Abstract
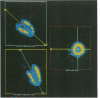
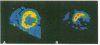
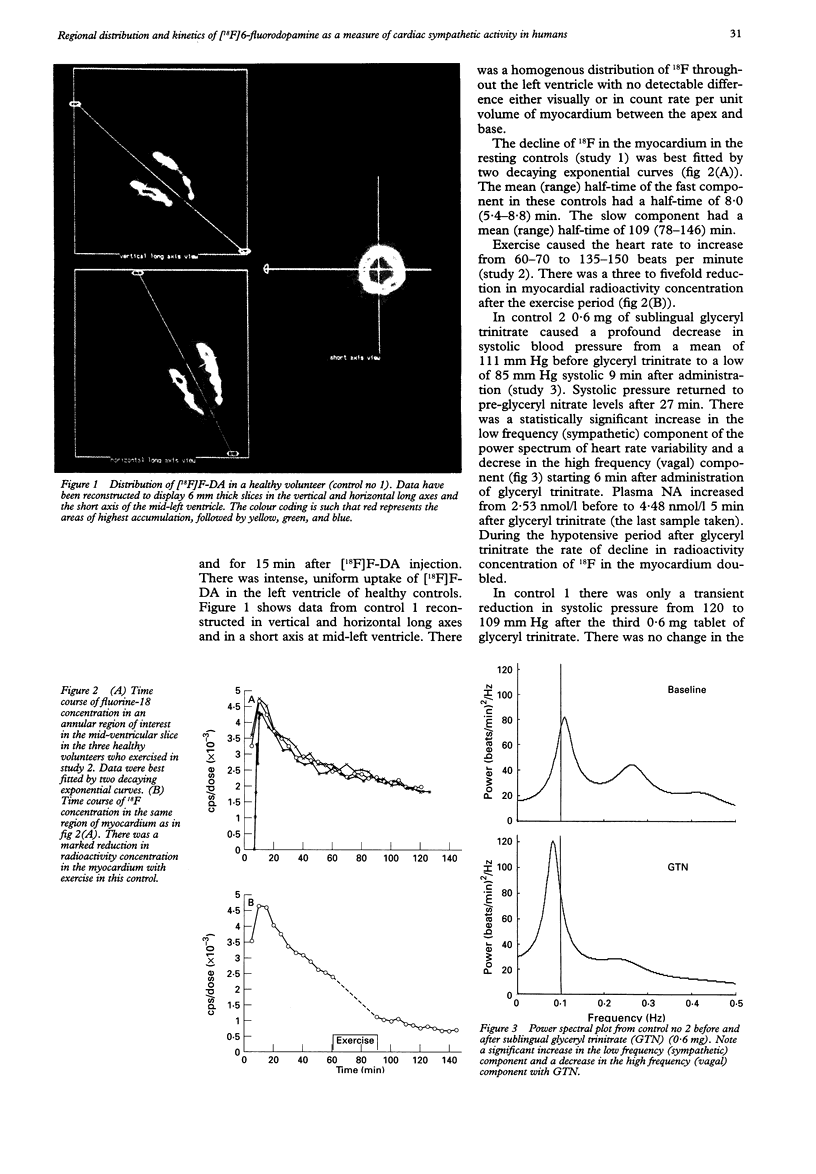
OBJECTIVES--To determine whether an increase in cardiac sympathetic activity produced by exercise or sublingual glyceryl trinitrate causes an increased rate of loss of fluorine-18 from the myocardium after intravenous [18F]6-fluorodopamine ([18F]F-DA) in normal volunteers. In addition, to determine the contribution of non-specific uptake of [18F]F-DA in the myocardium in patients with recent heart transplant. PROTOCOL--[18F]F was prepared by direct electrophilic fluorination of dopamine. Nine healthy volunteers each received 1.85 x 10(8) Bq (168-250 micrograms) [18F]F-DA over a period of 3 min and were scanned for 2 h in an ECAT 953/31 tomograph. Three controls were scanned before and after vigorous cycle exercise and two were scanned before and after sublingual glyceryl trinitrate. In addition, two patients (1 and 2 years post-heart transplant) underwent a myocardial perfusion study with ammonia labelled with nitrogen-13 followed by an [18F]F-DA study. RESULTS--There was intense uniform uptake of [18F]F-DA throughout the myocardium in the healthy volunteers. The time course of 18F in the myocardium under resting conditions fitted a biexponential function with mean half-times of 8.0 and 109 min. Vigorous exercise produced a three to fivefold increase in the rate of loss of 18F compared with that when resting. After glyceryl trinitrate, one control had a profound reduction in blood pressure (23%) and twofold increase in the rate of loss of myocardial 18F. The other control had no physiologically significant change in blood pressure, heart rate, or rate of loss of myocardial 18F. Uptake of [18F]F-DA in the two posttransplant patients was confined to a small anterobasal region adjacent to the atrioventricular groove, while blood flow, as measured with [13N] ammonia, was uniformly distributed throughout the myocardium. Partial reinnervation of the myocardium was confirmed by the presence of distinct low frequency spectral peaks of the heart rate power spectrum in both patients. CONCLUSIONS--These results suggest that the uptake of [18F]F-DA reflects the distribution of cardiac sympathetic innervation and that the rate of loss of 18F from the myocardium partially reflects spill over of noradrenaline. The technique may be useful in investigating various cardiac conditions in which the sympathetic system is compromised.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brush J. E., Jr, Eisenhofer G., Garty M., Stull R., Maron B. J., Cannon R. O., 3rd, Panza J. A., Epstein S. E., Goldstein D. S. Cardiac norepinephrine kinetics in hypertrophic cardiomyopathy. Circulation. 1989 Apr;79(4):836–844. doi: 10.1161/01.cir.79.4.836. [DOI] [PubMed] [Google Scholar]
- Chang P. C., Szemeredi K., Grossman E., Kopin I. J., Goldstein D. S. Fate of tritiated 6-fluorodopamine in rats: a false neurotransmitter for positron emission tomographic imaging of sympathetic innervation and function. J Pharmacol Exp Ther. 1990 Nov;255(2):809–817. [PubMed] [Google Scholar]
- Chiueh C. C., Zukowska-Grojec Z., Kirk K. L., Kopin I. J. 6-Fluorocatecholamines as false adrenergic neurotransmitters. J Pharmacol Exp Ther. 1983 Jun;225(3):529–533. [PubMed] [Google Scholar]
- Dae M. W., De Marco T., Botvinick E. H., O'Connell J. W., Hattner R. S., Huberty J. P., Yuen-Green M. S. Scintigraphic assessment of MIBG uptake in globally denervated human and canine hearts--implications for clinical studies. J Nucl Med. 1992 Aug;33(8):1444–1450. [PubMed] [Google Scholar]
- Dae M. W., O'Connell J. W., Botvinick E. H., Ahearn T., Yee E., Huberty J. P., Mori H., Chin M. C., Hattner R. S., Herre J. M. Scintigraphic assessment of regional cardiac adrenergic innervation. Circulation. 1989 Mar;79(3):634–644. doi: 10.1161/01.cir.79.3.634. [DOI] [PubMed] [Google Scholar]
- DeGrado T. R., Hutchins G. D., Toorongian S. A., Wieland D. M., Schwaiger M. Myocardial kinetics of carbon-11-meta-hydroxyephedrine: retention mechanisms and effects of norepinephrine. J Nucl Med. 1993 Aug;34(8):1287–1293. [PubMed] [Google Scholar]
- Dela F., Mikines K. J., Von Linstow M., Galbo H. Heart rate and plasma catecholamines during 24 h of everyday life in trained and untrained men. J Appl Physiol (1985) 1992 Dec;73(6):2389–2395. doi: 10.1152/jappl.1992.73.6.2389. [DOI] [PubMed] [Google Scholar]
- Delforge J., Syrota A., Lançon J. P., Nakajima K., Loc'h C., Janier M., Vallois J. M., Cayla J., Crouzel C. Cardiac beta-adrenergic receptor density measured in vivo using PET, CGP 12177, and a new graphical method. J Nucl Med. 1991 Apr;32(4):739–748. [PubMed] [Google Scholar]
- Ding Y. S., Fowler J. S., Dewey S. L., Logan J., Schlyer D. J., Gatley S. J., Volkow N. D., King P. T., Wolf A. P. Comparison of high specific activity (-) and (+)-6-[18F]fluoronorepinephrine and 6-[18F]fluorodopamine in baboons: heart uptake, metabolism and the effect of desipramine. J Nucl Med. 1993 Apr;34(4):619–629. [PubMed] [Google Scholar]
- Eisenhofer G., Hovevey-Sion D., Kopin I. J., Miletich R., Kirk K. L., Finn R., Goldstein D. S. Neuronal uptake and metabolism of 2- and 6-fluorodopamine: false neurotransmitters for positron emission tomographic imaging of sympathetically innervated tissues. J Pharmacol Exp Ther. 1989 Jan;248(1):419–427. [PubMed] [Google Scholar]
- Fallen E. L., Kamath M. V., Ghista D. N. Power spectrum of heart rate variability: a non-invasive test of integrated neurocardiac function. Clin Invest Med. 1988 Oct;11(5):331–340. [PubMed] [Google Scholar]
- Goldstein D. S., Chang P. C., Eisenhofer G., Miletich R., Finn R., Bacher J., Kirk K. L., Bacharach S., Kopin I. J. Positron emission tomographic imaging of cardiac sympathetic innervation and function. Circulation. 1990 May;81(5):1606–1621. doi: 10.1161/01.cir.81.5.1606. [DOI] [PubMed] [Google Scholar]
- Goldstein D. S., Chang P. C., Smith C. B., Herscovitch P., Austin S. M., Eisenhofer G., Kopin I. J. Dosimetric estimates for clinical positron emission tomographic scanning after injection of [18F]-6-fluorodopamine. J Nucl Med. 1991 Jan;32(1):102–110. [PubMed] [Google Scholar]
- Goldstein D. S., Eisenhofer G., Dunn B. B., Armando I., Lenders J., Grossman E., Holmes C., Kirk K. L., Bacharach S., Adams R. Positron emission tomographic imaging of cardiac sympathetic innervation using 6-[18F]fluorodopamine: initial findings in humans. J Am Coll Cardiol. 1993 Dec;22(7):1961–1971. doi: 10.1016/0735-1097(93)90786-z. [DOI] [PubMed] [Google Scholar]
- Hellmann G., Hertting G., Peskar B. Uptake kinetics and metabolism of 7-3H-dopamine in the isolated perfused rat heart. Br J Pharmacol. 1971 Feb;41(2):256–269. doi: 10.1111/j.1476-5381.1971.tb08026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Ninomiya I., Azumi T. Cardiac sympathetic nerve activity and catecholamine kinetics in cat hearts. Am J Physiol. 1987 May;252(5 Pt 2):H879–H885. doi: 10.1152/ajpheart.1987.252.5.H879. [DOI] [PubMed] [Google Scholar]
- Horwitz L. D., Gorlin R., Taylor W. J., Kemp H. G. Effects of nitroglycerin on regional myocardial blood flow in coronary artery disease. J Clin Invest. 1971 Aug;50(8):1578–1584. doi: 10.1172/JCI106645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath M. V., Fallen E. L., McKelvie R. Effects of steady state exercise on the power spectrum of heart rate variability. Med Sci Sports Exerc. 1991 Apr;23(4):428–434. [PubMed] [Google Scholar]
- Kline R. C., Swanson D. P., Wieland D. M., Thrall J. H., Gross M. D., Pitt B., Beierwaltes W. H. Myocardial imaging in man with I-123 meta-iodobenzylguanidine. J Nucl Med. 1981 Feb;22(2):129–132. [PubMed] [Google Scholar]
- Meredith I. T., Broughton A., Jennings G. L., Esler M. D. Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med. 1991 Aug 29;325(9):618–624. doi: 10.1056/NEJM199108293250905. [DOI] [PubMed] [Google Scholar]
- Merlet P., Delforge J., Syrota A., Angevin E., Mazière B., Crouzel C., Valette H., Loisance D., Castaigne A., Randé J. L. Positron emission tomography with 11C CGP-12177 to assess beta-adrenergic receptor concentration in idiopathic dilated cardiomyopathy. Circulation. 1993 Apr;87(4):1169–1178. doi: 10.1161/01.cir.87.4.1169. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Bunko H., Taki J., Shimizu M., Muramori A., Hisada K. Quantitative analysis of 123I-meta-iodobenzylguanidine (MIBG) uptake in hypertrophic cardiomyopathy. Am Heart J. 1990 Jun;119(6):1329–1337. doi: 10.1016/s0002-8703(05)80183-8. [DOI] [PubMed] [Google Scholar]
- Schwaiger M., Kalff V., Rosenspire K., Haka M. S., Molina E., Hutchins G. D., Deeb M., Wolfe E., Jr, Wieland D. M. Noninvasive evaluation of sympathetic nervous system in human heart by positron emission tomography. Circulation. 1990 Aug;82(2):457–464. doi: 10.1161/01.cir.82.2.457. [DOI] [PubMed] [Google Scholar]
- Sisson J. C., Wieland D. M., Sherman P., Mangner T. J., Tobes M. C., Jacques S., Jr Metaiodobenzylguanidine as an index of the adrenergic nervous system integrity and function. J Nucl Med. 1987 Oct;28(10):1620–1624. [PubMed] [Google Scholar]
- Stanton M. S., Tuli M. M., Radtke N. L., Heger J. J., Miles W. M., Mock B. H., Burt R. W., Wellman H. N., Zipes D. P. Regional sympathetic denervation after myocardial infarction in humans detected noninvasively using I-123-metaiodobenzylguanidine. J Am Coll Cardiol. 1989 Nov 15;14(6):1519–1526. doi: 10.1016/0735-1097(89)90391-4. [DOI] [PubMed] [Google Scholar]
- Wakasugi S., Wada A., Hasegawa Y., Nakano S., Shibata N. Detection of abnormal cardiac adrenergic neuron activity in adriamycin-induced cardiomyopathy with iodine-125-metaiodobenzylguanidine. J Nucl Med. 1992 Feb;33(2):208–214. [PubMed] [Google Scholar]
- Yamaguchi N., de Champlain J., Nadeau R. Correlation between the response of the heart to sympathetic stimulation and the release of endogenous catecholamines into the coronary sinus of the dog. Circ Res. 1975 May;36(5):662–668. doi: 10.1161/01.res.36.5.662. [DOI] [PubMed] [Google Scholar]