Abstract
Background & Aims
We evaluated differences in treatment of black vs white patients with colon cancer and assessed their effects on survival, based on cancer stage.
Methods
We collected data from the Surveillance, Epidemiology and End Results (SEER)-Medicare database and identified 6190 black and 61,951 white patients with colon cancer diagnosed from 1998 through 2009 and followed through 2011. Three sets of 6190 white patients were sequentially matched, using a minimum distance strategy, to the same set of 6190 black patients based on demographic (age, sex, diagnosis year, and SEER registry), tumor presentation (demographic plus comorbidities, tumor stage, grade, and size), and treatment (presentation plus therapies) variables. We conducted sensitivity analyses to explore the effects of socioeconomic status in a sub-cohort that included 2000 randomly selected black patients. Racial differences in treatment were assessed using a logistic regression model; their effects on racial survival disparity were evaluated using Kaplan-Meier method and Cox proportional hazards model.
Results
After patients were matched for demographic variables, the absolute 5 y difference in survival between black and white patients was 8.3% (white, 59.2% 5 y survival; blacks, 50.9% 5 y survival) (P<.0001); this value decreased significantly, to 5.0% (P<.0001), after patients were matched for tumor presentation, and decreased to 4.9% (P<.0001) when patients were matched for treatment. Differences in treatment therefore accounted for 0.1% of the 8.3% difference in survival between black and white patients. After patients were matched for tumor presentation, racial disparities were observed in almost all types of treatment; the disparities were most prominent for patients with advanced-stage cancer (stages III or IV, up to 11.1% difference) vs early-stage cancer (stages I or II, up to 4.3% difference). After patients were matched for treatment, there was a greater reduction in disparity for black vs white patients with advanced-stage compared with early-stage cancer. In sensitivity analyses, the 5 y racial survival disparity was 7.7% after demographic match, which was less than 8.3% observed in the complete cohort. This reduction was likely due to the differences between the sub-cohort and the complete cohort in those variables that were not included in the demographic match. This value was reduced to 6.5% (P=.0001) after socioeconomic status was included in the demographic match. The difference decreased significantly to 2.8% (P=.090) after tumor presentation match, but was not further reduced after treatment match.
Conclusions
We observed significant disparities in treatment and survival of black vs white patients with colon cancer. The disparity in survival appears to have been more strongly affected by tumor presentation at diagnosis than treatment. The effects of treatment differences on disparities in survival were greater for patients with advanced-stage, vs early-stage, cancer.
Keywords: race, colorectal cancer, CRC, outcome
INTRODUCTION
As the second leading cause of cancer death in the United States, colorectal cancer (CRC), including colon and rectal cancers, has disproportionally affected black men and women for over twenty years1–3. Black patients have higher CRC incidence, are often diagnosed at more advanced stages, and have worse overall outcomes than white patients3–5. Although some biological differences have been proposed to exist between black and white CRC patients 6, it has been shown that socioeconomic and sociodemographic status may be the major driving factors for disparities in both CRC incidence and outcomes7–10. For instance, sociodemographic status can affect many risk and protective factors for CRC such as lifestyle factors (diet and exercise) and health-care utilization (screening, diagnosis, and treatment) 11, 12. It is important to identify those factors relating to socioeconomic and sociodemographic status which can be modified, in order to facilitate the design and implementation of appropriate policies and interventions to minimize racial disparities in CRC patients.
Treatment is an important modifiable factor that may be affected by socioeconomic and sociodemographic status 11–13. There are many inconsistencies reported regarding treatment disparities and their impact on outcome disparities in CRC patients. The treatment disparities between black and white CRC patients have been reported mainly in population-based studies 14, 15. However, studies conducted in single institutions usually reported no apparent treatment disparities between black and white CRC patients 16–18. Moreover, how “equal treatment” affects disease outcomes is also inconsistently reported. For example, a recent study demonstrated that the survival disparity between black and white metastatic CRC patients might be fully explained by physician consultation and treatment differences 4. Mack et al. reported that in stage III colon cancer patients who received oxaliplatin, black patients appeared to have even better survival than white patients, indicating that differential receipt and effectiveness of oxaliplatin-containing regimens may not contribute to the poorer survival observed in black than white colon cancer patients 19. In contrast, several other studies found that black patients had lower survival rates even when similar treatments were provided 6, 16, 20 The controversial findings from these studies may be partially explained by differences in the types of treatments analyzed, number of confounding variables considered, and relatively small number of black patients included in model based studies. For example, Silber et al. suggested that a model based on a population dominated by white patients “would be a model that mostly describes what happens to whites” 21, 22. In this study, to address these controversies, we utilized the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to evaluate the treatment disparities between black and white colon cancer patients, as well as their impact on racial survival disparity. Taking advantage of an innovative minimum distance matching strategy, we drew from a pool of 61,951 white patients to match three distinct white comparison cohorts to a cohort of 6190 black patients. By achieving very close matches between black and white colon cancer patients, we bypassed the need for the model-based analyses used in most previous studies.
METHODS
Study population and three sets of matching variables (demographics, tumor presentation, and treatment)
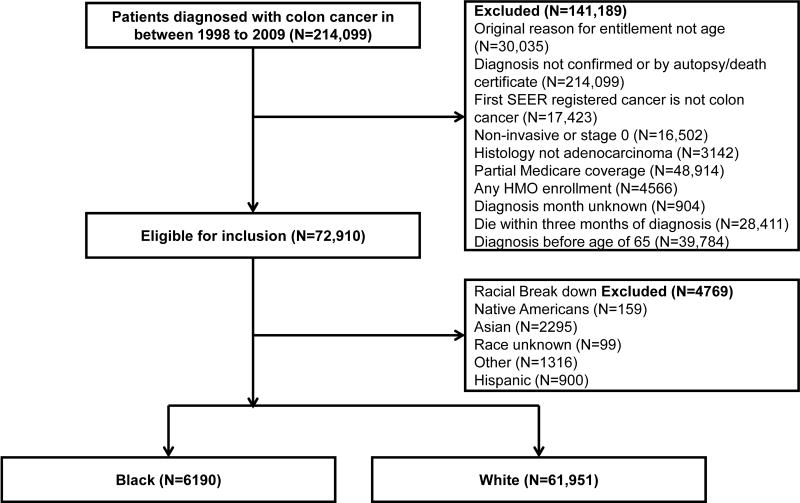
The details of the study population are described in Supplementary Material. Briefly, the study cohort included patients aged 66 years or older with histologically confirmed colon cancer diagnosed between 1998 and 2009 identified from the NCI SEER-Medicare database. The detailed patient selection procedure was summarized in Figure 1. Details on the three sets of matching variables are described in Supplementary Material. The corresponding codes to identify treatment procedures and drug usage used in this study are listed in Supplementary Table S1.
Figure 1.
Cohort definition and exclusions.
Survival outcomes
The primary outcomes were overall two-, three-, and five-year survivals. We set our study starting time to be 90 days after cancer diagnosis. Patients who died within 90 days were excluded due to the possible insufficient time for them to receive cancer-related treatments. Survival time was defined as the number of months from study starting time until the date of death from any cause, or until the end of the observation period. To ensure the completeness and accuracy of death information, we defined our study’s observation window until SEER’s last follow up date, December 31, 2011, which ensured an at least two-year follow-up time for each patient. Patients alive at the end of follow-up were censored.
Statistical analysis
A sequential matching process was conducted similar to the methodology described in Silber et al. 21. We included all 6190 black patients for each match, so the blacks were constant and fully representative of black patients in the SEER population. In comparison, the white comparison cohort changed according to the variables used in each match, and 61,951 white patients made it possible to achieve adequate matches to the 6190 black patients. We matched three sets of comparison cohorts by minimizing the overall distance between black and white patients based on demographic-, presentation-, and treatment-related variables. The variables in the demographic match included age, gender, year of diagnosis, and SEER registries. The variables in the presentation match included all the variables in the demographic match, plus individual comorbidities, overall comorbidity score, and tumor characteristics (tumor stage, grade, and size). The variables in the treatment match included all the variables in the presentation match, plus surgery, radiation therapy, targeted therapy, and individual types of common cytotoxic chemotherapy. This novel matching approach finds an optimal match that minimizes the total covariate distance within matched pairs 23, which is done by defining a special patterned distance matrix and passing it to a subroutine to implement the optimal assignment algorithm. The optimal assignment algorithm is implemented using the PROC OPTNET function in SAS which works by minimizing the overall distance between the blacks and whites 21 to ensure the matching of the optimal pairs of white and black patients. Propensity scores derived from the logistic regression of blacks vs whites on the variables to be controlled in the matching were used to calibrate the distance before assigning whites to blacks according to minimum distance. The quality of the matching was carefully verified (see Supplementary Tables S2 and S3). Racial differences in treatment were assessed using the logistic regression model. Prevalence ratios (PRs) and corresponding 95% confidence intervals (CIs) were estimated. The overall survival curve was plotted using the Kaplan-Meier method 24. Survival difference was tested with the log-rank test 25, 26. The association between ethnicity and five-year survival for colon cancer patients in each matching was evaluated using hazard ratios (HRs) and 95% CIs calculated by the Cox proportional hazards model. Sensitivity analyses were conducted to explore the impacts of socioeconomic status (SES) in a sub-cohort including 2000 randomly sampled black patients. SAS (Version 9.4, SAS Institute, Cary, NC) and STATA (Version 11.0, STATA Corp., College station, TX) software packages were used for the analyses in this study. All P values were 2-sided. A P value of less than 0.05 was considered to indicate a statistically significant difference.
RESULTS
Overall matching results
This study included a total of 68,141 patients who were newly diagnosed with colon cancer from 1998 to 2009 and met the inclusion criteria (Figure 1). Among them, 6190 (9.1%) were black and 61,951 (90.9%) were white (excluding “Caucasian with Spanish origin/surname”). Three sets of 6190 white comparison cohorts were identified and sequentially matched to the same set of 6190 black patients according to the approach as previously described 21. Supplementary Table S2 summarizes patient characteristics from the entire population of black patients and each of the three sets of white comparison cohorts selected from the entire population of white patients. Patient characteristics stratified by tumor stage are summarized in Supplementary Table S3. As suggested by Silber et al 21, a 10% standardized difference (SDD) (defined as the mean difference between black and white patients as a fraction of the standard deviation before matching) was used as a cut-off value to assess the balance of covariates after matching. The controlled variables in each match in this study were closely balanced with less than 0.1 SDDs to ensure that all matches were successful (Supplementary Tables S2 and S3). The aspects of disparity between black and white patients were sequentially removed in the three white matches. The characteristics that were not controlled in each match, and those characteristics unobservable in our dataset, reflected the possible sources of disparity between blacks and whites (refer to Supplementary Material for examples of how to interpret sequential matching results).
Treatment disparity in the overall population and stratified by cancer stage
Compared to presentation-matched white patients, black patients had a lower rate of receiving most treatments, including surgery (88.5% vs 91.4%, P<.0001), targeted therapy (3.3% vs 4.4%, P=.002), and most of the individual types of cytotoxic chemotherapies (Supplementary Table S2). There were very few patients (0.3%) who received capecitabine for both black and presentation-matched white patients; and it was the only chemotherapy agent that was not differentially received by black and white patients (Supplementary Table S2). Moreover, there was a significantly higher percentage of black patients who did not have evidence of receiving any anti-tumor treatment for their colon cancer (8.5%) than demographic-matched whites (4.8%) and presentation-matched whites (5.5%) (P<.0001) (Supplementary Table S2). Table 1 lists the detailed treatment disparities by cancer stage between black and presentation-matched whites. The matching quality was adequate as shown in Supplementary Table S3. After controlling for tumor characteristics and comorbidities, advanced-stage (III/IV) patients exhibited a more pronounced pattern of treatment disparity compared to early-stage (I/II) patients (Table 1). Stage I black patients had a lower surgery rate than presentation-matched white patients (92.5% vs 95.7%, P=.0003). There was no statistical difference in surgery in stage II, III, and IV patients. Stage II black patients received less chemotherapy than matched white patients (18.1% vs 22.4%, P=.001); although the magnitude of the difference was small (4.3%). Stage III black and white patients exhibited a significant difference in the use of chemotherapy (53.1% vs 64.2%, P<.0001) with a large absolute difference (11.1%), including fluorouracil/capecitabine alone (32.9% vs 41.2%, P<.0001) and fluorouracil/capecitabine plus oxaliplatin (15.2% vs 19.0%, P=.005). Remarkable differences were also observed in stage IV patients using chemotherapy (56.1% vs 63.3%, P=.001) including fluorouracil/capecitabine plus oxaliplatin (17.9% vs 24.7%, P=.0002) and irinotecan (12.1% vs 18.5%, P<.0001), as well as targeted therapy agents (15.6% vs 21.1%, P=.002, Table 1).
Table 1.
Treatment and SES disparities between black and presentation-matched white colon cancer patients by cancer stage#
| Variable | Black Patients (%) | Matched White Patients (%) | PR (95% CI) | P value |
|---|---|---|---|---|
| Stage I (N=1462, each) | ||||
| Surgery | 1353 (92.5) | 1399 (95.7) | 1.03 (1.02–1.05) | .0003 |
| SES | ||||
| High | 292 (20.0) | 835 (57.1) | 2.86 (2.56–3.20) | <.0001 |
|
| ||||
| Stage II (N=1818, each) | ||||
| Surgery | 1791 (98.5) | 1799 (99.0) | 1.00 (1.00–1.01) | .24 |
| Chemotherapy | 329 (18.1) | 408 (22.4) | 1.24 (1.09–1.41) | .001 |
| SES | ||||
| High | 344 (18.9) | 986 (54.2) | 2.87 (2.58–3.18) | <.0001 |
|
| ||||
| Stage III (N=1546, each) | ||||
| Surgery | 1536 (99.4) | 1541 (99.7) | 1.00 (1.00–1.01) | .20 |
| Chemotherapy | 821 (53.1) | 993 (64.2) | 1.21 (1.14–1.28) | <.0001 |
| Fluorouracil/capecitabine alone | 508 (32.9) | 637 (41.2) | 1.25 (1.14–1.38) | <.0001 |
| Fluorouracil/capecitabine plus oxaliplatin | 235 (15.2) | 294 (19.0) | 1.25 (1.07–1.46) | .005 |
| Irinotecan* | 38 (2.5) | 53 (3.4) | 1.39 (0.93–2.10) | .11 |
| Targeted Therapy | 33 (2.1) | 39 (2.5) | 1.18 (0.75–1.87) | .47 |
| SES | ||||
| High | 277 (17.9) | 842 (54.5) | 3.04 (2.71–3.41) | <.0001 |
|
| ||||
| Stage IV (N=983, each) | ||||
| Surgery | 703 (71.5) | 731 (74.4) | 1.04 (0.99–1.10) | .16 |
| Chemotherapy | 551 (56.1) | 622 (63.3) | 1.13 (1.05–1.21) | .001 |
| Fluorouracil/capecitabine alone | 290 (29.5) | 322 (32.8) | 1.11 (0.97–1.27) | .12 |
| Fluorouracil/capecitabine plus oxaliplatin | 176 (17.9) | 243 (24.7) | 1.38 (1.16–1.64) | .0002 |
| Irinotecan* | 119 (12.1) | 182 (18.5) | 1.53 (1.24–1.89) | <.0001 |
| Targeted Therapy | 153 (15.6) | 207 (21.1) | 1.35 (1.12–1.64) | .002 |
| SES | ||||
| High | 204 (20.8) | 537 (54.6) | 2.63 (2.30–3.01) | <.0001 |
SES: socioeconomic status; PR: prevalence ratio; CI: confidence interval; significant effects (P<.05) in bold.
The 381 black patients with unknown stage are not included in stage-stratified analyses.
The patients using irinotecan alone could not be distinguished from those using irinotecan with targeted therapy.
Survival disparity between black and sequentially matched white patients in the overall population
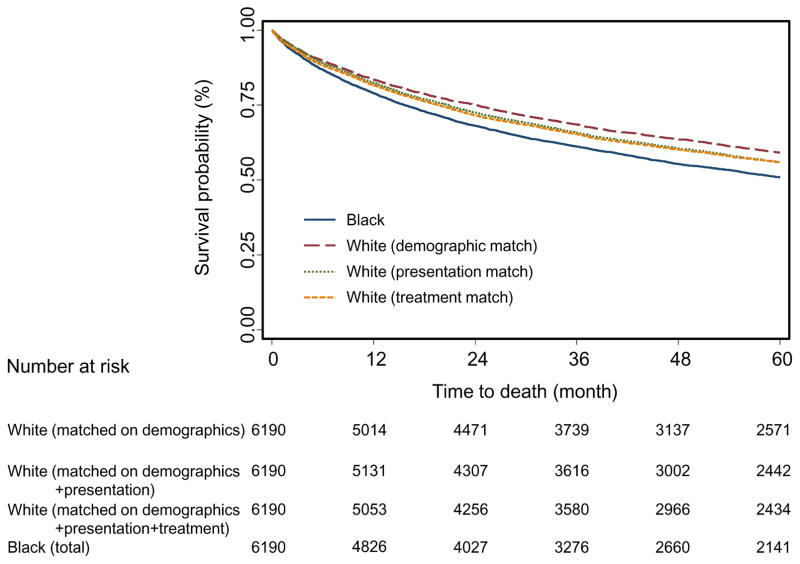
Figure 2 shows the survival curves of all black patients and three sets of sequentially matched white patients. Table 2 summarizes patient survival, with median survival time, two-, three-, and five-year survival rates, the absolute survival rate differences between each matched set of white and black patients, and HRs calculated with the Cox proportional model. As depicted in Figure 2, there was a significant survival difference between black and demographic-matched white patients (absolute five-year survival rate difference, 8.3%, P<.0001, Table 2). This difference was dramatically reduced after presentation match (5.0%, P<.0001, Table 2), but was only slightly reduced after further matching on treatment (4.9%, P<.0001, Table 2). Therefore, after demographic match, the percentage of the five-year survival disparity explained by treatment was much smaller (1.2%) than that explained by presentation (39.8%) (Table 2). Consistently, the five-year survival curve for treatment-matched whites almost overlapped with that of presentation-matched whites (Figure 2). Very similar patterns of survival disparity changes over sequential matching were also noted in the two- and three-year survival analyses (Table 2).
Figure 2.
Kaplan-Meier plot for the five-year survivals of all black colon cancer patients and the three sets of matched white patients diagnosed between 1998 and 2009. Blue solid line: all black patients; red long dashed line: demographic-matched white patients; green dotted line: presentation-matched white patients; orange dashed line: treatment-matched white patients.
Table 2.
Two-, three-, and five-year survival for black and matched white colon cancer patients
| Matched White Patients
|
||||||
|---|---|---|---|---|---|---|
| Outcome Measure | Black Patients (n=6190) | Treatment-Match (n=6190) | Presentation-Match (n=6190) | Demographic-Match (n=6190) | ||
| Survival, median (95% CI), mo | 61.5 (57.9–66.3) | 76.5 (72.6–81.3) | 77.2 (72.6–82.6) | 92.2 (87.2–100.0) | ||
| 2-yr Survival, % (95% CI) | 68.1 (67.0–69.3) | 71.6 (70.4–72.7) | 72.5 (71.4–73.6) | 75.0 (73.9–76.1) | ||
| Survival difference, % | 3.5* | 0.9 (13.0%)† | 4.4* | 2.5 (36.2%)# | 6.9* | |
| P value | <.0001 | <.0001 | <.0001 | |||
| 3-yr Survival, % (95% CI) | 61.1 (59.9–62.4) | 65.3 (64.1–66.5) | 65.8 (64.6–67.0) | 68.5 (67.4–69.7) | ||
| Survival difference, % | 4.2* | 0.5 (6.8%)† | 4.7* | 2.7 (36.5%)# | 7.4* | |
| P value | <.0001 | <.0001 | <.0001 | |||
| 5-yr Survival, % (95% CI) | 50.9 (49.6–52.2) | 55.8 (54.5–57.1) | 55.9 (54.6–57.2) | 59.2 (57.9–60.5) | ||
| Survival difference, % | 4.9* | 0.1 (1.2%)† | 5.0* | 3.3 (39.8%)# | 8.3* | |
| P value | <.0001 | <.0001 | <.0001 | |||
| Cox HR (95% CI) | 1 (reference) | 0.88 (0.84–0.93) | 0.87 (0.83–0.91) | 0.79 (0.75–0.83) | ||
mo: month; yr: year; CI: confidence interval; HR: hazard ratio; significant effects (P<.05) in bold.
Survival differences between black and matched white patients.
The absolute difference (percentage) of survival disparity after demographic match explained by presentation. For example, the absolute five-year difference of survival disparity after demographic match explained by presentation is 3.3% (8.3%-5.0%), and the percentage is 39.8% (3.3/8.3 × 100%).
The absolute difference (percentage) of survival disparity after demographic match explained by treatment.
Survival disparity between black and sequentially matched white patients by cancer stage
Supplementary Figure S1 shows the survival curves by cancer stage for black patients and the three sets of sequentially matched white patients. Table 3 lists the two-, three-, and five-year survivals for black and matched white patients in each stage as well as their survival differences. The detailed matching properties are listed in Supplementary Table S3. Survival disparity between black and demographic-matched white patients was apparent for all four stages (Supplementary Figure S1). The differences in survival were statistically significant in almost all analyses except for three- and five-year survivals in stage IV patients with borderline significances (P=.13 and .095, respectively), likely due to the heterogeneity (e.g., disease and treatment history) as well as the much smaller number of stage IV patients with a long survival. For stage I patients, presentation-match substantially reduced the racial survival difference (4.5% to 2.5%, 5.8% to 3.0%, and 7.3% to 4.7% for two-, three-, and five-year survivals, respectively), which was only modestly reduced on further treatment match (2.5% to 1.1%, 3.0% to 2.0%, and 4.7% to 4.2%, respectively) (Table 3, Supplementary Figure S1A). For stage II patients, the sequential match on presentation and then treatment resulted in sequential but modest reductions in racial survival disparity (4.7% to 2.7% to 2.8%, 5.8% to 4.1% to 3.6%, and 6.6% to 4.6% to 4.0% for two-, three-, and five-year survivals, respectively) (Table 3, Supplementary Figure S1B), with patterns close to those observed in stage I patients. In sharp contrast, for stage III patients, presentation match did not substantially narrow the survival disparity but further treatment match did (4.0% to 4.5% to 2.2%, 3.9% to 3.1% to 2.0%, and 5.1% to 4.3% to 2.8%, for two-, three-, and five-year survivals, respectively) (Table 3, Supplementary Figure S1C), suggesting that the observed treatment disparity (Table 1) had a dramatic effect on the survival disparity in stage III patients. A similar effect by treatment match on survival disparity was observed for stage IV patients (3.5% to 0.4%, 2.6% to 0.2%, and 0.5% to −0.2%, for two-, three-, and five-year survivals, respectively) (Table 3, Supplementary Figure S1D). Consistently, after demographic match, the percentage of the three-year survival disparity explained by presentation and treatment was 48.3% and 17.2%, respectively, for stage I patients, and 29.3% and 8.6% for stage II patients, whereas 20.5% and 28.2% for stage III patients, and 10.3% and 82.8% for stage IV patients (Table 3). These data further indicated that, compared to the survival disparity explained by tumor presentation, the survival disparity explained by treatment difference was much smaller in early-stage but larger in advanced-stage patients. Similar patterns of the stage-specific effect by presentation and treatment were also apparent in most two- and five-year analyses, with the only exception being in the five-year analysis for stage IV patients, most likely due to the small percentage of stage IV patients surviving for five years.
Table 3.
Two-, three-, and five-year survival for black and matched white colon cancer patients by stage
| Black Patients | Matched White Patients
|
|||||
|---|---|---|---|---|---|---|
| Treatment-Match | Presentation-Match | Demographic-Match | ||||
| Stage I | ||||||
| 2-yr survival, % (95% CI) | 84.3 (82.3–86.1) | 85.4 (83.4–87.1) | 86.8 (84.9–88.4) | 88.8 (87.1–90.3) | ||
| Survival difference, % | 1.1* | 1.4 (31.1%)† | 2.5* | 2.0 (44.4%)# | 4.5* | |
| P value | .43 | .062 | .0004 | |||
| 3-yr survival, % (95% CI) | 79.0 (76.8–81.0) | 81.0 (78.9–83.0) | 82.0 (79.9–83.9) | 84.8 (82.9–86.6) | ||
| Survival difference, % | 2.0* | 1.0 (17.2%)† | 3.0* | 2.8 (48.3%)# | 5.8* | |
| P value | .18 | .046 | <.0001 | |||
| 5-yr survival, % (95% CI) | 69.0 (66.5–71.5) | 73.2 (70.8–75.5) | 73.7 (71.3–76.0) | 76.3 (74.0–78.5) | ||
| Survival difference, % | 4.2* | 0.5 (6.8%)† | 4.7* | 2.6 (35.6%)# | 7.3* | |
| P value | .017 | .007 | <.0001 | |||
| Cox HR (95% CI) | 1 (reference) | 0.85 (0.74–0.97) | 0.82 (0.71–0.94) | 0.72 (0.63–0.84) | ||
|
| ||||||
| Stage II | ||||||
| 2-yr survival, % (95% CI) | 81.2 (79.3–82.9) | 84.0 (82.3–85.7) | 83.9 (82.2–85.5) | 85.9 (84.2–87.4) | ||
| Survival difference, % | 2.8* | −0.1 (−2.1%)† | 2.7* | 2.0 (42.6%)# | 4.7* | |
| P value | .022 | .028 | .0001 | |||
| 3-yr survival, % (95% CI) | 74.5 (72.4–76.5) | 78.1 (76.2–80.0) | 78.6 (76.7–80.5) | 80.3 (78.4–82.1) | ||
| Survival difference, % | 3.6* | 0.5 (8.6%)† | 4.1* | 1.7 (29.3%)# | 5.8* | |
| P value | .011 | .004 | <.0001 | |||
| 5-yr survival, % (95% CI) | 62.7 (60.3–65.1) | 66.7 (64.4–68.9) | 67.3 (65.0–69.6) | 69.3 (67.0–71.5) | ||
| Survival difference, % | 4.0* | 0.6 (9.1%)† | 4.6* | 2.0 (30.3%)# | 6.6* | |
| P value | .019 | .006 | .0001 | |||
| Cox HR (95% CI) | 1 (reference) | 0.86 (0.76–0.96) | 0.84 (0.75–0.94) | 0.77 (0.68–0.87) | ||
|
| ||||||
| Stage III | ||||||
| 2-yr survival, % (95% CI) | 69.0 (66.6–71.3) | 71.2 (68.9–73.4) | 73.5 (71.2–75.6) | 73.0 (70.7–75.1) | ||
| Survival difference, % | 2.2* | 2.3 (57.5%)† | 4.5* | −0.5 (−12.5%)# | 4.0* | |
| P value | .19 | .006 | .016 | |||
| 3-yr survival, % (95% CI) | 60.7 (58.1–63.1) | 62.7 (60.2–65.1) | 63.8 (61.3–66.2) | 64.6 (62.1–67.0) | ||
| Survival difference, % | 2.0* | 1.1 (28.2%)† | 3.1* | 0.8 (20.5%)# | 3.9* | |
| P value | .26 | .076 | .025 | |||
| 5-yr survival, % (95% CI) | 48.9 (46.2–51.5) | 51.7 (49.0–54.3) | 53.2 (50.5–55.8) | 54.0 (51.3–56.6) | ||
| Survival difference, % | 2.8* | 1.5 (29.4%)† | 4.3* | 0.8 (15.7%)# | 5.1* | |
| P value | .14 | .024 | .007 | |||
| Cox HR (95% CI) | 1 (reference) | 0.92 (0.83–1.02) | 0.87 (0.78–0.97) | 0.87 (0.78–0.96) | ||
|
| ||||||
| Stage IV | ||||||
| 2-yr survival, % (95% CI) | 25.4 (22.7–28.2) | 25.8 (23.1–28.7) | 28.9 (26.0–31.8) | 29.8 (26.9–32.8) | ||
| Survival difference, % | 0.4* | 3.1 (70.5%)† | 3.5* | 0.9 (20.5%)# | 4.4* | |
| P value | .83 | .092 | .033 | |||
| 3-yr survival, % (95% CI) | 16.9 (14.5–19.5) | 17.1 (14.7–19.7) | 19.5 (17.0–22.2) | 19.8 (17.2–22.5) | ||
| Survival difference, % | 0.2* | 2.4 (82.8%)† | 2.6* | 0.3 (10.3%)# | 2.9* | |
| P value | .91 | .16 | .13 | |||
| 5-yr survival, % (95% CI) | 9.9 (7.8–12.2) | 9.7 (7.6–12.0) | 10.4 (8.3–12.8) | 12.7 (10.4–15.2) | ||
| Survival difference, % | −0.2* | 0.7 (25.0%)† | 0.5* | 2.3 (82.1%)# | 2.8* | |
| P value | .88 | .75 | .095 | |||
| Cox HR (95% CI) | 1 (reference) | 0.99 (0.90–1.09) | 0.92 (0.84–1.01) | 0.88 (0.79–0.97) | ||
yr: year; CI: confidence interval; HR: hazard ratio; significant effects (P<.05) in bold.
Survival differences between black and matched white patients.
The absolute difference (percentage) of survival disparity after demographic match explained by presentation.
The absolute difference (percentage) of survival disparity after demographic match explained by treatment.
The confounding effects of SES, marital status, and urban/rural residence
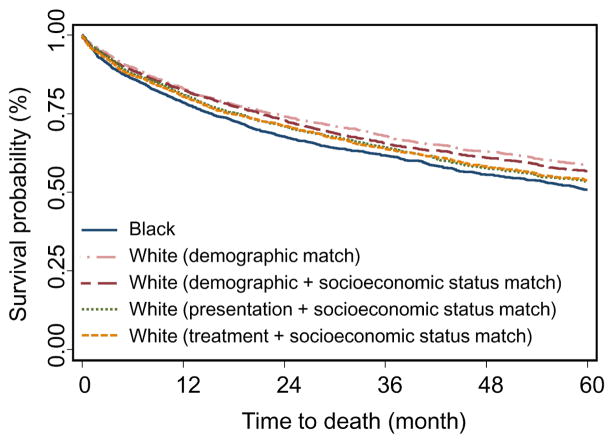
SES, marital status, and urban/rural residence are major factors that have been significantly associated with colon cancer racial disparity 8, 11, 27, 28 and thus are major confounders in our analyses. Because these variables are, as expected, highly different between blacks and whites (Table 1, Supplementary Tables S2 and S3) 8, 27, adding them to the matching made it impossible to achieve balanced matches if we used the full set of 6190 black patients. In order to evaluate their effects on our analyses, we conducted a sub-cohort analysis in which we randomly sampled 2000 black patients from the full set of 6190 black patients, and then used the entire white population (N=61,951) to match to the sampled black patients. By reducing the number of black patients, we increased the ratio of the number of white to black patients so that we were able to achieve adequate matches on all variables. The randomly sampled 2000 black patients are representative of the full set of 6190 black patients in terms of host characteristics (Supplementary Table S4) and survivals (Supplementary Table S5 and Supplementary Figure S2). It should be noted that after demographic match, the racial survival disparity of the sub-cohort reduced compared to the complete cohort. For example, the two-, three-, and five-year survival difference of the sub-cohort vs complete cohort was 6.5% vs 6.9%, 6.2% vs 7.4%, and 7.7% vs 8.3%, respectively. The reduction was likely due to the combined effects of the differences between the sub-cohort and the complete cohort in those variables that were not included in the demographic match. After computing an SES score based on neighborhood poverty, income, and education level to reflect SES status of the patients, we added SES to all three types of matches and the results of the sub-cohort analyses were highly consistent with those of the main analyses. As shown in Table 4 and Figure 3, when SES was added to the demographic match, the survival difference between blacks and matched whites was reduced from 6.5% to 5.9% (9.2% reduction), from 6.2% to 4.4% (29.0% reduction), and from 7.7% to 6.5% (15.6% reduction), for two-, three-, and five-year survivals, respectively. These changes, which were not surprising, support the view that SES plays an important role in colon cancer racial survival disparity. Importantly, tumor presentation match further significantly reduced the survival disparity by 35.4%, 24.2%, and 48.1%, for two-, three-, and five-year survivals, respectively, but treatment match did not (Table 4 and Figure 3). These findings are consistent with the results derived from the complete cohort (Table 2 and Figure 2). Similarly, marital status and urban/rural residence also reduced survival disparity in demographic matching, although to a less extent than SES (0%, 8.1%, 6.5% reduction for two-, three-, and five-year survivals, respectively, Supplementary Table S6 vs Table 4, Supplementary Figure S3 vs Figure 3). Consistently, we also observed that the survival disparity was largely reduced in presentation match (29.2%, 30.6%, and 44.2% for two-, three-, and five-year survivals, respectively), but not treatment match in the sub-cohort analysis that matched patients on marital status and urban/rural residence (Supplementary Table S6 and Supplementary Figure S3). We further explored the stage-specific effects of these analyses. Because early-stage patients exhibited similar survival disparity patterns and advanced-stage patients exhibited similar patterns (Table 3), we combined early-stage patients together and advanced-stage patients together, in order to increase the sample sizes and thus reliability of these analyses. Supplementary Table S7 showed that, in the sub-cohort analyses after matching on SES or marital status and urban/rural residence, racial difference in treatment still existed, especially for the use of chemotherapy (including fluorouracil/capecitabine alone and fluorouracil/capecitabine plus oxaliplatin) in advanced-stage patients (P<.0001). Furthermore, after demographic match plus SES status, the percentage of three-year survival disparity explained by tumor presentation and treatment was 36.0% and 0% for early-stage patients, whereas 10.4% and 45.8% for advanced-stage patients (Supplementary Table S8), respectively. These findings indicate that treatment difference mainly accounted for survival disparity in advanced-stage patients, consistent with the findings from the complete cohort (Table 3). Similar stage-specific effects were also observed when the analyses were done after controlling marital status and urban/rural residence, with the exceptions of the results of five-year analysis, an anomaly that is most likely due to the small number of patients surviving for five years in the sub-cohort analyses (Supplementary Table S8). The findings of stage-stratified sub-cohort analyses (Supplementary Tables S7 and S8) were consistent with those derived from the complete cohort (Tables 1 and 3).
Table 4.
Two-, three- and five-year survival for sampled black and matched white colon cancer patients in the sub-cohort analysis
| Outcome Measure | Black Patients (n=2000) | Matched White Patients
|
||||||
|---|---|---|---|---|---|---|---|---|
| Matching including SES | Excluding SES | |||||||
|
| ||||||||
| Treatment-Match (n=2000) | Presentation-Match (n=2000) | Demographic-Match (n=2000) | Demographic-Match (n=2000) | |||||
| Survival, median (95% CI), mo | 64.6 (57.0–75.4) | 70.6 (64.3–76.4) | 70.1 (62.5–76.1) | 90.3 (78.4–107.9) | 96.9 (86.5–114.9) | |||
| 2-yr Survival, % (95% CI) | 67.7 (65.6–69.7) | 71.4 (69.3–73.3) | 71.3 (69.3–73.3) | 73.6 (71.6–75.4) | 74.2 (72.2–76.1) | |||
| Survival difference, % | 3.7* | −0.1 (−1.5%)† | 3.6* | 2.3 (35.4%)# | 5.9* | 0.6 (9.2%)§ | 6.5* | |
| P value | .012 | .014 | .0001 | <.0001 | ||||
| 3-yr Survival, % (95% CI) | 61.7 (59.5–63.8) | 64.1 (61.9–66.2) | 64.6 (62.4–66.7) | 66.1 (64.0–68.2) | 67.9 (65.8–69.9) | |||
| Survival difference, % | 2.4* | 0.5 (8.1%)† | 2.9* | 1.5 (24.2%)# | 4.4* | 1.8 (29.0%)§ | 6.2* | |
| P value | .12 | .059 | .004 | <.0001 | ||||
| 5-yr Survival, % (95% CI) | 50.9 (48.5–53.2) | 54.2 (51.9–56.4) | 53.7 (51.4–56.0) | 57.4 (55.1–59.7) | 58.6 (56.3–60.8) | |||
| Survival difference, % | 3.3* | −0.5 (−6.5%)† | 2.8* | 3.7 (48.1%)# | 6.5* | 1.2 (15.6%)§ | 7.7* | |
| P value | .049 | .090 | .0001 | <.0001 | ||||
| Cox HR (95% CI) | 1 (reference) | 0.90 (0.82–0.99) | 0.91 (0.83–0.99) | 0.82 (0.74–0.90) | 0.79 (0.72–0.87) | |||
mo: month; yr: year; CI: confidence interval; HR: hazard ratio; SES: socioeconomic status; significant effects (P<.05) in bold.
Survival differences between black and matched white patients.
The absolute difference (percentage) of survival disparity after demographic match explained by SES.
The absolute difference (percentage) of survival disparity after demographic match explained by SES and presentation.
The absolute difference (percentage) of survival disparity after demographic match explained by SES and treatment.
Figure 3.
Kaplan-Meier plot for the five-year survivals of sampled black colon cancer patients and the four sets of matched white patients. Blue solid line: 2000 sampled black patients; pink long dashed and dotted line: demographic-matched white patients; red long dashed line: demographic plus socioeconomic status (SES)-matched white patient; green dotted line: presentation plus SES-matched white patients; orange dashed line: treatment plus SES-matched white patients.
Change in survival disparity over time
We next explored the change of survival disparity over time. Because the most important chemotherapy medicine oxaliplatin and targeted agent bevacizumab were both approved by the Food and Drug Administration in 2004 29, 30, these agents were frequently used in clinics since 2004 31, which is also consistent with the SEER-Medicare data (Supplementary Table S9). We then used the year of diagnosis in 2004 as the cut-off point to separate the patients into two groups: diagnosed before 2004 (1998–2003) and diagnosed in 2004 or after (2004–2009), similar to the approach used in the study of Silber et al 21. This approach divided all patients into approximately two halves (3039 black patients diagnosed before 2004 vs 3151 black patients diagnosed in 2004 or after). The change in survival disparity over time was illustrated in Supplementary Figure S4 and the detailed numbers were listed in Supplementary Table S10. From these new data, we could see several points clearly. First, as expected, significantly better survival was observed in both black and white patients who were diagnosed in 2004 or after than those diagnosed before 2004 (Supplementary Figure S4), which demonstrates the effectiveness of the use of oxaliplatin plus fluorouracil/capecitabine and target therapy in colorectal cancer treatment, and is consistent with the numerous published reports 32–38. Second, the racial survival disparity was smaller in the patients diagnosed in 2004 or after than diagnosed before 2004, indicating the use of oxaliplatin and/or bevacizumab helped to reduce the racial survival disparity (Supplementary Table S10, e.g., in demographic match, the two-, three-, and five-year survival disparity was 8.3%, 8.5%, and 9.6%, respectively, in the patients diagnosed before 2004, whereas 5.6%, 6.5%, and 7.1%, respectively, in those diagnosed in 2004 or after). This effect is consistent with a major finding of our study that the effect of treatment on survival disparity was much more apparent in advanced-stage patients, because both oxaliplatin and bevacizumab were approved and commonly used in advanced-stage but not early-stage patients. In addition, we found that tumor presentation had a much larger effect than treatment on survival disparity in the patients diagnosed both before and after 2004 (Supplementary Table S10), which was consistent with the major findings for the complete cohort.
DISCUSSION
The causes of the known disparities in incidence and outcomes for black as compared to white colon cancer patients have remained an unresolved issue for decades 3. Many efforts have been devoted to unravel the determinants of such disparities so that appropriate policies and interventions can be designed to minimize the racial disparity, and thereby improve outcomes for black patients. As a broadly recognized driver of colon cancer racial survival disparity, socioeconomic and sociodemographic status may affect many contributing factors, such as lifestyle, concurrent/comorbid medical conditions, stage at diagnosis, tumor characteristics, healthcare utilization, and treatment 17, 39. Despite many years of efforts, the survival disparity between black and white CRC patients is still widening 3. Therefore, better understanding of the role played by each possible mediator of racial survival disparity downstream of SES is necessary to help determine how interventions should be tailored to reduce this disparity.
Differences in the biological behavior of colon cancer may also contribute to survival disparity mediated by cancer stage at initial presentation. For example, colon cancer may occur at significantly younger age in black patients as compared to white patients, with black patients having approximately double the proportion of colon cancers which are diagnosed before the age of 50 (10.6% vs 5.5%) 40. This observation has prompted new recommendations from the American College of Gastroenterology such that the current standard of care for screening for colon cancer is actually a function of race, with white average-risk patients recommended to undergo first screening colonoscopy at age 50 but black average-risk patients at age 45 41. Although the observation that colon cancer may occur at an earlier age in black patients may partly be explained by factors related to SES, such as diet, further differences in distribution of colon cancer lesions in black vs white patients argue that there is likely a biological component as well. There is evidence that black patients may be predisposed to more proximal colon cancers in comparison to white patients who are more likely to develop more distal disease 42. Notwithstanding these lines of evidence suggesting that there may well be a difference in the biological behavior of colon cancer in black vs white patients, the relative importance of this contributor to the well-documented disparity in survival is unclear 43, and our current study is geared specifically to investigate those contributors driven by modifiable factors rather than genetic factors.
One important factor affected by SES is cancer stage at diagnosis. Patients with higher SES tend to have higher health-care utilization and higher CRC screening rates 44, 45, which makes them more likely to be diagnosed at an earlier stage compared to those with lower SES. Some studies suggest that SES affects CRC survival disparity through stage at diagnosis only, with no additional influence after stage is controlled 17, 39. Our results indicate that tumor presentation, including tumor stage, is indeed one of the most important factors contributing to the racial disparity in colon cancer survival. We observed that, after controlling for demographic factors, black patients in comparison to white patients had a significantly higher proportion of stage IV and lower proportions of stages I and II disease (Supplementary Table S2). Adequately matching on tumor presentation variables (e.g., stage, grade, size, and comorbidity) significantly reduced survival disparities (Table 2). These data are highly consistent with previous studies showing that tumor stage explains a significant portion, but not all, of CRC racial survival differences 17, 39, 45.
Treatment disparity is another possible source of colon cancer survival disparity related to SES 11–13. There is compelling evidence showing that treatment disparity exists between black and white CRC patients. Compared to whites, black CRC patients are less likely to receive surgical resection or radiation treatments 46, and are treated less frequently with standard chemotherapy 47–50. Disappointingly, the treatment gap between blacks and whites remained unaltered between 1992 and 2002, despite many mitigation efforts 50, 51. Our finding that the presentation-matched whites had higher rates of most treatments than blacks is consistent with previous studies suggesting that black CRC patients are likely to still be under-treated 46–50. However, it should be noted that the absolute differences in the receipt of some treatments are small, which were also reported in other studies such as that of Silber et al.21. These small absolute differences in treatment might be part of the reasons that, although treatment differences were significant in early-stage patients (Table 1), they had little effect on survival disparity. In contrast, the absolute differences of treatment disparities in advanced-stage patients were much larger. These data are in line with our conclusion that the impact of treatment differences on survival disparity was more prominent in advanced-stage patients.
Stratified analysis by cancer stage revealed sharp differences of treatment disparity between early- and advanced-stage patients, in that early-stage patients usually had disparity in surgery rate whereas advanced-stage patients exhibited heavy disparity in the use of chemotherapy and targeted therapy (Table 1). These findings were consistent with clinical practice guidelines that predominantly use surgery in early-stage and chemotherapy in advanced-stage patients. Although, after presentation match, white patients received more of some treatments in stage I (use of surgery) and stage II (use of chemotherapy) patients, most differences were modestly significant with small PRs (Table 1). In comparison, most differences in the use of chemotherapy agents in advanced-stage patients were highly significant with high PRs. For example, the use of fluorouracil/capecitabine plus oxaliplatin in both stages III and IV was significantly different in black vs white patients (Table 1). This data derived from 1998 to 2009 was similar to the previously reported racial difference in the use of standard chemotherapy from 1992 to 2002 51, disappointingly suggesting that despite of the many efforts made recently, the disparity in the use of chemotherapy in advanced-stage colon cancer patients has not been meaningfully improved. These data echo another important conclusion made in our study that the percentage of survival disparity after demographic match explained by treatment is much higher in advanced-stage than early-stage patients (Table 3 and Supplementary Figure S1). Taken together, these lines of evidence suggest that, in order to control survival disparity, more efforts may need to be tailored to minimize treatment disparities (especially chemotherapy use) in patients with advanced-stage disease. Nevertheless, it should be borne in mind that although it is ideal to eliminate all racial treatment disparities, there will be a point of diminishing return-to-efforts ratio to ensure the appropriate delivery of cancer treatment for all patients.
Our sub-cohort analyses which adjusted either SES or marital status and urban/rural residence provided us with important clues as to the effects on racial survival disparity by different contributing factors. Highly consistent with the results of the analyses derived from the complete cohort, tumor presentation also played a much stronger role on survival disparity than treatment in the sub-cohort analyses. For example, in the analyses that controlled SES, the two-, three-, and five-year survival after tumor presentation match was 35.4%, 24.2%, and 48.1%, respectively. After further treatment match, the survival disparity was −1.5%, 8.1%, and −6.5%, respectively. Very similar phenomenon was observed in the analyses that controlled marital status and rural/urban residence. Nonetheless, it should be noted that these analyses were derived from the sub-cohort of only 2000 black patients and needed to be confirmed in larger studies.
The underlying reasons for racial disparity are complex and difficult to decipher and need even larger prospective studies with more homogenous patient characteristics (ideally randomized controlled trials). However, currently it may not be feasible to conduct such studies, especially in prospective settings. The SEER-Medicare dataset, although not perfect, is one of the largest available resources that may help decipher some of the reasons for racial survival disparities. Our findings that larger treatment differences were observed in advanced-stage patients provided one stepping stone towards a better understanding of the complex natures of racial survival disparities in colon cancer. Nevertheless, treatment differences might be, to some extent, a reflection of other racial differences, such as physician-patient communication and patient attitude. Mitigating these disparities will require continued efforts in patient education and navigation that would help both physicians and patients to work together towards an informed decision making on their treatments.
The novel minimum distance-based matching strategy is a major strength of our study. Most racial disparity studies used model-based methods. With white patients constituting the majority of the study population, utilization of such models to explain racial disparity may be limited21. During the matching, we also used propensity scores to calibrate the distance before assigning whites to blacks according to minimum distance. This method is different from the propensity score matching method which tends to only balance all of the observed covariates used to define the score in a stochastic sense similar to random assignment 52, 53. By incorporating propensity score, the minimum distance method not only balances the observed covariates in a stochastic sense, but also achieves close matches on individual key covariates 54. Another strength of our study is the large patient cohort size in the SEER-Medicare database that allowed us to focus the analysis on colon cancer and excluding rectal cancer, eliminating potential confounding effects from the differences between these two subtypes, especially as regards of treatment 55.
A major limitation is that due to the drastic differences of SES, as well as other important variables such as marital status and urban/rural residence between black and white patients, we were unable to control all of them in the primary matching analyses. Therefore, the primary findings of this study are still subject to residual confounding which warrants further explorations. It is apparent that, even in the sub-cohort analyses, there is still remaining survival disparity after treatment match (3.7%, 2.4%, and 3.3%, respectively for two-, three-, and five-year survivals in Table 4, 4.7%, 4.3%, and 5.2%, respectively in Supplementary Table S6). The residual disparity could be explained by factors that were not controlled in the analyses. SEER data do not collect information on other potentially important confounder such as the uses of over-the-counter medications and oral prescription drugs 56, provider- and facility-related characteristics 57, as well as genetics 58, dietary 59, environmental60, and biological factors 17. A more complete assessment of major underlying factors is needed in order to inform the development of strategies that can further reduce or even completely eliminate the racial disparity in colon cancer survival.
In conclusion, we present evidence that in the SEER-Medicare population, black colon cancer patients received significantly less treatments than white patients, and this treatment disparity exhibited a dramatic impact on survival disparity in advanced-stage but not early-stage patients. For all stage combined patients, the differences in survival appear to be primarily related to tumor presentation characteristics at diagnosis rather than treatment differences. These findings suggest that reducing the treatment disparity is important to mitigate the racial survival disparity in advanced-stage colon cancer patients. However, for overall colon cancer population, tumor presentation may play a more prominent role. To reduce the survival disparity between black and white colon cancer patients, more effort is needed in areas such as disease biology and CRC screening to reduce the tumor presentation disparities between black and white patients 2, 3, 61.
Supplementary Material
Supplementary Table S1. Codes used to identify procedures and drug usage from Medicare files
Supplementary Table S2. Detailed overall patient characteristics and matching results
Supplementary Table S3. Detailed patient characteristics for black and white controls by cancer stage#
Supplementary Table S4. Characteristics of sampled and remaining black patients
Supplementary Table S5. Two-, three- and five-year survivals for the sampled, all, and remaining black patients in the sub-cohort analysis
Supplementary Table S6. Two-, three- and five-year survival for sampled black and matched white colon cancer patients in the sub-cohort analysis
Supplementary Table S7. Treatment and SES disparities between black and presentation-matched white colon cancer patients by cancer stage in the sub-cohort analysis#
Supplementary Table S8. Two- and three-year survival for black and matched white colon cancer patients by stage in the sub-cohort analysis
Supplementary Table S9. The numbers of patients using oxaliplatin, bevacizumab, and cetuximab in 68,141 colon cancer patients diagnosed from 1998 to 2009
Supplementary Table S10. Two-, three-, and five-year survival for black and matched white colon cancer patients diagnosed before 2004 (1998–2003) and diagnosed in 2004 or after (2004–2009)
Acknowledgments
Grant supports: The work was supported by grants from Pennsylvania Department of Health, Thomas Jefferson University, National Cancer Institute grant CA 162201, and American Cancer Society Research Scholar Grant 123741-RSG-13-003-01-CCE.
Abbreviations
- CRC
colorectal cancer
- SEER
the Surveillance, Epidemiology and End Results
- SES
socioeconomic status
- NCI
the National Cancer Institute
- CHF
congestive heart failure
- COPD
chronic obstructive pulmonary disease
- IQR
interquartile range
- CI
confidence interval
- HR
hazard ratio
- PR
prevalence ratio
- SDD
standardized differences
Footnotes
Disclosure: The authors report no conflict of interest.
Author contributions:
Hushan Yang has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hushan Yang, Yinzhi Lai, Chun Wang.
Acquisition, analysis, or interpretation of data: Hushan Yang, Yinzhi Lai, Chun Wang, Jesse M. Civan, Juan P. Palazzo, Terry Hyslop, Ronald E. Myers, Ashwin Sama, Bingshan Li, Binghua Jiang, Jinliang Xing.
Drafting of the manuscript: Hushan Yang, Yinzhi Lai, Chun Wang.
Critical revision of the manuscript for important intellectual content: Hushan Yang, Chun Wang, Jesse M. Civan, Juan P. Palazzo, Terry Hyslop, Jianqing Lin, Ronald E. Myers, Zhong Ye, Bing-Hua Jiang, Ashwin Sama, Jinliang Xing.
Statistical analysis: Hushan Yang, Yinzhi Lai, Chun Wang, Terry Hyslop, Bingshan Li.
Obtained funding: Hushan Yang.
Administrative, technical, or material support: Zhong Ye.
Study supervision: Hushan Yang.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Irby K, Anderson WF, Henson DE, et al. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975–2002) Cancer Epidemiol Biomarkers Prev. 2006;15:792–797. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 2.Grubbs SS, Polite BN, Carney J, et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013;31:1928–1930. doi: 10.1200/JCO.2012.47.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30:401–405. doi: 10.1200/JCO.2011.37.5527. [DOI] [PubMed] [Google Scholar]
- 4.Simpson DR, Martínez ME, Gupta S, et al. Racial disparity in consultation, treatment, and the impact on survival in metastatic colorectal cancer. J Natl Cancer Inst. 2013;105:1814–1820. doi: 10.1093/jnci/djt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975–2010. National Cancer Institute; Bethesda, MD: 2013. [Google Scholar]
- 6.Alexander D, Chatla C, Funkhouser E, et al. Postsurgical disparity in survival between African Americans and Caucasians with colonic adenocarcinoma. Cancer. 2004;101:66–76. doi: 10.1002/cncr.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102:538–546. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 9.Du XL, Meyer TE, Franzini L. Meta-analysis of racial disparities in survival in association with socioeconomic status among men and women with colon cancer. Cancer. 2007;109:2161–2170. doi: 10.1002/cncr.22664. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers GM, 3rd, Becker PS, Bennett CL, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw. 2008;6:536–564. doi: 10.6004/jnccn.2008.0042. [DOI] [PubMed] [Google Scholar]
- 11.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 12.Lemmens V, Van Halteren A, Janssen-Heijnen M, et al. Adjuvant treatment for elderly patients with stage III colon cancer in the southern Netherlands is affected by socioeconomic status, gender, and comorbidity. Ann Oncol. 2005;16:767–772. doi: 10.1093/annonc/mdi159. [DOI] [PubMed] [Google Scholar]
- 13.Doubeni CA, Field TS, Buist DS, et al. Racial differences in tumor stage and survival for colorectal cancer in an insured population. Cancer. 2007;109:612–620. doi: 10.1002/cncr.22437. [DOI] [PubMed] [Google Scholar]
- 14.Hynes D, Tarlov E, Lee T, et al. Examining disparities in colon cancer treatment patterns. J Clin Oncol (Meeting Abstracts) 2008 [Google Scholar]
- 15.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 16.Wudel LJ, Jr, Chapman WC, Shyr Y, et al. Disparate outcomes in patients with colorectal cancer: effect of race on long-term survival. Arch Surg. 2002;137:550–554. doi: 10.1001/archsurg.137.5.550. [DOI] [PubMed] [Google Scholar]
- 17.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer model of health disparities: understanding mortality differences in minority populations. J Clin Oncol. 2006;24:2179–2187. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 18.Laryea JA, Siegel E, Klimberg S. Racial disparity in colorectal cancer: The role of equal treatment. Dis Colon Rectum. 2014;57:295–302. doi: 10.1097/DCR.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 19.Mack CD, Carpenter W, Meyer AM, et al. Racial disparities in receipt and comparative effectiveness of oxaliplatin for stage III colon cancer in older adults. Cancer. 2012;118:2925–2934. doi: 10.1002/cncr.26622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominitz JA, Samsa GP, Landsman P, et al. Race, treatment, and survival among colorectal carcinoma patients in an equal-access medical system. Cancer. 1998;82:2312–2320. doi: 10.1002/(sici)1097-0142(19980615)82:12<2312::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310:389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 22.Daniel SR, Armstrong K, Silber JH, et al. An algorithm for optimal tapered matching, with application to disparities in survival. J Comput Graph Stat. 2008;17:914–924. doi: 10.1198/106186008X385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang D, Small DS, Silber JH, et al. Optimal matching with minimal deviation from fine balance in a study of obesity and surgical outcomes. Biometrics. 2012;68:628–636. doi: 10.1111/j.1541-0420.2011.01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.O’Brien PC, Fleming TR. A paired Prentice-Wilcoxon test for censored paired data. Biometrics. 1987:169–180. [Google Scholar]
- 26.Wei L, Ying Z, Lin D. Linear regression analysis of censored survival data based on rank tests. Biometrika. 1990;77:845–851. [Google Scholar]
- 27.Le H, Ziogas A, Lipkin SM, et al. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17:1950–1962. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed NU, Pelletier V, Winter K, et al. Factors explaining racial/ethnic disparities in rates of physician recommendation for colorectal cancer screening. Am J Public Health. 2013;103:e91–99. doi: 10.2105/AJPH.2012.301034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eloxatin: New or modified indication. Washington, DC: US Food and Drug Administration; 2004. [Google Scholar]
- 30.http://www.fda.gov/newsevents/newsroom/pressannouncements/2004/ucm108252.htm.
- 31.Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Petrelli F, Coinu A, Ghilardi M, et al. Efficacy of oxaliplatin-based chemotherapy + bevacizumab as first-line treatment for advanced colorectal cancer: a systematic review and pooled analysis of published trials. Am J Clin Oncol. 2015;38:227–233. doi: 10.1097/COC.0b013e3182a2d7b8. [DOI] [PubMed] [Google Scholar]
- 33.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 34.Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 35.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 36.Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 37.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 38.Kemeny N, Garay CA, Gurtler J, et al. Randomized multicenter phase II trial of bolus plus infusional fluorouracil/leucovorin compared with fluorouracil/leucovorin plus oxaliplatin as third-line treatment of patients with advanced colorectal cancer. J Clin Oncol. 2004;22:4753–4761. doi: 10.1200/JCO.2004.03.119. [DOI] [PubMed] [Google Scholar]
- 39.Mayberry RM, Coates RJ, Hill HA, et al. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87:1686–1693. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- 40.Theuer CP, Wagner JL, Taylor TH, et al. Racial and ethnic colorectal cancer patterns affect the cost-effectiveness of colorectal cancer screening in the United States. Gastroenterology. 2001;120:848–856. doi: 10.1053/gast.2001.22535. [DOI] [PubMed] [Google Scholar]
- 41.Rex DK, Johnson DA, Anderson JC, et al. American college of gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 42.Rex DK, Khan AM, Shah P, et al. Screening colonoscopy in asymptomatic average-risk African Americans. Gastrointest Endosc. 2000;51:524–527. doi: 10.1016/s0016-5107(00)70283-5. [DOI] [PubMed] [Google Scholar]
- 43.Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol. 2005;100:515–523. doi: 10.1111/j.1572-0241.2005.41829.x. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman-Goetz L, Breen N, Meissner H. The impact of social class on the use of cancer screening within three racial/ethnic groups in the United States. Ethn Dis. 1997;8:43–51. [PubMed] [Google Scholar]
- 45.Marcella S, Miller JE. Racial differences in colorectal cancer mortality: the importance of stage and socioeconomic status. J Clin Epidemiol. 2001;54:359–366. doi: 10.1016/s0895-4356(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 46.Morris AM, Billingsley KG, Baxter NN, et al. Racial disparities in rectal cancer treatment: a population-based analysis. Arch Surg. 2004;139:151–155. doi: 10.1001/archsurg.139.2.151. [DOI] [PubMed] [Google Scholar]
- 47.Berry J, Bumpers K, Ogunlade V, et al. Examining racial disparities in colorectal cancer care. J Psychosoc Oncol. 2009;27:59–83. doi: 10.1080/07347330802614840. [DOI] [PubMed] [Google Scholar]
- 48.Hao Y, Landrine H, Jemal A, et al. Race, neighbourhood characteristics and disparities in chemotherapy for colorectal cancer. J Epidemiol Community Health. 2011;65:211–217. doi: 10.1136/jech.2009.096008. [DOI] [PubMed] [Google Scholar]
- 49.Obeidat NA, Pradel FG, Zuckerman IH, et al. Racial/ethnic and age disparities in chemotherapy selection for colorectal cancer. Am J Manag Care. 2010;16:515–522. [PubMed] [Google Scholar]
- 50.White A, Liu CC, Xia R, et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008;113:3400–3409. doi: 10.1002/cncr.23924. [DOI] [PubMed] [Google Scholar]
- 51.Gross CP, Smith BD, Wolf E, et al. Racial disparities in cancer therapy. Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 53.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 54.Rosenbaum PR, Ross RN, Silber JH. Minimum distance matched sampling with fine balance in an observational study of treatment for ovarian cancer. J Am Stat Assoc. 2007;102:75–83. [Google Scholar]
- 55.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 56.Shiovitz S, Bansal A, Burnett-Hartman AN, et al. Cancer-Directed Therapy and Hospice Care for Metastatic Cancer in American Indians and Alaska Natives. Cancer Epidemiol Biomarkers Prev. 2015;24:1138–1143. doi: 10.1158/1055-9965.EPI-15-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joseph LJ, Goodman M, Higgins K, et al. Racial disparities in squamous cell carcinoma of the oral tongue among women: a SEER data analysis. Oral Oncol. 2015;51:586–592. doi: 10.1016/j.oraloncology.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Burnett T, Kono S, et al. Trans-ethnic genome-wide association study of colorectal cancer identifies a new susceptibility locus in VTI1A. Nat Commun. 2014;5:4613. doi: 10.1038/ncomms5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lian M, Schootman M, Doubeni CA, et al. Geographic variation in colorectal cancer survival and the role of small-area socioeconomic deprivation: a multilevel survival analysis of the NIH-AARP Diet and Health Study Cohort. Am J Epidemiol. 2011;174:828–838. doi: 10.1093/aje/kwr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexander DD, Waterbor J, Hughes T, et al. African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: an epidemiologic review. Cancer Biomark. 2007;3:301–313. doi: 10.3233/cbm-2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baldwin LM, Dobie SA, Billingsley K, et al. Explaining black–white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97:1211–1220. doi: 10.1093/jnci/dji241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Codes used to identify procedures and drug usage from Medicare files
Supplementary Table S2. Detailed overall patient characteristics and matching results
Supplementary Table S3. Detailed patient characteristics for black and white controls by cancer stage#
Supplementary Table S4. Characteristics of sampled and remaining black patients
Supplementary Table S5. Two-, three- and five-year survivals for the sampled, all, and remaining black patients in the sub-cohort analysis
Supplementary Table S6. Two-, three- and five-year survival for sampled black and matched white colon cancer patients in the sub-cohort analysis
Supplementary Table S7. Treatment and SES disparities between black and presentation-matched white colon cancer patients by cancer stage in the sub-cohort analysis#
Supplementary Table S8. Two- and three-year survival for black and matched white colon cancer patients by stage in the sub-cohort analysis
Supplementary Table S9. The numbers of patients using oxaliplatin, bevacizumab, and cetuximab in 68,141 colon cancer patients diagnosed from 1998 to 2009
Supplementary Table S10. Two-, three-, and five-year survival for black and matched white colon cancer patients diagnosed before 2004 (1998–2003) and diagnosed in 2004 or after (2004–2009)