Abstract
Although human association studies suggest a link between polymorphisms in the gene encoding transforming growth factor (TGF) β1 and differing blood pressure levels, a causative mechanism for this correlation remains elusive. Recently we have generated a series of mice with graded expression of TGFβ1, ranging from approximately 10% to 300% compared to normal. We have found that blood pressure and plasma volume are negatively regulated by TGFβ1. Of note, the 10% hypomorph exhibits primary aldosteronism and markedly impaired urinary excretion of water and electrolytes. We here review previous literature highlighting the importance of TGFβ signaling as a natriuretic system, which we postulate is a causative mechanism explaining how polymorphisms in TGFβ1 could influence blood pressure levels.
Keywords: Corticosteroid, collecting duct, epithelial sodium channel, endothelin, nitric oxide
INTRODUCTION
Transforming growth factor β1 (TGFβ1) is a pleiotropic cytokine, that is ubiquitously expressed in most tissues with a wide range of biological functions including senescence [1], cell proliferation [2], apoptosis [3], tumor suppression [2], differentiation [4], migration [5], immunity [6], osteogenesis [7], adipogenesis [8], and wound healing [9].
TGFβ1 plays a causative role in the development of cardiovascular-renal complications in many pathophysiological conditions [10]. Patients with chronic diseases, including hypertension, diabetes mellitus and hypercholesterolemia, develop end organ damage (e.g. cardiac dysfunction, arteriosclerosis and chronic renal failure) that substantially affects morbidity and mortality. Prior studies show that TGFβ1 is an important cause of fibrosis [11, 12], extracellular matrix accumulation [13] and epithelial/endothelial-mesenchymal transformation [14, 15], all of which are pathogenic in the development of end organ damage.
TGFβ1 is induced by components of the renin-angiotensin-aldosterone system (RAAS), and the RAAS plays an important role in the regulation of blood pressure. It is well established that blocking RAAS is effective in treating cardiovascular and renal complications that develop as a result of hypertension and diabetes mellitus [15–20]. Although induction of TGFβ1 by components of RAAS mediates many of the hypertrophic and fibrogenic changes leading to cardiovascular-renal complications, it is still controversial as to whether TGFβ1 can be a direct target to prevent such complications. Indeed, whether TGFβ1 plays a role in the regulation of blood pressure independent of RAAS is not clearly demonstrated yet. However, we will review recent data that suggest TGFβ1 may have an integral role in blood pressure regulation.
Previously, TGFβ1 was shown to profoundly suppress adrenal production of corticosteroids [21–23]. Recently, a set of C57BL/6 mice having 5 different levels of Tgfb1 mRNA expression, corresponding to ~10 %, ~60 %, 100 %, ~200 %, and ~300% of normal, have been generated by genetically modifying the 3’ untranslated regions (UTR) of the mRNA. Analysis of these mice showed that blood pressure is negatively regulated by TGFβ1 [24]. It is noteworthy that the mice with ~10 % wild type (WT) Tgfb1 expression exhibit impaired diuresis and natriuresis and primary aldosteronism, resulting in plasma volume expansion and hypertension [24].
These in vivo findings demonstrate that TGFβ1 directly suppresses the adrenocortical synthesis of mineralocorticoids and interferes with their activation of renal tubular sodium reabsorption, suggesting that TGFβ1 is critically maintaining sodium and water homeostasis and controlling blood pressure. Here we review recent findings, focusing on the role of TGFβ1 in regulation of fluid homeostasis and blood pressure.
TGFβ SIGNALING
The TGFβ superfamily, consisting of more than 30 members, contains two subgroups; the TGFβ-like subgroup includes TGFβs, Nodals, activins, as well as several growth factors, and the Bone Morphogenetic Protein (BMP)-like subgroup includes BMPs and anti-Muellerian hormone. TGFβ family proteins are encoded as large precursors containing a short secretory signal at the N-terminus, followed by a relatively large pro-peptide region that is cleaved to form the latency associated peptide (LAP), and a C-terminus encoding the mature protein. These genes are synthesized as inactive homodimeric precursors, from which the dimeric mature protein is cleaved. TGFβ1, the focus of this review, is one of three isoforms (TGFβ1, TGFβ2, and TGFβ3) of TGFβ. It is synthesized by virtually all cell types and secreted as an inactive precursor consisting of TGFβ1 and its cleaved but still bound latency associated peptide (LAP) [25, 26]. This “small latent complex” and latent TGFβ binding proteins (LTBP) bind and form the “large latent complex”. LTBP and LAP are enzymatically cleaved by plasmin, thrombospondin, matrix metalloproteinases (MMP) 2 and 9, reactive oxygen species, and two members of the integlin family αvβ6 and αvβ8 [27], to activate TGFβ.
TGFβ signaling of TGFβ family members are transmitted via transmembrane complexes consisting of type I and type II receptors. To date, they have identified seven type I receptors and five type II receptors. One layer of specificity in the TGFβ signaling is achieved by the ability of different ligands to bind to different combinations of type I and type II receptors. The signal transduction of TGFβs involves TGFβ type I receptor (TβRI) and TGFβ type II receptor (TβRII). Access to these receptors can be regulated by type III accessory receptors including endoglin and betaglycan. TGFβ type III receptors are not involved directly in TGFβ signal transduction but are thought to bind and retain TGFβ and facilitate delivery to TGFβ receptors.
Activation of the TGFβ signaling pathway begins with TGFβ binding to the TβRII dimer, this is in turn recruits a TβRI dimer, which forms a hetero-tetrameric complex with the ligand [28]. The serine/threonine kinase domains of the TβRII phosphorylate and subsequently activate the TβRI [27, 29]. Activation of TβRI leads to signal propagation by at least two routes: the SMAD-independent non-canonical pathways and the SMAD-dependent canonical pathway.
In the SMAD-dependent pathway, activation of TβRI facilitates phosphorylation of receptor regulated SMAD proteins (R-SMAD), such as SMAD2 and SMAD3. Upon phosphorylation by TβRI, R-SMADs obtain a high affinity for a co-SMAD (e.g. SMAD4) and form a complex. This R-SMAD/co-SMAD complex translocates to the nucleus, associates with other transcription factors, and regulates transcriptional responses.
In the non-canonical pathways, the signal from the activated TGFβ receptor complex is transmitted via other factors, including phosphoinositide 3-kinase (PI3K), p38 mitogen-activated protein kinase (MAPK), tumor necrosis factor (TNF) receptor-associated factor 4 (TRAF4), TRAF6, TGFβ-activated kinase 1 (TAK1/MAP3K7), Rho, Akt/protein kinase B, extracellular signal-regulated kinase (ERK), nuclear factor-κB (NF-κB), or c-jun N-terminal kinase (JNK) [27, 29, 30].
In addition to the complexity of canonical and non-canonical TGFβ signaling pathway, other signaling pathways, such as the Hedgehog, Wnt, Notch, Ras and interferon pathways, can influence TGFβ signaling.
HYPERTENSION AND TGFβ
A study at a U.S. outpatient clinic found that both African-American and Caucasian patients with hypertension have higher serum TGFβ1 levels than their respective normotensive controls [31]. In African-Americans, hypertension-associated end-stage renal disease is more frequent [32] and serum TGFβ1 levels are higher than in Caucasian hypertensive patients [31]. In addition, the allele encoding proline at codon 10 of TGFβ1 was more frequent in blacks compared with whites [31]. Higher steady-state levels of TGFβ1 mRNA also correlated with hypertension, though no direct causal effect has been proven [31]. Likewise, the serum concentration of TGFβ1 was higher in hypertensive patients with microalbuminuria and left ventricular hypertrophy than in patients without cardiorenal damage [33]. These results suggest that there is a correlation among serum TGFβ1 levels, polymorphisms for the TGFβ1 gene, and the severity of both hypertension as well as resultant hypertensive organ damage.
The 915C single nucleotide polymorphism (SNP) in human TGFB1, leading to proline at residue 25 within the signal peptide sequence, is associated with reduced risk of hypertension in both a U.S. and a European population [34, 35]. The 869C polymorphism, resulting in proline at residue 10 within the signal peptide sequence, is also associated with an increased risk of hypertension in an Asian population [36, 37]. Whether or not and how the polymorphisms affect blood pressure have not been elucidated.
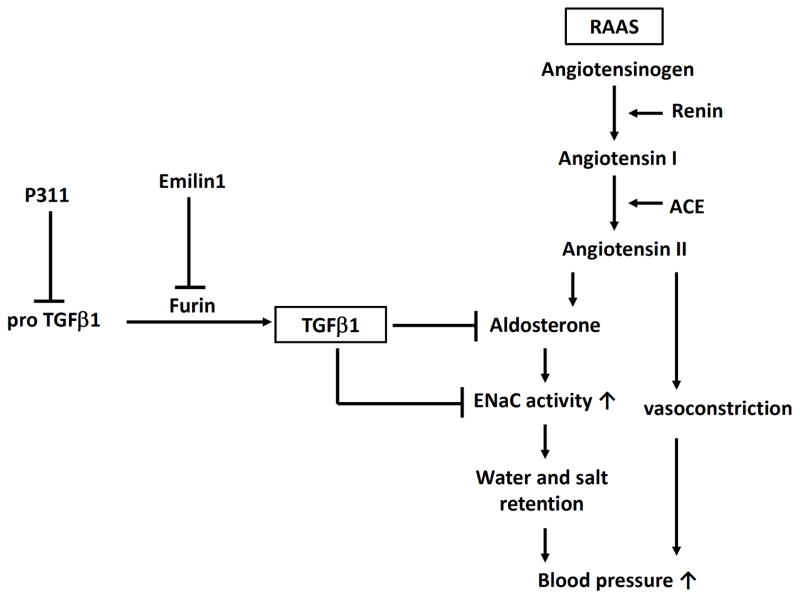
In animal studies, the pan-TGFβ neutralizing antibody against TGFβ1, TGFβ2 and TGFβ3 (1D11) attenuates hypertension in a rat model of hypertensive chronic kidney disease due to renal mass reduction [38]. Emilin1, which is a secreted glycoprotein associated with the extracellular matrix of blood vessels, regulates TGFβ availability [39]. Emilin1 binds only immature pro-TGFβ and prevents its maturation by the protein convertase furin. Therefore, lack of Emilin1 facilitates conversion of pro-TGFβ to the mature TGFβ, leading to a subsequent increase in TGFβ signaling (Fig. 1). Emilin1 knockout mice display increased TGFβ signaling in the vessel wall as well as arterial hypertension. These Emilin1 knockout mice have a reduction in the blood vessel diameter, which leads to increased peripheral vascular resistance and elevated blood pressure [39]. Hypertension was rescued to normal levels upon inactivation of a single Tgfb1 allele in Emilin1 knockout mice [39]. This study highlights the importance of modulation in TGFβ availability in the pathogenesis of hypertension by alteration of peripheral vascular resistance.
Fig 1.
Mechanisms whereby transforming growth factor (TGF) β1 regulates blood pressure and whereby pro-TGFβ1 is converted to mature TGFβ1. RAAS, renin-angiotensin-aldosterone system; ACE, angiotensin converting enzyme; ENaC, epithelial sodium channel.
In addition, Badri et al. have reported that P311-null (P311−/−) mice are markedly hypotensive with accompanying defects in vascular tone and contractility of vascular smooth muscle cells [40]. Functional abnormalities in P311−/− mice resulted from decreased total and active levels of TGFβ1, TGFβ2, and TGFβ3 that arose as a specific consequence of decreased translation. In contrast, P311-transgenic mice had elevated levels of TGFβ1–3 and subsequent hypertension.
On the other hand, Venkatesha et al. have reported that excess concentration of circulating soluble endoglin (sEng) contributes to the pathogenesis of preeclampsia, which is a pregnancy-specific hypertensive syndrome [41]. sEng impairs binding of TGFβ1 to its co-receptor endoglin, therefore their results suggest decreased TGFβ signaling acts to induce elevated blood pressure in preeclampsia.
Mice have been generated that completely lack TGFβ1 [42, 43], but their death from severe inflammatory disease around weaning precludes blood pressure measurements. Furthermore, maternal sources of TGFβ1 via the placenta and milk contribute to the normal appearance and perinatal survival of TGFβ1-null mice [44], suggesting that interpretation of the experimental results obtained from TGFβ1-null embryos and litters could be complicated. The inflammatory disease and early death can be circumvented if the mice also lack functional T and B cells as a result of inactivation of the recombination activating gene, Rag1 [45]. Unfortunately, lack of Rag1 blunts the hypertensive response to angiotensin II and deoxycorticosterone acetate, disturbing blood pressure regulation [46].
Given the contradictory results of the above studies looking at indirect alterations of TGFβ signaling and blood pressure, we developed a mouse model to study TGFβ signaling directly and its affect on blood pressure regulation. Since the nullizygote for TGFβ1 develops systemic inflammation and dies by 3 to 4 weeks of age [42, 43], we applied the strategy of replacing the 3’UTR of TGFβ1 gene into either the unstable Fos gene 3’UTR or the stable bovine growth hormone 3’UTR using gene targeting procedure [47]. Modifying the 3’UTR to change the expression of genes of interest in mice is based on the fact that the 3’UTR regulates the half-life of mRNAs [48], thus one can modify expression levels with no changes in the transcription and the structure of the gene product. Via this method we generated mice expressing TGFβ1 mRNA at levels of 10 ± 7 % (L/L), 62 ± 6 % (L/+), 182 ± 7 % (H/+) and 293 ± 7 % (H/H) WT mice, which likely covers the range of variation seen in the general human population due to its polymorphisms [24]. In contrast to the finding in the TGFβ1-null mice, no fatal infiltration of inflammatory cells was observed in any tissues studied of the TGFβ1 L/L mice.
The TGFβ1 L/L mice had a markedly higher systolic blood pressure than WT on a normal chow (0.29 % [w/w] sodium chloride), whether measured by the tail-cuff method (138 ± 3mmHg vs. 106 ± 3 mmHg; p < 10−5) or by telemetry (146 ± 3 mmHg vs. WT 120 ± 2 mmHg; p < 10−5). Hypertension in TGFβ1 L/L mice is associated with an increased plasma volume of approximately 50 % above WT. In the reverse direction, TGFβ1 H/H mice have a decreased plasma volume of approximately 50 % below WT, though their blood pressure is not significantly different from that of WT [24]. Notably, urine volume, osmolarity, sodium, chloride and potassium of TGFβ1 L/L mice were markedly less than normal, despite no significant difference in glomerular filtration rate or plasma creatinine compared with WT. These results suggest that TGFβ1 physiologically suppresses renal tubular sodium reabsorption and enhances urinary sodium excretion.
Indeed, TGFβ1 is expressed in all nephron segments in the kidney [49] and all tubular segments express Smad proteins (pSmad2 and Smad2, 3, and 4), the intermediates of the canonical TGFβ signaling [50], suggesting that TGFβ signaling is involved in all segments of the nephron.
Our findings demonstrate that in a mouse model with direct alteration of TGFβ signaling, elevated levels of TGFβ1 do not lead to hypertension, and in fact TGFβ1 insufficiency leads to hypertension. In this model, the hypertension associated with TGFβ1 insufficiency appears to be due sodium and water retention. Mice with increased TGFβ signaling develop volume contraction and have reduced salt and water retention.
HYPERTENSION AND SODIUM INTAKE
Humans have lived with a minimal sodium intake for several million years because of its low presence in natural foods. Sodium is one of the most essential minerals in mammalian physiology, particularly, to maintain extracellular fluid homeostasis. Therefore, physiological mechanisms to retain sodium in the body have been developed early in human evolution. One of the most important mechanisms for sodium retention is the RAAS, which is maximally activated in people with minimal sodium intake. The RAAS oversees the functions of cardiovascular system, renal system, and adrenal glands by regulating blood pressure, fluid volume, and sodium and potassium balance [51]. However, human diet has drastically and rapidly altered in recent years. The amount of sodium intake has been elevated by increased consumption of manufactured foods having high sodium content. Yanomamo Indians, an unacculturated native tribe in the Amazon rain forest, have little access to salt, alcohol, refined sugar, or dairy products. The Yanomamo have no hypertension and no increase in blood pressure by aging. Their average urinary sodium was 0.9 mmol/day and their blood pressure was 96.0/60.6 mmHg [52–54]. In contrast, in developed countries where hypertension is widespread, people consume about 100 times more sodium than its minimum requirement. These findings suggest that prevention of sodium loss which has conferred a survival advantage in ancient years now results in chronic hypertension on excessive dietary sodium. Hypertension serves as a major cause of chronic cardiovascular disease resulting in significant mortality and morbidity.
According to the Guyton hypothesis, renal salt handling mechanisms critically determines the control of the steady-state blood pressure on a long-term basis [55]. Guyton revealed that impaired renal excretory function of sodium in patients with salt-sensitive hypertension leads to an elevated blood pressure [56]. Many studies indicate that blood pressure response to sodium intake varies among individuals and that the degree of these responses can be grouped into categories of either salt sensitive or salt resistant. However, a consistent definition and precise mechanism of salt sensitivity have not yet been determined [57–59]. Recent studies suggest that nuclear mineralocorticoid and glucocorticoid receptors at different tubular segments increase sodium reabsorption and impair renal excretory function which results in salt sensitive hypertension.
In summary, excessive dietary sodium intake is essential for the development of hypertension. Since a sodium-deficient diet (~ 0.01 % [w/w]) almost completely abolished the hypertension seen in TGFβ1 L/L mice (unpublished observation), the hypertension in the L/L mice is considered to be salt sensitive in a broad sense. However, L/L mice developed hypertension on normal chow, when sodium intake was not different from that of WT, suggesting that sodium retention caused by enhanced tubular reabsorption due to genetic insufficiency of TGFβ signaling is important for the development of hypertension. TGFβ1 signaling plays a significant role in differential renal handling of sodium and water and thus could be a mechanism accounting for salt sensitivity and salt resistance.
HYPERTENSION AND SODIUM TRANSPORT IN THE KIDNEY
Each human kidney contains approximately 1 million nephrons. Each individual nephron, the renal functional unit, is composed of a single glomerulus, proximal convoluted tubule (PCT), proximal straight tubule (PST), loop of Henle, distal convoluted tubule (DCT), and a connecting tubule (CNT). In humans, roughly 180 L of fluid is filtered in the glomerulus per day. Of the glomerular filtrate approximately 99 % is reabsorbed, leaving 1.8 L per day to be excreted as urine. Around 60% of this reabsorption will take place in the proximal tubule, and 30% in the loop of Henle; the remaining 10% being absorbed in the DCT, CNT, and the collecting duct (CD). The distal nephron (DCT, CNT and CD) performs the final reabsoption of solute and water, which is mainly controlled by hormones such as vasopressin and aldosterone. The regulated reabsorption of filtered sodium by the distal tubules is important for control of blood pressure and extracellular fluid volume [57].
The Na+/K+-ATPase on the basolateral surface of tubular cells transfers sodium ions from the cytoplasm into the peritubular vessel, generating a concentration gradient of sodium between the urinary space and tubular cells. The sodium gradient created between tubular cells and the urinary space is the major driving force of sodium reabsorption. However, the actual velocity of sodium transport depends on the functional expression and opening rate of sodium channels on the surface of tubular cells (Table 1). In the proximal tubule these sodium channels include the sodium-hydrogen exchanger (NHE1~4/SLC9A1~4), sodium/glucose co-transporter (SGLT2/SLC5A2 in PCT and SGLT1/ SLC5A1 in PST), sodium/phosphate co-transporter (NPT2a/ SLC34A1, NPT2c/SLC34A3, NPT1/ SLC17A1), sodium/ bicarbonate co-transporter (NBCe1-A/SLC4A4), sodium/ iodide co-transporter (NIS/SLC5A5), sodium/monocarboxylate co-transporter (SMCT1/SLC5A8, SMCT2/SLC5A12), sodium/ sulfate co-transporter (NaS1/SLC13A1), sodium/multivitamine co-transporter (SMVT/SLC5A6), as well as other sodium-dependent co-transporters. The Na+/K+/2Cl− co-transporter 2 (NKCC2/SLC12A1) is furosemide-sensitive and expressed in the thick ascending limb of Henle’s loop (TAL), while the thiazide-sensitive Na+/Cl− co-transporter (NCC/SLC12A3) is expressed in the DCT. Aldosterone-sensitive distal nephron (ASDN) including late DCT2, CNT, and CD in the kidney performs the final reabsorption of sodium [60, 61], where the activity of epithelial Na+ channel (ENaC/SCNN1) is regulated by mineralocorticoids, angiotensin II, vasopressin, bradykinin, insulin/insulin-like growth factor, nitric oxide, adenosine triphosphate, prostaglandin E2, peroxisome proliferator-activated receptor γ and other hormonal or nonhormonal factors [62–69]. Despite the fact that the ASDN reabsorbs less than 10% of the filtered Na+, many of the genetic hypertensive syndromes have been demonstrated to be mediated by the enhanced activity of ENaC expressed in this portion of the kidney. In other words, the activity of ENaC in the ASDN is critical for the final adjustment of sodium excretion by the kidney and for the blood pressure regulation.
Table 1.
The sodium transporters in nephron segments.
| Segments | Apical Membrane | Basolateral Membrane |
|---|---|---|
|
| ||
| Proximal tubule | NHE 3/2 | Na+/K+-ATPase |
| Sodium/glucose co-transporter 1/2 | NHE 1/4 | |
| Sodium/phosphate co-transporter 1/2a/2c | Sodium/iodide co-transporter | |
| Sodium/sulfate co-transporter 1 | Sodium/bicarbonate co-transporter 1 | |
| Sodium/monocarboxylate co-transporter 1/2 | ||
| Sodium/multivitamine co-transporter | ||
|
| ||
| Loop of Henle | Na+/K+-ATPase | |
| Descending limb | NHE 3 | |
| Thin ascending limb | Na+/K+/2Cl− cotransporter 2 | NHE 1/4 |
| Thick ascending limb | NHE 3/2 | |
|
| ||
| Distal convoluted tubule | Na+/Cl− cotransporter | Na+/K+-ATPase NHE 1/4 |
|
| ||
| Collecting duct system | Epithelial Na+ channel | Na+/K+-ATPase NHE 1/4 |
NHE, Sodium-hydrogen exchanger.
ENaC belongs to the acid-sensing ion channel (ASIC)/ENaC/degenerin family and is an amiloride sensitive and highly sodium/lithium selective channel located on the apical membrane in sweat glands, colon, lung, and distal nephron of the kidney [70]. Functional ENaC consists of three different subunits (α-ENaC/SCNN1A, β-ENaC/ SCNN1B, and γ-ENaC/SCNN1G) in the kidney, which share similar structural features. Gain-of-function mutations in the β or γ subunits of ENaC result in a hypertensive phenotype (Liddle syndrome) [71], which is autosomal dominant and featured by suppressed aldosterone secretion (pseudoaldosteronism), low plasma renin activity, salt-sensitive hypertension, hypokalaemia, and metabolic alkalosis. On the other hand, loss-of-function mutations in the α, β, or γ subunits of ENaC lead to pseudohypoaldosteronism type 1 (PHA-1), a salt-losing syndrome with metabolic acidosis and hyperkalaemia with a Mendelian autosomal recessive mode of transmission [72]. Genetic deficiency in each individual subunit of the ENaC caused a lethal and early phenotype [73–75]. Interestingly, CD-specific inactivation of α-ENaC could maintain sodium balance, even on water deprivation, salt restriction, or potassium loading [76]; suggesting that the late DCT or CNT is important in sodium homeostasis.
It has been found that hypomorphic mutations in With-no-lysine kinase (WNK) 1 and 4 lead to pseudohypoaldosteronism type II (PHA-II), an autosomal dominant hereditary hyper- tension [77], by enhancing the activity of NCC [78]. WNK4 substantially reduces NCC abundance on the plasma membrane, and WNK1 can completely prevent the inhibition of NCC by WNK4 [78]. β-adrenoceptor stimulation-induced WNK4 downregulation is mediated by glucocorticoid receptors, but not by mineralocorticoid receptors [79]. In contrast, hypomorphic mutations in NCC result in Gitelman syndrome, which is an autosomal recessive salt-losing syndrome with hypokalaemia, hypocalciuria, hypomagnesemia and metabolic alkalosis [80].
These findings demonstrate that although only 10 % of the filtrated sodium is reabsorbed at the distal nephron (DCT, CNT, and CD), mutations in the genes involved in sodium reabsorption in this portion of the nephron are adequate to cause human familial hyper/hypotension syndromes.
TGFβ SIGNALING AND RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM (RAAS)
RAAS is the major system that controls blood pressure. Most components of RAAS have been demonstrated to up-regulate TGFβ signaling independently of blood pressure. For example, aliskiren, a (pro) renin inhibitor, prevents renal scarring and TGFβ1 expression without changing blood pressure in Col4a3-deficient mice [81] and in diabetic TG(mRen-2)27 rats [82]. Angiotensin II was found to increase TGFβ1 mRNA levels via the angiotensin II type 1 receptor (AT1R) in rat heart endothelial cells [83]. In proximal tubular cells, angiotensin II stimulates mRNA and protein expression of TβRII but not those of TβRI [84], leading to a further amplification of the stimulatory effects of RAAS on TGFβ signaling. Activator protein-1 (AP-1) sites in the promoter of the TβRII gene are necessary for the angiotensin II-induced transcriptional activity [84]. Angiotensin III, a degradation product of angiotensin II, also increased the mRNA levels of TGFβ1 in fibroblasts and mesangial cells [85].
Infusion of aldosterone even at doses that unalter systolic blood pressure caused an increase in urinary TGFβ1 excretion via the mineralcorticoid receptor [86]. However, no change in TGFβ1 mRNA occurred, suggesting a posttranscriptional effect of aldosterone on TGFβ1 [86]. Likewise, TGFβ1 mRNA expression was increased with an enhanced binding density of angiotensin converting enzyme (ACE) and AT1R in the kidney of rats subcutaneously receiving aldosterone [87]. Aldosterone increased mRNA and protein levels of plasminogen activator inhibitor-1 which was partially blocked by TGFβ neutralizing antibody in cultured rat renal mesangial and fibroblast cells [88]. Also, Han et al. have demonstrated that aldosterone stimulates TGFβ1 mRNA expression via ERK1/2, JNK, and AP-1 in rat mesangial cells [89]. On the other hand, Juknevicius et al. have shown that aldosterone provokes urinary excretion of TGFβ1 without influencing renal TGFβ1 transcripts, suggesting posttranscriptional enhancement of renal TGFβ1 [86]. In mice treated with NG-nitro-L-arginine methyl ester (L-NAME), a nitric oxide synthase (NOS) inhibitor, and angiotensin II, genetic deficiency of the mineralocorticoid receptor in myeloid cells significantly attenuated inflammation, fibrosis and the mRNA levels of TGFβ1 in the heart and aorta [90]. Aldosterone also increased the secretion of TGFβ1 in dendritic cells [91].
Increased renal production of TGFβ1 is associated with various diseases related to activation of RAAS, two of which are hypertension and diabetes mellitus. Urinary TGFβ1 excretion reflects renal TGFβ1 production. As such, urinary excretion of TGFβ1 is a useful marker in assessing the efficacy of RAAS inhibitors in clinical settings. For example, losartan, an AT1R blocker, has been reported to reduce urinary TGFβ1 excretion [92].
Although most of the active components of RAAS induce TGFβ1 expression, the effect of TGFβ1 on the activity of RAAS is not well understood. It has been shown that TGFβ1 at picomolar concentrations directly stimulates renin release in rat renal cortical slices, an effect which is abrogated by meclofenamate, an inhibitor of cyclooxygenase [93]. TGFβ1 also increased renin activity in cultured bovine zona glomerulosa cells [23]. In rat immortalized renal proximal tubular cells, addition of active human TGFβ1 or transfection of rat TGFβ1 cDNA increases the mRNA level of angiotensinogen; this action is at least in part mediated, via nicotinamide adenine dinucleotide oxidase, p38 MAPK and p53 [94]. An inhibitor of TβRI (SD-208) reduces plasma levels of active renin as well as cardiac and renal expression of angiotensinogen, ACE and AT1R in mice [95]. TGFβ1 also induces the protein levels and activity of ACE in rat cardiac ventricular fibroblasts [96]. In human lung fibroblasts, TGFβ1 has been shown to increase the transcript and protein levels of AT1R through the mitogen-activated protein kinase kinase 1 and 2 (MKK1/MKK2) signaling pathway [97].
We found that mice having genetically reduced TGFβ1 expression had lower plasma levels of active renin and angiotensin II and increased plasma volume compared with WT [24]. In the reverse direction, mice having genetically increased TGFβ1 expression had higher plasma levels of active renin and angiotensin II and reduced plasma volume compared with WT [24]. These findings suggest that TGFβ1 increases the activity and expression of RAAS components via both direct and indirect (changing plasma volume) mechanisms [24].
TGFβ1 SUPPRESSES SYNTHESIS OF CORTIC-OSTEROIDS
Although aldosterone stimulates the expression of TGFβ1, TGFβ1 potently inhibits aldosterone synthesis in cultured adrenocortical cells [21–23].
Hotta et al. have shown that TGFβ1 inhibits the formation of δ4-steroids including corticosterone, cortisol, androstenedione and aldosterone in cultured bovine adrenocortical cells [21]. This suggests that TGFβ1 suppresses the synthesis of a wide variety of corticosteroids. Indeed, TGFβ1 decreases the activity of cholesterol side-chain cleavage, which is the first enzymatic step of steroidogenesis, and reduces the protein levels for cytochrome P450 side-chain cleavage (P450scc, Cyp11a1), adrenodoxin and adrenodoxin reductase in cultured sheep adrenal cells [98]. TGFβ1 also decreases the transcript levels of steroidogenic acute regulatory protein (StAR), which assists the transport of cholesterol from the outer membrane to the inner membrane in the mitochondria where P450scc resides, in bovine adrenocortical cells [99]. Le Roy et al. reported that TGFβ1 reduced adrenocorticotropin-induced cortisol production and decreased the transcript levels of steroid 17α-monooxygenase (Cyp17a1), hydroxyl-δ-5-steroid dehydrogenase, 3β- and steroid δ-isomerase 1 (Hsd3b1), and StAR in the bovine adrenocortical cells [100]. These results indicate that TGFβ1 suppresses multiple enzymatic steps of steroidogenesis in the adrenal cortex.
Mineralocorticoids, including aldosterone and 11-deoxycorticosterone, are important for blood pressure regulation. Liakos et al. have reported that TGFβ1 reduced forskolin-induced production of cortisol and 11-hydroxyandrostenedione by 85% and angiotensin II-induced production of aldosterone by 80%. TGFβ1 also strongly inhibits forskolin-induced steroid 11β-hydroxylase (Cyp11b1) mRNA levels and the Cyp11b1 activity, as well as angiotensin II-induced aldosterone synthase (Cyp11b2) mRNA levels and the Cyp11b2 activity in NCI-H295R cells, derived from the human adrenocortical tumor [22]. The promoter activity of Cyp11b1, studied by the luciferase assay, was suppressed by TGFβ1; though the responsible site was different from the Smad-binding sequences, suggesting that the repression of Cyp11b1 promoter activity by TGFβ1 is indirect [22]. Indeed, TGFβ1 inhibits transcription of steroidogenic factor 1 [101], which is important for the development of adrenal and gonadal steroidogenic cells [102] and enhances transcription of steroidogenic enzymes including Cyp11a1, steroid 21-hydroxylase (Cyp21a1), Cyp11b1 and Cyp11b2 [103] by binding a shared promoter element [104].
These findings in cultured adrenocortical cells have been reproduced in mice with graded expression of TGFβ1 mRNA ranging from 10% to 300% of normal [24]. Genetically high levels of TGFβ1 lead to reduced plasma aldosterone and corticosterone levels despite the plasma volume being decreased. Increased TGFβ1 was also associated with decreased expression of Cyp11b1, Cyp11b2, Hsd3b1, and Star, whereas genetically low levels of TGFβ1 lead to increased plasma aldosterone and corticosterone levels despite the plasma volume being expanded, which is associated with increased expression of the aforementioned genes.
Plasma aldosterone and corticosterone levels were significantly higher than normal in TGFβ1 hypomorphic mice, whereas in hypermorphic mice plasma aldosterone levels were significantly lower than normal [24]. In contrast, plasma levels of active renin and angiotensin II were lower than normal in hypomorphic mice and higher than normal mice in hypermorphic mice. This seems to confirm that the suppressed adrenal functions in the TGFβ1 hypermorph and enhanced adrenal functions in the TGFβ1 hypomorph are due to primary effects of TGFβ1 on adrenocrtical cells in an autocrine and/or paracrine fashion, and not due to renin/ angiotensin influence. Additionally, hypomorphic mice had markedly higher systolic blood pressure as well as less diuresis and natriuresis than WT mice, which can be normalized by administration of spironolactone, a mineralocorticoid receptor blocker, or amiloride, an epithelial sodium channel (ENaC) blocker [24]. These results further suggest that TGFβ signaling is important for regulating fluid homeostasis at least partly via the suppression of corticosteroid synthesis and the reduced ENaC activity.
The inhibition of steroidogenesis by TGFβ1 can be dissociated from its effect on cell proliferation of bovine adrenocortical cells [21]. Qualitative histological abnormalities, including nodular hyperplasia, were not observed in the adrenal of TGFβ1 L/L mice. However, the weight of adrenal divided by body weight in TGFβ1 L/L mice was twice as large as that in WT mice. Therefore, the TGFβ1 L/L mice can reasonably be judged as having idiopathic bilateral adrenal hyperplasia and primary aldosteronism, which at least partly account for their hypertension.
Loss-of-function mutations in the PKA type I α regulatory subunit (PRKAR1A) causes Carney complex, a congenital multiple endocrine neoplasia syndrome characterized by pigmented adrenocortical nodules as the most frequent neoplasia. PRKAR1A-silenced NCI-H295R cells exhibit suppressed SMAD3 expression and resistance to TGFβ1-induced apoptosis [105], suggesting that TGFβ1 is also involved in preventing adrenocortical tumors in the multiple endocrine neoplasia syndrome. Indeed, in human adrenocortical tumors, the expression of SMAD3 varied inversely with Weiss scores for malignancy [106]. Likewise, TGFβ1 mRNA was abundantly expressed in normal adrenals and adrenocortical adenomas, but reduced in carcinomas [107].
In summary, many lines of evidence shows that TGFβ1 regulates adrenal development and inhibits steroidogenesis in the adrenal cortex. The interaction of TGFβ1 and steroido- genesis is another mechanism by which TGFβ signaling can influence blood pressure. Additionally, TGFβ1 appears to be involved in adrenocortical tumorigenesis.
TGFβ1 SUPPRESSES RENAL SODIUM REABSORPTION
In addition to the suppressive effect of TGFβ1 on adrenocortical function, TGFβ1 also directly inhibits aldosterone stimulated ENaC activity in the kidney. Aldosterone leads to elevated blood pressure via expansion of extracellular fluid volume through its stimulation of the ENaC activity, which is located in the ASDN. In the collecting duct, principal cells respond to aldosterone and absorb sodium. All of ENaCs, mineralocorticoid receptors, and Na+/K+-ATPase are necessary for this response to aldosterone and present along the ASDN. Both plasma aldosterone levels and dietary sodium intake regulate the activity of ENaC [62]. It appears that aldosterone stimulates Na+ transport by ENaC more than that by Na+/K+-ATPase.
Although aldosterone is extremely important for both Na+ homeostasis and blood pressure control via ENaC regulation, a variety of other hormones, including TGFβ1, regulate ENaC activity either independently or in parallel with aldosterone [61]. Frank et al. found that TGFβ1 decreases the expression of α-ENaC via the activation of ERK1/2 [108]. Husted et al. reported that TGFβ1 inhibits the action of mineralocorticoid and decreases Na+ entry across the apical membrane of inner medullary collecting duct cells [109, 110]. Peters et al. reported that TGFβ1 promotes internalization of β-ENaC, through its activation of phospholipase D1 (PLD1) [111]. Phosphatidic acid generated by PLD1 activates phosphatidylinositol-4-phosphate 5-kinase 1 α and generates phosphatidylinositol [4,5]-biphosphate, which stimulates NADPH oxidase 4 to generate reactive oxygen species that in turn target Cys43 residue of β-ENaC and facilitate its internalization [111]. The trafficking of β-ENaC from the cell surface to endocytic vesicles by TGFβ1 destabilizes the entire ENaC complex within the epithelial cell surface [111].
We found that the open probability, functional expression and total activity of ENaC in the collecting duct of cells microdissected from TGFβ1 hypomorphic mice, which have high plasma aldosterone levels, are significantly greater than those from WT mice [24]. In addition, collecting duct specific overexpression of TGFβ1 restores natriuresis in mice with low Tgfb1 expression despite high plasma aldosterone levels (unpublished observation). These findings indicate that TGFβ1 inhibits ENaC-mediated Na+ reabsorption not only indirectly via decreased aldosterone production in the adrenal gland, but also directly in collecting duct cells.
Protease nexin-1 (PN-1) is a member of the serpin (the abbreviation of serine protease inhibitor) family and inhibits plasmin, α-thrombin, prostasin [112] and plasminogen activators. PN-1 inhibits the activation of ENaC induced by prostasin in Xenopus oocytes, and suppression of PN-1 expression increased the baseline current of sodium in mouse cortical collecting duct cells [113]. Moreover, it has been demonstrated that aldosterone increases prostasin and decreases PN-1 and that TGFβ1 decreases prostasin and increases PN-1 [113]. Another study in a mouse collecting duct cell line (mpkCCDc14 cells) showed that TGFβ1 inhibited the transepithelial amiloride sensitive electrical current, which is accompanied by a decrease in α-ENaC expression [114]. A deletion mutant for Smad4 did not inhibit sodium current and abolished the TGFβ1-induced inhibition of sodium current. This suggests that TGFβ1 decreases ENaC functionality via a Smad4-dependent pathway.
It has been demonstrated that TGFβ1 and aldosterone differentially regulate renal sodium reabsorption in proximal tubules as well. Human, rat, and mouse proximal tubular cells express both mineralocorticoid receptors and Na+/K+-ATPase subunits [115]. In HKC11 cells, a human renal proximal tubulular cell line, aldosterone increases Na+/K+-ATPase-mediated 86Rb uptake [115]. In primary human proximal tubules, aldosterone stimulates Na+/H+ exchange activity accompanied by an increase in the expression of biotinylated Na+/H+ exchanger 3 [116]. In contrast, TGFβ1 induces dose dependent decrease in the activity of Na+/K+-ATPase [117] and the expression of α and β subunits of Na+/K+-ATPase in rabbit primary proximal tubules [118].
The precise molecular mechanism by which TGFβ1 reduces the renal actions of aldosterone is still poorly understood. However, it is probably multifactorial and likely involves differential metabolic effects of aldosterone and TGFβ1 on mitochondrial oxidative phosphorylation [117].
TGFβ1 AND NITRIC OXIDE
Nitric oxide (NO) is an endogenously generated molecule having a short half-life in vivo that regulates blood pressure by both reducing vascular resistance and increasing sodium excretion from the kidney [119]. After generation, NO quickly degrades into nitrite and nitrate, which are stable and can be measured by the Griess colorimetric method. However, since nitrite/nitrate are also derived from food, nitrite/nitrate measurement is an insensitive indicator of endogenous NO production. Three isoforms of NO synthases (NOSs) that catalyze the generation of NO from the substrate L-arginine have been identified: neuronal NOS (nNOS/NOS1), inducible NOS (iNOS/NOS2), and endothelial NOS (eNOS/NOS3). Both nNOS and eNOS are classified to constitutive NOSs, which are expressed in neurons and endothelial cells. The enzymatic activities of eNOS and nNOS are Ca++/calmodulin-dependent, and activated by various chemical and mechanical stimuli that elevate intracellular calcium. In contrast, iNOS is not expressed constitutively but can be induced in most types of cells under several pathophysiological conditions by various cytokines including interleukin-1, TNF-α and interferon-γ. The enzymatic activity of iNOS is calcium/ calmodulin-independent, so once the enzyme is induced, it can generate a large amount of NO and thus plays the major causative role in severe hypotension in septic shock.
Under normal physiological conditions, blood pressure is primarily determined by the constitutive NOS (nNOS and eNOS), as they are the primary source of endogenous NO. In animal experiments, mice lacking eNOS exhibit higher mean arterial pressure than WT by approximately 20 mmHg [120], clearly demonstrating that eNOS is involved in regulation of blood pressure. In eNOS deficient mice, a NOS inhibitor Nω-nitro-L-arginine caused a paradoxical decrease in blood pressure, suggesting that nNOS may maintain blood pressure. However, blood pressure observed in mutant nNOS mice is normal [121]. These seemingly conflicting results may suggest that nNOS significantly regulates blood pressure only when the function of eNOS is impaired. Though iNOS does not seem to be important in regulation of blood pressure under physiological conditions, blood pressure is decreased by the induction of iNOS in sepsis. The arterial pressure in iNOS deficient mice is comparable to WT, though lipopolysaccharide (LPS)-induced falls in blood pressure are completely averted [122].
Previous studies have suggested that TGFβ1 affects the production of NO by several mechanisms. Inoue et al. demonstrated that TGFβ1 dose-dependently increases both the eNOS transcript and protein levels as well as media nitrite/nitrate levels in cultured bovine aortic endothelial cells [123]. A region of the eNOS promoter containing Smad binding sites, spanning from -1269 to -935, mediates the effect of TGFβ1 on eNOS transcription [124]. In rat renal or mesenteric microvessels, TGFβ1 increases vascular diameters; this can be reversed by co-infusion of L-NAME or of sEng, a soluble form of a co-receptor of TGFβs, endoglin [41]. TGFβ1 does not change the amount of total eNOS protein or Ser1177-phosphorylated eNOS, but significantly decreased Thr495-phosphorylated eNOS, an inactive form of NOS, which was again reversed by sEng in cultured mouse endothelial cells [41]. In contrast, in human umbilical vein endothelial cells, TGFβ1 increases the protein levels of Ser1177-phosphorylated eNOS and nitrite/nitrate levels in the media, both of which can be abrogated by co-incubation with small interference RNA for phophatase and tensin homolog (PTEN) [125]. Since the siRNA for PTEN increases the amount of phosphorylated Akt, the increase in phophorylation of eNOS by TGFβ1 is likely due to the suppression of PTEN by TGFβ1 and via the subsequent Ser1177-phosphorylation of eNOS by the phosphorylation of Akt.
While TGFβ1 increases expression of eNOS, it was shown to suppress cytokine- stimulated iNOS expression in both RAW 264.7 cells and cultured smooth muscle cells [126, 127]. TGFβ1 reduces the stability and translational efficiency of iNOS mRNA and enhances the protein degradation of iNOS, but leaves transcription of iNOS unaltered [128]. In TGFβ1-null mice, iNOS mRNA and protein levels are increased in the kidney and heart, and the nitrite/nitrate levels in serum are elevated approximately fourfold over controls [129]. In transgenic mice with overexpression of TGFβ1 given LPS to induce septic shock, serum nitrite/nitrate levels are decreased and iNOS protein levels in peritoneal exudate cells are much less than in WT [130].
Endoglin is one of the TGFβ type III accessory receptors [131] and its mutation is associated with human hereditary telangiectasia type 1 [132]. The association of endoglin with the TGFβ receptor complex potentiates ALK1/Smad1 and ALK5/Smad2 and represses the ALK5/Smad3 in the TGFβ signaling pathway [133–136]. Jerkic et al. have demonstrated that in haploinsufficient endoglin (Eng+/−) mice the intravenous infusion of acetylcholine, which causes endothelium dependent vasodilation, decreases arterial pressure to a lesser extent than in WT. In addition, the pressor response to L-NAME in the Eng+/− mice is also smaller than that of WT [137]. The dilatory response of aortic rings to acetylcholine from the Eng+/− mice is smaller than that of WT, but the dilatory effect by an endothelium-independent vasodilator is comparable between Eng+/−and WT mice. These results suggest that endothelium-dependent vasodilation, which is considered to be due mainly to eNOS activation, is attenuated by the decreased expression of Eng. They also found that the protein levels of eNOS and the mRNA levels of TGFβ1 are also diminished in the Eng+/−mice. Toporsian et al. showed that the activity and expression of eNOS are decreased in the Eng+/−mice as compared with WT [138]; they also noted that the association of eNOS, endoglin and heat shock protein 90 is impaired in Eng+/− mice. This demonstrates that TGFβ signaling is important for blood pressure regulation via eNOS.
L-NAME elevated blood pressure to a similar extent in TGFβ1 L/L and WT mice. It was found that the renal protein levels for Ser1177-phosphorylated eNOS is more abundant in the TGFβ1 L/L mice than in WT, but the levels of total eNOS protein, Thr495-phosphorylated eNOS, and eNOS mRNA are not significantly different between the two groups. Similarly, the TGFβ1 L/L mice have the greater protein levels for serine473-phosphorylated Akt than WT, although TGFβ1 L/L and WT mice have comparable content of total Akt.
No significant differences between TGFβ1 L/L and WT mice were found in the serum nitrite/nitrate levels or the inducible NOS expression, although these parameters are markedly increased in TGFβ1-null mice as compared to WT mice [129]. Thus, it is unlikely that the decreased NO results in the hypertension of TGFβ1 L/L mice. Consequently, the increase in Ser1177-phosphorylated eNOS in the TGFβ1 hypomorph is unlikely to be because of the direct effect of decreased TGFβ1; though it may be due to the stimulatory effect of aldosterone on the Ser1177-phosphorylation of eNOS [139] or to the expanded plasma volume which may lead to increased shear stress [140].
Interestingly, the mRNA levels for nNOS are significantly decreased in the kidney of the TGFβ1 L/L mice. Dietary salt increases the protein expression of nNOS in freshly microdissected inner medullary collecting ducts [141]. It has been suggested that NO inhibits the activities of Na+/K+-ATPase and ENaC [142]. Since collecting duct-specific disruption of nNOS reduces urinary output of sodium and nitrite/nitrate and results in salt-dependent hypertension in mice [143], it is also possible that TGFβ1 increases natriuresis via nNOS in the collecting duct cells.
TGFβ1 AND ENDOTHELIN-1
Endothelin-1 was first discovered in cultured endothelial cells as a peptide with a function of potent vasoconstriction [144]. It was later found to exert a broad range of biological effects on cell proliferation [145], survival [146], differentiation [147], migration [148], immunity [149], osteogenesis [150], adipogenesis [151] and wound healing [152]. Endothelin-1 is formed from big endothelin-1 by endothelin converting enzymes [153]. It acts by binding to endothelin ETA [154] and ETB receptors [155], which participate in the Ca++/calmodulin- dependent [156] and phospholipase A2-dependent signaling pathways [157].
Although endothelin-1 is ubiquitously expressed in the body, endothelin-1 mRNA is most abundantly expressed in inner medullary collecting duct cells (IMCDs) among microdissected nephron segments [158, 159]. Likewise, ETB receptors are also most highly expressed in IMCDs [160]. Kohan et al. have shown that endothelin-1 inhibits arginine vasopressin-induced accumulation of adenosine 3’,5’-cyclic monophosphate (cAMP) and Na+/K+-ATPase activity in IMCDs via ETB receptors [161]. Bugaj et al. demonstrated that endothelin-1 decreases the activity of ENaC in isolated rat collecting ducts, through ETB, but not ETA receptors [162]. Endothelin-1 decreases sodium reabsorption in isolated mouse collecting ducts, through both ETA and ETB receptors [163]. The inhibition of ENaC by endothelin-1 is abolished by a selective Src kinase inhibitor, PP2 [164].β1Pix activated by endothelin-1 decreases the channel number of ENaC through the binding of 14-3-3β, which impairs the binding of 14-3-3β to Nedd4-2, a ubiquitin ligase; and hence ENaC is promoted to be ubiquitinated and degraded [165]. Although β type splice variant of nNOS is the only NOS isoform expressed in mouse IMCD segment-3 cells, endothelin-1 stimulates nitric oxide production via ETB receptors in those cells [166].
In gene targeting studies, mice completely lacking endothelin-1 have severe anomalies in the heart and aorta with craniofacial abnormalities and die at age 10–12 days post coitum [167]. However, endothelin-1 haplodeficient mice had an elevated blood pressure as compared with WT, suggesting that endothelin-1 plays a physiological role in decreasing blood pressure [167]. Likewise, collecting duct-specific endothelin-1 or ETB receptor knockout mice develop high blood pressure and show decreased urinary excretion of sodium on a high-salt diet [168, 169]. In contrast, collecting duct-specific ETA receptor knockout mice have normal blood pressure [169]. Endothelin-1 decreases the open probability of ENaC in collecting ducts isolated from WT and ETA receptor knockout mice, but not in those from ETB receptor knockout mice [170]. The basal activity of ENaC in collecting ducts isolated from ETB receptor knockout mice was elevated as compared with those from WT and ETA receptor knockout mice [170]. The differences in sodium excretion and blood pressure by the collecting duct-specific deletion of endothelin-1 were abolished by L-NAME administration [171]. Thus, endothelin-1 is important in natriuresis by stimulating ETB receptors and nitric oxide production in collecting duct cells.
Previous studies have demonstrated that TGFβ1 increases the expression and/or secretion of endothelin-1 in vascular endothelial cells [172], glomerular mesangial cells [173], vascular smooth muscle cells [174], and renal collecting duct cells [158, 175]. Activation of transcription in the promoter for endothelin-1 accounts for the TGFβ1-induced increase in endothelin-1 expression. The binding sites for Smad and AP-1 in the endothelin-1 promoter are indispensable for TGFβ1-induced transcription of endothelin-1 [176]. Binding of Smad3/Smad4, c-Jun, and the coactivator CREB-binding protein/p300 were found to be essential for induction of endothelin-1 [176]. Dibutyryl-cAMP increased and Rp-cAMP decreased TGFβ1-induced expression of endothelin-1 [177], suggesting that the effect of TGFβ1 on endothelin-1 expression occurs via cAMP. In contrast, a pan-TGFβ antibody caused a significant reduction in endothelin-1 mRNA levels and endothelin-1 protein secretion in two cell lines of renal cortical and inner medulary collecting ducts [178], suggesting that endogenous TGFβs are autocrine/ paracrine stimulators of endothelin-1 expression.
Thus, it is likely that TGFβ1 exerts its natriuretic effects partly via induction of endothelin-1 in collecting duct cells.
HIGH SALT INTAKE AND TGFβ1
RAAS is an important stimulator of TGFβ signaling and tissue fibrosis. Interestingly, Dahl salt-sensitive rats [179, 180], an animal model of hypertension with sodium retention and low circulating levels of both active renin and aldosterone, also exhibit enhanced TGFβ signaling. This suggests that sodium retention and volume expansion promote TGFβ1 activation and fibrosis, leading to cardio- vascular and renal complications independent of RAAS. Indeed, Ingenuity Pathway Analysis of gene expression profiles, determined by microarray, identified an enrichment of genes involved in the TGFβ signaling of primary cultured human chondrocytes under hyperosmotic condition obtained by adding sodium chloride [181]. However, the mechanisms for RAAS-independent activation of TGFβ signaling by salt-loading are not well understood.
Tamaki, et al. demonstrated that Dahl salt-sensitive rats develop marked hypertension and renal damage on a high-salt diet while showing increased cortical gene expression of TGFβ1, LTBP, and TGFβ receptors (Type I, II and III); this was accompanied by augmented expressions of genes acting downstream of TGFβ1: collagen I, fibronectin, and plasminogen activator inhibitor-1 [182]. The increased expression of TGFβ1 in salt-loaded Dahl salt-sensitive rats is important for developing renal injury. Reduced TGFβ1 expression, achieved either by anti-TGFβ neutralizing antibody (1D11) or haploinsufficiency of TGFβ1 gene attenuated urinary protein excretion, glomerular injury and renal interstitial fibrosis; these occurred without a change in blood pressure of Dahl-salt sensitive rats on a high-salt diet [183, 184].
The effect of salt loading on tissue damage is also observed in the absence of elevated blood pressure. An increase in sodium intake elevates mRNA levels of TGFβ1, TGFβ2 and TGFβ3 in the kidney and heart of Sprague-Dawley rats without an associated increase in arterial pressure [185]. In Sprague-Dawley rats receiving angiotensin II (100 ng/kg/min) for two weeks by subcutaneous minipump, a high sodium diet aggravated renal histological changes and the expression of TGFβ1 in the kidney without changing blood pressure [186]. Likewise, in young female transgenic (mRen2)27 rats a high-sodium diet for 3 weeks did not significantly alter blood pressure, but did exacerbate cardiac left ventricular diastolic dysfunction, proteinuria, periarterial and interstitial fibrosis; TGFβ1 expression in the kidney was increased as well [187, 188]. The same study found that salt loading increased fibrosis and TGFβ1 expression in the kidney even in normotensive Sprague-Dawley rats [187].
Several molecular mechanisms for the increased expression of TGFβ1 resulting from high salt intake have been reported. The enhanced glomerular production of TGFβ1 induced by the 8 % NaCl diet was inhibited by tetraethylammonium but not glibenclamide, suggesting that the mechanism includes voltage-activated potassium channels [185]. Iberiotoxin, an inhibitor of large-conductance calcium-activated potassium channels, also suppressed salt-induced production of TGFβ1 [189]. Ying et al. found that dietary salt activates c-Src and proline-rich tyrosine kinase-2 (Pyk2) in the aortic ring and glomerulus [190]; concurrent with downstream activation of ERK1/2 and p38, which induces TGFβ1 generation [190]. High Na+ diet increased the protein amount of phosphorylated Pyk2 and Pyk2 activity in the aorta and renal glomerulus. Both the total and active amount of TGFβ1 were enhanced on a high salt diet; this enhancement was abolished by tyrphostin Ap, an inhibitor of Pyk2 [190]. It was shown that two sorts of dominant negative Tat fusion proteins, which inhibit the respective binding of c-Src and PI3K to Pyk2, inhibit the dietary salt-induced increase in total and active TGFβ1 [190]; suggesting that both c-Src and PI3K are involved in the Pyk2 phosphorylation. Indeed, the amount of phosphorylated c-Src was augmented by high salt, and the high salt diet-induced increase in total and active TGFβ1 was abrogated by a c-Src inhibitor PP2 [190]. MAPK is a downstream target of Pyk2 [191]. The activities of p42/p44 MAPK and of p38 MAPK enhanced by high salt diet were also abolished by Tat fusion proteins which inhibit the binding of c-Src and PI3K to Pyk2 [190]. In agreement with these results, induction of genes involved in TGFβ signaling were also blocked by inhibitors for p38 MAPK and p42/p44 MAPK in cultured human chondrocytes under hyperosmolar conditions [181].
In the TGFβ1 L/L mice, urinary excretion of sodium and water was markedly impaired and the blood pressure and plasma volume were significantly increased as compared with WT [24]. In contrast, urinary excretion of sodium and water was unaltered and the blood pressure and plasma volume were significantly decreased in the TGFβ1 H/H mice. These results suggest that TGFβ1 is a natriuretic protein. In this context, the induction of TGFβ1 by salt loading as a negative regulatory mechanism is a reasonable physiological response to maintain sodium and fluid homeostasis. The restriction of salt intake seems to be more beneficial than pharmacological suppression of TGFβ signaling in minimizing salt-induced tissue fibrotic changes.
CONCLUSION
TGFβ1 is integrally involved in blood pressure regulation, salt and water balance, as well as fibrosis. While TGFβ1 develops fibrosis and end organ damage, we demonstrate that the increased expression of TGFβ1 in the kidney may in fact be an important counterregulatory mechanism in response to salt loading and sodium retention. In fact, TGFβ1 induction leads to reduction in mineralocorticoid/corticosteroid synthesis and increased salt and water excretion.
It is well established that blocking RAAS provides beneficial effects for the treatment of cardiovascular and renal complications in hypertension and diabetes [192]. Although the induction of TGFβ1 by RAAS mediates the fibrogenic changes in these complications, TGFβ1 is unlikely to be a viable target to manage hypertension, since the results of recent studies indicate that TGFβ1 suppresses mineralocorticoid production and maintaining normal diuresis and natriuresis by reducing renal tubular reabsorption of sodium.
Human studies looking at patients who have already developed end organ damage due to hypertension as well as hypertensive patients compared with normotensive patients show elevated TGFβ1 levels. But this may in fact be due to hypertension and salt loading rather than the cause of hypertension. Thus blocking TGFβ1 activity may actually be detrimental rather than beneficial to managing hypertension in the clinical setting.
It is clear that more work needs to be done to elucidate the relationship between TGFβ1 and renal sodium and water handling as well as fibrosis. In order to develop successful therapeutic strategies that target TGFβ signaling for the treatment of end organ damage, it will be necessary to find a way to avoid worsening sodium and water retention due to reduced TGFβ signaling.
Acknowledgments
Our studies were supported by National Institutes of Health Grants DK76131 and HL49277, and by Career Development Award 2006-2-106 from Juvenile Diabetes Research Foundation.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
References
- 1.Munoz-Espin D, Canamero M, Maraver A, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155(5):1104–18. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Barcellos-Hoff MH, Cucinotta FA. New tricks for an old fox: Impact of TGFbeta on the DNA damage response and genomic stability. Sci Signal. 2014;7(341):re5. doi: 10.1126/scisignal.2005474. [DOI] [PubMed] [Google Scholar]
- 3.Wiener Z, Band AM, Kallio P, et al. Oncogenic mutations in intestinal adenomas regulate Bim-mediated apoptosis induced by TGF-beta. Proc Natl Acad Sci U S A. 2014;111(21):E2229–36. doi: 10.1073/pnas.1406444111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han A, Zhao H, Li J, Pelikan R, Chai Y. ALK5-mediated transforming growth factor beta signaling in neural crest cells controls craniofacial muscle development via tissue-tissue interactions. Mol Cell Biol. 2014;34(16):3120–31. doi: 10.1128/MCB.00623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng YF, Yuan F, Guo H, Wu WZ. TGF-beta1 enhances SDF-1-induced migration and tube formation of choroid-retinal endothelial cells by up-regulating CXCR4 and CXCR7 expression. Mol Cell Biochem. 2014;397(1–2):131–8. doi: 10.1007/s11010-014-2180-6. [DOI] [PubMed] [Google Scholar]
- 6.Stephen TL, Rutkowski MR, Allegrezza MJ, et al. Transforming Growth Factor beta-Mediated Suppression of Antitumor T Cells Requires FoxP1 Transcription Factor Expression. Immunity. 2014;41(3):427–39. doi: 10.1016/j.immuni.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grafe I, Yang T, Alexander S, et al. Excessive transforming growth factor-beta signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014;20(6):670–5. doi: 10.1038/nm.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JG, Lee DH, Moon YS, Kim KH. Reversine increases the plasticity of lineage-committed preadipocytes to osteogenesis by inhibiting adipogenesis through induction of TGF-beta pathway in vitro. Biochem Biophys Res Commun. 2014;446(1):30–6. doi: 10.1016/j.bbrc.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 9.Wang YW, Liou NH, Cherng JH, et al. siRNA-Targeting Transforming Growth Factor-beta Type I Receptor Reduces Wound Scarring and Extracellular Matrix Deposition of Scar Tissue. J Invest Dermatol. 2014;134(7):2016–25. doi: 10.1038/jid.2014.84. [DOI] [PubMed] [Google Scholar]
- 10.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clouthier DE, Comerford SA, Hammer RE. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. J Clin Invest. 1997;100(11):2697–713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanderson N, Factor V, Nagy P, et al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995;92(7):2572–6. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts AB, Heine UI, Flanders KC, Sporn MB. Transforming growth factor-beta. Major role in regulation of extracellular matrix. Ann N Y Acad Sci. 1990;580:225–32. doi: 10.1111/j.1749-6632.1990.tb17931.x. [DOI] [PubMed] [Google Scholar]
- 14.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9(7):964–8. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 15.Wylie-Sears J, Levine RA, Bischoff J. Losartan inhibits endothelial-to-mesenchymal transformation in mitral valve endothelial cells by blocking transforming growth factor-beta-induced phosphorylation of ERK. Biochem Biophys Res Commun. 2014;446(4):870–5. doi: 10.1016/j.bbrc.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito S, Shimizu H, Yisireyili M, Nishijima F, Enomoto A, Niwa T. Indoxyl sulfate-induced activation of (pro)renin receptor is involved in expression of TGF-beta1 and alpha-smooth muscle actin in proximal tubular cells. Endocrinology. 2014;155(5):1899–907. doi: 10.1210/en.2013-1937. [DOI] [PubMed] [Google Scholar]
- 17.Saraswat MS, Addepalli V, Jain M, Pawar VD, Patel RB. Renoprotective activity of aliskiren, a renin inhibitor in cyclosporine A induced hypertensive nephropathy in dTG mice. Pharmacol Rep. 2014;66(1):62–7. doi: 10.1016/j.pharep.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Bae EH, Kim IJ, Joo SY, et al. Renoprotective effects of the direct renin inhibitor aliskiren on gentamicin-induced nephrotoxicity in rats. J Renin Angiotensin Aldosterone Syst. 2014;15(4):348–61. doi: 10.1177/1470320312474853. [DOI] [PubMed] [Google Scholar]
- 19.Gallo EM, Loch DC, Habashi JP, et al. Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124(1):448–60. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Shao L, Ma A, et al. Telmisartan delays myocardial fibrosis in rats with hypertensive left ventricular hypertrophy by TGF-beta1/Smad signal pathway. Hypertens Res. 2014;37(1):43–9. doi: 10.1038/hr.2013.119. [DOI] [PubMed] [Google Scholar]
- 21.Hotta M, Baird A. Differential effects of transforming growth factor type beta on the growth and function of adrenocortical cells in vitro. Proc Natl Acad Sci U S A. 1986;83(20):7795–9. doi: 10.1073/pnas.83.20.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liakos P, Lenz D, Bernhardt R, Feige JJ, Defaye G. Transforming growth factor beta1 inhibits aldosterone and cortisol production in the human adrenocortical cell line NCI-H295R through inhibition of CYP11B1 and CYP11B2 expression. J Endocrinol. 2003;176(1):69–82. doi: 10.1677/joe.0.1760069. [DOI] [PubMed] [Google Scholar]
- 23.Gupta P, Franco-Saenz R, Gentry LE, Mulrow PJ. Transforming growth factor-beta 1 inhibits aldosterone and stimulates adrenal renin in cultured bovine zona glomerulosa cells. Endocrinology. 1992;131(2):631–6. doi: 10.1210/endo.131.2.1322277. [DOI] [PubMed] [Google Scholar]
- 24.Kakoki M, Pochynyuk OM, Hathaway CM, et al. Primary aldosteronism and impaired natriuresis in mice underexpressing TGFbeta1. Proc Natl Acad Sci U S A. 2013;110(14):5600–5. doi: 10.1073/pnas.1302641110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(6659):465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 26.Pardali E, Ten Dijke P. TGFbeta signaling and cardiovascular diseases. Int J Biol Sci. 2012;8(2):195–213. doi: 10.7150/ijbs.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8(11):857–69. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 28.Wrana JL, Attisano L, Carcamo J, et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71(6):1003–14. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 29.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19(1):71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suthanthiran M, Li B, Song JO, et al. Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci U S A. 2000;97(7):3479–84. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. N Engl J Med. 1989;320(11):684–8. doi: 10.1056/NEJM198903163201102. [DOI] [PubMed] [Google Scholar]
- 33.Laviades C, Varo N, Diez J. Transforming growth factor beta in hypertensives with cardiorenal damage. Hypertension. 2000;36(4):517–22. doi: 10.1161/01.hyp.36.4.517. [DOI] [PubMed] [Google Scholar]
- 34.Cambien F, Ricard S, Troesch A, et al. Polymorphisms of the transforming growth factor-beta 1 gene in relation to myocardial infarction and blood pressure. The Etude Cas-Temoin de l'Infarctus du Myocarde (ECTIM) Study. Hypertension. 1996;28(5):881–7. doi: 10.1161/01.hyp.28.5.881. [DOI] [PubMed] [Google Scholar]
- 35.Li B, Khanna A, Sharma V, Singh T, Suthanthiran M, August P. TGF-beta1 DNA polymorphisms, protein levels, and blood pressure. Hypertension. 1999;33(1 Pt 2):271–5. doi: 10.1161/01.hyp.33.1.271. [DOI] [PubMed] [Google Scholar]
- 36.Niu W. Evaluation of Transforming Growth Factor Beta-1 Gene 869T/C Polymorphism with Hypertension: A Meta-Analysis. Int J Hypertens. 2011;2011:934265. doi: 10.4061/2011/934265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan-Yan L. Transforming growth factor beta1 +869T/C gene polymorphism and essential hypertension: a meta-analysis involving 2708 participants in the Chinese population. Intern Med. 2011;50(10):1089–92. doi: 10.2169/internalmedicine.50.4967. [DOI] [PubMed] [Google Scholar]
- 38.Lavoie P, Robitaille G, Agharazii M, Ledbetter S, Lebel M, Lariviere R. Neutralization of transforming growth factor-beta attenuates hypertension and prevents renal injury in uremic rats. J Hypertens. 2005;23(10):1895–903. doi: 10.1097/01.hjh.0000182521.44440.c5. [DOI] [PubMed] [Google Scholar]
- 39.Zacchigna L, Vecchione C, Notte A, et al. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell. 2006;124(5):929–42. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 40.Badri KR, Yue M, Carretero OA, et al. Blood pressure homeostasis is maintained by a P311-TGF-beta axis. J Clin Invest. 2013;123(10):4502–12. doi: 10.1172/JCI69884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 42.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90(2):770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science. 1994;264(5167):1936–8. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- 45.Schultz Jel J, Witt SA, Glascock BJ, et al. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109(6):787–96. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–60. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kakoki M, Tsai YS, Kim HS, et al. Altering the expression in mice of genes by modifying their 3' regions. Dev Cell. 2004;6(4):597–606. doi: 10.1016/s1534-5807(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 48.Shaw G, Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46(5):659–67. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 49.Ando T, Okuda S, Tamaki K, Yoshitomi K, Fujishima M. Localization of transforming growth factor-beta and latent transforming growth factor-beta binding protein in rat kidney. Kidney Int. 1995;47(3):733–9. doi: 10.1038/ki.1995.112. [DOI] [PubMed] [Google Scholar]
- 50.Banas MC, Parks WT, Hudkins KL, et al. Localization of TGF-beta signaling intermediates Smad2, 3, 4, and 7 in developing and mature human and mouse kidney. J Histochem Cytochem. 2007;55(3):275–85. doi: 10.1369/jhc.6A7083.2006. [DOI] [PubMed] [Google Scholar]
- 51.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98(1):121–8. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 52.Carvalho JJ, Baruzzi RG, Howard PF, et al. Blood pressure in four remote populations in the INTERSALT Study. Hypertension. 1989;14(3):238–46. doi: 10.1161/01.hyp.14.3.238. [DOI] [PubMed] [Google Scholar]
- 53.Mancilha-Carvalho JJ, de Oliveira R, Esposito RJ. Blood pressure and electrolyte excretion in the Yanomamo Indians, an isolated population. J Hum Hypertens. 1989;3(5):309–14. [PubMed] [Google Scholar]
- 54.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;297(6644):319–28. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252(5014):1813–6. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 56.Guyton AC. The surprising kidney-fluid mechanism for pressure control--its infinite gain! Hypertension. 1990;16(6):725–30. doi: 10.1161/01.hyp.16.6.725. [DOI] [PubMed] [Google Scholar]
- 57.Rossier BC. Negative regulators of sodium transport in the kidney: key factors in understanding salt-sensitive hypertension? J Clin Invest. 2003;111(7):947–50. doi: 10.1172/JCI18232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanbay M, Chen Y, Solak Y, Sanders PW. Mechanisms and consequences of salt sensitivity and dietary salt intake. Curr Opin Nephrol Hypertens. 2011;20(1):37–43. doi: 10.1097/MNH.0b013e32834122f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujita T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol. 2014;25(6):1148–55. doi: 10.1681/ASN.2013121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77(2):359–96. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 61.Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC) Pflugers Arch. 2009;458(1):111–35. doi: 10.1007/s00424-009-0656-0. [DOI] [PubMed] [Google Scholar]
- 62.Mamenko M, Zaika O, Doris PA, Pochynyuk O. Salt-dependent inhibition of epithelial Na+ channel-mediated sodium reabsorption in the aldosterone-sensitive distal nephron by bradykinin. Hypertension. 2012;60(5):1234–41. doi: 10.1161/HYPERTENSIONAHA.112.200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II Increases Activity of the Epithelial Na+ Channel (ENaC) in Distal Nephron Additively to Aldosterone. J Biol Chem. 2012;287(1):660–71. doi: 10.1074/jbc.M111.298919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Helms MN, Yu L, Malik B, Kleinhenz DJ, Hart CM, Eaton DC. Role of SGK1 in nitric oxide inhibition of ENaC in Na+-transporting epithelia. Am J Physiol Cell Physiol. 2005;289(3):C717–26. doi: 10.1152/ajpcell.00006.2005. [DOI] [PubMed] [Google Scholar]
- 65.Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol. 2008;294(1):F38–46. doi: 10.1152/ajprenal.00403.2007. [DOI] [PubMed] [Google Scholar]
- 66.Bugaj V, Pochynyuk O, Stockand JD. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol. 2009;297(5):F1411–8. doi: 10.1152/ajprenal.00371.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand JD. Acute regulation of the epithelial Na+ channel by phosphatidylinositide 3-OH kinase signaling in native collecting duct principal cells. J Am Soc Nephrol. 2007;18(6):1652–61. doi: 10.1681/ASN.2007010020. [DOI] [PubMed] [Google Scholar]
- 68.Wegmann M, Nusing RM. Prostaglandin E2 stimulates sodium reabsorption in MDCK C7 cells, a renal collecting duct principal cell model. Prostaglandins Leukot Essent Fatty Acids. 2003;69(5):315–22. doi: 10.1016/j.plefa.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Guan Y, Hao C, Cha DR, et al. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11(8):861–6. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 70.Kashlan OB, Kleyman TR. ENaC structure and function in the wake of a resolved structure of a family member. Am J Physiol Renal Physiol. 2011;301(4):F684–96. doi: 10.1152/ajprenal.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimkets RA, Warnock DG, Bositis CM, et al. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79(3):407–14. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 72.Chang SS, Grunder S, Hanukoglu A, et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet. 1996;12(3):248–53. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- 73.Hummler E, Barker P, Gatzy J, et al. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet. 1996;12(3):325–28. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 74.McDonald FJ, Yang B, Hrstka RF, et al. Disruption of the beta subunit of the epithelial Na+ channel in mice: hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci U S A. 1999;96(4):1727–31. doi: 10.1073/pnas.96.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barker PM, Nguyen MS, Gatzy JT, et al. Role of gammaENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest. 1998;102(8):1634–40. doi: 10.1172/JCI3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubera I, Loffing J, Palmer LG, et al. Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest. 2003;112(4):554–65. doi: 10.1172/JCI16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson FH, Disse-Nicodeme S, Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293(5532):1107–12. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 78.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest. 2003;111(7):1039–45. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mu S, Shimosawa T, Ogura S, et al. Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med. 2011;17(5):573–80. doi: 10.1038/nm.2337. [DOI] [PubMed] [Google Scholar]
- 80.Simon DB, Nelson-Williams C, Bia MJ, et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12(1):24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 81.Gross O, Girgert R, Rubel D, Temme J, Theissen S, Muller GA. Renal protective effects of aliskiren beyond its antihypertensive property in a mouse model of progressive fibrosis. Am J Hypertens. 2011;24(3):355–61. doi: 10.1038/ajh.2010.231. [DOI] [PubMed] [Google Scholar]
- 82.Feldman DL, Jin L, Xuan H, et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52(1):130–6. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- 83.Chua CC, Diglio CA, Siu BB, Chua BH. Angiotensin II induces TGF-beta 1 production in rat heart endothelial cells. Biochim Biophys Acta. 1994;1223(1):141–7. doi: 10.1016/0167-4889(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 84.Wolf G, Ziyadeh FN, Stahl RA. Angiotensin II stimulates expression of transforming growth factor beta receptor type II in cultured mouse proximal tubular cells. J Mol Med (Berl) 1999;77(7):556–64. doi: 10.1007/s001099900028. [DOI] [PubMed] [Google Scholar]
- 85.Ruiz-Ortega M, Lorenzo O, Egido J. Angiotensin III up-regulates genes involved in kidney damage in mesangial cells and renal interstitial fibroblasts. Kidney Int Suppl. 1998;68:S41–5. doi: 10.1046/j.1523-1755.1998.06811.x. [DOI] [PubMed] [Google Scholar]
- 86.Juknevicius I, Segal Y, Kren S, Lee R, Hostetter TH. Effect of aldosterone on renal transforming growth factor-beta. Am J Physiol Renal Physiol. 2004;286(6):F1059–62. doi: 10.1152/ajprenal.00202.2003. [DOI] [PubMed] [Google Scholar]
- 87.Sun Y, Zhang J, Zhang JQ, Ramires FJ. Local angiotensin II and transforming growth factor-beta1 in renal fibrosis of rats. Hypertension. 2000;35(5):1078–84. doi: 10.1161/01.hyp.35.5.1078. [DOI] [PubMed] [Google Scholar]
- 88.Huang W, Xu C, Kahng KW, Noble NA, Border WA, Huang Y. Aldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol. 2008;294(6):F1287–95. doi: 10.1152/ajprenal.00017.2008. [DOI] [PubMed] [Google Scholar]
- 89.Han JS, Choi BS, Yang CW, Kim YS. Aldosterone-induced TGF-beta1 expression is regulated by mitogen-activated protein kinases and activator protein-1 in mesangial cells. J Korean Med Sci. 2009;24(Suppl):S195–203. doi: 10.3346/jkms.2009.24.S1.S195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Usher MG, Duan SZ, Ivaschenko CY, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120(9):3350–64. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herrada AA, Contreras FJ, Marini NP, et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol. 2010;184(1):191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- 92.Houlihan CA, Akdeniz A, Tsalamandris C, Cooper ME, Jerums G, Gilbert RE. Urinary transforming growth factor-beta excretion in patients with hypertension, type 2 diabetes, and elevated albumin excretion rate: effects of angiotensin receptor blockade and sodium restriction. Diabetes Care. 2002;25(6):1072–7. doi: 10.2337/diacare.25.6.1072. [DOI] [PubMed] [Google Scholar]
- 93.Antonipillai I, Le TH, Soceneantu L, Horton R. Transforming growth factor-beta is a renin secretagogue at picomolar concentrations. Am J Physiol. 1993;265(4 Pt 2):F537–41. doi: 10.1152/ajprenal.1993.265.4.F537. [DOI] [PubMed] [Google Scholar]
- 94.Brezniceanu ML, Wei CC, Zhang SL, et al. Transforming growth factor-beta 1 stimulates angiotensinogen gene expression in kidney proximal tubular cells. Kidney Int. 2006;69(11):1977–85. doi: 10.1038/sj.ki.5000396. [DOI] [PubMed] [Google Scholar]
- 95.Ellmers LJ, Scott NJ, Medicherla S, et al. Transforming growth factor-beta blockade down-regulates the renin-angiotensin system and modifies cardiac remodeling after myocardial infarction. Endocrinology. 2008;149(11):5828–34. doi: 10.1210/en.2008-0165. [DOI] [PubMed] [Google Scholar]
- 96.Petrov VV, Fagard RH, Lijnen PJ. Transforming growth factor-beta(1) induces angiotensin-converting enzyme synthesis in rat cardiac fibroblasts during their differentiation to myofibroblasts. J Renin Angiotensin Aldosterone Syst. 2000;1(4):342–52. doi: 10.3317/jraas.2000.064. [DOI] [PubMed] [Google Scholar]
- 97.Renzoni EA, Abraham DJ, Howat S, et al. Gene expression profiling reveals novel TGFbeta targets in adult lung fibroblasts. Respir Res. 2004;5:24. doi: 10.1186/1465-9921-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naaman-Reperant E, Hales DB, Durand P. Effect of transforming growth factor beta-1 on the cholesterol side-chain cleavage system in the adrenal gland of sheep fetuses and newborns. Endocrinology. 1996;137(3):886–92. doi: 10.1210/endo.137.3.8603599. [DOI] [PubMed] [Google Scholar]
- 99.Brand C, Cherradi N, Defaye G, et al. Transforming growth factor beta1 decreases cholesterol supply to mitochondria via repression of steroidogenic acute regulatory protein expression. J Biol Chem. 1998;273(11):6410–6. doi: 10.1074/jbc.273.11.6410. [DOI] [PubMed] [Google Scholar]
- 100.Le Roy C, Li JY, Stocco DM, Langlois D, Saez JM. Regulation by adrenocorticotropin (ACTH), angiotensin II, transforming growth factor-beta, and insulin-like growth factor I of bovine adrenal cell steroidogenic capacity and expression of ACTH receptor, steroidogenic acute regulatory protein, cytochrome P450c17, and 3beta-hydroxysteroid dehydrogenase. Endocrinology. 2000;141(5):1599–607. doi: 10.1210/endo.141.5.7457. [DOI] [PubMed] [Google Scholar]
- 101.Lehmann TP, Biernacka-Lukanty JM, Trzeciak WH, Li JY. Steroidogenic factor 1 gene transcription is inhibited by transforming growth factor beta. Endocr Res. 2005;31(2):71–9. doi: 10.1080/07435800500229110. [DOI] [PubMed] [Google Scholar]
- 102.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–90. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 103.Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6(8):1249–58. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- 104.Rice DA, Mouw AR, Bogerd AM, Parker KL. A shared promoter element regulates the expression of three steroidogenic enzymes. Mol Endocrinol. 1991;5(10):1552–61. doi: 10.1210/mend-5-10-1552. [DOI] [PubMed] [Google Scholar]
- 105.Ragazzon B, Cazabat L, Rizk-Rabin M, et al. Inactivation of the Carney complex gene 1 (protein kinase A regulatory subunit 1A) inhibits SMAD3 expression and TGF beta-stimulated apoptosis in adrenocortical cells. Cancer Res. 2009;69(18):7278–84. doi: 10.1158/0008-5472.CAN-09-1601. [DOI] [PubMed] [Google Scholar]
- 106.Parviainen H, Schrade A, Kiiveri S, et al. Expression of Wnt and TGF-beta pathway components and key adrenal transcription factors in adrenocortical tumors: association to carcinoma aggressiveness. Pathol Res Pract. 2013;209(8):503–9. doi: 10.1016/j.prp.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boccuzzi A, Terzolo M, Cappia S, et al. Different immuno- histochemical patterns of TGF-beta1 expression in benign and malignant adrenocortical tumours. Clin Endocrinol (Oxf) 1999;50(6):801–8. doi: 10.1046/j.1365-2265.1999.00683.x. [DOI] [PubMed] [Google Scholar]
- 108.Frank J, Roux J, Kawakatsu H, et al. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem. 2003;278(45):43939–50. doi: 10.1074/jbc.M304882200. [DOI] [PubMed] [Google Scholar]
- 109.Husted RF, Matsushita K, Stokes JB. Induction of resistance to mineralocorticoid hormone in cultured inner medullary collecting duct cells by TGF-beta 1. Am J Physiol. 1994;267(5 Pt 2):F767–75. doi: 10.1152/ajprenal.1994.267.5.F767. [DOI] [PubMed] [Google Scholar]
- 110.Husted RF, Sigmund RD, Stokes JB. Mechanisms of inactivation of the action of aldosterone on collecting duct by TGF-beta. Am J Physiol Renal Physiol. 2000;278(3):F425–33. doi: 10.1152/ajprenal.2000.278.3.F425. [DOI] [PubMed] [Google Scholar]
- 111.Peters DM, Vadasz I, Wujak L, et al. TGF-beta directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc Natl Acad Sci U S A. 2014;111(3):E374–83. doi: 10.1073/pnas.1306798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen LM, Zhang X, Chai KX. Regulation of prostasin expression and function in the prostate. Prostate. 2004;59(1):1–12. doi: 10.1002/pros.10346. [DOI] [PubMed] [Google Scholar]
- 113.Wakida N, Kitamura K, Tuyen DG, et al. Inhibition of prostasin-induced ENaC activities by PN-1 and regulation of PN-1 expression by TGF-beta1 and aldosterone. Kidney Int. 2006;70(8):1432–8. doi: 10.1038/sj.ki.5001787. [DOI] [PubMed] [Google Scholar]
- 114.Chang CT, Hung CC, Chen YC, et al. Transforming growth factor-beta1 decreases epithelial sodium channel functionality in renal collecting duct cells via a Smad4-dependent pathway. Nephrol Dial Transplant. 2008;23(4):1126–34. doi: 10.1093/ndt/gfm786. [DOI] [PubMed] [Google Scholar]
- 115.Salyer SA, Parks J, Barati MT, et al. Aldosterone regulates Na(+), K(+) ATPase activity in human renal proximal tubule cells through mineralocorticoid receptor. Biochim Biophys Acta. 2013;1833(10):2143–52. doi: 10.1016/j.bbamcr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 116.Drumm K, Kress TR, Gassner B, Krug AW, Gekle M. Aldosterone stimulates activity and surface expression of NHE3 in human primary proximal tubule epithelial cells (RPTEC) Cell Physiol Biochem. 2006;17(1–2):21–8. doi: 10.1159/000091456. [DOI] [PubMed] [Google Scholar]
- 117.Nowak G, Schnellmann RG. Autocrine production and TGF-beta 1-mediated effects on metabolism and viability in renal cells. Am J Physiol. 1996;271(3 Pt 2):F689–97. doi: 10.1152/ajprenal.1996.271.3.F689. [DOI] [PubMed] [Google Scholar]
- 118.Tang MJ, Wang YK, Lin HH. Butyrate and TGF-beta downregulate Na,K-ATPase expression in cultured proximal tubule cells. Biochem Biophys Res Commun. 1995;215(1):57–66. doi: 10.1006/bbrc.1995.2433. [DOI] [PubMed] [Google Scholar]
- 119.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–42. [PubMed] [Google Scholar]
- 120.Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377(6546):239–42. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 121.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265(5180):1883–5. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 122.MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81(4):641–50. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 123.Inoue N, Venema RC, Sayegh HS, Ohara Y, Murphy TJ, Harrison DG. Molecular regulation of the bovine endothelial cell nitric oxide synthase by transforming growth factor-beta 1. Arterioscler Thromb Vasc Biol. 1995;15(8):1255–61. doi: 10.1161/01.atv.15.8.1255. [DOI] [PubMed] [Google Scholar]
- 124.Saura M, Zaragoza C, Cao W, et al. Smad2 mediates transforming growth factor-beta induction of endothelial nitric oxide synthase expression. Circ Res. 2002;91(9):806–13. doi: 10.1161/01.res.0000040397.23817.e5. [DOI] [PubMed] [Google Scholar]
- 125.Ying WZ, Aaron KJ, Sanders PW. Transforming growth factor-beta regulates endothelial function during high salt intake in rats. Hypertension. 2013;62(5):951–6. doi: 10.1161/HYPERTENSIONAHA.113.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Forstermann U, Schmidt HH, Kohlhaas KL, Murad F. Induced RAW 264.7 macrophages express soluble and particulate nitric oxide synthase: inhibition by transforming growth factor-beta. Eur J Pharmacol. 1992;225(2):161–5. doi: 10.1016/0922-4106(92)90096-e. [DOI] [PubMed] [Google Scholar]
- 127.Junquero DC, Scott-Burden T, Schini VB, Vanhoutte PM. Inhibition of cytokine-induced nitric oxide production by transforming growth factor-beta 1 in human smooth muscle cells. J Physiol. 1992;454:451–65. doi: 10.1113/jphysiol.1992.sp019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993;178(2):605–13. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vodovotz Y, Geiser AG, Chesler L, et al. Spontaneously increased production of nitric oxide and aberrant expression of the inducible nitric oxide synthase in vivo in the transforming growth factor beta 1 null mouse. J Exp Med. 1996;183(5):2337–42. doi: 10.1084/jem.183.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vodovotz Y, Kopp JB, Takeguchi H, et al. Increased mortality, blunted production of nitric oxide, and increased production of TNF-alpha in endotoxemic TGF-beta1 transgenic mice. J Leukoc Biol. 1998;63(1):31–9. doi: 10.1002/jlb.63.1.31. [DOI] [PubMed] [Google Scholar]
- 131.Lastres P, Letamendia A, Zhang H, et al. Endoglin modulates cellular responses to TGF-beta 1. J Cell Biol. 1996;133(5):1109–21. doi: 10.1083/jcb.133.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pece-Barbara N, Cymerman U, Vera S, Marchuk DA, Letarte M. Expression analysis of four endoglin missense mutations suggests that haploinsufficiency is the predominant mechanism for hereditary hemorrhagic telangiectasia type 1. Hum Mol Genet. 1999;8(12):2171–81. doi: 10.1093/hmg/8.12.2171. [DOI] [PubMed] [Google Scholar]
- 133.Lebrin F, Goumans MJ, Jonker L, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23(20):4018–28. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blanco FJ, Santibanez JF, Guerrero-Esteo M, Langa C, Vary CP, Bernabeu C. Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. J Cell Physiol. 2005;204(2):574–84. doi: 10.1002/jcp.20311. [DOI] [PubMed] [Google Scholar]
- 135.Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II. J Biol Chem. 2002;277(32):29197–209. doi: 10.1074/jbc.M111991200. [DOI] [PubMed] [Google Scholar]
- 136.Merta M, Reiterova J, Tesar V, Stekrova J, Viklicky O. Influence of the endothelial nitric oxide synthase polymorphism on the progression of autosomal dominant polycystic kidney disease and IgA nephropathy. Ren Fail. 2002;24(5):585–93. doi: 10.1081/jdi-120013961. [DOI] [PubMed] [Google Scholar]
- 137.Jerkic M, Rivas-Elena JV, Prieto M, et al. Endoglin regulates nitric oxide-dependent vasodilatation. FASEB J. 2004;18(3):609–11. doi: 10.1096/fj.03-0197fje. [DOI] [PubMed] [Google Scholar]
- 138.Toporsian M, Gros R, Kabir MG, et al. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res. 2005;96(6):684–92. doi: 10.1161/01.RES.0000159936.38601.22. [DOI] [PubMed] [Google Scholar]
- 139.Mutoh A, Isshiki M, Fujita T. Aldosterone enhances ligand-stimulated nitric oxide production in endothelial cells. Hypertens Res. 2008;31(9):1811–20. doi: 10.1291/hypres.31.1811. [DOI] [PubMed] [Google Scholar]
- 140.Boo YC, Sorescu G, Boyd N, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277(5):3388–96. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 141.Roczniak A, Zimpelmann J, Burns KD. Effect of dietary salt on neuronal nitric oxide synthase in the inner medullary collecting duct. Am J Physiol. 1998;275(1 Pt 2):F46–54. doi: 10.1152/ajprenal.1998.275.1.F46. [DOI] [PubMed] [Google Scholar]
- 142.Althaus M, Pichl A, Clauss WG, Seeger W, Fronius M, Morty RE. Nitric oxide inhibits highly selective sodium channels and the Na+/K+-ATPase in H441 cells. Am J Respir Cell Mol Biol. 2011;44(1):53–65. doi: 10.1165/2009-0335OC. [DOI] [PubMed] [Google Scholar]
- 143.Hyndman KA, Boesen EI, Elmarakby AA, et al. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension. 2013;62(1):91–8. doi: 10.1161/HYPERTENSIONAHA.113.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–5. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 145.Shichiri M, Hirata Y, Nakajima T, et al. Endothelin-1 is an autocrine/paracrine growth factor for human cancer cell lines. J Clin Invest. 1991;87(5):1867–71. doi: 10.1172/JCI115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shichiri M, Kato H, Marumo F, Hirata Y. Endothelin-1 as an autocrine/paracrine apoptosis survival factor for endothelial cells. Hypertension. 1997;30(5):1198–203. doi: 10.1161/01.hyp.30.5.1198. [DOI] [PubMed] [Google Scholar]
- 147.Reid K, Turnley AM, Maxwell GD, et al. Multiple roles for endothelin in melanocyte development: regulation of progenitor number and stimulation of differentiation. Development. 1996;122(12):3911–9. doi: 10.1242/dev.122.12.3911. [DOI] [PubMed] [Google Scholar]
- 148.Achmad TH, Rao GS. Chemotaxis of human blood monocytes toward endothelin-1 and the influence of calcium channel blockers. Biochem Biophys Res Commun. 1992;189(2):994–1000. doi: 10.1016/0006-291x(92)92302-e. [DOI] [PubMed] [Google Scholar]
- 149.Ishida K, Takeshige K, Minakami S. Endothelin-1 enhances superoxide generation of human neutrophils stimulated by the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine. Biochem Biophys Res Commun. 1990;173(2):496–500. doi: 10.1016/s0006-291x(05)80061-0. [DOI] [PubMed] [Google Scholar]
- 150.Kasperk CH, Borcsok I, Schairer HU, et al. Endothelin-1 is a potent regulator of human bone cell metabolism in vitro. Calcif Tissue Int. 1997;60(4):368–74. doi: 10.1007/s002239900245. [DOI] [PubMed] [Google Scholar]
- 151.Bhattacharya I, Ullrich A. Endothelin-1 inhibits adipogenesis: role of phosphorylation of Akt and ERK1/2. FEBS Lett. 2006;580(24):5765–71. doi: 10.1016/j.febslet.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 152.Takagi H, Reinach PS, Yoshimura N, Honda Y. Endothelin-1 promotes corneal epithelial wound healing in rabbits. Curr Eye Res. 1994;13(8):625–8. doi: 10.3109/02713689408999897. [DOI] [PubMed] [Google Scholar]
- 153.Xu D, Emoto N, Giaid A, et al. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78(3):473–85. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 154.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348(6303):730–2. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 155.Sakurai T, Yanagisawa M, Takuwa Y, et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348(6303):732–5. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 156.Wang Y, Simonson MS. Voltage-insensitive Ca2+ channels and Ca2+/calmodulin-dependent protein kinases propagate signals from endothelin-1 receptors to the c-fos promoter. Mol Cell Biol. 1996;16(10):5915–23. doi: 10.1128/mcb.16.10.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schramek H, Wang Y, Konieczkowski M, Simonson MS, Dunn MJ. Endothelin-1 stimulates cytosolic phospholipase A2 activity and gene expression in rat glomerular mesangial cells. Kidney Int. 1994;46(6):1644–52. doi: 10.1038/ki.1994.464. [DOI] [PubMed] [Google Scholar]
- 158.Ujiie K, Terada Y, Nonoguchi H, Shinohara M, Tomita K, Marumo F. Messenger RNA expression and synthesis of endothelin-1 along rat nephron segments. J Clin Invest. 1992;90(3):1043–8. doi: 10.1172/JCI115918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen M, Todd-Turla K, Wang WH, et al. Endothelin-1 mRNA in glomerular and epithelial cells of kidney. Am J Physiol. 1993;265(4 Pt 2):F542–50. doi: 10.1152/ajprenal.1993.265.4.F542. [DOI] [PubMed] [Google Scholar]
- 160.Terada Y, Tomita K, Nonoguchi H, Marumo F. Different localization of two types of endothelin receptor mRNA in microdissected rat nephron segments using reverse transcription and polymerase chain reaction assay. J Clin Invest. 1992;90(1):107–12. doi: 10.1172/JCI115822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kohan DE, Padilla E, Hughes AK. Endothelin B receptor mediates ET-1 effects on cAMP and PGE2 accumulation in rat IMCD. Am J Physiol. 1993;265(5 Pt 2):F670–6. doi: 10.1152/ajprenal.1993.265.5.F670. [DOI] [PubMed] [Google Scholar]
- 162.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295(4):F1063–70. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lynch IJ, Welch AK, Kohan DE, Cain BD, Wingo CS. Endothelin-1 inhibits sodium reabsorption by ET(A) and ET(B) receptors in the mouse cortical collecting duct. Am J Physiol Renal Physiol. 2013;305(4):F568–73. doi: 10.1152/ajprenal.00613.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gilmore ES, Stutts MJ, Milgram SL. SRC family kinases mediate epithelial Na+ channel inhibition by endothelin. J Biol Chem. 2001;276(45):42610–7. doi: 10.1074/jbc.M106919200. [DOI] [PubMed] [Google Scholar]
- 165.Pavlov TS, Chahdi A, Ilatovskaya DV, et al. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14-3-3/Nedd4-2. J Am Soc Nephrol. 2010;21(5):833–43. doi: 10.1681/ASN.2009080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hyndman KA, MacDonell AH, Pollock JS. Extracellular signal-regulated kinases 1/2 signaling pathways are not involved in endothelin regulation of mouse inner medullary collecting duct nitric oxide production. Life Sci. 2012;91(13–14):578–82. doi: 10.1016/j.lfs.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kurihara Y, Kurihara H, Suzuki H, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368(6473):703–10. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 168.Ahn D, Ge Y, Stricklett PK, et al. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114(4):504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ge Y, Bagnall A, Stricklett PK, et al. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291(6):F1274–80. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 170.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol. 2012;302(1):C188–94. doi: 10.1152/ajpcell.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension. 2008;51(6):1605–10. doi: 10.1161/HYPERTENSIONAHA.107.108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kurihara H, Yoshizumi M, Sugiyama T, et al. Transforming growth factor-beta stimulates the expression of endothelin mRNA by vascular endothelial cells. Biochem Biophys Res Commun. 1989;159(3):1435–40. doi: 10.1016/0006-291x(89)92270-5. [DOI] [PubMed] [Google Scholar]
- 173.Zoja C, Orisio S, Perico N, et al. Constitutive expression of endothelin gene in cultured human mesangial cells and its modulation by transforming growth factor-beta, thrombin, and a thromboxane A2 analogue. Lab Invest. 1991;64(1):16–20. [PubMed] [Google Scholar]
- 174.Resink TJ, Hahn AW, Scott-Burden T, Powell J, Weber E, Buhler FR. Inducible endothelin mRNA expression and peptide secretion in cultured human vascular smooth muscle cells. Biochem Biophys Res Commun. 1990;168(3):1303–10. doi: 10.1016/0006-291x(90)91171-n. [DOI] [PubMed] [Google Scholar]
- 175.Horie M, Uchida S, Yanagisawa M, Matsushita Y, Kurokawa K, Ogata E. Mechanisms of endothelin-1 mRNA and peptides induction by TGF-beta and TPA in MDCK cells. J Cardiovasc Pharmacol. 1991;17(Suppl 7):S222–5. doi: 10.1097/00005344-199100177-00064. [DOI] [PubMed] [Google Scholar]
- 176.Rodriguez-Pascual F, Redondo-Horcajo M, Lamas S. Functional cooperation between Smad proteins and activator protein-1 regulates transforming growth factor-beta-mediated induction of endothelin-1 expression. Circ Res. 2003;92(12):1288–95. doi: 10.1161/01.RES.0000078491.79697.7F. [DOI] [PubMed] [Google Scholar]
- 177.Lee SD, Lee DS, Chun YG, et al. Transforming growth factor-beta1 induces endothelin-1 in a bovine pulmonary artery endothelial cell line and rat lungs via cAMP. Pulm Pharmacol Ther. 2000;13(6):257–65. doi: 10.1006/pupt.2000.0252. [DOI] [PubMed] [Google Scholar]
- 178.Schnermann JB, Zhu XL, Shu X, et al. Regulation of endothelin production and secretion in cultured collecting duct cells by endogenous transforming growth factor-beta. Endocrinology. 1996;137(11):5000–8. doi: 10.1210/endo.137.11.8895374. [DOI] [PubMed] [Google Scholar]
- 179.Iwai J, Dahl LK, Knudsen KD. Genetic influence on the renin-angiotensin system: low renin activities in hypertension-prone rats. Circ Res. 1973;32(6):678–84. doi: 10.1161/01.res.32.6.678. [DOI] [PubMed] [Google Scholar]
- 180.Rapp JP, Tan SY, Margolius HS. Plasma mineralocorticoids, plasma renin, and urinary kallikrein in salt-sensitive and salt-resistant rats. Endocr Res Commun. 1978;5(1):35–41. doi: 10.3109/07435807809073634. [DOI] [PubMed] [Google Scholar]
- 181.Tew SR, Vasieva O, Peffers MJ, Clegg PD. Post-transcriptional gene regulation following exposure of osteoarthritic human articular chondrocytes to hyperosmotic conditions. Osteoarthritis Cartilage. 2011;19(8):1036–46. doi: 10.1016/j.joca.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 182.Tamaki K, Okuda S, Nakayama M, Yanagida T, Fujishima M. Transforming growth factor-beta 1 in hypertensive renal injury in Dahl salt-sensitive rats. J Am Soc Nephrol. 1996;7(12):2578–89. doi: 10.1681/ASN.V7122578. [DOI] [PubMed] [Google Scholar]
- 183.Murphy SR, Dahly-Vernon AJ, Dunn KM, et al. Renoprotective effects of anti-TGF-beta antibody and antihypertensive therapies in Dahl S rats. Am J Physiol Regul Integr Comp Physiol. 2012;303(1):R57–69. doi: 10.1152/ajpregu.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen CC, Geurts AM, Jacob HJ, Fan F, Roman RJ. Heterozygous knockout of transforming growth factor-beta1 protects Dahl S rats against high salt-induced renal injury. Physiol Genomics. 2013;45(3):110–8. doi: 10.1152/physiolgenomics.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-beta in rats. Am J Physiol. 1998;274(4 Pt 2):F635–41. doi: 10.1152/ajprenal.1998.274.4.F635. [DOI] [PubMed] [Google Scholar]
- 186.Zheng Y, Liu ZQ, Ye T, et al. Effects of high salt diet on blood pressure, renal morphology and osteopontin expression in Sprague-Dawley rat with Ang II-induced renal injury. Di Yi Jun Yi Da Xue Xue Bao. 2005;25(7):827–32. [PubMed] [Google Scholar]
- 187.Habibi J, Hayden MR, Ferrario CM, Sowers JR, Whaley-Connell AT. Salt Loading Promotes Kidney Injury via Fibrosis in Young Female Ren2 Rats. Cardiorenal Med. 2014;4(1):43–52. doi: 10.1159/000360866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Whaley-Connell AT, Habibi J, Aroor A, et al. Salt loading exacerbates diastolic dysfunction and cardiac remodeling in young female Ren2 rats. Metabolism. 2013;62(12):1761–71. doi: 10.1016/j.metabol.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ying WZ, Aaron K, Wang PX, Sanders PW. Potassium inhibits dietary salt-induced transforming growth factor-beta production. Hypertension. 2009;54(5):1159–63. doi: 10.1161/HYPERTENSIONAHA.109.138255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-beta1. Am J Physiol Renal Physiol. 2008;295(2):F406–14. doi: 10.1152/ajprenal.90294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12(3):123–33. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 192.Onozato ML, Tojo A, Kobayashi N, Goto A, Matsuoka H, Fujita T. Dual blockade of aldosterone and angiotensin II additively suppresses TGF-beta and NADPH oxidase in the hypertensive kidney. Nephrol Dial Transplant. 2007;22(5):1314–22. doi: 10.1093/ndt/gfl780. [DOI] [PubMed] [Google Scholar]