as mean ± standard error of the mean. Statistical significance (P < 0.05 for all analysis) was assessed by ANOVA using the Instat 3.05 software package (GraphPad Software, San Diego, CA, USA), followed by Student–Newman–Keuls analyses.
Results
Astrocytic TRPV1-derived CNTF protects degeneration of dopamine neurons from MPP+ neurotoxicity in vivo
To explore the potential function of TRPV1 in Parkinson’s disease, the rat unilateral MPP+-lesion model of Parkinson’s disease was used (Park et al., 2012). The loss of nigral dopamine neurons in the MPP+ model used here is 40% at 1 week post MPP+ and 63% at 2 week post MPP+ (Supplementary Fig. 1).
Amphetamine (5 mg/kg, intraperitoneally)-induced ipsilateral rotation (Jin and Iacovitti, 1995) was analysed at 1 week after MPP+ administration. Rats that exhibited ipsilateral rotations, indicative of an effective lesion were randomly selected for treatment with the TRPV1 agonist, capsaicin (1 mg/kg, intraperitoneally) (Veldhuis et al., 2003; Park et al., 2012) or vehicle each day for 7 days (Fig. 1A). Similar to our recent report (Park et al., 2012), capsaicin prevents the ongoing degeneration in this model.
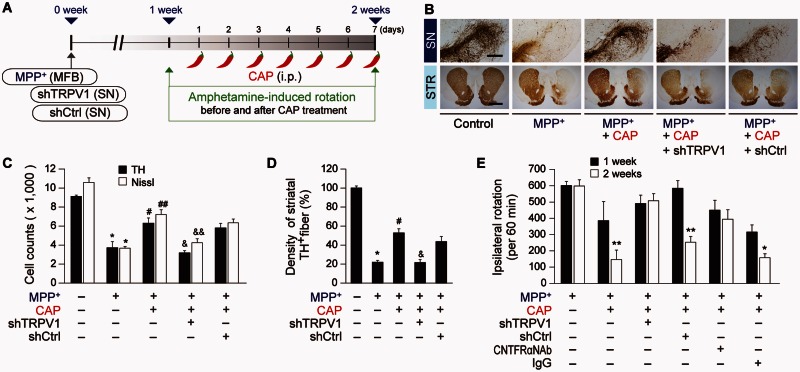
Figure 1.
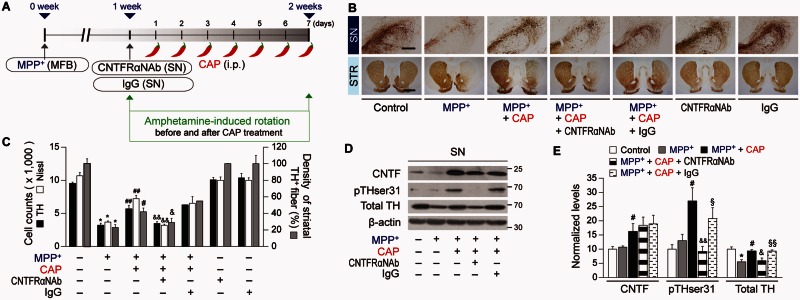
Capsaicin protects against degeneration of dopamine neurons from MPP+ neurotoxicity in vivo. (A) Diagram of the experimental design. Rats were given a unilateral medial forebrain bundle (MFB) injection of MPP+ followed by injection of shTRPV1 or shCtrl (control) into the substantia nigra (SN). All rats intraperitoneally (i.p.) received capsaicin (CAP; 1 mg/kg) or vehicle at 8 days post MPP+ and a continuous single injection per day for 7 days. Rats were transcardially perfused after the last rotation experiment. (B) Photomicrographs of TH+ cells in the SN and TH+ fibres in the striatum (STR). Scale bars: 400 µm (SN), 2 mm (STR). (C) Number of TH+ or Nissl+ cells in the SN pars compacta (SNpc). *P < 0.001, significantly different from control. #P < 0.01, ##P < 0.001, significantly different from MPP+. &P < 0.01, &&P < 0.001 significantly different from MPP+ + capsaicin. (D) Optical density of TH+ fibres in the striatum, *P < 0.001, significantly different from control. #P < 0.01, significantly from different from MPP+. &P < 0.01, significantly different from MPP+ + capsaicin. (E) Cumulative amphetamine-induced ipsilateral rotations, *P < 0.05, **P < 0.01, significantly different from 1 week in each group. shTRPV1 = TRPV1 shRNA lentivirus; shCtrl = scrambled shRNA lentivirus. Mean ± SEM; A, D, n = 5 to 13 in each group. E, n = 8 to 12 in each group. ANOVA and Student-Newman-Keuls analysis.
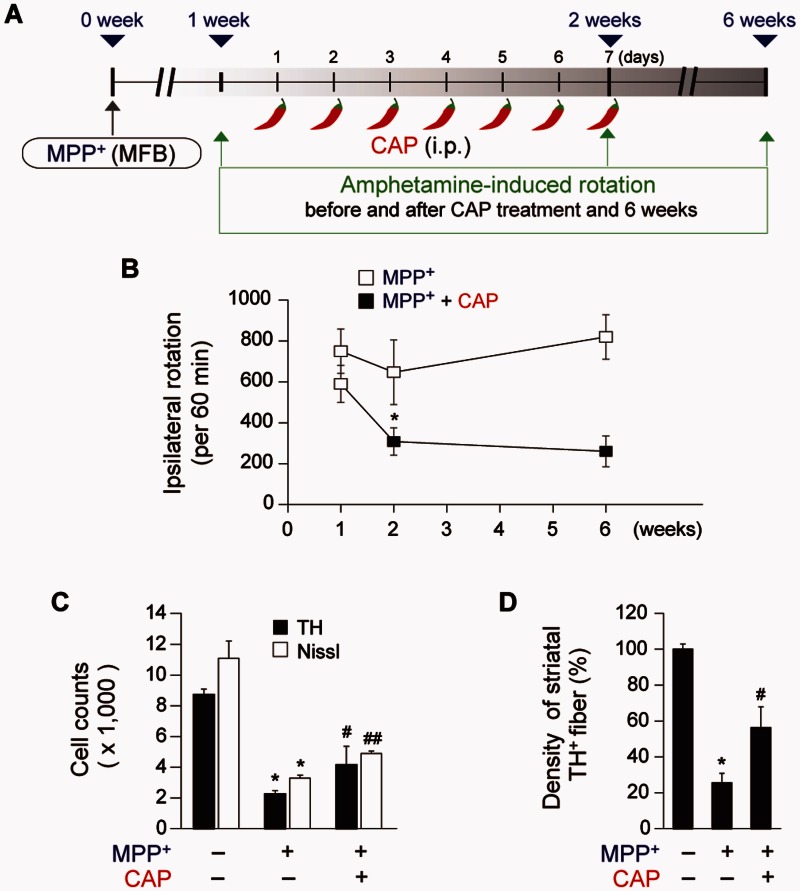
The number of TH+ and Nissl+ cells as assessed by stereology in the SNpc and the density of TH+ fibres in the striatum are significantly higher in the MPP+-lesioned rats treated with capsaicin, compared with the vehicle-treated rats (Fig. 1B–D). Capsaicin also significantly attenuates amphetamine-induced rotations indicative of behavioural rescue (Fig. 1E). These behavioural and neuroprotective effects were prolonged up to 4 weeks after the last capsaicin treatment (Fig. 2).
Figure 2.
Prolonged neuroprotective effects of capsaicin. (A) Diagram of the experimental design. Rats were given a unilateral medial forebrain bundle (MFB) injection of MPP+. All rats intraperitoneally (i.p.) received capsaicin (CAP; 1 mg/kg) or vehicle at 8 days post MPP+ and a continuous single injection per day for 7 days. Amphetamine-induced rotation was analysed at 1, 2, or 6 weeks post MPP+ and rats were transcardially perfused after the last rotation experiment. (B) Cumulative amphetamine-induced ipsilateral rotations, *P < 0.05, significantly different from 2 weeks MPP+. (C) Number of TH+ or Nissl+ stained cells in the SNpc, *P < 0.001, significantly different from control, #P < 0.05, ##P < 0.01, significantly different from MPP+. (D) Optical density of TH+ fibres in the striatum (STR), *P < 0.01, significantly from different control, #P < 0.05, significantly different from MPP+. Mean ± SEM; B–D, n = 3–7 in each group. ANOVA and Student-Newman-Keuls analysis.
To confirm that capsaicin is mediating protection via activation of TRPV1, we selectively inhibited TRPV1 function with a lentivirus carrying a small hairpin-forming interference RNA (shRNA) targeted against TRPV1 (shTRPV1) (Christoph et al., 2008; Lee et al., 2010). This virus also contained DNA encoding a fluorescent marker, mCherry, which permitted visualization of the location and amount of viral infection. When shTRPV1 or control scrambled shRNA (shCtrl) was injected into the rat SN immediately after the unilateral medial forebrain bundle injection of MPP+ (Fig. 1A), shTRPV1 efficiently reduced TRPV1 protein expression (Supplementary Fig. 2A and B) mainly within astrocytes in the SNpc of MPP+-lesioned rats at 1 week post MPP+ (Supplementary Fig. 2C and F). Compared to GFAP, there was relatively minimal mCherry expression in TH+ neurons or OX-42+ microglia (Supplementary Fig. 2D–F). Knockdown of TRPV1 exacerbates the MPP+-induced loss of TH+ neurons as assessed by stereological counting of TH+ and Nissl+ cells (Supplementary Fig. 2G and H), suggesting that TRPV1 might function as endogenous neuroprotective machinery in vivo. In the capsaicin-treated MPP+-lesioned rats, knockdown of TRPV1 expression by shTRPV1 significantly inhibited capsaicin neuroprotection by reducing the number of TH+ and Nissl+ cells in the SNpc (Fig. 1B and C) and density of TH+ fibres in the striatum (Fig. 1B–D) compared to the shCtrl-injected rats at 2 weeks post MPP+. Accompanying the lack of neuroprotection is an attenuation of the effects of capsaicin on amphetamine-induced ipsilateral rotations compared to the shCtrl injection (Fig. 1E). Thus, capsaicin, via activation of TRPV1 on astrocytes, produces a functional recovery and protects dopamine neurons in vivo from MPP+ toxicity.
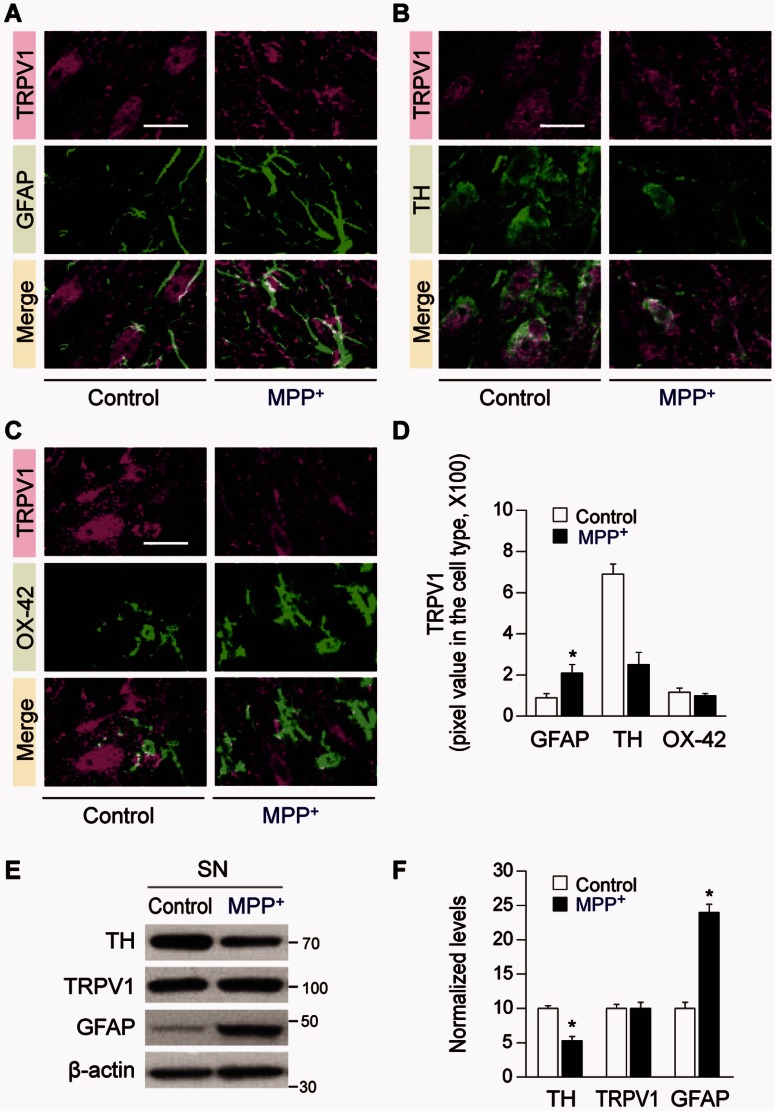
Expression of TRPV1 was analysed in GFAP+ astrocytes, TH+ neurons and OX-42+ microglia at 1 week post MPP+. Expression of GFAP and TRPV1 in GFAP+ astrocytes was significantly higher in the rat SNpc at 1 week post MPP+ compared to the control, whereas TRPV1 in TH+ neurons was significantly reduced and TRPV1 in OX-42+ microglia was relatively unchanged (Fig. 3A–D). Western blot analysis shows a higher expression of GFAP with total TRPV1 levels unchanged in the SN, whereas TH levels are significantly reduced by 48% at 1 week post MPP+ compared to the control (Fig. 3E and F). Taken together these results indicate that following MPP+-induced degeneration of dopamine neurons there is a reduction of TRPV1 in TH+ neurons and a significant upregulation in GFAP+ astrocytes, which is consistent with capsaicin acting primarily on astrocytic TRPV1 receptors (Fig. 1).
Figure 3.
TRPV1 expression in the substantia nigra of MPP+-lesioned rat. The rats were given a unilateral medial forebrain bundle (MFB) injection of MPP+ and brain tissues were processed for immunohistochemical and western blot analysis at 1 week post MPP+. (A–D) Fluorescence images of TRPV1 (magenta; A) and GFAP (green; A), or TRPV1 (magenta; B) and TH (green; B) or TRPV1 (magenta; C) and OX-42 (green; C) and both images are merged (yellow; A–C) in the SNpc of MPP+-lesioned rat brain. (D) Quantification of TRPV1 expression in each cell type, *P < 0.01, **P < 0.001, significantly different from control. (E and F) Western blot analysis of TH, TRPV1 and GFAP (E), and quantification (F) in the SN of MPP+-lesioned rat brain, *P < 0.001, significantly different from control. Scale bars = 20 µm. Mean ± SEM; D, n = 6–7; F, n = 4–6. ANOVA and Student-Newman-Keuls analysis.
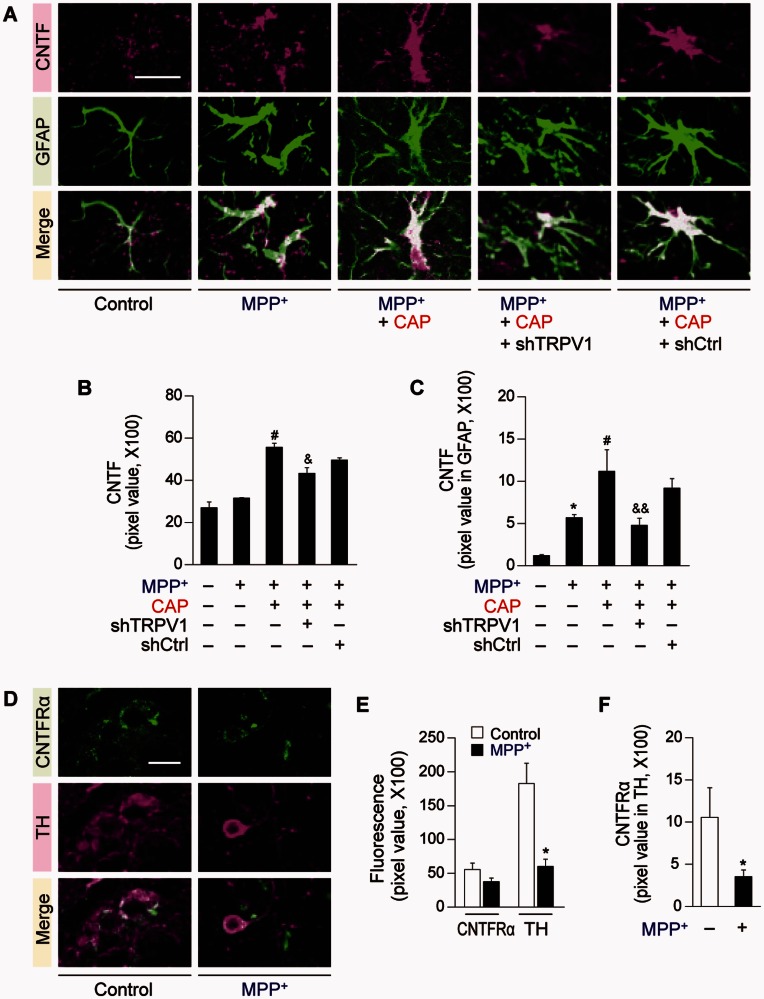
As capsaicin seems to be primarily exerting its protective function via astrocytic TRPV1 receptors, we wondered if it could exert neuroprotection via CNTF, which is expressed in astrocytes upon brain injury (Stockli et al., 1989; Lee et al., 1998; Park et al., 2000; Leibinger et al., 2009; Kang et al., 2012). Immunohistochemical analysis reveals a significant increase in expression of CNTF and particular, in GFAP+ astrocytes in the SNpc of capsaicin-treated MPP+-lesioned rat brain (Fig. 4A–C) compared to vehicle treated control at 2 weeks post MPP+ (Fig. 4A–C). shTRPV1 attenuated capsaicin-induced expression of CNTF and CNTF in GFAP+ astrocytes (Fig. 4A–C) in the SNpc of MPP+-lesioned rats compared to shCtrl (Fig. 4A–C). Immunohistochemical analysis indicates that the CNTFRα is expressed in TH+ cells in the SNpc of intact or MPP+-lesioned rats (Fig. 4D–F). Accordingly, we tested the effect of CNTFRα on the survival of dopamine neurons. CNTFRα neutralizing antibody was unilaterally injected to block CNTF actions in the ipsilateral rat SNpc at 1 week after the unilateral medial forebrain bundle injection of MPP+ (Fig. 5A). The capsaicin-induced increase in number of TH+ and Nissl+ cells in the SNpc and density of TH+ fibres in the striatum was significantly reduced in MPP+-lesioned rats treated with CNTFRα neutralizing antibody compared with the non-specific IgG-treated rats as a control (Fig. 5B and C). Accompanying the lack of neuroprotection is an attenuation of the effects of capsaicin on amphetamine-induced ipsilateral rotations compared with the IgG injection (Fig. 1E).
Figure 4.
CNTF and CNTFRα expression in the substantia nigra of MPP+-lesioned rat. Brain sections adjacent to those used in Fig. 1 were immunostained. (A) Fluorescence images of CNTF (magenta) and GFAP (green) and both images are merged in the SNpc of MPP+-lesioned rat brain. Florescence images of CNTF (from green to magenta) and GFAP (from blue to green) were pseudo-coloured in MPP+ +capsaicin + shTRPV1- or MPP+ + capsaicin + shCtrl-treated tissues. (B and C) Quantification of CNTF (B) or CNTF expression in GFAP+ astrocytes (C) of rat SNpc at 2 weeks post MPP+, *P < 0.001, significantly different from control, #P < 0.01, significantly different from MPP +, &P < 0.01, &&P < 0.001, significantly different from MPP+ + capsaicin. (D) Fluorescence images of CNTFRα (green) and TH+ dopamine neurons (magenta), and both images are merged in the rat SNpc at 1 week after a unilateral medial forebrain bundle (MFB) injection of MPP+. (E and F) Quantification of CNTFRα or TH expression (E) and CNTFRα expression in TH+ dopamine neurons (F), *P < 0.05, significantly different from control. Scale bars = 20 µm. Mean ± SEM; B and C, n = 4–8. E and F, n = 5. ANOVA and Student-Newman-Keuls analysis.
Figure 5.
Astrocytic TRPV1 induced CNTF exerts neuroprotection on dopamine neurons in vivo. (A) Diagram of the experimental design showing various treatments as indicated. After the last rotation experiment, brain tissues were prepared for immunohistochemical staining or western blot analysis. (B) Photomicrographs of TH+ cells in the SN and TH+ fibres in the striatum (STR). (C) Number of TH+ or Nissl+ cells in the SNpc, and optical density of TH+ fibres in the striatum, *P < 0.001, significantly different from control, #P < 0.05, ##P < 0.001, significantly different from MPP+, &P < 0.05, &&P < 0.01, significantly different from MPP+ + capsaicin. (D and E) Western blot analysis (D), and quantification (E) in the rat substantia nigra (SN) at 2 weeks post MPP+, *P < 0.01, significantly different from control, #P < 0.01, significantly different from MPP+, &P < 0.01, &&P < 0.001, significantly different from MPP+ + capsaicin, §P < 0.05, §§P < 0.01, significantly different from MPP+ + capsaicin + CNTFRα neutralizing antibody (CNTFRαNAb). Scale bars = 400 µm (SN); 2 mm (STR). Mean ± SEM; C, n = 3–13 in each group. D and E, n = 5–7 in each group. ANOVA and Student-Newman-Keuls analysis.
CNTF produced by astrocytes can stimulate TH enzyme activity via phosphorylation of TH at Ser31 (Shi et al., 2012) in vivo, eventually contributing to functional recovery. To test this possibility, western blot analysis was performed to measure changes in CNTF levels and examine the state of TH phosphorylation at Ser31 (pTHser31) (Shi et al., 2012). In capsaicin-treated MPP+-lesioned rats, CNTF levels are increased (Fig. 5D and E) compared to vehicle-treated MPP+-lesioned rats consistent with the immunohistochemical data (Fig. 4A–C). In parallel, in the capsaicin-treated MPP+-lesioned rat, pTHser31 levels are increased in the SN (Fig. 5D and E) compared to vehicle-treated MPP+-lesioned rats, indicating activation of TH enzyme activity. In the capsaicin-treated MPP+-lesioned rat, total TH levels are also increased (Fig. 5D and E) compared to vehicle-treated MPP+-lesioned rats, reflecting increased survival of dopamine neurons in the SN. CNTFRα neutralizing antibody (Fig. 5D and E) prevents the increase in total TH levels and pTHser31 levels compared control IgG-treated rats (Fig. 5D and E). In parallel, CNTFRα neutralizing antibody attenuated the effects of capsaicin on amphetamine-induced ipsilateral rotations compared with the IgG injection (Fig. 1E). Taken together these results provide evidence that astrocyte-derived endogenous CNTF can act on CNTFRα and prevent MPP+-induced degeneration of dopamine neurons and stimulate TH enzyme activity in vivo.
Astrocytic TRPV1-derived CNTF prevents degeneration of dopamine neurons in an α-synuclein model of Parkinson’s disease
To determine whether these findings extend to a genetic model of Parkinson’s disease, the rat unilateral α-synuclein lesion model of Parkinson’s disease was used (Kirik et al., 2002). Recombinant adeno-associated virus serotype 2 (AAV2) containing the human wild-type SNCA gene (AAV2-α-synuclein or α-synuclein) or enhanced green fluorescent protein (AAV2-eGFP or eGFP) were stereotaxically injected into the SN. At 7 weeks post α-synuclein, immunohistochemical analysis shows an efficient transduction of α-synuclein into the nigrostriatal dopamine neurons (Supplementary Fig. 3A and B). The loss of nigral TH+ neurons in the α-synuclein model used here is 44% at 7 weeks and 64% at 8 weeks post α-synuclein (Supplementary Fig. 3C).
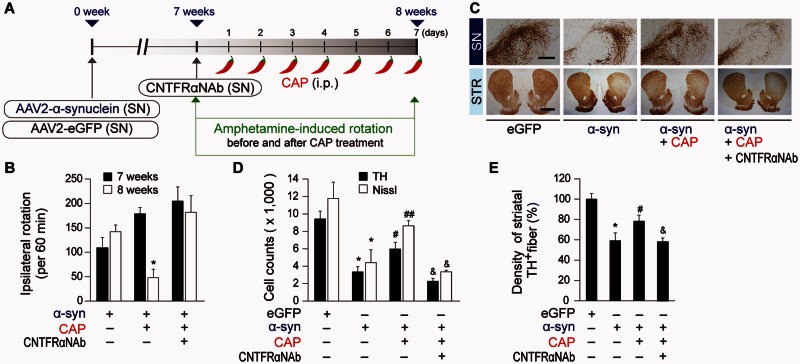
In α-synuclein-injected rats, expression pattern of TRPV1 and CNTF on astrocytes or of CNTFRα on dopamine neurons was the same as those of MPP+-treated rats compared to eGFP (Supplementary Fig. 3D–L). At 7 weeks post AAV2-α-synuclein rats were treated with capsaicin (intraperitoneal 1 mg/kg) or vehicle (Fig. 6A). In α-synuclein-lesioned rats, capsaicin treatment significantly enhanced the expression of CNTF (Supplementary Fig. 3J and K) and CNTF in GFAP+ astrocytes (Supplementary Fig. 3J and L) in the SNpc compared to vehicle-treated control at 8 weeks post α-synuclein, similar to capsaicin treatment in the MPP+-lesioned model (Fig. 4). Capsaicin-treatment of α-synuclein-lesioned rats significantly reduces amphetamine-induced rotations at 8 weeks post α-synuclein compared to vehicle-treated α-synuclein-lesioned rats (Fig. 6B). Accompanying with behavioural improvement, capsaicin increases number of TH+ and Nissl+ cells in the SNpc and density of TH+ fibres in the striatum at 8 weeks post α-synuclein compared with vehicle-treated rats (Fig. 6C–E). EGFP (control) had no effects on amphetamine-induced rotations (data not shown) and degeneration of dopamine neurons (Fig. 6C–E). CNTFRα neutralizing antibody (Fig. 6A) prevents the behavioural recovery (Fig. 6B) and the rescue of TH+ and Nissl+ cells in the SNpc and density of TH+ fibres in the striatum (Fig. 6C-E). Thus, consistent with the toxin (MPP+)-based Parkinson’s disease model, TRPV1 activated by capsaicin contributes to production of CNTF from astrocytes and prevents α-synuclein-induced degeneration of dopamine neurons in vivo.
Figure 6.
Capsaicin prevents α-synuclein-induced degeneration of dopamine neurons in vivo. (A) Diagram of the experimental design. Rats were given a unilateral injection of AAV2-α-synuclein (α-syn) or AAV2-eGFP (eGFP, control) into the rat SN and injection of CNTFRα neutralizing antibody (CNTFRαNAb) into the SN at 7 weeks post AAV2-α-synuclein. All rats received capsaicin (CAP; 1 mg/kg, intraperitoneally) or vehicle at 7 weeks and 1 day after injection of AAV2 and a continuous single injection per day for 7 days. Rats were transcardially perfused after the last rotation experiment. (B) Cumulative amphetamine-induced ipsilateral rotations, *P < 0.01, significantly different from 7weeks α-synuclein. (C) Photomicrographs of TH+ cells in the SN and TH+ fibres in the striatum (STR). (D) Number of TH+ or Nissl+ cells in the SNpc, *P < 0.001, significantly different from eGFP, #P < 0.05, ##P < 0.01, significantly different from α-synuclein, &P < 0.01, significantly different from α-synuclein + capsaicin. (E) Optical density of TH+fibres in the striatum, *P < 0.01, significantly different from eGFP, #P < 0.05, significantly different from α-synuclein, &P < 0.05, significantly different from α-synuclein + capsaicin. Scale bars: 400 µm (SN), 2 mm (STR). Mean ± SEM; B–E, n = 4–10 in each group. ANOVA and Student-Newman-Keuls analysis.
Expression of TH, TRPV1, GFAP, CNTF and CNTFRα in the substantia nigra of human Parkinson’s disease brain
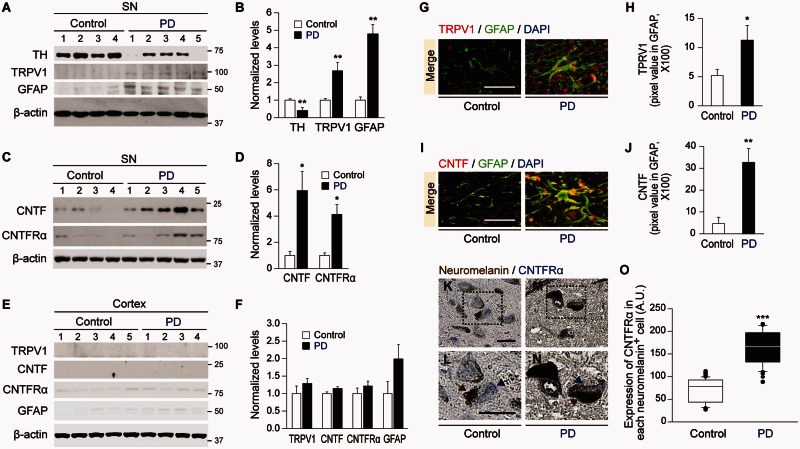
To ascertain whether these findings might have relevance to Parkinson’s disease, human post-mortem SN from control versus Parkinson’s disease patients was examined. Western blot analysis reveals a significant increase in TRPV1 and GFAP levels in Parkinson’s disease SN versus control SN (Fig. 7A and B). In addition, there is a significant increase in the levels of CNTF and CNTFRα of Parkinson’s disease SN versus control SN (Fig. 7C and D). These changes seem to be specific to the SN as there is no significant difference in the levels of TRPV1, CNTF, CNTFRα and GFAP in the cortex, which is relatively unaffected in Parkinson’s disease (Fig. 7E and F). Immunohistochemical analysis reveals a higher expression of TRPV1 in GFAP+ astrocytes (Fig. 7G and H) and a higher expression of CNTF in GFAP+ astrocytes in the SNpc of human Parkinson’s disease (Fig. 7I and J). Immunohistochemical analysis also reveals a significant increase in levels of CNTFRα expression in each remaining dopamine neuron in the SNpc of Parkinson’s disease brain compared to human control brain (Fig. 7K–O). Overall these changes are similar to the changes observed in the MPP+ and the α-synuclein rat models of Parkinson’s disease.
Figure 7.
Expression of TH, TRPV1, GFAP, CNTF and CNTFRα in the substantia nigra of human Parkinson’s disease brain. (A–F) Western blot analysis of TH, TRPV1, GFAP, CNTF, and CNTFRα in the SN (A–D) or cortex (E and F) of human control and Parkinson’s disease brain. (G–J) Immunohistochemical analysis of TRPV1 (red, G) or CNTF (red, I) in GFAP+ astrocytes (green, G and I) in the SNpc of human control or Parkinson’s disease brain. DAPI (blue) stained nucleus. Quantification of TRPV1 (H) or CNTF (J) expression co-localized in GFAP+ astrocytes in the SNpc of human brain, respectively (K–O) Photomicrographs of neuromelain+ dopamine neurons (brown) and CNTFRα+ cells (blue) in the SNpc of human control (K and L) and Parkinson’s disease (M and N) brain. L and N show enlargement of areas depicted in the boxes of K and M, respectively. Quantification of CNTFRα expression in each neuromelanin+ dopamine neuron in the SNpc of human brain (O). One hundred and fifty-six (control) or 65 (Parkinson’s disease) neuromelanin+ cells were analysed. *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from the corresponding control. Scale bars: G and I, 20 µm; K–M, 40 µm. Mean ± SEM; A–F, n = 4–5 in each group. G–O, n = 3 for TRPV1, CNTF and CNTFRα. ANOVA and Student-Newman-Keuls analysis.
Discussion
The major finding of this manuscript is the observation that TRPV1 activation by capsaicin produces endogenous CNTF, which acts on CNTFRα on nigral dopamine neurons protecting against degeneration of dopamine neurons and eliciting behavioural recovery in the MPP+ rat model of Parkinson’s disease (Supplementary Fig. 4). This occurs through activation of TRPV1 on astrocytes since the protection afforded by capsaicin in the MPP+ rat model of Parkinson’s disease is reduced by knockdown of TRPV1 in astrocytes. In addition, TRPV1 activation by capsaicin also protects against loss of dopamine neurons in the α-synuclein rat model of Parkinson’s disease and provides behavioural recovery via increases in astrocyte-derived CNTF levels acting on CNTFRα on nigral dopamine neurons. These findings may have relevance to human Parkinson’s disease as TRPV1 and CNTF levels in GFAP+ astrocytes and CNTFRα on neuromelanin+ nigral dopamine neurons are increased in human Parkinson’s disease SN similar to the MPP+ and the α-synuclein rat models of Parkinson’s disease. TRPV1 and CNTF expressed in astrocytes might function as endogenous neuroprotective machinery in vivo as MPP+-induced neurotoxicity was more aggravated in the SNpc when TRPV1 was knocked down.
Our current results demonstrate that astrocytic TRPV1 mediates production of endogenous CNTF, which prevents MPP+-induced degeneration of dopamine neurons in vivo. In addition, endogenous CNTF derived from astrocytes increased TH enzyme activity in vivo as assessed by pTHser31 suggesting that it not only protects against the loss of dopamine neurons, but it also contributes to behavioural recovery through increased TH enzymatic activity. Consistent with the notion that CNTF is neuroprotective is the observation that exogenous administration of CNTF prevents degeneration of nigral dopamine neurons in nigrostriatal pathway-transected rat (Hagg and Varon, 1993). Because TRPV1 and CNTF are significantly expressed in astrocytes in the SN of Parkinson’s disease brain and of Parkinson’s disease animal models, the present results suggest a path for pharmacologically promoting the utilization of endogenous CNTF in Parkinson’s disease for neuroprotection via capsaicin activation of TRPV1 receptors. This approach has the promising potential to bypass the low CNS bioavailability and side effects of exogenously administered CNTF (Thoenen and Sendtner, 2002).
Neurotrophic factors, promising disease-modifying agents are commonly explored for use in the therapy of Parkinson’s disease (Ramaswamy et al., 2009; Aron and Klein, 2011). Although various neurotrophic factors have powerful protective restorative effects on dopamine neurons, the experimental and clinical data suggest that improved brain delivery of neurotrophic factors (sustained and localized neurotrophic factor delivery by crossing the blood–brain barrier) is crucial for producing beneficial results. One of the promising approaches to achieve this is to search for an endogenous system locally producing neurotrophic factors in brain and/or small molecules that can activate this endogenous system producing neurotrophic factors by passing through the blood–brain barrier. Furthermore, neurotrophic factor use is a safer and efficient gene therapy when expressed in astrocytes compared to its expression in neurons in MPTP and 6-OHDA models of Parkinson’s disease (Drinkut et al., 2012). Taken together, astrocytic TRPV1-derived endogenous CNTF production by capsaicin described in our current data has the in vivo neuroprotective properties both in toxin (MPP+)- or gene- (α-synuclein, SNCA) based models of Parkinson’s disease, suggesting that this endogenous system producing CNTF in astrocytes can overcome the substantial problems for clinical application of neurotrophic factors and might be a promising therapeutic target for the treatment of Parkinson’s disease.
In summary, the present work describes the beneficial effects of astrocytic TRPV1-derived endogenous CNTF (neuroprotection and stimulation of TH enzymatic activity) in the rat MPP+ and α-synuclein models of Parkinson’s disease. Thus, astrocytic TRPV1 activation by capsaicin or related compounds may have therapeutic potential for the treatment of Parkinson’s disease to prevent the progressive degeneration of dopamine neurons and to enhance TH enzyme activity needed for functional recovery.
Supplementary Material
Acknowledgements
The authors thank Dr Bobby Thomas, Medical College of Georgia for assistance with the stereology. Human brain Tissues were received from the Victorian Brain Bank Network, supported by The Florey Institute of Neuroscience and Mental Health, The Alfred and the Victorian Forensic Institute of Medicine and funded in part by Australia’s National Health & Medical Research Council and Parkinson’s Victoria.
Glossary
Abbreviations
- AAV
adeno-associated virus
- CNTFRα
CNTFR alpha
- MPP+
1-methyl-4-phenylpyridinium
- SN
substantia nigra
- SNpc
substantia nigra pars compacta
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2008-0061888). The authors acknowledge the Adrienne Helis Malvin and Diana Helis Henry Medical Research Foundations and their direct engagement in the continuous active conduct of medical research in conjunction with The Johns Hopkins Hospital and The Johns Hopkins University School of Medicine and the Foundation’s Parkinson’s Disease Programs M-1, M-2014, H-2013. This work was also supported by NIH/NINDS P50NS038377, the JPB Foundation. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases.
Supplementary material
Supplementary material is available at Brain online.
References
- Aron L, Klein R. Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci 2011; 34: 88–100. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J, et al. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson’s disease and nonhuman primate brains. Mov Disord 2011; 26: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 2007; 8: 57–69. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 2004; 318: 121–34. [DOI] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson’s disease? Exp Neurol 2008; 209: 22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Joe EH, Kim SU, Jin BK. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J Neurosci 2003; 23: 5877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph T, Bahrenberg G, De Vry J, Englberger W, Erdmann VA, Frech M, et al. Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Mol Cell Neurosci 2008; 37: 579–89. [DOI] [PubMed] [Google Scholar]
- Chung YC, Kim SR, Jin BK. Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson’s disease. J Immunol 2010; 185: 1230–7. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron 2003; 39: 889–909. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis 2012; 45: 939–53. [DOI] [PubMed] [Google Scholar]
- Drinkut A, Tereshchenko Y, Schulz JB, Bahr M, Kugler S. Efficient gene therapy for Parkinson’s disease using astrocytes as hosts for localized neurotrophic factor delivery. Mol Ther 2012; 20: 534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episcopo FL, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B. Reactive astrocytes are key players in nigrostriatal dopaminergic neurorepair in the MPTP mouse model of Parkinson’s disease: focus on endogenous neurorestoration. Curr Aging Sci 2013; 6: 45–55. [DOI] [PubMed] [Google Scholar]
- Fischer D, Hauk TG, Muller A, Thanos S. Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci 2008; 37: 471–9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aparicio R, Moratalla R. Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson s disease. Neurobiol Dis 2014; 62: 416–25. [DOI] [PubMed] [Google Scholar]
- Guler AD, Rainwater A, Parker JG, Jones GL, Argilli E, Arenkiel BR, et al. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat Commun 2012; 3: 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Szallasi A. Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharm Des 2008; 14: 32–41. [DOI] [PubMed] [Google Scholar]
- Hagg T, Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci USA 1993; 90: 6315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin BK, Iacovitti L. Dopamine differentiation factors produce partial motor recovery in 6-hydroxydopamine lesioned rats. Neurobiol Dis 1995; 2: 1–12. [DOI] [PubMed] [Google Scholar]
- Kang SS, Keasey MP, Cai J, Hagg T. Loss of neuron-astroglial interaction rapidly induces protective CNTF expression after stroke in mice. J Neurosci 2012; 32: 9277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci 2009; 32: 215–24. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci 2002; 22: 2780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Di Marzo V, Brotchie JM. A role for vanilloid receptor 1 (TRPV1) and endocannabinnoid signalling in the regulation of spontaneous and L-DOPA induced locomotion in normal and reserpine-treated rats. Neuropharmacology 2006; 51: 557–65. [DOI] [PubMed] [Google Scholar]
- Lee MY, Kim CJ, Shin SL, Moon SH, Chun MH. Increased ciliary neurotrophic factor expression in reactive astrocytes following spinal cord injury in the rat. Neurosci Lett 1998; 255: 79–82. [DOI] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, et al. Channel-mediated tonic GABA release from glia. Science 2010; 330: 790–6. [DOI] [PubMed] [Google Scholar]
- Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci 2009; 29: 14334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AR, Dias MM, Vasconcelos TM, Caldo H, Costa MC, Chimelli L, et al. Microwave-stimulated recovery of myosin-V immunoreactivity from formalin-fixed, paraffin-embedded human CNS. J Neurosci Methods 1999; 92: 25–9. [DOI] [PubMed] [Google Scholar]
- Morgese MG, Cassano T, Cuomo V, Giuffrida A. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson’s disease: role of CB(1) and TRPV1 receptors. Exp Neurol 2007; 208: 110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Schwartz JP. Gene expression profiles of reactive astrocytes in dopamine-depleted striatum. Brain Pathol 2004; 14: 275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JH, Leem E, Jeon MT, Jeong KH, Park JW, Jung UJ, et al. Induction of GDNF and BDNF by hRheb(S16H) transduction of SNpc neurons: neuroprotective mechanisms of hRheb(S16H) in a model of Parkinson’s disease. Mol Neurobiol 2015; 51: 487–99. [DOI] [PubMed] [Google Scholar]
- Park CK, Ju WK, Hofmann HD, Kirsch M, Ki Kang J, Chun MH, et al. Differential regulation of ciliary neurotrophic factor and its receptor in the rat hippocampus following transient global ischemia. Brain Res 2000; 861: 345–53. [DOI] [PubMed] [Google Scholar]
- Park ES, Kim SR, Jin BK. Transient receptor potential vanilloid subtype 1 contributes to mesencephalic dopaminergic neuronal survival by inhibiting microglia-originated oxidative stress. Brain Res Bull 2012; 89: 92–6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press: San Diego, CA; 1998. [Google Scholar]
- Ramaswamy S, Soderstrom KE, Kordower JH. Trophic factors therapy in Parkinson’s disease. Prog Brain Res 2009; 175: 201–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold PM, Tieu K. Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics 2010; 7: 413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Investig 2006; 116: 1744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Sun A, Wang Y, Cha D, Wang H, Wang F, et al. Upregulation of mesencephalic astrocyte-derived neurotrophic factor in glial cells is associated with ischemia-induced glial activation. J Neuroinflamm 2012; 9: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Woodward WR, Habecker BA. Ciliary neurotrophic factor stimulates tyrosine hydroxylase activity. J Neurochem 2012; 121: 700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Cristino L, Di Marzo V. TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications. Curr Pharm Des 2008; 14: 42–54. [DOI] [PubMed] [Google Scholar]
- Stockli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Gotz R, et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature 1989; 342: 920–3. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nature neuroscience 2002; 5: 1046–50. [DOI] [PubMed] [Google Scholar]
- Veldhuis WB, van der Stelt M, Wadman MW, van Zadelhoff G, Maccarrone M, Fezza F, et al. Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases. J Neurosci 2003; 23: 4127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Guegan C, Wu DC, Teismann P, Choi DK, et al. The role of glial cells in Parkinson’s disease. Curr Opin Neurol 2001; 14: 483–9. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec 1991; 231: 482–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.