Abstract
Early stages of glaucoma and optic neuropathies are thought to show inner retina remodeling and functional changes of retinal ganglion cells (RGCs) before they die. To assess RGC functional plasticity, we investigated the contrast-gain control properties of the pattern electroretinogram (PERG), a sensitive measure of RGC function, as an index of spatio-temporal integration occurring in the inner retina circuitry subserving PERG generators. We studied the integrative properties of the PERG in mice exposed to different conditions of neurotrophic support. We also investigated the effect of genotypic differences among mouse strains with different susceptibility to glaucoma (C57BL/6J, DBA/2J, DBA/2.Gpnmb+). Results show that the integrative properties of the PERG recorded in the standard C57BL/6J inbred mouse strain are impaired after deficit of neurotrophic support and partially restored after exogenous neurotrophic administration. Changes in PERG amplitude, latency, and contrast-dependent responses differ between mouse strains with different susceptibility to glaucoma. Results represent a proof of concept that the PERG could be used as a tool for in-vivo monitoring of RGC functional plasticity before RGC death, the effect of neuroactive treatments, as well as for high-throughput tool for phenotypic screening of different mouse genotypes.
Keywords: retinal ganglion cell function, pattern electroretinogram, contrast gain control, retinal plasticity, glaucoma susceptibility, mouse
Introduction
Early stages of optic neuropathies, including glaucoma, are characterized by loss of axonal and synaptic function (Adalbert and Coleman, 2013; Almasieh et al., 2012), as well as by compensatory changes in surviving neurons (Samuel et al., 2011). Dendritic shrinkage/remodeling preceding cell death and loss of retinal ganglion cell (RGC) function is well documented in ex-vivo retinal preparations (Buckingham et al., 2008; Enriquez-Algeciras et al., 2013; Liu et al., 2011; Morquette and Di Polo, 2008; Williams et al., 2013; Williams et al., 2012) (Della Santina et al., 2013) (Pang et al., 2015). An in vivo, non-invasive method to assess RGC functional plasticity would greatly improve our ability to detect/monitor early stages of the disease and the effects of neuroprotective treatments. To this aim, we have investigated the integrative properties of the pattern electroretinogram (PERG), a sensitive measure of RGC function (Porciatti, 2015). The PERG is reversibly altered in DBA/2J mouse glaucoma before histological loss of RGC bodies and axons (Howell et al., 2007b; Nagaraju et al., 2007; Porciatti and Nagaraju, 2010; Saleh et al., 2007). The PERG has been shown to be altered in the absence of cell death when target-derived neurotrophic support to RGCs is impaired (Chou et al., 2013; Yang et al., 2013). The PERG may also display differences between common mouse strains (Porciatti et al., 2010).
Here we specifically investigated in the mouse how PERG amplitude and latency change as a function of stimulus strength (contrast). The PERG signal integrates the activity of a large number of RGCs that includes cellular function as well as connectivity of the inner retina circuitry impinging on RGCs (Porciatti, 2015). Signal integration requires time (manifested as response latency after stimulus onset), which typically decreases as the strength of the stimulus increases. Also, the response gain (strength of response relative to the strength of the stimulus) may change for different levels of stimulus strengths to adapt the limited dynamic range of neurons to the spatio-temporal statistics of the stimulus. Changes in both response latency and gain with contrast have been explained within neural gain control modeling frameworks (Beaudoin et al., 2008; Carandini and Heeger, 2012; Demb, 2008; Demb et al., 1999; Shapley and Enroth-Cugell, 1984). We show that the integrative properties of the PERG recorded in the standard C57BL/6J inbred mouse strain are modifiable in response to alterations of neurotrophic support. PERG integrative properties may also differ between mouse strains with different susceptibility to glaucoma.
Methods
Animals and husbandry
All procedures were performed in compliance with the Association for Research in Vision and Ophthalmology (ARVO) statement for use of animals in ophthalmic and vision research. The experimental protocol was approved by the Animal Care and Use Committee of the University of Miami. A total of 88 mice were used to investigate the effect of neurotrophic support and genotype on PERG at different stimulus contrast. All mice were maintained in a cyclic light environment (12 h light: 50 lux – 12 h: dark) and fed with Grain Based Diet (Lab Diet: 500, Opti-diet, PMI Nutrition International, Inc., Brentwood, MO). All procedures and testing were performed under anesthesia by means of intraperitoneal injections (0.5–0.7 ml/kg) of a mixture of ketamine (42.8 mg/ml) and xylazine (8.6 mg/ml). For the experiments on the role of neurotrophic support, PERGs were recorded before and one week after intravitreal injection of BDNF (2 μL, 5 μg/μL) or anti-BDNF antibody (2 μL, 1 μg/μL) using a Hamilton syringe connected to a fine needle (33 gauge/0.375 inch/point style 4). For the experiments on chronic deficiency of target-derived factors, the bone overlying the right superior colliculus (SC, 0.5 mm lateral to the central suture, 2.9 mm posterior to the bregma) was drilled under sterile conditions to aspirate a narrow column of cortical tissue and expose the SC. Then the superficial layers of the SC were also removed by aspiration (Yang et al., 2013). PERGs were recorded from the left eye –contralateral to the lesioned SC– one month after surgery.
Pattern Electroretinogram – PERG
RGC function was assessed by Pattern Electroretinogram (PERG), an electrical signal that specifically depends on the presence of functional RGCs (Chou et al., 2013; Miura et al., 2009; Porciatti, 2015; Porciatti et al., 1996; Xia et al., 2014)and is commonly used in human and experimental models of glaucoma and optic neuropathies (Enriquez-Algeciras et al., 2013; Howell et al., 2007a; Porciatti, 2015; Yu et al., 2015; Yu et al., 2012). Anesthetized mice were gently restrained in a holder allowing unobstructed vision as previously described (Porciatti et al., 2007) and kept at a constant body temperature of 37.0 °C using a rectal probe and fee dback-controlled heating pad (TCAT-2LV; Physitemp Instruments, Inc. Clifton, NJ). Pupils were natural and had a diameter smaller than 1 mm at the mean luminance of the visual stimulus (Nagaraju et al., 2007); eyes were not refracted for the viewing distance since the mouse eye with natural pupil has a large depth of focus (Artal et al., 1998; Remtulla and Hallett, 1985; Schmucker and Schaeffel, 2004). A small drop of balanced saline was topically applied every 15 min to prevent corneal drying. A PERG electrode (0.25 mm diameter silver wire configured to a semi-circular loop of 2 mm radius) was placed on the extrapupillary corneal surface by means of a micromanipulator. Reference and ground electrodes were subcutaneous stainless steel needles inserted in the scalp behind the interaural line and the base of tail, respectively. At the end of the recording session the pupil was dilated to check for possible lens opacification, which was not detectable in all eyes tested as assessed with a 2.7x surgical loupe.
Visual stimuli consisted of contrast-reversing (2 reversal/s) horizontal bars (0.05 cycles/deg, mean luminance 50 cd/m2 of two different Michelson contrasts – high, 1.0 and low, 0.2 – generated by a programmable graphic card (VSG- Cambridge Research Systems, Rochester, UK) on a CRT display (Sony Multiscan 500) whose center was aligned with the projection of the pupil. At the viewing distance of 15 cm, the stimulus field covered an area of 69.4 × 63.4 deg (Porciatti et al., 2010). Occluded visual stimuli of 1.0 contrast were also used to record noise responses.
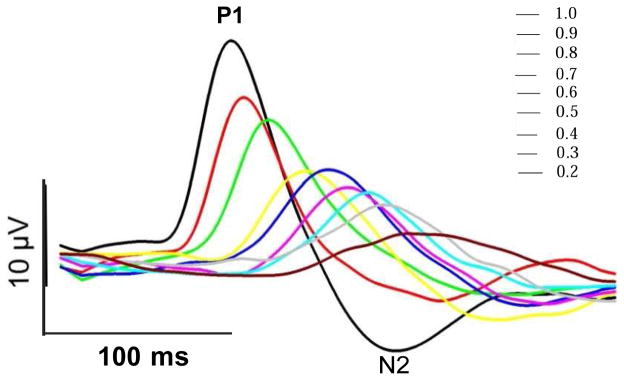
Retinal signals were averaged in sync with each contrast-reversal over 1800 epochs to retrieve PERG waveforms. In all mouse groups, PERG waveforms (examples in Figure 1 and 2) consisted of a positive wave (defined as P1) followed by a slower negative wave with a broad trough (defined as N2) (Chou et al., 2014; Porciatti, 2015; Porciatti et al., 2007; Xia et al., 2014). The positive peak and the negative trough of PERG waveforms were automatically identified using a macro written in Sigmaplot 11.2 language (Systat Software, San Jose, CA USA) to measure the peak-to-trough (P1-N2) amplitude (defined as PERG amplitude) and the time-to-peak of the P1 wave (defined as PERG latency). Typically, the PERG has its largest amplitude at maximum contrast and progressively decreases with decreasing contrast while the PERG latency progressively increases (Porciatti, 2015; Porciatti et al., 1996). The relationship relating PERG amplitude with contrast may be non-linear (Porciatti et al., 2010). Departure from linearity implies that mechanisms of contrast gain control are at play in the PERG generators (Beaudoin et al., 2008; Carandini and Heeger, 2012; Demb, 2008; Demb et al., 1999; Shapley and Enroth-Cugell, 1984), which this study seeks to quantify. Quantitative analysis of non-linearities in contrast response functions is a laborious procedure that requires recording a series of PERG waveforms at close-spaced contrasts and fitting corresponding amplitude/latency data with suitable mathematical functions (e.g., (Albrecht and Hamilton, 1982)). A preset mathematical function may sub-optimally fit contrast response functions in different mouse genotypes/interventions. In addition, it requires assumptions on the relationship between stimulus and response. For the purposes of the present study, we introduced a simplification by assessing PERG amplitude and latency at high (1.0) and low (0.2) contrast only. Our rationale was, if the relationship between PERG amplitude and contrast were linear, the amplitude at low contrast would be lower than that at high contrast by a 0.2/1.0 factor. The normalized contrast amplitude ratio (NAR) would be [amplitude @ 0.2 contrast ÷ amplitude @ 1.0 contrast) ÷ 0.2 = 1]. An NAR >1 would mean that the relationship between PERG amplitude and contrast is non-linear of compressive type (the response at low contrast is relatively higher than that at high contrast). An NAR <1 would mean that the relationship between PERG amplitude and contrast is non-linear of expansive type (the response at high contrast is relatively higher that that at low contrast). Thus, we calculated the NAR to have a quantitative index of relative amplitude gain at low contrast in different mouse groups. We also calculated the latency difference between PERG at low and high contrast (latency delta = latency @ 0.2 minus latency @ 1.0) to have an index of latency change at low contrast. In absence of contrast gain control, the expected NAR and latency delta are respectively 1 and 0.
Figure 1.
Examples of PERG waveforms at different contrasts. PERGs were recorded in one representative C57BL/6J mouse (B6) in response to black-white reversing (2/s) gratings of fixed spatial frequency (0.05 cycles/deg) and different contrasts. At all contrasts, the PERG waveform consists of a major positive wave (P1) followed by a negative wave (N2). PERG amplitude was measured peak-to- trough (P1-N2) and PERG latency was the time-to-peak of the P1 wave. Note that by decreasing contrast, the PERG amplitude progressively decreases whereas the PERG latency progressively increases.
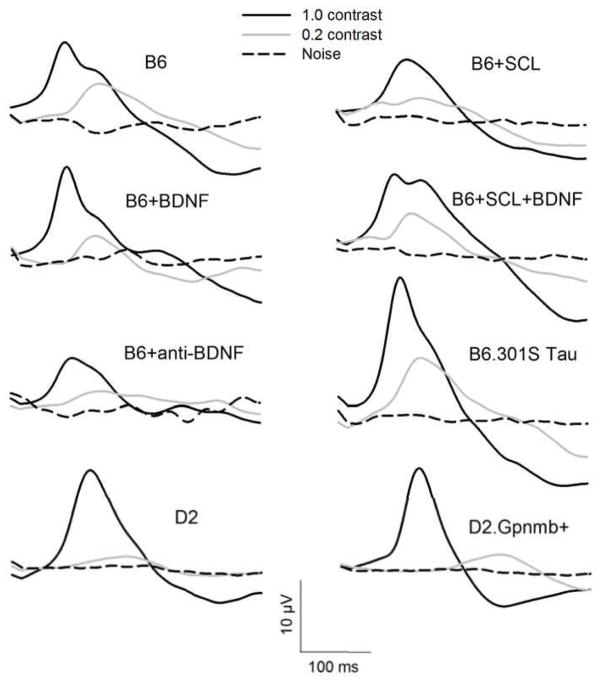
Figure 2.
Population PERG waveforms for different mouse groups. Waveforms represent the grand-average of PERG waveforms recorded in individual mice. Black continuous line, PERG in response to 1.0 contrast pattern-reversal stimuli; gray continuous line, PERG in response to 0.2 contrast pattern reversal stimuli; black-dashed line, Noise signal in response to an occluded pattern stimulus.
Statistics
Statistical differences between mouse groups have been analyzed by means of multivariate analysis of variance (MANOVA) including all role variables (PERG amplitude and latency and high and low contrast, NAR, latency delta). Association between PERG amplitude and latency variables have been analyzed with Pearson correlation coefficient. All statistical tests were performed with JMP pro statistical package (SAS Institute, NC).
Results
Figure 1 shows representative examples of PERG waveforms recorded in one B6 mouse in response to black-white reversing (2/s) gratings of fixed spatial frequency (0.05 cycles/deg) and different contrasts, ranging between 1.0 and 0.2 in steps of 0.1 contrast. Note that by decreasing contrast, the PERG amplitude progressively decreased whereas the PERG latency progressively increased. The latency at 0.2 contrast was considerably longer than the latency at 1.0 contrast, suggesting that the temporal integration properties of PERG generators were highly dependent on stimulus contrast.
Figure 2 shows population PERG waveforms (grand-average of waveforms recorded in individual mice) at high and low contrast for all mouse groups. Note in all groups that at 0.2 contrast, compared to 1.0 contrast, the PERG signal had a smaller amplitude and longer latency. Also note that the PERG signal at 0.2 contrast was larger than the noise.
In order to investigate whether PERG integrative properties depended on either neurotrophic support or genotype, we tested the following hypotheses in young adult (~4 month old) mice:
Effect of neurotrophic support in mice of the same C57BL/6J (B6) genetic background
If RGC functional plasticity depends on neurotrophic support, then intravitreal injection of either BDNF or antibody against BDNF should modify integrative properties of the PERG. The integrative properties of the PERG should also be altered after chronic deficit of target-derived factors from the superior colliculus (Superior Colliculus Lesion, SCL) and perhaps restored after intravitreal BDNF injection. Finally, a transgenic mouse for human mutant P301S Tau with constitutionally reduced axonal transport in the optic nerve (Bull et al., 2012) and possible chronic deficit of target-derived factors, may display altered integrative properties of the PERG.
Effect of genotype
Because the PERG shows functional changes in pre-glaucomatous stages, PERG integrative properties may differ between mouse strains known to display different vulnerability to IOP elevation and glaucoma development. DBA/2J mice (D2) develop a pigment-dispersing iris disease caused by mutations in two genes, Gpnmb and Tyrp1, which leads to age-related IOP elevation and RGC loss (Howell et al., 2007a; Libby et al., 2005). D2 mice are the most used genetic model of glaucoma (Fernandes et al., 2015). However, a congenic strain of B6 mice that has the same Tyrp1 and Gpnmb mutations as DBA/2J mice and develops the same degree of iris disease, is resistant to IOP elevation and does not develop glaucoma (Anderson et al., 2006; Howell et al., 2007a). Coisogenic D2.Gpnmb+ mice have a Gpnmb gene with normal function and do not develop elevated IOP or glaucoma with age (Howell et al., 2007b).
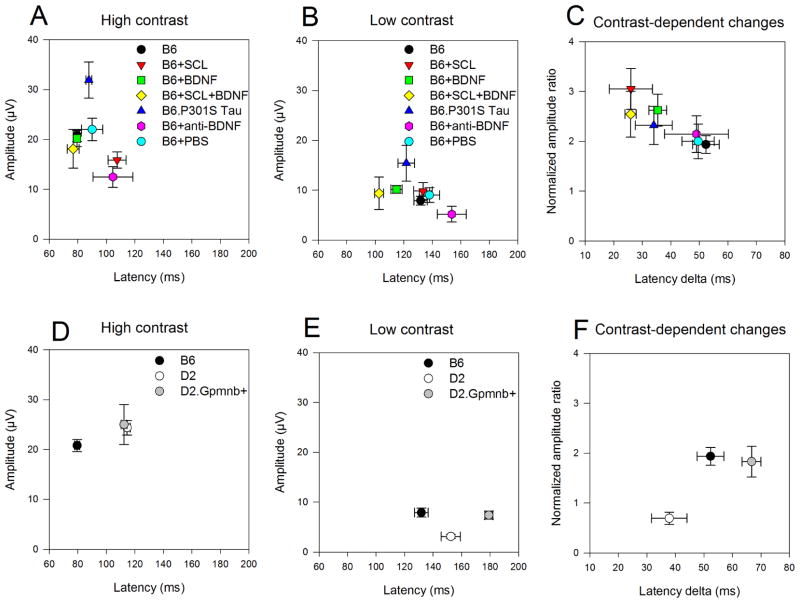
PERG amplitudes and corresponding latencies for high and low contrast measured in individual mice are displayed in Figure 3 as bi-directional scattergrams of group means (±SEM). The upper panel (Fig. 3A–C) includes mice of the same B6 genetic background but exposed to different conditions of neurotrophic support. The lower panel (Fig. 3D–F) includes mice of different genotype with different susceptibility to glaucoma. High contrast stimuli (Fig. 3A, 3D) showed a substantial separation among group means as indicated by non- overlapping error bars of either amplitude, latency, or both. Reduction of stimulus contrast (Fig. 3B, 3E) resulted in a substantially reduced PERG amplitude and a much delayed PERG latency. The low-contrast PERG showed a substantial separation among group means. However, the distribution of means appeared to differ from that of high-contrast PERG (compare Fig. 3A, with Fig. 3B, and Fig. 3D with Fig. 3E), suggesting that diverse mouse groups may have different contrast-dependent properties.
Figure 3.
Effect of contrast on PERG amplitude and latency for different mouse groups. Top panels: B6 mice exposed to different conditions of neurotrophic support (A–C). Bottom panels: Mice with different genotype (D–F). A, D: mean (±SEM) amplitudes as a function of corresponding mean (±SEM) latencies for stimuli of 1.0 contrast. B, E: mean (±SEM) amplitudes as a function of corresponding mean (±SEM) latencies for stimuli of 0.2 contrast. C, F: Normalized mean (±SEM) amplitude ratios [(amplitude at 0.2 contrast ÷ amplitude at 1.0 contrast) ÷ 0.2] as a function of mean (±SEM) latency deltas (latency at 0.2 contrast minus latency at 1.0 contrast). Note in all panels that there is a sizeable separation between mouse groups, and that the distribution of mouse groups may depend on the contrast level. B6, control C57BL/6J; B6+SCL, lesion of the contralateral superior colliculus one month before; B6+BDNF, 2 weeks after intravitreal injection of BDNF; B6+SCL+BDNF, 2 weeks after intravitreal injection of BDNF in mice with lesion of the contralateral superior colliculus; B6.P301S Tau, mice transgenic for human mutant P301S Tau; B6+anti-BDNF, 2 weeks after intravitreal injection of anti-BDNF; B6+PBS, 1 week after intravitreal injection of PBS; D2, DBA/2J mice 4 months old; D2.Gpnmb+, mice coisogenic with D2 that have a Gpnmb gene with normal function.
Contrast-dependent PERG changes were isolated (Fig. 3C, 3F) by displaying amplitude changes as normalized amplitude ratios, (NAR = amplitude @ 0.2/amplitude @ 1.0/0.2) as a function of corresponding latency changes (Latency delta = latency @ 0.2 minus latency @ 1.0). Fig. 3C compares contrast-dependent PERG changes between mice with the same genetic B6 background but exposed to different conditions of neurotrophic support. In absence of contrast gain control, the expected NAR and latency delta should have been 1 and 0, respectively. As all NARs were larger than 1 and all latency deltas had positive values, this would mean that contrast gain control mechanisms were at play and regulating electrical responsiveness of PERG generators. Interestingly, the distribution of means appeared to be part of a continuum, with control B6 having the lowest NAR associated with largest latency delta. There was a significant inverse correlation between NAR and latency delta (R2 = 0.74, P= 0.027), suggesting a trade-off between amplitude gain and temporal integration in contrast gain control mechanisms (Shapley and Enroth-Cugell, 1984). Overall, manipulation of neurotrophic support appeared to be able to regulate integrative properties of RGC responsiveness. The strongest effect was observed in SC-lesioned wild type mice, conceivably due to compensatory mechanisms after chronic deficit of target-derived factors (Yang et al., 2013).
Fig. 3F compares contrast-dependent PERG changes between mice with different genotype and different susceptibility to glaucoma. There was a clear separation between B6, pre-glaucomatous D2, and D2.Gpnmb+ strains, conceivably reflecting intrinsic differences in inner retina PERG generators.
Data shown in fig. 3A–F were submitted to multivariate analysis of variance (MANOVA) including all PERG role variables (amplitude and latency at high and low contrast, NAR, latency delta) to isolate significant differences between mouse groups. Results are summarized in Table 1. Two effects were investigated in relevant mouse groups, 1) Effect of neurotrophic support in the same B6 genotype, 2) Effect of different genotype in glaucoma-relevant strains.
Table 1.
| MANOVA comparisons | |||||
|---|---|---|---|---|---|
| Effect of neurotrophic support | |||||
| B6+BDNF* (n=6) | B6+anti-BDNF (n=8) | B6+SCL (n=12) | B6+SCL+BDNF* (n=5) | B6.P301STau (n=6) | |
| B6* (n=23) | P = 0.06 | P = 0.034 | P < 0.0001 | P = 0.1 | P = 0.0006 |
| B6+SCL (n=12) | P < 0.0001 | ||||
| Effect of genotype | |||||
| D2 (n=10) | D2.Gpnmb+ (n=12) | ||||
| B6 (n=23) | P < 0.0001 | P < 0.0001 | |||
| D2 (n=10) | P = 0.0012 | ||||
Legend. B6, control C57BL/6J; B6+SCL, lesion of the contralateral superior colliculus one month before; B6+BDNF, 2 weeks after intravitreal injection of BDNF; B6+SCL+BDNF, 2 weeks after intravitreal injection of BDNF in mice with lesion of the contralateral superior colliculus; B6.P301S Tau, mice transgenic for human mutant P301S Tau; B6+anti-BDNF, 2 weeks after intravitreal injection of anti-BDNF; D2, DBA/2J mice 4 months old; D2.Gpnmb+, mice coisogenic with D2 that have a Gpnmb gene with normal function.
An initial 2-factor analysis including B6, B6+BDNF, B6+SCL, and B6+SCL+BDNF identified a statistically significant interaction (p=0.005) of BDNF and SCL. Since these effects were not additive, the pairwise group comparisons appearing in this table were performed. Details appear in the text.
Relevant within-group comparisons were made based on appreciable visual differences in Fig. 3 and statistical analysis in Table 1.
1 – Effect of neurotrophic support, Fig. 3A–C
1.1 – B6 (black circle) vs B6+BDNF (green square)
BDNF injection in the healthy retina tended to reduce PERG latency at low contrast (Fig. 3B) but not at high contrast (Fig. 3A). These trends were reflected in an increased amplitude gain and reduced latency delta (Fig. 3C). The overall effect was borderline significant (MANOVA, P=0.06), and may mean that exogenous BDNF had a modest effect on RGC electrical responsiveness in the healthy retina.
1.2 – B6 (black circle) vs B6+anti-BDNF (magenta hexagon)
Antibody against BDNF should reduce available BDNF support in the retina. Injection of anti-BDNF tended to reduce PERG amplitude at both high (Fig. 3A) and low contrast (Fig. 3B), without altering amplitude gain and latency delta at low contrast (Fig. 3C). The overall effect was significant (MANOVA, P=0.034). Note that injection of PBS (cyan circles in Fig. 3A, 3B and 3C) did not cause significant changes in PERG amplitude and latency compared to non-injected controls (black circles).
1.3 – B6 (black circle) vs B6+SCL (red triangle down)
Chronic lesion to the superior colliculus tended to reduce PERG amplitude and increase latency at high contrast (Fig. 3A) but less so at low contrast (Fig. 3B), resulting in substantial difference in amplitude gain and reduction in latency delta (Fig. 3C). The overall effect was highly significant (MANOVA, P <0.0001). The inner retina of B6+SCL mice has been shown to thin and undergo molecular changes after chronic deficit of target-derived neurotrophic factors (Yang et al., 2013). This has been also shown to be associated with altered PERG at high contrast (Yang et al., 2013). The present results show in addition a remarkable alteration of contrast gain control properties (Fig. 3C). The effects of SCL on the PERG on B6 mice could not be replicated with intravitreal injection of anti-BDNF on B6 mice (compare with section 1.2).
1.4 – B6+SCL (red triangle down) vs B6+SCL+BDNF (yellow diamond)
Injection of BDNF in mice with chronic lesion of the superior colliculus tended to shorten PERG latency at both high (Fig. 3A) and low contrast (Fig. 3B) without noticeable differences in contrast gain control (Fig. 3C). Overall, the effect of BDNF injection in B6+SCL mice was highly significant (MANOVA, P< 0.0001). The PERG of BDNF-treated SCL mice was not significantly different from that of control B6 mice (MANOVA, P=0.1). Thus, while BDNF did not have a strong effect on healthy retina (mentioned in section 1.1), it was able to induce substantial restorative changes in the inner retina of mice chronically deficient of target-derived factors.
1.5 – B6 (black circle) vs B6.P301A Tau (blue triangle up)
Transgenic P301S Tau mice have constitutionally reduced axonal transport in the optic nerve. The PERG amplitude of B6.P3101A Tau mice tended to be higher than that of B6 at both high and low contrast, while the PERG latency was similar at both contrasts (Fig. 3A, 3B), resulting in a reduction of latency delta (Fig. 3C). Overall, the difference between the two groups was highly significant (MANOVA, P= 0.0006), and may be related to overcompensatory changes in the inner retina of mice transgenic for human mutant P301S Tau in response to constitutionally reduced axonal transport in the optic nerve (Bull et al., 2012).
2 – Effect of genotype, Fig. 3D–F
2.1 – B6 (black circle) vs D2 (white circle)
At both high and low contrast (Fig. 3D, 3E) B6 and D2 mice had similar PERG amplitudes, however the latency was substantially longer in D2 mice. In D2 mice the amplitude gain was considerably lower than that of B6, and the latency delta was shorter (fig. 3F). Overall, the difference between B6 and D2 was highly significant (MANOVA, P < 0.0001). The difference between the PERG of B6 and pre-glaucomatous D2 mice may be due to the relatively higher number of RGCs in D2 mice (Williams et al., 1996) resulting in difference in inner retina connectivity and longer integration time.
2.2 – D2 (white circle) vs D2.Gpnmb+ (grey circle)
At high contrast (Fig. 3D), D2 and D2.Gpnmb+ mice had overlapping amplitude and latency. At low contrast (Fig. 3E), however, D2.Gpnmb+ tended to have relatively larger amplitude and longer latency, resulting in different contrast-dependent changes (Fig. 3F). Overall, the difference between D2 and D2.Gpnmb+ mice was highly significant (MANOVA, P = 0.0012). D2 mice have a mutant Gpnmb gene and develop a form of age-related pigmentary glaucoma. Coisogenic D2.Gpnmb+ mice have a Gpnmb gene with normal function and do not develop IOP elevation and glaucoma (Howell et al., 2007b). As the Gpnmb gene encodes a transmembrane protein whose function(s) remain largely unknown, it is possible that differences in GPNMB expression in the inner retina (Kompass et al., 2008) alter RGC function and this is reflected in the PERG. The PERG of D2.Gpnmb+ mice were also significantly different from that of B6 mice (MANOVA, P < 0.0001).
Discussion
The PERG is dominated by the summed activity of inner retina neurons with receptive field organized in antagonistic regions, as they respond to each contrast-reversal transition whereas the summed activity of photoreceptors is absent in the PERG at any contrast level (Porciatti, 2007). Thus, it is possible to investigate whether the integrative properties of inner retina neurons and their response dynamics depend on the stimulus contrast without the confounding effects of outer retina activity. Contrast gain control mechanisms are known to play a major role at sites where there is a large convergence of neural inputs to target neurons (e.g., (Beaudoin et al., 2008)) as in the case of mouse RGCs (Jeon et al., 1998), which may adjust their responsiveness at different contrasts to allow more efficient use of their dynamic range.
The results of this study show that PERG assessment at high and low contrast can reveal phenotypic differences among mouse strains of similar genetic background but exposed to different conditions of neurotrophic support. Also, phenotypic differences could be detected in mice with different genotype and different susceptibility to glaucoma. Contrast-dependent PERG differences among groups may be thought to reflect changes occurring in the integration field of inner retina circuitry impinging on RGCs and regulating RGC electrical responsiveness.
Since neurotrophins are involved in synaptic plasticity (Blum et al., 2002; Leal et al., 2014; Poo, 2001) we tested the hypothesis that modulation of neurotrophic support would alter contrast-dependent PERG changes. Converging results of this study supported this hypothesis. First, chronic deficiency of target-derived neurotrophic factors in superior colliculus lesioned mice reduced and delayed the PERG signal and also altered contrast gain control by increasing amplitude gain and reducing latency delta at low contrast. Changes in contrast gain control might be interpreted as a compensatory response to neurotrophic deficiency associated with inner retina shrinkage (Yang et al., 2013). Increased gain at low stimulus intensity has been also reported in other sensory systems, such as seen in the auditory system in the case of tinnitus to compensate for neural loss (Parra and Pearlmutter, 2007). More generally, changes in gain control have been suggested in a number of disorders and in normal aging (e.g., (Betts et al., 2005; Butler et al., 2008; Porciatti et al., 2000; Rosenberg et al., 2015)).
Second, injection of BDNF in superior colliculus lesioned mice restored, at least in part, altered PERG integrative properties. Third, injection of anti-BDNF antibody reduced and delayed the PERG signal, showing that BDNF is necessary to sustain PERG responsiveness. Fourth, in B6.301S Tau mice with constitutionally reduced axon transport (Bull et al., 2012) the PERG signal was larger than that of control B6 at both high and low contrast. This may be interpreted as developmental overcompensation and maladaptive neuronal reorganization as it has been postulated for human tinnitus (Rauschecker et al., 2010). Altogether, these results demonstrate that integrative properties of PERG are modifiable by modulation of neurotrophic support and are detectable with PERG.
Our results also show that PERG integrative properties may also display substantial differences between mouse strains with different susceptibility to glaucoma. In particular, the PERG of D2 mice had a longer latency than that of B6 at high contrast as well as different contrast gain control properties. D2 mice are known to have significantly higher numbers of RGCs compared to B6 (Williams et al., 1996), and this may result in different inner retina connectivity and longer integration time. Dramatic reorganization of inner retina connectivity has been previously shown in transgenic mice expressing the antiapoptotic gene bcl-2 (Strettoi and Volpini, 2002) that had a larger number of RGCs due to inhibition of developmental cell death. Reorganization of inner retina connectivity in Bcl-2 overexpressing mice did not impact behavioral visual acuity (Gianfranceschi et al., 1999). Significant differences were also detectable between pre-glaucomatous D2 and coisogenic D2.Gpnmb+ mice, in agreement with previous results (Porciatti et al., 2010). D2 and D2.Gpnmb+ mice had overlapping PERG amplitude and latency at high contrast, but different contrast gain control characteristics. As these differences cannot be explained based on different number of RGCs, they may be due to different GPNMB expression in the inner retina as well as to other unknown factors that impact PERG integrative properties.
In conclusion, the integrative properties of the PERG recorded in the standard C57Bl/6J inbred mouse strain are impaired after deficit of neurotrophic support, and partially restored after exogenous neurotrophic administration, conceivably reflecting plastic changes in RGC electrical responsiveness. This further supports the critical role of neurotrophic support on neuronal electrical activity. PERG integrative properties also differ between mice with different genotype and with different susceptibility to glaucoma. Recently, several studies have shown in mouse models of glaucoma significant changes in dendritic structure associated with loss of function in distinct RGC and amacrine cell types (Della Santina et al., 2013; El-Danaf and Huberman, 2015; Pang et al., 2015). Whether similar mechanisms subserve the contrast-dependent PERG changes identified in the present study, will require further investigation. Nevertheless, the present results represent a promising proof of concept that the PERG could be used as a tool for in-vivo monitoring of the effect of neurotrophic support on electrical activity of inner retina, as well as for phenotypic screening of different mouse genotypes. These pursuits may be greatly facilitated by the use of high- throughput PERG methods (Chou et al., 2014) that are now commercially available.
We propose an in-vivo method to quantify retinal ganglion cell (RGC) functional plasticity.
We examine the integrative properties of the Pattern Electroretinogram (PERG) in mice.
PERG integrative properties are modifiable with neurotrophic support.
Mouse genotypes differently susceptible to glaucoma have distinct PERG integration.
Acknowledgments
Financial support: NIH-NEI RO1 EY019077, NIH center grant P30-EY014801, unrestricted grant to Bascom Palmer Eye Institute from Research to Prevent Blindness, Inc.
Footnotes
Financial disclosure: THC, WJF, OS, MR, JKR, VP, none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adalbert R, Coleman MP. Axon pathology in age-related neurodegenerative disorders. Neuropathology and Applied Neurobiology. 2013;39:90–108. doi: 10.1111/j.1365-2990.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Progress in Retinal and Eye Research. 2012;31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Libby RT, Mao M, Cosma IM, Wilson LA, Smith RS, John SW. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006;4:20. doi: 10.1186/1741-7007-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artal P, Herreros de Tejada P, Munoz Tedo C, Green DG. Retinal image quality in the rodent eye. Vis Neurosci. 1998;15:597–605. doi: 10.1017/s0952523898154020. [DOI] [PubMed] [Google Scholar]
- Beaudoin DL, Manookin MB, Demb JB. Distinct expressions of contrast gain control in parallel synaptic pathways converging on a retinal ganglion cell. J Physiol. 2008;586:5487–5502. doi: 10.1113/jphysiol.2008.156224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron. 2005;45:361–366. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Blum R, Kafitz KW, Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature. 2002;419:687–693. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull ND, Guidi A, Goedert M, Martin KR, Spillantini MG. Reduced axonal transport and increased excitotoxic retinal ganglion cell degeneration in mice transgenic for human mutant P301S tau. PLoS One. 2012;7:e34724. doi: 10.1371/journal.pone.0034724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biological psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TH, Bohorquez J, Toft-Nielsen J, Ozdamar O, Porciatti V. Robust mouse pattern electroretinograms derived simultaneously from each eye using a common snout electrode. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.14-13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TH, Park KK, Luo X, Porciatti V. Retrograde signaling in the optic nerve is necessary for electrical responsiveness of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2013;54:1236–1243. doi: 10.1167/iovs.12-11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina L, Inman DM, Lupien CB, Horner PJ, Wong RO. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci. 2013;33:17444–17457. doi: 10.1523/JNEUROSCI.5461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB. Functional circuitry of visual adaptation in the retina. J Physiol. 2008;586:4377–4384. doi: 10.1113/jphysiol.2008.156638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Haarsma L, Freed MA, Sterling P. Functional circuitry of the retinal ganglion cell’s nonlinear receptive field. J Neurosci. 1999;19:9756–9767. doi: 10.1523/JNEUROSCI.19-22-09756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Danaf RN, Huberman AD. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J Neurosci. 2015;35:2329–2343. doi: 10.1523/JNEUROSCI.1419-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Algeciras M, Ding D, Mastronardi FG, Marc RE, Porciatti V, Bhattacharya SK. Deimination restores inner retinal visual function in murine demyelinating disease. J Clin Invest. 2013;123:646–656. doi: 10.1172/JCI64811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, Williams PA, Rausch RL, Kiernan AE, Nair KS, Anderson MG, John SW, Howell GR, Libby RT. Using genetic mouse models to gain insight into glaucoma: Past results and future possibilities. Exp Eye Res. 2015 doi: 10.1016/j.exer.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfranceschi L, Fiorentini A, Maffei L. Behavioural visual acuity of wild type and bcl2 transgenic mouse. Vision Res. 1999;39:569–574. doi: 10.1016/s0042-6989(98)00169-2. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, MN, Porciatti V, Whitmore AV, Masland RH, John SW. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007a;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Marchant JK, Wilson LA, Cosma IM, Smith RS, Anderson MG, John SW. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet. 2007b;8:45. doi: 10.1186/1471-2156-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompass KS, Agapova OA, Li W, Kaufman PL, Rasmussen CA, Hernandez MR. Bioinformatic and statistical analysis of the optic nerve head in a primate model of ocular hypertension. BMC Neurosci. 2008;9:93. doi: 10.1186/1471-2202-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76(Part C):639–656. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SW. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22:637–648. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- Liu M, Duggan J, Salt TE, Cordeiro MF. Dendritic changes in visual pathways in glaucoma and other neurodegenerative conditions. Exp Eye Res. 2011;92:244–250. doi: 10.1016/j.exer.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Miura G, Wang MH, Ivers KM, Frishman LJ. Retinal pathway origins of the pattern ERG of the mouse. Exp Eye Res. 2009;89:49–62. doi: 10.1016/j.exer.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquette JB, Di Polo A. Dendritic and synaptic protection: is it enough to save the retinal ganglion cell body and axon? J Neuroophthalmol. 2008;28:144–154. doi: 10.1097/WNO.0b013e318177edf0. [DOI] [PubMed] [Google Scholar]
- Nagaraju M, Saleh M, Porciatti V. IOP-Dependent Retinal Ganglion Cell Dysfunction in Glaucomatous DBA/2J Mice. Invest Ophthalmol Vis Sci. 2007;48:4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Frankfort BJ, Gross RL, Wu SM. Elevated intraocular pressure decreases response sensitivity of inner retinal neurons in experimental glaucoma mice. Proceedings of the National Academy of Sciences. 2015;112:2593–2598. doi: 10.1073/pnas.1419921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra LC, Pearlmutter BA. Illusory percepts from auditory adaptation. The Journal of the Acoustical Society of America. 2007;121:1632–1641. doi: 10.1121/1.2431346. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Porciatti V. The mouse pattern electroretinogram. Doc Ophthalmol. 2007;115:145–153. doi: 10.1007/s10633-007-9059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V. Electrophysiological assessment of retinal ganglion cell function. Exp Eye Res. 2015 doi: 10.1016/j.exer.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Bonanni P, Fiorentini A, Guerrini R. Lack of cortical contrast gain control in human photosensitive epilepsy. Nat Neurosci. 2000;3:259–263. doi: 10.1038/72972. [DOI] [PubMed] [Google Scholar]
- Porciatti V, Chou TH, Feuer WJ. C57BL/6J, DBA/2J, and DBA/2J.Gpnmb mice have different visual signal processing in the inner retina. Mol Vis. 2010;16:2939–2947. [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Nagaraju M. Head-up tilt lowers IOP and improves RGC dysfunction in glaucomatous DBA/2J mice. Exp Eye Res. 2010;90:452–460. doi: 10.1016/j.exer.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Pizzorusso T, Cenni MC, Maffei L. The visual response of retinal ganglion cells is not altered by optic nerve transection in transgenic mice overexpressing Bcl-2. Proc Natl Acad Sci U S A. 1996;93:14955–14959. doi: 10.1073/pnas.93.25.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Saleh M, Nagaraju M. The pattern electroretinogram as a tool to monitor progressive retinal ganglion cell dysfunction in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2007;48:745–751. doi: 10.1167/iovs.06-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remtulla S, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985;25:21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg A, Patterson JS, Angelaki DE. A computational perspective on autism. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1510583112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Nagaraju M, Porciatti V. Longitudinal Evaluation of Retinal Ganglion Cell Function and IOP in the DBA/2J Mouse Model of Glaucoma. Invest Ophthalmol Vis Sci. 2007;48:4564–4572. doi: 10.1167/iovs.07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Zhang Y, Meister M, Sanes JR. Age-related alterations in neurons of the mouse retina. J Neurosci. 2011;31:16033–16044. doi: 10.1523/JNEUROSCI.3580-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004;44:1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Shapley R, Enroth-Cugell C. Visual adaptation and retinal gain controls. Progress in Retinal Research. 1984;3:263–346. [Google Scholar]
- Strettoi E, Volpini M. Retinal organization in the bcl-2-overexpressing transgenic mouse. J Comp Neurol. 2002;446:1–10. doi: 10.1002/cne.10177. [DOI] [PubMed] [Google Scholar]
- Williams PA, Howell GR, Barbay JM, Braine CE, Sousa GL, John SW, Morgan JE. Retinal ganglion cell dendritic atrophy in DBA/2J glaucoma. PLoS One. 2013;8:e72282. doi: 10.1371/journal.pone.0072282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Piechota M, von Ruhland C, Taylor E, Morgan JE, Votruba M. Opa1 is essential for retinal ganglion cell synaptic architecture and connectivity. Brain. 2012;135:493–505. doi: 10.1093/brain/awr330. [DOI] [PubMed] [Google Scholar]
- Williams RW, Strom RC, Rice DS, Goldowitz D. Genetic and environmental control of variation in retinal ganglion cell number in mice. J Neurosci. 1996;16:7193–7205. doi: 10.1523/JNEUROSCI.16-22-07193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Wen R, Chou TH, Li Y, Wang Z, Porciatti V. Protection of pattern electroretinogram and retinal ganglion cells by oncostatin M after optic nerve injury. PLoS One. 2014;9:e108524. doi: 10.1371/journal.pone.0108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chou TH, Ruggeri M, Porciatti V. A New Mouse Model of Inducible, Chronic Retinal Ganglion Cell Dysfunction Not Associated with Cell Death. Investigative Ophthalmology & Visual Science. 2013;54:1898–1904. doi: 10.1167/iovs.12-11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Koilkonda RD, Chou TH, Porciatti V, Mehta A, Hentall ID, Chiodo VA, Boye SL, Hauswirth WW, Lewin AS, Guy J. Consequences of zygote injection and germline transfer of mutant human mitochondrial DNA in mice. Proc Natl Acad Sci U S A. 2015;112:E5689–5698. doi: 10.1073/pnas.1506129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Ozdemir SS, Koilkonda RD, Chou TH, Porciatti V, Chiodo V, Boye SL, Hauswirth WW, Lewin AS, Guy J. Mutant NADH dehydrogenase subunit 4 gene delivery to mitochondria by targeting sequence-modified adeno-associated virus induces visual loss and optic atrophy in mice. Mol Vis. 2012;18:1668–1683. [PMC free article] [PubMed] [Google Scholar]