Abstract
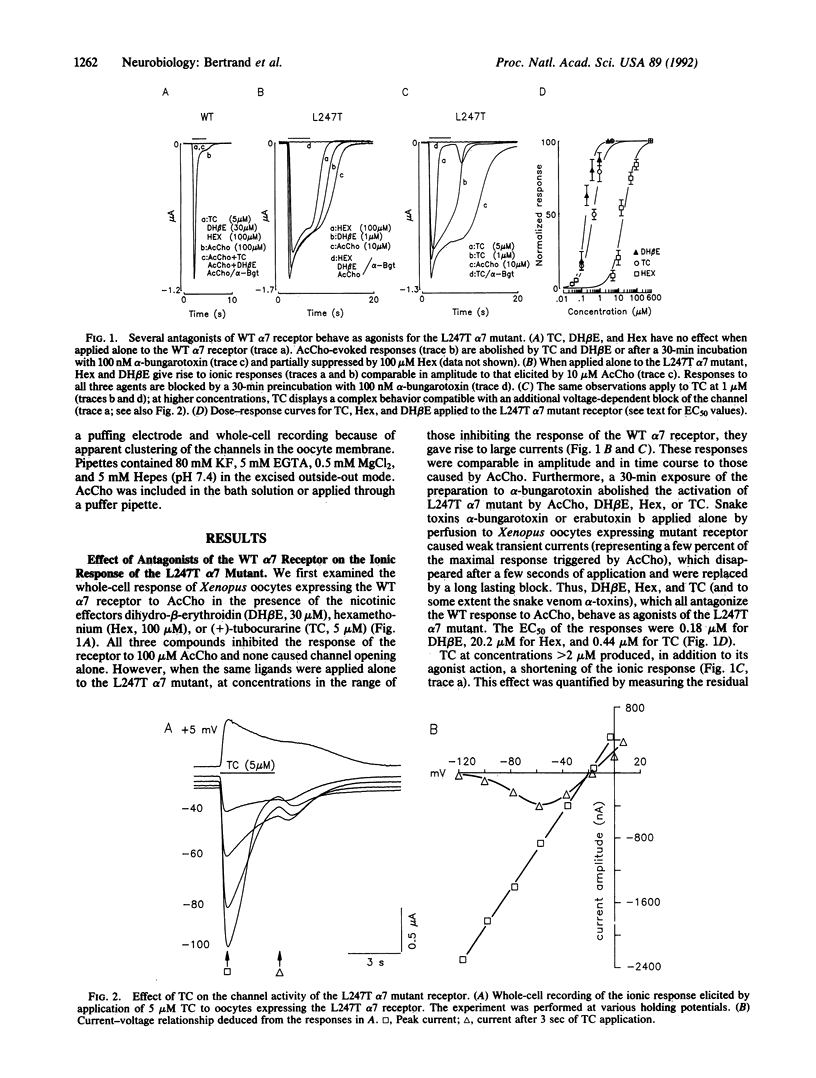
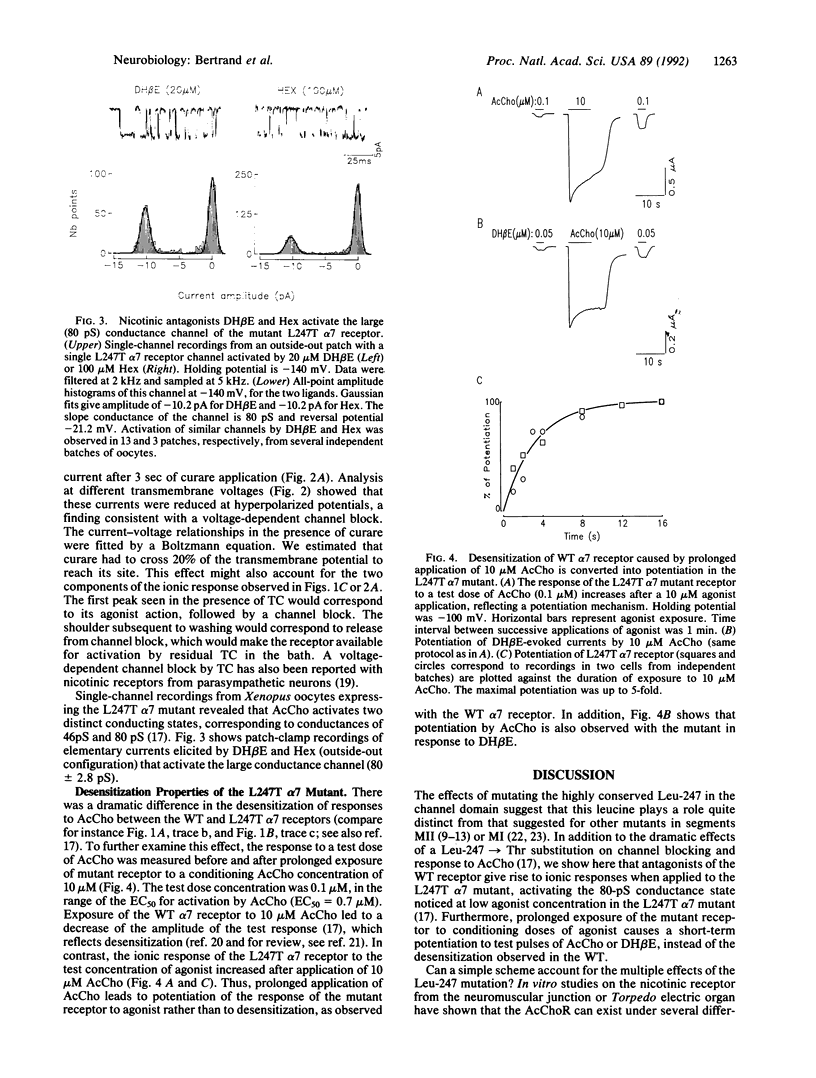
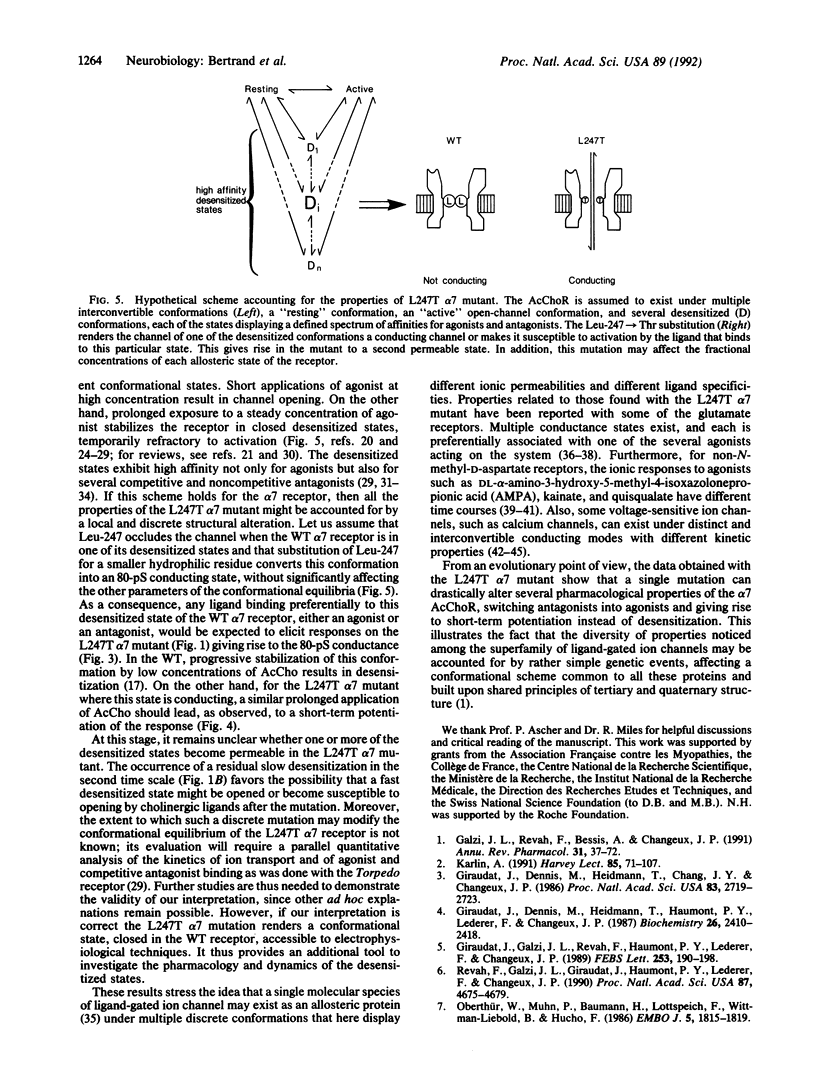
The putative channel-forming MII domains of the nicotinic, gamma-aminobutyric acid type A, and glycine receptors contain a highly conserved leucine residue. Mutation of this hydrophobic amino acid in the neuronal nicotinic receptor alpha 7 (Leu-247), reconstituted in Xenopus oocytes, modifies the ionic response to acetylcholine and alters desensitization. Furthermore, the Leu----Thr (L247T) mutant has two conducting states (46 pS and 80 pS), in contrast with the wild-type (WT) receptor, which has only one (45 pS). We now show that this mutant possesses a rather paradoxical pharmacology: antagonists of the WT receptor such as dihydro-beta-erythroidin, hexamethonium, or (+)-tubocurarine elicit ionic currents when applied to the L247T alpha 7 mutant and these responses are blocked by alpha-bungarotoxin. Furthermore, prolonged application of acetylcholine causes desensitization in the WT but leads to a potentiation of the responses to acetylcholine or dihydro-beta-erythroidin in the mutant. These data are consistent with a scheme in which mutation of Leu-247 renders a desensitized state in the WT channel a conducting state. They also strengthen the proposal that, in the WT, some competitive antagonists may stabilize desensitized states. Finally, these observations may shed light on properties of other ion channels, in particular the glutamate receptors, which display multiple conductance levels associated with various pharmacological agents.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Leonard R. J., Vogelaar N. J., Czyzyk L., Gouin A., Davidson N., Lester H. A. An open-channel blocker interacts with adjacent turns of alpha-helices in the nicotinic acetylcholine receptor. Neuron. 1990 Jan;4(1):87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Couturier S., Bertrand D., Matter J. M., Hernandez M. C., Bertrand S., Millar N., Valera S., Barkas T., Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990 Dec;5(6):847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Elmslie K. S., Zhou W., Jones S. W. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990 Jul;5(1):75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Bessis A., Changeux J. P. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu Rev Pharmacol Toxicol. 1991;31:37–72. doi: 10.1146/annurev.pa.31.040191.000345. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Chang J. Y., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: serine-262 of the delta subunit is labeled by [3H]chlorpromazine. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2719–2723. doi: 10.1073/pnas.83.8.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Haumont P. Y., Lederer F., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the beta and delta chains. Biochemistry. 1987 May 5;26(9):2410–2418. doi: 10.1021/bi00383a003. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Gali J., Revah F., Changeux J., Haumont P., Lederer F. The noncompetitive blocker [(3)H]chlorpromazine labels segment M2 but not segment M1 of the nicotinic acetylcholine receptor alpha-subunit. FEBS Lett. 1989 Aug 14;253(1-2):190–198. doi: 10.1016/0014-5793(89)80957-3. [DOI] [PubMed] [Google Scholar]
- Grünhagen H. H., Changeux J. P. Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. V. Qualitative correlation between pharmacological effects and equilibration processes of the cholinergic receptor protein as revealed by the structural probe quinacrine. J Mol Biol. 1976 Sep 25;106(3):517–535. doi: 10.1016/0022-2836(76)90250-3. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Bernhardt J., Neumann E., Changeux J. P. Rapid kinetics of agonist binding and permeability response analyzed in parallel on acetylcholine receptor rich membranes from Torpedo marmorata. Biochemistry. 1983 Nov 8;22(23):5452–5459. doi: 10.1021/bi00292a029. [DOI] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Hucho F., Oberthür W., Lottspeich F. The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett. 1986 Sep 1;205(1):137–142. doi: 10.1016/0014-5793(86)80881-x. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991 Aug;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. Explorations of the nicotinic acetylcholine receptor. Harvey Lect. 1989;85:71–107. [PubMed] [Google Scholar]
- Konno T., Busch C., Von Kitzing E., Imoto K., Wang F., Nakai J., Mishina M., Numa S., Sakmann B. Rings of anionic amino acids as structural determinants of ion selectivity in the acetylcholine receptor channel. Proc Biol Sci. 1991 May 22;244(1310):69–79. doi: 10.1098/rspb.1991.0053. [DOI] [PubMed] [Google Scholar]
- Krodel E. K., Beckman R. A., Cohen J. B. Identification of a local anesthetic binding site in nicotinic post-synaptic membranes isolated from Torpedo marmorata electric tissue. Mol Pharmacol. 1979 Mar;15(2):294–312. [PubMed] [Google Scholar]
- Leonard R. J., Labarca C. G., Charnet P., Davidson N., Lester H. A. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988 Dec 16;242(4885):1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Lo D. C., Pinkham J. L., Stevens C. F. Influence of the gamma subunit and expression system on acetylcholine receptor gating. Neuron. 1990 Dec;5(6):857–866. doi: 10.1016/0896-6273(90)90345-g. [DOI] [PubMed] [Google Scholar]
- Lo D. C., Pinkham J. L., Stevens C. F. Role of a key cysteine residue in the gating of the acetylcholine receptor. Neuron. 1991 Jan;6(1):31–40. doi: 10.1016/0896-6273(91)90119-k. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Shneider N. A., Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990 Nov;5(5):569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Boyd N. D., Cohen J. B. Conformations of Torpedo acetylcholine receptor associated with ion transport and desensitization. Biochemistry. 1982 Jul 6;21(14):3460–3467. doi: 10.1021/bi00257a032. [DOI] [PubMed] [Google Scholar]
- Oberthür W., Muhn P., Baumann H., Lottspeich F., Wittmann-Liebold B., Hucho F. The reaction site of a non-competitive antagonist in the delta-subunit of the nicotinic acetylcholine receptor. EMBO J. 1986 Aug;5(8):1815–1819. doi: 10.1002/j.1460-2075.1986.tb04431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa E. L., Chattopadhyay A., McNamee M. G. Desensitization of the nicotinic acetylcholine receptor: molecular mechanisms and effect of modulators. Cell Mol Neurobiol. 1989 Jun;9(2):141–178. doi: 10.1007/BF00713026. [DOI] [PubMed] [Google Scholar]
- Patneau D. K., Mayer M. L. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991 May;6(5):785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Hess P. Reversible uncoupling of inactivation in N-type calcium channels. Nature. 1991 Jun 20;351(6328):657–659. doi: 10.1038/351657a0. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Revah F., Bertrand D., Galzi J. L., Devillers-Thiéry A., Mulle C., Hussy N., Bertrand S., Ballivet M., Changeux J. P. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991 Oct 31;353(6347):846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- Revah F., Galzi J. L., Giraudat J., Haumont P. Y., Lederer F., Changeux J. P. The noncompetitive blocker [3H]chlorpromazine labels three amino acids of the acetylcholine receptor gamma subunit: implications for the alpha-helical organization of regions MII and for the structure of the ion channel. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4675–4679. doi: 10.1073/pnas.87.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schoepfer R., Conroy W. G., Whiting P., Gore M., Lindstrom J. Brain alpha-bungarotoxin binding protein cDNAs and MAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990 Jul;5(1):35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- Villarroel A., Herlitze S., Koenen M., Sakmann B. Location of a threonine residue in the alpha-subunit M2 transmembrane segment that determines the ion flow through the acetylcholine receptor channel. Proc Biol Sci. 1991 Jan 22;243(1306):69–74. doi: 10.1098/rspb.1991.0012. [DOI] [PubMed] [Google Scholar]
- Weiland G., Taylor P. Ligand specificity of state transitions in the cholinergic receptor: behavior of agonists and antagonists. Mol Pharmacol. 1979 Mar;15(2):197–212. [PubMed] [Google Scholar]



