Abstract
Traumatic brain injury (TBI) results in varying degrees of disability in a significant number of persons annually. The mechanisms of cognitive dysfunction after TBI have been explored in both animal models and human clinical studies for decades. Dopaminergic, serotonergic, and noradrenergic dysfunction has been described in many previous reports. In addition, cholinergic dysfunction has also been a familiar topic among TBI researchers for many years. Although pharmacological agents that modulate cholinergic neurotransmission have been used with varying degrees of success in previous studies, improving their function and maximizing cognitive recovery is an ongoing process. In this article, we review the previous findings on the biological mechanism of cholinergic dysfunction after TBI. In addition, we describe studies that use both older agents and newly developed agents as candidates for targeting cholinergic neurotransmission in future studies.
Key words: : acetylcholine, cholinergic, nicotinic, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a common cause of death and disability worldwide. Among the survivors of TBI in the United States, 70,000 to 90,000 have substantial long-term loss of cognitive function that results in a lifetime of disability.1 Neuropsychological tests that assess various aspects of behavior such as social function, cognitive abilities, and psychiatric symptoms at 10–20 years after TBI show significant behavioral impairment at such chronic time points.2 Specifically, there are problems in memory, attention, and information processing among many others. Much has been discovered about the biological mechanisms of injury, which includes oxidative stress, inflammation, neurotransmitter dysfunction, and mitochondrial dysfunction. Development of pharmacological treatment for persons with TBI, however, has been difficult because of the wide heterogeneity of disease and mechanisms of injury.3
Acetylcholine (ACh) is a neurotransmitter that is composed of an ester of acetic acid and choline, and it is effective in regulating plasticity and arousal among many other functions. Cholinergic neurotransmission is a crucial factor in regulation of cognitive function, specifically in learning and memory,4 as well as attention.5 Both the septo-hippocampal cholinergic and the nucleus basalis-neocortical cholinergic pathways are important components of the neural circuitry of cognition. In the septo-hippocampal pathway, neurons of the medial septum and the diagonal band of Broca innervate the hippocampus via the fimbria–fornix bundle and the supracallosal striae. The nucleus basalis consists of a collection of magnacellular cholinergic neurons in the basal forebrain that provide diffuse, predominantly ipsilateral, projections to most of the cerebral cortex. Cholinergic inputs to the medial prefrontal cortex of rats mediate attentional processing,6 and cholinergic inputs to hippocampus regulate memory consolidation.7
In neurodegenerative diseases, such as Alzheimer's disease, loss of cholinergic functions is believed to be an important contributor to cognitive deficits. Similarly, TBI induces dysregulation of the cholinergic system, and this is believed to be one of the significant underlying causes of impairment of cognitive functions. Because of ACh's major role in numerous cognitive processes, the relationship between alterations in cholinergic neurotransmission and cognitive deficits after TBI has been investigated in many studies. The role of cholinergic dysfunction after TBI leading to long-term deficits in learning and memory have been described previously.8,9
As reviewed previously,9,10 the cholinergic system exhibits an acute surge in activity leading to massive release of ACh immediately after TBI.11 Early studies documented acute cholinergic excess in cerebrospinal fluid (CSF) after TBI in humans.12,13 At later times, however, there is a persistent reduction in cholinergic function.14,15 In agreement with this, several studies have shown that TBI causes direct injury to cholinergic projections. Cholinergic neuronal loss is found in several areas of the forebrain such as the medial septal nucleus and nucleus of the diagonal band of Broca, which have major projections to the hippocampus.16,17 Human postmortem studies after TBI reported loss of ACh neurons in the nucleus basalis of Meynert, reflecting a general deficit in the cholinergic neurotramission,18 and functional imaging of the brain after TBI suggested long-term cholinergic deficits.19 Overall, the cholinergic system undergoes drastic change throughout the days to months after TBI. Therapeutically attenuating the acute injury to the cholinergic system and enhancing its function chronically has been the challenge of TBI researchers.
In this review, we will outline the alterations in various components of the cholinergic system after TBI in both animal models and human clinical studies. Pharmacological agents targeting ACh neurotransmission have been shown in many studies to attenuate cognitive deficits in both neurodegenerative diseases as well as acute injury. For example, nicotinic agonists have shown promise in Alzheimer's disease.20–22 Similarly, acetylcholinesterase (AChE) inhibitors have been shown to be beneficial in Alzheimer's disease.23,24 The therapeutic effects of cholinergic agents on cognitive function were also evidenced in studies of TBI25–29 as well as stroke.30,31
The importance of cholinergic signaling in TBI has been explored by a fair number of studies and reviews. An updated review is currently warranted, however, given the introduction of new pharmacological agents that are receptor specific and recent clinical trials. By reviewing and reinterpreting the studies that used pharmacological agents in the setting of TBI, both the cholinergic pathobiology of TBI as well as future strategies for targeting specific aspects of cholinergic deficit after TBI will be explored.
Muscarinic Receptors
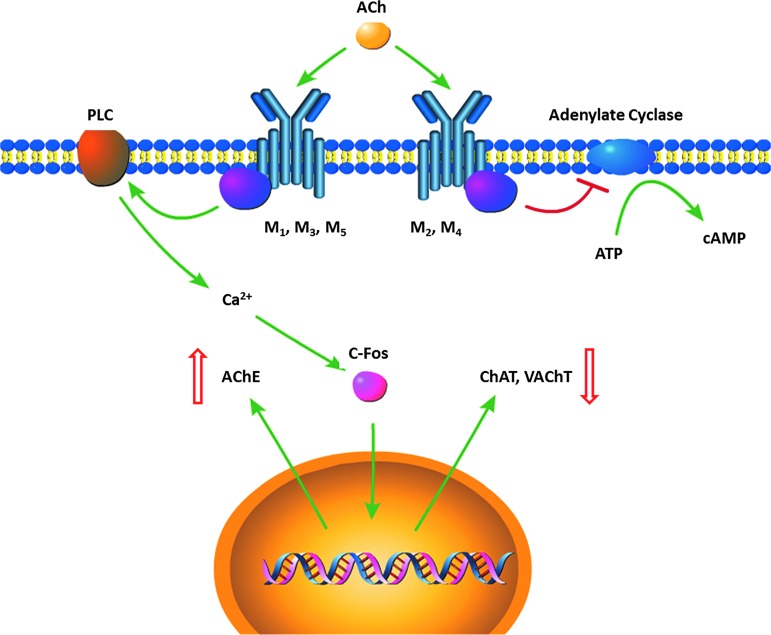
Muscarinic acetylcholine receptors (mAChR) are G-protein coupled receptors that are important for neurogenesis,32 survival of newborn neurons,33 and long-term potentiation (Fig. 1).34,35 These receptors are categorized to five subtypes (M1–M5). The M1, M3, and M5 subtypes mediate excitatory function, whereas M2 and M4 subtypes mediate inhibitory function. Of particular interest is the M2 autoreceptor, which is found presynaptically and inhibits ACh release. By activation of this receptor, ACh signaling is modulated by presynaptic feedback inhibition.
FIG. 1.
Muscarinic acetylcholine receptor (AChR) regulation of ACh regulating enzymes. Activation of M1, M3, and M5 are excitatory, leading to activation of phospholipase C (PLC), then subsequent calcium mediated C-Fos activation. This upregulates acetylcholinesterase (AChE) expression and downregulates choline acetylransferase (ChAT) and vesicular ACh transporter (vAChT) expression. Activation of M2 and M4 are inhibitory, however, and can inhibit cyclic AMP (cAMP) mediated signaling. Color image is available online at www.liebertpub.com/neu
Alterations in mAChRs after TBI
Although human postmortem study of inferior temporal gyrus tissue showed no alteration in muscarinic receptor binding,36 many animal studies showed reduction of mAChR at an early time point after injury ranging from hours to days. Newborn piglets subjected to fluid percussion injury (FPI) had reduced mAChR determined by autoradiography at 6 h after injury.37 At as early as 2 h after FPI in rats, there was decreased affinity of muscarinic receptor to positron emission tomography (PET) radiotracer in the hippocampus.38 An autoradiography study in rats also showed early decrease in mAChR affinity in the hippocampus and brainstem at 1 h.39 Studies using the controlled cortical impact (CCI) model of injury, however, showed a decrease in muscarinic receptors at slightly later time points: the earliest time of mAChR reduction was at 24 h, but no significant decrease was found at 2 h.40 Similarly, no decrease was detected between the 1–24 h interval, and earliest mAChR deficit was found at 3 days by radioligand binding.41 This difference in timeline of mAChR downregulation is possibly because of the differences in the nature of injury: FPI induces more diffuse mechanical injury compared with CCI.
Although both types of injury produce diffuse axonal injury and cortical contusion injury, CCI's more focal distribution of energy may lead to delayed deficits in the pericontusional regions. In contrast, direct injury to a wider volume of tissue by FPI may lead to earlier changes in mAChR at pericontusional areas. mAChR, however, may also show compensatory increases at later time points, because an increased number of binding sites of mAChR was reported in the hippocampus and neocortex at 15 days after injury.42
Most of these studies used PET tracer or radioactive ligand nonspecific to muscarinic receptor subtypes, but several studies indicated that this mAChR reduction may be largely because of a decrease in M2 subtype. A specific autoradiography study differentiating M1 and M2 receptors showed that while there was no change in M1 receptors, there was a significant decrease of M2 receptors in several regions of the hippocampus.43 Also, by immunohistochemistry, there was decreased M2 staining at 2 and 4 weeks after CCI in the hippocampus.44 This delayed decrease of M2 receptors in the hippocampus was also confirmed at 4 weeks after CCI by Western blot analysis. Because M2 receptors are autoreceptors inhibiting ACh release presynaptically, one possible explanation of this specific decrease is a compensatory downregulation of inhibitory autoreceptors to maintain high ACh release chronically.
Pharmacological agents targeting mAChRs
It is not clear whether these changes in mAChR are compensatory changes induced by injury or if they are direct consequences of injury. Regardless of the cause, several studies have suggested that mAChR blockade acutely after injury may be neuroprotective.45,46 TBI leads to acute elevations of ACh,47 and excessive muscarinic cholinergic receptor activation can lead to epileptic damage.48,49 In these studies, injection of cholinergic agents into limbic regions or the hippocampus induced seizure activity as well as local necrosis of the tissue.48,50 Thus, administration of scopolamine, a muscarinic antagonist, at early time points after TBI may be neuroprotective by preventing the effects of acute elevations of ACh. Early administration of scopolamine, which has equal affinity for all five mAChR subtypes,51 attenuates motor deficits,46,52 mortality, and weight loss52,53 after TBI in rats.
Another possible benefit of scopolamine after TBI is by enhancing ACh release at chronic time points after TBI by blockade of the M2 autoreceptor. Several microdialysis studies showed that scopolamine administration can evoke ACh release in the hippocampus15,54 and neocortex14 at 14 days post-injury, likely by blocking presynaptic autoreceptors. Although the effect of scopolamine on ACh release is compromised in the setting of TBI compared with sham animals,55 enhancing ACh release at chronic time points when there is decreased cholinergic activity may theoretically improve cognitive function. Thus, depending on the time frame of application, scopolamine can enhance cholinergic signaling by preventing injury (acute) or enhancing neurotransmission (chronic).
M1 vs. M2 specific agents
Similar to the effect of scopolamine administration, specific blockade of M1 receptors using dicyclomine 15 min before injury reduced motor deficits.56 In another study, dicyclomine treatment 5 min before injury attenuated the increase in spectrin breakdown products in the CSF after TBI, but it did not reduce neuronal degeneration assessed by fluoro-jade staining.57 As discussed by Cox and colleagues,57 functional deficits after TBI can occur in the absence of neuronal death. Thus, the behavioral improvement by dicyclomine treatment may not be because of preventing neuronal death but rather by preventing deficits in cholinergic neurotransmission by reversing sublethal cellular dysfunction such as deficits in long-term potentiation. Although dicyclomine did not affect the level of neuronal death, these findings from behavioral and biomarker studies support the idea that attenuating the effects of acute and excessive activation of ACh receptors (specifically M1 receptors) is neuroprotective.
Similarly, administration of muscarinic M2 autoreceptor antagonist BIBN99 improves cognitive function as tested by Morris water maze (MWM) after FPI in rats.25 Unlike the previous studies using dicyclomine, which was administered at the time of the injury, the administration of BIBN99 in this study was continuous for days after injury. Since specific block of M2 autoreceptors can cause ACh release in the neocortex and hippocampus,58,59 the mechanism of BIBN99's effect on behavioral improvement may involve enhancement of ACh release. The therapeutic effect of BIBN99, however, is likely a combination of preventing ACh receptor activation both initially as well as chronically after injury. There was a therapeutic effect when animals were injected from 24 h after TBI and during the time of the MWM task from days 11–15, but not if injections were performed just during the days of MWM task. Previously mentioned scopolamine studies also used early administration after injury to achieve neuroprotection,45,52,53 indicating that muscarinic antagonism early after injury is important in inhibiting secondary injury.
Targeting both M1 and M2 receptors further enhances the therapeutic effect. A partial M1 agonist and M2 antagonist, Lu 25-109-T, reduces TBI-induced choline acetyltransferase (ChAT) loss in the forebrain60 and improves performance in the MWM61 after TBI. This agent would theoretically activate understimulated M1 receptors while inhibiting M2 autoreceptors, thereby exogenously activating ACh receptors and amplifying endogenous ACh release at the same time. In this study, Lu 25-109-T was subcutaneously injected until 15 days post-injury, indicating that maintaining the cholinergic tone may be beneficial during the recovery phase of trauma.
In summary, the inhibition of cholinergic receptors at acute time points after TBI by using mAChR specific antagonists such as scopolamine would inhibit the damage induced by excessive ACh release. At later time points, when there is general cholinergic hypofunction, agents with agonist properties for each subtype of receptor should be used to enhance ACh signaling.
Nicotinic Receptors
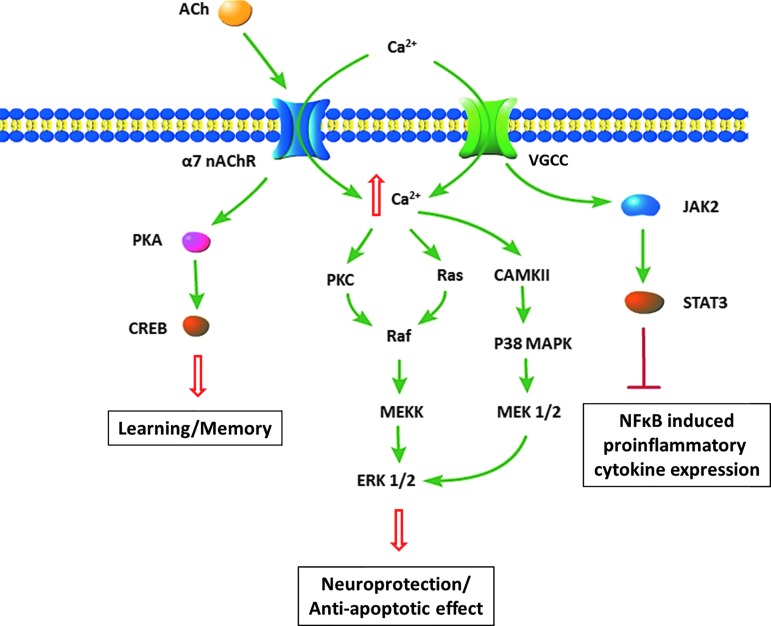
Activation of nicotinic ACh receptor (nAChR) by binding of an agonist leads to influx of Na+ and Ca2+ (Fig. 2). The degree of permeability of Ca2+ depends on the subunit composition of the nAChR, because the α7 subunit containing nAChRs has a much higher Ca2+ permeability than the α3 or α4 subunit containing receptors.62,63 This property of regulating the intracellular Ca2+ level makes it an important mediator of learning and memory, which depends on signal transduction pathways involving extracellular signal-regulated kinase 1/2 (ERK 1/2) and cAMP response element binding protein (CREB).64 In addition, Ca2+ permeability makes α7 receptors also a possible contributor to excitotoxicity, because excessive influx of Ca2+ activated by glutamate release can lead to neuronal death.
FIG. 2.
Activation of nicotinic α7 receptor and its effects. There are multiple effects of α7 receptor activation, including downstream pathways activating long-term potentiation as well as anti-apoptotic effects. In microglia, there is inhibition of pro-inflammatory cytokine expression. Color image is available online at www.liebertpub.com/neu
Alterations of nAChRs after TBI
Many previous studies have explored nAChR changes in various brain regions after TBI using autoradiography similar to studies exploring mAChR changes. Although one study reported no change in binding of [3H]epibatidine, which nonselectively binds to α3 and α4 subunit containing receptors in newborn piglets,37 other studies report significant alteration in receptor levels. A rat model of injury using [3H]epibatidine showed reduction of α3 and α4 subunits in various regions such as the thalamus, hypothalamus, olfactory tubercle, gigantocellular reticular nucleus, and motor cortex40 as well as subregions within the hippocampus.41
Similar reduction of α7 nAChR levels were reported by a decrease in [125I] α-bungarotoxin binding in newborn piglets after FPI in the CA1 region of the hippocampus, thalamus, and superior colliculus.65 This finding was also correlated with a generalized decrease in α7 nAChR levels in all the brain regions of adult rats after FPI at 2 and 24 h post-injury in the same study. Decreased binding of α7 receptors was also shown after CCI in adult rats in the hippocampus, somatosensory cortex, stratum oriens, and superior colliculus as early as 1 h to 21 days after injury.41 Compared with this, α3, α4 reduction is delayed, occurring at 72 h to 21 days.
These changes in α7 receptors have numerous implications on the mechanisms of secondary injury. Several studies showed that activation of α7 receptors attenuates release of inflammatory mediators from macrophages and microglia.66–68 Also, α7 receptors have an anti-apoptotic effect mediated by pathways involving phosphatidylinositol 3-kinase-Akt69 as well as by ERK1/2.70 Based on these studies reporting TBI induced changes in nAChR levels, many groups have used pharmacological agents targeting these receptors, aiming to improve molecular and behavioral outcomes.
Agents targeting nAChRs
Nicotine administration, which would nonspecifically activate both α7 as well as α3 and α4 containing nAChRs, improves spatial learning and memory retention71 and reverses α7 receptor deficits.72 Other studies showed therapeutic potential of agents that specifically activate α7 receptors—namely, choline. Dietary treatment using choline, a selective α7 nAChR agonist, for 2 weeks before TBI leads to improved spatial memory function, reduced cortical tissue loss, and microglial activation after TBI.73
There are multiple effects of choline, however, besides α7 nAChR activation that may aid in neurorecovery. Choline is also an intermediary in ACh formation, and its administration may enhance ACh synthesis. Accordingly, injection of a naturally occurring compound, cytidine 5'-diphosphocholine (CDP-choline), which is metabolized to form choline, attenuates TBI induced ACh surge in the hippocampus and neocortex as well as enhances spatial memory in rats.27 In addition, CDP-choline treatment can reduce hippocampal neuronal loss and cortical lesion volume.74 In a double-blind, placebo-controlled, multicenter trial using CDP-choline for 90 days,75 no significant cognitive benefit was found, however. The reasons for this lack of agreement between animal studies and human trial are unclear, but may be because of the complexity of human TBI and heterogeneity of the injury mechanism.
Choline is also a main building block for phospholipid, which is important for membrane formation, and it is released from damaged cellular membranes after TBI,76 likely because of phospholipid degradation and decreased circulatory clearance. Thus, its abundant supply after TBI by CDP-choline treatment is believed to aid in stabilization and repair of damaged membranes.77 Other mechanisms to explain the neuroprotective effects of CDP-choline have been suggested, however, such as increased levels of antioxidant glutathione,78 which reduces oxidative damage.
α7 receptor specific agents
Newer pharmacological agents with specific affinity to α7 receptors were used in various brain injury studies.79–84 In rats with intracerebral hemorrhage in the striatum, intraperitoneal injection of α7 agonist PNU-282987 reduced the number of activated microglia/macrophages and neuronal loss.82 In contrast, α4β2 specific agonist RJR-2403 did not ameliorate these neuronal losses induced by intracerebral hemorrhage, supporting the crucial role the α7 receptor has in attenuating brain injury. Because the majority of nAChRs in the striatum are composed of α4β2 and α6β2 subunits, α7 is a minority in neuronal cells. The beneficial effect of α7 activation is thus likely because of its effect on microglia, reducing inflammatory response. Similarly, PNU-282987 was neuroprotective in a subarachnoid hemorrhage model of rats.83 Its administration decreased cleaved caspase-3 and neuronal cell death. In addition, it improved neurological deficits assessed by spontaneous activity, limb movements, climbing, and various other functions.
Aside from enhancing ACh neurotransmission, attenuating inflammation, and enhancing membrane synthesis, α7 receptor activation may be important targets for improving learning and memory. The α7 receptors have high Ca2+ permeability and are known to activate ERK ½ signaling70 and CREB signaling85 pathways, which are central components of learning and memory.64 A wide variety of α7 agonists such as choline, GTS-21, SSR-180711A, and PNU-282987 are able to activate this pathway,86 and positive allosteric modulator PNU-12596 enhances this α7 receptor activation effect, which will be reviewed later. Accordingly, behavioral experiments with α7 agonist AR-R 17779 given to adult rats resulted in improved learning and memory tested in radial maze.87 These agents have not been tested in a TBI setting but could serve as potential candidates for cognitive function enhancing agents during recovery.
Alterations in Intracellular Cholinergic Enzymes after TBI
Changes in choline acetytransferase and vesicular acetylcholine transporter
Changes among the cholinergic neurons have been described in many TBI studies. A commonly used marker of cholinergic neuron is ChAT, a presynaptic enzyme for ACh synthesis and a marker of integrity of presynaptic cholinergic function and structure. Most of the regions of the brain have decrease in either ChAT levels or its activity after TBI. In rats, there is a moderate reduction of ChAT activity in the dorsal hippocampus, frontal and temporal cortices 1 h after injury (25, 32, and 23%, respectively), but there is greater than a 50% increase in ChAT activity in the parietal cortex.88
Similarly, other studies in rats after FPI showed decreased ChAT positive neurons in the basal forebrain16 and medial septal nucleus, nucleus of the diagonal band of Broca, and nucleus basalis of Meynert in 10–15 days.17
Human postmortem studies also show decrease in ChAT activity—there is a 50% reduction of ChAT activity in the inferior temporal gyrus samples after TBI.36 Reduction of ChAT activity was also found in bilateral cingulate, inferior temporal, and posterior parietal regions.89 Histological analysis of neurons in the nucleus basalis of Meynert showed significant damage in patients who died after TBI, and decreased intensity of ChAT immunoreactivity was also found in these damaged neurons (survival times 1–300 h, median=27 h).18
Such decrease in ChAT levels and activity may not only indicate cholinergic neuronal death; ChAT immunoreactivity decrease was not accompanied by changes in cresyl violet-staining in rats after TBI.60 In support of this, the decrease in ChAT immunoreactive cells after TBI in rats was only transient, and there was no significant difference by 28 days.16 The decrease in level and activity of ChAT is likely a combination of cholinergic neuronal loss as well as downregulation of the ChAT protein.
Another important regulator of ACh neurotransmission is vesicular ACh transporter (vAChT), an enzyme that is responsible for loading ACh into secretory vesicles. This enzyme also has time dependent alteration in levels after TBI: downregulation acutely, but upregulation at subacute to chronic time points. At acute time points after injury (2 h–72 h) vAChT expression is reduced in multiple brain regions including the thalamus, hypothalamus, motor cortex, and basal forebrain in adult rats.40
Starting at weeks after injury, however, there is an upregulation. Increased hippocampal vAChT protein has been reported using immunohistochemistry and Western blot at 2–4 weeks (but not at 1 day or 1 week after injury).44 At 4 weeks after injury, there is an increase in mRNA as well as protein levels of vAChT in the hippocampus.90 This increase is also present at least up to 1 year after injury in the hippocampus as well as the cortex of rats.91
Both ChAT and vAChT are crucial for the function of presynaptic ACh release. The reported alterations in these studies may reflect a combination of pathological process and compensatory changes. Given the ACh surge at immediate time points after injury, the decrease in vAChT levels may reflect compensatory downregulation to prevent excessive ACh receptor activation. Also, a direct effect of the injury leading to compromised function of presynaptic cholinergic neuronal function may underlie these changes. The increase in vAChT levels at weeks to 1 year after injury, however, correlates with the general behavioral recovery of animals at chronic time points,91 indicating the compensatory nature of this change.
AChE Changes after TBI
AChE is a crucial regulator of cholinergic neurotransmission that metabolizes acetycholine after its release in the synaptic terminals. By decreasing or increasing its function, the level of ACh activating its receptors can be changed, and thus the strength of ACh signaling is affected. Because the activity level of AChE has been shown to be associated to attention and working memory,92 there have been several efforts to characterize activity of AChE in different brain regions after TBI to understand the basis of TBI-induced cognitive deficits. Immediately after TBI, massive release of ACh and glutamate causes excitotoxic damage as well as compensatory changes in the regulators of the cholinergic system such as AChE.
Alterations in AChE after TBI
Several studies reported short-term alterations in AChE activity after TBI. Acutely after injury, basal forebrain showed an increase in AChE activity at 2–24 h, which normalized by 72 h. This increase in basal forebrain AChE activity also occurred in newborn pigs at 6 h after TBI.93 After exposure to acute stress, such as a forced swim protocol, acute ACh release in the basal forebrain leads to upregulation of AChE mRNA to possibly restore physiological levels of ACh.94 Thus, AChE expression and activity may be upregulated in the basal forebrain as a compensatory response to acutely increased cholinergic neurotransmission.
Stress from restraining also transiently increases ACh levels in the hippocampus of rats.95 In addition, cold and immobilization stress increased AChE activity in the cerebrum of rats.96 This increase in AChE activity may have an important contribution to functional losses, because transgenic mice that overexpress AChE have spatial learning and memory deficits.97 Because application of AChE inhibitors in patients with Alzheimer's disease and enhancing ACh signaling can improve cognitive function,98 this increase in AChE and subsequent decrease in ACh signaling may underlie post-TBI cognitive deficits.
Although the explanation of this AchE activity change has not been thoroughly explored by TBI researchers, a possible mechanism can be gleaned from a previous study using corticohippocampal brain slice. Application of AChE inhibitors to a corticohippocampal brain slice to increase ACh levels and subsequent ACh signaling caused alterations in cholinergic enzymes.94 AChE mRNA levels were increased, but ChAT and vAChT mRNA were decreased. These alterations occurred 20 min after increase in c-Fos levels, correlating with the expression of enzymes that regulate cholinergic signaling. This bidirectional modulation of cholinergic gene regulation was presumed to be because of the increase in c-Fos levels. The increase in c-Fos has previously been shown to occur in other studies where muscarinic agonists were administered: c-Fos was increased in frontal, cingulate, and retrosplenial cortex.99,100 Thus, after TBI, acute increase in ACh have been shown to correlate with signaling cascades leading to AChE overexpression and ChAT and vAChT underexpression via c-Fos.
Another study, however, has shown that hippocampus, hypothalamus, and motor cortex has decreased AChE activity acutely (between 2–24 h).101 AChE activity deficit was also present in the hippocampus in a blast injury model of TBI.102 AChE activity may not be regulated just by transcriptional change affecting its concentration, but it likely involves additional mechanisms such as release of soluble form of AChE, which has been reported in the setting of hypoxic damage.103 The regional differences in AChE activity may reflect different mechanisms of control, as well as different levels of cholinergic neurotransmission in these regions.
At chronic periods after TBI, there is a general hypofunction of the cholinergic system as evidenced by reduction of ACh synthesis15 and release.54 In addition, there is a general decrease in the AChE activity in several cortical areas, likely as a compensatory change for decreased ACh release. PET imaging determined the decrease in AChE activity in human subjects with TBI more than 1 year after injury, with the most prominent decrease found in parieto-occipital regions of the neocortex.104 These cholinergic deficits may be in part because of dysfunction of the basal forebrain cholinergic system, which has been demonstrated in the past.16–18
To reverse these deficits in ACh neurotransmission, cholinesterase inhibitors have been used to increase the availability of cortical ACh by inhibiting enzymatic catabolism of ACh. These agents have been well characterized and studied widely in Alzheimer's disease animal models and clinical patients. These include ENA713, rivastigmine, physostigmine, tacrine, and donepezil. These agents have various differences in central nervous system (CNS) specificity, hepatotixicity, and other peripheral side effects.
AChE inhibitor studies in animals and humans
The efficacy of physostigmine in treating cognitive deficits after TBI has been observed in many animal studies as well as patients with TBI. In rats with TBI that were given physostigmine by continuous infusion, there was an improvement in locomotor function assessed by rotarod task,105 attenuation of brain tissue loss, as well as spatial learning and memory deficit assessed by MWM.106 This study also indicated that not only AChE inhibition alone, but AChE inhibition in combination with continuous training is important for reversing cognitive deficits. Early case studies of TBI patients with physostigmine enhanced several aspects of cognitive function such as improving verbal recall and attention, as well as reducing confusion.107–109 Physostigmine also improved memory after TBI assessed by standardized neuropsychological tests.110
One possible mechanism of physostigmine induced behavioral improvement after TBI is regulation of cerebral blood flow (CBF). As previously shown by Scremin and associates,111 measurement of CBF with autoradiography techniques showed that the site of impact after TBI has decreased CBF at 2–24 h after injury. Cerebral cortex contralateral to focus of contusion was shown to have hyperemia in this study. Physostigmine reversed this decrease in CBF after TBI in the ipsilateral side and also increased CBF in the contralateral side to the trauma.
Because cholinergic activation enhances CBF in the neocortex,112 hyperemia contralateral to contusion was postulated to be because of increased ACh synthesis and turnover.76,113 Thus, the behavioral benefit of physostigmine after TBI may be because of enhancing perfusion after TBI and enhancing neurological function secondarily. Physostigmine, however, has an extremely short half-life and narrow therapeutic window. It has significant systemic side effects, such as reducing heart rate and blood pressure, which has discouraged its use in patients and led to developments of newer AChE inhibitors that have more CNS specific effects.
Another AChE inhibitor, ENA713, which diffuses mainly into the CNS, has been used in animal TBI experiments. Its administration improved reflex and motor function in a closed head injury model in rats.28 Aside from increasing neuronal cholinergic function, ENA713 reduced the disruption of the blood–brain barrier after injury and thus reduced vasogenic edema. The mechanism of this reduction in blood–brain barrier disruption has not been clarified, but this effect is also found with rivastigmine treatment, another AChE inhibitor with central specificity.
Rivastigmine ameliorates spatial memory impairments, motor deficits, and edema in a closed head injury in mice.29 This neuroprotection was dependent on both nicotinic and muscarinic receptors, because using either mecamylamine (nicotinic antagonist) or scopolamine (muscarinic antagonist) prevented neuroprotection. These studies by Chen and coworkers28,29 used one-time injection of AChE inhibitor acutely after injury (5 min–2 h). Because these agents would have inhibited AChE for a short term, the improvement in spatial learning ability was attributed to attenuation of damage in the cholinergic system, rather than increased intrasynaptic ACh levels during the days of behavioral testing.29
Rivastigmine, however, has only a very modest effect that was shown in clinical studies. A randomized, prospective, double-blind study showed no difference between the placebo and rivastigmine treated group.114 Only a subgroup analysis among moderate to severe injured patients treated with rivastigmine showed improvements in verbal learning/memory and information processing. Similarly, in an open-labeled, multicenter study that was performed as a follow-up, rivastigmine has shown mostly no benefit except in ra apid visual information processing test, a measure of sustained attention, using a subgroup analysis.115
Tetrahydroaminoacridine (tacrine) is also a centrally acting AChE inhibitor with disappointing results in the previous studies. Administration of tacrine did not improve MWM performance in rats after TBI.26 Even in sham rats, tacrine worsened MWM performance in a dose dependent manner. This result, however, may be because of the NMDA receptor antagonist property of tacrine.116 Because inhibition of NMDA receptor after TBI results in profound memory deficits,117,118 tacrine's failure to enhance memory is possibly because of its interaction with the NMDA receptors. In addition to this lack of effects, it also has poor tolerance and significant hepatic toxicity, discouraging clinical trials with this drug in patients with TBI.119
Donepezil: centrally specific agent with less systemic effects
Donepezil, a centrally acting AChE inhibitor with a much milder side effect profile than other AChE inhibitors,120 has recently gained attention as a treatment option for patients with TBI in several initial case studies more than a decade ago.121,122 The clinical use of donepezil for cognitive deficits in Alzheimer's disease and anecdotal evidence from several case reports122,123 have indicated a therapeutic effect of donepezil in improving cognitive function after TBI.
As extensively reviewed by Ballesteros and colleagues,124 the effect of donepezil was assessed by various neuropsychological tests in several small case series and case reports, as well as randomized controlled trials.124 Donepezil was shown to improve cognitive function by mini-mental status examinations122 as well as subjective improvement in vigilance and attention.125 Other tests have also shown improvements in intelligence quotient,126 visual memory,127 verbal learning and memory,128 affective-behavioral function,129 and attention and auditory/visual memory.130
Several studies, however, reported no significant change in cognitive functions after donepezil treatment.131–133 The evidence for effectiveness of donepezil was deemed uncertain because of scarcity of data and poor methodological quality of many of these studies.124 Despite its specificity to central AChE and low toxicity, more studies are needed to validate donepezil's efficacy in patients with TBI.
Among these studies using AChE inhibitors, simply increasing the intrasynaptic concentration of ACh or activation of ACh receptors may not be sufficient to result in behavioral improvements after TBI. Repeated AChE inhibitor administration may reduce ACh synthesis, because constant activation of the presynaptic M2 autoreceptors may inhibit presynaptic release of ACh.26 Also, excessive stimulation of mAChRs may lead to M1 receptor downregulation.134,135 Because normal signaling leading to cognitive benefit will involve complex and synchronous neurotransmitter release, simply increasing the absolute concentration of synaptic ACh may not be therapeutic. As such, continuous physostigmine infusion with subcutaneous osmotic pump resulted in progressive impairment of locomotor performance at higher doses, whereas a lower dose was able to improve the performance.105 Also, a higher rate of physostigmine infusion did not lead to improvement in MWM performance after TBI, but there was improvement compared with controls only at lower rate of infusion.106
With chronic treatment using high dosages, cholinergic neurotransmission may not be enhanced but instead compromised possibly because of the effects on M2 autoreceptors as well as M1 receptor downregulation. Aside from several studies having poor design or too few subjects, the heterogeneity of the effects of AChE inhibitors in human trials are also likely because of some studies using optimal doses and other using excessive doses, which compromises cholinergic signaling.
Time dependent changes in the cholinergic system: excitotoxicity and chronic traumatic encephalopathy
Time dependent changes in cholinergic signaling were mentioned in previous sections of this review. At early time points around TBI, blockade of mAChR was shown to be neuroprotective.45,46,52,53,56
Antagonists to muscarinic receptors such as scopolamine and dicyclomine were shown to reduce mortality or behavioral deficits. An important explanation of this neuroprotection is that mAChR blockade prevented cholinergic excitotoxicity. With the excessive activation of these receptors, there is depolarization and elevation of intracellular Ca2+ that leads to damaging effects to the neuron.
Cholinergic excitotoxicity may also have a role in other major pathologies of the CNS such as ischemic stroke and epilepsy. Application of AChE inhibitors or muscarinic receptor agonists can induce seizures, which can lead to neuronal injury48,136,137 suggesting a possible role of excessive cholinergic signaling in epilepsy. In addition, a major pathologic mechanism in ischemic stroke is glutamate excitotoxicity, which can in turn lead to secondary release of excessive ACh and neuronal injury.138 Although ACh excitotoxicity has been implicated in epilepsy and stroke, the role of this pathologic mechanism will need further future research.
At chronic time points, the opposite effect occurs in the cholinergic signaling, because there is a general hypofunction of the system. Reduction of scopolamine evoked ACh release54 and decrease in nAChR that contain α7, α3, and α2 subunits have been reported.40,41 These changes show that there is a degeneration of specific components of the cholinergic neuronal circuitry. After TBI, chronic traumatic encephalopathy (CTE) ensues. Thus, degeneration of specific components of cholinergic signaling (e.g., α7 nAChR) may occur in CTE. The details of which components of the cholinergic signaling pathway degenerate in CTE and the timeline of these events will need further clarification in future studies.
Future Directions: Using Novel Agents to Enhance Cholinergic Function
Future research with agents targeting the cholinergic system will show significant progress as newer agents with receptor specific agonist/antagonist properties are developed. Muscarinic agents that have receptor subtype specific properties, such as BIBN99 and dicyclomine, can be optimally used during times of recovery and rehabilitation. Agents that have a combination of agonist and antagonist properties, such as Lu 25-109-T, could also be used to inhibit M2 autoreceptors while activating M1 postsynaptic receptors. In addition, many studies are now showing cognitive enhancement and attenuation of neuroinflammation by selective targeting of α7 nAChRs. Agents such as GTS-21, SSR-180711A, AR-R17779, and PNU-282987 may be useful in future TBI studies to show cognitive enhancement. In addition, novel agents such as allosteric modulators that have even more complex function in activation of cholinergic signaling may show significant benefit.
Simple injection of nicotinic agonists into an animal may lead to nonspecific activation of cholinergic neurotransmission throughout all the regions of the brain that contain nicotinic receptors. Positive allosteric modulators (PAMs), however, cause more specific activation of cholinergic receptors by enhancing a pre-existing cholinergic signaling. These agents function by enhancing the potency of activation by endogenous nicotinic receptor activation without directly activating or desensitizing the target receptor.
There are specific advantages of enhancing cholinergic signaling by PAMs than nonspecific activation by a direct application of nicotinic agonist, as previously reviewed.139 For example, activation by α7 nAChR specific PAMs preserves the spatiotemporal patterns of endogenous α7 nAChR activation. In addition, unlike direct activation of nicotinic receptors by nicotinic agonists, which is prone to desensitization, the neurocognitive effects induced by α7 PAMs are not reduced by desensitization. Various benefits of PAMs have been demonstrated in previous studies.140–147
There are two subtypes of PAMs: Type I, which increases the peak amplitude of agonist induced response, and Type II, which increases the peak in addition to prolonging the current decay. Several of these agents have been used in either hippocampal slice study141 or behavioral studies to improve cognitive function.142,146,147 In addition, α7 nAChR PAM can reduce infarct volume143 and attenuate motor deficit 144 in a mouse model of stroke. The exact mechanism of PAM in reducing stroke-induced damage has not been clarified, and future studies are needed to clarify this.
These novel agents have not been used in the setting of TBI, but may be potential candidates for future studies given their unique advantages over cholinergic agonists. In the past, nonspecific receptor agonists have been used in an attempt to modulate the cholinergic system after TBI. Newer pharmacological developments, however, continue to provide a variety of agents that are receptor subtype specific and have varying agonist/antagonist properties. Using these agents individually or in combination may be helpful in preventing secondary injury as well as reversing chronic cognitive deficits after TBI.
Several lines of clinical and laboratory evidence demonstrate that TBI can produce acute and chronic alterations in cholinergic systems. While therapies that target the cholinergic system have met with some laboratory success, improved therapies are needed for clinical translation in the future.
Author Disclosure Statement
The authors thank The Pittsburgh Foundation Walter Copeland Fund for their support of our TBI research. No competing financial interests exist.
References
- 1.(1999). Consensus conference. Rehabilitation of persons with traumatic brain injury. NIH Consensus Development Panel on Rehabilitation of Persons With Traumatic Brain Injury. JAMA 282, 974–983 [PubMed] [Google Scholar]
- 2.Hoofien D., Gilboa A., Vakil E., and Donovick P.J. (2001). Traumatic brain injury (TBI) 10-20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj. 15, 189–209 [DOI] [PubMed] [Google Scholar]
- 3.Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., and Manley G.T., Workshop Scientific Team and Advisory Panel Members. (2008). Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 25, 719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aigner T.G. (1995). Pharmacology of memory: cholinergic-glutamatergic interactions. Curr. Opin. Neurobiol. 5, 155–160 [DOI] [PubMed] [Google Scholar]
- 5.Blokland A. (1995). Acetylcholine: a neurotransmitter for learning and memory? Brain Res. Brain Res. Rev. 21, 285–300 [DOI] [PubMed] [Google Scholar]
- 6.Gill T.M., Sarter M., and Givens B. (2000). Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J. Neurosci. 20, 4745–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasselmo M.E. (1999). Neuromodulation: acetylcholine and memory consolidation. Trends Cogn. Sci. 3, 351–359 [DOI] [PubMed] [Google Scholar]
- 8.Arciniegas D., Adler L., Topkoff J., Cawthra E., Filley C.M., and Reite M. (1999). Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 13, 1–13 [DOI] [PubMed] [Google Scholar]
- 9.Arciniegas D.B. (2003). The cholinergic hypothesis of cognitive impairment caused by traumatic brain injury. Curr. Psychiatry Rep. 5, 391–399 [DOI] [PubMed] [Google Scholar]
- 10.Hayes R.L., Stonnington H.H., Lyeth B.G., Dixon C.E., and Yamamoto T. (1986). Metabolic and neurophysiologic sequelae of brain injury: a cholinergic hypothesis. Cent. Nerv. Syst. Trauma 3, 163–173 [DOI] [PubMed] [Google Scholar]
- 11.Saija A., Hayes R.L., Lyeth B.G., Dixon C.E., Yamamoto T., and Robinson S.E. (1988). The effect of concussive head injury on central cholinergic neurons. Brain Res. 452, 303–311 [DOI] [PubMed] [Google Scholar]
- 12.Tower D.B., and McEachern D. (1949). Acetylcholine and neuronal activity; cholinesterase patterns and acetylcholine in the cerebrospinal fluids of patients with craniocerebral trauma. Can. J. Res. 27, 105–119 [DOI] [PubMed] [Google Scholar]
- 13.Tower D.B., and McEachern D. (1948). Acetylcholine and neuronal activity in craniocerebral trauma. J. Clin. Invest. 27, 558. [PubMed] [Google Scholar]
- 14.Dixon C.E., Ma X., and Marion D.W. (1997). Reduced evoked release of acetylcholine in the rodent neocortex following traumatic brain injury. Brain Res. 749, 127–130 [DOI] [PubMed] [Google Scholar]
- 15.Dixon C.E., Bao J., Johnson K.M., Yang K., Whitson J., Clifton G.L., and Hayes R.L. (1995). Basal and scopolamine-evoked release of hippocampal acetylcholine following traumatic brain injury in rats. Neurosci. Lett. 198, 111–114 [DOI] [PubMed] [Google Scholar]
- 16.Leonard J.R., Maris D.O., and Grady M.S. (1994). Fluid percussion injury causes loss of forebrain choline acetyltransferase and nerve growth factor receptor immunoreactive cells in the rat. J. Neurotrauma 11, 379–392 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt R.H., and Grady M.S. (1995). Loss of forebrain cholinergic neurons following fluid-percussion injury: implications for cognitive impairment in closed head injury. J. Neurosurg. 83, 496–502 [DOI] [PubMed] [Google Scholar]
- 18.Murdoch I., Nicoll J.A., Graham D.I., and Dewar D. (2002). Nucleus basalis of Meynert pathology in the human brain after fatal head injury. J. Neurotrauma 19, 279–284 [DOI] [PubMed] [Google Scholar]
- 19.Huang M.X., Yurgil K.A., Robb A., Angeles A., Diwakar M., Risbrough V.B., Nichols S.L., McLay R., Theilmann R.J., Song T., Huang C.W., Lee R.R., and Baker D.G. (2014). Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. NeuroImage Clin. 5, 408–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahakian B., Jones G., Levy R., Gray J., and Warburton D. (1989). The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br. J. Psychiatry 154, 797–800 [DOI] [PubMed] [Google Scholar]
- 21.Jones G.M., Sahakian B.J., Levy R., Warburton D.M., and Gray J.A. (1992). Effects of acute subcutaneous nicotine on attention, information processing and short-term memory in Alzheimer's disease. Psychopharmacology 108, 485–494 [DOI] [PubMed] [Google Scholar]
- 22.Wilson A.L., Langley L.K., Monley J., Bauer T., Rottunda S., McFalls E., Kovera C., and McCarten J.R. (1995). Nicotine patches in Alzheimer's disease: pilot study on learning, memory, and safety. Pharmacol. Biochem. Behav. 51, 509–514 [DOI] [PubMed] [Google Scholar]
- 23.Rosler M., Anand R., Cicin-Sain A., Gauthier S., Agid Y., Dal-Bianco P., Stahelin H.B., Hartman R., and Gharabawi M. (1999). Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ 318, 633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knapp M.J., Knopman D.S., Solomon P.R., Pendlebury W.W., Davis C.S., and Gracon S.I. (1994). A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. The Tacrine Study Group. JAMA 271, 985–991 [PubMed] [Google Scholar]
- 25.Pike B.R., and Hamm R.J. (1995). Post-injury administration of BIBN 99, a selective muscarinic M2 receptor antagonist, improves cognitive performance following traumatic brain injury in rats. Brain Res. 686, 37–43 [DOI] [PubMed] [Google Scholar]
- 26.Pike B.R., Hamm R.J., Temple M.D., Buck D.L., and Lyeth B.G. (1997). Effect of tetrahydroaminoacridine, a cholinesterase inhibitor, on cognitive performance following experimental brain injury. J. Neurotrauma 14, 897–905 [DOI] [PubMed] [Google Scholar]
- 27.Dixon C.E., Ma X., and Marion D.W. (1997). Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J. Neurotrauma 14, 161–169 [DOI] [PubMed] [Google Scholar]
- 28.Chen Y., Shohami E., Bass R., and Weinstock M. (1998). Cerebro-protective effects of ENA713, a novel acetylcholinesterase inhibitor, in closed head injury in the rat. Brain Res. 784, 18–24 [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., Shohami E., Constantini S., and Weinstock M. (1998). Rivastigmine, a brain-selective acetylcholinesterase inhibitor, ameliorates cognitive and motor deficits induced by closed-head injury in the mouse. J. Neurotrauma 15, 231–237 [DOI] [PubMed] [Google Scholar]
- 30.Paolucci S., Bureca I., Multari M., Nocentini U., and Matano A. (2010). An open-label pilot study of the use of rivastigmine to promote functional recovery in patients with unilateral spatial neglect due to first ischemic stroke. Funct. Neurol. 25, 195–200 [PubMed] [Google Scholar]
- 31.Whyte E.M., Lenze E.J., Butters M., Skidmore E., Koenig K., Dew M.A., Penrod L., Mulsant B.H., Pollock B.G., Cabacungan L., Reynolds C.F., 3rd, and Munin M.C. (2008). An open-label pilot study of acetylcholinesterase inhibitors to promote functional recovery in elderly cognitively impaired stroke patients. Cerebrovasc. Dis. 26, 317–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Kampen J.M.– and Eckman C.B. (2010). Agonist-induced restoration of hippocampal neurogenesis and cognitive improvement in a model of cholinergic denervation. Neuropharmacology 58, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotani S., Yamauchi T., Teramoto T. and Ogura H. (2006). Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience 142, 505–514 [DOI] [PubMed] [Google Scholar]
- 34.Auerbach J.M., and Segal M. (1996). Muscarinic receptors mediating depression and long-term potentiation in rat hippocampus. J. Physiol. 492, 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgard E.C., and Sarvey J.M. (1990). Muscarinic receptor activation facilitates the induction of long-term potentiation (LTP) in the rat dentate gyrus. Neurosci. Lett. 116, 34–39 [DOI] [PubMed] [Google Scholar]
- 36.Dewar D., and Graham D.I. (1996). Depletion of choline acetyltransferase activity but preservation of M1 and M2 muscarinic receptor binding sites in temporal cortex following head injury: a preliminary human postmortem study. J. Neurotrauma 13, 181–187 [DOI] [PubMed] [Google Scholar]
- 37.Donat C.K., Walter B., Deuther-Conrad W., Wenzel B., Nieber K., Bauer R., and Brust P. (2010). Alterations of cholinergic receptors and the vesicular acetylcholine transporter after lateral fluid percussion injury in newborn piglets. Neuropathol. Appl. Neurobiol. 36, 225–236 [DOI] [PubMed] [Google Scholar]
- 38.Sihver S., Marklund N., Hillered L., Langstrom B., Watanabe Y., and Bergstrom M. (2001). Changes in mACh, NMDA and GABA(A) receptor binding after lateral fluid-percussion injury: in vitro autoradiography of rat brain frozen sections. J. Neurochem. 78, 417–423 [DOI] [PubMed] [Google Scholar]
- 39.Lyeth B.G., Jiang J.Y., Delahunty T.M., Phillips L.L., and Hamm R.J. (1994). Muscarinic cholinergic receptor binding in rat brain following traumatic brain injury. Brain Res. 640, 240–245 [DOI] [PubMed] [Google Scholar]
- 40.Donat C.K., Schuhmann M.U., Voigt C., Nieber K., Deuther-Conrad W., and Brust P. (2008). Time-dependent alterations of cholinergic markers after experimental traumatic brain injury. Brain Res. 1246, 167–177 [DOI] [PubMed] [Google Scholar]
- 41.Verbois S.L., Scheff S.W. and Pauly J.R. (2002). Time-dependent changes in rat brain cholinergic receptor expression after experimental brain injury. J. Neurotrauma 19, 1569–1585 [DOI] [PubMed] [Google Scholar]
- 42.Jiang J.Y., Lyeth B.G., Delahunty T.M., Phillips L.L., and Hamm R.J. (1994). Muscarinic cholinergic receptor binding in rat brain at 15 days following traumatic brain injury. Brain Res. 651, 123–128 [DOI] [PubMed] [Google Scholar]
- 43.DeAngelis M.M., Hayes R.L., and Lyeth B.G. (1994). Traumatic brain injury causes a decrease in M2 muscarinic cholinergic receptor binding in the rat brain. Brain Res. 653, 39–44 [DOI] [PubMed] [Google Scholar]
- 44.Ciallella J.R., Yan H.Q., Ma X., Wolfson B.M., Marion D.W., DeKosky S.T., and Dixon C.E. (1998). Chronic effects of traumatic brain injury on hippocampal vesicular acetylcholine transporter and M2 muscarinic receptor protein in rats. Exp. Neurol. 152, 11–19 [DOI] [PubMed] [Google Scholar]
- 45.Lyeth B.G., Ray M., Hamm R.J., Schnabel J., Saady J.J., Poklis A., Jenkins L.W., Gudeman S.K., and Hayes R.L. (1992). Postinjury scopolamine administration in experimental traumatic brain injury. Brain Res. 569, 281–286 [DOI] [PubMed] [Google Scholar]
- 46.Lyeth B.G., Liu S., and Hamm R.J. (1993). Combined scopolamine and morphine treatment of traumatic brain injury in the rat. Brain Res. 617, 69–75 [DOI] [PubMed] [Google Scholar]
- 47.Lyeth B.G., Jiang J.Y., Robinson S.E., Guo H., and Jenkins L.W. (1993). Hypothermia blunts acetylcholine increase in CSF of traumatically brain injured rats. Mol. Chem. Neuropathol. 18, 247–256 [DOI] [PubMed] [Google Scholar]
- 48.Olney J.W., de Gubareff T., and Labruyere J. (1983). Seizure-related brain damage induced by cholinergic agents. Nature 301, 520–522 [DOI] [PubMed] [Google Scholar]
- 49.Turski W.A., Czuczwar S.J., Kleinrok Z., and Turski L. (1983). Cholinomimetics produce seizures and brain damage in rats. Experientia 39, 1408–1411 [DOI] [PubMed] [Google Scholar]
- 50.Turski W.A., Cavalheiro E.A., Turski L., and Kleinrok Z. (1983). Intrahippocampal bethanechol in rats: behavioural, electroencephalographic and neuropathological correlates. Behav. Brain Res. 7, 361–370 [DOI] [PubMed] [Google Scholar]
- 51.Billard W., Binch H., 3rd, Crosby G., and McQuade R.D. (1995). Identification of the primary muscarinic autoreceptor subtype in rat striatum as m2 through a correlation of in vivo microdialysis and in vitro receptor binding data. J. Pharmacol. Exp. Ther. 273, 273–279 [PubMed] [Google Scholar]
- 52.Lyeth B.G., Dixon C.E., Jenkins L.W., Hamm R.J., Alberico A., Young H.F., Stonnington H.H., and Hayes R.L. (1988). Effects of scopolamine treatment on long-term behavioral deficits following concussive brain injury to the rat. Brain Res. 452, 39–48 [DOI] [PubMed] [Google Scholar]
- 53.Lyeth B.G., Dixon C.E., Hamm R.J., Jenkins L.W., Young H.F., Stonnington H.H., and Hayes R.L. (1988). Effects of anticholinergic treatment on transient behavioral suppression and physiological responses following concussive brain injury to the rat. Brain Res. 448, 88–97 [DOI] [PubMed] [Google Scholar]
- 54.Dixon C.E., Bao J., Long D.A., and Hayes R.L. (1996). Reduced evoked release of acetylcholine in the rodent hippocampus following traumatic brain injury. Pharmacol. Biochem. Behav. 53, 679–686 [DOI] [PubMed] [Google Scholar]
- 55.Dixon C.E., Hamm R.J., Taft W.C., and Hayes R.L. (1994). Increased anticholinergic sensitivity following closed skull impact and controlled cortical impact traumatic brain injury in the rat. J. Neurotrauma 11, 275–287 [DOI] [PubMed] [Google Scholar]
- 56.Robinson S.E., Foxx S.D., Posner M.G., Martin R.M., Davis T.R., Guo H.Z., and Enters E.K. (1990). The effect of M1 muscarinic blockade on behavior and physiological responses following traumatic brain injury in the rat. Brain Res. 511, 141–148 [DOI] [PubMed] [Google Scholar]
- 57.Cox C.D., West E.J., Liu M.C., Wang K.K., Hayes R.L., and Lyeth B.G. (2008). Dicyclomine, an M1 muscarinic antagonist, reduces biomarker levels, but not neuronal degeneration, in fluid percussion brain injury. J. Neurotrauma 25, 1355–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoss W., Messer W.S., Jr., Monsma F.J., Jr., Miller M.D., Ellerbrock B.R., Scranton T., Ghodsi-Hovsepian S., Price M.A., Balan S., Mazloum Z., et al. (1990). Biochemical and behavioral evidence for muscarinic autoreceptors in the CNS. Brain Res. 517, 195–201 [DOI] [PubMed] [Google Scholar]
- 59.Lapchak P.A., Araujo D.M., Quirion R., and Collier B. (1989). Binding sites for [3H]AF-DX 116 and effect of AF-DX 116 on endogenous acetylcholine release from rat brain slices. Brain Res. 496, 285–294 [DOI] [PubMed] [Google Scholar]
- 60.Pike B.R., and Hamm R.J. (1997). Chronic administration of a partial muscarinic M1 receptor agonist attenuates decreases in forebrain choline acetyltransferase immunoreactivity following experimental brain trauma. Exp. Neurol. 147, 55–65 [DOI] [PubMed] [Google Scholar]
- 61.Pike B.R., and Hamm R.J. (1997). Activating the posttraumatic cholinergic system for the treatment of cognitive impairment following traumatic brain injury. Pharmacol. Biochem. Behav. 57, 785–791 [DOI] [PubMed] [Google Scholar]
- 62.Dani J.A. (2001). Overview of nicotinic receptors and their roles in the central nervous system. Biol. Psychiatry 49, 166–174 [DOI] [PubMed] [Google Scholar]
- 63.Burnashev N. (1998). Calcium permeability of ligand-gated channels. Cell calcium 24, 325–332 [DOI] [PubMed] [Google Scholar]
- 64.Thomas G.M., and Huganir R.L. (2004). MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 5, 173–183 [DOI] [PubMed] [Google Scholar]
- 65.Hoffmeister P.G., Donat C.K., Schuhmann M.U., Voigt C., Walter B., Nieber K., Meixensberger J., Bauer R., and Brust P. (2011). Traumatic brain injury elicits similar alterations in alpha7 nicotinic receptor density in two different experimental models. Neuromolecular Med. 13, 44–53 [DOI] [PubMed] [Google Scholar]
- 66.Carnevale D., De Simone R., and Minghetti L. (2007). Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrenergic systems. CNS Neurol. Disord. Drug Targets 6, 388–397 [DOI] [PubMed] [Google Scholar]
- 67.Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C.J., and Tracey K.J. (2003). Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388 [DOI] [PubMed] [Google Scholar]
- 68.Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., Al-Abed Y., Wang H., Metz C., Miller E.J., Tracey K.J., and Ulloa L. (2004). Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10, 1216–1221 [DOI] [PubMed] [Google Scholar]
- 69.Takada-Takatori Y., Kume T., Sugimoto M., Katsuki H., Sugimoto H., and Akaike A. (2006). Acetylcholinesterase inhibitors used in treatment of Alzheimer's disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology 51, 474–486 [DOI] [PubMed] [Google Scholar]
- 70.Ren K., Puig V., Papke R.L., Itoh Y., Hughes J.A., and Meyer E.M. (2005). Multiple calcium channels and kinases mediate alpha7 nicotinic receptor neuroprotection in PC12 cells. J. Neurochem. 94, 926–933 [DOI] [PubMed] [Google Scholar]
- 71.Verbois S.L., Hopkins D.M., Scheff S.W., and Pauly J.R. (2003). Chronic intermittent nicotine administration attenuates traumatic brain injury-induced cognitive dysfunction. Neuroscience 119, 1199–1208 [DOI] [PubMed] [Google Scholar]
- 72.Verbois S.L., Scheff S.W., and Pauly J.R. (2003). Chronic nicotine treatment attenuates alpha 7 nicotinic receptor deficits following traumatic brain injury. Neuropharmacology 44, 224–233 [DOI] [PubMed] [Google Scholar]
- 73.Guseva M.V., Hopkins D.M., Scheff S.W., and Pauly J.R. (2008). Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J. Neurotrauma 25, 975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dempsey R.J., and Raghavendra Rao V.L. (2003). Cytidinediphosphocholine treatment to decrease traumatic brain injury-induced hippocampal neuronal death, cortical contusion volume, and neurological dysfunction in rats. J. Neurosurg. 98, 867–873 [DOI] [PubMed] [Google Scholar]
- 75.Zafonte R.D., Bagiella E., Ansel B.M., Novack T.A., Friedewald W.T., Hesdorffer D.C., Timmons S.D., Jallo J., Eisenberg H., Hart T., Ricker J.H., Diaz-Arrastia R., Merchant R.E., Temkin N.R., Melton S., and Dikmen S.S. (2012). Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA 308, 1993–2000 [DOI] [PubMed] [Google Scholar]
- 76.Scremin O.U., Li M.G., Roch M., Booth R., and Jenden D.J. (2006). Acetylcholine and choline dynamics provide early and late markers of traumatic brain injury. Brain Res. 1124, 155–166 [DOI] [PubMed] [Google Scholar]
- 77.Adibhatla R.M., and Hatcher J.F. (2002). Citicoline mechanisms and clinical efficacy in cerebral ischemia. J. Neurosci. Res. 70, 133–139 [DOI] [PubMed] [Google Scholar]
- 78.Barrachina M., Dominguez I., Ambrosio S., Secades J., Lozano R., and Ferrer I. (2003). Neuroprotective effect of citicoline in 6-hydroxydopamine-lesioned rats and in 6-hydroxydopamine-treated SH-SY5Y human neuroblastoma cells. J. Neurol. Sci. 215, 105–110 [DOI] [PubMed] [Google Scholar]
- 79.Van Kampen M., Selbach K., Schneider R., Schiegel E., Boess F. and Schreiber R. (2004). AR-R 17779 improves social recognition in rats by activation of nicotinic alpha7 receptors. Psychopharmacology 172, 375–383 [DOI] [PubMed] [Google Scholar]
- 80.Cannon C.E., Puri V., Vivian J.A., Egbertson M.S., Eddins D., and Uslaner J.M. (2013). The nicotinic alpha7 receptor agonist GTS-21 improves cognitive performance in ketamine impaired rhesus monkeys. Neuropharmacology 64, 191–196 [DOI] [PubMed] [Google Scholar]
- 81.Jones K.M., McDonald I.M., Bourin C., Olson R.E., Bristow L.J., and Easton A. (2014). Effect of alpha7 nicotinic acetylcholine receptor agonists on attentional set-shifting impairment in rats. Psychopharmacology 231, 673–683 [DOI] [PubMed] [Google Scholar]
- 82.Hijioka M., Matsushita H., Ishibashi H., Hisatsune A., Isohama Y. and Katsuki H. (2012). alpha7 Nicotinic acetylcholine receptor agonist attenuates neuropathological changes associated with intracerebral hemorrhage in mice. Neuroscience 222, 10–19 [DOI] [PubMed] [Google Scholar]
- 83.Duris K., Manaenko A., Suzuki H., Rolland W.B., Krafft P.R., and Zhang J.H. (2011). alpha7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke 42, 3530–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng Q., and Yakel J.L. (2014). Presynaptic alpha7 nicotinic acetylcholine receptors enhance hippocampal mossy fiber glutamatergic transmission via PKA activation. J. Neurosci. 34, 124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roman J., Ritzenthaler J.D., Gil-Acosta A., Rivera H.N., and Roser-Page S. (2004). Nicotine and fibronectin expression in lung fibroblasts: implications for tobacco-related lung tissue remodeling. FASEB J. 18, 1436–1438 [DOI] [PubMed] [Google Scholar]
- 86.Gubbins E.J., Gopalakrishnan M., and Li J. (2010). Alpha7 nAChR-mediated activation of MAP kinase pathways in PC12 cells. Brain Res. 1328, 1–11 [DOI] [PubMed] [Google Scholar]
- 87.Levin E.D., Bettegowda C., Blosser J., and Gordon J. (1999). AR-R17779, and alpha7 nicotinic agonist, improves learning and memory in rats. Behav. Pharmacol. 10, 675–680 [DOI] [PubMed] [Google Scholar]
- 88.Gorman L.K., Fu K., Hovda D.A., Murray M., and Traystman R.J. (1996). Effects of traumatic brain injury on the cholinergic system in the rat. J. Neurotrauma 13, 457–463 [DOI] [PubMed] [Google Scholar]
- 89.Murdoch I., Perry E.K., Court J.A., Graham D.I., and Dewar D. (1998). Cortical cholinergic dysfunction after human head injury. J. Neurotrauma 15, 295–305 [DOI] [PubMed] [Google Scholar]
- 90.Shao L., Ciallella J.R., Yan H.Q., Ma X., Wolfson B.M., Marion D.W., Dekosky S.T., and Dixon C.E. (1999). Differential effects of traumatic brain injury on vesicular acetylcholine transporter and M2 muscarinic receptor mRNA and protein in rat. J. Neurotrauma 16, 555–566 [DOI] [PubMed] [Google Scholar]
- 91.Dixon C.E., Kochanek P.M., Yan H.Q., Schiding J.K., Griffith R.G., Baum E., Marion D.W., and DeKosky S.T. (1999). One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma 16, 109–122 [DOI] [PubMed] [Google Scholar]
- 92.Bohnen N.I., Kaufer D.I., Hendrickson R., Ivanco L.S., Lopresti B., Davis J.G., Constantine G., Mathis C.A., Moore R.Y., and DeKosky S.T. (2005). Cognitive correlates of alterations in acetylcholinesterase in Alzheimer's disease. Neurosci. Lett. 380, 127–132 [DOI] [PubMed] [Google Scholar]
- 93.Donat C.K., Walter B., Kayser T., Deuther-Conrad W., Schliebs R., Nieber K., Bauer R., Hartig W., and Brust P. (2010). Effects of lateral fluid percussion injury on cholinergic markers in the newborn piglet brain. Int. J. Dev. Neurosci. 28, 31–38 [DOI] [PubMed] [Google Scholar]
- 94.Kaufer D., Friedman A., Seidman S. and Soreq H. (1998). Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 393, 373–377 [DOI] [PubMed] [Google Scholar]
- 95.Imperato A., Puglisi-Allegra S., Casolini P., and Angelucci L. (1991). Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res. 538, 111–117 [DOI] [PubMed] [Google Scholar]
- 96.Tsakiris S., and Kontopoulos A.N. (1993). Time changes in Na+,K(+)-ATPase, Mg(++)-ATPase, and acetylcholinesterase activities in the rat cerebrum and cerebellum caused by stress. Pharmacol. Biochem. Behav. 44, 339–342 [DOI] [PubMed] [Google Scholar]
- 97.Beeri R., Andres C., Lev-Lehman E., Timberg R., Huberman T., Shani M., and Soreq H. (1995). Transgenic expression of human acetylcholinesterase induces progressive cognitive deterioration in mice. Curr. Biol. 5, 1063–1071 [DOI] [PubMed] [Google Scholar]
- 98.Farlow M.R., and Evans R.M. (1998). Pharmacologic treatment of cognition in Alzheimer's dementia. Neurology 51, Suppl 1, S36–S44, S65–S67 [DOI] [PubMed] [Google Scholar]
- 99.Bucci D.J., Rosen D.L., and Gallagher M. (1998). Effects of age on pilocarpine-induced c-fos expression in rat hippocampus and cortex. Neurobiol. Aging 19, 227–232 [DOI] [PubMed] [Google Scholar]
- 100.Hughes P., and Dragunow M. (1993). Muscarinic receptor-mediated induction of Fos protein in rat brain. Neurosci. Lett. 150, 122–126 [DOI] [PubMed] [Google Scholar]
- 101.Donat C.K., Schuhmann M.U., Voigt C., Nieber K., Schliebs R., and Brust P. (2007). Alterations of acetylcholinesterase activity after traumatic brain injury in rats. Brain Inj. 21, 1031–1037 [DOI] [PubMed] [Google Scholar]
- 102.Valiyaveettil M., Alamneh Y., Oguntayo S., Wei Y., Wang Y., Arun P., and Nambiar M.P. (2012). Regional specific alterations in brain acetylcholinesterase activity after repeated blast exposures in mice. Neurosci. Lett. 506, 141–145 [DOI] [PubMed] [Google Scholar]
- 103.Bond C.E., Patel P., Crouch L., Tetlow N., Day T., Abu-Hayyeh S., Williamson C., and Greenfield S.A. (2006). Astroglia up-regulate transcription and secretion of ‘readthrough’ acetylcholinesterase following oxidative stress. Eur. J. Neurosci. 24, 381–386 [DOI] [PubMed] [Google Scholar]
- 104.Ostberg A., Virta J., Rinne J.O., Oikonen V., Luoto P., Nagren K., Arponen E., and Tenovuo O. (2011). Cholinergic dysfunction after traumatic brain injury: preliminary findings from a PET study. Neurology 76, 1046–1050 [DOI] [PubMed] [Google Scholar]
- 105.Holschneider D.P., Guo Y., Roch M., Norman K.M., and Scremin O.U. (2011). Acetylcholinesterase inhibition and locomotor function after motor-sensory cortex impact injury. J. Neurotrauma 28, 1909–1919 [DOI] [PubMed] [Google Scholar]
- 106.Scremin O.U., Norman K.M., Roch M., Holschneider D.P., and Scremin A.M. (2012). Acetylcholinesterase inhibition interacts with training to reverse spatial learning deficits after cortical impact injury. J. Neurotrauma 29, 2457–2464 [DOI] [PubMed] [Google Scholar]
- 107.Eames P., and Sutton A. (1995). Protracted post-traumatic confusional state treated with physostigmine. Brain Inj. 9, 729–734 [DOI] [PubMed] [Google Scholar]
- 108.Goldberg E., Gerstman L.J., Mattis S., Hughes J.E., Sirio C.A., and Bilder R.M., Jr. (1982). Selective effects of cholinergic treatment on verbal memory in posttraumatic amnesia. J. Clin. Neuropsychol. 4, 219–,234 [DOI] [PubMed] [Google Scholar]
- 109.Levin H.S., Peters B.H., Kalisky Z., High W.M., Jr., von Laufen A., Eisenberg H.M., Morrison D.P., and Gary H.E., Jr. (1986). Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent. Nerv. Syst. Trauma 3, 333–342 [DOI] [PubMed] [Google Scholar]
- 110.Cardenas D.D., McLean A., Jr., Farrell-Roberts L., Baker L., Brooke M., and Haselkorn J. (1994). Oral physostigmine and impaired memory in adults with brain injury. Brain Inj. 8, 579–587 [DOI] [PubMed] [Google Scholar]
- 111.Scremin O.U., Li M.G., and Jenden D.J. (1997). Cholinergic modulation of cerebral cortical blood flow changes induced by trauma. J. Neurotrauma 14, 573–586 [DOI] [PubMed] [Google Scholar]
- 112.Scremin O.U., Rovere A.A., Raynald A.C., and Giardini A. (1973). Cholinergic control of blood flow in the cerebral cortex of the rat. Stroke 4, 233–239 [PubMed] [Google Scholar]
- 113.Scremin O.U., and Jenden D.J. (1996). Cholinergic control of cerebral blood flow in stroke, trauma and aging. Life Sci. 58, 2011–2018 [DOI] [PubMed] [Google Scholar]
- 114.Silver J.M., Koumaras B., Chen M., Mirski D., Potkin S.G., Reyes P., Warden D., Harvey P.D., Arciniegas D., Katz D.I. and Gunay I. (2006). Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology 67, 748–755 [DOI] [PubMed] [Google Scholar]
- 115.Silver J.M., Koumaras B., Meng X., Potkin S.G., Reyes P.F., Harvey P.D., Katz D.I., Gunay I., and Arciniegas D.B. (2009). Long-term effects of rivastigmine capsules in patients with traumatic brain injury. Brain Inj. 23, 123–132 [DOI] [PubMed] [Google Scholar]
- 116.Albin R.L., Young A.B., and Penney J.B. (1988). Tetrahydro-9-aminoacridine (THA) interacts with the phencyclidine (PCP) receptor site. Neurosci. Lett. 88, 303–307 [DOI] [PubMed] [Google Scholar]
- 117.Hamm R.J., Pike B.R., O'Dell D.M., and Lyeth B.G. (1994). Traumatic brain injury enhances the amnesic effect of an NMDA antagonist in rats. J. Neurosurg. 81, 267–271 [DOI] [PubMed] [Google Scholar]
- 118.Hamm R.J., O'Dell D.M., Pike B.R., and Lyeth B.G. (1993). Cognitive impairment following traumatic brain injury: the effect of pre- and post-injury administration of scopolamine and MK-801. Brain Res. Cogn. Brain Res. 1, 223–226 [DOI] [PubMed] [Google Scholar]
- 119.Soares J.C., and Gershon S. (1995). THA—historical aspects, review of pharmacological properties and therapeutic effects. Dementia 6, 225–234 [DOI] [PubMed] [Google Scholar]
- 120.Whitlock J.A., Jr. (1999). Brain injury, cognitive impairment, and donepezil. J. Head Trauma Rehabil. 14, 424–427 [DOI] [PubMed] [Google Scholar]
- 121.Taverni J.P., Seliger G., and Lichtman S.W. (1998). Donepezil medicated memory improvement in traumatic brain injury during post acute rehabilitation. Brain Inj. 12, 77–80 [DOI] [PubMed] [Google Scholar]
- 122.Bourgeois J.A., Bahadur N., and Minjares S. (2002). Donepezil for cognitive deficits following traumatic brain injury: a case report. J. Neuropsychiatry Clin. Neurosci. 14, 463–464 [DOI] [PubMed] [Google Scholar]
- 123.Foster M., and Spiegel D.R. (2008). Use of donepezil in the treatment of cognitive impairments of moderate traumatic brain injury. J. Neuropsychiatry Clin Neurosci. 20, 106. [DOI] [PubMed] [Google Scholar]
- 124.Ballesteros J., Guemes I., Ibarra N., and Quemada J.I. (2008). The effectiveness of donepezil for cognitive rehabilitation after traumatic brain injury: a systematic review. The J. Head Trauma Rehabil. 23, 171–180 [DOI] [PubMed] [Google Scholar]
- 125.Tenovuo O. (2005). Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury-clinical experience in 111 patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 61–67 [DOI] [PubMed] [Google Scholar]
- 126.Whelan F.J., Walker M.S., and Schultz S.K. (2000). Donepezil in the treatment of cognitive dysfunction associated with traumatic brain injury. Ann. Clin. Psychiatry 12, 131–135 [DOI] [PubMed] [Google Scholar]
- 127.Morey C.E., Cilo M., Berry J. and Cusick C. (2003). The effect of Aricept in persons with persistent memory disorder following traumatic brain injury: a pilot study. Brain Inj. 17, 809–815 [DOI] [PubMed] [Google Scholar]
- 128.Trovato M., Slomine B., Pidcock F., and Christensen J. (2006). The efficacy of donepezil hydrochloride on memory functioning in three adolescents with severe traumatic brain injury. Brain Inj. 20, 339–343 [DOI] [PubMed] [Google Scholar]
- 129.Khateb A., Ammann J., Annoni J.M., and Diserens K. (2005). Cognition-enhancing effects of donepezil in traumatic brain injury. Eur. Neurol. 54, 39–45 [DOI] [PubMed] [Google Scholar]
- 130.Zhang L., Plotkin R.C., Wang G., Sandel M.E., and Lee S. (2004). Cholinergic augmentation with donepezil enhances recovery in short-term memory and sustained attention after traumatic brain injury. Arch. Phys. Med. Rehabil. 85, 1050–1055 [DOI] [PubMed] [Google Scholar]
- 131.Masanic C.A., Bayley M.T., VanReekum R. and Simard M. (2001). Open-label study of donepezil in traumatic brain injury. Arch. Phys. Med. Rehabil. 82, 896–901 [DOI] [PubMed] [Google Scholar]
- 132.Walker W., Seel R., Gibellato M., Lew H., Cornis-Pop M., Jena T., and Silver T. (2004). The effects of Donepezil on traumatic brain injury acute rehabilitation outcomes. Brain Inj. 18, 739–750 [DOI] [PubMed] [Google Scholar]
- 133.Kaye N.S., Townsend J.B., 3rd, and Ivins R. (2003). An open-label trial of donepezil (aricept) in the treatment of persons with mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 15, 383–385 [DOI] [PubMed] [Google Scholar]
- 134.Marks M.J., Artman L.D., Patinkin D.M., and Collins A.C. (1981). Cholinergic adaptations to chronic oxotremorine infusion. J. Pharmacol Exp. Ther. 218, 337–343 [PubMed] [Google Scholar]
- 135.Ben-Barak J., Gazit H., Silman I., and Dudai Y. (1981). In vivo modulation of the number of muscarinic receptors in rat brain by cholinergic ligands. Eur. J. Pharmacol. 74, 73–81 [DOI] [PubMed] [Google Scholar]
- 136.Turski L., Ikonomidou C., Turski W.A., Bortolotto Z.A., and Cavalheiro E.A. (1989). Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse 3, 154–171 [DOI] [PubMed] [Google Scholar]
- 137.Savolainen K., Hirvonen M.R., and Naarala J. (1994). Phosphoinositide second messengers in cholinergic excitotoxicity. Neurotoxicology 15, 493–502 [PubMed] [Google Scholar]
- 138.Tsuda K., Tsuda S., Goldstein M., Nishio I., and Masuyama Y. (1996). Glutamatergic regulation of [3H]acetylcholine release in striatal slices of normotensive and spontaneously hypertensive rats. Neurochem. Int. 29, 231–237 [DOI] [PubMed] [Google Scholar]
- 139.Uteshev V.V. (2014). The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur. J. Pharmacol. 727, 181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomsen M.S., El-Sayed M., and Mikkelsen J.D. (2011). Differential immediate and sustained memory enhancing effects of alpha7 nicotinic receptor agonists and allosteric modulators in rats. PloS One 6, e27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dinklo T., Shaban H., Thuring J.W., Lavreysen H., Stevens K.E., Zheng L., Mackie C., Grantham C., Vandenberk I., Meulders G., Peeters L., Verachtert H., De Prins E., and Lesage A.S. (2011). Characterization of 2-[[4-fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol (JNJ-1930942), a novel positive allosteric modulator of the {alpha}7 nicotinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 336, 560–574 [DOI] [PubMed] [Google Scholar]
- 142.Faghih R., Gopalakrishnan S.M., Gronlien J.H., Malysz J., Briggs C.A., Wetterstrand C., Ween H., Curtis M.P., Sarris K.A., Gfesser G.A., El-Kouhen R., Robb H.M., Radek R.J., Marsh K.C., Bunnelle W.H., and Gopalakrishnan M. (2009). Discovery of 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744) as a novel positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor. J. Med. Chem. 52, 3377–3384 [DOI] [PubMed] [Google Scholar]
- 143.Kalappa B.I., Sun F., Johnson S.R., Jin K., and Uteshev V.V. (2013). A positive allosteric modulator of alpha7 nAChRs augments neuroprotective effects of endogenous nicotinic agonists in cerebral ischaemia. Br. J. Pharmacol. 169, 1862–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun F., Jin K., and Uteshev V.V. (2013). A type-II positive allosteric modulator of alpha7 nAChRs reduces brain injury and improves neurological function after focal cerebral ischemia in rats. PloS One 8, e73581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McLean S.L., Idris N.F., Grayson B., Gendle D.F., Mackie C., Lesage A.S., Pemberton D.J., and Neill J.C. (2012). PNU-120596, a positive allosteric modulator of alpha7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine-induced cognitive deficit in the attentional set-shifting task in female rats. J. Psychopharmacol. 26, 1265–1270 [DOI] [PubMed] [Google Scholar]
- 146.Callahan P.M., Hutchings E.J., Kille N.J., Chapman J.M., and Terry A.V., Jr. (2013). Positive allosteric modulator of alpha7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology 67, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hurst R.S., Hajos M., Raggenbass M., Wall T.M., Higdon N.R., Lawson J.A., Rutherford-Root K.L., Berkenpas M.B., Hoffmann W.E., Piotrowski D.W., Groppi V.E., Allaman G., Ogier R., Bertrand S., Bertrand D., and Arneric S.P. (2005). A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J. Neurosci. 25, 4396–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]