Abstract
Self-grooming is a complex innate behaviour with an evolutionary conserved sequencing pattern and is one of the most frequently performed behavioural activities in rodents. In this Review, we discuss the neurobiology of rodent self-grooming, and we highlight studies of rodent models of neuropsychiatric disorders — including models of autism spectrum disorder and obsessive compulsive disorder — that have assessed self-grooming phenotypes. We suggest that rodent self-grooming may be a useful measure of repetitive behaviour in such models, and therefore of value to translational psychiatry. Assessment of rodent self-grooming may also be useful for understanding the neural circuits that are involved in complex sequential patterns of action.
Professor John C. Fentress (1939–2015) passed away suddenly after this manuscript was initially submitted. The remaining authors dedicate this Review to him as a tribute to a brilliant scientist, good friend and a true pioneer of neurobiology research.
Self-grooming in animals is an innate behaviour that is involved in hygiene maintenance and other physiologically important processes, including thermoregulation, social communication and de-arousal1–6. It is one of the most frequently observed behaviours in awake rodents and has a patterned, sequential organization with characteristic cephalocaudal progression7–11 (FIG. 1). Self-grooming is remarkably similar across species in several taxa1–5. Humans engage in self-grooming, and this behaviour shows some similarity to that seen in other animals12,13. However, human self-grooming behaviour can become pathological, for example, during stressful conditions or in certain neuropsychiatric disorders7–11,14,15.
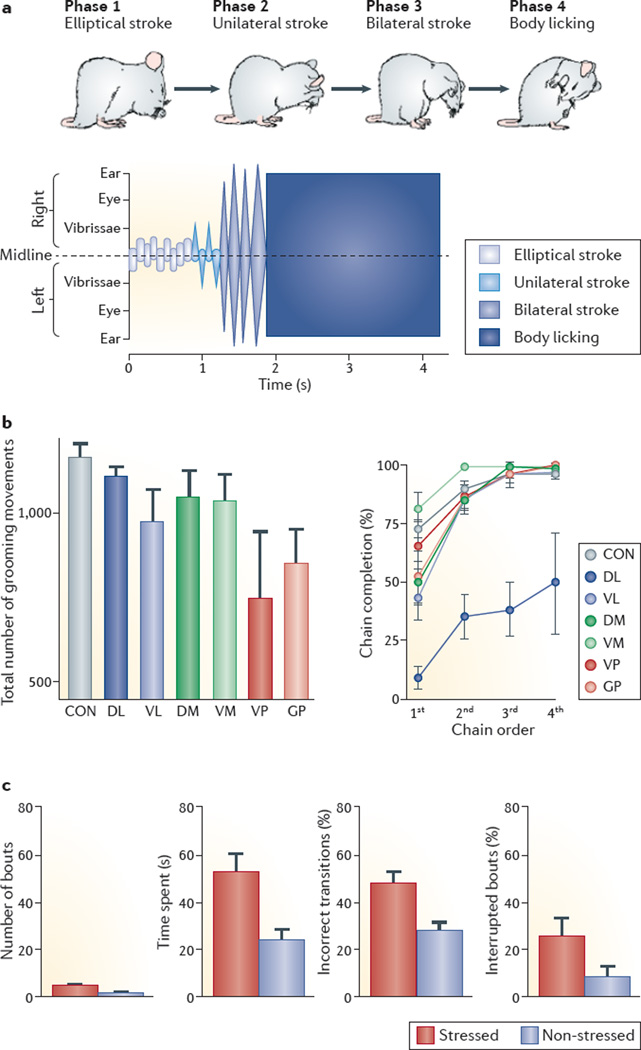
Figure 1. Rodent self-grooming behaviour.
a | Mouse self-grooming has a complex sequenced structure that consists of repeated stereotyped movements known as syntactic chains9 (see Supplementary information S1 and S2 (movies)). Phase 1 of a syntactic chain consists of a series of elliptical bilateral paw strokes made near the nose (paw and nose grooming); phase 2 consists of a series of unilateral strokes (each made by one paw) from the mystacial vibrissae to below the eye (face grooming); phase 3 comprises a series of bilateral strokes backwards and upwards made by both paws simultaneously (head grooming); and phase 4 consists of body licking (denoted by the blue box), which is preceded by a postural cephalocaudal transition from paw–head grooming to body grooming7. In addition, grooming and scratching of the tail and genitals are frequently seen in rodents within the general cephalocaudal grooming pattern (not shown as they do not form a syntactic chain7). Although chain grooming is important for assessing the sequencing of self-grooming, note that the majority (approximately 90%) of self-grooming behaviour is represented by a more flexible, non-chain self-grooming behaviour7. b | Distinct localized brain lesions affect rat self-grooming behaviour in different ways34. Rats with lesions of the ventral pallidum (VP) display fewer grooming behaviours than control rats, but rats with lesions of the anterior dorsolateral striatum (DL) do not show this deficit (left). By contrast, lesions of the anterior DL result in the overt disruption of grooming sequencing (right). This is indicated by a decrease in the percentage of the first, second, third and fourth grooming chains that were begun within a session and that were completed syntactically, that is, the frequency of phases 1, 2, 3 and 4 (as illustrated in the mouse in part a) completed without interruption, but this parameter is not affected in rats with ventromedial striatal lesions. c | Example of the effects of acute stress (a 5-minute ‘aversive’ exposure to bright light) on self-grooming sequencing in rats, assessed by the ethological global analysis of chain and non-chain bouts7, including the amount of grooming activity (number of bouts and time spent grooming) and number of incorrect transitions (that is, transitions that violate the cephalocaudal rule) as a percentage of total transitions, and the number of interrupted grooming bouts as a percentage of the total. CON, control; DM, dorsomedial striatum; G P, globus pallidus; VL, ventrolateral striatum; VM, ventromedial striatum. Figure part a is adapted with permission from REF. 29, BMC. Figure part b is adapted with permission from REF. 34, Society for Neuroscience. Figure part c is from REF. 7, Nature Publishing Group.
The assessment of rodent self-grooming is potentially useful for translational neuroscience research, as aberrant rodent self-grooming can be related to human disorders in which abnormal self-grooming is a symptom. However, it is important to note that animal self-grooming cannot be considered an exact model of any particular human pathology. Rather, the broader value of rodent self-grooming is as a model of complex repetitive, self-directed and sequentially patterned behaviours. Therefore, rather than viewing rodent self-grooming behaviour as a direct correlate of a particular symptom, it may be best considered as an indirect index of several behavioural phenomena that may be relevant to human brain disorders, including chains of motor action and complex patterning of motor activities. From this broad viewpoint, the analysis of rodent self-grooming may help in understanding the neural mechanisms of hierarchical motor control14–26 that underlie complex sequential behaviours in general, and may also provide valuable mechanistic insights into their dysregulation.
Neurophysiology, genetics and pharmacology have been used to study this interesting complex behaviour in rodents14–26. In this Review, we discuss findings from this work and highlight the potential implications of assessing rodent self-grooming behaviour for understanding human brain disorders. We propose that rodent self-grooming is an important behavioural phenotype that can be used to understand the neural basis of complex action patterns in other species, including humans, in both normal and abnormal conditions. This Review does not discuss heterogrooming (a form of grooming behaviour that is directed towards another animal, which occurs in other contexts, such as maternal, sexual and aggressive or social behaviours), or peripheral and brainstem or spinal coordination mechanisms that are the ultimate targets of the forebrain control networks involved in grooming.
Neurobiology of rodent self-grooming
Behavioural complexity
Self-grooming in mice and rats shows a high level of behavioural complexity and organization (grooming microstructure)7,27,28, which involves a series of individual movements that form functional sequences, including highly stereotyped patterns9 (FIG. 1a). In the first postnatal days, rodent self-grooming behaviour targets the face and consists of either temporally isolated grooming strokes with the front paws or bouts of strokes with varying amplitude and symmetry. During the following weeks, self-grooming behaviour develops to include symmetrical, double-handed lower amplitude movements and finally matures into the species-typical sequencing of short and long symmetrical and asymmetrical strokes10. Thus, in such early stages of development, self-grooming consists of facial grooming alone, but over time comes to include grooming of the entire head, neck and trunk. In addition to displaying the stereotyped grooming that is also present in young animals, adults show more flexible, less stereotyped facial grooming movements10.
Mature rodent grooming behaviour consists of specific and highly stereotyped patterns of sequential movements, known as a syntactic chain pattern7, which often occurs during the transition between facial and body grooming (FIG. 1a,b). Syntactic chains of self-grooming have features similar to those of other fixed-action patterns, such as sexual or aggressive behaviours, in that they are highly stereotyped in order, and, once begun, they proceed to completion without requiring sensory feedback7. A typical self-grooming syntactic chain in rodents, which is often embedded in other forms of grooming behaviours, serially links 20 or more grooming movements into four distinct, predictable phases that follow the same cephalocaudal (head-to-body) rule9,29. The serial structure of such chains is repetitive and consistent in terms of order and time, so that once the first phase begins, the entire remaining sequential pattern reliably continues through all four phases. This syntactic chain pattern accounts for approximately 10–15% of all observed self-grooming behaviours in rodents, the remainder of which follow less predictable sequential patterning rules (FIG. 1; see Supplementary information S1 (movie))7. Self-grooming sequencing, chain initiation and chain completion in rodents can be bidirectionally affected by experimental manipulation, including lesions of the dopamine-containing nigrostriatal tract, administration of various dopaminergic drugs, genetic mutations and psychological stress7. The syntactic chains are usually interspersed with more flexible ‘non-chain’ grooming (that is, flexibly ordered mixtures of strokes, licks or scratches that are not components of syntactic chains), which accounts for approximately 85–90% of all grooming behaviours7 (see Supplementary information S2 (movie)). Ethologically based analyses of grooming behaviours, including both chain and non-chain bouts, are widely used in neurobiological research to assess their global adherence to the cephalocaudal rule7. Correct and incorrect cephalocaudal transitions between stages can be studied in this way (FIG. 1c), along with interruptions in grooming bouts (as an index of disturbed self-grooming) and their regional distribution over the body7,28,30. Such analyses demonstrate the high sensitivity of grooming sequencing to genetic, pharmacological and psychological challenges7,27,28,30–33.
Neural circuitry of self-grooming
Because of its highly patterned nature, grooming is particularly suitable for studying how various neural circuits regulate both the key aspects — motor and sequencing — of this behaviour34. Studies of rats decerebrated at successively lower levels of the neuraxis have demonstrated that rats that underwent mesencephalic decerebration, in which the midbrain is intact, have a normal sequential pattern of self-grooming chains, although such animals have difficulty in completing the full pattern35,36. By contrast, a gradual degradation of the sequential pattern itself is seen in rats that have been decerebrated at more caudal (that is, metencephalic and myelencephalic) levels, suggesting that the brainstem circuitry is necessary for the execution of fully patterned grooming sequences35,36 (FIG. 2).
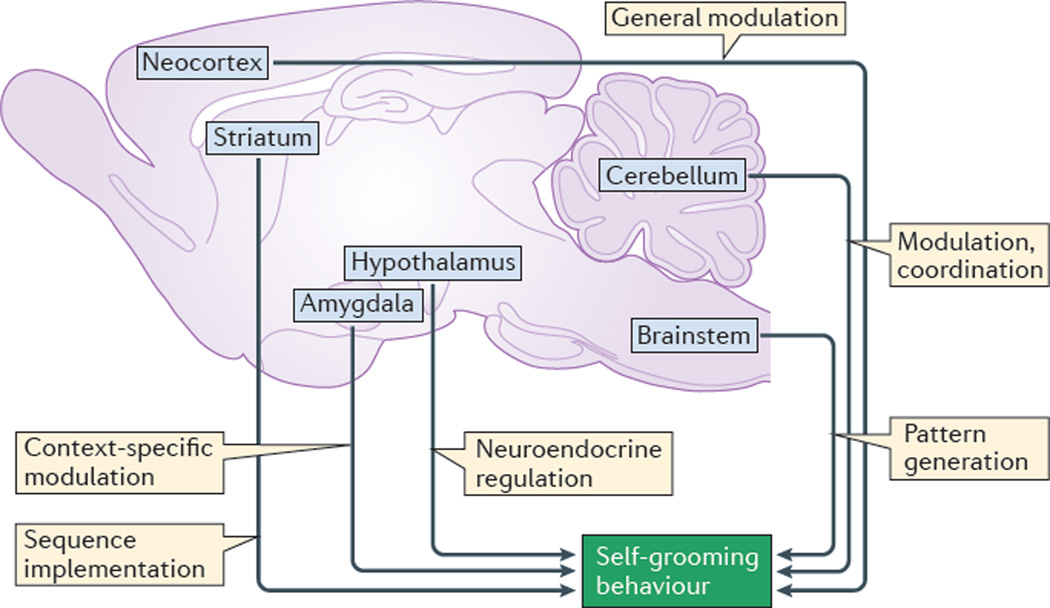
Figure 2. Brain regions involved in the regulation of rodent self-grooming.
A simplified overview of the key brain regions that are involved in different aspects of rodent self-grooming. The basal ganglia, especially the striatum and its dopaminergic inputs, control rodent self-grooming motor behaviour and its sequencing (see REF. 50 for details of the major neural connections of the basal ganglia), even for sequential patterns that are generated in the brainstem. The neocortex is involved in the general modulation of self-grooming movements, sending excitatory projections to the striatum, and receiving excitatory projections from the thalamus and amygdala. The cerebellum (including its multiple projections to the basal ganglia, thalamus, cortex, amygdala, hypothalamus, brainstem and spinal cord) is involved in motor control and coordination, and in fine-tuning of self-grooming movements. The amygdala (with its neural connections with the cortex, thalamus, hypothalamus, basal ganglia and brainstem) is involved in context-specific modulation of self-grooming (for example, during stress or in competition with social interactions)47. The hypothalamus (interconnected with the cerebellum, cortex and amygdala) is important for the neuroendocrine regulation of grooming, as stimulation of the paraventricular nucleus and the dorsal hypothalamus elicits robust self-grooming52. The hypothalamic–pituitary system also modulates self-grooming, as several hypothalamic and pituitary hormones, especially the stress-related peptides corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), potently induce rodent self-grooming56,58. Finally, the brainstem is crucial for initiating self-grooming movements and for generating basic sequential patterns (such as a syntactic chain), although by itself the brainstem is not sufficient to fully implement those patterns (as this requires striatal involvement). Figure is adapted with permission from REF. 50, Frontiers.
Within the forebrain, circuits that incorporate the basal ganglia and allied nuclei, including the striatum, globus pallidus, substantia nigra, nucleus accumbens and subthalamic nucleus, have been strongly implicated in hierarchical motor control and sequencing of behaviour, including self-grooming. The striatum is the main input region of the basal ganglia. Striatal circuits are involved in learning, motivation and motor sequencing. For example, the basal ganglia37,38 and, in particular, the striatum, are required for the execution of full sequential patterns of grooming chains and other types of sequential behaviour in mice and rats18,39,40 (FIG. 2). Lesions of the striatum result in a permanent deficit in the ability to complete sequential syntactic self-grooming chains34 (FIG. 1b). Extensive work using localized striatal lesions has shown that it is the anterior dorsolateral region of the striatum that is essential for this normal grooming behaviour. Damage to this striatal region impairs the completion of (but not the ability to initiate) syntax patterns of grooming movements34. Rats with such striatal lesions completed only ~50% of the syntactic chains, a completion rate that is similar to that of rats with full mesencephalic decerebration (that is, transection above the midbrain)34, whereas control rats completed ~90% of the chains. Thus, similar deficits of pattern completion are produced by anterior dorsolateral striatal lesions and by decerebration, but both mesencephalic and pontine decerebrates can still produce the basic sequential self-grooming pattern34.
These results suggest that the essential pattern generator for syntactic chains is in the brainstem, and that the dorsolateral striatum may act as a forebrain controller to coordinate the normal completion of the chain pattern. By contrast, lesions that affect the major output nuclei of the basal ganglia, including the ventral pallidum and globus pallidus, disrupt the movements that are required for grooming but not the syntax of self-grooming34. These studies suggest that distinct striatal pathways may regulate self-grooming activity and its patterning and sequencing. Neurons in the dorsolateral striatum and the substantia nigra pars reticulata also show distinct spiking patterns during different types of grooming. For example, some dorsolateral striatal neurons that are active during syntactic grooming sequences are unresponsive during kinematically similar movements that occur during flexible grooming41. Because striatal neurons can code various types of naturally sequenced behaviours, it is likely that the basal ganglia have a crucial role in the control of sequential movement not only in self-grooming but also in the complex natural patterns of other sequenced behaviour41–43.
Lesions made in the neocortex or in the cerebellum produce timing deficits and abnormalities in the individual movements of self-grooming without affecting the sequential pattern of grooming chains44. Other manipulations in the cerebellum also affect self-grooming. For example, electrical stimulation of the cerebellum elicits self-grooming in rats45, whereas Lurcher mutant mice, which have cerebellar degeneration, display reduced duration, but unaltered sequencing, of self-grooming compared with wild-type mice46. Given that the striatum, the neocortex and the cerebellum are directly and indirectly interconnected with one another in movement-control networks, this difference in the amount versus the patterning of self-grooming suggests that the striatum and its associated neural pathways are particularly important for the sequencing of grooming patterns44. This conclusion is in accord with other evidence supporting the importance of the striatum-based circuits in sequential behaviours in general42,43.
Self-grooming behaviour is also modulated by the limbic circuitry, including the amygdala and the hypothalamus (FIG. 2). The amygdala is a limbic brain structure that is involved in the regulation of modulating motivational states, such as fear, anxiety and desire47. Studies have demonstrated correlations between increased anxiety-like behaviour and reduced dopamine release within the amygdala in selectively bred high-grooming versus low-grooming rats48. The extended amygdala is an anatomical system that forms a continuum stretching from the amygdala, to the bed nucleus of the stria terminalis (BNST), and to the nucleus accumbens shell. This complex is involved in the regulation of reward and affect. The extended amygdala contains a medial division, which includes the medial nucleus of amygdala (MeA) and the medial BNST, and a lateral division, which includes the central nucleus of the amygdala (CeA) and the lateral BNST. Both divisions are implicated in self-grooming and seem to act together. For example, stimulation of glutamatergic neurons in the posterior dorsal part of the MeA (MeApd) induced repetitive self-grooming in mice and suppressed social interaction, whereas stimulation of GABAergic neurons of the MeApd inhibited mouse self-grooming and promoted social interaction47. Within the lateral division of the extended amygdala, microinjections of orexin-B into the CeA evoke moderate increases in grooming frequency in hamsters49, collectively supporting the role of both the MeA and the CeA in modulating self-grooming.
Further study is required to obtain a full understanding of amygdala-related grooming circuitry. For example, in addition to its connections to the striatum, the amygdala — primarily its basolateral nucleus (BLA) — projects to the prefrontal cortex, which together with other cortical regions in turn projects to the striatum. Although it is known that corticostriatal connections can modulate self-grooming behaviour50 (FIG. 2), the potential functions of indirect amygdalo-corticostriatal networks in grooming remain to be investigated. The connectivity between the striatum and the amygdala also raises the possibility of a distinction between the locomotor and sequencing control of self-grooming (that is linked to basal ganglia circuits) versus self-grooming related to affective states (that is modulated by the amygdala-related limbic circuits). However, because affective state is central to striatal state modulation, this contrast may be an over-simplification, and therefore the functional and anatomical diversity of both amygdala circuits and striatal circuits must be considered. For example, complex context-specific modulation of grooming behaviour may involve both the BLA–CeA–anterior BNST circuits (that mediate stress, anxiety and conditioned defence) and the MeA–posterior BNST circuits projecting to the hypothalamus (that are responsible for innate social and predator-defence behaviours)47,51.
The hypothalamus — a forebrain region that coordinates neural and endocrine regulation of brain functions and behaviour — is another limbic region that has been implicated in the regulation of rodent self-grooming52. Local electrical stimulation or injection of a wide range of drugs in the hypothalamus evokes robust self-grooming in rats, suggesting that the paraventricular nucleus and the dorsal hypothalamus may be part of a specific region that is responsible for grooming52. The paraventricular nucleus projects to the MeApd52, and glutamatergic neurons within the lateral hypothalamic area adjacent to the MeApd contribute to repetitive self-grooming in mice47. Both the CeA and MeA project to respective divisions of the BNST — the main connector between the amygdala and the hypothalamus53,54. Nuclei of the amygdala, most notably the MeApd (that is implicated in self-grooming52), also project to the medial hypothalamus47. Finally, the hypothalamic–pituitary system has now been implicated in the modulation of self-grooming, as several hypothalamic and pituitary hormones (including the stress-related peptides corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH)) are known to induce self-grooming55–58 (see Supplementary information S3 (table)). The effects of these hormones on grooming are partly dependent on the mesolimbic dopaminergic system59–61 (as we emphasize below). Collectively, this evidence indicates that the hypothalamus (and its connections to the pituitary) is an important brain region that incorporates neural and endocrine regulation of self-grooming2,62.
Pharmacological modulation of self-grooming
Pharmacological manipulations can potently modulate rodent self-grooming. Dopamine, which is a major modulator in the nigrostriatal and mesolimbic systems, is critical for locomotor function, self-grooming and other complex patterned behaviours29,34,40,44. In rodents, systemic administration of dopamine D1 receptor (D1) agonists amplifies complex behavioural super-stereotypy, leading both to excessive production of self-grooming chains, and to more rigid self-grooming chains8,63,64. Systemic co-administration of the dopamine D2 receptor (D2) antagonist haloperidol prevents sequential super-stereotypy that is induced by the D1 agonist SKF38393 (REF. 64), and the activation of grooming by SKF83959, a D1 agonist and D2 partial agonist, is eliminated in knockout mice lacking the D1 (but not the D2) gene65. Collectively, these results illustrate the importance of a balance between the D1 and the D2 systems of the striatum in the regulation of self-grooming.
Striatal circuits can also be characterized in terms of the compartmental architecture of the striatum. Within the striatum of humans and other mammals, chemically specialized macroscopic zones known as striosomes (‘striatal bodies’) form a distributed labyrinthine system within the large volume of the striatum that constitutes the extra-striosomal matrix. This architecture is known as the striosome-matrix architecture, which governs the distribution of nearly all neurotransmitters and their receptors as well as the relative distributions of projection neurons and interneurons in the striatum66. Studies have shown that, following dopaminergic challenge, striosomes are strongly activated and express early response genes that code for transcription factors, and that this heightened striosomal activation is highly correlated with increased repetitive behaviours, including self-grooming, in both non-human primates and rodents42,67–70.
Pharmacological studies have shown that glutamate is also involved in the regulation of self-grooming71. For example, the systemic administration of anti-glutamatergic agents, such as an NMDA receptor antagonist phencyclidine (PCP), is a well-established experimental method for inducing grooming in rodents72. In addition, PCP induces generalized hyperlocomotion and other stereotypic behaviours in rodents73–75. Notably, although PCP increases the duration of experimentally evoked self-grooming, it disrupts the sequencing of self-grooming only when the animals are under stress72, further indicating that self-grooming activity and its detailed patterning are controlled differently by the CNS.
GABAergic neurotransmission also contributes to the regulation of self-grooming. Drugs that enhance GABAergic tone, such as benzodiazepines and allo-pregnanolone, generally reduce rodent self-grooming at non-sedative doses76–78. By contrast, GABA-inhibiting drugs often increase grooming in rodents and can also reverse the anti-grooming effects that are produced by GABA-enhancing agents76,77. The GABAergic system is also a key modulator of stress and anxiety-related behaviours in rodents32,79. Drugs that enhance GABAergic tone exert anxiolytic effects and may be useful as augmentation agents for the treatment of obsessive compulsive disorder (OCD)80. Thus, these GABA-enhancing drugs and other anxiolytic drugs may suppress stress-induced grooming through attenuating the intensity of the perception of anxiogenic stimuli81, as anxiety-like states alter rodent self-grooming and its sequencing28,30,82. The cephalocaudal patterning of rodent self-grooming is sensitive to GABAergic drugs: drugs that inhibit GABA signalling generally disorganize cephalocaudal patterning and drugs that enhance GABA signalling tend to normalize this response32,76,83.
Given the ubiquity of GABA and glutamate in the CNS, region-specific manipulations are required to provide further insights into their role in grooming. For example, the injection of the GABA type A receptor (GABAA) agonist zolpidem into the hamster CeA did not affect orexin B-evoked grooming behaviour, whereas co-infusion of an NMDA receptor agonist potentiated the effect of orexin B49. Injection of the GABAA agonist muscimol into the BNST (but not into the BLA) strongly reduced the self-grooming response that is evoked by cat urine exposure81, suggesting that this region may be crucial for anxiogenic responses in general, including increased self-grooming. Administration of muscimol to the ventral tegmental area potentiates the excessive self-grooming behaviour that is evoked by α-melanocyte-stimulating hormone84. By contrast, treatment with an NMDA receptor antagonist, memantine, ameliorates pathological self-grooming in mice that lack the astrocyte-specific excitatory amino acid transporter 2 (also known as GLT1), which display aberrant excitatory transmission at corticostriatal synapses85. Taken together, this evidence implicates key central neurotransmitters and their circuits in the regulation of grooming.
Self-grooming in CNS disorders
Self-grooming in rodents can be used to model normal or pathological human grooming behaviours86, but study of this behaviour also has a much broader value, as it can be relevant to the neurobiology of complex, repetitive and sequentially patterned behaviours6,7,24. Different aspects of self-grooming in rodents can be used to mimic phenotypes across a range of human conditions (FIG. 3), only some of which manifest themselves as aberrant grooming. In line with recently introduced research domain criteria (RDoC)87,88, we take a dimensional approach and discuss the dysregulation of rodent self-grooming and its value for modelling dimensions of human psychopathology that may cross traditional diagnoses.
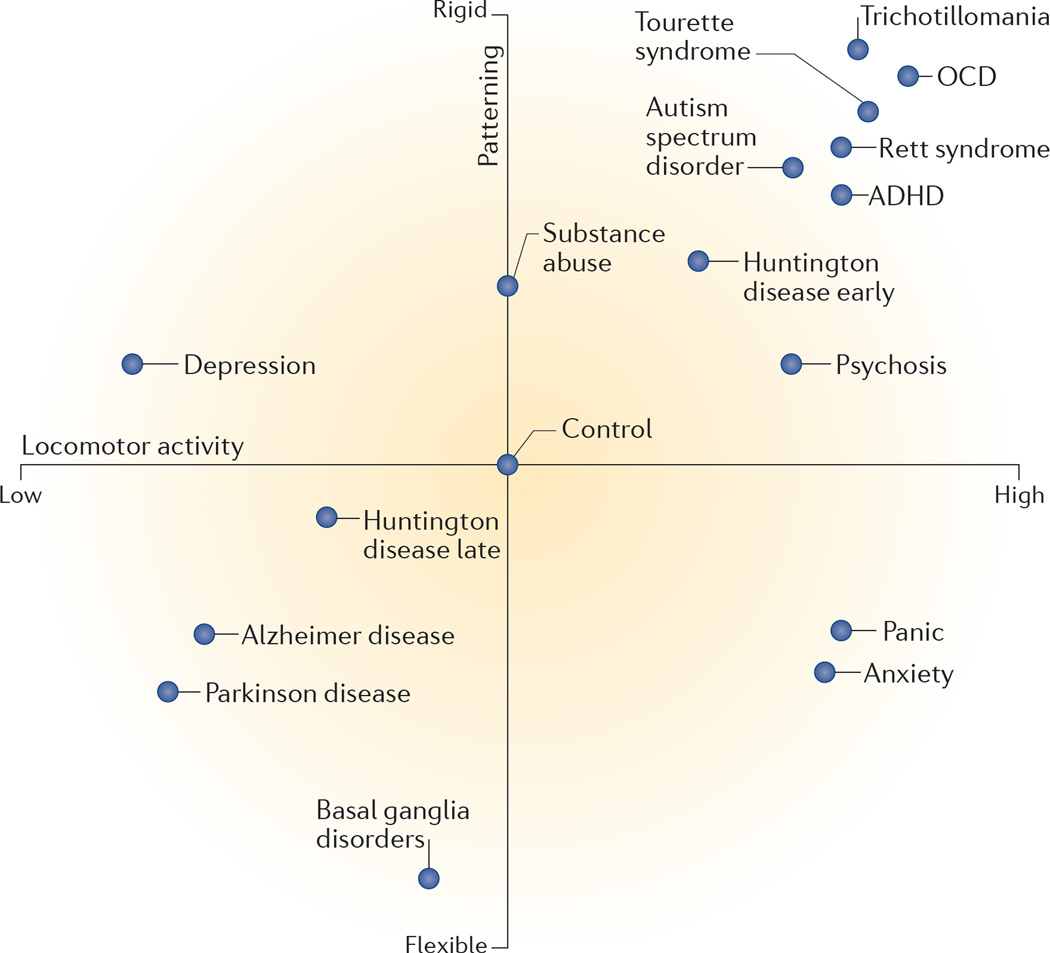
Figure 3. Expected self-grooming behaviour in rodent models of neuropsychiatric and neurodegenerative disorders.
Aberrant self-grooming is observed in anima models of several neuropsychiatric disorders, but each disease model is expected to have distinct grooming phenotypes. Shown are the expected rodent self-grooming phenotypes relevant to particular disease models. The x-axis represents the amount of self-grooming activity that can be assessed as ranging from low frequency to high frequency, or from short to long duration, and the y-axis represents the degree of sequential patterning, ranging from rigid and repetitive to more flexible behaviour7. The expected behaviour of a wild-type control animal with normal self-grooming behaviour is shown at the centre. In this diagram, a ‘rigid’ patterning of rodent grooming, based on high adherence to the cephalocaudal progression of grooming sequence (FIG. 1), will be maximal for the stereotyped ‘chain’ grooming. By contrast, ‘flexible’ patterning denotes frequent deviations from the cephalocaudal rule, and will be maximal in ‘non-chain’ grooming. For example, Sapap3−/− mice, which display an obsessive compulsive disorder (OCD)-like phenotype, spend more time self-grooming, and their self-grooming behaviour is highly repetitive, compared with wild-type control mice40. Rodent models of anxiety (such as stress-exposed rats or mice treated with anxiogenic drugs) also display an increase in the amount of time spent grooming, but patterning of their self-grooming is impaired (see REFS 7,32 for examples). By contrast, animal models of Alzheimer disease and Parkinson disease are likely to show global progressive deficits in their grooming owing to motor impairments (for example, see REF. 160). Note that in animal models of Huntington disease, self-grooming is increased in the early stages of the animal model (see REFS 162,167 for examples), but progressive ataxia and global motor deficits (and thus decreased self-grooming) are likely to be observed at later stages, paralleling the clinical trajectory of Huntington disease. ADHD, attention deficit hyperactivity disorder.
Autism spectrum disorder
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with CNS aetiology and complex symptoms, including difficulties with communication, repetitive behaviours and social deficits89–92. There is considerable interest in developing experimental animal models of ASD90,91,93. Because self-grooming episodes in rodents are thought to recapitulate pathological repetitive behaviours (behavioural perseveration), strains of rodents that exhibit these phenotypes have been investigated, with the goal of identifying neural circuits and genes relevant to ASD33,90,94. We discuss rodent self-grooming as a measure of behavioural perseveration rather than as a specific model of an ASD phenotype. Notably, many of the mouse strains discussed here also display other phenotypes that are relevant to ASD (for example, they also show non-grooming behavioural perseverations and/or deficits in other relevant domains, such as social impairments and anxiety).
The inbred BTBR T+Itpr3tf/J (BTBR) mouse strain, which exhibits agenesis of the corpus callosum, displays several aberrant behaviours that resemble symptoms of ASD, including social deficits, anxiety and general behavioural inflexibility19,95,41–43. Peer rearing with a different (‘non-ASD’) strain improved social deficits in BTBR mice but did not improve their repetitive self-grooming96, raising the possibility that different ASD behavioural domains may be regulated by distinct brain mechanisms. However, increased self-grooming in these animals can be corrected pharmacologically. For example, cholinergic agents (which may be useful in correcting postulated cholinergic deficits in ASD97,98 and/or some of its clinical symptoms99) reduce self-grooming19 and other ASD-like behaviours100 in BTBR mice. Furthermore, repetitive self-grooming behaviour in BTBR mice is rescued by the inhibition of glutamatergic metabotropic mGluR5 receptors90,101 and by the stimulation of NMDA receptors by d-cycloserine102 (which has also been shown to ameliorate some behavioural deficits in individuals with ASD103,104). Environmental enrichment reduces the duration, but not the rigid patterning, of abnormal self-grooming in BTBR mice33. The ability to modulate the quantity (amount) and the quality (degree of sequencing) of self-grooming in these mice by distinct interventions raises the possibility that there are also distinctions between these different aspects of self-grooming behaviour at the level of circuits and molecular pathways. Consistent with the goal of defining psychiatric diseases as circuit disorders105,106, this work further emphasizes the value of a nuanced understanding of grooming phenotypes, including self-grooming, in preclinical biological psychiatry research.
The genetic mechanisms underlying ASD have been unclear to date, owing to its highly polygenic nature. Currently, the number of genes associated with ASD is estimated to be ~700, according to the Simons Foundation Autism Research Initiative gene database. Individuals with ASD have heterogeneous behavioural and neuromorphological phenotypes91,106–109. Three examples are used here to illustrate how assessing self-grooming in transgenic mice can be useful in investigating the role of particular genes associated with autism. SHANK1 (SH3 and multiple ankyrin repeat domains 1), SHANK2 and SHANK3 encode postsynaptic scaffolding proteins that are crucial for synaptic function in the brain110, and mutations in these genes are strongly implicated in ASD. In addition to exhibiting ASD-like social deficits and repetitive behaviours, mice with mutations in different Shank genes show aberrant self-grooming phenotypes111 (TABLE 1). For example, Shank1+/− (and, to a lesser extent, Shank1−/−) mice demonstrate mildly increased self-grooming behaviour as adults, but not as juveniles21. Female, but not male, Shank2−/− mice lacking exon 7 show increased duration of self-grooming bouts16, and male Shank2−/− mice lacking exons 6 and 7 spend more time engaged in self-grooming during a novel object recognition (but not during the open field) test112. Increased duration of self-grooming bouts in Shank3−/− mice has also been reported by several groups89,113,114. Taken together, these findings establish a link between disruptions in Shank genes, aberrant synaptic function in the brain and ASD-related behaviours in mice88,106,107,111, suggesting the Shank-mutant mice, and their self-grooming phenotypes in particular, are good models of ASD.
Table 1.
Selected rodent strains that display aberrant self-grooming behaviour
| Model | Aberrant self-grooming phenotype | Refs |
|---|---|---|
| Dat1−/− mice | Increased stereotypy | 29 |
| Drd1a−/− mice | Increased frequency and disrupted sequencing | 148 |
| Hoxb8−/− mice | Excessive self-grooming | 17,25 |
| Sapap3−/− mice | Increased frequency and duration* | 23,40,39 |
| Shank1+/− and Shank1−/− mice | Increased duration | 21 |
| Shank2−/− mice | Partially increased in females (lacking exon 7) and in males (lacking exons 6 and 7) |
16,112 |
| Shank3+/− and Shank3−/− mice | Mildly increased duration | 88,106,107 |
| Syn2−/− mice | Increased duration | 22 |
| Hdc−/− mice | Increased duration | 150 |
| Vdr−/− mice | Increased duration and disrupted sequencing | 31,158 |
| Astrocyte-specific inducible Glt1−/− mice |
Increased duration | 85 |
| Striatum-specific Gad1−/− mice | Increased duration | 118 |
| MAO-ANeo mice | Increased frequency and duration | 192 |
| BTBR mice‡ | Increased duration and repetition | 19,33,94 |
| RLA rats | Increased duration | 159,193,194 |
| LY and HY rats | Different patterning in HY rats compared with LY rats | 195,196 |
Dat1, dopamine transporter; Drd1a, dopamine receptor 1A; Gad1, glutamic acid decarboxylase 1; Glt1, excitatory amino acid transporter 2; Hdc, histidine decarboxylase; Hoxb8, homeobox protein Hox-b8; HY, selectively bred high-yawning rats; LY, selectively bred low-yawning rats; MAO-A, monoamine oxidase A; RLA, selectively bred Roman low avoidance rats; Sapap3, SAP90/PSD-95-associated protein 3; Shank, SH3 and multiple ankyrin repeat domains; Syn2, Synapsin II; Vdr, vitamin D receptor.
Phenotype can be reversed by genetic deletion of the gene encoding melanocortin 4 receptor, or by optogenetic stimulation of the orbitofrontal cortex and its striatal terminals.
Ephrin A ligands and ephrin A receptors are strongly implicated in neurodevelopment115. Ephrin A ligands are membrane-anchored cellular proteins that bind to ephrin A receptors, members of the receptor tyrosine kinase superfamily. During development, ephrin A-mediated signalling modulates neuronal differentiation and synaptic plasticity115. Because ASD is a neurodevelopmental disorder, ephrin A ligands and their receptors may be relevant to ASD and modelling its pathogenesis in animals115. For example, mice that lack both the ephrin A2 and the ephrin A3 receptors display robust repetitive self-grooming in addition to motor retardation, increased prepulse inhibition and social deficits (impaired social interaction and preference), thereby paralleling in their phenotypes some of the clinical symptoms of ASD115. The search for novel molecular anti-ASD drug targets is a recognized priority19,93,106,116,117, and the utility of grooming-based analyses for exploring novel candidate pathways of this disorder (for example, ephrin A receptor agonists) continues to emerge.
Another example of rodents with specific mutations displaying an aberrant self-grooming phenotype are mice lacking the GABA-synthesizing enzyme glutamate decarboxylase 1 (GAD1; also known as GAD67) in striatal neurons. These mice display behavioural abnormalities that resemble symptoms of ASD, including stereotypic grooming and impaired spatial learning and social behaviour118, suggesting that GABAergic output from the striatum might contribute to behavioural deficits in ASD118.
A deletion on human chromosome 16p11.2, spanning approximately 30 genes, is associated with ASD and other neurodevelopmental disorders119–121. Notably, mice heterozygous for a deletion of the syntenic region on chromosome 7F3 (16p11+/− mice) show reduced self-grooming behaviour, but the mice also display hyperactivity and behavioural perseverations, such as increased circling108. 16p11+/− mice also have increased numbers of striatal medium spiny neurons expressing the dopamine D2 receptor, fewer cortical neurons expressing D1 dopamine receptors, and synaptic defects indicating abnormal basal ganglia circuitry108. The behavioural phenotype of these mice is of particular note, because the decreased self-grooming is observed alongside increased non-grooming stereotypies108, thereby suggesting further distinctions between the activity and patterning aspects of grooming (see TABLE 1 and Supplementary information S4 (table) for more information on genetic models of mouse self-grooming). Finally, studying self-grooming improves the development of animal models of ASD, because when other ASD-like phenotypes are present, the co-occurrence of a self-grooming phenotype as a measure of repetitive behaviour considerably strengthens the validity of the models.
Disorders of the basal ganglia
Excessive self-grooming is a feature of some forms of OCD24,86 and related illnesses, such as body dysmorphic disorder, excoriation (compulsive skin-picking) and trichotillomania (compulsive hair-pulling)92. Studying aberrant self-grooming in rodents may therefore be relevant to modelling such conditions, and may also be useful for modelling the OCD-spectrum disorders that, although they are not associated with abnormal self-grooming, are characterized by excessive repetitiveness of behavioural actions122,123.
OCD is a common heterogeneous psychiatric disorder that is characterized by obsessions and compulsions124,125. Obsessions are intrusive, recurrent and persistent unwanted thoughts, and are often associated with elevated anxiety124,125. Compulsions include a range of repetitive behaviours or thoughts. A conventional view is that these are performed to relieve obsessions124, but this link between obsessions and compulsions is not certain. Compulsions are sometimes focused on aspects of personal hygiene, which can involve self-cleaning or self-grooming behaviours (such as hand-washing), and behaviours to avoid perceived contamination from the individual’s surroundings44,86. Evidence from studies of individuals with OCD syndromes, including neuroimaging and clinical genetics, and from studies of a wide range of animal models of repetitive behaviour68, has suggested that basal ganglia-related circuit dysfunction contributes to these syndromes.
A growing number of genetic mutations have been shown to affect self-grooming behaviour in rodents68 (TABLE 1; see Supplementary information S4 (table)). Some of these may be useful in modelling self-grooming-related symptoms of OCD, including compulsive hand-washing19,22,25 and obsessive hair-pulling126–128,129 (TABLE 2). For example, serotonergic drugs that are effective in treating some symptoms of clinical OCD71 are also successful in reducing aberrant self-grooming phenotypes in some of these mutant mice (TABLE 3). Such findings support the value of rodent self-grooming behaviours in mimicking human OCD. They also raise the possibility that the serotonergic system contributes to the regulation of normal and pathological grooming in both humans and rodents. Although direct support for this notion remains elusive71, clinical and experimental evidence continues to implicate serotonergic function in various OCD-like symptoms130–136.
Table 2.
Disease symptoms that may be modelled in rodents by the assessment of self-grooming behaviour
| Human disease | Symptom | Relevant rodent self- grooming phenotype |
Refs |
|---|---|---|---|
| OCD | Compulsive hand washing | Increased self-grooming | 37,143–145 |
| Trichotillomania | Compulsive hair pulling | Increased self-grooming | 128,199 |
| Body dysmorphic disorder | Obsessive cosmetic grooming | Increased self-grooming | 92 |
| Excoriation | Compulsive skin-picking | Increased self-grooming | 92 |
| ASD | Behavioural perseveration | Increased self-grooming | 16,19–22,33 |
| Tourette syndrome | Tics | Increased self-grooming | 29 |
| Anxiety disorders and panic disorder |
Stress-induced displacement behaviour |
Increased self-grooming | 7,27,31,158 |
| Schizophrenia | Hyperarousal | Increased self-grooming | 92 |
| Trichotillomania | Compulsive hair-pulling | Increased self-barbering* | 27,45 |
| ASD | Behavioural perseveration | Grooming patterning rigidity | 89–91 |
| Depression | Behavioural perseveration | Grooming patterning rigidity | 92 |
| Anxiety disorders and panic disorder |
Hyperarousal | Disrupted grooming patterning | 27,28,159 |
| Basal ganglia disorders | Impaired action sequencing | Disrupted grooming patterning | 64 |
| Depression | Anhedonia and poor hygiene | Reduced grooming activity | 92 |
| Neurodegenerative disorders |
General decline in motor function | Reduced grooming activity | 160 |
ASD, autism spectrum disorder; OCD, obsessive compulsive disorder.
Self-inflicted hair and whisker loss frequently seen in laboratory rodents in different contexts126. This grooming-related behaviour is an important rodent phenotype sensitive to various environmental and genetic manipulations (see Supplementary information S4 (table)).
Table 3.
Sensitivity of rodent self-grooming to pharmacological manipulation
| Model | Effect on self-grooming behaviour | Refs |
|---|---|---|
| Chronic fluoxetine | ||
| Chronic corticostriatal stimulation in mice |
Evoked grooming reversed | 15 |
| Sapap3−/− mice | Over-grooming and facial lesions corrected | 40 |
| Slitrk5−/− mice | Over-grooming and facial lesions corrected | 18 |
| Chronic clomipramine | ||
| Rats selectively bred for high anxiety-like behaviour |
Reduced activity | 200 |
| Rats displaying stress-evoked self-grooming |
Reduced activity | 157 |
| Acute memantine | ||
| Astrocyte-specific inducible Glt1−/− mice |
Over-grooming and body lesions corrected | 85 |
| Mice prenatally exposed to valproate |
Reduced over-grooming | 201 |
| Acute MPEP | ||
| BTBR mouse strain | Reduced activity | 90 |
| Acute risperidone | ||
| BTBR mouse strain | Reduced activity | 90 |
| Acute diazepam* | ||
| Wild-type mice and rats | Reduced activity, normalized patterning during novelty-based tests |
32,76 |
| Acute clonazepam* | ||
| Wild-type mice and rats | Reduced activity, normalized patterning during novelty-based tests |
32,76 |
Glt1, excitatory amino acid transporter 2; MPEP, methyl-6-phenylethynyl-pyridine; Sapap3, SAP90/PSD-95-associated protein 3; Slitrk5, SLIT and NTRK-like family, member 5.
Benzodiazepines in general can be used as augmentation agents for nonspecific anxiolytic treatment in anti-obsessive compulsive disorder pharmacotherapy80.
Mutations in SAPAP3, which encodes synapse-associated protein 90/postsynaptic density protein 95-associated protein 3, have been implicated, though only weakly, in OCD and self-grooming disorders, such as pathologic skin picking, nail biting and hair pulling122,137,138. SAPAP3 binds to SHANK3, another post-synaptic scaffolding protein that, as discussed above, is linked to ASD44. In rodents, SAPAP3 is primarily expressed in neurons in the striatum — a key brain region that is involved in the control of self-grooming. Sapap3−/−mice display robust increased self-grooming that is rescued by the re-expression of Sapap3 in the striatum40,139. Because Sapap3 is expressed in striatal glutamatergic synapses, these findings suggest that excitatory neurotransmission in this region is important for the regulation of normal self-grooming behaviour139. Interestingly, although Sapap3 deletion reduces corticostriatal synaptic transmission, it does not affect thalamostriatal activity139, providing an excellent opportunity to use the abnormal grooming phenotype of the Sapap3−/− mice to dissect the role of thalamostriatal versus corticostriatal circuits in mediating excessive repetitive behaviours in individuals with OCD. The over-grooming phenotype observed in the Sapap3−/− mice can be rescued by optogenetic stimulation of the corticostriatal pathway originating in the orbitofrontal cortex39,68,140,141. The mechanism underlying this rescue seems to involve striatal high-firing interneurons (that are impaired in this genetic mouse model), directly implicating intrastriatal network activity in the aetiology of the compulsive grooming behaviour. Furthermore, repeated daily stimulation of a nearby part of the orbitofrontal cortex in wild-type mice can evoke a prolonged increase in self-grooming behaviour15. These results emphasize the importance of corticostriatal circuits, and potentially intrastriatal microcircuits, in the control of self-grooming in rodents, which may also be relevant to modelling compulsions in individuals with OCD.
Tourette syndrome is another common, highly her-itable, childhood-onset neuropsychiatric disorder that is characterized by motor and phonic tics114,142,127. This syndrome is frequently comorbid with OCD and attention deficit hyperactivity disorder (ADHD), and can be accompanied by affective disorders, such as anxiety and depression92,143. Albeit related to OCD and grooming disorders (such as trichotillomania), Tourette syndrome differs from them genetically and phenotypically142,144–146. Owing to its complex repetitive nature, rodent self-grooming behaviour is a logical phenotype to investigate in putative models of Tourette syndrome29, especially given that the nigrostriatal dopaminergic system has been implicated in sequential stereotypy of behaviour, which manifests itself as inflexible actions or stereotyped ‘rigid’ thought in individuals with OCD, individuals with Tourette syndrome, or individuals with both OCD and Tourette syndrome. Therefore, rodents with abnormal dopaminergic signalling can be good candidates for modelling aspects of these disorders29,147.
Dopamine transporter (DAT)-deficient mice, which have elevated levels of dopamine, exhibit more stereotyped and predictable syntactic grooming sequences than their wild-type counterparts, with fewer disruptions of syntactic patterns and a sequential ‘super-stereotypy’ in the complex fixed-action patterns29. Dopamine receptor subtypes may mediate different effects of dopamine on self-grooming phenotypes8,148. Mutant mice that lack dopamine D1A receptors (TABLE 1) exhibit shorter self-grooming bouts and more disrupted, incomplete sequential patterns148. This phenotype suggests that dopamine D1A receptors can specifically modulate the sequencing of grooming behaviour in rodents, which supports findings from human studies suggesting that dopamine receptor subtypes have distinct roles in patients with Tourette syndrome and related basal ganglia disorders29. In addition, transgenic mice that express a form of cholera toxin that potentiates neurotransmission selectively within corticolimbic D1-expressing neurons (D1 neurons) exhibit elevated self-grooming, as well as various juvenile-onset tics, and so mimic aspects of comorbid OCD and Tourette syndrome149. Similarly, mutations in the histidine decarboxylase gene (HDC) have been implicated in Tourette syndrome, and Hdc−/− mice display tic-like behaviours that recapitulate certain aspects of Tourette syndrome, including stereotypic self-grooming150.
Rodents with impaired motor behaviour and impaired motor sequencing (such as pathologically reduced self-grooming) can also be useful for understanding basal ganglia disorders in general (TABLE 1). For example, the weaver (wv/wv) mouse possesses a naturally occurring mutation in the Girk2 gene that encodes a G protein-activated inwardly rectifying potassium ion channel30,31. This mutation markedly affects cerebellar and striatal pathways151–154 (crucial for motor performance), resulting in an aberrant self-grooming phenotype that consists of more frequent, but shorter, grooming bouts with smaller forelimb strokes and less complete sequences30,31. Because they display deficits in the two critical CNS circuits, the context- and age-specific neurological defects in wv/wv mice provide a useful tool for examining how the two systems control self-grooming during development155. For example, although the mutant mice initially spend less time self-grooming, after day 15 they initiate more frequent, briefer grooming bouts, which are more likely to be associated with striatal control of sequencing155. Taken together, these models illustrate the important role of the basal ganglia in the modulation of normal and pathological self-grooming behaviour in rodents, thereby potentially offering translational insights into human basal ganglia disorders.
Other disorders
It has long been known that acute stressors (for example, exposure to a novel environment or to predators) can potently modulate self-grooming, often increasing the frequency and/or total duration of bouts1,28,95,156,157 and inducing displacement activity3,4. In addition, stressors also result in disorganized grooming patterning7 by impairing cephalocaudal progression (for example, increasing incorrect transitions that do not follow this pattern), evoking more incomplete bouts, causing more interruptions in bouts of self-grooming, and by disrupting its regional distribution (changing how different parts of the body are being groomed)7,27,31,158. High chronic baseline anxiety in certain mouse and rat strains is often accompanied by increased self-grooming and disorganized patterning7,27,159, whereas anxiolytic treatments tend to reduce rodent self-grooming activity and normalize its sequential organization7,28,32,76. The neurobiological bases of the interaction between stress and self-grooming remain poorly understood, but the brain regions that are involved in affect, especially the amygdala, are likely to be involved (FIG. 2). As stress and anxiety seem to modulate rodent self-grooming, it is possible that abnormal grooming behaviour could be used as a measure of stress or anxiety in various experimental models and tests7.
Motor deficits are a feature of major neurodegener-ative disorders, and as a complex patterned behaviour, self-grooming is a logical candidate behaviour to assess such deficits in rodent models. Parkinson disease, which produces debilitating impoverishment of voluntary movement, is neuropathologically characterized by the presence of Lewy bodies, which are mostly composed of α-synuclein160. The A53T missense mutation in the α-synuclein gene is strongly implicated in the pathogenesis of parkinsonian states161. Transgenic mice that express the human A53T variant under the control of the mouse prion promoter display progressive motor and cognitive deficits, including impaired grooming that is observed as early as 1–2 months old160, before the onset of spatial memory deficits (at 6–12 months) or abnormal gait (at 12 months). Combined with aberrant synaptic neurotransmission, behavioural analyses of self-grooming in this mouse strain may therefore be useful for assessing novel therapeutic interventions for Parkinson disease160.
Several rodent models of other neurodegenerative diseases also display aberrant self-grooming phenotypes, including recently developed rodent models of Huntington disease162–167, familial Danish dementia168, Krabbe disease169 and other types of neurodegeneration170,171. Collectively, these studies illustrate the utility of self-grooming phenotypes for modelling various neurodegenerative disorders and dissecting their pathobiological mechanisms. Interestingly, aberrant hyper-grooming is observed in the early stages of the disease in a rat model of Huntington disease that is induced by the striatal injection of quinolinic acid164, and impaired grooming in A53T-mutant mice appears before parkinsonian-like cognitive or gait deficits160. These observations raise the possibility that altered self-grooming may represent an early behavioural hallmark162,169,172 in these disease models, although this remains to be tested.
Novel approaches and future directions
Recognizing the importance of neuromorphological endophenotypes related to brain disorders173,174, it is logical to apply similar approaches and imaging techniques to rodents with aberrant self-grooming phenotypes. For example, using functional MRI, decreased fronto-cortical, occipital and thalamic grey matter volume and decreased cortical thickness have been detected in hyper-grooming BTBR mice compared with a low-grooming control C57BL/6J mouse strain175. In addition, diffusion tensor tractography has confirmed callosal agenesis and impaired hippocampal commissure formation in BTBR mice, whereas resting-state brain activity using cerebral blood volume weighted fMRI revealed reduced corticothalamic function175.
Given the complexity and polygenic nature of most brain disorders, research is increasingly focused on identifying sets of genes that contribute to several CNS dis-orders176. For example, although repetitive behaviours (including self-grooming) and increased anxiety are both observed in OCD177,178, most clinical and animal studies examine the genetic and physiological correlates of these two behavioural domains separately179–181 (see also REFS 182,183). Applying large-scale bioinformatics and pathway analyses to complex behavioural endophenotypes and the interactions between them, rather than targeting only individual endophenotypes (for example, assessing genes that are related to pathologically increased self-grooming and the dysregulation of the neural circuits involved in controlling complex movements in a single study), can markedly enrich the landscape of genes related to neuropsychiatric disorders184, including those involved in the regulation of self-grooming-related behaviours.
Optogenetic manipulations are a useful tool for understanding the circuits that are involved in rodent self-grooming. As noted above, repeated (but not acute) stimulation of the medial orbitofrontal cortex-ventromedial striatum pathway in mice can trigger pathological self-grooming that lasts for weeks, a condition that can be reversed by the chronic administration of the selective serotonin reuptake inhibitor fluoxetine15. Stimulating the nearby orbitofrontal cortex (or its intrastriatal terminals) can block compulsive self-grooming in Sapap3−/− mice39. These findings are important, as they provide strong experimental evidence for circuit-level control of repetitive episodes of grooming. Optogenetic approaches to modulate grooming cannot yet be translated to the clinic, but these studies suggest that future circuit modulation methods may become valuable therapeutic tools in the treatment of disorders that are associated with repetitive behaviour39.
In-depth analyses of self-grooming behaviour are now an important part of behavioural phenomics (BOX 1). Several automated tools are currently available for both quantity-based and patterning-based studies of grooming phenotypes in laboratory rodents (see also REFS 95,185; Supplementary information S5 (figure)), and their future refinement is expected to contribute to progress in this field.
Box 1 | Behavioural phenomics and high-throughput analyses of grooming.
Several problems are often associated with rodent behavioural tests: they are highly time-, space- and labour-consuming, they may be expensive and they are low-medium-throughput in nature95,189. Some rodent behaviours also require a prolonged testing time to emerge, whereas others necessitate special conditions (for example, homecage testing) and/or long-term assessment to be detected. Furthermore, the behavioural response to novel drugs or genetic mutations may spontaneously appear when the animals are not being observed. These issues are particularly problematic when studying complex behaviours, such as grooming, which involves movements of multiple body points with elaborate spatiotemporal organization. However, recent advances in behavioural phenotyping have provided some timely and efficient solutions to empower grooming research. Behavioural phenomics is a rapidly developing field that merges phenomics and neuroscience, and links behavioural phenotypes to genetic and environmental factors189,190. For example, automated tools that record force, vibration or visual signals have recently been developed to allow non-invasive assessment of self-grooming that can be implemented without prior training of animals to evaluate grooming in different experimental conditions (Supplementary information S5 (figure)). Currently, such analyses cannot assess all the stages of self-grooming but can be markedly improved by using multiple cameras, three-dimensional spatial imaging of multiple body points and increased IT-based signal integration. Even more powerful tools for analysing grooming activity are likely to become available soon. For example, systems that allow the detection and integration of several different behavioural signals simultaneously (such as vibration and image) have already improved the phenotyping of rodent self-grooming95. As better signal detection and behaviour recognition capabilities continue to improve the automated analyses of grooming and affiliated behaviours, this may lead to the increased use of self-grooming analyses in high-throughput phenotyping131. A typical self-grooming patterning analysis, which previously took several days and two or three investigators to complete, can now be carried out much faster using these new technologies131,191 (Supplementary information S5 (figure)).
Given the established role of dopamine-containing neurons in movement initiation and sequencing, the regulation (and dysregulation) of the dopaminergic system in numerous brain disorders will be of particular interest for further study8,148. For example, future research may examine larger networks of molecular interactors that are related to dopaminergic genes (genes encoding proteins that directly control dopamine signalling and metabolism, cytoskeletal processes, synaptic release, Ca2+, adenosine, and glutamatergic and GABA signalling), evaluate the role of these genes in rodent self-grooming behaviour, and relate these findings to the genes that have been implicated in human brain disorders116.
Studies in rodents (including studies of rats treated with D1 agonists, mice with increased neurotransmission in the D1 circuit and DAT-deficient mice) have also shown that the activation of D1-expressing neural circuits results in the generation of excessively stereotyped, but sequentially complex, grooming patterns. This suggests that the direct output circuits of the basal ganglia are particularly important in compulsive behavioural patterns related to serial perseveration and sequential rigidity42,67–70,186. It is conceivable that basal ganglia circuitry, which is evolutionarily embedded in the control of mammalian self-grooming, could also contribute to the content of pathological human super-stereotypies. For example, in humans, mesocorticostriatal disorders that result in washing rituals or self-purification compulsions that aim to escape from perceived contamination may share similar mechanisms to self-grooming in rodents.
Conclusions
The study of rodent self-grooming offers researchers important insights into how complex behaviours are regulated by the brain under normal circumstances and how they are affected in pathological conditions (FIG. 3). Therefore, understanding the neural circuitry, genetic determinants and associated molecular pathways that are involved in rodent self-grooming can facilitate better understanding of neurological disorders in which repetitive behaviours are expressed. It is also possible that the brain circuitry that originally evolved to control the sequence and coordination of self-grooming as an instinctive behaviour could have been utilized throughout human evolution and cultural expansion, to extend to ritualistic behaviours, cognitive functions, and even linguistic syntax and serially ordered streams of thought123,187,188. Although this speculation remains untested, it is already clear that studies of rodent self-grooming are likely to have implications that extend beyond the motor aspects of grooming, to include the sequential control of complex behaviours in general.
Supplementary Material
Acknowledgments
This Review is a tribute to John C. Fentress (1939–2015), a brilliant scientist, good friend and a true pioneer of ethology and neurobiology research. This study is supported by the ZENEREI Research Center (A.V.K., A.M.S.), Guangdong Ocean University (A.V.K., C.S.), St. Petersburg State University grant 1.38.201.2014 (A.V.K.), as well as by the US National Institutes of Health grants NS025529, HD028341, MH060379 (A.M.G.) and MH63649, DA015188 (K.B.). A.V.K. research is supported by the Government of Russian Federation (Act 211, contract 02.A03.21.0006 with Ural Federal University). The authors thank M. Nguyen, E. J. Kyzar and Y. Kubota for their assistance with this manuscript. They wish to acknowledge helpful suggestions from D. J. Anderson (California Institute of Technology, USA) regarding the roles of amygdala-related circuitry in grooming behaviour. The authors also thank manufacturers of neurophenotyping tools for providing information used in Supplementary information S5 (figure).
Glossary
- Cephalocaudal progression
A general direction (or rule) of rodent self-grooming behaviour that begins at the nose, then continues to the face and head, the body, the tail and the genitals.
- Grooming microstructure
The complex sequential organization (patterning) of self-grooming movements.
- Fixed-action patterns
Instinctive species-specific behavioural sequences that, once begun, run to their completion.
- Basal ganglia
A group of subcortical nuclei involved in motor control, motivation and organizing movements into behavioural sequences.
- Ventral tegmental area
A midbrain region (implicated in reward, anxiety and aversion) that contains the dopaminergic cell bodies of the mesocorticolimbic system.
- Research domain criteria (RDoC)
A strategy in translational mental health research that aims to explore the basic mechanisms of brain deficits to understand symptom sets that are observed across multiple disorders.
- Behavioural perseveration
The repetition of a specific behaviour that becomes inappropriate in the absence of behaviour-evoking stimuli.
- Stereotypies
Repetitive behaviours involving an abnormal or excessive repetition of a behavioural action in the same way over time.
- Tics
Sudden, repetitive, involuntary movements or vocalizations with varying intensity and frequency.
- Displacement
Behaviour that is seemingly irrelevant to the context, which is displayed during a conflict of motivations or when the animal is unable to perform an activity for which it is motivated.
- Krabbe disease
(Also known as globoid cell leukodystrophy). A rare, fatal neurodegenerative disorder that is due to genetic defect causing aberrant brain myelination.
Footnotes
Competing interests
The authors declare no competing interests.
DATABASES
Simons Foundation Autism Research Initiative gene database: http://gene.sfari.org/autdb/Welcome.do
SUPPLEMENTARY INFORMATION
See online article: S1 (movie) | S2 (movie) | S3 (table) | S4 (table) | S5 (figure)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Spruijt BM, Welbergen P, Brakkee J, Gispen WH. An ethological analysis of excessive grooming in young and aged rats. Ann. NY Acad. Sci. 1988;525:89–100. doi: 10.1111/j.1749-6632.1988.tb38598.x. [DOI] [PubMed] [Google Scholar]
- 2. Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol. Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. This article provides an excellent introduction to the neurobiology of grooming behaviour.
- 3.Fentress JC. Interrupted ongoing behaviour in two species of vole (Microtus agrestis and Clethrionomys britannicus). II. Extended analysis of motivational variables underlying fleeing and grooming behaviour. Anim. Behav. 1968;16:154–167. doi: 10.1016/0003-3472(68)90125-5. [DOI] [PubMed] [Google Scholar]
- 4.Fentress JC. Interrupted ongoing behaviour in two species of vole (Microtus agrestis and Clethrionomys britannicus). I. Response as a function of preceding activity and the context of an apparently “irrelevant” motor pattern. Anim. Behav. 1968;16:135–153. doi: 10.1016/0003-3472(68)90124-3. [DOI] [PubMed] [Google Scholar]
- 5.Leonard ST, Alizadeh-Naderi R, Stokes K, Ferkin MH. The role of prolactin and testosterone in mediating seasonal differences in the self-grooming behavior of male meadow voles, Microtus pennsylvanicus. Physiol. Behav. 2005;85:461–468. doi: 10.1016/j.physbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 6. Kalueff A, LaPorte JL, Bergner C. Neurobiology of Grooming Behavior. Cambridge Univ. Press; 2010. This book provides a comprehensive overview of animal self-grooming and its relevance to human behaviours.
- 7.Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat. Protoc. 2007;2:2538–2544. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- 8.Berridge KC, Aldridge JW. Super-stereotypy II: enhancement of a complex movement sequence by intraventricular dopamine D1 agonists. Synapse. 2000;37:205–215. doi: 10.1002/1098-2396(20000901)37:3<205::AID-SYN4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9. Berridge KC, Fentress JC, Parr H. Natural syntax rules control action sequence of rats. Behav. Brain Res. 1987;23:59–68. doi: 10.1016/0166-4328(87)90242-7. An important description of natural sequence ‘syntax’ in rodent grooming.
- 10.Golani I, Fentress JC. Early ontogeny of face grooming in mice. Dev. Psychobiol. 1985;18:529–544. doi: 10.1002/dev.420180609. [DOI] [PubMed] [Google Scholar]
- 11.Spruijt BM, Gispen WH. Behavioral sequences as an easily quantifiable parameter in experimental studies. Physiol. Behav. 1985;32:707–710. doi: 10.1016/0031-9384(84)90182-3. [DOI] [PubMed] [Google Scholar]
- 12.Prokop P, Fancovicova J, Fedor P. Parasites enhance self-grooming behaviour and information retention in humans. Behav. Processes. 2014;107:42–46. doi: 10.1016/j.beproc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Mansfield J, Jensen B. Dressing and grooming: preferences of community-dwelling older adults. J. Gerontol. Nurs. 2007;33:31–39. doi: 10.3928/00989134-20070201-07. [DOI] [PubMed] [Google Scholar]
- 14.Roth A, et al. Potential translational targets revealed by linking mouse grooming behavioral phenotypes to gene expression using public databases. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;40:312–325. doi: 10.1016/j.pnpbp.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahmari SE, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. An important study that demonstrated that chronic optogenetic stimulation of the cortico-striatal pathway induces repetitive OCD-like grooming behaviour in mice.
- 16. Schmeisser MJ, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. A critical, authoritative evaluation of the behaviour of mice with mutations in Shank genes, which suggests that these genes have a role in psychiatric diseases.
- 17.Chen SK, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shmelkov SV, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat. Med. 2010;16:598–602. doi: 10.1038/nm.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amodeo DA, Yi J, Sweeney JA, Ragozzino ME. Oxotremorine treatment reduces repetitive behaviors in BTBR T+ tf/J mice. Front. Synaptic Neurosci. 2014;6:17. doi: 10.3389/fnsyn.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moy SS, et al. Repetitive behavior profile and supersensitivity to amphetamine in the C58/J mouse model of autism. Behav. Brain Res. 2014;259:200–214. doi: 10.1016/j.bbr.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sungur AO, Vorckel KJ, Schwarting RK, Wohr M. Repetitive behaviors in the Shank1 knockout mouse model for autism spectrum disorder: developmental aspects and effects of social context. J. Neurosci. Methods. 2014;234:92–100. doi: 10.1016/j.jneumeth.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Greco B, et al. Autism-related behavioral abnormalities in synapsin knockout mice. Behav. Brain Res. 2013;251:65–74. doi: 10.1016/j.bbr.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu P, et al. Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proc. Natl Acad. Sci. USA. 2013;110:10759–10764. doi: 10.1073/pnas.1308195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33:1–2. doi: 10.1016/s0896-6273(01)00575-x. [DOI] [PubMed] [Google Scholar]
- 25.Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- 26.Fentress JC. Expressive contexts, fine structure, and central mediation of rodent grooming. Ann. NY Acad. Sci. 1988;525:18–26. doi: 10.1111/j.1749-6632.1988.tb38592.x. [DOI] [PubMed] [Google Scholar]
- 27.Kalueff AV, Tuohimaa P. Contrasting grooming phenotypes in C57Bl/6 and 129S1/SvImJ mice. Brain Res. 2004;1028:75–82. doi: 10.1016/j.brainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Kalueff AV, Tuohimaa P. Grooming analysis algorithm for neurobehavioural stress research. Brain Res. Protoc. 2004;13:151–158. doi: 10.1016/j.brainresprot.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Berridge KC, Aldridge JW, Houchard KR, Zhuang X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: a model of obsessive compulsive disorder and Tourette’s. BMC Biol. 2005;3:4. doi: 10.1186/1741-7007-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalueff AV, Tuohimaa P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J. Neurosci. Methods. 2005;143:169–177. doi: 10.1016/j.jneumeth.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Kalueff AV, Lou YR, Laaksi I, Tuohimaa P. Increased grooming behavior in mice lacking vitamin D receptors. Physiol. Behav. 2004;82:405–409. doi: 10.1016/j.physbeh.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Kalueff AV, Tuohimaa P. Mouse grooming microstructure is a reliable anxiety marker bidirectionally sensitive to GABAergic drugs. Eur. J. Pharmacol. 2005;508:147–153. doi: 10.1016/j.ejphar.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds S, Urruela M, Devine DP. Effects of environmental enrichment on repetitive behaviors in the BTBR T+tf/J mouse model of autism. Autism Res. 2013;6:337–343. doi: 10.1002/aur.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J. Neurosci. 1996;16:3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. This study provided a thorough dissection of the neural circuitry underlying rodent grooming using striatal lesions.
- 35. Berridge KC. Progressive degradation of serial grooming chains by descending decerebration. Behav. Brain Res. 1989;33:241–253. doi: 10.1016/s0166-4328(89)80119-6. This study used progressive decerebration in rodents to show that rodent grooming is controlled by brain regions in a hierarchical manner.
- 36.Berntson GG, Jang JF, Ronca AE. Brainstem systems and grooming behaviors. Ann. NY Acad. Sci. 1988;525:350–362. doi: 10.1111/j.1749-6632.1988.tb38619.x. [DOI] [PubMed] [Google Scholar]
- 37.Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat. Neurosci. 2014;17:423–430. doi: 10.1038/nn.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friend DM, Kravitz AV. Working together: basal ganglia pathways in action selection. Trends Neurosci. 2014;37:301–303. doi: 10.1016/j.tins.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. An important study showing that pathological OCD-like grooming in Sapap3-mutant mice can be corrected by optogenetic stimulation of orbitostriatal pathways, including the lateral orbitofrontal cortex and its terminals in the striatum.
- 40.Welch JM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aldridge JW, Berridge KC, Rosen AR. Basal ganglia neural mechanisms of natural movement sequences. Can. J. Physiol. Pharmacol. 2004;82:732–739. doi: 10.1139/y04-061. A detailed summary of the role of the basal ganglia in the sequencing of complex behaviours such as self-grooming.
- 42. Graybiel AM. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. This article discusses an important conceptual argument linking habits and rituals to normal and pathological behaviours, with a useful expert discussion of their respective underlying circuits.
- 43.Graybiel AM, Grafton ST. The striatum: where skills and habits meet. Cold Spring Harb. Perspect. Biol. 2015;7:a021691. doi: 10.1101/cshperspect.a021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berridge KC, Whishaw IQ. Cortex, striatum and cerebellum: control of serial order in a grooming sequence. Exp. Brain Res. 1992;90:275–290. doi: 10.1007/BF00227239. A comprehensive summary of the role of cortical, striatal and cerebellar structures in controlling the sequencing and motor activity underlying rodent grooming.
- 45.Watson PJ. Behavior maintained by electrical stimulation of the rat cerebellum. Physiol. Behav. 1978;21:749–755. doi: 10.1016/0031-9384(78)90014-8. [DOI] [PubMed] [Google Scholar]
- 46.Strazielle C, Lalonde R. Grooming in Lurcher mutant mice. Physiol. Behav. 1998;64:57–61. doi: 10.1016/s0031-9384(98)00014-6. [DOI] [PubMed] [Google Scholar]
- 47.Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158:1348–1361. doi: 10.1016/j.cell.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Homberg JR, et al. Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Eur. J. Neurosci. 2002;15:1542–1550. doi: 10.1046/j.1460-9568.2002.01976.x. [DOI] [PubMed] [Google Scholar]
- 49.Alo R, Avolio E, Mele M, Di Vito A, Canonaco M. Central amygdalar nucleus treated with orexin neuropeptides evoke differing feeding and grooming responses in the hamster. J. Neurol. Sci. 2015;351:46–51. doi: 10.1016/j.jns.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 50. Obeso JA, Lanciego JL. Past, present, and future of the pathophysiological model of the basal ganglia. Front. Neuroanat. 2011;5:39. doi: 10.3389/fnana.2011.00039. A comprehensive review of basal ganglia circuitry and functions in the brain, with a particular focus on complex patterned behaviours.
- 51.Friedman A, et al. A corticostriatal path targeting striosomes controls decision-making under conflict. Cell. 2015;161:1320–1333. doi: 10.1016/j.cell.2015.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roeling TA, Veening JG, Peters JP, Vermelis ME, Nieuwenhuys R. Efferent connections of the hypothalamic “grooming area” in the rat. Neuroscience. 1993;56:199–225. doi: 10.1016/0306-4522(93)90574-y. [DOI] [PubMed] [Google Scholar]
- 53.Heimer L, Van Hoesen GW, Trimble M, Zahm DS. Anatomy of Neuropsychiatry: The New Anatomy of the Basal Forebrain and Its Implications for Neuropsychiatric Illness. Academic Press; 2007. [Google Scholar]
- 54.Swanson LW. Quest for the basic plan of nervous system circuitry. Brain Res. Rev. 2007;55:356–372. doi: 10.1016/j.brainresrev.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roseberry AG, Stuhrman K, Dunigan AI. Regulation of the mesocorticolimbic and mesostriatal dopamine systems by alpha-melanocyte stimulating hormone and agouti-related protein. Neurosci. Biobehav Rev. 2015;56:15–25. doi: 10.1016/j.neubiorev.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Dunn AJ. Studies on the neurochemical mechanisms and significance of ACTH-induced grooming. Ann. NY Acad. Sci. 1988;525:150–168. doi: 10.1111/j.1749-6632.1988.tb38603.x. [DOI] [PubMed] [Google Scholar]
- 57.Dunn AJ, Green EJ, Isaacson RL. Intracerebral adrenocorticotropic hormone mediates novelty-induced grooming in the rat. Science. 1979;203:281–283. doi: 10.1126/science.216073. [DOI] [PubMed] [Google Scholar]
- 58.Dunn AJ, Berridge CW, Lai YI, Yachabach TL. CRF-induced excessive grooming behavior in rats and mice. Peptides. 1987;8:841–844. doi: 10.1016/0196-9781(87)90069-6. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto M, Nagawa Y. Mesolimbic involvement in the locomotor stimulant action of thyrotropin-releasing hormone (TRH) in rats. Eur. J. Pharmacol. 1977;44:143–152. doi: 10.1016/0014-2999(77)90100-5. [DOI] [PubMed] [Google Scholar]
- 60.Gargiulo PA. Thyrotropin releasing hormone injected into the nucleus accumbens septi selectively increases face grooming in rats. Braz. J. Med. Biol. Res. 1996;29:805–810. [PubMed] [Google Scholar]
- 61.Sanchez MS, Barontini M, Armando I, Celis ME. Correlation of increased grooming behavior and motor activity with alterations in nigrostriatal and mesolimbic catecholamines after alpha-melanotropin and neuropeptide glutamine-isoleucine injection in the rat ventral tegmental area. Cell. Mol. Neurobiol. 2001;21:523–533. doi: 10.1023/A:1013871407464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kruk MR, et al. The hypothalamus: cross-roads of endocrine and behavioural regulation in grooming and aggression. Neurosci. Biobehav. Rev. 1998;23:163–177. doi: 10.1016/s0149-7634(98)00018-9. A detailed review of the ‘dual’ role of the hypothalamus in the neural and endocrine regulation of grooming behaviours.
- 63.Berridge KC, Aldridge JW. Super-stereotypy I: enhancement of a complex movement sequence by systemic dopamine D1 agonists. Synapse. 2000;37:194–204. doi: 10.1002/1098-2396(20000901)37:3<194::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 64.Taylor JL, Rajbhandari AK, Berridge KC, Aldridge JW. Dopamine receptor modulation of repetitive grooming actions in the rat: potential relevance for Tourette syndrome. Brain Res. 2010;1322:92–101. doi: 10.1016/j.brainres.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frederick AL, et al. Evidence against dopamine D1/D2 receptor heteromers. Mol. Psychiatry. 2015;20:1373–1385. doi: 10.1038/mp.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graybiel AM, Ragsdale CW., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc. Natl Acad. Sci. USA. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crittenden JR, Graybiel AM. Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front. Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Burguiere E, Monteiro P, Mallet L, Feng G, Graybiel AM. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr. Opin. Neurobiol. 2015;30:59–65. doi: 10.1016/j.conb.2014.08.008. An excellent summary of striatal mechanisms in the formation of normal and pathological habits, with a particular reference to the pathogenesis of OCD.
- 69.Canales JJ, Graybiel AM. Patterns of gene expression and behavior induced by chronic dopamine treatments. Ann. Neurol. 2000;47:S53–S59. [PubMed] [Google Scholar]
- 70.Saka E, Goodrich C, Harlan P, Madras BK, Graybiel AM. Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J. Neurosci. 2004;24:7557–7565. doi: 10.1523/JNEUROSCI.1072-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodman WK, Grice DE, Lapidus KA, Coffey BJ. Obsessive-compulsive disorder. Psychiatr. Clin. North Am. 2014;37:257–267. doi: 10.1016/j.psc.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Audet MC, Goulet S, Dore FY. Repeated subchronic exposure to phencyclidine elicits excessive atypical grooming in rats. Behav. Brain Res. 2006;167:103–110. doi: 10.1016/j.bbr.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 73.Williams HJ, Zamzow CR, Robertson H, Dursun SM. Effects of clozapine plus lamotrigine on phencyclidine-induced hyperactivity. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:239–243. doi: 10.1016/j.pnpbp.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Abekawa T, Honda M, Ito K, Koyama T. Effects of NRA0045, a novel potent antagonist at dopamine D4, 5-HT2A, and α1 adrenaline receptors, and NRA0160, a selective D4 receptor antagonist, on phencyclidine-induced behavior and glutamate release in rats. Psychopharmacology (Berl.) 2003;169:247–256. doi: 10.1007/s00213-003-1517-8. [DOI] [PubMed] [Google Scholar]
- 75.Iwamoto ET. An assessment of the spontaneous activity of rats administered morphine, phencyclidine, or nicotine using automated and observational methods. Psychopharmacology (Berl.) 1984;84:374–382. doi: 10.1007/BF00555216. [DOI] [PubMed] [Google Scholar]
- 76.Nin MS, et al. Anxiolytic effect of clonazepam in female rats: grooming microstructure and elevated plus maze tests. Eur. J. Pharmacol. 2012;684:95–101. doi: 10.1016/j.ejphar.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 77.Barros HM, Tannhauser SL, Tannhauser MA, Tannhauser M. The effects of GABAergic drugs on grooming behaviour in the open field. Pharmacol. Toxicol. 1994;74:339–344. doi: 10.1111/j.1600-0773.1994.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 78.Dunn AJ, Guild AL, Kramarcy NR, Ware MD. Benzodiazepines decrease grooming in response to novelty but not ACTH or p-endorphin. Pharmacol. Biochem. Behav. 1981;15:605–608. doi: 10.1016/0091-3057(81)90217-3. [DOI] [PubMed] [Google Scholar]
- 79.Kalueff A, Nutt DJ. Role of GABA in memory and anxiety. Depress. Anxiety. 1996;4:100–110. doi: 10.1002/(SICI)1520-6394(1996)4:3<100::AID-DA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 80.Leonard HL, et al. Clonazepam as an augmenting agent in the treatment of childhood-onset obsessive-compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1994;33:792–794. doi: 10.1097/00004583-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Xu HY, et al. Inactivation of the bed nucleus of the stria terminalis suppresses the innate fear responses of rats induced by the odor of cat urine. Neuroscience. 2012;221:21–27. doi: 10.1016/j.neuroscience.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 82.Denmark A, et al. The effects of chronic social defeat stress on mouse self-grooming behavior and its patterning. Behav. Brain Res. 2010;208:553–559. doi: 10.1016/j.bbr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 83.Nin MS, et al. The effect of intra-nucleus accumbens administration of allopregnanolone on 5 and y2 GABAA receptor subunit mRNA expression in the hippocampus and on depressive-like and grooming behaviors in rats. Pharmacol. Biochem. Behav. 2012;103:359–366. doi: 10.1016/j.pbb.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 84.De Barioglio SR, Lezcano N, Celis ME. Alpha MSH-induced excessive grooming behavior involves a GABAergic mechanism. Peptides. 1991;12:203–205. doi: 10.1016/0196-9781(91)90189-v. [DOI] [PubMed] [Google Scholar]
- 85.Aida T, et al. Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology. 2015;40:1569–1579. doi: 10.1038/npp.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rapoport JL. The Boy Who Couldn’t Stop Washing: The Experience and Treatment of Obsessive-Compulsive Disorder. Penguin Publishing Group; 1989. An excellent description of aberrant grooming behaviours in individuals with OCD.
- 87.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am. J. Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 88.Casey BJ, et al. DSM-5 and RDoC: progress in psychiatry research? Nat. Rev. Neurosci. 2013;14:810–814. doi: 10.1038/nrn3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang M, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J. Neurosci. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. This study assessed social and repetitive behaviours that are relevant to autism in the BTBR mouse model and their pharmacological modulation.
- 91.Kas MJ, et al. Assessing behavioural and cognitive domains of autism spectrum disorders in rodents: current status and future perspectives. Psychopharmacology (Berl.) 2014;231:1125–1146. doi: 10.1007/s00213-013-3268-5. [DOI] [PubMed] [Google Scholar]
- 92.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. American Psychiatric Association; 2013. [Google Scholar]
- 93.Silverman JL, Crawley JN. The promising trajectory of autism therapeutics discovery. Drug Discov. Today. 2014;19:838–844. doi: 10.1016/j.drudis.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Pearson BL, et al. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brodkin J, et al. Validation and implementation of a novel high-throughput behavioral phenotyping instrument for mice. J. Neurosci. Methods. 2014;224:48–57. doi: 10.1016/j.jneumeth.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deutsch SI, Urbano MR, Neumann SA, Burket JA, Katz E. Cholinergic abnormalities in autism: is there a rationale for selective nicotinic agonist interventions? Clin. Neuropharmacol. 2010;33:114–120. doi: 10.1097/WNF.0b013e3181d6f7ad. [DOI] [PubMed] [Google Scholar]
- 98.Perry EK, et al. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am. J. Psychiatry. 2001;158:1058–1066. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- 99.Van Schalkwyk GI, et al. Reduction of aggressive episodes after repeated transdermal nicotine administration in a hospitalized adolescent with autism spectrum disorder. J. Autism Dev. Disord. 2015;45:3061–3066. doi: 10.1007/s10803-015-2471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karvat G, Kimchi T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology. 2014;39:831–840. doi: 10.1038/npp.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silverman JL, et al. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci. Transl Med. 2012;4:131ra51. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burket JA, Benson AD, Tang AH, Deutsch SI. D-Cycloserine improves sociability in the BTBR T+ Itpr3tf/J mouse model of autism spectrum disorders with altered Ras/Raf/ERK1/2 signaling. Brain Res. Bull. 2013;96:62–70. doi: 10.1016/j.brainresbull.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Urbano M, et al. A trial of D-cycloserine to treat stereotypies in older adolescents and young adults with autism spectrum disorder. Clin. Neuropharmacol. 2014;37:69–72. doi: 10.1097/WNF.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Posey DJ, et al. A pilot study of D-cycloserine in subjects with autistic disorder. Am. J. Psychiatry. 2004;161:2115–2117. doi: 10.1176/appi.ajp.161.11.2115. [DOI] [PubMed] [Google Scholar]
- 105.Insel TR, Freund M. Shedding light on brain circuits. Biol. Psychiatry. 2012;71:1028–1029. doi: 10.1016/j.biopsych.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 106.Kas MJ, Modi ME, Saxe MD, Smith DG. Advancing the discovery of medications for autism spectrum disorder using new technologies to reveal social brain circuitry in rodents. Psychopharmacolology (Berl.) 2014;231:1147–1165. doi: 10.1007/s00213-014-3464-y. [DOI] [PubMed] [Google Scholar]
- 107.Ellegood J, et al. Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol. Psychiatry. 2015;20:118–125. doi: 10.1038/mp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Portmann T, et al. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell Rep. 2014;7:1077–1092. doi: 10.1016/j.celrep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bruining H, et al. Behavioral signatures related to genetic disorders in autism. Mol. Autism. 2014;5:11. doi: 10.1186/2040-2392-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Guilmatre A, Huguet G, Delorme R, Bourgeron T. The emerging role of SHANK genes in neuropsychiatric disorders. Dev. Neurobiol. 2014;74:113–122. doi: 10.1002/dneu.22128. A rigorous analysis of the association between mutations in SHANK genes and brain disorders with a particular focus on the genetics of repetitive behaviours.
- 111.Schmeisser MJ. Translational neurobiology in Shank mutant mice — model systems for neuropsychiatric disorders. Ann. Anat. 2015;200:115–117. doi: 10.1016/j.aanat.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 112.Won H, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 113.Peca J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum. Mol. Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wurzman R, Forcelli PA, Griffey CJ, Kromer LF. Repetitive grooming and sensorimotor abnormalities in an ephrin-A knockout model for autism spectrum disorders. Behav. Brain Res. 2014;278:115–128. doi: 10.1016/j.bbr.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen M, et al. Decoding the contribution of dopaminergic genes and pathways to autism spectrum disorder (ASD) Neurochem. Int. 2014;66:15–26. doi: 10.1016/j.neuint.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 117.Stewart AM, Nguyen M, Wong K, Poudel MK, Kalueff AV. Developing zebrafish models of autism spectrum disorder (ASD) Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;50:27–36. doi: 10.1016/j.pnpbp.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 118.Zhang K, Hill K, Labak S, Blatt GJ, Soghomonian JJ. Loss of glutamic acid decarboxylase (Gad67) in Gpr88-expressing neurons induces learning and social behavior deficits in mice. Neuroscience. 2014;275:238–247. doi: 10.1016/j.neuroscience.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 119.Blumenthal I, et al. Transcriptional consequences of 16p11.2 deletion and duplication in mouse cortex and multiplex autism families. Am. J. Hum. Genet. 2014;94:870–883. doi: 10.1016/j.ajhg.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maillard AM, et al. The 16p11.2 locus modulates brain structures common to autism, schizophrenia and obesity. Mol. Psychiatry. 2015;20:140–147. doi: 10.1038/mp.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moreno-De-Luca A, et al. The role of parental cognitive, behavioral, and motor profiles in clinical variability in individuals with chromosome 16p11.2 deletions. JAMA Psychiatry. 2015;72:119–126. doi: 10.1001/jamapsychiatry.2014.2147. [DOI] [PubMed] [Google Scholar]
- 122.Bienvenu OJ, et al. Sapap3 and pathological grooming in humans: results from the OCD collaborative genetics study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;150B:710–720. doi: 10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 124.Bokor G, Anderson PD. Obsessive-compulsive disorder. J. Pharm. Pract. 2014;27:116–130. doi: 10.1177/0897190014521996. [DOI] [PubMed] [Google Scholar]
- 125.Murphy DL, Timpano KR, Wheaton MG, Greenberg BD, Miguel EC. Obsessive-compulsive disorder and its related disorders: a reappraisal of obsessive-compulsive spectrum concepts. Dialogues Clin. Neurosci. 2010;12:131–148. doi: 10.31887/DCNS.2010.12.2/dmurphy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kalueff AV, Minasyan A, Keisala T, Shah ZH, Tuohimaa P. Hair barbering in mice: implications for neurobehavioural research. Behav. Processes. 2006;71:8–15. doi: 10.1016/j.beproc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 127.Garner JP, et al. Reverse-translational biomarker validation of abnormal repetitive behaviors in mice: an illustration of the 4P’s modeling approach. Behav. Brain Res. 2011;219:189–196. doi: 10.1016/j.bbr.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garner JP, Weisker SM, Dufour B, Mench JA. Barbering (fur and whisker trimming) by laboratory mice as a model of human trichotillomania and obsessive-compulsive spectrum disorders. Comp. Med. 2004;54:216–224. [PubMed] [Google Scholar]
- 129.Feusner JD, Hembacher E, Phillips KA. The mouse who couldn’t stop washing: pathologic grooming in animals and humans. CNS Spectr. 2009;14:503–513. doi: 10.1017/s1092852900023567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat. Rev. Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 131.Kyzar EJ, et al. Alterations in grooming activity and syntax in heterozygous SERT and BDNF knockout mice: the utility of behavior-recognition tools to characterize mutant mouse phenotypes. Brain Res. Bull. 2012;89:168–176. doi: 10.1016/j.brainresbull.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 132.Kalueff AV, Olivier JD, Nonkes LJ, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci. Biobehav. Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 133.Moya PR, et al. Common and rare alleles of the serotonin transporter gene, SLC6A4, associated with Tourette’s disorder. Mov. Disord. 2013;28:1263–1270. doi: 10.1002/mds.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murphy DL, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wendland JR, et al. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum. Mol. Genet. 2008;17:717–723. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
- 136.Voyiaziakis E, et al. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Mol. Psychiatry. 2011;16:108–120. doi: 10.1038/mp.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zuchner S, et al. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Mol. Psychiatry. 2009;14:6–9. doi: 10.1038/mp.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boardman L, et al. Investigating SAPAP3 variants in the etiology of obsessive-compulsive disorder and trichotillomania in the South African white population. Compr. Psychiatry. 2011;52:181–187. doi: 10.1016/j.comppsych.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 139. Wan Y, et al. Circuit-selective striatal synaptic dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder. Biol. Psychiatry. 2014;75:623–630. doi: 10.1016/j.biopsych.2013.01.008. This study identified the striatal mechanisms responsible for the behavioural phenotype in the Sapap3 −/− mouse.
- 140.Monteiro P, Feng G. Learning from animal models of obsessive-compulsive disorder. Biol. Psychiatry. 2016;79:7–16. doi: 10.1016/j.biopsych.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ahmari SE, Dougherty DD. Dissecting OCD circuits: from animal models to targeted treatments. Depress. Anxiety. 2015;32:550–562. doi: 10.1002/da.22367. This recent review provides an excellent summary of circuits involved in OCD pathogenesis in preclinical and translational models.
- 142.Yu D, et al. Cross-disorder genome-wide analyses suggest a complex genetic relationship between Tourette’s syndrome and OCD. Am. J. Psychiatry. 2015;172:82–93. doi: 10.1176/appi.ajp.2014.13101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Felling RJ, Singer HS. Neurobiology of tourette syndrome: current status and need for further investigation. J. Neurosci. 2011;31:12387–12395. doi: 10.1523/JNEUROSCI.0150-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Crane J, et al. Family-based genetic association study of DLGAP3 in Tourette Syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:108–114. doi: 10.1002/ajmg.b.31134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McGrath LM, et al. Copy number variation in obsessive-compulsive disorder and tourette syndrome: a cross-disorder study. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:910–919. doi: 10.1016/j.jaac.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Davis LK, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS Genet. 2013;9:e1003864. doi: 10.1371/journal.pgen.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Denys D, et al. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 2013;23:1423–1431. doi: 10.1016/j.euroneuro.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 148.Cromwell HC, Berridge KC, Drago J, Levine MS. Action sequencing is impaired in D1A–deficient mutant mice. Eur. J. Neurosci. 1998;10:2426–2432. doi: 10.1046/j.1460-9568.1998.00250.x. [DOI] [PubMed] [Google Scholar]
- 149.Nordstrom EJ, Burton FH. A transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder circuitry. Mol. Psychiatry. 2002;7:617–625. doi: 10.1038/sj.mp.4001144. [DOI] [PubMed] [Google Scholar]
- 150.Xu M, Li L, Ohtsu H, Pittenger C. Histidine decarboxylase knockout mice, a genetic model of Tourette syndrome, show repetitive grooming after induced fear. Neurosci. Lett. 2015;595:50–53. doi: 10.1016/j.neulet.2015.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roffler-Tarlov S, Martin B, Graybiel AM, Kauer JS. Cell death in the midbrain of the murine mutation weaver. J. Neurosci. 1996;16:1819–1826. doi: 10.1523/JNEUROSCI.16-05-01819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Graybiel AM, Ohta K, Roffler-Tarlov S. Patterns of cell fiber vulnerability in the mesostriatal system of the mutant mouse weaver. I. Gradients and compartments. J. Neurosci. 1990;10:720–733. doi: 10.1523/JNEUROSCI.10-03-00720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roffler-Tarlov S, Graybiel AM. Expression of the weaver gene in dopamine-containing neural systems is dose-dependent and affects both striatal and nonstriatal regions. J. Neurosci. 1986;6:3319–3330. doi: 10.1523/JNEUROSCI.06-11-03319.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Roffler-Tarlov S, Graybiel AM. Weaver mutation has differential effects on the dopamine-containing innervation of the limbic and nonlimbic striatum. Nature. 1984;307:62–66. doi: 10.1038/307062a0. [DOI] [PubMed] [Google Scholar]
- 155.Bolivar VJ, Danilchuk W, Fentress JC. Separation of activation and pattern in grooming development of weaver mice. Behav. Brain Res. 1996;75:49–58. doi: 10.1016/0166-4328(96)00156-8. [DOI] [PubMed] [Google Scholar]
- 156.Spruijt BM, Welbergen P, Brakkee J, Gispen WH. Behavioral changes in ACTH-(1–24)-induced excessive grooming in aging rats. Neurobiol. Aging. 1987;8:265–270. doi: 10.1016/0197-4580(87)90011-x. [DOI] [PubMed] [Google Scholar]
- 157.Rodriguez Echandia EL, Broitman ST, Foscolo MR. Effect of the chronic ingestion of chlorimipramine and desipramine on the hole board response to acute stresses in male rats. Pharmacol. Biochem. Behav. 1987;26:207–210. doi: 10.1016/0091-3057(87)90106-7. [DOI] [PubMed] [Google Scholar]
- 158.Kalueff AV, Lou YR, Laaksi I, Tuohimaa P. Abnormal behavioral organization of grooming in mice lacking the vitamin D receptor gene. J. Neurogenet. 2005;19:1–24. doi: 10.1080/01677060590949683. [DOI] [PubMed] [Google Scholar]
- 159.Estanislau C, et al. Context-dependent differences in grooming behavior among the NIH heterogeneous stock and the Roman high- and low-avoidance rats. Neurosci. Res. 2013;77:187–201. doi: 10.1016/j.neures.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 160.Paumier KL, et al. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson’s disease. PLoS ONE. 2013;8:e70274. doi: 10.1371/journal.pone.0070274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kasten M, Klein C. The many faces of α-synuclein mutations. Mov. Disord. 2013;28:697–701. doi: 10.1002/mds.25499. [DOI] [PubMed] [Google Scholar]
- 162.Steele AD, Jackson WS, King OD, Lindquist S. The power of automated high-resolution behavior analysis revealed by its application to mouse models of Huntington’s and prion diseases. Proc. Natl Acad. Sci. USA. 2007;104:1983–1988. doi: 10.1073/pnas.0610779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reddy PH, et al. Transgenic mice expressing mutated full-length HD cDNA: a paradigm for locomotor changes and selective neuronal loss in Huntington’s disease. Phil. Trans. R. Soc. Lond. B. 1999;354:1035–1045. doi: 10.1098/rstb.1999.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Scattoni ML, et al. Progressive behavioural changes in the spatial open-field in the quinolinic acid rat model of Huntington’s disease. Behav. Brain Res. 2004;152:375–383. doi: 10.1016/j.bbr.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 165.Dorner JL, Miller BR, Barton SJ, Brock TJ, Rebec GV. Sex differences in behavior and striatal ascorbate release in the 140 CAG knock-in mouse model of Huntington’s disease. Behav. Brain Res. 2007;178:90–97. doi: 10.1016/j.bbr.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Andre VM, et al. Differential electrophysiological changes in striatal output neurons in Huntington’s disease. J. Neurosci. 2011;31:1170–1182. doi: 10.1523/JNEUROSCI.3539-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hickey MA, Reynolds GP, Morton AJ. The role of dopamine in motor symptoms in the R6/2 transgenic mouse model of Huntington’s disease. J. Neurochem. 2002;81:46–59. doi: 10.1046/j.1471-4159.2002.00804.x. [DOI] [PubMed] [Google Scholar]
- 168.Vidal R, Barbeito AG, Miravalle L, Ghetti B. Cerebral amyloid angiopathy and parenchymal amyloid deposition in transgenic mice expressing the Danish mutant form of human BRI2. Brain Pathol. 2009;19:58–68. doi: 10.1111/j.1750-3639.2008.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Scruggs BA, et al. High-throughput screening of stem cell therapy for globoid cell leukodystrophy using automated neurophenotyping of twitcher mice. Behav. Brain Res. 2013;236:35–47. doi: 10.1016/j.bbr.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bubenikova-Valesova V, Balcar VJ, Tejkalova H, Langmeier M, St’astny F. Neonatal administration of N-acetyl-L-aspartyl-L-glutamate induces early neurodegeneration in hippocampus and alters behaviour in young adult rats. Neurochem. Int. 2006;48:515–522. doi: 10.1016/j.neuint.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 171.Glynn D, Drew CJ, Reim K, Brose N, Morton AJ. Profound ataxia in complexin I knockout mice masks a complex phenotype that includes exploratory and habituation deficits. Hum. Mol. Genet. 2005;14:2369–2385. doi: 10.1093/hmg/ddi239. [DOI] [PubMed] [Google Scholar]
- 172.Ferrante RJ. Mouse models of Huntington’s disease and methodological considerations for therapeutic trials. Biochim. Biophys. Acta. 2009;1792:506–520. doi: 10.1016/j.bbadis.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol. Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- 174.Prasad KM, Keshavan MS. Structural cerebral variations as useful endophenotypes in schizophrenia: do they help construct “extended endophenotypes”? Schizophr. Bull. 2008;34:774–790. doi: 10.1093/schbul/sbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dodero L, et al. Neuroimaging evidence of major morpho-anatomical and functional abnormalities in the BTBR T+TF/J mouse model of autism. PLoS ONE. 2013;8:e76655. doi: 10.1371/journal.pone.0076655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 177.Pigott TA, L’Heureux F, Dubbert B, Bernstein S, Murphy DL. Obsessive compulsive disorder: comorbid conditions. J. Clin. Psychiatry. 1994;55(Suppl):15–27. [PubMed] [Google Scholar]
- 178.Welkowitz LA, Struening EL, Pittman J, Guardino M, Welkowitz J. Obsessive-compulsive disorder and comorbid anxiety problems in a national anxiety screening sample. J. Anxiety Disord. 2000;14:471–482. doi: 10.1016/s0887-6185(00)00034-7. [DOI] [PubMed] [Google Scholar]
- 179.McGrath MJ, Campbell KM, Veldman MB, Burton FH. Anxiety in a transgenic mouse model of cortical-limbic neuro-potentiated compulsive behavior. Behav. Pharmacol. 1999;10:435–443. doi: 10.1097/00008877-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 180.Sturm V, et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J. Chem. Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 181.Schneier FR, et al. Striatal dopamine D2 receptor availability in OCD with and without comorbid social anxiety disorder: preliminary findings. Depress. Anxiety. 2008;25:1–7. doi: 10.1002/da.20268. [DOI] [PubMed] [Google Scholar]
- 182.Kalueff AV, Stewart AM. Modeling neuropsychiatric spectra to empower translational biological psychiatry. Behav. Brain Res. 2015;276:1–7. doi: 10.1016/j.bbr.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 183.Laporte JL, Ren-Patterson RF, Murphy DL, Kalueff AV. Refining psychiatric genetics: from ‘mouse psychiatry’ to understanding complex human disorders. Behav. Pharmacol. 2008;19:377–384. doi: 10.1097/FBP.0b013e32830dc09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stewart AM, Kalueff AV. Developing better and more valid animal models of brain disorders. Behav. Brain Res. 2015;276:28–31. doi: 10.1016/j.bbr.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 185.Xu M, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc. Natl Acad. Sci. USA. 2015;112:893–898. doi: 10.1073/pnas.1419533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 187.Lieberman P. Human Language and Our Reptilian Brain: The Subcortical Bases of Speech, Syntax, and Thought. Harvard Univ. Press; 2000. [DOI] [PubMed] [Google Scholar]
- 188.Graybiel AM. The basal ganglia and cognitive pattern generators. Schizophr. Bull. 1997;23:459–469. doi: 10.1093/schbul/23.3.459. [DOI] [PubMed] [Google Scholar]
- 189.Tecott LH, Nestler EJ. Neurobehavioral assessment in the information age. Nat. Neurosci. 2004;7:462–466. doi: 10.1038/nn1225. [DOI] [PubMed] [Google Scholar]
- 190.Kalueff AV, Wheaton M, Murphy DL. What’s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav. Brain Res. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 191. Kyzar E, et al. Towards high-throughput phenotyping of complex patterned behaviors in rodents: focus on mouse self-grooming and its sequencing. Behav. Brain Res. 2011;225:426–431. doi: 10.1016/j.bbr.2011.07.052. This paper describes the first successful application of automated behaviour-recognition protocols to quantify rodent self-grooming behaviours and their sequential microstructure, and emphasizes the value of grooming analyses in high-throughput rodent phenotyping.
- 192.Bortolato M, et al. Social deficits and perseverative behaviors, but not overt aggression, in MAO-A hypomorphic mice. Neuropsychopharmacology. 2011;36:2674–2688. doi: 10.1038/npp.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Escorihuela RM, et al. Inbred Roman high- and low-avoidance rats: differences in anxiety, novelty-seeking, and shuttlebox behaviors. Physiol. Behav. 1999;67:19–26. doi: 10.1016/s0031-9384(99)00064-5. [DOI] [PubMed] [Google Scholar]
- 194.Ferre P, et al. Behavior of the Roman/Verh high- and low-avoidance rat lines in anxiety tests: relationship with defecation and self-grooming. Physiol. Behav. 1995;58:1209–1213. doi: 10.1016/0031-9384(95)02068-3. [DOI] [PubMed] [Google Scholar]
- 195.Eguibar JR, Romero-Carbente JC, Moyaho A. Behavioral differences between selectively bred rats: D1 versus D2 receptors in yawning and grooming. Pharmacol. Biochem. Behav. 2003;74:827–832. doi: 10.1016/s0091-3057(02)01082-1. [DOI] [PubMed] [Google Scholar]
- 196.Eguibar JR, Moyaho A. Inhibition of grooming by pilocarpine differs in high- and low-yawning sublines of Sprague-Dawley rats. Pharmacol. Biochem. Behav. 1997;58:317–322. doi: 10.1016/s0091-3057(97)00108-1. [DOI] [PubMed] [Google Scholar]
- 197.Rossi-Arnaud C, Ammassari-Teule M. Modifications of open field and novelty behaviours by hippocampal and amygdaloid lesions in two inbred strains of mice: lack of strain × lesion interactions. Behav. Processes. 1992;27:155–164. doi: 10.1016/0376-6357(92)90171-9. [DOI] [PubMed] [Google Scholar]
- 198.Kalueff AV, Tuohimaa P. Contrasting grooming phenotypes in three mouse strains markedly different in anxiety and activity (129S1, BALB/c and NMRI) Behav. Brain Res. 2005;160:1–10. doi: 10.1016/j.bbr.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 199.Dufour BD, et al. Nutritional up-regulation of serotonin paradoxically induces compulsive behavior. Nutr. Neurosci. 2010;13:256–264. doi: 10.1179/147683010X12611460764688. [DOI] [PubMed] [Google Scholar]
- 200.Rogel-Salazar G, Lopez-Rubalcava C. Evaluation of the anxiolytic-like effects of clomipramine in two rat strains with different anxiety vulnerability (Wistar and Wistar-Kyoto rats): participation of 5-HT1A receptors. Behav. Pharmacol. 2011;22:136–146. doi: 10.1097/FBP.0b013e328343d7c5. [DOI] [PubMed] [Google Scholar]
- 201.Kang J, Kim E. Suppression of NMDA receptor function in mice prenatally exposed to valproic acid improves social deficits and repetitive behaviors. Front. Mol. Neurosci. 2015;8:17. doi: 10.3389/fnmol.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.