Abstract
Purpose: To develop a preoperative prediction model using a computer-assisted volumetric assessment of potential spared parenchyma to estimate the probability of chronic kidney disease (CKD, estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) 6 months from extirpative renal surgery (nephron-sparing surgery [NSS] or radical nephrectomy [RN]).
Patients and Methods: Retrospective analysis of patients who underwent NSS or RN at our institution from January 2000 to June 2013 with a compatible CT scan 6-month renal function follow-up was performed. Primary outcome was defined as the accuracy of 6-month postoperative eGFR compared with actual postoperative eGFR based on root mean square error (RMSE). Models were constructed using renal volumes and externally validated. A clinical tool was developed on the best model after a given surgical procedure using area under the curve (AUC).
Results: We identified 130 (51 radical, 79 partial) patients with a median age of 58 years (interquartile range [IQR] 48–67) and preoperative eGFR of 82.1 (IQR 65.9–104.3); postoperative CKD (eGFR <60) developed in 42% (55/130). We performed various linear regression models to predict postoperative eGFR. The Quadratic model was the highest performing model, which relied only on preoperative GFR and the volumetric data for a RMSE of 15.3 on external validation corresponding to a clinical tool with an AUC of 0.89.
Conclusion: Volumetric-based assessment provides information to predict postoperative eGFR. A tool based on this equation may assist surgical counseling regarding renal functional outcomes before renal tumor surgical procedures.
Introduction
Despite improved imaging leading to discovery of smaller tumors more feasible to nephron-sparing surgery (NSS) and multiple guideline recommendations encouraging NSS for clinical T1 renal cortical lesion, the adoption of such surgery has been underutilized.1–5 Compared with radical nephrectomy (RN), NSS reduces risk of development of chronic kidney disease (CKD) and may reduce potential of cardiovascular and metabolic sequelae,6–8 while conferring equivalent oncologic outcomes to RN.9,10
Various patient and surgeon-specific factors, however, may be influencing clinical decision-making regarding offering NSS.11–13 NSS is associated with increased risk of perioperative complications, such as bleeding or urinoma.2 Therefore, clinical tools are needed to more precisely identify patients who would receive a renal function benefit.
Previous studies have shown preoperative kidney volume derived from computer-assisted techniques correlate with postoperative estimated glomerular filtration rate (eGFR); however, none has shown a predictive model that can be used as a decision tool.14–16 We developed an integrative model composed of preoperative patient risk factors combined with three-dimensional (3D) renal imaging characteristics to estimate postoperative renal function, with the aim that this model may eventually be used as a decision aid for clinicians at the point of care to help identify patients who may or may not benefit from a higher-risk intervention such as NSS.
Patients and Methods
Population
After Institutional Review Board approval, we queried our renal mass database for patients who underwent surgical excision (RN or NSS) from January 1, 2000 to June 1, 2013. We included patients with 6-month renal functional outcomes who had undergone a contrast-enhanced multiphase CT scan that was compatible with our volumetric imaging software. Other demographic data were collected including R.E.N.A.L. (radius; exophytic/endophytic; nearness; anterior/posterior; location) nephrometry score assessment,17 and renal function was calculated by the Modification of Diet in Renal Disease (MDRD) equation.18
Volumetric assessment
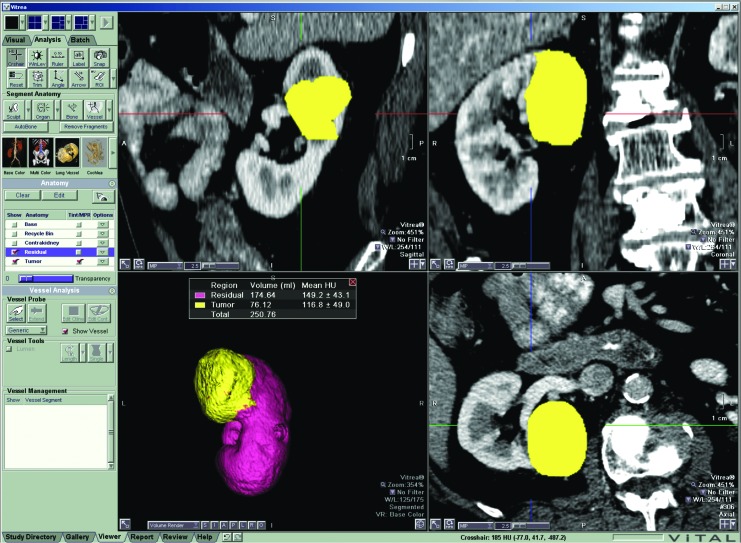
CT was performed on both 16- and 64-detector multidetector CT scanners (LightSpeed Plus, LightSpeed 16, Discovery CT750HD; GE Healthcare, Milwaukee, WI). Images were reconstructed to 2.5-mm thin sections when available with slices ranging up to 5 mm. We uploaded images into a commercially available 3D volume rendering and calculation program (Vitrea v. 6.3; Vital Images, Inc., Minnetonka, MN), which uses Hounsfield units to recognize different tissues creating a 3D volume-rendered image providing volume in cubic centimeters19 (Fig. 1). A 1-cm margin volume was obtained on a subset of patients to examine as a variable to improve model performance.
FIG. 1.
Volumetric acquisition from the Vitrea v. 6.3 software. Software used to produce the volumes of the tumor, ipsilateral uninvolved kidney, and the contralateral kidney was based on computer recognition of Houndsfield units. The recognition allowed for automatic highlighting of the different aspects of the kidney for faster manual correction.
Statistical analyses
Univariate analysis
Demographic data were summarized in aggregate with median and interquartile ranges (IQRs), and compared across postoperative CKD using the Student t test, chi-square, or Fisher exact test when appropriate. Statistical analysis was performed using the software program R with a significance level of alpha <0.05.
Defined outcomes
We defined the primary outcome as the accuracy of predicted 6-month postoperative eGFR compared with the actual 6-month eGFR. Accuracy was defined as the lowest root mean square error (RMSE) found after various combinations of predictors were used to develop possible predictive models. We used area under the curve (AUC) to define the accuracy of the final model used as a tool to predict the binary event of CKD (defined eGFR <60 mL/min/1.73 m2).18
Creation of multivariate prediction model
We constructed multiple linear regression models using preoperative kidney volumes, eGFR, and various demographic risk factors. Models were either designed based on clinical variables expected to perform well or to make comparisons between other predictors. One such comparison is the use of quadratic terms or certain inputs such as the R.E.N.A.L. nephrometry score. Although the MDRD calculation for GFR includes age, race, and sex in a conglomerate, these factors may have other influences on the prediction model and may also be used independently because colinearity is not a factor in predictive modeling.
Model validation and performance
Models initially are evaluated in simple linear regression (R2). The primary performance metric was leave-one-out cross-validation to provide an expected root mean square error (RMSE) cross validation, chosen specifically to avoid overfitting. We also performed confirmatory testing by splitting the data into thirds using two-thirds to train the model and one-third to test the model (RMSE testing). We also compared the Akaike information criterion as a secondary confirmatory analysis to select the best models.
Development of clinical decision tool
The primary goal of predictive modeling is to develop a decision tool to predict the probability of CKD (eGFR <60 mL/min/1.73 m2) at 6 months depending on which surgical procedure would be performed. Six-month renal function outcome is the chosen time because the most common practice is to perform a contrast-enhanced CT scan at that time, and renal function is clinically important to potential risk of acute renal failure from the contrast.
Using the best model, we would then generate two results: (1) eGFR if RN were performed and a separate (2) eGFR if NSS were performed. We refined this output by using the model estimate of prediction error, which incorporates estimated variance of model error with the uncertainty around the predicted eGFR to calculate probability that any given patient will maintain a postoperative eGFR above a certain threshold—for example, > 60. AUCs are obtained on the internal data using the various models to provide a predicted accuracy of the models.
External validation
To test the model, a separate dataset of 98 patients who underwent partial nephrectomy at the University Hospitals of Leuven (Belgium) was obtained from a previously published cohort.20 External validation was performed to test for overfitting on our training dataset and to test the generalizability of our model. The volumes were obtained using CT images, and postprocessing was performed on MeVisLab software (MeVis Medical Solutions, Bremen, Germany). We compared this out-of-sample performance using RMSE external validation with the results from our training data both directly and using leave-one-out cross-validation.
Results
Population and univariate analysis
After identifying 690 patients in the University of California, San Diego renal cancer database, 130 patients met the inclusion criteria (Supplementary Fig. 1; supplementary data are available online at www.liebertpub.com/end). To determine significant selection bias, we compared the demographics of the excluded and included patients noting no differences age, sex, body mass index, race, hypertension, diabetes, malignancy of neoplasms, R.E.N.A.L. nephrometry score, preoperative GFR or postoperative GFR (all P > 0.05, data not shown).
The median age in our selected cohort was 58 years (IQR 48–67) with median preoperative eGFR of 82.1 (IQR 65.9–104.2). The surgical groups (RN vs NSS) differed significantly in median tumor size (5.3 cm and 2.1 cm), R.E.N.A.L. score (10 vs 7), and contralateral renal volume (175 cm3 vs 140 cm3; all P < 0.001, Table 1). Preoperative eGFR was not statistically different between the RN and NSS (84.8 vs 81.6; P = 0.17), but the change in eGFR postoperatively was greater for RN patients (25.6 vs 13.1: P < 0.01, Welch Two Sample t test). Patients with postoperative CKD had older age (65 vs 53; P < 0.001), lower baseline eGFR (66.2 vs 92.8; P < 0.001), and were more likely to have diabetes (70.9% vs 48%; P = 0.009).
Table 1.
Demographics of Nephron-Sparing Surgery Versus Radical Nephrectomy
| Radical nephrectomy n = 51 | Partial nephrectomy n = 79 | P value | ||
|---|---|---|---|---|
| Preoperative | ||||
| Age | * | 56 (48–67) | 60 (50–67) | 0.466 |
| BMI | * | 29 (24–33) | 27 (24–31) | 0.292 |
| Male sex | + | 33 (64.7) | 50 (63.3) | 0.87 |
| Caucasian | + | 27 (52.9) | 50 (63.3) | 0.241 |
| Hypertension | + | 31 (60.8) | 44 (55.7) | 0.566 |
| Diabetes | + | 14 (27.5) | 18 (22.8) | 0.546 |
| Cardiovascular disease | + | 1 (1) | 2 (1) | N/A |
| Smoker | + | 0.579 | ||
| Never | 19 (37.3) | 25 (31.6) | ||
| Former | 11 (21.6) | 22 (27.8) | ||
| Current | 9 (19.6) | 10 (26.6) | ||
| Preop glomerular filtration rate | * | 84.8 (68.0–112.7) | 81.6 (64.8–99.4) | 0.17 |
| Clinical tumor stage | + | 0.014 | ||
| T1 | 39 (76) | 73 (92.4) | ||
| T2 | 9 (18) | 6 (7.6) | ||
| T3 | 3 (6) | 0 (0) | ||
| Tumor size (CT measured in cm) | * | 5.3 (3.7–7.0) | 2.1 (1.5–4.0) | <0.001 |
| R.E.N.A.L. nephrometry score | * | 10 (8–11) | 7 (5–9) | <0.001 |
| Preoperative volumes | ||||
| Ipsilateral | * | 139.6 (112.4–215.0) | 141.0 (117–180) | 0.307 |
| Tumor | * | 50.4 (27.8–145.3) | 9.1 (2.4–39) | 0.031 |
| Contralateral | * | 175.0 (144.0–214.2) | 140 (117.4–182.8) | <0.001 |
| Intraoperative | ||||
| Warm ischemia time | + | N/A | N/A | N/A |
| Cold ischemia time | + | N/A | N/A | N/A |
| Postoperative | ||||
| 6 mos postop glomerular filtration rate | * | 58.7 (48.9–77.3) | 67.6 (52.2–84.3) | 0.131 |
| Malignant histology | + | 50 (98.0) | 64 (81.0) | 0.004 |
| Pathologic tumor stage | + | 0.001 | ||
| T1 | 30 (62.) | 58 (90.6) | ||
| T2 | 5 (10.4) | 4 (6.3) | ||
| T3 | 13 (27.1) | 2 (3.1) | ||
| Length of follow-up (ms) | * | 5.8 (4.8–7.3) | 6.2 (5.3–7.3) | 0.426 |
Demographics of patients included in the retrospective study who underwent nephron-sparing surgery (NSS, i.e., partial nephrectomy) compared with those who underwent radical nephrectomy (RN).
Continuous data represented by median and interquartile range and analyzed using the unpaired, two-tailed Student t test.
BMI = body mass index; preop = preoperative; CT = computed tomography; R.E.N.A.L. = radius; exophytic/endophytic; nearness; anterior/posterior; location; postop = postoperative.
Creation of multivariable prediction model
Besides other preoperative factors, we noted that adding a tumor margin volume did not improve model performance (AUC R2 of 0.79 vs 0.78 for margin vs no margin, respectively), that volumetric assessment outperformed the R.E.N.A.L. nephrometry score in model performance (R2 [0.66 vs 0.71], respectively), and that separate models for RN and NSS were not needed (see Supplementary Table 1 for model selection; supplementary data are available online at www.liebertpub.com/end). The final model selected and equation is displayed in Table 2.
Table 2.
Final Multivariate Model for Prediction of 6-Month Estimated Glomerular Filration Rate
| Formula Postoperative eGFR ∼ Preoperative eGFR + surgery + contralateral volume + ipsilateral volume + tumor volume + (ipsilateral volume) + tumor volume) surgery + ipsilateral volume2 + age + gender + race + hypertension + diabetes | ||||
|---|---|---|---|---|
| Coefficients | Estimate | SE | t value | P value |
| (Intercept) | 0.79 | 18.75 | 0.04 | 0.97 |
| Preoperative eGFR | 0.52 | 0.06 | 9.05 | <0.001 |
| Contralateral volume (cm3) | 0.07 | 0.04 | 1.87 | 0.06 |
| Ipsilateral volume (cm3) | 0.43 | 0.15 | 2.92 | <0.001 |
| Tumor volume (cm3) | 0.01 | 0.00 | 1.42 | 0.16 |
| Ipsilateral volume (cm3) × surgery | 0.01 | 0.00 | 2.76 | <0.01 |
| Tumor volume (cm3) × surgery | −0.11 | 0.03 | −4.30 | <0.001 |
| Ipsilateral volume2 | −0.00 | 0.00 | −3.89 | <0.001 |
| Age | −0.19 | 0.12 | −1.55 | 0.12 |
| Sex | 5.63 | 2.87 | 1.96 | 0.05 |
| Race (black) | −15.30 | 5.27 | −2.91 | <0.01 |
| Race (white) | −7.31 | 2.70 | −2.71 | <0.01 |
| Hypertension | −5.85 | 2.68 | −2.18 | 0.03 |
| Diabetes | −5.64 | 2.94 | −1.92 | 0.06 |
| Statistics | ||||
| Residual standard error: 13.64 on 115 degrees of freedom | ||||
| Multiple R-squared: 0.6819 | ||||
| Adjusted R-squared: 0.6431 | ||||
| F-statistic: 17.61 on 14 and 115 DF | P < 2.2e–16 | |||
The quadratic model is the final model with the best prediction of 6-month estimated glomerular filration rate (eGFR). The full equation is written at the top of the chart. Each particular preoperative characteristic is displayed with an associated P value giving the indication of how much weight each variable will contribute to the predictive eGFR.
SE = standard error.
Model validation and performance
After the creation of multiple models (equations) using various demographics, we determined that using the quadratic model had the best performance, and the variables of the equation are shown in Table 1. Essentially, the volumes add the most information when the values are squared (quadratic) and added to the demographic information. The final equation includes the preoperative GFR, age, sex, race, hypertension, diabetes, and the volumes (tumor, ipsilateral kidney, and contralateral kidney).
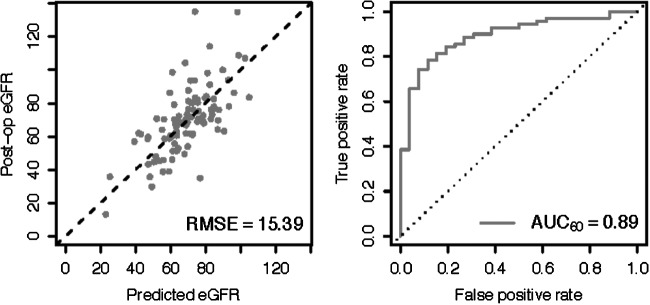
The most important factor in predicting postoperative renal function is the preoperative GFR (t value 9.05) followed by the squared tumor volume (t value − 4.30). The model predicted eGFR compared with patient postoperative eGFRs for all patients (R2 of 0.68, RMSE = 12.8, Supplementary Fig. 1A). We used leave-one-out cross-validation to confirm these predictions and noted similar values (R2 = 0.57, RMSE = 15, Supplementary Fig. 2). Model characteristics and cut-off points are displayed in Supplementary Figures 3 and 4 (supplementary data are available online at www.liebertpub.com/end). Because these values are similar, we assume internal validation of this predictive model. Using the cutoff GFR >60, the performance would have an AUC of 0.89 (Fig. 3).
FIG. 3.
External validation. Our model was tested on an external dataset of 98 patients obtained from University of Leuven, Belgium. The receiver operating characteristic curve displays accuracy of the model, and the area under the curve (AUC) is 0.89. RMSE = root mean square error.
Clinical decision tool performance
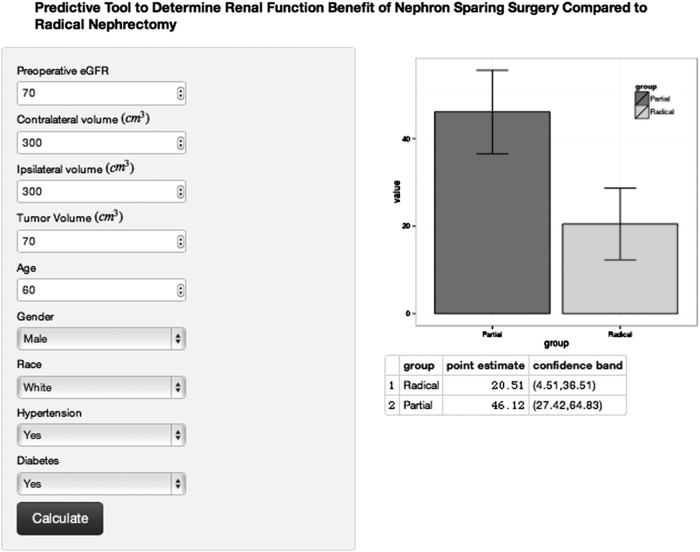
The clinical decision tool refers using the equation in Table 2 to predict the postoperative CKD. By automatically changing the surgical variable in the equation, one can produce two proportions corresponding to the prediction of postoperative GFR if a RN or NSS is performed. We have created an online calculator to perform this calculation automatically and to be used at the point of care potentially to inform decisions (https://kidneycancersurgery.shinyapps.io/webapp/) (screen shot Fig. 2).
FIG. 2.
Screen shot of Online Calculator Clinical Tool using the quadratic linear model for the prediction of estimated glomerular filtration rate (eGFR). After entry of the clinical and volumetric information, a bar graph will display the predicted postoperative eGFR if a radical nephrectomy was performed compared with partial nephrectomy with associated confidence bands. The actual numbers for the eGFR are provided at the bottom of the screen for convenience and future study validation (https://kidneycancersurgery.shinyapps.io/webapp/).
External validation
To verify that the model was sufficiently generalizable, we applied it to a set of patients from University Hospitals Leuven, Belgium. All these patients received partial nephrectomies, and their demographics have been published previously.20 The RMSE of the model eGFR predictions on this out-of-sample dataset was 15.98, which is only 0.98 above our expected performance of 15.00 as calculated using leave-one-out cross-validation and a given AUC of 0.89, which is the same as the cross-validation of the original dataset (Fig. 3).
Discussion
We have formulated a predictive online calculator that may be used at point of care to predict postoperative eGFR before renal surgical procedure for a localized renal mass. The equation was reliable and has a favorable ablity to predict CKD after surgery. The value in this approach is twofold. First, radiologists or urologists can provide nonsubjective volumetric information to the urologic surgeon by simply postprocessing a CT scan already obtained for patient care. Radiologists can use a 3D rederning postprocessing Current Procedural Terminology code, and nearly all radiology departments have software to provide three simple volumes needed for the calculator (our study used one software for the model and a separate software for validation).
Second, the data can be used in an online calculator at the point of care to assist in surgical counseling and understanding the risk of renal functional outcomes. In a recent report, Russo and associates21 described that patients did not fully understand the association between nephron loss and the development of CKD and that some patients never were given the option of NSS for the management of their early-stage kidney cancer.
Moreover, recent studies suggest the most important factor in predicting postoperative eGFR is the volume of paranchyma preserved and is potentially more important than ischemia time.22,23 Therefore, a tool such as a calculator that may assist the surgeon in formulating an expectation of CKD with the individual patient could be an important tool in the patient-physician discussion regarding renal function outcomes after renal surgery.
A limited amount of individualized, patient-specific tools are available to guide clinical decisions regarding surgical approach (RN vs NSS). The R.E.N.A.L. nephrometry score is an anatomic characterization of renal masses developed to standardize reporting, not necessarily to guide clinical decisions.17 Previous studies have concluded the R.E.N.A.L. nephrometry score correlates with surgeon preference, surgical complications, and renal volume loss with postoperative renal function.24–26 Therefore, R.E.N.A.L. nephrometry score may help surgeons determine the feasability of NSS but did not outperform our volume-based model in predicting CKD.
The only multicenter randomized clinical trial (EORTC 30904) regarding NSS comparing partial nephrectomy and RN oncologic outcomes did show a signficant reduction in CKD.27 Significant improvement in all-cause mortality, however, was not seen as anticipated from a previous study showing increased mortality rates for each stage of CKD because of medical disease (CKD-M).7
The concept of CKD-M and CKD from a surgical cause was recently described by Lane and colleagues28 indicating that patients presenting with CKD-M who undergo renal surgery are at much higher risk for renal decline.28 The long-term sequelae of patients who do not have CKD before renal extirpative surgery and later form CKD-M is unknown. An emphasis should be placed, however, regarding the shared decision-making and discussion of anticipated CKD after surgery with potential anticipatory nephrology specialist involvement. We urge the use of these tools in the context of a prospective clinical trial.29
Limitations of our study include the inherent bias of a retrospective analysis and that physicians' preference and size of the tumor will play a large role in influencing the outcomes. From a technical standpoint, the Vitrea program was unable to evaluate CT scans from certain consulting hospitals, ultrasound images, or MRI images. In addition, the images can be obtained with a single mouse click options on each image based on Hounsfield units if the intravenous contrast was used in the image. If no contrast was used, the borders of the mass could be too difficult to determine for volume rendering.
Unfortunately, this technically excludes many patients with preexisting CKD, which in turn biases our study toward normal preoperative renal function. Further study is needed to determine if these results could be translated to that particular population.
We recommend using a Hounsfield unit computer assistance technique that can take on average 5 minutes (Fig. 1) compared with the manual technique (30 min). While a larger analysis may have demonstrated the effect of a few millimeters or a centimeter margin, we did not demonstrate this. Therefore, while margin added to the tumor volume may improve predictive accuracy in an incremental fashion, it also substantially adds to the processing time, suggesting the need to develop a computer program to automate this process for further evaluation.
Moreover, the results were externally validated; therefore, if a large selection bias affected the cohort, the accurracy defined by AUC would not have been identical. While we did perform external validation, the external data set only had NSS, and further validation with larger samples are needed.
Nonetheless, this analysis represents the most accurate and quantitative rendering of an equation to predict postoperative renal function for RN and NSS, with external validation. We believe this tool may be used in answering the first question that should be asked when offering partial nephrectomy to a patient—would performing NSS reduce risk of developing CKD? If the renal functional benefit of proposed elective NSS is negligible, this informaiton would be essential in an informed discussion with the patient regarding the predicted renal outcome. Along with this information, the increased risk of morbidity associated NSS can be placed in context with an individualized renal function outcome.
Conclusion
We developed an externally validated equation by which preoperative eGFR and computer assisted renal volume measurement was able to provide accurate predictions for 6-month postoperative eGFR whether NSS or RN was performed. This model may have utility in and impact on shared decision-making, although further evaluation potentially in the context of a prospective study.
Supplementary Material
Abbreviations Used
- AUC
area under the curve
- CKD
chronic kidney disease
- CKD-M
chronic kidney disease because of medical disease
- CT
computed tomography
- eGFR
estimated glomerular filtration rate
- IQR
interquartile range
- MDRD
Modification of Diet in Renal Disease
- NSS
nephron sparing surgery
- R.E.N.A.L.
radius; exophytic/endophytic; nearness; anterior/posterior; location
- RMSE
root mean square error
- RN
radical nephrectomy
- 3D
three dimensional
Acknowledgment
We would like to thank Dr. Florin Vaida for his statistical review.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: The 2010 update. Eur Urol 2010;58:398–406 [DOI] [PubMed] [Google Scholar]
- 2.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009;182:1271–1279 [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Mallin K, Kane CJ, Carroll PR. Treatment trends for stage I renal cell carcinoma. J Urol 2011;186:394–399 [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: A need to reassess treatment effect. J Natl Cancer Inst 2006;98:1331–1334 [DOI] [PubMed] [Google Scholar]
- 5.Woldrich JM, Palazzi K, Stroup SP, et al. Trends in the surgical management of localized renal masses: Thermal ablation, partial and radical nephrectomy in the USA, 1998–2008. BJU Int 2013;111:1261–1268 [DOI] [PubMed] [Google Scholar]
- 6.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol 2000;163:442–445 [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 8.Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J Urol 2012;188:51–57 [DOI] [PubMed] [Google Scholar]
- 9.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2007;51:1606–1615 [DOI] [PubMed] [Google Scholar]
- 10.Antonelli A, Ficarra V, Bertini R, et al. Elective partial nephrectomy is equivalent to radical nephrectomy in patients with clinical T1 renal cell carcinoma: Results of a retrospective, comparative, multi-institutional study. BJU Int 2012;109:1013–1018 [DOI] [PubMed] [Google Scholar]
- 11.Weight CJ, Crispen PL, Breau RH, et al. Practice-setting and surgeon characteristics heavily influence the decision to perform partial nephrectomy among American Urologic Association surgeons. BJU Int 2013;111:731–738 [DOI] [PubMed] [Google Scholar]
- 12.Tanagho YS, Figenshau RS, Sandhu GS, Bhayani SB. Is there a financial disincentive to perform partial nephrectomy? J Urol 2012;187:1995–1999 [DOI] [PubMed] [Google Scholar]
- 13.Lane BR, Golan S, Eggener S, et al. Differential use of partial nephrectomy for intermediate and high complexity tumors may explain variability in reported utilization rates. J Urol 2013;189:2047–2053 [DOI] [PubMed] [Google Scholar]
- 14.Gong IH, Hwang J, Choi DK, et al. Relationship among total kidney volume, renal function and age. J Urol 2012;187:344–349 [DOI] [PubMed] [Google Scholar]
- 15.Jeon HG, Gong IH, Hwang JH, et al. Prognostic significance of preoperative kidney volume for predicting renal function in renal cell carcinoma patients receiving a radical or partial nephrectomy. BJU Int 2012;109:1468–1473 [DOI] [PubMed] [Google Scholar]
- 16.Tobert CM, Boelkins B, Culver S, Mammen L, Kahnoski RJ, Lane BR: Surgeon assessment of renal preservation with partial nephrectomy provides information comparable to measurement of volume preservation with 3D image analysis. J Urol 2014;191:1218–1224 [DOI] [PubMed] [Google Scholar]
- 17.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844–853 [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 19.Derweesh IH, Herts B, Novick AC. Three-dimensional image reconstruction for preplanning of renal surgery. Urol Clin North Am 2003;30:515–528 [DOI] [PubMed] [Google Scholar]
- 20.Aertsen M, De Keyzer F, Van Poppel H, et al. Tumour-related imaging parameters predicting the percentage of preserved normal renal parenchyma following nephron sparing surgery: A retrospective study. Eur Radiol 2013;23:280–286 [DOI] [PubMed] [Google Scholar]
- 21.Russo P, Szczech LA, Torres GS, Swartz M. Patient and caregiver knowledge and utilization of partial versus radical nephrectomy: Results of a National Kidney Foundation survey to assess educational needs of kidney cancer patients and caregivers. Am J Kidney Dis 2013;61:939–946 [DOI] [PubMed] [Google Scholar]
- 22.Mir MC, Campbell RA, Sharma N, et al. Parenchymal volume preservation and ischemia during partial nephrectomy: Functional and volumetric analysis. Urology 2013;82:263–268 [DOI] [PubMed] [Google Scholar]
- 23.Becker F, Van Poppel H, Hakenberg OW, et al. Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol 2009;56:625–634 [DOI] [PubMed] [Google Scholar]
- 24.Stroup SP, Palazzi K, Kopp RP, et al. RENAL nephrometry score is associated with operative approach for partial nephrectomy and urine leak. Urology 2012;80:151–156 [DOI] [PubMed] [Google Scholar]
- 25.Rosevear HM, Gellhaus PT, Lightfoot AJ, et al. Utility of the RENAL nephrometry scoring system in the real world: Predicting surgeon operative preference and complication risk. BJU Int 2012;109:700–705 [DOI] [PubMed] [Google Scholar]
- 26.Simmons MN, Hillyer SP, Lee BH, et al. Nephrometry score is associated with volume loss and functional recovery after partial nephrectomy. J Urol 2012;188:39–44 [DOI] [PubMed] [Google Scholar]
- 27.Scosyrev E, Messing EM, Sylvester R, et al. Renal function after nephron-sparing surgery versus radical nephrectomy: Results from EORTC randomized trial 30904. Eur Urol 2014;65:372–377 [DOI] [PubMed] [Google Scholar]
- 28.Lane BR, Campbell SC, Demirjian S, Fergany AF. Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol 2013;189:1649–1655 [DOI] [PubMed] [Google Scholar]
- 29.Weight CJ, Miller DC, Campbell SC, et al. The management of a clinical t1b renal tumor in the presence of a normal contralateral kidney. J Urol 2013;189:1198–1202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.