Abstract
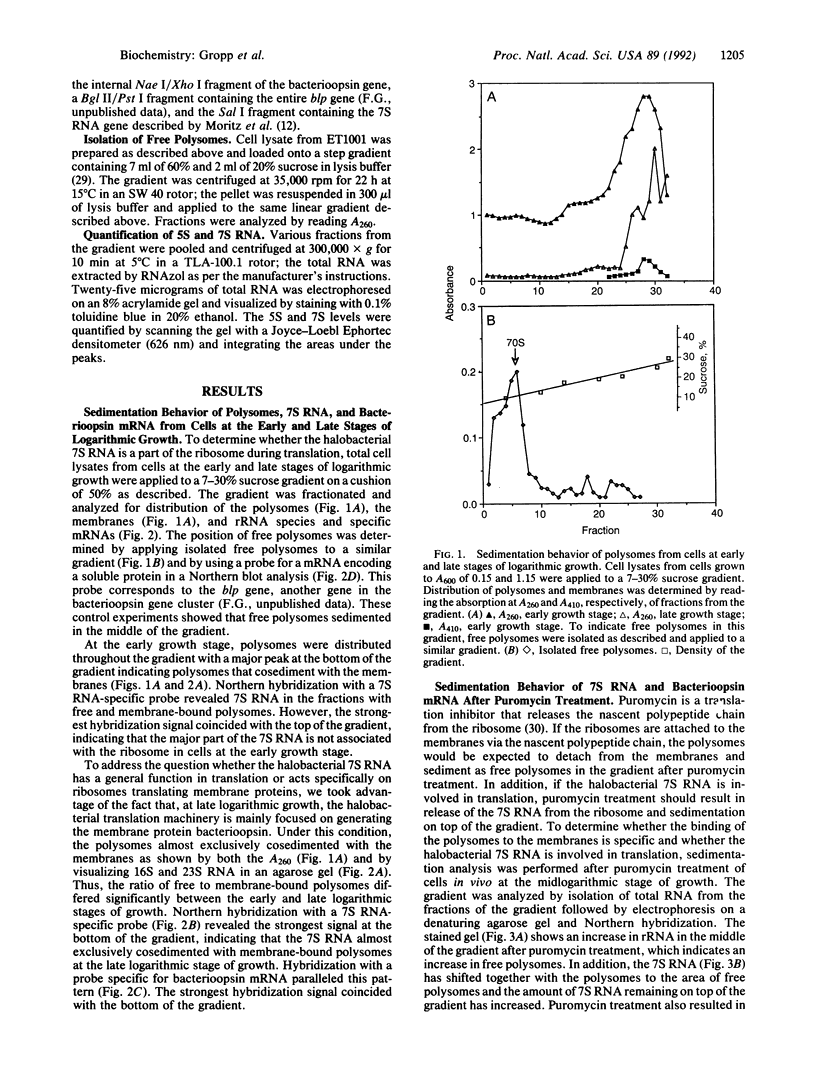
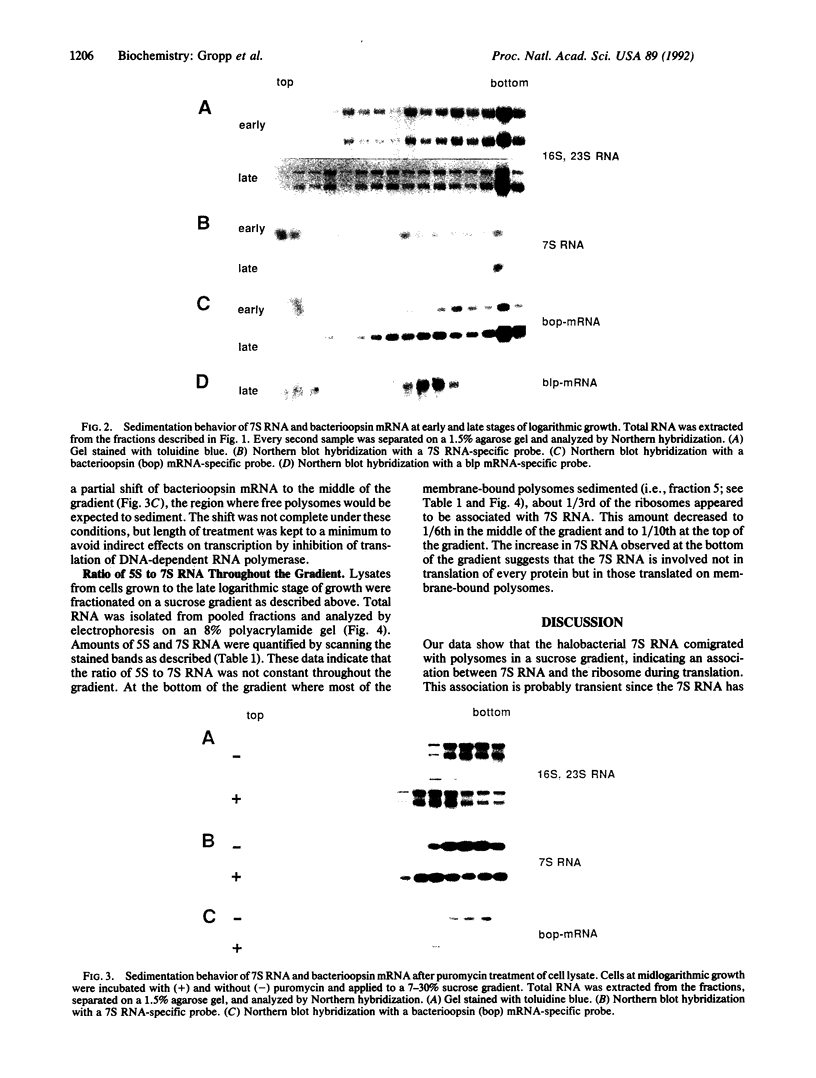
The sedimentation behavior of the halobacterial 7S RNA and bacterioopsin mRNA was assessed after application of total cell lysates to sucrose gradients. These two RNAs cosedimented predominantly with membrane-bound polysomes, and the quantity of 7S RNA bound to the ribosomes was directly correlated with the expression of bacterioopsin. Puromycin treatment released the 7S RNA from the polysomes, indicating that it is transiently associated with protein translation. We suggest that halobacteria contain a signal-recognition-like particle involved in translation of membrane-associated proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanck A., Oesterhelt D., Ferrando E., Schegk E. S., Lottspeich F. Primary structure of sensory rhodopsin I, a prokaryotic photoreceptor. EMBO J. 1989 Dec 20;8(13):3963–3971. doi: 10.1002/j.1460-2075.1989.tb08579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck A., Oesterhelt D. The halo-opsin gene. II. Sequence, primary structure of halorhodopsin and comparison with bacteriorhodopsin. EMBO J. 1987 Jan;6(1):265–273. doi: 10.1002/j.1460-2075.1987.tb04749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. Genes for 7S RNAs can replace the gene for 4.5S RNA in growth of Escherichia coli. J Bacteriol. 1991 Mar;173(5):1835–1837. doi: 10.1128/jb.173.5.1835-1837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. Mutations in the gene for EF-G reduce the requirement for 4.5S RNA in the growth of E. coli. Cell. 1987 Jun 19;49(6):825–833. doi: 10.1016/0092-8674(87)90620-9. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Majumdar A., Dunn R., Makabe O., RajBhandary U. L., Khorana H. G., Ohtsuka E., Tanaka T., Taniyama Y. O., Ikehara M. Bacteriorhodopsin: partial sequence of mRNA provides amino acid sequence in the precursor region. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3398–3402. doi: 10.1073/pnas.78.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellweg H. G., Sumper M. Identification of a bacterio-opsin species with a N-terminally extended amino acid sequence. FEBS Lett. 1980 Jul 28;116(2):303–306. doi: 10.1016/0014-5793(80)80668-5. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Schekman R. The role of stress proteins in membrane biogenesis. Trends Biochem Sci. 1988 Oct;13(10):384–388. doi: 10.1016/0968-0004(88)90180-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaine B. P. Structure of the archaebacterial 7S RNA molecule. Mol Gen Genet. 1990 May;221(3):315–321. doi: 10.1007/BF00259394. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Duschl A., Hatfield G. W., May K., Oesterhelt D. The primary structure of a halorhodopsin from Natronobacterium pharaonis. Structural, functional and evolutionary implications for bacterial rhodopsins and halorhodopsins. J Biol Chem. 1990 Jan 25;265(3):1253–1260. [PubMed] [Google Scholar]
- Lechner J., Sumper M. The primary structure of a procaryotic glycoprotein. Cloning and sequencing of the cell surface glycoprotein gene of halobacteria. J Biol Chem. 1987 Jul 15;262(20):9724–9729. [PubMed] [Google Scholar]
- Meyer D. I. Preprotein conformation: the year's major theme in translocation studies. Trends Biochem Sci. 1988 Dec;13(12):471–474. doi: 10.1016/0968-0004(88)90233-2. [DOI] [PubMed] [Google Scholar]
- Moritz A., Lankat-Buttgereit B., Gross H. J., Goebel W. Common structural features of the genes for two stable RNAs from Halobacterium halobium. Nucleic Acids Res. 1985 Jan 11;13(1):31–43. doi: 10.1093/nar/13.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J. Two pumps, one principle: light-driven ion transport in halobacteria. Trends Biochem Sci. 1989 Feb;14(2):57–61. doi: 10.1016/0968-0004(89)90044-3. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Bernstein H. D., Strub K., Zopf D., Wilhelm H., Walter P. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science. 1990 Nov 23;250(4984):1111–1117. doi: 10.1126/science.1701272. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Siegel V., Hansen W., Walter P. Small ribonucleoproteins in Schizosaccharomyces pombe and Yarrowia lipolytica homologous to signal recognition particle. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4315–4319. doi: 10.1073/pnas.85.12.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poritz M. A., Strub K., Walter P. Human SRP RNA and E. coli 4.5S RNA contain a highly homologous structural domain. Cell. 1988 Oct 7;55(1):4–6. doi: 10.1016/0092-8674(88)90003-7. [DOI] [PubMed] [Google Scholar]
- Ramírez C., Matheson A. T. A gene in the archaebacterium Sulfolobus solfataricus that codes for a protein equivalent to the alpha subunits of the signal recognition particle receptor in eukaryotes. Mol Microbiol. 1991 Jul;5(7):1687–1693. doi: 10.1111/j.1365-2958.1991.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Synthesis of exported proteins by membrane-bound polysomes from Escherichia coli. Eur J Biochem. 1977 May 2;75(1):43–53. doi: 10.1111/j.1432-1033.1977.tb11502.x. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Unity in function in the absence of consensus in sequence: role of leader peptides in export. Science. 1989 Mar 3;243(4895):1156–1159. doi: 10.1126/science.2646712. [DOI] [PubMed] [Google Scholar]
- Ribes V., Römisch K., Giner A., Dobberstein B., Tollervey D. E. coli 4.5S RNA is part of a ribonucleoprotein particle that has properties related to signal recognition particle. Cell. 1990 Nov 2;63(3):591–600. doi: 10.1016/0092-8674(90)90454-m. [DOI] [PubMed] [Google Scholar]
- Seehra J. S., Khorana H. G. Bacteriorhodopsin precursor. Characterization and its integration into the purple membrane. J Biol Chem. 1984 Apr 10;259(7):4187–4193. [PubMed] [Google Scholar]
- Sugiyama Y., Maeda M., Futai M., Mukohata Y. Isolation of a gene that encodes a new retinal protein, archaerhodopsin, from Halobacterium sp. aus-1. J Biol Chem. 1989 Dec 15;264(35):20859–20862. [PubMed] [Google Scholar]
- Suissa M., Schatz G. Import of proteins into mitochondria. Translatable mRNAs for imported mitochondrial proteins are present in free as well as mitochondria-bound cytoplasmic polysomes. J Biol Chem. 1982 Nov 10;257(21):13048–13055. [PubMed] [Google Scholar]
- Sumper M., Herrmann G. Studies on the biosynthesis of bacterio-opsin. Demonstration of the existence of protein species structurally related to bacterio-opsin. Eur J Biochem. 1978 Aug 15;89(1):229–235. doi: 10.1111/j.1432-1033.1978.tb20917.x. [DOI] [PubMed] [Google Scholar]
- Uegaki K., Sugiyama Y., Mukohata Y. Archaerhodopsin-2, from Halobacterium sp. aus-2 further reveals essential amino acid residues for light-driven proton pumps. Arch Biochem Biophys. 1991 Apr;286(1):107–110. doi: 10.1016/0003-9861(91)90014-a. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Wölfer U., Dencher N. A., Büldt G., Wrede P. Bacteriorhodopsin precursor is processed in two steps. Eur J Biochem. 1988 May 16;174(1):51–57. doi: 10.1111/j.1432-1033.1988.tb14061.x. [DOI] [PubMed] [Google Scholar]
- Zopf D., Bernstein H. D., Johnson A. E., Walter P. The methionine-rich domain of the 54 kd protein subunit of the signal recognition particle contains an RNA binding site and can be crosslinked to a signal sequence. EMBO J. 1990 Dec;9(13):4511–4517. doi: 10.1002/j.1460-2075.1990.tb07902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Analysis of the distribution of charged residues in the N-terminal region of signal sequences: implications for protein export in prokaryotic and eukaryotic cells. EMBO J. 1984 Oct;3(10):2315–2318. doi: 10.1002/j.1460-2075.1984.tb02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]