Abstract
Anthropogenic salinization of rivers is an emerging issue of global concern, with significant adverse effects on biodiversity and ecosystem functioning. Impacts of freshwater salinization on biota are strongly mediated by evolutionary history, as this is a major factor determining species physiological salinity tolerance. Freshwater insects dominate most flowing waters, and the common lotic insect orders Ephemeroptera (mayflies), Plecoptera (stoneflies) and Trichoptera (caddisflies) are particularly salt-sensitive. Tolerances of existing taxa, rapid adaption, colonization by novel taxa (from naturally saline environments) and interactions between species will be key drivers of assemblages in saline lotic systems. Here we outline a conceptual framework predicting how communities may change in salinizing rivers. We envision that a relatively small number of taxa will be saline-tolerant and able to colonize salinized rivers (e.g. most naturally saline habitats are lentic; thus potential colonizers would need to adapt to lotic environments), leading to depauperate communities in these environments.
Keywords: major ions, salinity, freshwater, novel ecosystems, osmoregulation, adaptation
1. Introduction
Salinization of rivers is occurring in every inhabited continent as a result of various human activities, and is expected to worsen owing to climate change and increased water demand [1]. Anthropogenic increases in riverine salinization usually result in modest salinities (1–10 mS cm−1 range [1,2]) compared with natural saline lakes, but rivers occasionally increase to the levels of highly saline lakes (> 100 mS cm−1 [3]). Short-term responses to increased salinization are clear: reduced biodiversity [4,5], altered ecosystem processes [6], changed composition [7,8] and altered trait composition of communities [9,10].
Anthropogenic salinization of rivers is creating novel environments by introducing a strong selective force (salinity) to systems dominated by another strong selective force (unidirectional flow). While natural inland saline lakes are quite common, especially in Mediterranean and (semi-)arid climates [11], natural saline rivers are much rarer and seldom reach the high salinities of lakes (>100 mS cm−1) [12,13]. Long-term evaporation can concentrate salts in terminal lakes even with low salt inputs, but naturally saline rivers require a continued and large input of salt. For example, in wadeable streams in the eastern USA, 73% of ecoregions had a median electrical conductivity, EC < 0.2 mS cm−1 [13]. Salinity was higher in the western USA but only 3% of ecoregions had a median EC > 1 mS cm−1 [13]. Furthermore, naturally saline rivers usually have low discharge and mostly do not flow permanently [12], so we would expect saline rivers with high discharges to be rare in nature (figure 1a).
Figure 1.
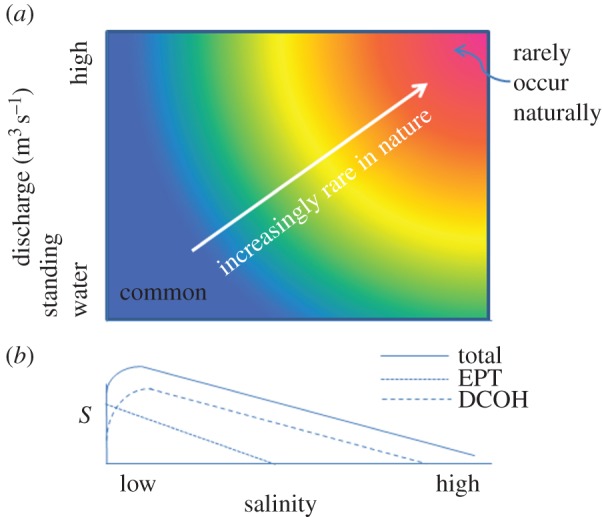
Conceptual diagrams of the relationships between salinity and (a) discharge (m3 s−1)—with red indicating the salinity–flow combination is relatively rare, and blue that it is relatively common; (b) species richness (S) (i) for total invertebrates, (ii) Ephemeroptera, Plecoptera and Trichoptera (EPT) and (iii) Diptera, Coleoptera, Odonata and Hemiptera (DCOH).
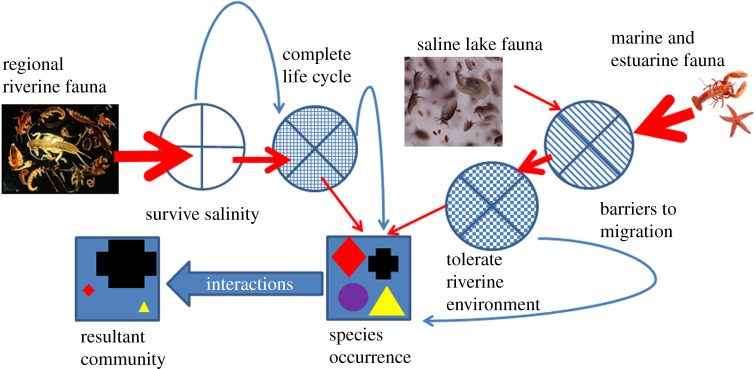
The long-term consequences of anthropogenic river salinization are unclear. Here we explore the potential for organisms to adapt to or exploit these novel ecosystems, and develop a conceptual model for predicting the ecological attributes of salinized rivers. We consider future species composition of salinized rivers as a function of (i) the ability of the original fauna to survive and complete their life cycles at elevated salinity; (ii) the potential for salt-tolerant fauna from elsewhere to colonize the new saline environment; (iii) the potential for species initially unable to tolerate the salinity rise to rapidly adapt to increased salinity; and (iv) the outcome of changing interactions among species (figure 2).
Figure 2.
Conceptual diagram of environmental filters determining the presence and absence of species in salinized rivers. The fauna that inhabit salinized rivers will be influenced by the ability of regional riverine fauna to survive and complete their life cycles in the new salinity, perhaps following adaption. The ability of species from existing saline environments to disperse to and tolerate riverine environments (perhaps following adaption) will also be important. Red straight arrows indicate species movement through filters, the reduced size of arrows indicates that some species did not pass the filters (shown by circles). Blue curved arrows indicate species that indirectly pass through a filter after rapid adaptation. The word ‘interactions’ refers to ecological interactions between organisms, e.g. competition and predation. The shapes in the blue squares indicate different species, with their sizes in the final square indicating relative population size.
2. Surviving salinity increase
Modest salinity increases (to ca 0.3–0.5 mS cm−1) [4] may actually slightly increase invertebrate species richness (figure 1b). Nevertheless, the richness of three orders of insects, Ephemeroptera (mayflies), Plecoptera (stoneflies) and Trichoptera (caddisflies) (EPT), declines with increasing salinity [4], as does the abundance of Ephemeroptera [5] (figure 1b), although a few trichopteran species can inhabit saline environments [14,15]. Turbellaria (flatworms), Oligochaeta (segmented freshwater worms) and Hirudinea (leeches) also contain some salt-sensitive species [16] as well as salt-tolerant species. Riverine Diptera (true flies), Coleoptera (beetles), Odonata (dragon- and damselflies) and Hemiptera (true bugs; DCOH) can vary from sensitive to very tolerant of salinity [1,3,16], and some DCOH species have independently evolved to inhabit highly saline lakes and marine environments [15]. Nevertheless, increases in salinity above about 1.5 mS cm−1 can lead to a decline in insect species richness in streams, with the ratio of EPT to DCOH species declining with increasing salinity [4], matching the results from laboratory experiments [17].
Taxa that are sensitive to salinity tend to decline in abundance in salinized streams. Nevertheless, the taxa that dominate salinized streams can vary widely. In naturally saline Spanish streams, Diptera, Coleoptera and Heteroptera are the most diverse macroinvertebrate taxa, with Ephemeroptera, Trichoptera, Odonata, Crustacea, Hydrachnidia (water mites) and gastropods scarce and mostly restricted to less than 30 mS cm−1 [12]. In Australian agriculturally salinized streams, the dominant groups are generally Diptera, Coleoptera and crustaceans [3,18], whereas in a French stream salinized by industry, introduced crustaceans and bivalves dominate [19].
The flow environment and water permanency may affect salinity tolerance. Species of two genera of Coleoptera inhabiting temporary saline standing waters tolerated higher salinity than congeneric species inhabiting permanent saline-flowing waters [20]. This may be because species inhabiting temporary water bodies experience a wider range of salinities.
The evolutionary distance between freshwater taxa and their marine/estuarine ancestors could explain differences in salinity tolerance and the potential for taxa to evolve tolerance, where NaCl is the dominant salt. Many freshwater crustaceans, molluscs and fishes are closely related to marine or estuarine species and can be salinity-tolerant [16]. At least 33 extant lineages of gastropods have colonized freshwater from marine environments [21], and freshwater fish most likely evolved from marine ancestors, although some lineages have been long confined to freshwater [22]. Only 2% of amphibian species have been documented inhabiting saline environments [23]. Freshwater insects, which dominate streams and rivers in terms of biomass, abundance and richness, evolved on multiple occasions from terrestrial insects, and saline-tolerant insects evolved from freshwater ancestors in multiple lineages [24]. Insects, along with rotifers [25], are among the few invertebrate taxa with much higher species richness in freshwater than marine environments [15,26]. While multiple lineages of some insect orders are tolerant of highly saline waters, there are far fewer insect species in saline environments compared with freshwater environments. A lack of evolutionary exposure to saline conditions may mean that most, but not all, insects are poorly equipped to cope with salinization.
Organisms in (semi-)arid regions may have higher salinity tolerance than those in other regions owing to past aridity-driven salinization [3,27,28]. However, the salinity sensitivity of macroinvertebrates is better explained by taxonomic identity than the aridity of the region they were collected from [16,29], with the possible exception of southwest Australia [3].
3. Exposure duration
Salinity dose–response relationships depend on exposure period, and invertebrate species are typically found at salinity levels lower than acute lethal levels [30,31]. While organisms may survive short salinity change, they may not complete their life cycle. The eggs and early-life stages of freshwater invertebrates [32,33] and fish [34] are often more salt-sensitive than older-life stages. Non-lethal elevated salinity levels can also slow development, growth and/or fecundity of freshwater invertebrates [2,35,36] and fish [37]. However, relationships between sublethal effects and salinity are often non-monotonic, with some freshwater species performing optimally at intermediate salinity [37–39]. In contrast, an increase in growth or development was not observed with slightly elevated salinity in three ephemeropteran species [2,35].
4. Osmoregulation
The effect of salinity on freshwater organisms depends on their ability to osmoregulate. In most marine invertebrates, the extracellular osmotic pressure equals that in their external environment [24], but in freshwater this would result in internal fluids being too dilute for normal metabolic processes. Freshwater animals therefore must expend energy to maintain a higher internal osmotic pressure than their external environment [40]. As salinity increases the difference in osmotic pressure between the internal and external environment decreases, which would be expected to lower osmoregulation energy needs, possibly explaining why some freshwater species achieve maximum growth at elevated salinity [37,41]. However, salt-sensitive EPT species studied do not perform optimally at slightly elevated salinity and their osmoregulation may differ in important respects [2,35,42]. If external osmotic pressure continues to rise, organisms must either tolerate the increase in osmotic pressure or hyporegulate [43]. However, many EPT species die at salinities well below that of their extracellular fluids.
Osmoregulation is relatively well understood in freshwater crustaceans, molluscs and fish [24], but not in freshwater insects, especially the salt-sensitive EPT orders. Ephemeroptera and Plecoptera appear to have never evolved salinity tolerance despite being among the oldest insects. There are important differences in osmoregulation between freshwater insects and other groups. In terrestrial insects, osmoregulation is fundamentally internal: midgut uptake and sequestration, Malpighian tubule primary urine formation, hindgut/rectum ion resorption [44,45]. Freshwater insects have two additional complexities. (i) They have evolved the capacity to apically uptake ions from the surrounding water, via ionocytes (chloride cells), chloride epithelia or anal papillae, depending on lineage [46]. The organism's surface area to volume ratio is important, as smaller taxa within a given lineage tend to have faster mass-specific ion uptake [47]. (ii) Some aquatic insects, including lineages of Diptera, Hemiptera and Coleoptera, obtain atmospheric air through open tracheae, maintaining a relatively impermeable cuticle, like their terrestrial ancestors [48]. Others, including the EPT, have evolved water-breathing gills and other modified surfaces, which, as a consequence of being gas permeable, are potentially more prone to ion loss and water influx [48]. Thus, in addition to body size, respiratory strategy strongly affects osmotic permeability [47,48] and likely the response of species to salinization.
Most obligate freshwater insects maintain haemolymph osmolarity at ca 300–420 mOsm [10,49], whereas freshwater is less than 25 mOsm [24]. Few studies have examined the effects of increased salinity on haemolymph homeostasis in aquatic insects, although some data suggest extreme salinities are required to increase haemolymph osmolarity [50]. The cycling of ions—uptake from dilute water to the more osmotically rich internal environment, and back to dilute water—requires energy. Nevertheless, the energy demands of osmotic changes do not appear to affect oxygen consumption in aquatic insects [42]. Rather, developmental delays are observed in response to increasing salinity [2].
5. Rapid adaptation
Some populations faced with new abiotic stressors, including pesticides and thermal shocks, have rapidly adapted to these stressors [51]; what is the potential for a similar response with salinization? The potential for rapid evolutionary adaptation to salinity stress will depend on the generation time of the species, plasticity and heritability of the relevant physiological traits, as well as genetic constraints arising from interactions among traits. Most of the data on adaptation associated with salinity come from fish moving from high to low salinity, e.g. sticklebacks [52,53], Atlantic killifish [54] and alewives [55]. The mechanisms behind these adaptive shifts are likely to be complex and involve genes relating to membrane structure and regulation, ATPase activity and other components of osmoregulation [52,56]. There are fewer cased documented in invertebrates, e.g. the invasive copepod Eurytemora affinis [57], adapting from marine to low salinity lentic systems.
In contrast, there is limited documentation of the evolutionary adaptation of freshwater taxa to saline conditions. Variation in salinity tolerance has been observed between individuals or populations of amphibian species inhabiting saline and freshwater, and in some cases, this variation is heritable [23]. Lentic cladocerans (water fleas) differ in their ability to adapt to salinity change, with Daphnia pulex adapting [58] but apparently not Daphnia magna [59] and Simocephalus vetulus [60]. The ability of freshwater invertebrates to rapidly adapt to saline conditions may be quite limited, especially for insects, given their evolutionary origin as terrestrial taxa [61], although more evidence is required. This could be due to strong trade-offs between salinity tolerance and other traits (e.g. respiration mode, gill permeability, osmolality of extracellular fluids), but estimates of genetic correlations and heritable variation across traits are lacking.
Non-genetic adaptive shifts may also occur through plastic changes involving acclimation within generations and acclimatory responses across generations, including epigenetic changes. Exposure to saline conditions failed to alter osmoregulatory responses in Daphnia exilis [62]. However, some freshwater fish [34] and salt-tolerant amphibians [23] tolerate higher salinity when increases are gradual. Transgenerational effects increase the low salinity tolerance of the marine ascidian Ciona intestinalis [63]. They may also occur in freshwater water striders. Nevertheless, there appears to be a trade-off as flight activity of offspring from adults exposed to brackish water is lowered when these offspring encounter freshwater [64]. Overall, there is little evidence to suggest that freshwater lotic species can adapt rapidly to increasing salinity, although the topic has been poorly studied.
6. Dispersal from saline environments
Already salt-adapted species might disperse to and colonize salinizing rivers from nearby oceans, estuaries and naturally saline lakes and rivers (figure 2). There are examples of marine/estuarine species colonizing salinized rivers in southwest Australia [65], France [19] and Germany [66]. Nevertheless, disregarding salinity, riverine environments differ from saline environments in many ways, so that colonizing saline-adapted species will need to cope with other aspects of river life, including unidirectional flow, greater variability in dissolved oxygen, pH, ionic proportions and water temperature [67], and differences in habitat structure. The fact that so few organisms from marine/estuarine habitats live in salinized rivers, even when salinities are similar [18,28,65], suggests that barriers to dispersal and colonization of rivers are formidable [20].
Naturally saline inland waters can have a wide range of taxa (including Diptera, Coleoptera, Odonata and Hemiptera) that could potentially colonize anthropogenic salinized streams. However, colonization might be limited because naturally saline inland aquatic habitats are only common in Mediterranean and (semi-)arid climates. Most saline habitats are lentic [11] and the colonization of more unstable lotic habitats might be difficult [20]. Although there is limited knowledge on the dispersal capacity of species in naturally saline systems, one study showed that the coleopteran Ochthebius glaber had high spatial genetic variation, suggesting limited dispersal [68]. Finally, many naturally saline systems have salt concentrations much higher than expected in anthropogenic salinized streams (but see [3] for an exception), and many organisms of naturally saline systems may not be adapted to lower salinities [69]. The capacity of a species-poor salt-tolerant fauna to colonize anthropogenic saline rivers from natural saline systems needs further investigation.
7. Interactions between species
Within the osmoregulatory capacity [70] of species their distribution and abundance will be influenced by many other factors, including biotic interactions (figure 2). Extreme salinity can restrict species interactions, providing a refuge for saline-tolerant species with limited competitive and predator avoidance ability [70]. Salinities in most rivers, however, are not expected to reach these levels, so interspecific interactions will be important.
Interactions among species can mediate indirect effects of salinity. Three types of indirect effects are relevant. (i) Individuals stressed by competition, food scarcity or predator avoidance can become more susceptible to stressors [71,72], including possibly salinity. (ii) Increasing salinity can reduce the impact of one species on another by reducing levels of predation or competition, and the death or loss of function of salinity-sensitive species may free up resources for more tolerant species. Elevated salinity reduced the feeding ability and interspecific aggression of the invasive mosquitofish Gambusia holbrooki, favouring the native Aphanius fasciatus [73] (see also [25]). Reduced predator efficiency caused by salinization could trigger tropic cascades, although none was detected in an experiment manipulating both salinity and predator occurrence [74]. (iii) Rapidly evolved tolerance to stressful environments can come at the cost of reduced competitive ability [75] or reduced tolerance of other stressors. Such trade-offs may be common, but few have been documented [76].
8. Variation in ionic proportions
Salinity is the product of all dissolved inorganic ions, chiefly: Cl−, HCO3−, SO42−, Na+, Ca2+, Mg2+ and K+ [13]. NaCl is an important cause of salinization but there are exceptions, especially with effluents from resource extraction and industry [2,77]. Saline toxicity in freshwater species depends on ionic concentrations and ratios, not just total salinity [78–80] and interactions between ions [81]. Furthermore, saline effluents can cause non-salinity water quality issues [82]. Our conceptual framework (figure 2) provides a guide to understanding the effects of salinization on rivers, regardless of ionic proportions. However, the ecological effects of a particular salinity will vary with ionic ratios because of different levels of toxicity on organisms, and species tolerant of one set of ionic proportions may be sensitive to another [80,83]. Organisms from marine/estuarine environments may be less likely to invade, and freshwater species recently evolved from estuarine/marine species may be less able to tolerate, or adapt to, non-NaCl-dominated saline waters. More studies of non-NaCl-dominated saline waters are urgently needed.
9. Conclusion
Humans are increasing salinization in flowing freshwaters and these systems will change as a result. The dominant taxa in rivers are insects, many of which have a limited ability to adapt to salinization owing to their evolutionary history. Likely consequences include loss of biodiversity and changes in community composition. Loss of EPT with a relative increase in DCOH in slightly salinizing rivers is expected, and a decline in the species richness of all insects in more salinized rivers.
Acknowledgements
The comments of three anonymous reviewers and the editor, Surayya Johar, improved this paper.
Authors' contributions
Developed the conceptual framework: B.J.K., R.T., R.P.D., J.D. and D.B. Reviewed literature and drafted sections of the text: B.J.K., A.H., D.B., J.D. and M.C.-A. Compiled text into one document: B.J.K. Edited the manuscript and approved the final version: all authors.
Competing interests
We have no competing interests.
Funding
M.C.-A. was supported by the People Programme (Marie Curie Actions) of the Seventh Framework Programme of the European Union (FP7/2007-2013) under grant agreement no. 600388 of REA (TECNIOSpring programme) and the Agency for Competitiveness and Business of the Government of Catalonia, ACCIÓ.
References
- 1.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schäfer RB, Schulz C-J. 2013. Salinisation of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 2.Johnson BR, Weaver PC, Nietch CT, Lazorchak JM, Struewing KA, Funk DH. 2015. Elevated major ion concentrations inhibit larval mayfly growth and development. Environ. Toxicol. Chem. 34, 167–172. ( 10.1002/etc.2777) [DOI] [PubMed] [Google Scholar]
- 3.Kay WR, Halse SA, Scanlon MD, Smith WJ. 2001. Distribution and environmental tolerances of aquatic macroinvertebrate families in the agricultural zone of southwestern Australia. J. N. Am. Benthol. Soc. 20, 182–199. ( 10.2307/1468193) [DOI] [Google Scholar]
- 4.Kefford BJ, Marchant R, Schäfer RB, Metzeling L, Dunlop JE, Choy SC, Goonan P. 2011. The definition of species richness used by species sensitivity distributions approximates observed effects of salinity on stream macroinvertebrates. Environ. Pollut. 159, 302–310. ( 10.1016/j.envpol.2010.08.025) [DOI] [PubMed] [Google Scholar]
- 5.Pond GJ. 2010. Patterns of Ephemeroptera taxa loss in Appalachian headwater streams (Kentucky, USA). Hydrobiologia 641, 185–201. ( 10.1007/s10750-009-0081-6) [DOI] [Google Scholar]
- 6.Schäfer RB, Bundschuh M, Roach D, Szöcs E, von der Ohe PC, Pettigrove V, Schulz R, Nugegoda D, Kefford BJ. 2012. Relationships of selected ecosystem functions in streams with pesticide toxicity, salinity and other environmental variables and the relevance for ecosystem services. Sci. Total Environ. 415, 69–78. ( 10.1016/j.scitotenv.2011.05.056) [DOI] [PubMed] [Google Scholar]
- 7.Short TM, Black JA, Birge WJ. 1991. Ecology of a saline stream: community responses to spatial gradients of environmental conditions. Hydrobiologia 226, 167–178. ( 10.1007/BF00006858) [DOI] [Google Scholar]
- 8.Cormier SM, Suter GW II, Zheng L. 2013. Derivation of a benchmark for freshwater ionic strength. Environ. Toxicol. Chem. 32, 263–271. ( 10.1002/etc.2064) [DOI] [PubMed] [Google Scholar]
- 9.Piscart C, Usseglio-Polatera P, Moreteau J-C, Beisel J-N. 2006. The role of salinity in the selection of biological traits of freshwater invertbrates. Arch. Hydrobiol. 166, 185–198. ( 10.1127/0003-9136/2006/0166-0185) [DOI] [Google Scholar]
- 10.Szöcs E, Coring E, Bäthe J, Schäfer RB. 2014. Effects of anthropogenic salinization on biological traits and community composition of stream macroinvertebrates. Sci. Total Environ. 468–469, 943–949. ( 10.1016/j.scitotenv.2013.08.058) [DOI] [PubMed] [Google Scholar]
- 11.Williams WD. 2002. Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025. Environ. Conserv. 29, 154–167. ( 10.1017/S0376892902000103) [DOI] [Google Scholar]
- 12.Millán A, Velasco J, Gutiérrez-Cánovas C, Arribas P, Picazo F, Sánchez-Fernández D, Abellán P. 2011. Mediterranean saline streams in southeast Spain: what do we know? J. Arid Environ. 75, 1352–1359. ( 10.1016/j.jaridenv.2010.12.010) [DOI] [Google Scholar]
- 13.Griffith MB. 2014. Natural variation and current reference for specific conductivity and major ions in wadeable streams of the conterminous USA. Freshwat. Sci. 33, 1–17. ( 10.1086/674704) [DOI] [Google Scholar]
- 14.Winterbourn M, Anderson NH. 1980. The life history of Philanisus plebeius Walker (Trichoptera: Chathamiidae), a caddisfly whose eggs were found in a starfish. Ecol. Entomol. 5, 293–303. ( 10.1111/j.1365-2311.1980.tb01151.x) [DOI] [Google Scholar]
- 15.Cheng L. 1976. Marine insects. Amsterdam, The Netherlands: North-Holland Publishing Company. [Google Scholar]
- 16.Kefford BJ, Piscart C, Hickey HL, Gasith A, Ben-David E, Dunlop JE, Palmer CG, Allan K, Choy SC. 2012. Global scale variation in the salinity sensitivity of riverine macroinvertebrates: eastern Australia, France, Israel and South Africa. PLoS ONE 7, e35224 ( 10.1371/journal.pone.0035224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schäfer RB, Kefford BJ, Metzeling L, Liess M, Burgert S, Marchant R, Pettigrove V, Goonan P, Nugegoda D. 2011. A trait database of stream invertebrates for the ecological risk assessment of single and combined effects of salinity and pesticides in South-East Australia. Sci. Total Environ. 409, 2055–2063. ( 10.1016/j.scitotenv.2011.01.053) [DOI] [PubMed] [Google Scholar]
- 18.Kefford BJ. 1998. The relationship between electrical conductivity and selected macroinvertebrate communities in four river systems of south-west Victoria, Australia. Int. J. Salt Lake Res. 7, 153–170. ( 10.1007/BF02441884) [DOI] [Google Scholar]
- 19.Piscart C, Moreteau J-C, Beisel J-N. 2005. Biodiversity and structure of macroinvertebrate communities along a small permanent salinity gradient (Meurthe River, France). Hydrobiologia 551, 227–236. ( 10.1007/s10750-005-4463-0) [DOI] [Google Scholar]
- 20.Céspedes V, Pallarés S, Arribas P, Millán A, Velasco J. 2013. Water beetle tolerance to salinity and anionic composition and its relationship to habitat occupancy. J. Insect Physiol. 59, 1076–1084. ( 10.1016/j.jinsphys.2013.08.006) [DOI] [PubMed] [Google Scholar]
- 21.Strong EE, Colgan DJ, Healy JM, Lydeard C, Ponder WF, Glaubrecht M. 2011. Phylogeny of the gastropod superfamily Cerithioidea using morphology and molecules. Zool. J. Linn. Soc. 162, 43–89. ( 10.1111/j.1096-3642.2010.00670.x) [DOI] [Google Scholar]
- 22.Betancur-R R, Ortí G, Pyron RA. 2015. Fossil-based comparative analyses reveal ancient marine ancestry erased by extinction in ray-finned fishes. Ecol. Lett. 18, 441–450. ( 10.1111/ele.12423) [DOI] [PubMed] [Google Scholar]
- 23.Hopkins GR, Brodie ED Jr. 2015. Occurrence of amphibians in saline habitats: a review and evolutionary perspective. Herpetol. Monogr. 29, 1–27. ( 10.1655/HERPMONOGRAPHS-D-14-00006) [DOI] [Google Scholar]
- 24.Bradley TJ. 2009. Animal osmoregulation. Oxford, UK: Oxford University Press. [Google Scholar]
- 25.Sarma SSA, Elguea-Sanchez B, Nandini S. 2002. Effect of salinity on competition between the rotifers Brachionus rotundiformis Tschugunoff and Hexarthra jenkinae (De Beauchamp) (Rotifera). Hydrobiologia 474, 183–188. ( 10.1023/A:1016535821741) [DOI] [Google Scholar]
- 26.Williams DD. 1999. Why are there so few insects in the sea? Trends Entomol. 2, 63–70. [Google Scholar]
- 27.Nielsen DL, Hillman TJ. 2000. Ecological effects of dryland salinity on aquatic ecosystems. Albury: CRC for Freshwater Ecology, Murray Darling Freshwater Research Centre.
- 28.Williams WD, Taaffe RG, Boulton AJ. 1991. Longitudinal distribution of macroinvertebrates in two rivers subject to salinization. Hydrobiologia 210, 151–160. ( 10.1007/BF00014329) [DOI] [Google Scholar]
- 29.Kefford BJ, Palmer CG, Nugegoda D. 2005. Relative salinity tolerance of freshwater macroinvertebrates, from the south-east of the Eastern Cape, South Africa compared to the Barwon Catchment, Victoria, Australia. Mar. Freshw. Res. 56, 163–171. ( 10.1071/MF04098) [DOI] [Google Scholar]
- 30.Kefford BJ, Nugegoda D, Metzeling L, Fields EJ. 2006. Validating species sensitivity distributions using salinity tolerance of riverine macroinvertebrates in the southern Murray–Darling Basin (Victoria, Australia). Can. J. Fish. Aquat. Sci. 63, 1865–1877. ( 10.1139/f06-080) [DOI] [Google Scholar]
- 31.Kefford BJ, Papas PJ, Metzeling L, Nugegoda D. 2004. Do laboratory salinity tolerances of freshwater animals correspond with their field salinity? Environ. Pollut. 129, 355–362. ( 10.1016/j.envpol.2003.12.005) [DOI] [PubMed] [Google Scholar]
- 32.Kefford BJ, Dalton A, Palmer CG, Nugegoda D. 2004. The salinity tolerance of eggs and hatchlings of selected aquatic macroinvertebrates in south-east Australia and South Africa. Hydrobiologia 517, 179–192. ( 10.1023/B:HYDR.0000027346.06304.bc) [DOI] [Google Scholar]
- 33.Kefford BJ, Nugegoda D, Zalizniak L, Fields EF, Hassell KL. 2007. The salinity tolerance of freshwater macroinvertebrate eggs and hatchlings in comparison to their older life-stages. Aquat. Ecol. 41, 335–348. ( 10.1007/s10452-006-9066-y) [DOI] [Google Scholar]
- 34.James KR, Cant B, Ryan T. 2003. Responses of freshwater biota to rising salinity levels and implications for saline water managements: a review. Aust. J. Bot. 51, 703–713. ( 10.1071/BT02110) [DOI] [Google Scholar]
- 35.Hassell KL, Kefford BJ, Nugegoda D. 2006. Sub-lethal and chronic lethal salinity tolerance of three freshwater insects: Cloeon sp. and Centroptilum sp. (Ephemeroptera: Baetidae) and Chironomus sp. (Diptera: Chironomidae). J. Exp. Biol. 209, 4024–4032. ( 10.1242/jeb.02457) [DOI] [PubMed] [Google Scholar]
- 36.Dowse R, Palmer CG, Kefford BJ. 2013. Risk assessment using the species sensitivity distribution method: data quality versus data quantity. Environ. Toxicol. Chem. 32, 1360–1369. ( 10.1002/etc.2190) [DOI] [PubMed] [Google Scholar]
- 37.Boeuf G, Payan P. 2001. How should salinity influence fish growth? Comp. Biochem. Physiol. C 130, 411–423. ( 10.1016/S1532-0456(01)00268-X) [DOI] [PubMed] [Google Scholar]
- 38.Kefford BJ, Zalizniak L, Nugegoda D. 2006. Growth of the damselfly Ischnura heterosticta is better in saline water than freshwater. Environ. Pollut. 141, 409–419. ( 10.1016/j.envpol.2005.08.064) [DOI] [PubMed] [Google Scholar]
- 39.Kefford BJ, Fields EJ, Nugegoda D, Clay C. 2007. The salinity tolerance of riverine microinvertebrates from the southern Murray–Darling Basin. Mar. Freshw. Res. 58, 1019–1031. ( 10.1071/MF06046) [DOI] [Google Scholar]
- 40.Potts WTW. 1954. The energetics of osmotic regulation in brackish- and fresh-water animals. J. Exp. Biol. 31, 618–630. [Google Scholar]
- 41.Kefford BJ, Nugegoda D. 2005. No evidence for a critical salinity threshold for growth and reproduction in the freshwater snail Physa acuta. Environ. Pollut. 54, 755–765. ( 10.1016/j.envpol.2004.09.018) [DOI] [PubMed] [Google Scholar]
- 42.Edwards HA. 1982. Aedes aegypti: energetics of osmoregulation. J. Exp. Biol. 101, 135–141. [Google Scholar]
- 43.Bayly IAE. 1972. Salinity tolerance and osmotic behaviour of animals in athalassic saline and marine hypersaline waters. Annu. Rev. Ecol. Syst. 3, 233–268. ( 10.1146/annurev.es.03.110172.001313) [DOI] [Google Scholar]
- 44.Dow J, Harvey WR. 1988. Role of midgut electrogenic K+ pump potential difference in regulating lumen K+ and pH in larval Lepidoptera. J. Exp. Biol. 140, 455–463. [DOI] [PubMed] [Google Scholar]
- 45.Donini A, O'Donnell MJ, Orchard I. 2008. Differential actions of diuretic factors on the Malpighian tubules of Rhodnius prolixus. J. Exp. Biol. 211, 42–48. ( 10.1242/jeb.011882) [DOI] [PubMed] [Google Scholar]
- 46.Komnick H. 1977. Chloride cells and chloride epithelia of aquatic insects. Int. Rev. Cytol. 49, 285–329. ( 10.1016/S0074-7696(08)61951-8) [DOI] [Google Scholar]
- 47.Poteat M, Buchwalter DB. 2013. Calcium uptake in aquatic insects: influences of phylogeny and metals (Cd and Zn). J. Exp. Biol. 217, 1180–1186. ( 10.1242/jeb.097261) [DOI] [PubMed] [Google Scholar]
- 48.Buchwalter DB, Jenkins JJ, Curtis LR. 2002. Respiratory strategy is a major determinant of [3H]water and [14C]chlorpyrifos uptake in aquatic insects. Can. J. Fish Aquat. Sci. 59, 1315–1322. ( 10.1139/f02-107) [DOI] [Google Scholar]
- 49.Sutcliffe DW. 1962. The composition of haemolymph in aquatic insects. J. Exp. Biol. 39, 325–343. [Google Scholar]
- 50.Sutcliffe DW. 1961. Studies on salt and water balance in caddis larvae (Trichoptera): I. Osmotic and ionic regulation of body fluids in Limnephilus affinis Curtis. J. Exp. Biol. 38, 501–519. [Google Scholar]
- 51.Hoffmann AA, Willi Y. 2008. Detecting genetic responses to environmental change. Nat. Rev. Genet. 9, 421–432. ( 10.1038/nrg2339) [DOI] [PubMed] [Google Scholar]
- 52.DeFaveri J, Merila J. 2014. Local adaptation to salinity in the three-spined stickleback? J. Evol. Biol. 27, 290–302. ( 10.1111/jeb.12289) [DOI] [PubMed] [Google Scholar]
- 53.McCairns RJS, Bernatchez L. 2010. Adaptive divergence between freshwater and marine sticklebacks: insights into the role of phenotypic plasticity from an integrated analysis of candidate gene expression. Evolution 64, 1029–1047. ( 10.1111/j.1558-5646.2009.00886.x) [DOI] [PubMed] [Google Scholar]
- 54.Whitehead A, Roach JL, Zhang SJ, Galvez F. 2011. Genomic mechanisms of evolved physiological plasticity in killifish distributed along an environmental salinity gradient. Proc. Natl Acad. Sci. USA 108, 6193–6198. ( 10.1073/pnas.1017542108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Velotta JP, McCormick SD, O'Neill RJ, Schultz ET. 2014. Relaxed selection causes microevolution of seawater osmoregulation and gene expression in landlocked Alewives. Oecologia 175, 1081–1092. ( 10.1007/s00442-014-2961-3) [DOI] [PubMed] [Google Scholar]
- 56.Kozak GM, Brennan RS, Berdan EL, Fuller RC, Whitehead A. 2014. Functional and population genomic divergence within and between two species of killifish adapted to different osmotic niches. Evolution 68, 63–80. ( 10.1111/evo.12265) [DOI] [PubMed] [Google Scholar]
- 57.Posavi M, Gelembiuk GW, Larget B, Lee CE. 2014. Testing for beneficial reversal of dominance during salinity shifts in the invasive copepod Eurytemora affinis, and implications for the maintenance of genetic variation. Evolution 68, 3166–3183. ( 10.1111/evo.12502) [DOI] [PubMed] [Google Scholar]
- 58.Weider LJ, Hebert PDN. 1987. Ecological and physiological differentiation among low-arctic clones of Daphnia pulex. Ecology 68, 188–198. ( 10.2307/1938819) [DOI] [Google Scholar]
- 59.Ortells R, Reusch TBH, Lampert W. 2005. Salinity tolerance in Daphnia magna: characteristics of genotypes hatching from mixed sediments. Oecologia 143, 509–516. ( 10.1007/s00442-005-0027-2) [DOI] [PubMed] [Google Scholar]
- 60.Loureiro C, Castro BB, Cuco AP, Pedrosa MA, Goncalves F. 2013. Life-history responses of salinity-tolerant and salinity-sensitive lineages of a stenohaline cladoceran do not confirm clonal differentiation. Hydrobiologia 702, 73–82. ( 10.1007/s10750-012-1308-5) [DOI] [Google Scholar]
- 61.Stoks R, Geerts AN, De Meester L. 2014. Evolutionary and plastic responses of freshwater invertebrates to climate change: realized patterns and future potential. Evol. Appl. 7, 42–55. ( 10.1111/eva.12108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heine-Fuster I, Vega-Retter C, Sabat P, Ramos-Jiliberto R. 2010. Osmoregulatory and demographic responses to salinity of the exotic cladoceran Daphnia exilis. J. Plankton Res. 32, 1405–1411. ( 10.1093/plankt/fbq055) [DOI] [Google Scholar]
- 63.Renborg E, Johannesson K, Havenhand J. 2014. Variable salinity tolerance in ascidian larvae is primarily a plastic response to the parental environment. Evol. Ecol. 28, 561–572. ( 10.1007/s10682-013-9687-2) [DOI] [Google Scholar]
- 64.Kishi M, Harada T, Fujisaki K. 2009. Responses of life-history traits of brackish- and freshwater populations of the water strider to NaCl Aquarius paludum (Hemiptera: Gerridae). Eur. J. Entomol. 106, 43–48. ( 10.14411/eje.2009.006) [DOI] [Google Scholar]
- 65.Pinder AM, Halse SA, McRae JM, Shiel RJ. 2004. Aquatic invertebrate assemblages of wetlands and rivers in the wheatbelt region of Western Australia. Rec. Western Aust. Suppl. 67, 7–37. [Google Scholar]
- 66.Bäthe J. 1997. Decreasing salinity in Werra and Weser (Germany): reactions of the phytoplankton and the macrozoobenthos. Limnologica 27, 111–119. [Google Scholar]
- 67.Lee CE, Bell MA. 1999. Causes and consequences of recent freshwater invasions by saltwater animals. Trends Ecol. Evol. 14, 284–288. ( 10.1016/S0169-5347(99)01596-7) [DOI] [PubMed] [Google Scholar]
- 68.Abellán P, Gómez-Zurita J, Millán A, Sánchez-Fernández D, Velasco J, Galián J, Ribera I. 2007. Conservation genetics in hypersaline inland waters: mitochondrial diversity and phylogeography of an endangered Iberian beetle (Coleoptera: Hydraenidae). Conserv. Genet. 8, 79–88. ( 10.1007/s10592-006-9150-9) [DOI] [Google Scholar]
- 69.Velasco J, Millán A, Hernández J, Cayetano G, Abellán P, Sánchez D, Ruiz M. 2006. Response of biotic communities to salinity changes in a Mediterranean hypersaline stream. Saline Syst. 2, 12 ( 10.1186/1746-1448-2-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herbst DB. 2001. Gradients of salinity stress, environmental stability and water chemistry as a templet for defining habitat types and physiological strategies in inland salt waters. Hydrobiologia 466, 209–219. ( 10.1023/A:1014508026349) [DOI] [Google Scholar]
- 71.Liess M, Champeau O, Riddle M, Schulz R, Duquesne S. 2001. Combined effects of ultraviolet-B radiation and food shortage on the sensitivity of the Antarctic amphipod Paramoera walkeri to copper. Environ. Toxicol. Chem. 20, 2088–2092. ( 10.1002/etc.5620200931) [DOI] [PubMed] [Google Scholar]
- 72.Beketov M, Liess M. 2005. Acute contamination with esfenvalerate and food limitation: chronic effects on the mayfly Cloeon dipterum. Environ. Toxicol. Chem. 24, 1281–1286. ( 10.1897/04-256R1.1) [DOI] [PubMed] [Google Scholar]
- 73.Alcaraz C, Bisazza A, García-Berthou E. 2008. Salinity mediates the competitive interactions between invasive mosquitofish and an endangered fish. Oecologia 155, 205–213. ( 10.1007/s00442-007-0899-4) [DOI] [PubMed] [Google Scholar]
- 74.Cañedo-Argüelles M, Sala M, Peixoto G, Prat N, Faria M, Soares AMVM, Barata C, Kefford B. 2015. Can salinity trigger cascade effects on streams? A mesocosm approach. Sci. Total Environ. 540, 3–10. ( 10.1016/j.scitotenv.2015.03.039) [DOI] [PubMed] [Google Scholar]
- 75.Becker JM, Liess M. 2015. Biotic interactions govern genetic adaptation to toxicants. Proc. R. Soc. B 282, 20150071 ( 10.1098/rspb.2015.0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Badyaev AV, Ghalambor CK. 2001. Evolution of life histories along elevational gradients: trade-off between parental care and fecundity. Ecology 82, 2948–2960. ( 10.1890/0012-9658(2001)082[2948:EOLHAE]2.0.CO;2) [DOI] [Google Scholar]
- 77.Cormier SM, Suter GW II, Zheng L, Pond GJ. 2013. Assessing causation of the extirpation of stream macroinvertebrates by a mixture of ions. Environ. Toxicol. Chem. 32, 277–287. ( 10.1002/etc.2059) [DOI] [PubMed] [Google Scholar]
- 78.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. 1997. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (flathead minnows). Environ. Toxicol. Chem. 16, 2009–2019. ( 10.1002/etc.5620161005) [DOI] [Google Scholar]
- 79.Kefford BJ, Palmer CG, Pakhomova L, Nugegoda D. 2005. Comparing different approaches to measuring the salinity tolerance of freshwater invertebrates. Water SA 30, 499–506. ( 10.4314/wsa.v30i4.5102) [DOI] [Google Scholar]
- 80.Kunz JL, Conley JM, Buchwalter DB, Norberg-King TJ, Kemble NE, Wang N, Ingersoll CG. 2013. Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates. Environ. Toxicol. Chem. 32, 2826–2835. ( 10.1002/etc.2391) [DOI] [PubMed] [Google Scholar]
- 81.Van Dam RA, Hogan AC, Mccullough CD, Houston MA, Humphrey CL, Harford AJ. 2010. Aquatic toxicity of magnesium sulfate, and the influence of calcium, in very low ionic concentration water. Environ. Toxicol. Chem. 29, 410–421. ( 10.1002/etc.56) [DOI] [PubMed] [Google Scholar]
- 82.Kefford BJ. 2000. The effect of saline water disposal: implications for monitoring programs and management. Environ. Monit. Assess. 63, 313–327. ( 10.1023/A:1006201512469) [DOI] [Google Scholar]
- 83.Vera CL, Hyne RV, Patra R, Ramasamy S, Pablo F, Julli M, Kefford BJ. 2014. Bicarbonate toxicity to Ceriodaphnia dubia and the freshwater shrimp Paratya australiensis and its influence on zinc toxicity. Environ. Toxicol. Chem. 33, 1179–1186. ( 10.1002/etc.2545) [DOI] [PubMed] [Google Scholar]