Abstract
Background
The impact that the absence of expression of NeuGc in pigs might have on pig organ or cell transplantation in humans has been studied in vitro, but only using red blood cells (pRBCs) and peripheral blood mononuclear cells (pPBMCs) as the target cells for immune assays. We have extended this work in various in vitro models and now report our initial results.
Methods
The models we have used involve GTKO/hCD46 and GTKO/hCD46/NeuGcKO pig aortas and corneas, and pRBCs, pPBMCs, aortic endothelial cells (pAECs), corneal endothelial cells (pCECs), and isolated pancreatic islets. We have investigated the effect of the absence of NeuGc expression on (i) human IgM and IgG binding, (ii) the T cell proliferative response, (iii) human platelet aggregation, and (iv) in an in vitro assay of the instant blood-mediated inflammatory reaction (IBMIR) following exposure of pig islets to human blood/serum.
Results
The lack of expression of NeuGc on some pig tissues (aortas, corneas) and cells (RBCs, PBMCs, AECs) significantly reduces the extent of human antibody binding. In contrast, the absence of NeuGc expression on some pig tissues (CECs, isolated islet cells) does not reduce human antibody binding, possibly due to their relatively low NeuGc expression level. The strength of the human T cell proliferative response may also be marginally reduced, but is already weak to GTKO/hCD46 pAECs and islet cells. We also demonstrate that the absence of NeuGc expression on GTKO/hCD46 pAECs does not reduce human platelet aggregation, and nor does it significantly modify the IBMIR to pig islets.
Conclusion
The absence of NeuGc on some solid organs from GTKO/hCD46/NeuGcKO pigs should reduce the human antibody response after clinical transplantation when compared to GTKO/hCD46 pig organs. However, the clinical benefit of using certain tissue (e.g., cornea, islets) from GTKO/hCD46/NeuGcKO pigs is questionable.
Keywords: α1,3-galactosyltransferase gene-knockout; Cytidine monophospho-N-acetylneuraminic acid hydroxylase; Galactose-α1,3-galactose; Islets, pancreatic; N-glycolylneuraminic acid; Pig; Xenotransplantation
INTRODUCTION
For approximately 20 years it has been known that humans, who do not express galactose-α1,3-galactose (Gal) or N-glycolylneuraminic acid (NeuGc), develop natural antibodies directed to these two oligosaccharide antigens that are expressed on wild-type (i.e., genetically-unmodified) pig cells (1-5). Apes and Old World nonhuman primates also lack Gal expression and so represent an experimental surrogate for humans in this respect (6, 7), but they do express NeuGc and so do not provide a model for studying the role of anti-NeuGc antibodies in xenotransplantation (5). Several studies have investigated the potential deleterious role of anti-NeuGc antibody, but these have been carried out in NeuGcKO mice (8, 9).
It has been well-documented that the binding of human or nonhuman primate anti-Gal antibodies to Gal antigens on pig cells initiates complement activation, resulting in hyperacute or early pig graft destruction (10-12). The production of α1,3-galactosyltransferase gene-knockout (GTKO) pigs has overcome this immune barrier (13-16), particularly when combined with transgenic expression of a human complement-regulatory protein, e.g., human CD46 (hCD46) or human CD55 (hCD55) (17, 18).
More recently, the gene for the enzyme cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) has been knocked out, resulting in the generation of pigs that do not express NeuGc (which we here term ‘NeuGcKO pigs’) (19). Studies have been reported showing that WT porcine tissue (e.g., skin, ligament) or cells (e.g., leukocytes) elicited a significant anti-nonGal or anti-NeuGc antibody response in human recipients (20-22). However, organs or cells from these pigs have not been tested in an in vivo model, although this may now become possible as it has been reported that New World monkeys (that express Gal) do not express NeuGc, and, therefore, should produce anti-NeuGc antibodies (23).
The impact that the absence of expression of NeuGc in pigs might have on pig organ or cell transplantation in humans has been studied previously in vitro, but only using red blood cells (pRBCs) and peripheral blood mononuclear cells (pPBMCs) as the target cells for immune assays (24-26). We have extended this work in various in vitro models and now report our initial results.
The models we have used involve NeuGcKO pig aortas and corneas, and RBCs, PBMCs, aortic endothelial cells (AECs), corneal endothelial cells (CECs), and isolated pancreatic islet cells. We have investigated the effect of the absence of NeuGc expression on human IgM and IgG binding, the T cell proliferative response, and on human platelet aggregation. There is evidence that preexisting and elicited nonGal antibodies contribute to the development of thrombotic microangiopathy through activation of vascular endothelial cells (27). Genetic modification (e.g., GTKO/CD46) of pAECs significantly reduces human platelet aggregation in vitro compared to WT pAECs (28), and deletion of an additional carbohydrate antigen might prove beneficial.
Finally, as early islet loss secondary to the instant blood-mediated inflammatory reaction (IBMIR) is a major obstacle to successful engraftment of pig islets following xenotransplantation, we have investigated this response in an established in vitro model (29-31).
MATERIALS AND METHODS
Ethics
All animal procedures used in this study conformed to the University of Pittsburgh Institutional Animal Care and Use Committee guidance for laboratory animals (IACUC13082323), and the animals were humanely euthanized. In addition, all in vitro studies using human serum or blood were approved by the Research Ethics Committee at the University of Pittsburgh. The samples were obtained in accordance with the Declaration of Helsinki. Participants gave informed consent per the guidelines of the Institutional Review Board of the University of Pittsburgh (IRB0608179).
Generation of NeuGcKO pigs
A programmable meganuclease (zinc finger nucleases, ZFNs) gene-targeting strategy together with somatic cell nuclear transfer (SCNT) cloning was used to generate male and female homozygous NeuGcKO pigs. The mRNAs for the ZFNs [designed to target and cleave the pig CMAH gene sequence AAACTCCTGAACTACaaggcTCGGCTGGTGAAGGA, beginning at position 1,341 of Ensembl transcript ENSSSCT0000-0001195] were purchased from Sigma-Aldrich (St. Louis, MO). Semi-confluent porcine fetal fibroblasts (~ 1×106 cells/transfection) were harvested and mixed with a pair of ZFN mRNAs, 2μg each and electroporated at 500V, 1msec, 3 pulses. Cells were then cultured in DMEM with 20% FBS (Hyclone, UT) in a 6-well tissue culture plate at 32°C for 24h, as a cold shock treatment, then cultured for a further 24h at 38°C for recovery. Single-cell limiting dilutions were then carried out in 96-well tissue culture plates in DMEM supplemented with 20% FBS and media changed every 3-4 days. Confluent colonies were trypsinized and half the cells from each colony used for PCR analysis for indels (insertions/deletions). The other half of each colony was transferred to 24-well plates for freezing for SCNT cloning and further analysis of indels as needed. Biallelic NeuGcKO pigs were produced from frozen-thawed colonies pooled for SCNT cloning. The background genetics of the cells used to generate these NeuGcKO were GTKO/hCD46.
Cell sources
Adult pig (p) AECs were collected from wild-type (WT) pigs and from two different genetically-modified pigs provided by Revivicor, Blacksburg, VA - (i) GTKO pigs expressing the human complement-regulatory protein CD46 (32) (GTKO/hCD46 pigs) and (ii) GTKO/hCD46 pigs with additional knockout of NeuGc (GTKO/hCD46/NeuGcKO pigs). Expression of hCD46 was constitutive via an endogenous or CAG promoter system. All pigs were of blood type O (non-A) and of Large White/Landrace/Duroc cross-breed (but were not from identical clones).
pAECs were isolated from fresh aortas and cultured in collagen I-coated 25- or 75-cm2 tissue culture flasks (BD Biosciences, San Jose, CA) in pAEC culture medium (10% heat-inactivated FBS [Sigma, St. Louis, MO], antibiotic–antimycotic [Invitrogen, Carlsband, CA] and endothelial growth factor [30 μg/ml, BD Biosciences (BD), San Jose, CA]) at 37°C in a humidified atmosphere of 5% CO2, as previously described (33). Cell aliquots were frozen and stored at −80°C until used for in vitro assays.
Human aortic endothelial cells (hAECs) (as an allograft control), were purchased from Lonza (Walkersville, MD), and cultured to monolayers in EBM-2 medium (which included endothelial growth medium-2), under the same conditions. Confluent cultures of both pAECs and hAECs were characterized by their cobblestone morphology and were used before passage 7 in all experiments.
Preparation of various tissues
Small pieces of heart, lung, kidney, liver, spleen, and lymph nodes were collected from GTKO/hCD46/NeuGcKO pigs (n=2) to confirm the lack of NeuGc expression. The same samples from a GTKO/hCD46 pig (n=1) were used as controls. Each piece of tissue was embedded in optimal cutting temperature compound (Tissue-Tek, Miles Laboratories, Naperville, IL), frozen, and sectioned for immunofluorescence staining.
Preparation of red blood cells (RBCs), peripheral blood mononuclear cells (PBMCs), and cultured pig islet cells
Pig blood from WT (n=2), GTKO/hCD46 (n=2), GTKO/hCD46/NeuGcKO (n=2) pigs was provided by Revivicor, and human blood was drawn from healthy volunteers (AB blood type. RBCs and PBMCs were isolated as previously described (Hara, 2008 #397)(34). Pancreatic islets from one-month-old GTKO/hCD46 (n=1) and GTKO/hCD46/NeuGcKO (n=2) pigs were isolated and cultured, as previously described (35, 36).
Preparation of corneas and cultured corneal endothelial cells (CECs)
Eyes from 6 month-old WT pigs (n=3) were obtained from a local slaughterhouse. Eyes from GTKO/hCD46 (n=3) and GTKO/hCD46/NeuGcKO (n=6) pigs were provided by Revivicor. Corneas (that were not suitable for clinical allotransplantation) were obtained from deceased humans (blood type O) from the Pittsburgh Center for Organ Recovery and Education (CORE) with the approval of the University of Pittsburgh Committee for Oversight of Research Involving the Dead, and in accordance with the guidelines of the Declaration of Helsinki for research involving the use of human tissues. Tissue sections for immunofluorescence staining and isolation of CECs for all in vitro assays were as previously described (37, 38).
Flow cytometric analysis of expression of Gal, NeuGc, and hCD46 on RBCs, PBMCs, AECs, CECs and cultured pig islets
Surface expression of Gal, NeuGc, and hCD46 was investigated by flow cytometry, as previously described (33, 34, 38). Cultured porcine islet cells were dissociated into single-cell suspensions by gentle agitation in 0.25% trypsin-EDTA (Invitrogen) for flow cytometric analysis, as previously described (36).
Flow cytometric analysis for human IgM/IgG binding to RBCs, PBMCs, AECs, CECs and cultured pig islets
IgM and IgG binding assays using human sera (n=6, including all ABO blood types) were carried out as previously described (33, 34, 38, 39). FITC-conjugated goat-derived anti-human IgM (μ chain–specific) or IgG (γ chain–specific) polyclonal antibodies (concentration 1:100; Invitrogen) were used as secondary antibodies. Since these secondary antibodies bind to human B cells, this could possibly result in false positivity and so human PBMCs were not used in this assay.
Immunofluorescence staining for Gal and NeuGc expression on various tissues
Staining for expression of Gal and NeuGc was carried out as previously described (38).
Mixed cell culture to determine the human proliferative lymphocyte response
Human PBMC proliferation in response to pAECs or islet cells was evaluated in a mixed lymphocyte reaction (MLR)-based assay (40). Human PBMC were labeled with carboxyfluorescein succinimidyl ester (CFSE) before coculture. Stimulators were pAECs obtained from either GTKO/hCD46 or GTKO/hCD46/NeuGcKO pigs (responder stimulator ratio was 1:10), or isolated islet cells obtained from either GTKO/hCD46 or GTKO/hCD46/NeuGcKO pigs (responder stimulator ratio 1:1). Responders and stimulators were co-cultured for 5 days. PHA activation of human PBMC (1μg/mL) was used as a positive control. The strength of proliferation was determined by CFSE dilution using flow cytometry.
Human platelet aggregation assay
After the cells had grown to confluence in their respective media, 3.2% trisodium citrated fresh whole blood (1ml) drawn from a healthy human volunteer was added to the adherent monolayers of pAECs and hAECs and incubated for 2h at 37°C. Supernatant fluid (500μl) from co-incubation of blood and AECs was collected, mixed with saline (500μl) in a plastic cuvette, and used in the platelet aggregation assay by platelet aggregometry (two-sample, four-channel, model 592 Whole Blood Aggregometer, Chrono-log, Havertown, PA), as previously described (28). Calcium (15μl, 9.3 mg Ca++ per ml) was added in each assay, and no aggregation occurred in the absence of calcium. We compared human platelet aggregation to WT, GTKO/hCD46, and GTKO/hCD46/NeuGcKO pAECs with that to hAECs.
In vitro IBMIR assay
Following short-term culture, isolated islets from pancreases from juvenile GTKO/hCD46/NeuGcKO pigs (n=2) were exposed in vitro to blood or plasma from three blood type-matched human donors, as previously described (30, 31). Time to clotting was recorded. Thirty and 60min after exposure of the islets to blood, release of C-peptide (by ELISA, Mercodia, Uppsala, Sweden) was determined. Following 3h exposure to human plasma, the viability of islets was evaluated using a dual fluorescence dye (calcein-AM, propidium iodide, 1mg/ml; Molecular Probes, Eugene, Oregon, USA) and quantification of viable/dead cells was measured using Image J software (National Institutes of Health, Bethesda, MD). As controls, islets from a GTKO/hCD46 pig were similarly tested.
Statistical methods
The statistical significance of differences was determined by paired Student's t-test for two groups or nonparametric analysis of variance by Kruskal-Wallis test for multiple comparisons. Statistical analysis was performed using GraphPad Prism version 4 (GraphPad Software, San Diego, CA). Values are presented as mean ± SEM value. Differences were considered to be significant at p<0.05.
RESULTS
Expression of Gal and NeuGc on various tissues of GTKO/hCD46 and GTKO/hCD46/NeuGcKO pigs
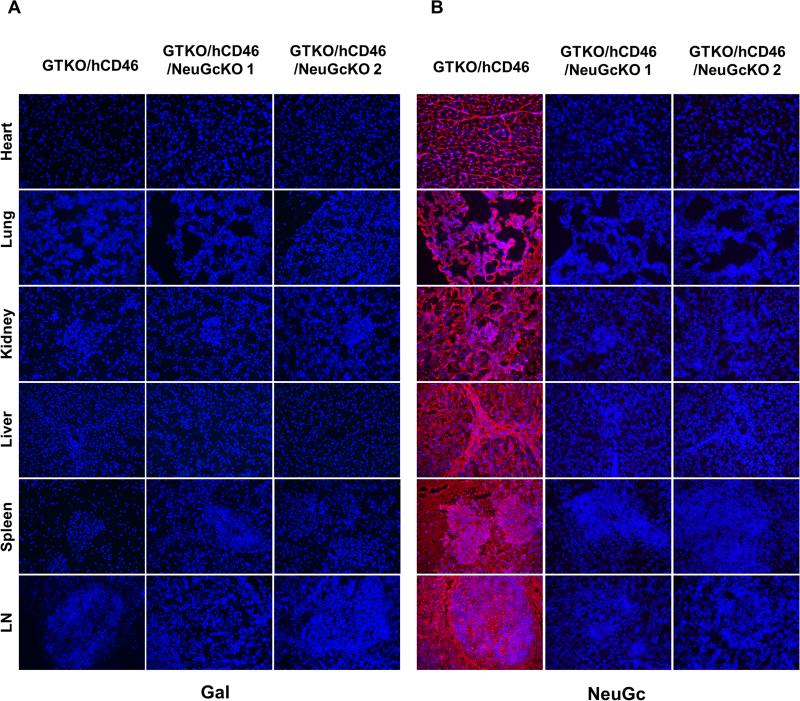
It is well-established that WT pig tissue expresses both Gal and NeuGc, as previously described [(41), data not shown]. NeuGc was expressed on all tissues from GTKO/hCD46 pigs, but neither Gal nor NeuGc expression was detected on any of the tissues from GTKO/hCD46/NeuGcKO pigs (Figure 1).
Figure 1.
Expression of Gal and NeuGc on heart, lung, kidney, liver, spleen, and lymph nodes of GTKO/hCD46 and GTKO/CD46/NeuGcKO pigs. All tissues from GTKO/hCD46 pigs were negative for Gal, but strongly positive for NeuGc, whereas tissues from two GTKO/hCD46/NeuGcKO pigs were negative for both antigens. (Magnification x200; nuclei, blue; Gal, green; NeuGc, red)
Expression of Gal, NeuGc, and hCD46 on pig and human RBCs and PBMCs by flow cytometry
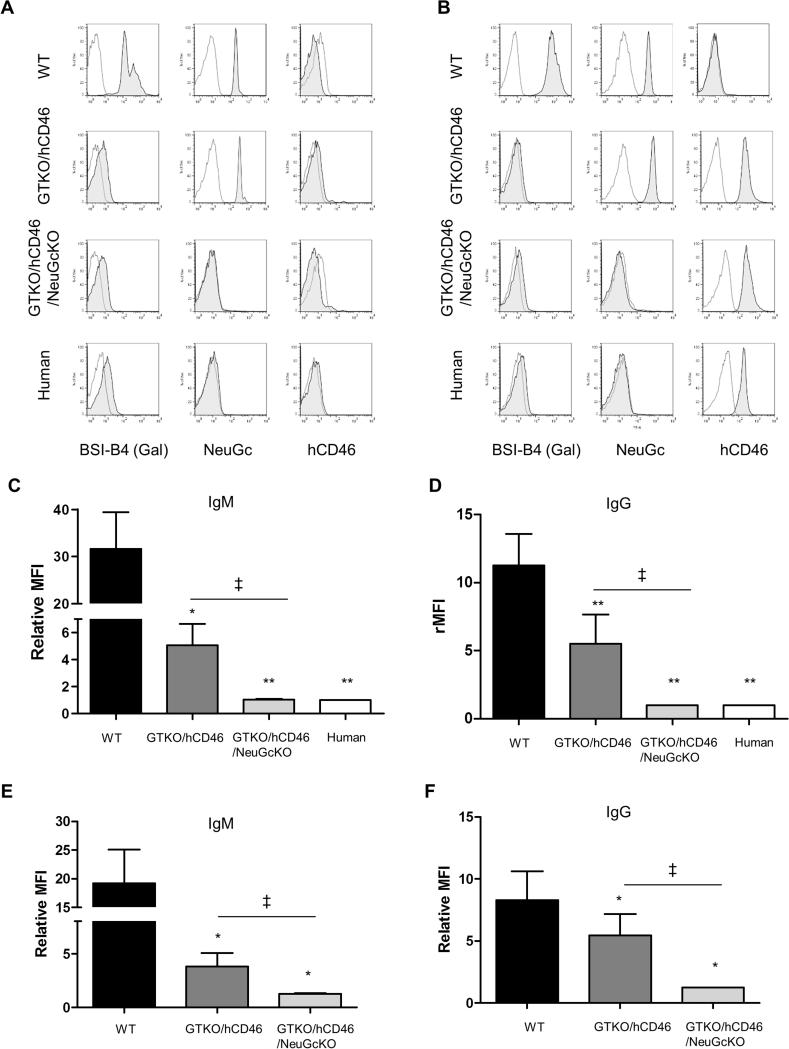
WT pig RBCs (Figure 2A) and PBMCs (Figure 2B) expressed Gal and NeuGc. GTKO/CD46 pig RBCs (Figure 2A), PBMCs (Figure 2B), and cultured islet cells (Figure 3A) were negative for Gal expression but positive for NeuGc. GTKO/hCD46/NeuGcKO pig RBCs (Figure 2A), PBMCs (Figure 2B), and cultured islet cells (Figure 3A) did not express either Gal or NeuGc, as was the case for human RBCs and PBMCs (Figure 2A,B). Although no RBCs expressed hCD46, significant levels of hCD46 were expressed on PBMCs and cultured islet cells from GTKO/hCD46 and GTKO/hCD46/NeuGcKO pigs (Figure 2A,B, and Figure 3A).
Figure 2.
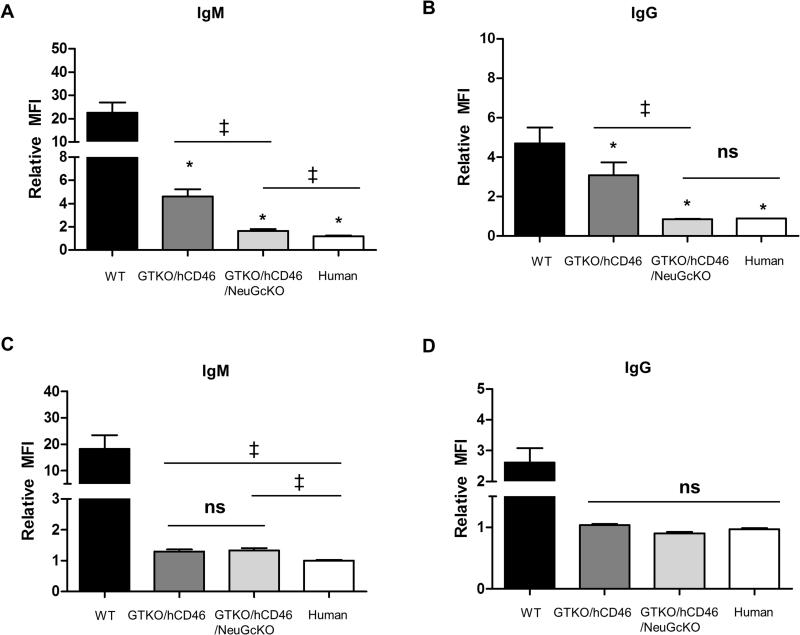
Expression of Gal, NeuGc, and hCD46 on WT, GTKO/hCD46, and GTKO/hCD46/NeuGcKO pig cells and on human cells - (A) RBCs, (B) PBMCs. Human IgM (C) and IgG (D) binding to WT, GTKO/hCD46, and GTKO/hCD46/NeuGcKO pig and human RBCs. Human IgM (E) and IgG (F) binding to WT, GTKO/hCD46, and GTKO/hCD46/NeuGcKO pig PBMCs. (To prevent a false positive result from alloantibody binding, human PBMC were not tested for IgM/IgG binding.) (A, B) Gal was detected only on WT RBCs and PBMCs. NeuGc was detected on RBCs and PBMCs from WT and GTKO/hCD46 pigs. RBCs and PBMCs from GTKO/hCD46/NeuGcKO pigs and humans were negative for both Gal and NeuGc. The hCD46 molecule is not expressed on the surface of RBCs, but it is detected on the PBMCs from GTKO/hCD46 and GTKO/hCD46/NeuGcKO pigs and from humans. (C, D) There was a significant difference in human IgM and IgG binding between WT vs. GTKO/hCD46, GTKO/hCD46/NeuGcKO pRBCs, and human RBCs (*p<0.05, **p<0.01). There was also a significant difference in binding between GTKO/hCD46 and GTKO/hCD46/NeuGcKO pRBCs (‡p<0.05). There was no IgM/IgG binding to GTKO/CD46/NeuGcKO pig and human RBCs (a relative MFI<1 indicates no significant binding of IgM or IgG). (E,F) There were significant differences in human IgM and IgG binding between WT and GTKO/hCD46 pPBMCs (*p<0.05) and GTKO/hCD46 and GTKO/hCD46/NeuGcKO pPBMCs (‡p<0.05).
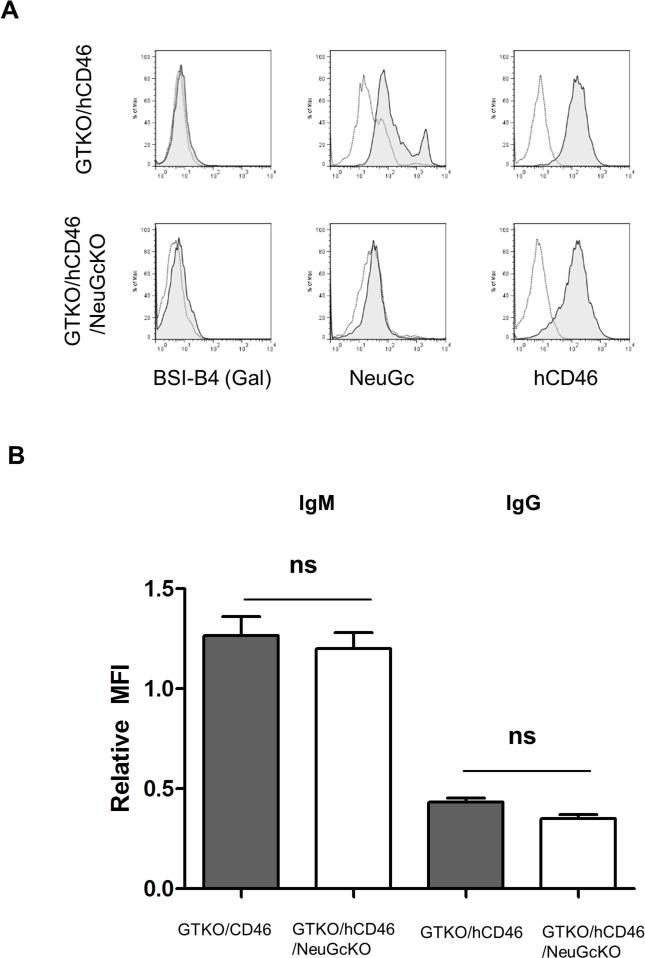
Figure 3.
(A) Expression of Gal, NeuGc, and hCD46 on GTKO/hCD46, and two GTKO/hCD46/NeuGcKO pigs islet cells. GTKO/hCD46 pig islets were negative for Gal, but positive for NeuGc and hCD46. GTKO/hCD46/NeuGcKO pig islets were negative for both Gal and NeuGc expression, and positive for hCD46. (B) Human IgM and IgG binding to GTKO/hCD46, and GTKO/hCD46/NeuGcKO pig islet cells. There was no statistical significance in IgM and IgG binding to islets between the two groups.
Human IgM and IgG antibody binding to RBCs, PBMCs, and cultured pig islet cells by flow cytometry
Binding of human IgM and IgG to GTKO/hCD46 pRBCs was greatly reduced compared to that to WT pRBCs (Figure 2C,D), and there was significantly further reduction of binding to GTKO/CD46/NeuGcKO pRBCs (Figure 2C,D). There was a significant difference in human IgM/IgG binding between GTKO/hCD46 and GTKO/hCD46/NeuGcKO pRBCs (both p<0.05). There was no significant difference in IgM/IgG binding between GTKO/CD46/NeuGcKO pig and human RBCs.
Binding of human IgM and IgG to GTKO/hCD46 pPBMCs was greatly reduced compared to that to WT pPBMCs (Figure 2E,F), and there was further reduction of binding to GTKO/CD46/NeuGcKO pPBMCs (Figure 2E,F). Both IgM and IgG showed significant reduction in human IgM/IgG binding to pPBMCs of GTKO/hCD46/NeuGcKO pigs compared to those of GTKO/hCD46 pigs (both p<0.05).
In contrast to pig RBCs and PBMCs, there was less human IgM antibody binding, and no IgG antibody binding to either GTKO/hCD46 or GTKO/hCD46/NeuGcKO cultured pig islet cells (Figure 3B). There was no significant difference in human IgM antibody binding to the cultured islet cells from GTKO/hCD46 and GTKO/hCD46/NeuGcKO pigs.
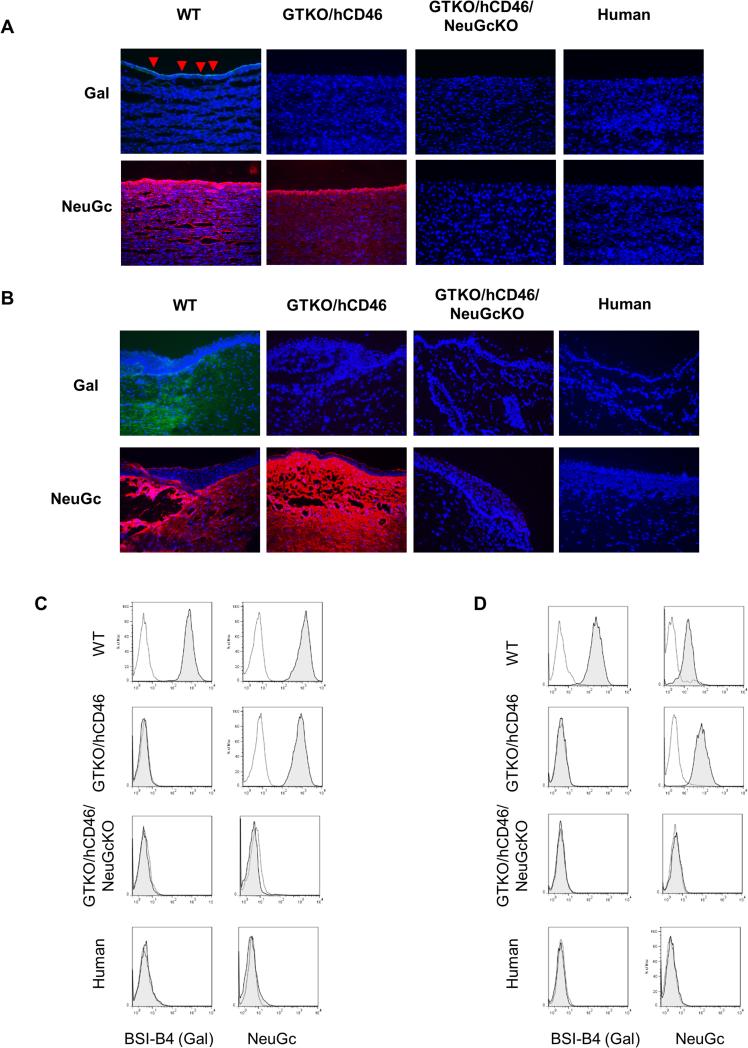
Expression of Gal and NeuGc on aortas and corneas by immunofluorescence and on AECs and CECs by flow cytometry
The results relating to CECs have been detailed elsewhere (38). In summary, the tissue structure and cell morphology of corneas from genetically-engineered pigs, including GTKO/hCD46/NeuGcKO pigs, were not different from those of WT pigs (38). WT pig aortas (Figure 4A) and corneas [Figure 4B, detailed in (38)] expressed Gal and NeuGc as did corresponding cultured pAECs and pCECs (Figure 4C,D). GTKO/hCD46 pig aortas and corneas were negative for Gal expression, but positive for NeuGc, as were GTKO/hCD46 AECs and CECs. GTKO/hCD46/NeuGcKO pig aortas and corneas (Figure 4A,B), and cultured pAECs and pCECs (Figures 4C,D) did not express either Gal or NeuGc, as was the case for human corneas and CECs (38).
Figure 4.
Expression of Gal and NeuGc on pig and human aortas (A) and corneas (B) by immunofluorescence, and on cultured AECs (C) and CECs (D) by flow cytometry. (A, B) Gal was detected on the endothelium of WT pig aortas (red arrows) and epithelium and stroma of WT pig corneas. NeuGc was strongly detected on aortas and corneas from WT and GTKO/hCD46 pigs (Magnification x200; nuclei, blue; Gal, green; NeuGc, red). (C, D) Gal was detected on AECs and CECs of WT pigs, whereas NeuGc was detected on AECs from WT and GTKO/hCD46 pigs. Cells from GTKO/hCD46/NeuGcKO pigs and humans were negative for both Gal and NeuGc antigens.
Human IgM and IgG antibody binding to aortas and corneas by immunofluorescence and to AECs and CECs by flow cytometry
Human IgM and IgG binding to the aortic and corneal tissues have been detailed elsewhere (38). In summary, human IgM and IgG bound primarily to the endothelium of pig aortas and to all layers of the corneas, which appeared to be related to Gal and NeuGc expression (not shown). Compared to binding to both WT pig aortas and corneas, human IgM and IgG binding to GTKO/hCD46 pig tissues was greatly decreased, and it was further decreased to GTKO/hCD46/NeuGcKO pig tissues (38).
Binding of human IgM and IgG to GTKO/CD46 pAECs was greatly reduced compared to that to WT pAECs, and there was significant further reduction of human IgM/IgG binding to GTKO/hCD46/NeuGcKO pAECs (Figure 5A,B). In contrast to pAECs, there was no obvious difference in human IgM and IgG binding to the CECs from GTKO/hCD46 and GTKO/hCD46/NeuGcKO pigs (Figure 5C,D) (38).
Figure 5.
Human IgM and IgG antibody binding to pig and human AECs (A, B) and CECs (C, D) by flow cytometry. (A, B) Human IgM and IgG binding to GTKO/hCD46 pAECs was significantly decreased compared to WT pAECs (*p<0.05), and was further decreased to GTKO/hCD46/NeuGcKO pAECs (*p<0.05). Also, there was a significant difference in IgM and IgG binding to GTKO/hCD46 and GTKO/hCD46/NeuGcKO pAECs (‡p<0.05). There was significantly greater IgM binding to GTKO/hCD46/NeuGcKO pAECs than human AECs (‡p<0.05), but there was no statistical significance in the extent of IgG binding between them. (C, D) Human IgM and IgG binding to WT pCECs was significantly greater than to CECs from the other pigs (*p<0.05), but there was no significant difference in binding between these other pigs.
Mixed cell culture to determine the human proliferative lymphocyte response
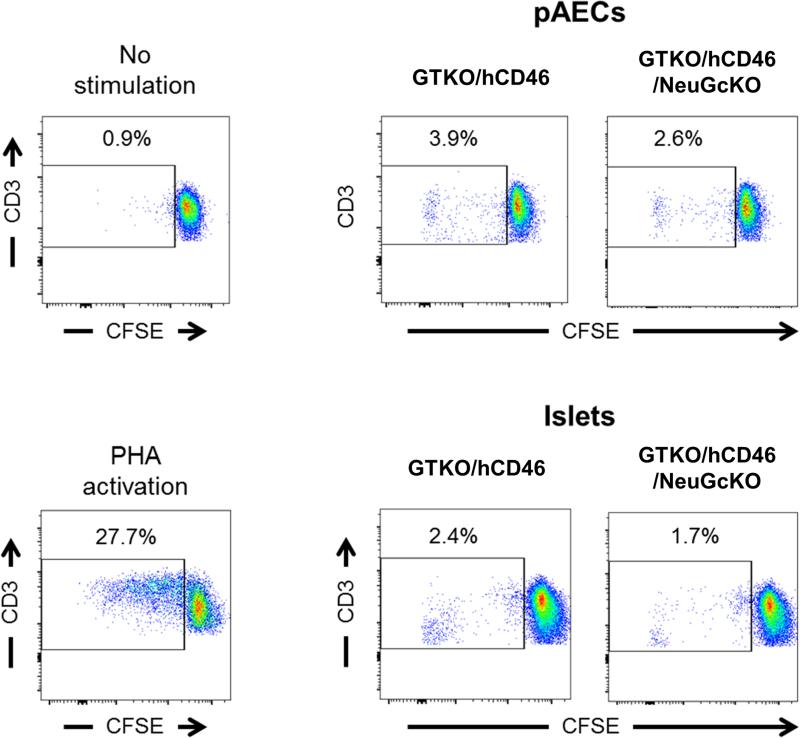
Following 5 days culture, proliferation of human PBMC to GTKO/hCD46 and GTKO/hCD46/NeuGcKO pAECs was comparable and markedly lower compared to positive control, i.e., PHA activation (Figure 6). Similarly, human PBMC proliferation to both GTKO/hCD46 and GTKO/hCD46/NeuGcKO islet cells was comparable and weaker than to the positive control (Figure 6).
Figure 6.
Human PBMC proliferative response to GTKO/hCD46 and GTKO/hCD46/NeuGcKO pAECs and islet cells. CFSE-labeled human PBMC were cocultured with either GTKO/hCD46 or GTKO/hCD46/NeuGcKO pAECs at 1:10 ratio for 5 days. Similarly, human PBMC were cocultured with islet cells at 1:1 ratio. The proliferative response to pAECs and islet cells was weak. The response to GTKO/hCD46/NeuGcKO AECs was slightly weaker than to GTKO/hCD46 AECs, as was the response to the respective isolated islet cells. (Representative data from experiments using PBMCs from two different humans)
Effect of genetic modification of pAECs on human platelet aggregation
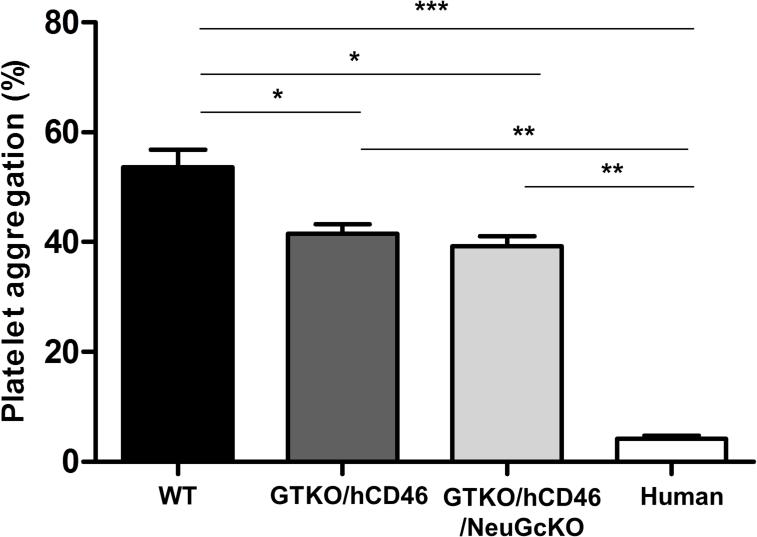
Human whole blood co-incubated with WT pAECs resulted in a prompt aggregation of platelets (54%) (Figure 7). In contrast, hAECs induced minimal platelet aggregation (4%). The difference in platelet aggregation between WT pAECs and hAECs was statistically significant (p<0.001). GTKO/hCD46 (42%) and GTKO/hCD46/NeuGcKO (39%) pAECs induced significantly less aggregation than WT pAECs (both p<0.05). However, there was no significant difference in platelet aggregation between GTKO/hCD46 and GTKO/hCD46/NeuGcKO pAECs, and platelet aggregation around both types of cells remained statistically greater than around hAECs (both p<0.01).
Figure 7.
The effect of genetic modification on pAEC-induced human platelet aggregation. When human platelet aggregation associated with WT pAEC (54%) was compared with that to hAEC (4%), a significant difference was observed (***p<0.001). GTKO/hCD46 (42%) and GTKO/hCD46/NeuGcKO (39%) pAEC significantly reduced the aggregation compared to WT pAEC (*p<0.05), but aggregation remained significantly greater than to hAEC (**p<0.01). There was no significant difference in platelet aggregation between GTKO/hCD46 and GTKO/hCD46/NeuGcKO.
Effect of NeuGcKO on the in vitro IBMIR assay
Regardless of their genetic background, all porcine islets triggered coagulation of human blood within 6min. When blood was exposed to GTKO/hCD46 islets, clotting occurred between 5min 05sec and 5min 55sec (mean 315±16 sec). When exposed to GTKO/hCD46/NeuGcKO pig islets, clotting occurred between 3min 19sec and 4min 48sec (mean 233±40 sec) (p>0.05). A non-physiologic increase in C-peptide is typically an indication of islet cell leakage/damage. In 5 of 6 combinations of human blood and GTKO/hCD46 islets (after 30 or 60 min incubation) C-peptide increased over control (autologous combination) by 5,177±2,639pmol/L (mean±STD). In contrast, C-peptide increased in only 3 of the 12 combinations when GTKO/hCD46/NeuGcKO islets were employed (mean±STD increase 6,263±6,230 pmol/L) (p>0.05). During the first 3h following exposure of islets to human plasma, the number of dead cells in GTKO/hCD46 islets increased 23% (23±13%, n=6) over control (autologous combination), whereas for GTKO/hCD46/NeuGcKO islets the increase was significantly lower (15±11%, n=12, p<0.05).
DISCUSSION
Three carbohydrate antigens have been identified on WT pig vascular endothelial cells to which humans develop natural (or preformed) antibodies – (i) Gal (1), (ii) NeuGc (3), and (iii) β4GalNT2 (through the activity of β1,4 N-acetylgalactosaminyl transferase) (42). Searches for other pig carbohydrate targets for human anti-pig antibodies have been otherwise unsuccessful (43). Anti-Gal antibodies develop during the first few months of infancy (44-46) probably as a response to colonization of the gastrointestinal tract by microorganisms and viruses that express Gal (47), and it is likely that antibodies to the other two antigens develop similarly. Several years ago Tangvoranuntakul et al., reported that consumption of red meat (that contains NeuGc) may result in intestinal absorption of NeuGc and could therefore increase production of anti-NeuGc antibodies (48, 49). Whether absorption of NeuGc from meat will sensitize subjects to a pig organ or cell transplant needs more investigation (49).
To extend pig graft survival, knockout of the gene responsible for producing the enzyme that attaches the oligosaccharide to the underlying structures was suggested (50) and has subsequently been carried out in all three cases (13, 14, 19, 26).
The results of our initial studies reported here add to the observations of the previous studies (24-26), and demonstrate that the lack of expression of NeuGc on some pig tissues and cells (RBCs, PBMCs, AECs) reduced the extent of human antibody binding, but not significantly to other cells (CECs, isolated islet cells) to which antibody binding was already very low. The strength of the human T cell proliferative response may also be weaker to cells that do not express NeuGc, but again the response to GTKO/hCD46 cells was already very weak. It appears that the reduction in human IgM and IgG binding is largely dependent on the tissue or cell type, possibly due to different carbohydrate expression levels.
For example, Lutz et al. reported that the median MFI of human IgM and IgG binding to GTKO/NeuGcKO pPBMCs decreased by 65% and 70% respectively, compared to GTKO pPBMCs (19). In the present study, we observed similar reductions in binding to PBMCs, pAECs, and pRBCs. In contrast, we could detect no statistical difference of antibody binding to GTKO/hCD46 and GTKO/hCD46/NeuGcKO pCECs or pig islet cells. The reason why can be explained by the relatively lower NeuGc expression level of pCECs (38) or pig islets (rMFI of NeuGc expression on PBMCs = 76.8, RBCs = 93.6, and AECs =111.7 vs. rMFI of NeuGc expression on CECs = 5.1 and islets = 6.2; representative data from one GTKO/CD46 pig).
We also demonstrate that the absence of NeuGc expression on GTKO/hCD46 pAECs does not reduce human platelet aggregation, and nor does it reduce the rapidity or strength of the IBMIR to pig islets. We would suggest that each organ/tissue expresses different levels/structures of carbohydrate antigens (51), and so needs to be investigated separately. Although there was a significant reduction in platelet aggregation using GTKO/hCD46±NeuGcKO pAECs compared to WT pAECs, a significant difference remained when compared to hAECs. As microvascular thrombosis is closely related to xenograft rejection, additional genetic modification (e.g., expression of human thrombomodulin and/or endothelial protein C receptor) and/or the administration of a thrombin inhibitory agent (e.g., hirudin) will almost certainly be necessary.
In respect to IBMIR, our previous studies have indicated that islets from GTKO pigs transgenic for one or more human complement-regulatory proteins have improved islet performance in vivo (36), but in vitro exposure of these islets to human blood demonstrated no evident protection against early islet loss (30, 31). Our results using GTKO/hCD46/NeuGcKO islets are inconclusive.
There are some limitations to our studies. Although human IgM and IgG antibody binding should not be affected by the expression of hCD46 on the pig cells, the human T cell proliferative response is reduced by expression of hCD46 (52). Expression of hCD46 also affects human platelet aggregation (28), and this may be the reason why we could not detect any significant difference in aggregation between GTKO/hCD46 and GTKO/hCD46/NeuGcKO pAECs. Therefore, it may have been preferable to carry out the studies on GTKO and GTKO/NeuGcKO cells (in the absence of hCD46 expression), but these latter cells were not available to us.
Furthermore, the T cell proliferative response to allogeneic (human) cells or to WT pig cells was not measured. However, there are data in the literature that throw light on this point. The human T cell response to a WT pig xenograft is generally accepted as being stronger than to an allograft (53). Recently, Ezzelarab and his colleagues have demonstrated that the absence of Gal expression and/or the expression of hCD46 significantly reduces the proliferative T cell response when compared to that of WT pig cells (40, 52). The present study suggests that the T cell proliferative response to cells from GTKO/hCD46/NeuGcKO pigs is weak, which encourages us that clinical trials of pig organ, and particularly islet, xenotransplantation will be successful without the need for excessive or intensive immunosuppressive therapy. The low level of human antibody binding and weak T cell proliferative response to GKO/hCD46/NeuGcKO cells have considerable clinical relevance.
In contrast to their results using human serum, Estrada et al. demonstrated more antibody binding to PBMCs from GTKO/NeuGcKO pigs when exposed to baboon or rhesus monkey serum (when compared to binding to cells from GTKO pigs) (26). Similarly, there was greater hemagglutination and hemolysis of erythrocytes from GTKO/NeuGcKO pigs when exposed to baboon serum, but hemagglutination and hemolysis of GTKO/NeuGcKO erythrocytes were dramatically decreased when exposed to human serum (24). As baboons and rhesus monkeys do not produce anti-NeuGc antibodies (as they express NeuGc on their tissues), these interesting observations would suggest that the deletion of NeuGc from the pig cells resulted in an increased expression of other (or newly-exposed) antigens on the pig cells against which these nonhuman primate species have antibodies. Old World nonhuman primate species are therefore not suitable for preclinical transplantation studies designed to investigate lack of expression on NeuGc in pigs.
Nevertheless, the previous studies by Tector and his colleagues and from the present report strongly suggest that when pig solid organs are transplanted clinically, the absence of NeuGc expression should reduce the human antibody response. In this respect, Burlak et al. reported that human antibody binding to GTKO/NeuGcKO pig PBMCs was less than to chimpanzee PBMCs in four of five human serum samples tested, regardless their blood type (25). This encouraging observation suggests that the antigenicity of GTKO/NeuGcKO pig tissues may now be no stronger than that of concordant nonhuman primate species. Nevertheless, there are many more biologic differences between pig and human than between nonhuman primate and human that may adversely influence the outcome.
We would suggest that the next step in investigation of the role that anti-NeuGc antibodies may play in pig xenograft rejection is the transplantation of tissues or organs from GTKO/hCD46 pigs (as controls) and GKO/hCD46/NeuGcKO pigs into New World monkeys (23). The artery patch model would appear ideal to monitor and compare the elicited antibody and T cell proliferative responses (54, 55).
ACKNOWLEDGEMENTS
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute at the University of Pittsburgh has been supported in part by NIH grants U01 AI068642, and U19 AI090959, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
ABBREVIATIONS
- AEC
aortic endothelial cell
- CEC
corneal endothelial cell
- CMAH
cytidine monophospho-N-acetylneuraminic acid hydroxylase
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- IBMIR
instant blood-mediated inflammatory reaction
- NeuGc
N-glycolylneuraminic acid
- NeuGcKO
cytidine monophospho-N-acetylneuraminic acid hydroxylase gene-knockout
- SCNT
somatic cell nuclear transfer
- WT
wild-type
- ZFNs
zinc finger nucleases
Footnotes
CONFLICTS OF INTEREST
JR and DA are employees of Revivicor, Inc. The other authors have no conflict of interest.
References
- 1.GOOD AH, COOPER DK, MALCOLM AJ, IPPOLITO RM, KOREN E, NEETHLING FA, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 2.COOPER DK, GOOD AH, KOREN E, ORIOL R, MALCOLM AJ, IPPOLITO RM, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 3.BOUHOURS D, POURCEL C, BOUHOURS J-F. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Galα1–3Gal), blood group H determinant andN-glycolylneuraminic acid. Glycoconj J. 1996;13:947–953. doi: 10.1007/BF01053190. [DOI] [PubMed] [Google Scholar]
- 4.ZHU A, HURST R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 5.PADLER-KARAVANI V, VARKI A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GALILI U, SHOHET SB, KOBRIN E, STULTS CL, MACHER BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 7.GALILI U. Evolution of alpha 1,3galactosyltransferase and of the alpha-Gal epitope. Subcell Biochem. 1999;32:1–23. doi: 10.1007/978-1-4615-4771-6_1. [DOI] [PubMed] [Google Scholar]
- 8.BASNET NB, IDE K, TAHARA H, TANAKA Y, OHDAN H. Deficiency of N-glycolylneuraminic acid and Galalpha1-3Galbeta1-4GlcNAc epitopes in xenogeneic cells attenuates cytotoxicity of human natural antibodies. Xenotransplantation. 2010;17:440–448. doi: 10.1111/j.1399-3089.2010.00610.x. [DOI] [PubMed] [Google Scholar]
- 9.TAHARA H, IDE K, BASNET NB, TANAKA Y, MATSUDA H, TAKEMATSU H, et al. Immunological property of antibodies against N-glycolylneuraminic acid epitopes in cytidine monophospho-N-acetylneuraminic acid hydroxylase-deficient mice. J Immunol. 2010;184:3269–3275. doi: 10.4049/jimmunol.0902857. [DOI] [PubMed] [Google Scholar]
- 10.LEXER G, COOPER DK, ROSE AG, WICOMB WN, REES J, KERAAN M, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant. 1986;5:411–418. [PubMed] [Google Scholar]
- 11.COOPER DK, HUMAN PA, LEXER G, ROSE AG, REES J, KERAAN M, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988;7:238–246. [PubMed] [Google Scholar]
- 12.YE Y, NEETHLING FA, NIEKRASZ M, KOREN E, RICHARDS SV, MARTIN M, et al. Evidence that intravenously administered alpha-galactosyl carbohydrates reduce baboon serum cytotoxicity to pig kidney cells (PK15) and transplanted pig hearts. Transplantation. 1994;58:330–337. [PubMed] [Google Scholar]
- 13.PHELPS CJ, KOIKE C, VAUGHT TD, BOONE J, WELLS KD, CHEN SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.KOLBER-SIMONDS D, LAI L, WATT SR, DENARO M, ARN S, AUGENSTEIN ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KUWAKI K, TSENG YL, DOR FJ, SHIMIZU A, HOUSER SL, SANDERSON TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 16.YAMADA K, YAZAWA K, SHIMIZU A, IWANAGA T, HISASHI Y, NUHN M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 17.AZIMZADEH AM, KELISHADI SS, EZZELARAB MB, SINGH AK, STODDARD T, IWASE H, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation. 2015;22:310–316. doi: 10.1111/xen.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MCGREGOR CG, RICCI D, MIYAGI N, STALBOERGER PG, DU Z, OEHLER EA, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93:686–692. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LUTZ AJ, LI P, ESTRADA JL, SIDNER RA, CHIHARA RK, DOWNEY SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 20.SCOBIE L, PADLER-KARAVANI V, LE BAS-BERNARDET S, CROSSAN C, BLAHA J, MATOUSKOVA M, et al. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J Immunol. 2013;191:2907–2915. doi: 10.4049/jimmunol.1301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.STONE KR, ABDEL-MOTAL UM, WALGENBACH AW, TUREK TJ, GALILI U. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83:211–219. doi: 10.1097/01.tp.0000250598.29377.13. [DOI] [PubMed] [Google Scholar]
- 22.MAGNUSSON S, MANSSON JE, STROKAN V, JUSSILA R, KOBAYASHI T, RYDBERG L, et al. Release of pig leukocytes during pig kidney perfusion and characterization of pig lymphocyte carbohydrate xenoantigens. Xenotransplantation. 2003;10:432–445. doi: 10.1034/j.1399-3089.2003.02052.x. [DOI] [PubMed] [Google Scholar]
- 23.SPRINGER SA, DIAZ SL, GAGNEUX P. Parallel evolution of a self-signal: humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics. 2014;66:671–674. doi: 10.1007/s00251-014-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WANG ZY, BURLAK C, ESTRADA JL, LI P, TECTOR MF, TECTOR AJ. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation. 2014;21:376–384. doi: 10.1111/xen.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.BURLAK C, PARIS LL, LUTZ AJ, SIDNER RA, ESTRADA J, LI P, et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant. 2014;14:1895–1900. doi: 10.1111/ajt.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ESTRADA JL, MARTENS G, LI P, ADAMS A, NEWELL KA, FORD ML, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22:194–202. doi: 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.COWAN PJ, ROUSSEL JC, D'APICE AJ. The vascular and coagulation issues in xenotransplantation. Curr Opin Organ Transplant. 2009;14:161–167. doi: 10.1097/mot.0b013e3283279591. [DOI] [PubMed] [Google Scholar]
- 28.IWASE H, EKSER B, HARA H, PHELPS C, AYARES D, COOPER DK, et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation. 2014;21:72–83. doi: 10.1111/xen.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.BENNET W, GROTH CG, LARSSON R, NILSSON B, KORSGREN O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105:125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 30.VAN DER WINDT DJ, MARIGLIANO M, HE J, VOTYAKOVA TV, ECHEVERRI GJ, EKSER B, et al. Early islet damage after direct exposure of pig islets to blood: has humoral immunity been underestimated? Cell Transplant. 2012;21:1791–1802. doi: 10.3727/096368912X653011. [DOI] [PubMed] [Google Scholar]
- 31.NAGARAJU S, BERTERA S, TANAKA T, HARA H, RAYAT GR, WIJKSTROM M, et al. In vitro exposure of pig neonatal isletlike cell clusters to human blood. Xenotransplantation. 2015;22:317–324. doi: 10.1111/xen.12178. [DOI] [PubMed] [Google Scholar]
- 32.LOVELAND BE, MILLAND J, KYRIAKOU P, THORLEY BR, CHRISTIANSEN D, LANTERI MB, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–183. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 33.HARA H, LONG C, LIN YJ, TAI HC, EZZELARAB M, AYARES D, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 34.LONG C, HARA H, PAWLIKOWSKI Z, KOIKE N, D'ARVILLE T, YEH P, et al. Genetically engineered pig red blood cells for clinical transfusion: initial in vitro studies. Transfusion. 2009;49:2418–2429. doi: 10.1111/j.1537-2995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 35.BOTTINO R, BALAMURUGAN AN, SMETANKA C, BERTERA S, HE J, ROOD PP, et al. Isolation outcome and functional characteristics of young and adult pig pancreatic islets for transplantation studies. Xenotransplantation. 2007;14:74–82. doi: 10.1111/j.1399-3089.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 36.BOTTINO R, WIJKSTROM M, VAN DER WINDT DJ, HARA H, EZZELARAB M, MURASE N, et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14:2275–2287. doi: 10.1111/ajt.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FUJITA M, MEHRA R, LEE SE, ROH DS, LONG C, FUNDERBURGH JL, et al. Comparison of proliferative capacity of genetically-engineered pig and human corneal endothelial cells. Ophthalmic Res. 2013;49:127–138. doi: 10.1159/000342978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LEE W, MIYAGAWA Y, LONG C, EKSER B, WALTERS E, RAMSOONDAR J, et al. Expression of NeuGc on pig corneas and its potential significance in pig corneal xenotransplantation. Cornea. 2016;35:105–113. doi: 10.1097/ICO.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.HARA H, EZZELARAB M, ROOD PP, LIN YJ, BUSCH J, IBRAHIM Z, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006;13:357–365. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 40.WILHITE T, EZZELARAB C, HARA H, LONG C, AYARES D, COOPER DK, et al. The effect of Gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation. 2012;19:56–63. doi: 10.1111/j.1399-3089.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.COHEN D, MIYAGAWA Y, MEHRA R, LEE W, ISSE K, LONG C, et al. Distribution of non-gal antigens in pig cornea: relevance to corneal xenotransplantation. Cornea. 2014;33:390–397. doi: 10.1097/ICO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 42.BYRNE GW, DU Z, STALBOERGER P, KOGELBERG H, MCGREGOR CG. Cloning and expression of porcine beta1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21:543–554. doi: 10.1111/xen.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.YEH P, EZZELARAB M, BOVIN N, HARA H, LONG C, TOMIYAMA K, et al. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 2010;17:197–206. doi: 10.1111/j.1399-3089.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- 44.NEETHLING F, COOPER DK, XU H, MICHLER RE. Newborn baboon serum anti-alpha galactosyl antibody levels and cytotoxicity to cultured pig kidney (PK15) cells. Transplantation. 1995;60:520–521. doi: 10.1097/00007890-199509000-00023. [DOI] [PubMed] [Google Scholar]
- 45.DONS EM, MONTOYA C, LONG CE, HARA H, ECHEVERRI GJ, EKSER B, et al. T-cell-based immunosuppressive therapy inhibits the development of natural antibodies in infant baboons. Transplantation. 2012;93:769–776. doi: 10.1097/TP.0b013e3182481168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ROOD PP, TAI HC, HARA H, LONG C, EZZELARAB M, LIN YJ, et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transpl Int. 2007;20:1050–1058. doi: 10.1111/j.1432-2277.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 47.GALILI U, MANDRELL RE, HAMADEH RM, SHOHET SB, GRIFFISS JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56:1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.TANGVORANUNTAKUL P, GAGNEUX P, DIAZ S, BARDOR M, VARKI N, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.SAMRAJ AN, PEARCE OM, LAUBLI H, CRITTENDEN AN, BERGFELD AK, BANDA K, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA. 2015;112:542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.COOPER DK, KOREN E, ORIOL R. Genetically engineered pigs. Lancet. 1993;342:682–683. doi: 10.1016/0140-6736(93)91791-j. [DOI] [PubMed] [Google Scholar]
- 51.MIWA Y, KOBAYASHI T, NAGASAKA T, LIU D, YU M, YOKOYAMA I, et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation. 2004;11:247–253. doi: 10.1111/j.1399-3089.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 52.EZZELARAB MB, AYARES D, COOPER DK. Transgenic expression of human CD46: does it reduce the primate T-cell response to pig endothelial cells? Xenotransplantation. 2015;22:487–489. doi: 10.1111/xen.12209. [DOI] [PubMed] [Google Scholar]
- 53.BUHLER LH, COOPER DK. How strong is the T cell response in the pig-to-primate model? Xenotransplantation. 2005;12:85–87. doi: 10.1111/j.1399-3089.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 54.EZZELARAB MB, EKSER B, ECHEVERRI G, HARA H, EZZELARAB C, LONG C, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.IWASE H, EKSER B, SATYANANDA V, ZHOU H, HARA H, BAJONA P, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol. 2015;32:99–108. doi: 10.1016/j.trim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]