Abstract
Background & Aims
Tumor necrosis factor (TNF) receptor 2 (TNFR2, Tnfrsf1b) regulates multiple aspects of immune function; but little is known about its role in the immunopathogenesis of inflammatory bowel disease (IBD). We investigated whether TNFR2 restricts the activity of specific immune cell subtypes to protect against the development of colitis in mice.
Methods
Tnfr2−/− mice were crossed with Il10−/− mice, which spontaneously develop colitis, to generate Il10−/−Tnfr2−/− mice. Colonic tissues were collected from Il10−/−Tnfr2−/− mice along with Il10−/− mice (controls), and analyzed by flow cytometry and histology. Bone marrow was transplanted into Il10−/− and Il10−/−Tnfr2−/− mice from Il10−/− or Il10−/−Tnfr2−/− donors by intravenous injection. CD8+ T cells were neutralized in Il10−/−Tnfr2−/− mice by intraperitoneal injection of anti- CD8 or isotype control antibodies. Colitis was induced in Rag2−/− mice by intravenous injections of naïve CD8+ T cells isolated from C57BL/6 or Tnfr2−/− mice.
Results
Il10−/−Tnfr2−/− mice spontaneously developed more severe colitis compared to Il10−/− controls, characterized by selective expansion of colonic CD8+ T cells. Transplant of TNFR2- deficient bone marrow resulted in significantly increased incidence and severity of colitis. Transcriptome analyses showed that the expression of genes regulated by TNFR2 were specific to CD8+ T cells, and included genes associated with risk for IBD. Depletion of CD8+ T cells from Il10−/−Tnfr2−/− mice prevented colonic inflammation. Adoptive transfer of TNFR2-null naïve CD8+ T cells, compared with CD8+ T cells from control mice, increased the severity of colitis that developed in Rag2−/− mice.
Conclusion
TNFR2 protects mice from colitis by inhibiting the expansion of colonic CD8+ T cells. TNFR2 regulates expression of genes that regulate CD8+ T cells and have been associated with susceptibility to IBD. Disruption in TNFR2 signaling might therefore be associated with pathogenesis. Strategies to increase levels or activity of TNFR2, and thereby reduce the activity of CD8+ T cells, might be developed to treat IBD patients with CD8+ T cell dysfunction.
Keywords: mouse model, cytokine, ulcerative colitis, Crohn’s disease
Tumor necrosis factor (TNF) is a pleiotropic cytokine that regulates multiple aspects of the mucosal immune system and the gastrointestinal (GI) epithelium. TNF signals through two receptors, TNFR1 (Tnfrsf1a) and TNFR (Tnfrsf1b)1. Although increased TNF production is associated with inflammatory bowel disease (IBD)2, and TNF-directed therapies are an integral part of therapeutic regimes for IBD3,4, the specific role of TNF and its receptors in IBD pathogenesis is unclear5. Several TNFR2 polymorphisms associated with loss of function have been identified in subsets of Crohn’s disease and ulcerative colitis patients6,7; however, the specific mechanistic role of TNFR2 in the pathogenesis of colitis remains unknown. TNFR2 is constitutively expressed on numerous immune cells, and its expression is induced on colonic epithelial cells in response to inflammation8, and we have shown that TNFR2 enhances epithelial proliferation, migration and wound closure9,10. These data suggest that TNFR2 contributes to immune and/or epithelial responses during inflammation; however, the role of TNFR2 in these various cell types is unclear. Studies with Tnfr2−/− mice have yielded conflicting results regarding the role of TNFR2 in colitis, with differential protective versus deleterious roles in both mucosal damage and immune defect mouse models of colitis5, suggesting that TNFR2 may differentially regulate colitis in a cell type-specific manner. TNFR2 contributes regulatory roles for both myeloid, as well as CD4+ T cells in colitis11–15; however, the majority of these studies have been performed in lymphopenic mice and it is unclear how TNFR2 may influence colitis pathogenesis in the setting of normal adaptive immunity. Of particular note, TNFR2 is a critical negative regulator of CD8+ T cells by promoting activation-induced cell death16, and recent data have emphasized the potential importance of CD8+ T cells in IBD pathology17. This suggests that TNFR2 may be protective in IBD immunopathogenesis through the inhibition of CD8+ T cells.
In this study, we determined a novel role for TNFR2 to ameliorate colitis by restricting CD8+ T cells. We evaluated if TNFR2 differentially regulates colonic inflammation through immune cell-or non-hematopoietic cell-specific mechanisms. Herein, we report that TNFR2 plays a protective role through a hematopoietic cell-specific mechanism in which TNFR2 on CD8+ T cells suppresses colitis. Our data show that TNFR2 is a critical disease modifier that alters CD8+ T cell responses during inflammation. This improved appreciation of the anti-inflammatory functions of TNFR2 in specific immune cell subtypes may lead to refined therapeutic avenues for IBD.
Materials and Methods
Animals
All mice were C57BL/6 background and housed in microisolator cages in a specific pathogen-free facility in the Children’s Hospital Los Angeles Animal Care Facility. Wild type, Il10−/−, Tnfr2−/− and Rag2−/− mice were from The Jackson Laboratory (Bar Harbor, ME). Il10−/− mice were crossed to Tnfr2−/− mice to generate Il10−/−Tnfr2−/− mice. Animal studies were approved by the Children’s Hospital Los Angeles Institutional Animal Care and Use Committee.
Bone Marrow Transplantation
Recipient mice were treated with neosporin irrigant (Monarch Pharmaceuticals, Bristol, TN) in drinking water for 1 week prior to transplant. 6 week-old Il10−/− and Il10−/−Tnfr2−/− recipient mice were subjected to a single total body dose of 9 Gy from a 137Cs radiation source, 5 h prior to transplant. 5 x106 bone marrow cells from Il10−/− or Il10−/−Tnfr2−/− donor mice were injected via tail veins of irradiated mice. Mice were sacrificed 5 weeks post-transplant and colitis assessed by scoring colon inflammation and injury, as before18.
CD8+ T cell neutralization
Purified anti-mouse CD8a (clone 53-6.7) and control isotype antibody (rat IgG2a, κ) were purchased from Biolegend (San Diego, CA). Anti-CD8 or isotype control were injected i.p. starting at 8 weeks of age, at a dose of 20 µg/g, weekly for 4 weeks, as published19.
Naïve CD8+ T cell adoptive transfer model
8 week-old male RAG2−/− mice were intravenously injected with 7.5x105 naïve CD8+ T cells (FACS-sorted CD44lowCD62L+CD8+ naïve CD8+ T cells), as reported20, isolated from lymph nodes of wildtype or Tnfr2−/− mice. Mice were euthanized 4 weeks post-adoptive transfer.
Other details and additional experimental procedures are provided in Supplementary Materials and Methods.
Results
TNFR2 inhibits colitis in Il10−/− mice
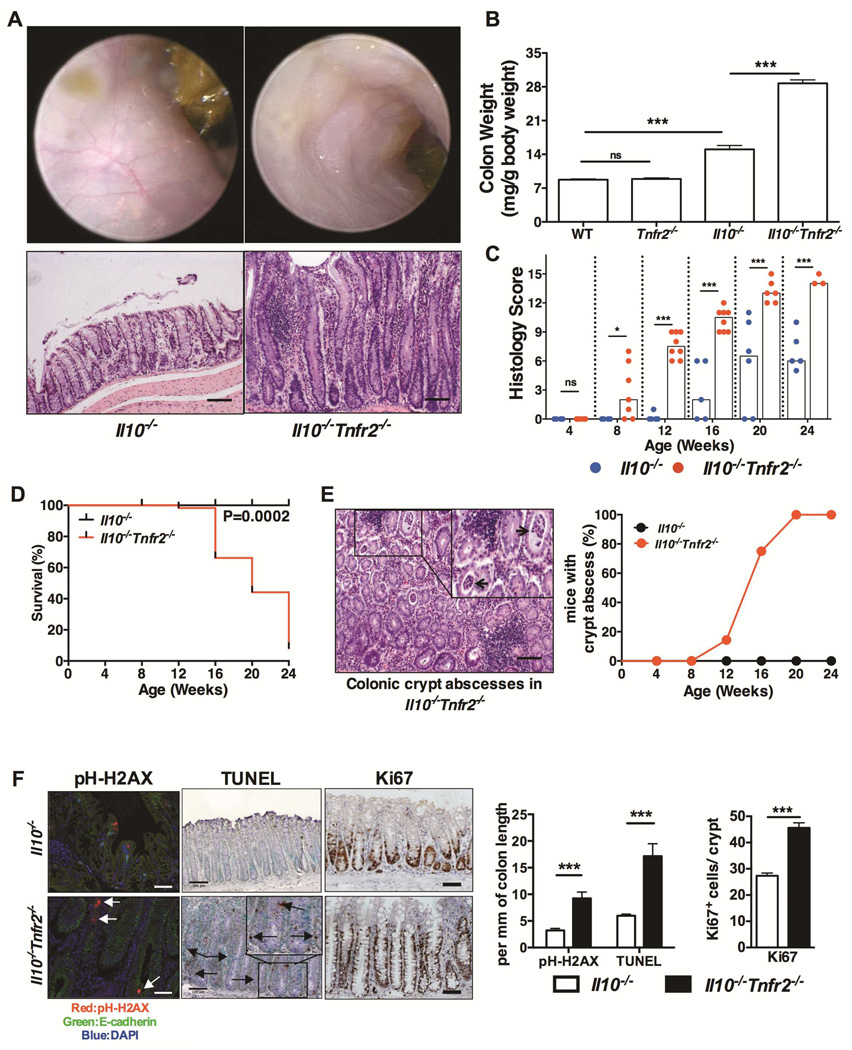
To determine the role of TNFR2 in a spontaneous model of colonic inflammation24,25, Tnfr2−/− mice were crossed to Il10−/− mice. The Il10−/− model recapitulates aspects of disease in certain IBD patients,21 and is of particular relevance to early-onset IBD driven by IL10 signaling defects22,23. Since TNF is elevated Il10−/− colons24, we hypothesized that TNFR2 would be protective in this model given our previous findings that TNFR2 was protective in the colon epithelium, and reports of TNFR2 polymorphisms in patients with Crohn’s disease and ulcerative colitis6,7. Indeed, Il10−/−Tnfr2−/− mice developed highly penetrant colitis, which was more severe and with earlier onset compared to Il10−/− controls on the same C57Bl/6 background (Fig 1). Endoscopy and histology revealed that Il10−/−Tnfr2−/− colons were inflamed, with mucosal thickening, mucosal granularity, vascular obfuscation and edema with marked epithelial hyperplasia with increased inflammatory infiltrate. This severe colitis phenotype was apparent by 8 weeks of age, worsened with age, and significantly decreased survival compared to Il10−/− controls (Fig 1C&1D). A prominent feature of inflammation in Il10−/−Tnfr2−/− mice was the development of crypt abscesses (Fig 1E). Interestingly, there was no difference in the permeability of Il10−/−Tnfr2−/− vs. Il10−/− colons, as assessed by colonic FITC-dextran absorption (Fig S1A), suggesting that a functional barrier defect was not responsible for worsened disease. Loss of TNFR2 increased epithelial DNA damage and apoptosis, irrespective of coincident inflammation both in vivo (Fig 1F) and in vitro (S1B-S1D). Furthermore, the lack of TNFR2 significantly increased epithelial proliferation (Fig 1F & S1D). Thus, the loss of TNFR2 altered colonic epithelial homeostasis by restricting DNA-damage, promoting survival and attenuating inflammation-induced hyperproliferation. Collectively, these data demonstrate that the loss of TNFR2 exacerbates colitis in Il10−/− mice with subsequent effects on both inflammation and epithelial dysfunction.
Figure 1. TNFR2 protects against colitis in Il10−/− mice.
(A) Representative colonoscopic and H&E sections of colons from 16 week-old mice of the indicated genotypes; scale bar: 100µm. (B) Colon weights of 16 week-old mice; n= 5 – 9; ***P < 0.001. (C) Colitis scores of Il10−/− and Il10−/−Tnfr2−/− mice; the score for each mouse is denoted by a dot, bars indicate median scores; n= 5 – 9; *P < 0.05, ***P < 0.001. (D) Kaplan-Meier survival curves compared by the log-rank test. (E) Representative H&E section & incidence of mice with colonic crypt abscesses (black arrows); scale bar: 100µm. (F) Representative images and quantification of epithelial DNA-damage (p-H2AX), apoptosis (TUNEL) and proliferation (Ki67); n=5; ***P < 0.001; scale bars: 100µm.
TNFR2 protects against colitis-associated tumorigenesis
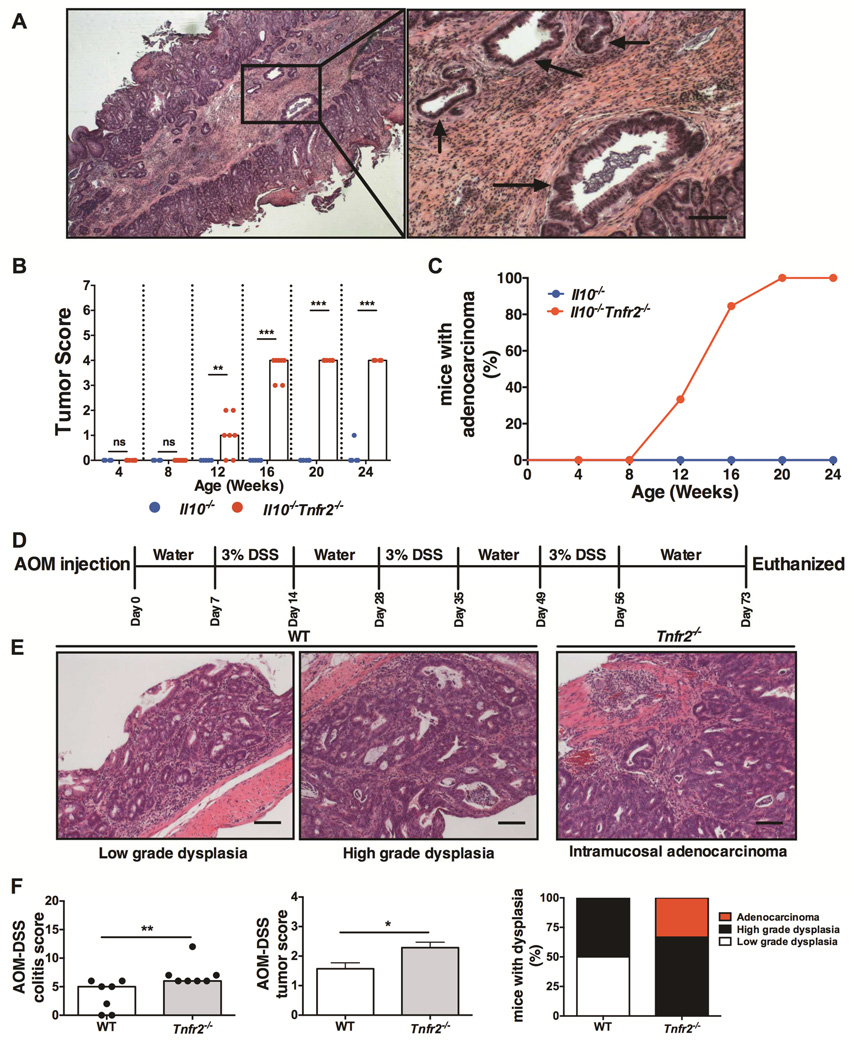
Since the loss of TNFR2 in Il10−/− mice resulted in severe colitis, we further determined the role of TNFR2 in inflammation-induced tumorigenesis. Loss of TNFR2 significantly increased the incidence and progression of adenomas and carcinomas in Il10−/−Tnfr2−/− mice (Fig 2A-2C). Dysplastic lesions were first noted in histological sections at 12 weeks of age and progressed to invasive adenocarcinoma by 16 weeks (Fig 2B). The incidence of adenocarcinoma invasive into the sub-mucosa increased to 84% by 16 weeks and 100% by 20 weeks of age in Il10−/−Tnfr2−/− mice (Fig 2C). This exacerbated tumor progression coincided with the decreased survival of these mice, as noted above, which may explain why more deeply invasive adenocarcinomas (i.e. invasion into the muscularis or serosa) were not observed (Fig 2B). In agreement with our previous findings in the Il10−/− model25, there were no detectable signs of tumorigenesis in Il10−/− mice prior to 24 weeks of age (Fig 2B-2C). Thus, loss of TNFR2 expression resulted in increased incidence and progression of CAC in Il10−/− mice.
Figure 2. TNFR2 inhibits colitis-associated colorectal cancer in mice.
(A) Representative H&E sections from a 16 week-old Il10−/−Tnfr2−/− colon showing adenocarcinoma invasive into the submucosa (arrows); scale bar: 200µm. (B) Tumor scores quantifying progression of adenomas and adenocarcinomas; lines indicate median scores; n= 7 – 13; **P < 0.01, ***P < 0.001. (C) Incidence of invasive adenocarcinoma. (D) Schematic representation of AOM/DSS protocol. (E) Representative H&E sections showing low-grade, high-grade dysplasia and intramucosal adenocarcinoma in WT and Tnfr2−/− colons (scoring system, see Supplementary Methods and Materials); scale bar: 100µm (F) Colitis, tumor progression scores and incidence of low-grade, high-grade dysplasia and adenocarcinoma in WT and Tnfr2−/− colons following AOM/DSS treatment; bar indicates median scores; n = 7; *P < 0.05.
We next determined if TNFR2 provided a similar protective role in the azoxymethane (AOM)/DSS CAC model. WT and Tnfr2−/− mice were injected with a single dose of AOM followed by three seven-day cycles of 3% DSS (Fig 2D)26. Consistent with our observation in Il10−/−Tnfr2−/− mice, the loss of TNFR2 increased tumor burden and progression (Fig 2E-2F), which was associated with more severe colitis, and increased mortality (45% of Tnfr2−/− mice vs. 10% of the WT mice died during this period). One-third of Tnfr2−/− mice developed intramucosal adenocarcinoma and two-thirds had high-grade dysplasia, while 50% of the wildtype mice displayed high-grade dysplasia with no evidence of adenocarcinoma (Figs 2F). Collectively, our data from two distinct models of CAC suggest a critical role for TNFR2 in protection against colitis-associated tumorigenesis.
TNFR2 in hematopoietic lineages protects against colitis and CAC
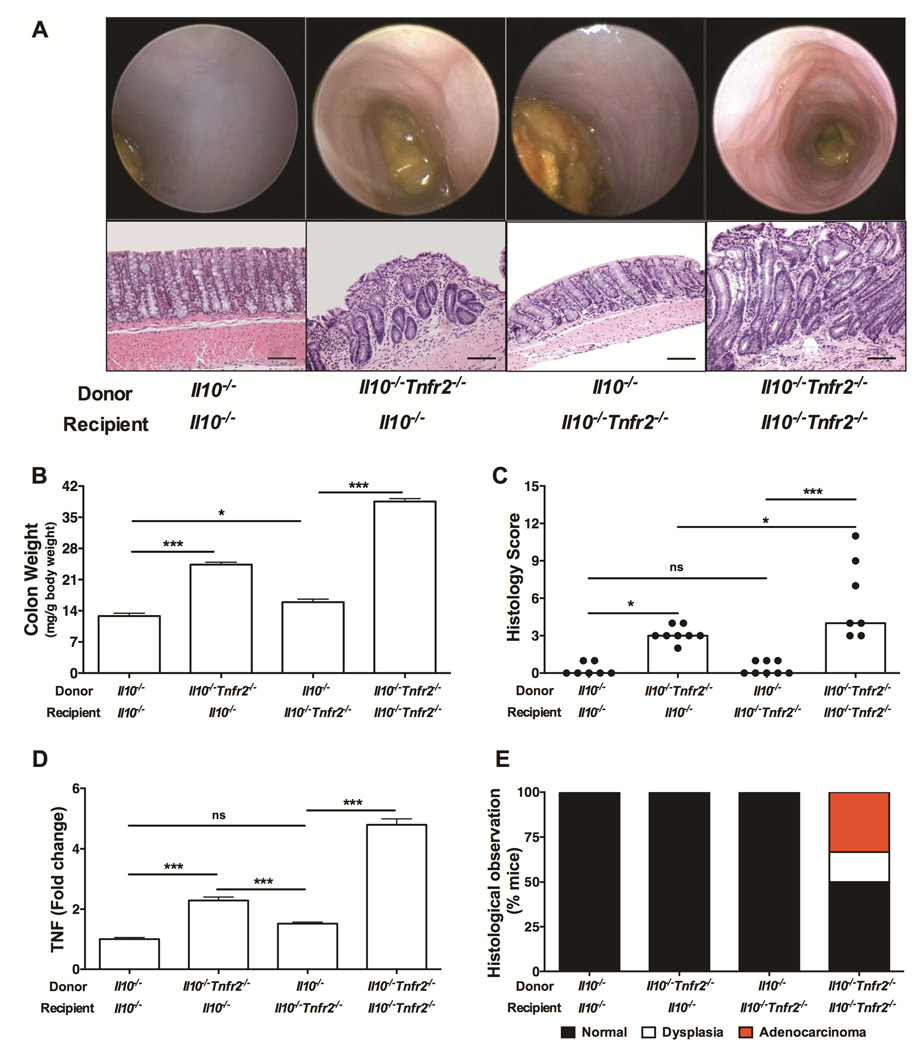
Since TNFR2 is expressed primarily on hematopoietic cells, we determined whether TNFR2 deficiency in bone marrow-derived cells was required for the worsened colitis and CAC in the Il10−/− model. Irradiated 6 week-old Il10−/− and Il10−/−Tnfr2−/− mice were engrafted with either Il10−/− or Il10−/−Tnfr2−/− bone marrow. The presence of TNFR2 in the bone marrow protected both Il10−/− and Il10−/−Tnfr2−/− recipients from colitis (Fig 3). In contrast, the absence of TNFR2 in the hematopoietic compartment significantly aggravated inflammation, as indicated by endoscopy, histology, colon weights and colitis scores (Fig 3A-3C). Furthermore, only Il10−/−Tnfr2−/− mice reconstituted with Il10−/−Tnfr2−/− bone marrow died during the experiment (33% mortality rate). Reconstitution of Il10−/− or Il10−/−Tnfr2−/− mice with Il10−/−Tnfr2−/− bone marrow resulted in significantly increased TNF mRNA expression (Fig 3D). Thus, TNFR2 in the bone marrow-derived compartment is critical to inhibit inflammation in colitis. Interestingly, only Il10−/−Tnfr2−/− mice reconstituted with Il10−/−Tnfr2−/− bone marrow developed adenocarcinoma, which was invasive into the muscularis propria (Fig 3A&3E), whereas Il10−/− mice reconstituted with Il10−/−Tnfr2−/− bone marrow did not show any evidence of dysplasia. This suggests differential roles for TNFR2 in hematopoietic versus non-hematopoietic cells; although the loss of TNFR2 in bone marrow exacerbated inflammation, the additional absence of TNFR2 in non-hematopoietic cells was also required for tumor development. These data suggest that TNFR2 regulates disparate functions in hematopoietic verses non-hematopoietic cells during inflammation, which may include protective effects of TNFR2 on epithelial cells, as noted above and as previously published10. Therefore, our data strongly support protective functions for hematopoietic TNFR2 in regulating colonic inflammation.
Figure 3. TNFR2 in hematopoietic lineages protects against colitis.
(A) Representative colonoscopic and H&E sections images; bars are 100 µm. (B) Colon weights (C) colitis scores of the indicated mice; n= 7 – 10; *P < 0.05, ***P < 0.001. (D) Colonic TNF mRNA expression; n= 6 – 9; ***P < 0.001. (E) Incidence of colonic dysplasia and adenocarcinoma.
Selective expansion of colonic CD8+ T cells in the absence of TNFR2
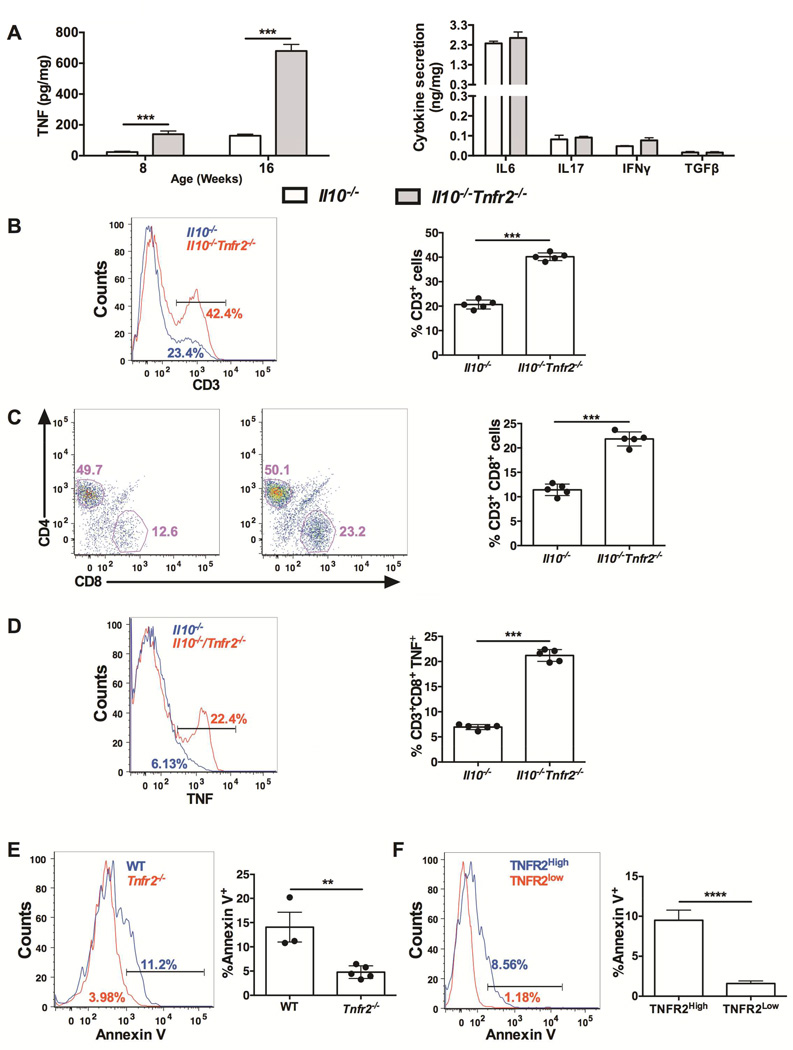
TNFR2 expression on hematopoietic cells provided a substantial degree of protection against inflammation in Il10−/− mice. To determine how loss of TNFR2 altered colonic inflammation, we next characterized how loss of TNFR2 altered cytokine production and immune cell populations in the colon. To determine how TNFR2 regulates the colonic cytokine milieu, we measured cytokine release from colonic explants of Il10−/−Tnfr2−/− and Il10−/− mice. There were no differences in the levels of Th1, Th2 or Th17 cytokine production, with the exception of TNF (Fig 4A). We next determined how loss of TNFR2 altered immune cell populations by flow cytometry. There was a 2-fold increase in CD3+ T cells in the lamina propria of Il10−/−Tnfr2−/− mice compared to Il10−/− controls (Fig 4B), and this increase was due to a specific expansion of the CD8+ T cell population (Fig 4C). This increase in CD8+ T cells was also observed in Tnfr2−/− colons (19.3%±1.2% vs. 9.0%±1.8% in wildtype colons, as a percentage of CD3+ cells; P<0.001, n=5). The majority of wildtype CD8+ T cells (~75%) expressed TNFR2, by flow cytometry (Fig S3A). Although published reports have shown that CD4+ T cells initiate inflammation in the Il10−/− colon27, these data suggest a role for CD8+ T cells to promote inflammation and are in accordance with data of increased CD8+ T cells in the intestinal mucosa of patients with CD and UC28. CD8+ T cells in Il10−/−Tnfr2−/− colons expressed significantly elevated levels of TNF (Fig 4D), whereas other immune cell subsets expressed equivalent levels of TNF (data not shown). TNF is increased during CD8+ T cell activation29, and TNF-TNFR2 signaling mediates activation-induced cell death in CD8+ T cells as part of a negative feedback mechanism16,30,31. This suggested that the increase in CD8+ T cells in the absence of TNFR2 occurred because these cells were resistant to activation-induced cell death. Therefore, we determined how loss of TNFR2 affected apoptosis in colonic CD8+ T cells. Colonic Tnfr2−/− CD8+ T cells had decreased apoptosis compared to wildtype CD8+ T cells (Fig. 4E). Furthermore, amongst wildtype CD8+ T cells, a high level of TNFR2 expression was associated with a 6-fold increase in apoptosis versus TNFR2-low CD8+ T cells (Fig. 4F). Together, these data demonstrate that TNFR2 inhibits colonic CD8+ T cell survival, potentially involving autocrine signaling through increased TNF production in these cells. Thus, TNFR2-mediated inhibition of colonic CD8+ T cells is a critical factor to dampen colonic inflammation.
Figure 4. Selective expansion of TNF-producing colonic CD8+ T cells in Il10−/−Tnfr2−/− mice.
(A) Colon explant cultures from 16 week-old Il10−/− and Il10−/−Tnfr2−/− mice; TNF levels; n=5; ***P < 0.001, other cytokines level detected by ELISA array; n=4. (B) Representative histogram and quantification showing lamina propria T cells in Il10−/− and Il10−/−Tnfr2−/− mice; cells were gated on positive CD3+ staining; n=5; ***P < 0.001. (C) Representative scatter plot and quantification showing the number of CD4+ and CD8+ cells within the CD3+ population; data are represented as % of CD3+CD8+ population; n=5; ***P < 0.001. (D) Representative histogram showing TNF production by CD8+ T cells; data are represented as % of CD8+TNF+ population; n=5; ***P < 0.001. (E) Representative histogram and quantification showing baseline apoptotic Annexin V+ cells (%) in CD3+CD8+ population in 16 week-old WT and Tnfr2−/− mice in lamina propria; n=3-5; **P < 0.01. (F) Representative histogram and quantification showing baseline apoptotic Annexin V+ cells (%) in CD3+CD8+TNFR2High and CD3+CD8+TNFR2low population in 8 week-old WT mice; n=5; ****P < 0.0001.
TNFR2 regulates IBD-associated CD8+ T cell-specific genes
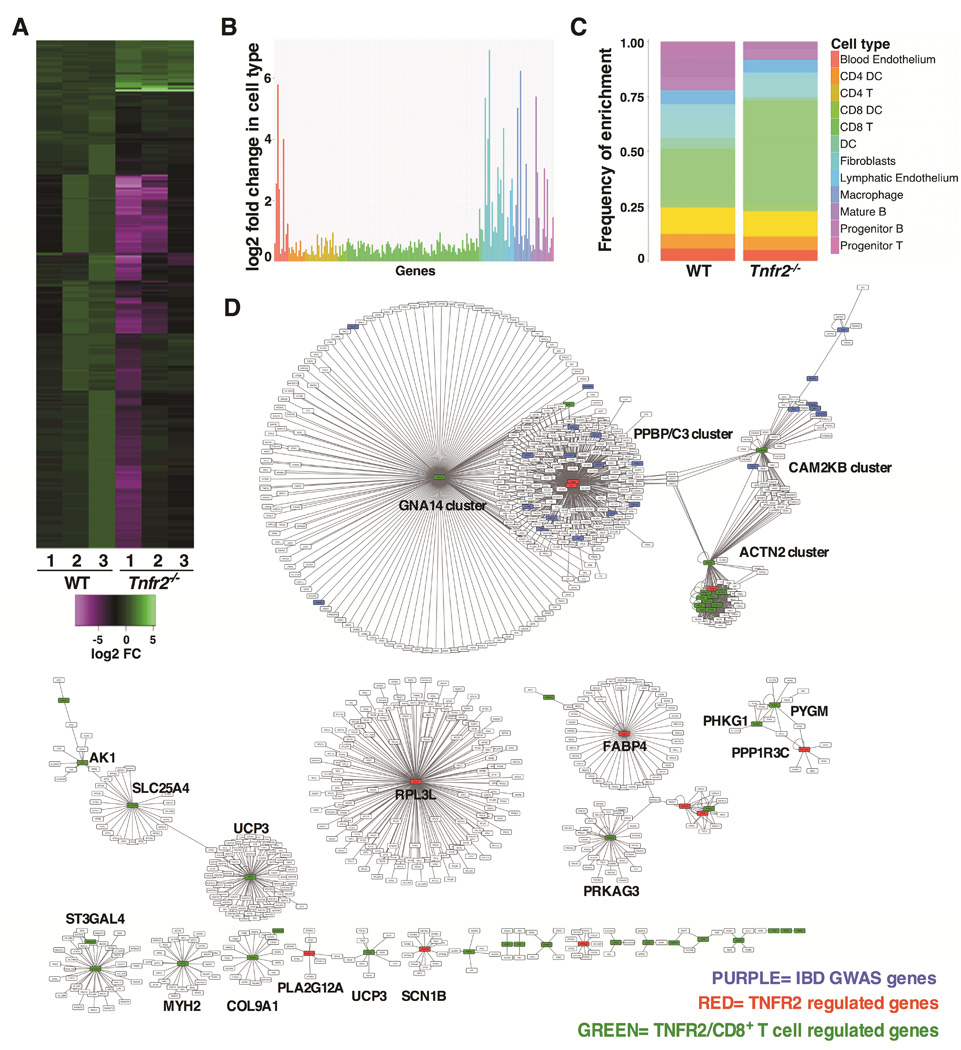
To further explore the biological role for TNFR2 in the colon, we determined what genes were regulated by TNFR2 by performing RNA sequencing analysis (RNAseq) of mRNA from whole colons of WT and Tnfr2−/− mice (deposited in NCBI's Gene Expression Omnibus; accession number GSE65408). We identified 252 differentially expressed transcripts that were significantly altered by the loss of TNFR2 (adjusted P < 0.05, n=3, Fig 5A, Fig S2A-2B and Suppl. Table 1). The majority of these transcripts (228 of 252, ~90%) were downregulated in Tnfr2−/− colons. Validation of selected transcripts was confirmed by qPCR (Fig S2C-2D) of independent colon samples. Given that this analysis was performed on whole colonic tissue, it was possible to determine how TNFR2 regulates disparate populations of cells in an unbiased manner. Since we observed a selective expansion of colonic CD8+ T cells in the absence of TNFR2 (Fig 4), we tested whether this TNFR2-regulated gene set was enriched for transcripts that were either specific to CD8+ T cells or involved in CD8+ T cell biology. Using data from the Immunological Genome Project (www.immgen.org)32, we determined that almost half (47%) of the TNFR2- regulated genes were most highly enriched in CD8+ T cells compared to other immune cell types (Fig 5B-C and Suppl. Table 2). Furthermore, gene set enrichment analysis33 and comparisons with published transcriptomics data34 revealed that 64 of the TNFR2-regulated transcripts are directly involved in the process of CD8+ T cell activation (Suppl. Table 3). This suggested that TNFR2 might be integral to the biology of CD8+ T cells in the colon. Intriguingly, transcriptional profiles in CD8+ T cells have recently been associated with differential prognosis in both CD and UC patients17, and TNFR2-regulated genes overlap with 12 of those transcripts linked to severe disease in patients (Suppl. Table 3). Reactome pathway analysis (reactome.org)35 predicted numerous interactions between TNFR2-regulated genes, including those specific to CD8+ T cells and IBD susceptibility genes (Fig 5D). In total, TNFR2-regulated genes were involved in 22 networks ranging in size and complexity. These data emphasize a role for TNFR2 to impact multiple networks in colonic CD8+ T cells, and provide a valuable avenue for the future study of TNFR2 regulation of CD8+ T cell function in the colon.
Figure 5. TNFR2 inhibits pro-inflammatory gene signature in the colon.
(A) Expression heatmap (log2 fold-change) of differentially expressed genes identified in RNAseq. The x-axis indicates 3 biological replicates for each of WT and Tnfr2−/− colons. (B) Enrichment of differentially expressed genes regulated by TNFR2 in various immune cell types. Each bar represents the cell type specific fold change over the median expression level, and the colors represent different cell type groupings. (C) Stack bar graph showing a higher enrichment of TNFR2-regulated genes in CD8+ T cells, as compared to the background enrichment rate of all genes in this cell type. (D) Network analysis showing interactions between TNFR2-regulated genes (RED), TNFR2/CD8+ T cells-regulated genes (GREEN), and IBD risk loci from GWAS (PURPLE).
CD8+ T cells drive inflammation in Il10−/−Tnfr2−/− colitis
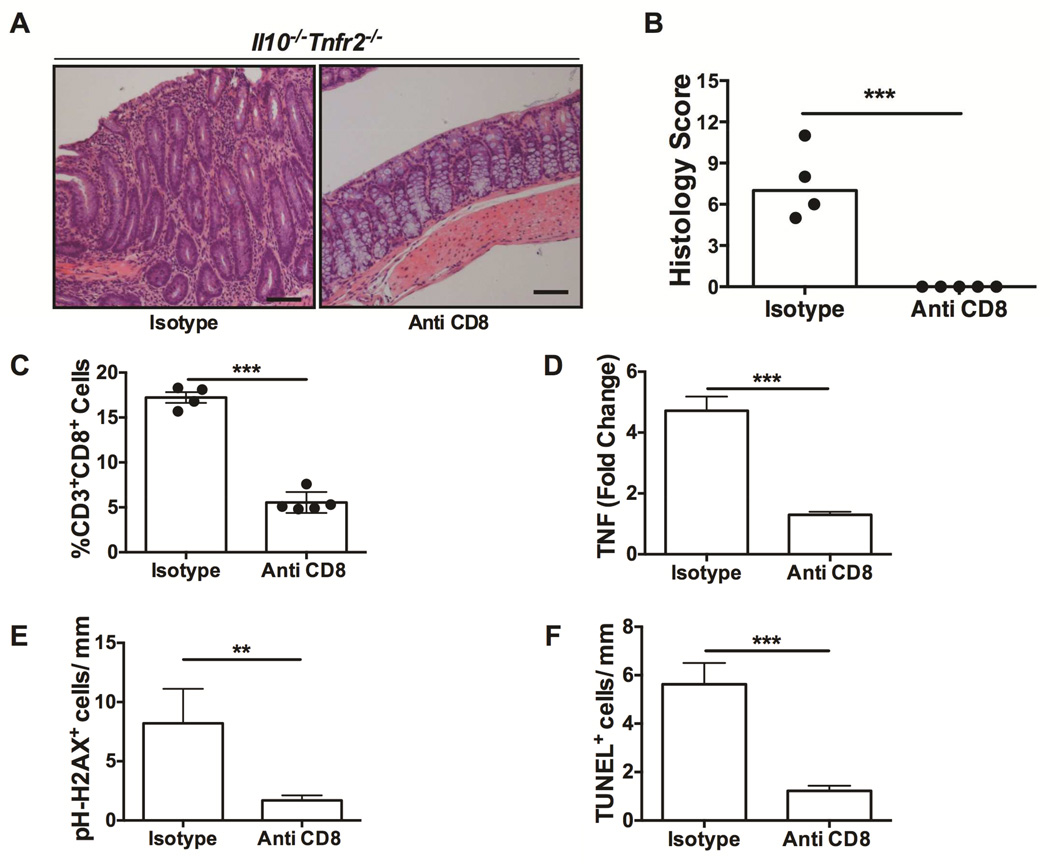
Given the prominence of CD8+ T cells in the colitis phenotype of Il10−/−Tnfr2−/− mice, we next determined if CD8+ T cells are necessary for this exacerbated colitis. Antibody-mediated CD8+ T cell neutralization19 was accomplished by administering anti-CD8 or isotype-specific control antibodies weekly to Il10−/−Tnfr2−/− mice starting at 8 weeks of age for 4 weeks. CD8+ T cell neutralization completely suppressed colitis in Il10−/−Tnfr2−/− mice (Fig 6). Il10−/−Tnfr2−/− mice treated with anti-CD8 did not show any discernable histological signs of colonic inflammation (Fig 6A-B), and, although CD8+ T cells were not completely ablated, they were significantly reduced versus isotype control (Fig 6C). Of interest, anti-CD8 significantly reduced the colonic mRNA expression of TNF (Fig 6D), confirming that these cells were responsible for excess TNF production. Furthermore, anti-CD8 also decreased the level of epithelial DNA damage and apoptosis to near wildtype levels (Fig 6E&F). Therefore, CD8+ T cells are necessary for colitis severity in Il10−/−Tnfr2−/− mice.
Figure 6. CD8+ T cells are required for colitis in Il10−/−Tnfr2−/− mice.
(A) Representative H&E colon sections; scale bar: 100µm. (B) Colitis scores; n=4-5; ***P < 0.001. (C) Numbers of CD8+ T cells (CD8+ as a percentage of CD3+) in the colons of the indicated mice; n=4 – 5; ***P < 0.001. (D) Levels of colonic TNF mRNA expression, ***P < 0.001. Indices for epithelial (E) DNA damage (pH-H2AX+) and (F) apoptosis (TUNEL+) of the indicated mice, determined as in Figure 1; **P < 0.01, ***P < 0.001.
Loss of TNFR2 on CD8+ T cells is sufficient to worsen colitis
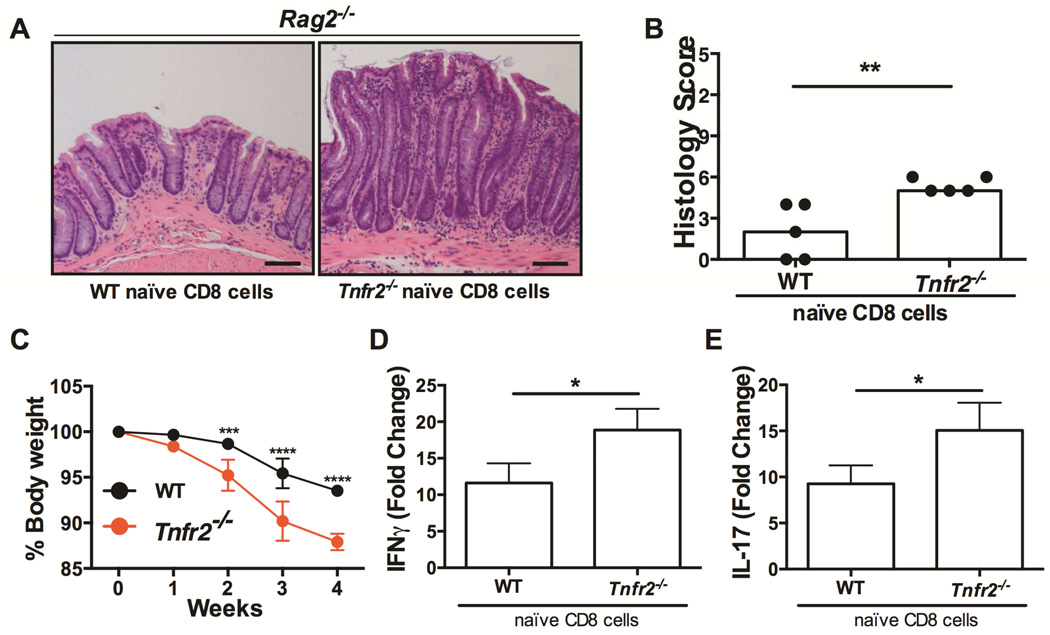
We then directly tested the hypothesis that TNFR2 on CD8+ T cells inhibits inflammation using a colitis model involving the adoptive transfer of naïve CD8+ T cells (CD44low CD62L+ CD8+) into lymphopenic Rag2−/− mice20. Naïve CD8+ T cells were isolated from peripheral lymph nodes of wildtype or Tnfr2−/− mice and injected into RAG2−/− recipients. As in Fig S3A, the majority of these naïve CD8 T cells (~75%) were positive for TNFR2 (Fig S3B). Mice receiving Tnfr2−/− CD8+ T cells had worse colitis compared to Rag2−/− mice receiving wildtype cells (Fig 7). Colons from Rag2−/− mice following Tnfr2−/− CD8+ T cell transfer had markedly elongated crypts and increased lamina propria inflammatory infiltrate (Fig 7A). Adoptive transfer of Tnfr2−/− naïve CD8+ T cells, worsened colitis as evident by increased histological scores (Fig 7B), and exacerbated weight loss in Rag2−/− recipients through week 2-4 of the experiment (Fig 7C). Moreover, there was a significant increase in the colonic mRNA levels of IFNγ and IL17 in mice receiving Tnfr2−/− naïve CD8+ T cells compared to wildtype (Fig 7D&E), consistent with the pathological role of CD8+ T cell-derived IL17 in this model20. Taken together with our observation of increased CD8+ T cell apoptosis in Tnfr2−/− colons (Fig 4), these data show that the loss of TNFR2 on CD8+ T cells is sufficient to exacerbate colitis, potentially through a deficit in TNFR2-mediated CD8+ T cell activation-induced cell death. Together, these experiments establish a central role for TNFR2 in the regulation CD8+ T cells to ameliorate colitis, and thus identify TNFR2 as a crucial disease modifier for IBD.
Figure 7. TNFR2 on CD8+ T cells restricts disease in an adoptive transfer colitis model.
(A) Representative H&E sections of colons of recipient Rag2−/− mice; scale bar: 100µm. (B) Colitis scores of these mice, wherein each mouse is represented by a dot and the bar indicates the median score; n=5; ***P < 0.001. (C) Body weight loss (% baseline); ***P < 0.001, ****P < 0.0001. Levels of (D) IFNγ and (E) IL17 mRNA in Rag2−/− colons following transfer of wildtype or Tnfr2−/− naïve CD8+ T cells; actin was used as the reference gene; n=5; *P < 0.05.
Discussion
In the present study, we determined that TNFR2 is protective in the pathogenesis of colitis through its inhibitory effects on CD8+ T cells. The loss of TNFR2 worsened disease in both the Il10−/− and AOM/DSS colitis models, with exacerbated inflammation and subsequent tumorigenesis. Although TNFR2 regulated multiple aspects of colonic epithelial homeostasis during chronic inflammation, this protective effect largely required the expression of TNFR2 in hematopoietic cells. TNFR2 promoted the apoptosis of colonic CD8+ T cells, and CD8+ T cells were necessary to promote colitis in the absence of TNFR2. Furthermore, the lack TNFR2 on CD8+ T cells was sufficient to worsen colitis in an adoptive transfer model. TNFR2-regulated colonic genes were highly enriched within CD8+ T cells, regulated CD8+ T cell activation, and interacted with multiple IBD risk genes. A subset of these genes have been implicated in CD8+ T cell-driven disease in IBD patients, emphasizing a role for TNFR2 as a disease susceptibility modifier for IBD involving a CD8+ T cell-mediated mechanism.
Impaired mucosal immune homeostasis plays a central role in IBD pathology. While deletion of TNFR2 alone is insufficient to induce colitis in mice in this study and others, we identified major alterations in CD8+ T cells following loss of TNFR2, which may interact with additional triggers to modify disease outcome in IBD. In the absence of IL10-induced immune tolerance, the protective role of TNFR2 was evident; Il10−/−Tnfr2−/− mice developed severe and highly penetrant early onset colitis that provided a unique opportunity to study how the loss of TNFR2 alters the mucosal immune system to induce colonic inflammation and CAC. The absence of TNFR2 in Il10−/−Tnfr2−/− mice increased colonic CD8+ T cells by decreasing apoptosis, which was presumably due to a lack of TNF-TNFR2-induced activation-induced cell death. This is the first report showing that TNFR2 inhibits CD8+ T cell driven pathogenicity in colonic inflammation. These findings are in agreement with the known role for TNFR2 to promote activation-induced cell death in CD8+ T cells16. While it is unclear if there are deficiencies in the balance between CD8+ T cell apoptosis and activation in IBD, there is evidence that autoreactive CD8+ T cells are involved in pathogenesis of both IBD and CAC28. Furthermore, differential expression patterns within CD8+ T cells have been associated with either mild disease, or poor prognosis and relapse in both UC and CD patients17. We identified TNFR2-regulated colonic transcripts (Fig 5), which not only overlap with the genes in this differential CD8+ T cell signature, but also are involved in CD8+ T cell activation and include IBD susceptibility loci (Suppl. Table 3). Our data show that CD8+ T cells were necessary for colitis severity in Il10−/−Tnfr2−/− mice and that TNFR2 on CD8+ T cells attenuated colitis in a CD8+ T cell adoptive transfer model (Fig 6 & 7). Therefore, our results uncover a novel role for TNFR2 on CD8+ T cells to modify disease outcomes in colitis.
Using bone marrow chimeras in which Il10−/− or Il10−/−Tnfr2−/− bone marrow was engrafted into either Il10−/− or Il10−/−Tnfr2−/− mice, we determined that TNFR2 in the hematopoietic compartment protected against inflammation. However, Tnfr2−/− in radioresistant cells was also required for tumor development. Thus, we conclude that hematopoietic expression of TNFR2 ameliorates chronic colonic inflammation, and subsequent CAC, by regulating CD8+ T cells. The specific role for non-hematopoietic TNFR2 may involve effects specifically in the colonic epithelium, as TNFR2 prevented epithelial hyperproliferation, apoptosis, and DNA damage during inflammation, in agreement with our previous results showing a protective role for TNFR2 in colonic epithelial cells10. However, we cannot rule out a role for TNFR2 in other non-immune cell types. Therefore, our data provide novel evidence uncovering a critical mechanism for TNFR2 to protect from colonic inflammation and CAC in mice.
The basis for the therapeutic effect of anti-TNF monoclonal antibodies is complex, and appears to involve mechanisms other than simply blocking TNF receptor signaling5. Multiple studies have shown that anti-TNF therapies effectively reduce lamina propria CD8+ T cells, but that this effect is limited to responsive IBD patients36–38. Furthermore, this action on CD8+ T cells may be independent of TNFR231; thus, it is likely that anti-TNF treatments would reduce CD8+ T cells, regardless of TNFR2. Understanding how TNFR2 restrains CD8+ T cells and induces apoptosis in health and disease may lead to novel IBD therapies, particularly for those patients who are refractory to anti-TNF treatment.
In addition to its role in IBD, TNFR2 may be protective in several other diseases. For instance, TNFR2 agonists induce autoreactive CD8+ T cell apoptosis39 and pancreatic regeneration40,41 in type I diabetes. TNFR2 may also promote the proliferation of neural and cardic stem cells42,43. TNFR2 expression also defines a highly suppressive subset of CD4+Foxp3+ Tregs, which suppress inflammation and tumorigenesis44. Given its restricted cellular distribution, TNFR2 activation may hold promise as a targeted therapeutic approach for IBD. Based on our data, this approach may target pathogenic CD8+ T cells, and potentially epithelial cells at sites of active inflammation.
In summary, we have identified a novel protective mechanism through which TNFR2 inhibits CD8+ T cells to inhibit colitis and colitis-associated colon cancer. TNFR2 inhibits the expansion and pathogenic role of colonic CD8+ T cells, and, in the absence of TNFR2, CD8+ T cells drive aggressive inflammation. Furthermore, TNFR2 regulates CD8+ T cell-specific genes that act as genetic susceptibility modifiers for IBD and mitigates the development of a pro-colitogenic milieu. This study on the anti-inflammatory functions of TNFR2 in colitis and CAC bears immense significance as it provides ample opportunities for future scientific discovery and refined therapeutic approaches for these diseases, especially in subclasses of IBD patients that have CD8+ T cell dysfunction.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01DK056008 and R01DK54993 (to D.B.P.), a fellowship from the Canadian Institutes of Health Research (to P.E.D.), a fellowship from the Crohn’s and Colitis Foundation of America (to P.E.D.), a fellowship from the California Institute for Regenerative Medicine TG-01168 (to C.Y.L.), and an NIH award P30DK058404 to the Vanderbilt Digestive Diseases Research Center. We thank Drs. Wendy Garrett and Vanja Lazarevic for critical review of the manuscript.
Study design was done by SP, PED and DBP. Data acquisition and analysis was done by SP and CYL. Data interpretation was done by SP, PED, CYL and DBP. Statistical analysis was done by SP and CYL. Manuscript writing was done by SP and DBP, with critical revisions by PED, MKW and DBP. Technical support was provided by NG and MKW. Study supervision was conducted by PED and DBP. This work was supported by NIH grants R01DK056008 and R01DK54993 (to D.B.P.), a fellowship from the Canadian Institutes of Health Research (to P.E.D.), a fellowship from the Crohn’s and Colitis Foundation of America (to P.E.D.), a fellowship from the California Institute for Regenerative Medicine TG-01168 (to C.Y.L.), and an NIH award P30DK058404 to the Vanderbilt Digestive Diseases Research Center. There are no conflicts of interests to declare for any author. RNA sequencing data has been deposited in Gene Expression Omnibus (Accession GSE65408, private reviewer link: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=qxyrsycmrzoztsn&acc=GSE65408).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loetscher H, Schlaeger EJ, Lahm HW, et al. Purification and partial amino acid sequence analysis of two distinct tumor necrosis factor receptors from HL60 cells. J Biol Chem. 1990;265:20131–20138. [PubMed] [Google Scholar]
- 2.Murch SH, Lamkin VA, Savage MO, et al. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991;32:913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 4.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 5.Dube P, Punit S, Polk DB. Redeeming an old foe: Protective as well as pathophysiological roles for tumor necrosis factor in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2014 doi: 10.1152/ajpgi.00142.2014. :ajpgi 00142 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sashio H, Tamura K, Ito R, et al. Polymorphisms of the TNF gene and the TNF receptor superfamily member 1B gene are associated with susceptibility to ulcerative colitis and Crohn's disease, respectively. Immunogenetics. 2002;53:1020–1027. doi: 10.1007/s00251-001-0423-7. [DOI] [PubMed] [Google Scholar]
- 7.Waschke KA, Villani AC, Vermeire S, et al. Tumor necrosis factor receptor gene polymorphisms in Crohn's disease: association with clinical phenotypes. Am J Gastroenterol. 2005;100:1126–1133. doi: 10.1111/j.1572-0241.2005.40534.x. [DOI] [PubMed] [Google Scholar]
- 8.Mizoguchi E, Mizoguchi A, Takedatsu H, et al. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122:134–144. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser GC, Polk DB. Tumor necrosis factor alpha regulates proliferation in a mouse intestinal cell line. Gastroenterology. 1997;112:1231–1240. doi: 10.1016/s0016-5085(97)70135-5. [DOI] [PubMed] [Google Scholar]
- 10.Corredor J, Yan F, Shen CC, et al. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol. 2003;284:C953–C961. doi: 10.1152/ajpcell.00309.2002. [DOI] [PubMed] [Google Scholar]
- 11.Su L, Nalle SC, Shen L, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizoguchi E, Hachiya Y, Kawada M, et al. TNF receptor type I-dependent activation of innate responses to reduce intestinal damage-associated mortality. Gastroenterology. 2008;134:470–480. doi: 10.1053/j.gastro.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 13.Ebach DR, Newberry R, Stenson WF. Differential role of tumor necrosis factor receptors in TNBS colitis. Inflamm Bowel Dis. 2005;11:533–540. doi: 10.1097/01.mib.0000163698.34592.30. [DOI] [PubMed] [Google Scholar]
- 14.Dayer Schneider J, Seibold I, Saxer-Sekulic N, et al. Lack of TNFR2 expression by CD4(+) T cells exacerbates experimental colitis. Eur J Immunol. 2009;39:1743–1753. doi: 10.1002/eji.200839132. [DOI] [PubMed] [Google Scholar]
- 15.Nakai M, Sudo K, Yamada Y, et al. The role of the tumor necrosis factor receptor in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice. Dig Dis Sci. 2005;50:1669–1676. doi: 10.1007/s10620-005-2913-1. [DOI] [PubMed] [Google Scholar]
- 16.Teh HS, Seebaran A, Teh SJ. TNF receptor 2-deficient CD8 T cells are resistant to Fas/Fas ligand-induced cell death. J Immunol. 2000;165:4814–4821. doi: 10.4049/jimmunol.165.9.4814. [DOI] [PubMed] [Google Scholar]
- 17.Lee JC, Lyons PA, McKinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest. 2011;121:4170–4179. doi: 10.1172/JCI59255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goettel JA, Scott Algood HM, Olivares-Villagomez D, et al. KSR1 protects from interleukin-10 deficiency-induced colitis in mice by suppressing T-lymphocyte interferon-gamma production. Gastroenterology. 2011;140:265–274. doi: 10.1053/j.gastro.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grcevic D, Lee SK, Marusic A, et al. Depletion of CD4 and CD8 T lymphocytes in mice in vivo enhances 1,25-dihydroxyvitamin D3-stimulated osteoclast-like cell formation in vitro by a mechanism that is dependent on prostaglandin synthesis. J Immunol. 2000;165:4231–4238. doi: 10.4049/jimmunol.165.8.4231. [DOI] [PubMed] [Google Scholar]
- 20.Tajima M, Wakita D, Noguchi D, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med. 2008;205:1019–1027. doi: 10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy RJ, Hoper M, Deodhar K, et al. Interleukin 10-deficient colitis: new similarities to human inflammatory bowel disease. Br J Surg. 2000;87:1346–1351. doi: 10.1046/j.1365-2168.2000.01615.x. [DOI] [PubMed] [Google Scholar]
- 22.Franke A, Balschun T, Karlsen TH, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 23.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rennick D, Davidson N, Berg D. Interleukin-10 gene knock-out mice: a model of chronic inflammation. Clin Immunol Immunopathol. 1995;76:S174–S178. doi: 10.1016/s0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 25.Dube PE, Yan F, Punit S, et al. Epidermal growth factor receptor inhibits colitis-associated cancer in mice. J Clin Invest. 2012;122:2780–2792. doi: 10.1172/JCI62888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 27.Davidson NJ, Fort MM, Muller W, et al. Chronic colitis in IL-10−/− mice: insufficient counter regulation of a Th1 response. Int Rev Immunol. 2000;19:91–121. doi: 10.3109/08830180009048392. [DOI] [PubMed] [Google Scholar]
- 28.Muller S, Lory J, Corazza N, et al. Activated CD4+ and CD8+ cytotoxic cells are present in increased numbers in the intestinal mucosa from patients with active inflammatory bowel disease. Am J Pathol. 1998;152:261–268. [PMC free article] [PubMed] [Google Scholar]
- 29.Brehm MA, Daniels KA, Welsh RM. Rapid production of TNF-alpha following TCR engagement of naive CD8 T cells. J Immunol. 2005;175:5043–5049. doi: 10.4049/jimmunol.175.8.5043. [DOI] [PubMed] [Google Scholar]
- 30.Kim EY, Teh SJ, Yang J, et al. TNFR2-deficient memory CD8 T cells provide superior protection against tumor cell growth. J Immunol. 2009;183:6051–6057. doi: 10.4049/jimmunol.0803482. [DOI] [PubMed] [Google Scholar]
- 31.Twu YC, Gold MR, Teh HS. TNFR1 delivers pro-survival signals that are required for limiting TNFR2-dependent activation-induced cell death (AICD) in CD8+ T cells. Eur J Immunol. 2011;41:335–344. doi: 10.1002/eji.201040639. [DOI] [PubMed] [Google Scholar]
- 32.Heng TS, Painter MW Immunological Genome Project C. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Windgassen D, Papoutsakis ET. Comparative analysis of transcriptional profiling of CD3+, CD4+ and CD8+ T cells identifies novel immune response players in T-cell activation. BMC Genomics. 2008;9:225. doi: 10.1186/1471-2164-9-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Croft D, O'Kelly G, Wu G, et al. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 2011;39:D691–D697. doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ten Hove T, van Montfrans C, Peppelenbosch MP, et al. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut. 2002;50:206–211. doi: 10.1136/gut.50.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eder P, Lykowska-Szuber L, Krela-Kazmierczak I, et al. The influence of infliximab and adalimumab on the expression of apoptosis-related proteins in lamina propria mononuclear cells and enterocytes in Crohn's disease - an immunohistochemical study. J Crohns Colitis. 2013;7:706–716. doi: 10.1016/j.crohns.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Ferkolj I, Ihan A, Markovic S, et al. Infliximab reduces the number of activated mucosal lymphocytes in patients with Crohn's disease. J Gastrointestin Liver Dis. 2006;15:231–235. [PubMed] [Google Scholar]
- 39.Ban L, Zhang J, Wang L, et al. Selective death of autoreactive T cells in human diabetes by TNF or TNF receptor 2 agonism. Proc Natl Acad Sci U S A. 2008;105:13644–13649. doi: 10.1073/pnas.0803429105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9:482–493. doi: 10.1038/nrd3030. [DOI] [PubMed] [Google Scholar]
- 41.Kodama S, Kuhtreiber W, Fujimura S, et al. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- 42.Arnett HA, Mason J, Marino M, et al. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 43.Al-Lamki RS, Lu W, Wang J, et al. TNF, acting through inducibly expressed TNFR2, drives activation and cell cycle entry of c-Kit+ cardiac stem cells in ischemic heart disease. Stem Cells. 2013;31:1881–1892. doi: 10.1002/stem.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Subleski JJ, Kopf H, et al. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.