Abstract
We assessed exercise performance, coronary blood flow and cardiac reserve of female ApoE/LDLR−/− mice with advanced atherosclerosis compared with age-matched, wild-type C57BL6/J mice. Exercise capacity was assessed as whole body maximal oxygen consumption (V’O2max), maximum running velocity (vmax) and maximum distance (DISTmax) during treadmill exercise. Cardiac systolic and diastolic function in basal conditions and in response to dobutamine (mimicking exercise-induced cardiac stress) were assessed by Magnetic Resonance Imaging (MRI) in vivo. Function of coronary circulation was assessed in isolated perfused hearts. In female ApoE/LDLR−/− mice V’O2max, vmax and DISTmax were not impaired as compared with C57BL6/J mice. Cardiac function at rest and systolic and diastolic cardiac reserve were also preserved in female ApoE/LDLR−/− mice as evidenced by preserved fractional area change and similar fall in systolic and end diastolic area after dobutamine. Moreover, endothelium-dependent responses of coronary circulation induced by bradykinin (Bk) and acetylcholine (ACh) were preserved, while endothelium-independent responses induced by NO-donors were augmented in female ApoE/LDLR−/− mice. Basal COX-2-dependent production of 6-keto-PGF1α was increased. Concluding, we suggest that robust compensatory mechanisms in coronary circulation involving PGI2- and NO-pathways may efficiently counterbalance coronary atherosclerosis-induced impairment in V’O2max and exercise capacity.
Atherosclerosis is a major cause of death worldwide (for review see)1. It is linked to development of endothelial dysfunction, lipid retention in subendothelial space of arteries and vascular inflammation that leads to intravascular thrombosis and its clinical consequences such as coronary artery disease2. Atherosclerotic changes are not only present in disease states (i.e. coronary heart disease, peripheral arterial disease or type 2 diabetes), but also accompany physiological human aging and may be clinically silent for long periods of time3.
Atherosclerosis usually is associated with worse exercise capacity in men4,5,6. Furthermore, impaired exercise tolerance was reported to be a prognostic tool to predict the development of atherosclerotic changes in patients7,8,9. In single knockout ApoE−/− mice, representing the most frequently used murine model of spontaneous atherosclerosis10, exercise capacity was also impaired compared to healthy mice11,12,13,14. Moreover, cardiac structure and function in ApoE−/− mice was altered15,16, including reduction of their cardiac contractility reserve after dobutamine stimulation17. On the other hand, single knockout LDL−/− mice covered distances comparable to their wild-type counterparts when accommodated in cages equipped with wheels that suggested their everyday exercise capacity was not compromised (please compare daily covered distances in Lightfoot et al.18 and O’Bryan et al.19).
Double ApoE−/− and LDLR−/− knockouts constitute a reliable model of human atherosclerosis that displays some advantages over single ApoE−/− and LDLR−/− knockouts20,21. Namely, in ApoE/LDLR−/− mice plasma concentration of low density lipoprotein cholesterol fraction is very high, and spontaneous atherosclerosis develops more rapidly compared to ApoE−/− mice. Moreover, a western diet is not needed to induce atherosclerosis as in LDLR−/− mice. Furthermore, arterial plaques become advanced atherosclerotic plaques earlier and resemble those observed in humans22. Surprisingly, even though ApoE/LDLR−/− mice develop intensive coronary atherosclerosis and some authors report higher susceptibility of their hearts to hypoxic stress23,24, no reports published so far provide evidence of impaired cardiac function in ApoE/LDLR−/− mice25. Furthermore, there are no data in the literature regarding exercise capacity and cardiac haemodynamic response to exercise in ApoE/LDLR−/− mice. Therefore, we examined whole body maximal oxygen uptake (V’O2max) and exercise running capacity, as well as NO- and PGI2-dependent function of the coronary circulation of female ApoE/LDLR−/− vs C57BL6/J mice in relationship to atherosclerosis development. We also measured basal cardiac function and dobutamine-stimulated cardiac performance that mimics exercise-induced haemodynamic changes in 6–8-month old female ApoE/LDLR−/− mice with advanced atherosclerosis as compared with age-matched C57BL6/J mice.
Results
Exercise capacity of female ApoE/LDLR−/− and C57BL6/J mice
To measure effects of atherosclerosis development on exercise capacity of female ApoE/LDLR−/− mice we measured whole body maximal oxygen consumption (V’O2max), maximal velocity (vmax) achieved and maximal distance (DISTmax) covered during incremental test for V’O2max measurement by young 3-month old female ApoE/LDLR−/− mice with early atherosclerosis and older 6–8-month old female ApoE/LDLR−/− mice with advanced atherosclerosis26 as compared with age-matched control C57BL6/J mice.
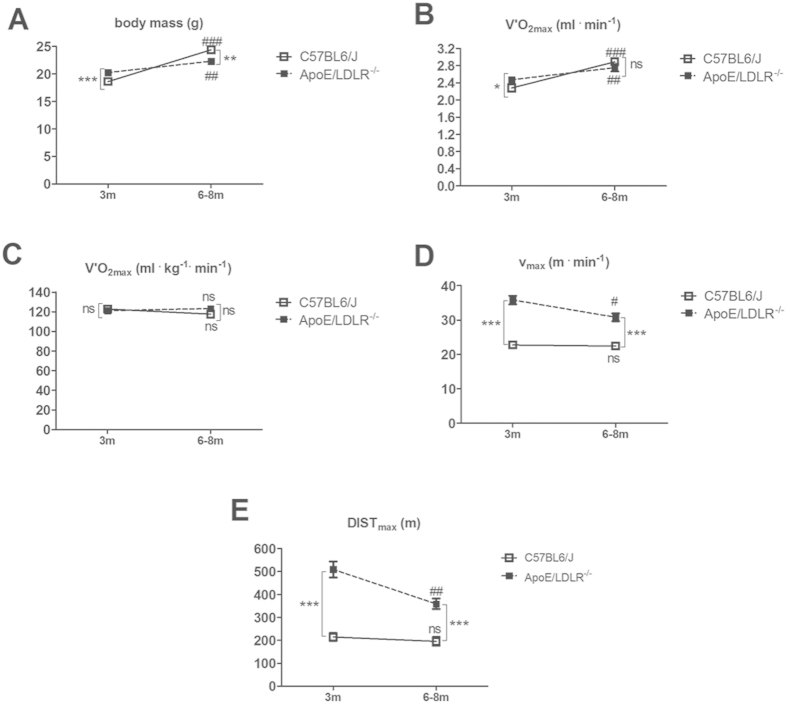
Body mass of young female ApoE/LDLR−/− mice was higher than control C57BL6/J mice while for older female ApoE/LDLR−/− and C57BL6/J mice we found the opposite (Fig. 1A) and it increased with age in both mouse strains. Therefore, the data regarding V’O2max are presented both in absolute (Fig. 1B) and relative values (Fig. 1C). There was no difference in V’O2max expressed in relative values between the mouse strains (Fig. 1C). However, when expressed in absolute values, changes in V’O2max reflected changes in body weight (please compare Fig. 1A,B).
Figure 1. Running performance in female ApoE/LDLR−/− mice and C57BL6/J mice.
(A) Body mass, (B,C) whole body maximal oxygen consumption (V’O2max) during incremental test, (D) maximal velocity (vmax) and (E) maximal distance (DISTmax) in running incremental test in 3-month (3m) and 6–8-month (6-8m) old ApoE/LDLR−/− and C57BL6/J mice. Statistical analysis was performed using two-sided t-test or non-parametric Mann-Whitney test depending on the results of D’Agostino and Pearson omnibus normality test. *indicates statistically significant difference between C57BL6/J and ApoE/LDLR−/− mice, #indicates statistically significant age-dependent difference within the strain. *,#P < 0.05, ##P < 0.01, ***,###P < 0.001; n = 16–20 for 3-month old C57BL6/J mice, n = 16–19 for 3-month old ApoE/LDLR−/− mice, n = 9–11 for 6–8-month old C57BL6/J mice and n = 11 for 6–8-month old ApoE/LDLR−/− mice. Data are presented as the mean ± SEM.
Surprisingly, maximal velocities (vmax) achieved by female ApoE/LDLR−/− were higher than for age-matched C57BL6/J mice (35.84 m·min−1 vs 22.8 ± 0.84 m·min−1, n = 19–20, P < 0.0001 for younger female ApoE/LDLR−/− and C57BL6/J mice, respectively, and 30.82 ± 1.12 m·min−1 vs 22.45 ± 0.97 m·min−1, n = 11, P < 0.0001 for older female ApoE/LDLR−/− and C57BL6/J mice, respectively) (Fig. 1D). Similarly, maximal distances (DISTmax) covered by female ApoE/LDLR−/− and C57BL6/J mice were higher for female ApoE/LDLR−/− mice than for age-matched C57BL6/J mice (509.2 ± 34.97 m vs 214.8 ± 0.8417.15 m, n = 19–20, P < 0.0001 for younger female ApoE/LDLR−/− and C57BL6/J mice, respectively, and 359.4 ± 22.9 m vs 195.9 ± 19.04 m, n = 11, P < 0.0001 for older female ApoE/LDLR−/− and C57BL6/J mice, respectively) (Fig. 1E). Interestingly, vmax and DISTmax declined with age only in female ApoE/LDLR−/− mice but not in C57BL6/J mice (35.84 m·min−1 for younger vs 30.82 ± 1.12 m·min−1 for older female ApoE/LDLR−/− mice, n = 11−19, P = 0.0118 and 509.2 ± 34.97 m for younger vs 359.4 ± 22.9 m for older female ApoE/LDLR−/− mice, n = 11, P = 0.0052, respectively) probably due to atherosclerosis progression (Fig. 1D,E).
Measurement of blood count revealed that both younger and older female ApoE/LDLR−/− mice had higher number of circulating erythrocytes when compared with age-matched C57BL6/J mice (Table 1). However, number of erythrocytes declined with age in female ApoE/LDLR−/− mice what corresponded with age-dependent decline in vmax and DISTmax covered by female ApoE/LDLR−/− mice in the incremental test for V’O2max measurement (Fig. 1D,E).
Table 1. Blood count.
| C57BL6/J 3m | ApoE/LDLR−/− 3m | C57BL6/J 6-8m | ApoE/LDLR−/− 6-8m | |
|---|---|---|---|---|
| WBC [K·μl−1] | 1.77 ± 0.28(n = 10) | 3.66 ± 1.31(n = 5) | 1.80 ± 0.23(n = 7) | 2.48 ± 0.47(n = 5) |
| LYM% [%] | 78.89 ± 0.5(n = 9) | 71.88 ± 3.42*(n = 5) | 73.74 ± 2.61# (n = 8) | 73.00 ± 1.93(n = 5) |
| MON% [%] | 5.98 ± 0.3(n = 9) | 8.72 ± 0.83*(n = 5) | 6.49 ± 0.53(n = 8) | 7.32 ± 0.68(n = 5) |
| GRA% [%] | 15.13 ± 0.32(n = 9) | 19.26 ± 2.85(n = 5) | 19.77 ± 2.57(n = 7) | 19.68 ± 2.07(n = 5) |
| RBC [M·μl−1] | 10.36 ± 0.12(n = 10) | 11.51 ± 0.019***(n = 5) | 9.85 ± 0.38(n = 8) | 10.83 ± 0.18*##(n = 6) |
| HGB [g·dL−1] | 15.08 ± 0.26(n = 10) | 17.14 ± 0.14**(n = 5) | 14.42 ± 0.49(n = 5) | 15.28 ± 0.42#(n = 4) |
| HCT [%] | 54.61 ± 0.65(n = 10) | 63.48 ± 0.31**(n = 5) | 52.30 ± 1.49(n = 8) | 57.13 ± 1.46*##(n = 6) |
| PLT [K·μl−1] | 902 ± 97.09(n = 10) | 895.4 ± 35.27(n = 5) | 1202 ± 67.88#(n = 7) | 1089 ± 62.90#(n = 6) |
WBC, white blood cells (LYM% (% of lymphocytes), MON% (% of monocytes), GRA% (% of granulocytes); RBC, red blood cells; HGB, haemoglobin; HCT, haematocrit; PLT, platelets count in young 3-month old (3m) and older 6-8-month old (6-8m) C57BL6/J and ApoE/LDLR−/− mice. Statistical analysis was performed using Mann-Whitney test based on the results of D’Agostino and Pearson omnibus normality test. Data are presented as mean ± SEM. *indicates statistically significant difference between female C57BL6/J and ApoE/LDLR−/− mice, #indicates statistically significant age-dependent difference within the strain. *,#P < 0.05, **,##P < 0.01, ***P < 0.001.
NO- and PGI2-dependent function of coronary circulation in female ApoE/LDLR−/− and C57BL6/J mice
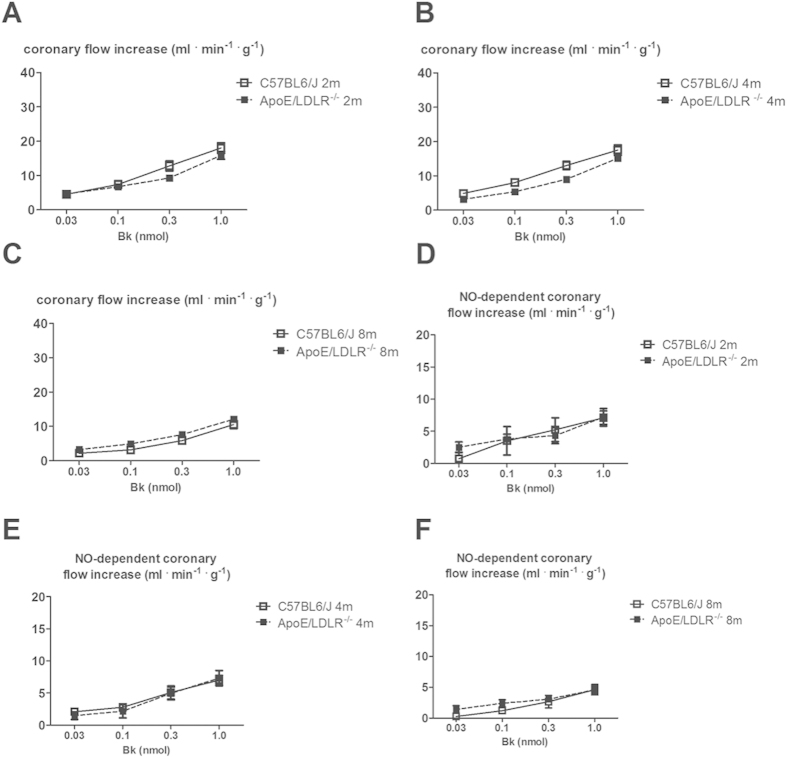
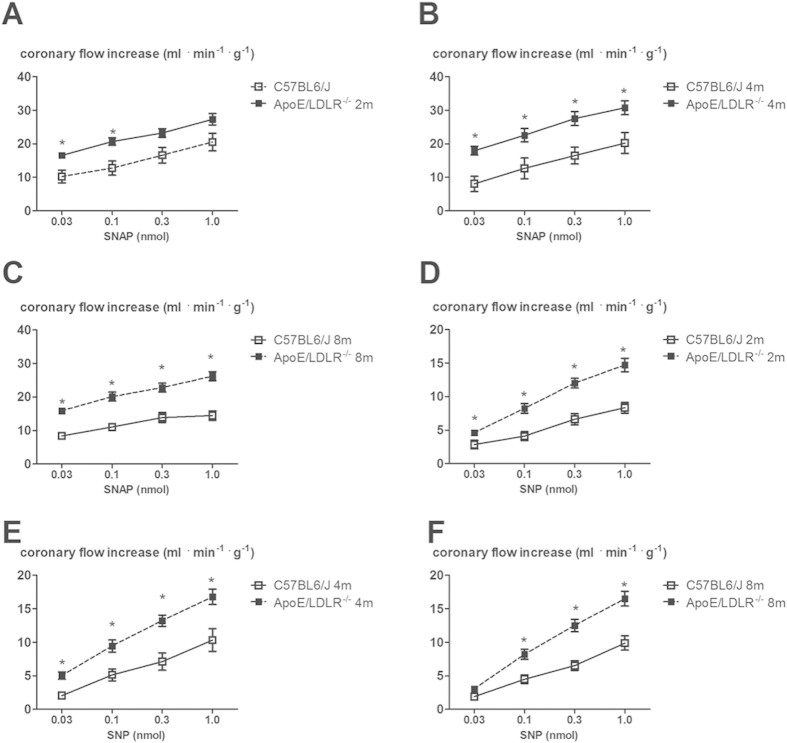
We previously showed that in mice, in contrast to humans27 and guinea pigs28, acetylcholine-induced response in coronary circulation was mediated mainly by prostacyclin (PGI2)29 whereas Bk-induced response was mediated to a large extent by NO. In this study we found that, in spite of age-dependent peripheral atherosclerosis progression described earlier26 and coronary atherosclerosis progression described in this paper, changes in coronary flow induced by Bk (0.03, 0.1, 0.3, 1.0 nmol) in isolated hearts from female ApoE/LDLR−/− mice were not impaired compared with age-matched C57BL6/J mice (Fig. 2A–C). Moreover, the inhibitory effect of L-NAME on the changes in coronary flow after Bk in C57BL6/J and in female ApoE/LDLR−/− mice hearts was similar (Fig. 2D–F). Interestingly, SNAP and SNP-induced coronary flow responses were augmented in 2, 4 and 8-month old female ApoE/LDLR−/− mice compared with age-matched C57BL6/J mice (Fig. 3).
Figure 2. Bk–induced vasodilation responses in the coronary circulation of isolated heart in female ApoE/LDLR−/− mice.
(A–C) Preservation of coronary flow responses evoked by Bk in the isolated hearts of 2, 4 and 8-month old ApoE/LDLR−/− mice compared with age-matched C57BL6/J mice; n = 11 for 2-month old C57BL6/J mice, n = 13 for 2-month old ApoE/LDLR−/− mice, n = 14 for 4-month old C57BL6/J mice and n = 10 for 4-month old ApoE/LDLR−/− mice, n = 9 for 8-month old C57BL6/J mice and n = 18 for 8-month old ApoE/LDLR−/− mice. (D–F) NO-dependent part of coronary flow responses evoked by Bk in the isolated heart of ApoE/LDLR−/− mice compared with controls subjected to L-NAME (500 μM) treatment; n = 6 for 2-month old C57BL6/J mice, n = 6 for 2-month old ApoE/LDLR−/− mice, n = 7 for 4-month old C57BL6/J mice and n = 8 for 4-month old ApoE/LDLR−/− mice, n = 5 for 8-month old C57BL6/J mice and n = 8 for 8-month old ApoE/LDLR−/− mice. Statistical analysis was performed using t-test or non-parametric Mann-Whitney test, when assumptions were not completed. Data are presented as the mean ± SEM.
Figure 3. SNAP and SNP–induced vasodilation responses in the coronary circulation of isolated heart in female ApoE/LDLR−/− mice.
(A–C) Up-regulation of SNAP-induced coronary flow response in the isolated hearts of in 2, 4 and 8-month old ApoE/LDLR−/− mice compared with age-matched C57BL6/J mice; n = 12 for 2-month old C57BL6/J mice, n = 7 for 2-month old ApoE/LDLR−/− mice, n = 6 for 4-month old C57BL6/J mice and n = 9 for 4-month old ApoE/LDLR−/− mice, n = 7 for 8-month old C57BL6/J mice and n = 13 for 8-month old ApoE/LDLR−/− mice. (D–F) SNP-induced coronary flow response in isolated hearts from ApoE/LDLR−/− mice compared with age-matched C57BL6/J mice; n = 7 for 2-month old C57BL6/J mice, n = 14 for 2-month old ApoE/LDLR−/− mice, n = 5 for 4-month old C57BL6/J mice and n = 14 for 4-month old ApoE/LDLR−/− mice, n = 10 for 8-month old C57BL6/J mice and n = 15 for 8-month old ApoE/LDLR−/− mice. Statistical analysis was performed using t-test or non-parametric Mann-Whitney test, when assumptions were not completed. Data are presented as the mean ± SEM.
ACh-induced coronary flow response in 2, 4 and 8-month old female ApoE/LDLR−/− mice was not impaired (Fig. 4A), but ACh-induced 6-keto-PGF1α release into cardiac effluent increased (18.18 ± 12.58 mg·ml−1 .ml·min−1 for 8-month old female ApoE/LDLR−/− mice, n = 11, compared to 4.99 ± 2.65 mg·ml−1.ml·min−1 for controls, n = 15, P < 0.05, Fig. 4B). It is worth to note that 6-keto-PGF1α release into cardiac effluent tended to be higher also for 2 and 4-month old female ApoE/LDLR−/− mice compared with control mice but the difference did not reach statistical significance. Basal 6-keto-PGF1α production in the hearts of female ApoE/LDLR−/− mice was also increased (238.6 ± 193.10 mg·ml−1.ml·min−1 for 8 months old female ApoE/LDLR−/− mice, n = 9, compared to 41.3 ± 17.02 mg·ml−1·ml·min−1 for controls, n = 11, P < 0.05) (Fig. 4C) and the difference in PGI2 production between female ApoE/LDLR−/− and C57BL6/J mice was abolished by COX-2 selective inhibitor, rofecoxib.
Figure 4. PGI2-dependent coronary vasodilation in the coronary circulation of isolated heart in female ApoE/LDLR−/− mice.
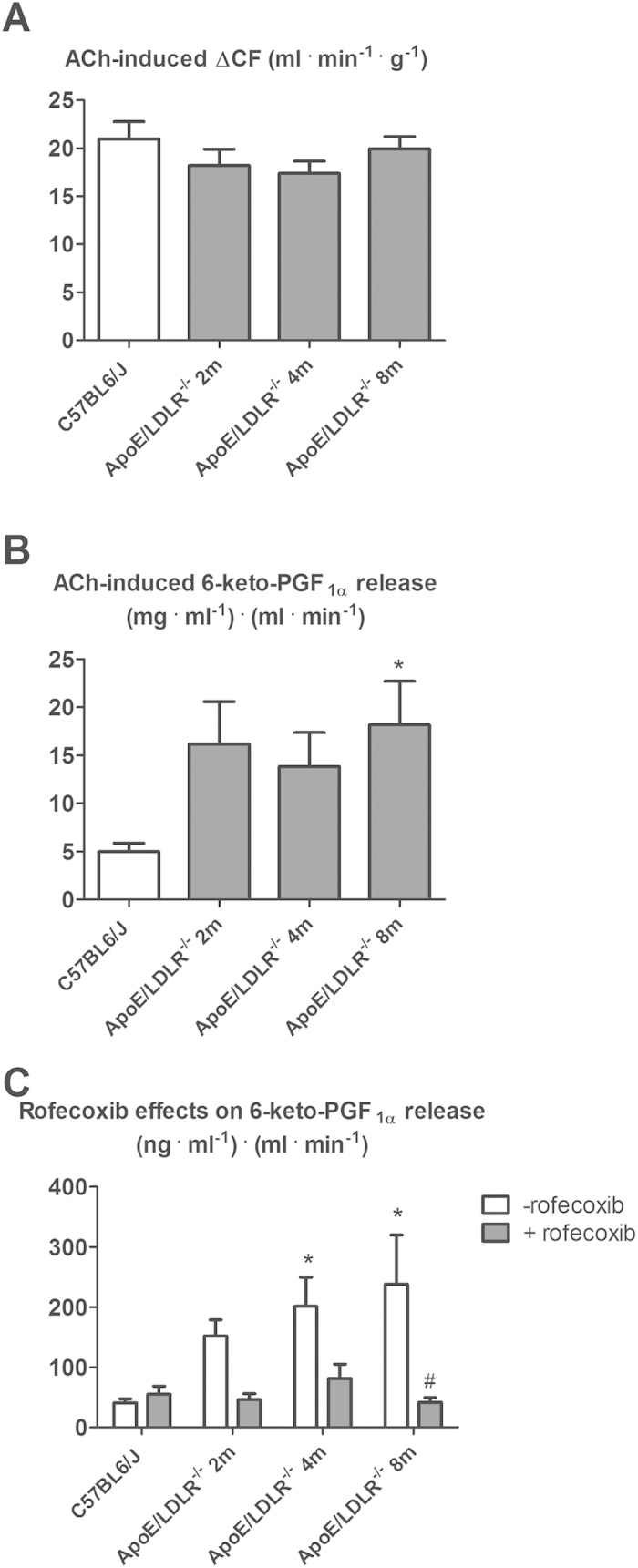
(A) PGI2-dependent coronary flow response evoked by ACh (0.3 nmol) in isolated hearts of ApoE/LDLR−/− mice compared with control C57BL6/J mice. Since there was no statistically significant difference in Ach—induced changes in coronary blood flow between 2, 4 and 8-month old C57BL6/J mice, the results for C57BL6/J mice were averaged; n = 25 for C57BL6/J mice, n = 23 for 2-month old ApoE/LDLR−/− mice, n = 16 for 4-month old ApoE/LDLR−/− mice, n = 22 for 8-month old ApoE/LDLR−/− mice. (B) ACh-induced 6-keto-PGF1α release in isolated heart from ApoE/LDLR−/− mice compared with age-matched C57BL6/J mice. Since there was no statistically significant difference in Ach—induced changes of 6-keto-PGF1α release between 2, 4 and 8-month old C57BL6/J mice, the results for C57BL6/J mice were averaged; n = 15 for C57BL6/J mice, n = 7 for 2-month old ApoE/LDLR−/− mice, n = 9 for 4-month old ApoE/LDLR−/− mice, n = 11 for 8-month old ApoE/LDLR−/− mice. (C) Effects of COX-2 inhibitor (rofecoxib, 10 μM) on basal 6-keto-PGF1α production in the isolated heart of ApoE/LDLR−/− mice. Since there was no statistically significant difference in changes of 6-keto-PGF1α release between 2, 4 and 8-month old C57BL6/J mice (basal and after rofecoxib treatment), the results for C57BL6/J mice were averaged; n = 11 and 5 for C57BL6/J mice, n = 8 and 6 for 2-month old ApoE/LDLR−/− mice, n = 9 and 6 for 4-month old ApoE/LDLR−/− mice, n = 9 and 6 for 8-month old ApoE/LDLR−/− mice with respect to basal and after-rofecoxib 6-keto-PGF1α release. Statistical analysis was performed using t-test or non-parametric Mann-Whitney test (if the assumptions were not completed). *indicates P < 0.05 for ApoE/LDLR−/− vs. control mice. #indicates P < 0.05 with rofecoxib vs. without. Data are presented as the mean ± SEM.
Since there was no statistically significant difference in Ach—induced changes in coronary flow, Ach—induced changes in 6-keto-PGF1α release and changes in 6-keto-PGF1α release (basal and after rofecoxib treatment) between 2, 4 and 8-month old C57BL6/J mice, the results for C57BL6/J mice were averaged.
Atherosclerotic lesions in coronary arteries of female ApoE/LDLR−/− mice
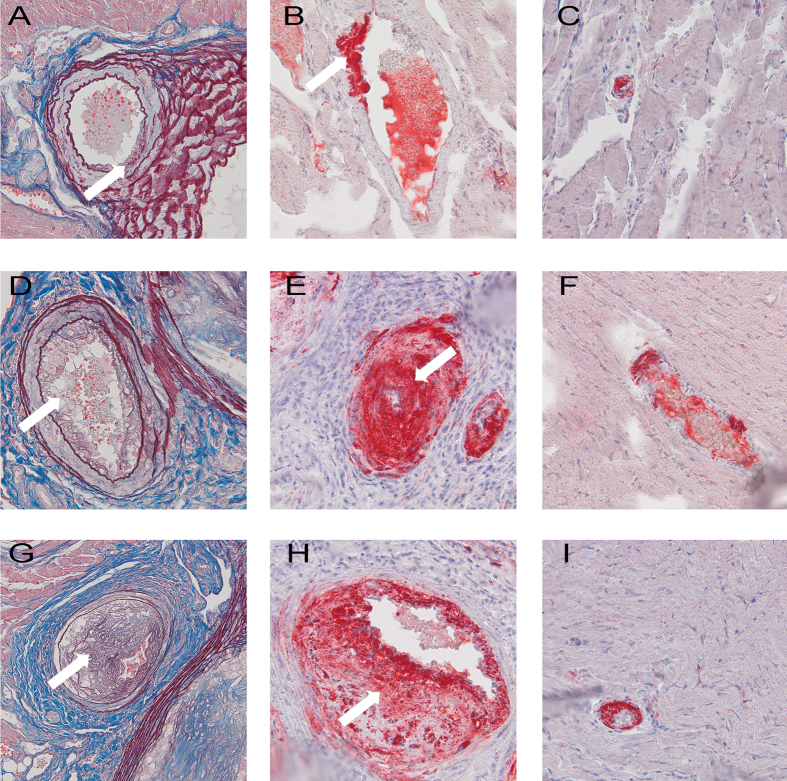
Histological analysis of atherosclerotic plaques in coronary circulation of 2, 4 and 6–8 month old female ApoE/LDLR−/− mice revealed that in initial parts of large coronary arteries branching from the aorta (proximal) age-related development of atherosclerotic lesions was found (A and B, D and E, G and H, respectively) whereas small intramuscular coronary arteries (distal) (Fig. 5C,F,I) displayed advanced atherosclerotic lesions largely occluding the vessel lumen. The middle parts of the coronary arteries were less affected (results not shown).
Figure 5. Coronary atherosclerosis in coronary arteries of female ApoE/LDLR−/− mice.
Large coronary arteries with atherosclerotic plaques in 2-month old (A), 4-month old (D) and 6–8-month old (G) female ApoE/LDLR−/− mice and their lipid components ((B,E,H), respectively); small atherosclerotic lesions are only occasionally found in large coronary arteries of 2-month old ApoE/LDLR−/− mice (A,B) but become evident in older mice (D,E,G,H) (white arrows). The lumen of small intramuscular coronary arteries of 2, 4 and 6–8month old ApoE/LDLR−/− mice ((C,F,I), respectively) is almost completely occluded by atherosclerotic plaque. OMSB staining was used to depict the morphology of atherosclerotic plaques (A,D,G) and ORO was used to visualise significant lipid deposits within the plaques (B,C,E,F,H,I). Magnification 400×.
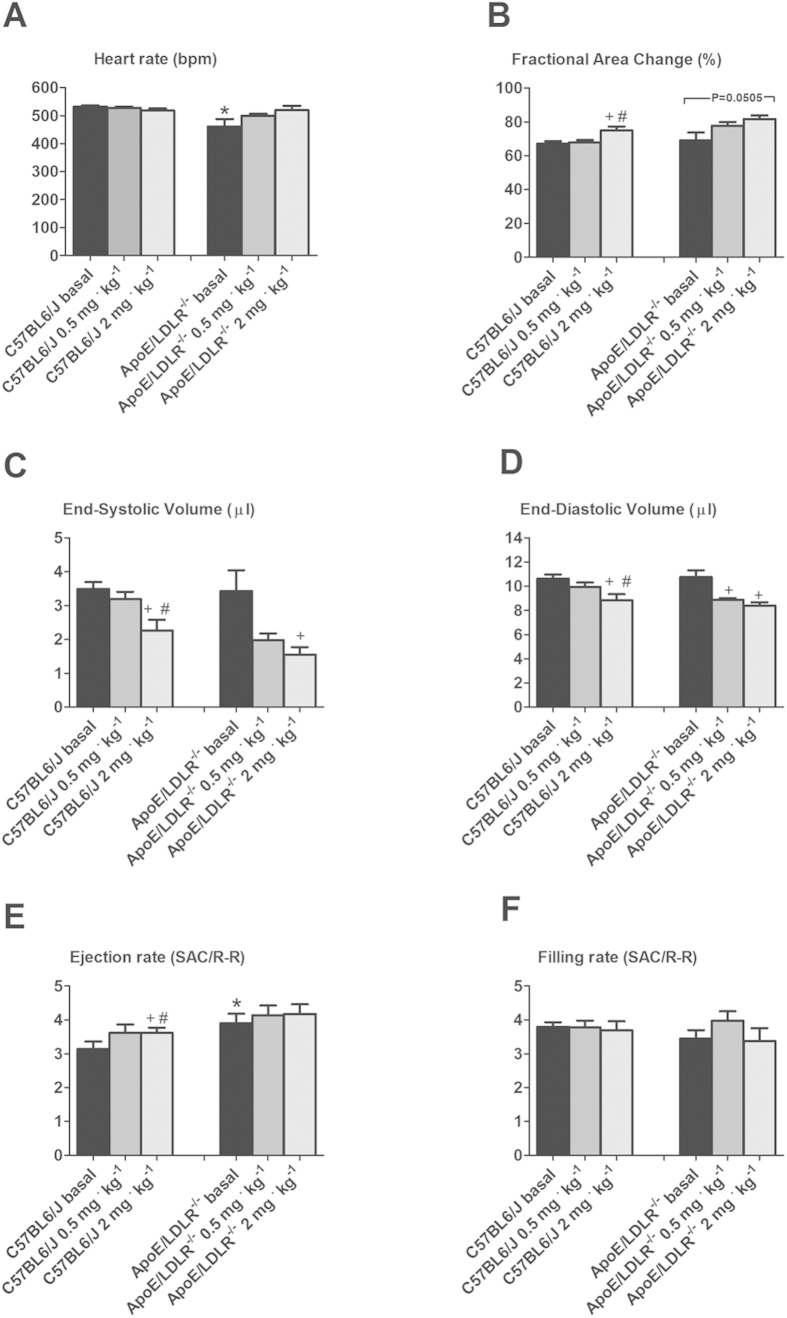
Cardiac performance at rest and under dobutamine stimulation in female ApoE/LDLR−/− and C57BL6/J mice
Basal left ventricle function including fractional area change (FAC), end systolic and end diastolic volume (ESV, EDV) and filling rate (FR) were not changed in 6–8-month old female ApoE/LDLR−/− mice compared to age-matched C57BL6/J mice (P < 0.05, Fig. 6B,C,D,F). The only difference was that the heart rate was accelerated in C57BL6/J mice and basal ejection rate was higher in female ApoE/LDLR−/− mice (P < 0.05, Fig. 6A,E, respectively). Not only was basal cardiac function not compromised in female ApoE/LDLR−/− mice as compared to age-matched C57BL6/J mice but also response to dobutamine stress was fully preserved. As shown in Fig. 6, dobutamine stimulation at low (0.5 mg·kg−1) and high dose (2 mg·kg−1) demonstrated fully preserved, inotropic and lusitropic response in female ApoE/LDLR−/− mice. In fact, the increase in FAC (from basal 0.67 ± 0.03 to 0.75 ± 0.06 and from 0.68 ± 0.11 to 0.82 ± 0.04 for controls vs. female ApoE/LDLR−/− mice respectively, P < 0.05, Fig. 6B–D) and decrease in ESA (from 3.49 ± 0.53 to 2.27 ± 0.81 and from 3.43±1.51 to 1.55 ± 0.53 for controls vs. female ApoE/LDLR−/− mice respectively, P < 0.05) and EDA (from 10.63 ± 0.9 to 8.85 ± 1.31 and from 10.75 ± 1.38 to 8.39 ± 0.67 for controls vs. female ApoE/LDLR−/− mice respectively, P < 0.05) were all comparable for female ApoE/LDLR−/− and age-matched C57BL6/J mice. In contrast, chronotropic reserve was observed only in female ApoE/LDLR−/− mice (P < 0.05, not significantly, Fig. 6A). No reserve in ejection rate was observed in female ApoE/LDLR−/− mice, however their basal ejection rate was higher than in C57BL6/J mice (3.91 ± 0.68 for female ApoE/LDLR−/− mice as regards 3.35 ± 0.46 for controls, P < 0.05, Fig. 6E). Both groups responded similarly as regards filling rate showing no changes under dobutamine stress (P < 0.05, Fig. 6F).
Figure 6. Cardiac reserve for low (0.5 mg·kg−1) and high (2.0 mg·kg−1) dose of dobutamine in female ApoE/LDLR−/− mice.
Dobutamine stress test uncovered well-preserved cardiac reserve in ApoE/LDLR−/− mice. *indicates statistical significance in comparison of the ApoE/LDLR−/− and the control mice at basal conditions (P < 0.05, t-test; n = 7 in each group), whereas changes after dobutamine were marked as + for comparisons between the given dose of dobutamine (low or high) and the basal condition, and as # for comparisons between the high and low dose (P < 0.05, ANOVA and Tukey post-hoc test); n = 7–8 for C57BL6/J mice and 6 for ApoE/LDLR−/− mice. Data are presented as the mean ± SEM.
Discussion
In this study we characterized whole body maximal oxygen uptake (V’O2max), exercise capacity and NO- and PGI2-dependent coronary endothelial function of female ApoE/LDLR−/− mice with respect to age-related peripheral atherosclerosis progression described elsewhere26 and age-related coronary atherosclerosis progression shown here. We also characterized cardiac haemodynamic response to dobutamine-induced stress in 6–8 month old female ApoE/LDLR−/− mice with advanced coronary atherosclerosis in comparison with age-matched C57BL6/J mice. Surprisingly, we found that V’O2max considered a key index of cardio-respiratory capacity and running exercise capacity of female ApoE/LDLR−/− mice were preserved irrespective of atherosclerosis progression (Fig. 1). Moreover, both younger (3-month old) female ApoE/LDLR−/− mice and older (6–8-month old) female ApoE/LDLR−/− mice with early and advanced peripheral (as reported earlier26) and early and advanced coronary (as shown here) atherosclerosis achieved higher velocities and covered longer distances than age-matched C57BL6/J mice (Fig. 1D,E). Excellent running exercise capacity of female ApoE/LDLR−/− mice was associated with preserved NO-dependent function of coronary circulation (Fig. 2) and increased COX-2-dependent PGI2 production (Fig. 4C). Moreover, cardiac performance at rest as well as its inotropic and lusitropic reserve were well preserved in 6–8-month old female ApoE/LDLR−/− mice (Fig. 6) with advanced coronary atherosclerosis (Fig. 5G–I) compared with age-matched C57BL6/J mice as evidenced by similar FAC, and slice-derived ESV and EDV in basal conditions as well as in response to dobutamine. Altogether, the present work demonstrated that development of atherosclerosis did not compromise V’O2max of female ApoE/LDLR−/− mice and did not influence their cardiac function even at the advanced stage of atherosclerosis with advanced atherosclerotic plaques in the coronary circulation and even during stress conditions. Running exercise capacity of 6–8-month old female ApoE/LDLR−/− mice was only slightly lower than in young female ApoE/LDLR−/− mice (Fig. 1D,E). Nevertheless, it was still better than in age-matched C57BL6/J mice. We claim that robust compensatory mechanisms in coronary circulation, including increased vascular responsiveness to NO (Fig. 3) and increased generation of PGI2 (Fig. 4B,C) that are triggered in the early phase of atherosclerosis development, could, at least partly, account for preserved V’O2max and running exercise capacity of female ApoE/LDLR−/− mice as well as their full cardiac adaptation to exercise occurring even at the stage of advanced atherosclerosis at the age of 6–8 months.
Given that it is widely known that atherosclerosis progression severely impairs cardiac reserve and exercise capacity in humans4,5,6 and in atherosclerotic ApoE−/− mice of both genders11,13,14,17, our results regarding V’O2max, running exercise capacity and cardiac function of female ApoE/LDLR−/− mice are surprising. Indeed, exercise capacity of female ApoE/LDLR−/− mice was preserved despite peripheral (as reported previously26) and coronary (Fig. 5) atherosclerosis progression what could be a factor that could limit their exercise capacity30. These results can be only explained by important compensatory mechanisms triggered by double knockout of ApoE and Ldlr genes that preserved cardiac haemodynamic response to exercise of female ApoE/LDLR−/− mice (Fig. 6) and assured sufficient adaptation of blood flow and oxygen delivery to their cardiac muscle during exercise which is the major determinant of V’O2max (for review see31). These compensatory mechanisms could be, at least partly, associated with estrogens known to exert cardioprotective and vasoprotective effects. In fact, it was shown that in female atherosclerotic LDLR−/− mice estrogens up-regulate cyclooxygenase 2 (COX-2) –derived production of PGI2 via estrogen receptor subtype alpha32.
It is also worth noting that oxygen extraction ratio within coronary circulation is already nearly maximal at rest and, thus, the major mechanism involved in increasing oxygen delivery during exercise is based on coronary vasodilation (and increased coronary blood flow), while in the skeletal muscles an increase in oxygen delivery to working muscles is brought about by an increase in its extraction ratio and recruitment of capillaries, as well as increased blood flow31. Considering this, it is the coronary and but not the skeletal muscle circulation that represents a locus minoris resistantiae in the regulation of V’O2max and exercise capacity. Accordingly, compensatory mechanisms aimed to preserve V’O2max in female ApoE/LDLR−/− mice should be, first of all, present within the coronary circulation to preserve cardiac haemodynamic response to exercise. Indeed, our results fully support this assumption. In line with fully preserved basal cardiac function and stress response to dobutamine (Fig. 6), NO- and PGI2-dependent function of coronary circulation in female ApoE/LDLR−/− mice was not compromised (Figs 2,3 and 4). On the contrary, clear-cut compensatory mechanisms in these mice were detected such as increased COX-2-dependent basal PGI2 production, increased COX-1-dependent PGI2 release in response to ACh as well as increased coronary vessel responsiveness to exogenous NO most likely linked to upregulation of soluble guanylate cyclase (sGC) activity in coronary vessels. Interestingly, these mechanisms were already evident in hearts of female ApoE/LDLR−/− mice as early as at two months of age, suggesting that these compensatory mechanisms of coronary circulation are triggered before peripheral atherosclerotic plaques development26 and are probably induced by early coronary atherosclerosis (Fig. 5A–C). Considering vasoprotective and anti-atherosclerotic activity of NO-sGC and COX-2-PGI2 pathways, it is tempting to speculate that these compensatory changes within coronary circulation could mitigate the development of symptomatic ischaemic heart disease in female ApoE/LDLR−/− mice and explain why their cardiac function was not compromised (Fig. 6) despite extensive coronary atherosclerosis at the age of 6–8 months (Fig. 5G–I).
To counteract detrimental results of peripheral endothelial dysfunction in female ApoE/LDLR−/− mice, compensatory mechanisms induced by atherosclerosis also developed within peripheral circulation26. Namely, the cyclooxygenase-2 (COX-2)/PGI2 pathway was upregulated in the aorta of female ApoE/LDLR−/− mice as early as at two months of age and remained elevated in older animals. Although we did not analyse endothelium-dependent regulation of blood flow in skeletal muscles, it could well be that exercise-induced increase in blood flow in skeletal muscles was also preserved due to compensatory mechanisms triggered by NO-deficiency in female ApoE/LDLR−/− mice.
The mechanisms controlling coronary and muscle blood flow during exercise are not entirely understood and involve multiple endothelium-dependent vasodilatory pathways compensating one another. NO, PGI2 and RBC-derived ATP were claimed to be the major mediators involved31. It is widely known that NO is an important coronary and peripheral vasodilator during exercise33. However, in view of the available data, NO action alone could not explain the huge exercise-induced increase in skeletal muscles34 and coronary blood flow35. There is growing body of evidence that exercise-induced PGI2 might play an important role in regulation of coronary and muscle blood flow. It was demonstrated that the magnitude of exercise-induced PGI2 release correlated positively with V’O2max in healthy humans36, while plasma nitrite concentration did not increase even upon vigorous exercise (for review see)31. Moreover, training-induced increase in V’O2max was also associated with increased exercise-induced PGI2 release37. Furthermore, administration of PGI2 to patients with pulmonary hypertension increased their exercise capacity38. Extrapolating from human studies, increased PGI2 release into coronary (Fig. 4C) and peripheral26 circulation of female ApoE/LDLR−/− mice could account for their preserved V’O2max (Fig. 1B,C) and, paradoxically better running exercise capacity (Fig. 1D,E) as well as their preserved basal and dobutamine-stimulated cardiac function (Fig. 6).
Interestingly, there was also a higher number of circulating erythrocytes in female ApoE/LDLR−/− mice compared with age-matched C57BL6/J control mice (Table 1). This could represent yet another mechanism that assured adequate oxygen supply to working tissues. Apart from oxygen transport, haemoglobin inside red blood cells reduces plasma nitrite (NO2−) to NO in hypoxic conditions that is subsequently released and contributes to local vasodilation31,39,40,41. When oxygen tension decreases within coronary microvasculature, red blood cells also release ATP which is a factor increasing coronary blood flow during exercise42. Increased number of circulating erythrocytes in female ApoE/LDLR−/− mice points to increased erythropoesis in these animals (probably to counteract hypoxia). Indeed, erythropoietin production was shown to be higher in mice with atherosclerosis43 and it activated mitochondrial biogenesis to couple red cell mass to mitochondrial mass in the heart44. Therefore, even though we claim that upregulation of PGI2-dependent pathways within the coronary circulation(Fig. 4B,C) seems to represent the major compensatory mechanism in female ApoE/LDLR−/− mice that contribute to their cardiac haemodynamic adaptation to exercise, many other possible mechanisms (i.e. enhanced RBC-driven ATP release, erythropoietin-mediated mechanisms, mitochondrial biogenesis in the heart, and shift in substrate metabolism in heart and skeletal muscles) could also contribute to their extraordinary exercise capacity. Moreover, higher maximal running velocity found in female ApoE/LDLR−/− mice compared with age-matched C57BL6/J mice was accompanied by similar or even higher V’O2max in female ApoE/LDLR−/− mice (compare Fig. 1D,B,C, respectively). This could be explained by higher muscle mechanical efficiency (for overview see45) in female ApoE/LDLR−/− mice, similar to in the trained vs. untrained humans (see46) and/or by higher capacity of anaerobic glycolysis and grater fatigue resistance in the locomotor muscles of the female ApoE/LDLR−/− mice allowing them to maintain the exercise long after reaching their V’O2max. Indeed, compensatory mechanisms might operate also in the skeletal muscles of female ApoE/LDLR−/− mice enabling them to run on the treadmill much better than their age-matched C57BL6/J counterparts with similar V’O2max.
To conclude, we claim that robust compensatory mechanisms in the hearts of female ApoE/LDLR−/− mice including increased vascular responsiveness to NO and increased generation of PGI2 could, at least partly, explain preserved whole body V’O2max as well as full cardiac adaptation to exercise of female ApoE/LDLR−/− mice despite peripheral and coronary atherosclerosis progression. Our results demonstrate surprisingly efficient adaptive mechanisms in the coronary circulation in female ApoE/LDLR−/− mice which when understood better, may be exploited therapeutically to limit impending impairment of cardiac output and the risk of perfusion deficit of the heart with coronary atherosclerosis.
Methods
Animals
ApoE/LDLR−/− female mice20 were obtained from Jackson Lab (USA) and bred in-house. To asses exercise capacity and in vivo cardiac reserve, we used 6–8-month old female ApoE/LDLR−/−(n = 22) with advanced atherosclerosis and their age-matched wild-type female C57BL6/J (n = 26) littermates obtained from Charles River (Germany)47. For isolated hearts studies, 2–8 month old (2 months old, n = 79; 4 months old, n = 76; and 8 months old, n = 95) female ApoE/LDLR−/− mice were used and compared to the controls (2–8 months C57BL6/J, n = 113). Mice were housed 3–5 per cage, in a temperature-controlled environment (22–25 °C) maintained on a 12 hour light/day cycle and given unlimited access to food (standard chow diet Altromin 1324 TPF (Altromin, Germany)) and water. All experimental protocols were conducted according to the Guidelines for Animal Care and Treatment of the European Union and approved by the First Local Ethical Committee on Animal Testing at the Jagiellonian University in Krakow (approvals No. 49/2009, 53/2009, 27/2014) and the Second Local Ethical Committee on Animal Testing at the Institute of Pharmacology of the Polish Academy of Sciences in Krakow (approval No. 914/2012).
Incremental exercise for V’O2max measurement
All animals were acclimatized to the closed treadmill for metabolic measurements (Columbus Instruments, Columbus, OH, USA) for three days before the experiment. Randomly selected mice from all groups (n > 9) underwent maximal incremental testing with measurement of respiratory metabolic performance according to the protocol described before48. Briefly, mice were run on a single-line metabolic treadmill equipped with a shock grid at 0°. The exercise protocol started at 5 m·min−1 and the speed was increased by 4 m·min−1 every 3 min until exhaustion. Data on whole body oxygen consumption (V’O2) were collected.
Blood collection
Mice were anesthetized by sodium pentobarbital/pentobarbital (5:1) overdose (1 ml·kg−1, intraperitoneally) and the rib cage was cut along the sides to expose the heart. Blood was collected from the right ventricle into a syringe with nadroparin (10 U·ml−1) and the blood count was performed at animal blood counter Vet abc (Horiba Medical, France).
Measurements in isolated perfused heart
The detailed technique of isolated heart perfusion to study alterations in coronary flow in mouse model according to Langendorff is described elsewhere49. The animals were anesthetized with mixture of ketamine (100 mg·kg−1) and xylazine (10 mg·kg−1). The hearts were precisely excised from the animal body and placed in ice-cold Krebs-Hanseleit bicarbonate buffer, where extraneous tissues were quickly removed. The aorta was attached to the prepared aortic cannula and isolated hearts were retrograde perfused with Krebs-Hanseleit buffer under 80 mmHg pressure and at 37 °C. It took approximately less than 30 seconds to mount the heart for Langendorff perfusion (hearts that required longer time for mounting were discarded from analysis). Coronary flow (CF) during the perfusion time was monitored by ultrasonic flow meter (Transit Time Flowmeter TTFM, HSE-Harvard and Transonic System Inc., USA) and flow measurements were displayed throughout the experiment at all time points. Two electrodes were placed on the right atrium of the heart model generating 400 impulses per minute. To characterize the responsiveness of coronary vessels to vasodilation several drugs were given at the volume of 10 μL each: bradykinin (Bk: NO – dependent, in 2 (n = 10), 4 (n = 10) and 8 (n = 13) months old female ApoE/LDLR−/− mice and 2–4 controls (n = 16)), Acetylcholine (ACh: PGI2 – dependent, in 2 (n = 23), 4 (n = 16) and 8 (n = 22) months old female ApoE/LDLR−/− mice and 2–4 controls(n = 25)) as well as two exogenous, endothelium-independent NO – donors with different mechanism of NO release sodium nitroprusside (SNP in 2 (n = 7), 4 (n = 8) and 8 (n = 12) months old female ApoE/LDLR−/− mice and 2–4 months old controls (n = 14) and S-Nitroso-N-acetylpenicillamine (SNAP in 2 (n = 14), 4 (n = 14) and 8 (n = 15) months old female ApoE/LDLR−/− mice and 2–4 months old controls (n = 16)). ACh (0.3 nmol) was injected as one bolus, whereas Bk, SNAP and SNP were injected as multiple doses (0.03; 0.1; 0.3 and 1.0 nmol). To study the contribution of NOS and cyclooxygenase (COX) to the coronary flow response separately, the nonselective NO - synthase inhibitors, NG – nitro-L-arginine methyl ester (L-NAME 500 μM in 2 (n = 6), 4 (n = 6) and 8 (n = 8) months old female ApoE/LDLR−/− mice and 2–4 months old controls (n = 16)) and COX-2 selective cyclooxygenase inhibitors – Rofecoxib, 10 μM (in 2 (n = 6), 4 (n = 6) and 8 (n = 6) months old female ApoE/LDLR−/− mice and 2–4 months old controls (n = 5) were used. After experiments all hearts were weighed and alterations in coronary flow were expressed in ml·min·g−1 of the wet ventricular weight. For determination of the concentration of the 6-keto-PGF1α in effluent from the coronary vessels, samples of the effluent (500 μl) were collected and stored at −70 °C until analysed by commercially available enzyme immunoassay kits (Cayman Chemical Co, Ann Arbor, MI). 6-keto-PGF1α concentration was expressed in mg·l−1 (or ng·ml−1) of coronary flow (ml·min−1).
Assessment of coronary atherosclerosis in coronary arteries
The hearts were dissected, embedded in the OCT compound (CellPath, Oxford, UK), and snap-frozen or fixed in 4% paraformaldehyde (pH 7.4), paraffin embedded and serially sectioned. Then, 10 μm thick serial cross-section slides of the heart were cut in the apex region, at the middle (at papillary muscle level) and upper part of the heart (aortic roots), to evaluate the coronary atherosclerosis in distal, intramuscular and proximal parts of coronary arteries. The sections were stained with the oil red-O for lipids detection in the atherosclerotic plaque or with the OMSB staining (Orceine with Martius, Scarlet and Blue) for histological characterisation. Photographs were acquired using the Carl Zeiss HmR monochromatic digital camera.
Cardiac performance
Cardiac function was assessed using cardiac magnetic resonance imaging (MRI) with a 9.4T Bruker BioSpec system (Bruker, Ettlingen, Germany) equipped with BFG-113/60-S gradient system and 36 mm 1H quadrature volume resonator. Physiological parameters were monitored and gated using Model 1025 Monitoring and Gating System (SA Inc., Stony Brook, NY). Mice were measured under anesthesia maintained at 1.7% of isoflurane (Aerrane, Baxter) in a 1/2 oxygen/air mixture delivered via nose mask with monitoring of ECG, respiration and body temperature (37 °C). MRI cine scans were acquired with retrospectively gated IntraGate FLASH 2D sequences with the following parameters: echo time 1.49 ms, repetition time 4.3 ms, flip angle 18°, number of repetitions 250, field of view 30 × 30 mm2, matrix 256 × 256, slice thickness 1.0 mm and reconstructed with IntraGate 1.2.b.2 macro (Bruker) to 60 frames per cardiac cycle. LV images were analysed using the Segment package17 (v.1.9 R2626, Medviso AB, Sweden) and papillary muscles were included in the cavity volume. Single-slice volume was plotted against the time and used for regional cardiac functional parameters: end-diastolic/end-systolic volume (EDV/ESV), fractional area change (FAC), stroke area change (SAC) and LV filling and ejection rates (FR, ER normalized to the individual SAC and R–R values).
Statistical analysis
Statistical analyses were performed in GraphPad Prism Software (GraphPad Software, USA) and STATISTICA 10 software (Stat-Soft Inc., USA). Results from exercise performance test, body mass and blood count were analysed with two-sided t test or non-parametric Mann-Whitney test based on the results of D’Agostino and Pearson omnibus normality test. Results from in vivo basic cardiac performance as well as from isolated perfused hearts were analysed by the t-test for independent variables. If parametric tests assumptions were not fulfilled (normality of distributions and homogeneity of variance), non-parametric Mann-Whitney U test or Friedman test was used. For in vivo cardiac reserve assessment, a repeated-measures ANOVA followed by a Tukey post-hoc test was used. All data were presented as mean values ± SEM.
Additional Information
How to cite this article: Wojewoda, M. et al. Exercise capacity and cardiac hemodynamic response in female ApoE/LDLR−/− mice: a paradox of preserved V’O2max and exercise capacity despite coronary atherosclerosis. Sci. Rep. 6, 24714; doi: 10.1038/srep24714 (2016).
Acknowledgments
This research was supported by the European Union from the resources of the European Regional Development Fund under the Innovative Economy Programme (grant coordinated by JCET-UJ) No. POIG.01.01.02-00-069/09) and partially by the National Science Centre grant No DEC-2015/16/W/NZ4/00070.
Footnotes
Author Contributions S.C. and J.A.Z. conceived the original idea and reviewed the manuscript. S.C., M.W. and U.T. designed experiments, M.W., U.T., P.G., T.S., M.J., A.O., K.J., A.J. and K.P. performed the experiments. R.K. provided experimental tools, M.W. and M.J. performed statistical analysis. M.W., U.T., S.C. and J.A.Z. wrote the manuscript.
References
- Jamkhande P. G. et al. Therapeutic approaches to drug targets in atherosclerosis. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 22, 179–90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Garcia-Cardena G. & Owens G. K. The cell biology of disease: Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 209, 13–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A. et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am. J. Hypertens. 15, 1101–8 (2002). [DOI] [PubMed] [Google Scholar]
- Hamburg N. M. & Balady G. J. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 123, 87–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner J. G. Type 2 diabetes mellitus and cardiovascular exercise performance. Rev. Endocr. Metab. Disord. 5, 269–76 (2004). [DOI] [PubMed] [Google Scholar]
- Mark D. B. & Lauer M. S. Exercise capacity: the prognostic variable that doesn’t get enough respect. Circulation 108, 1534–6 (2003). [DOI] [PubMed] [Google Scholar]
- Mégnien J.-L. & Simon A. Exercise tolerance test for predicting coronary heart disease in asymptomatic individuals: A review. Atherosclerosis 205, 579–83 (2009). [DOI] [PubMed] [Google Scholar]
- Ramos L. M. Cardiac diagnostic testing: what bedside nurses need to know. Crit. Care Nurse 34, 16–27, quiz 28 (2014). [DOI] [PubMed] [Google Scholar]
- Myers J. et al. Supervision of exercise testing by nonphysicians: a scientific statement from the American Heart Association. Circulation 130, 1014–27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz G. S. & Reardon C. A. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 1104–15 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell A. J., Schauble E., Bernstein D. & Cooke J. P. Limb blood flow during exercise is dependent on nitric oxide. Circulation 98, 369–74 (1998). [DOI] [PubMed] [Google Scholar]
- Niebauer J. et al. Impaired aerobic capacity in hypercholesterolemic mice: partial reversal by exercise training. Am. J. Physiol. 276, H1346–54 (1999). [DOI] [PubMed] [Google Scholar]
- Maxwell A. J. et al. L-arginine enhances aerobic exercise capacity in association with augmented nitric oxide production. J. Appl. Physiol. 90, 933–8 (2001). [DOI] [PubMed] [Google Scholar]
- Maxwell A. J. et al. Hypercholesterolemia impairs exercise capacity in mice. Vasc. Med. 14, 249–57 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C. J. et al. Hemodynamic changes in apolipoprotein E-knockout mice. Am. J. Physiol. Heart Circ. Physiol. 279, H2326–34 (2000). [DOI] [PubMed] [Google Scholar]
- Wang Y.-X. Cardiovascular functional phenotypes and pharmacological responses in apolipoprotein E deficient mice. Neurobiol. Aging 26, 309–16 (2005). [DOI] [PubMed] [Google Scholar]
- Vincelette J., Martin-McNulty B., Vergona R., Sullivan M. E. & Wang Y.-X. Reduced cardiac functional reserve in apolipoprotein E knockout mice. Transl. Res. 148, 30–6 (2006). [DOI] [PubMed] [Google Scholar]
- Lightfoot J. T., Turner M. J., Daves M., Vordermark A. & Kleeberger S. R. Genetic influence on daily wheel running activity level. Physiol. Genomics 19, 270–6 (2004). [DOI] [PubMed] [Google Scholar]
- Murphy M. O. B. The Role of Exercise in Polychlorinated Biphenyl Induced Cardiovascular Disease, PhD thesis, University of Kentucky (2014).
- Ishibashi S., Herz J., Maeda N., Goldstein J. L. & Brown M. S. The two-receptor model of lipoprotein clearance: tests of the hypothesis in ‘knockout’ mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc. Natl. Acad. Sci. USA 91, 4431–5 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W. et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J. Lipid Res. 53, 51–65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman S. C. A practical approach to using mice in atherosclerosis research. Clin. Biochem. Rev. 25, 81–93 (2004). [PMC free article] [PubMed] [Google Scholar]
- Caligiuri G., Levy B., Pernow J., Thorén P. & Hansson G. K. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc. Natl. Acad. Sci. USA 96, 6920–4 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemdahl A.-L., Falk E., Thorén P. & Hansson G. K. Thrombin inhibitor reduces myocardial infarction in apoE−/− × LDLR−/− mice. Am. J. Physiol. Heart Circ. Physiol. 287, H872–7 (2004). [DOI] [PubMed] [Google Scholar]
- Grönros J., Wikström J., Hägg U., Wandt B. & Gan L. Proximal to middle left coronary artery flow velocity ratio, as assessed using color Doppler echocardiography, predicts coronary artery atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 26, 1126–31 (2006). [DOI] [PubMed] [Google Scholar]
- Csányi G. et al. Functional alterations in endothelial NO, PGI2 and EDHF pathways in aorta in ApoE/LDLR-/- mice. Prostaglandins Other Lipid Mediat. 98, 107–15 (2012). [DOI] [PubMed] [Google Scholar]
- Nishikawa Y. & Ogawa S. Importance of nitric oxide in the coronary artery at rest and during pacing in humans. J. Am. Coll. Cardiol. 29, 85–92 (1997). [DOI] [PubMed] [Google Scholar]
- Kozlovski V. I., Lomnicka M. & Chlopicki S. Nebivovol and carvedilol induce NO-dependent coronary vasodilatation that is unlikely to be mediated by extracellular ATP in the isolated guinea pig heart. Pharmacol. Rep. 58 Suppl, 103–10 (2006). [PubMed] [Google Scholar]
- Gwóźdź P., Drelicharz L., Kozlovski V. I. & Chlopicki S. Prostacyclin, but not nitric oxide, is the major mediator of acetylcholine-induced vasodilatation in the isolated mouse heart. Pharmacol. Rep. 59, 545–52 (2007). [PubMed] [Google Scholar]
- Patel A. R. et al. Peripheral vascular endothelial function correlates with exercise capacity in women. Clin. Cardiol. 28, 433–6 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz J. A., Majerczak J., Duda K. & Chlopicki S. Coronary and muscle blood flow during physical exercise in humans; heterogenic alliance. Pharmacol. Rep. 67, 719–27 (2015). [DOI] [PubMed] [Google Scholar]
- Egan K. M. et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science 306, 1954–7 (2004). [DOI] [PubMed] [Google Scholar]
- Joyner M. J. & Casey D. P. Muscle blood flow, hypoxia, and hypoperfusion. J. Appl. Physiol. 116, 852–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B. In search of a vasodilator: is ATP the answer? J. Physiol. 590, 5261–2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tune J. D., Gorman M. W. & Feigl E. O. Matching coronary blood flow to myocardial oxygen consumption. J. Appl. Physiol. 97, 404–15 (2004). [DOI] [PubMed] [Google Scholar]
- Zoladz J. A., Majerczak J., Duda K. & Chłopicki S. Exercise-induced prostacyclin release positively correlates with V’O2max in young healthy men. Physiol. Res. 58, 229–38 (2009). [DOI] [PubMed] [Google Scholar]
- Zoladz J. A., Majerczak J., Duda K. & Chlopicki S. Endurance training increases exercise-induced prostacyclin release in young, healthy men–relationship with V’O2max. Pharmacol. Rep. 62, 494–502 (2010). [DOI] [PubMed] [Google Scholar]
- Paramothayan N. S., Lasserson T. J., Wells A. U. & Walters E. H. Prostacyclin for pulmonary hypertension in adults. Cochrane database Syst. Rev. CD002994 doi: 10.1002/14651858.CD002994.pub2 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin M. T. Role of the red blood cell in nitric oxide homeostasis and hypoxic vasodilation. Adv. Exp. Med. Biol. 588, 189–205 (2006). [DOI] [PubMed] [Google Scholar]
- Mairbäurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 4, 332 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonikar P., Abu-Soud H. M. & Kavdia M. Computational analysis of nitric oxide biotransport to red blood cell in the presence of free hemoglobin and NO donor. Microvasc. Res. 95, 15–25 (2014). [DOI] [PubMed] [Google Scholar]
- Farias M., Gorman M. W., Savage M. V. & Feigl E. O. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am. J. Physiol. Heart Circ. Physiol. 288, H1586–90 (2005). [DOI] [PubMed] [Google Scholar]
- Arcasoy M. O. Non-erythroid effects of erythropoietin. Haematologica 95, 1803–05 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway M. S. et al. Erythropoietin activates mitochondrial biogenesis and couples red cell mass to mitochondrial mass in the heart. Circ. Res. 106, 1722–30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B., Rossiter H. B. & Zoladz J. A. Skeletal muscle fatigue and decreased efficiency: two sides of the same coin? Exerc. Sport Sci. Rev. 43, 75–83 (2015). [DOI] [PubMed] [Google Scholar]
- Majerczak J. et al. Endurance training decreases the non-linearity in the oxygen uptake-power output relationship in humans. Exp. Physiol. 97, 386–99 (2012). [DOI] [PubMed] [Google Scholar]
- Vaillant F. et al. Mouse strain differences in metabolic fluxes and function of ex vivo working hearts. Am. J. Physiol. Heart Circ. Physiol. 306, H78–87 (2014). [DOI] [PubMed] [Google Scholar]
- Wojewoda M. et al. Running performance at high running velocities is impaired but V’O2max and peripheral endothelial function are preserved in IL-6−/− mice. PLoS One 9, e88333 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chłopicki S. & Gryglewski R. J. The endothelium-dependent and the endothelium-independent vasodilators in the isolated, perfused guinea pig heart. J. Physiol. Pharmacol. 43, 353–65 (1992). [PubMed] [Google Scholar]