Abstract
Plants exposed to excess metals are challenged by an increased generation of reactive oxygen species (ROS) such as superoxide (), hydrogen peroxide (H2O2) and the hydroxyl radical (•OH). The mechanisms underlying this oxidative challenge are often dependent on metal-specific properties and might play a role in stress perception, signaling and acclimation. Although ROS were initially considered as toxic compounds causing damage to various cellular structures, their role as signaling molecules became a topic of intense research over the last decade. Hydrogen peroxide in particular is important in signaling because of its relatively low toxicity, long lifespan and its ability to cross cellular membranes. The delicate balance between its production and scavenging by a plethora of enzymatic and metabolic antioxidants is crucial in the onset of diverse signaling cascades that finally lead to plant acclimation to metal stress. In this review, our current knowledge on the dual role of ROS in metal-exposed plants is presented. Evidence for a relationship between H2O2 and plant metal tolerance is provided. Furthermore, emphasis is put on recent advances in understanding cellular damage and downstream signaling responses as a result of metal-induced H2O2 production. Finally, special attention is paid to the interaction between H2O2 and other signaling components such as transcription factors, mitogen-activated protein kinases, phytohormones and regulating systems (e.g. microRNAs). These responses potentially underlie metal-induced senescence in plants. Elucidating the signaling network activated during metal stress is a pivotal step to make progress in applied technologies like phytoremediation of polluted soils.
Keywords: metals, hydrogen peroxide, oxidative stress, damage, signaling, crosstalk
The relationship between metals and oxidative stress in plants
Pollution of soils, air, (ground)water and sediments with toxic metals is one of the major problems our industrialized world is currently facing. Naturally occurring levels of these metals have been significantly exceeded by anthropogenic activities over the past two centuries. Mining and industry, as well as the use of phosphate fertilizers and sewage sludge in agriculture have jointly contributed to an increased production and emission of metals. As opposed to many organic contaminants, metals are non-biodegradable, resulting in their extended persistence in the environment. In addition, food and feed crop plants facilitate the entry of toxic metals into the food chain, thereby leading to bio-enrichment and enhanced risks for human health (Cuypers et al., 2009; Sharma and Dietz, 2009). The latter has been demonstrated by a plethora of in vitro, in vivo and epidemiological studies, revealing that the highest health risks are associated with exposure to cadmium (Cd), lead (Pb) and mercury (Hg). Adverse metal-induced health effects are wide-ranging, for example with kidney damage, bone effects and cancer related to human Cd exposure (Järup, 2003; Nair et al., 2013). Nevertheless, metal exposure persists and even increases in less developed countries (Järup, 2003), urging the need to remediate metal-polluted soils.
Metals are categorized as essential or non-essential for plant growth, with different dose-response curves for both classes (Lin and Aarts, 2012). Essential micronutrients such as copper (Cu), iron (Fe), nickel (Ni) and zinc (Zn) function as cofactors in over 1500 proteins crucial for the plant's metabolism. For example, Cu is cardinal for photosynthesis and mitochondrial respiration, while Zn-containing enzymes are important regulators of transcription and translation. For that reason, either too low or high levels of these essential metals would adversely affect plant growth and development (Hänsch and Mendel, 2009; Pilon et al., 2009). To avoid both deficiency and excess, plant cells possess different mechanisms to tightly control the concentrations of essential metals (Lin and Aarts, 2012). However, even low concentrations of non-essential metals such as Cd, Pb and Hg disturb biochemical and physiological processes and decrease plant productivity (Lin and Aarts, 2012).
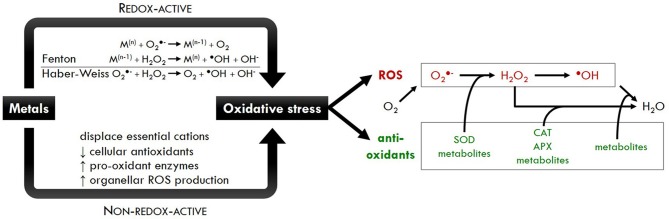
Sharma and Dietz (2009) have described three major mechanisms underlying metal toxicity in plants. First, different metals show a high affinity toward sulfur or nitrogen donors within proteins, which might interfere with cellular metabolism. Metals are also able to displace essential cations from their specific binding sites within an enzyme. For example, Cd2+ was suggested to competitively bind to the essential calcium (Ca2+) site in photosystem II during photoactivation (Faller et al., 2005). Finally, multiple studies have demonstrated that exposure of plants to a diverse array of metals elicits oxidative stress, indicating a misbalance between the production and neutralization of reactive oxygen species (ROS) such as superoxide (), hydrogen peroxide (H2O2) and the hydroxyl radical (•OH) (Schützendübel and Polle, 2002; Sharma and Dietz, 2009). In view of the different chemical properties of metals, two modes of action can be distinguished. Under physiological conditions, redox-active metals such as Cu and Fe exist in different oxidation states (Cu+∕2+ and Fe2+∕3+). This enables both metals to directly participate in the Fenton and Haber-Weiss reactions, finally leading to the formation of highly toxic •OH radicals from H2O2 (Figure 1; Schützendübel and Polle, 2002; Hänsch and Mendel, 2009; Sharma and Dietz, 2009). On the other hand, physiologically non-redox-active metals such as Cd, Hg, and Zn only indirectly contribute to increased ROS production, for example by depleting or inhibiting cellular antioxidants (Figure 1; Schützendübel and Polle, 2002; Sharma and Dietz, 2009).
Figure 1.
Schematic overview of metal-induced oxidative stress. Redox-active metals such as Cu and Fe can participate in the Fenton and Haber-Weiss reactions, finally leading to the formation of highly toxic •OH radicals from H2O2. On the other hand, non-redox-active metals such as Cd and Zn can only indirectly contribute to ROS production by (1) displacing essential cations, (2) depleting cellular antioxidants, (3) increasing the activity of ROS producing enzymes and/or (4) enhancing ROS production in organelles. The net result for both classes of metals is the induction of oxidative stress, an imbalance between ROS and antioxidants in favor of the former. Abbreviations: APX, ascorbate peroxidase; CAT, catalase; H2O2, hydrogen peroxide; M(n), oxidized redox-active metal; M(n−1), reduced redox-active metal; , superoxide; OH−, hydroxide ion; •OH, hydroxyl radical; SOD, superoxide dismutase.
The term “oxidative stress” implies a harmful process, which is mainly related to the oxidizing nature of ROS. However, intense research over the past decades has shifted this paradigm, pointing toward a dual role for ROS as damaging vs. signaling compounds (Foyer and Noctor, 2005). Currently, ROS and H2O2 in particular are considered as essential components of signal transduction used by plants to respond to developmental and environmental cues. In this review, it is our intent to provide an overview of the experimental evidence underlying a dual role for H2O2 during metal stress in plants. Within this framework, both H2O2-induced damage and signaling—including its targets and interaction with other signaling pathways and regulating systems—are highlighted. Ultimately, the term “oxidative challenge” is preferred, as this implies the harmful vs. beneficial effects of H2O2 produced in metal-exposed plants (Figure 2).
Figure 2.
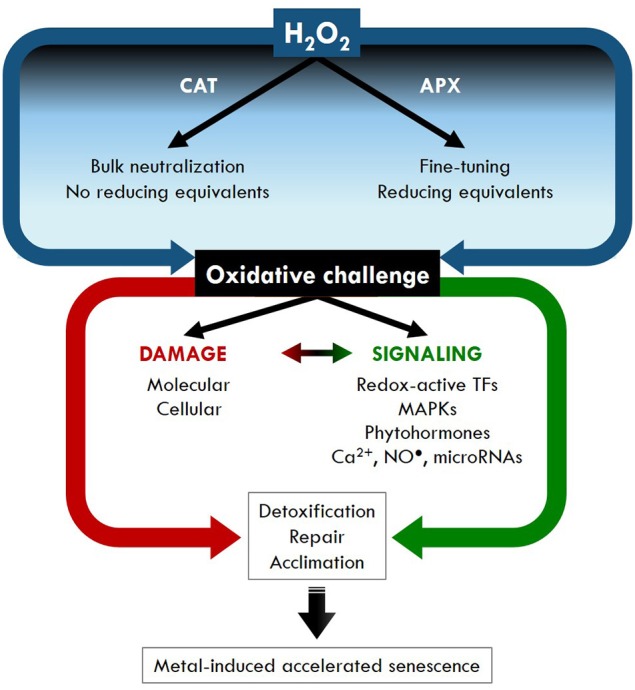
Generalized model for the central role of hydrogen peroxide (H2O2) in metal-induced oxidative damage vs. signaling responses. Ascorbate peroxidase (APX) and catalase (CAT) are the two most important enzymes that counterbalance metal-induced H2O2 production in plants. However, APX has a much higher affinity for H2O2 as compared to CAT. Therefore, the former enzyme is considered to be primarily involved in the fine-tuning of H2O2 levels crucial for their signaling function. Conversely, CAT is important for the bulk removal of excess H2O2 produced in stressed plants. In addition, while APX uses AsA as a reducing agent to detoxify H2O2, CAT does not need any reducing equivalents. Depending on its extent, the metal-induced rise in H2O2 content can lead to molecular and cellular damage and/or signaling. Different studies highlight the interaction between ROS/H2O2 and signaling components such as redox-active transcription factors (TFs), mitogen-activated protein kinases (MAPKs), phytohormones, Ca2+, NO• and regulating systems like miRNAs. Finally, detoxification, repair and acclimation responses are activated, with accelerated senescence as a potential last resort in metal-exposed plants.
Hydrogen peroxide, a signaling molecule in disguise
Both energy transfer to as well as incomplete reduction of O2 generate ROS such as singlet oxygen (1O2) and , H2O2 and •OH respectively. These reactive intermediates are byproducts of physiological processes such as photosynthesis and respiration, with a high oxidizing potential toward DNA, lipids and proteins. However, not all ROS are equally reactive, with and H2O2 being rather selective in their reactions and •OH attacking all molecules in its surroundings (Halliwell, 2006; Møller et al., 2007). Under steady-state conditions, antioxidant enzymes and metabolites tightly control ROS concentrations in different cellular compartments to prevent oxidative damage (Mittler et al., 2004, 2011). In addition, plants have developed a way to employ low levels of ROS as signaling compounds to appropriately and coordinately respond to developmental as well as environmental cues (Petrov and Van Breusegem, 2012). It has long been known that different biotic (e.g. pathogen attack) and abiotic (e.g. drought, salinity, heat and metal stress) stimuli increase ROS generation in plants, leading to a misbalance between ROS and antioxidants in favor of the former (Dat et al., 2000; Apel and Hirt, 2004). Especially under these conditions, the use of ROS in signal transduction can contribute to acclimation and eventually tolerance to various stressors (Hossain et al., 2015).
Among all ROS, H2O2 is often put forward as the most attractive signaling molecule (Neill S. et al., 2002; Neill S. J. et al., 2002; Foyer and Noctor, 2005; Petrov and Van Breusegem, 2012). It is produced by a two-step reduction of molecular O2. Superoxide—generated after the first reduction step—is converted into H2O2, for example by superoxide dismutase (SOD). Subsequently, H2O2 can give rise to highly toxic •OH radicals through the Fenton and Haber-Weiss reactions with the help of free redox-active metal ions (Figure 1; Halliwell, 2006). With a half-life of 1 ms, H2O2 is relatively stable as compared to and •OH that have a half-life of only 1 μs and 1 ns, respectively (Møller et al., 2007). Additional advantages are its high cellular abundance (up to the low millimolar range) (Cheeseman, 2006; Møller et al., 2007), its small size (Petrov and Van Breusegem, 2012) and its ability to cross cellular membranes through aquaporins and thereby migrate to different cellular compartments (Bienert et al., 2006, 2007; Bienert and Chaumont, 2014). Furthermore, H2O2 is an uncharged non-radical with an intermediate oxidation number (−1 for each oxygen atom), implying both oxidizing and reducing properties (Bienert et al., 2007; Bienert and Chaumont, 2014). With regard to H2O2 scavenging, it is important to keep in mind the unique property of catalase (CAT) among all antioxidative enzymes: it is able to convert H2O2 to H2O2 and O2 without the use of reducing equivalents (see Section “Production and Scavenging of H2O2 in Metal-Exposed Plants”) (Bienert et al., 2007; Das and Roychoudhury, 2014). The fact that H2O2 scavenging by CAT occurs in an energy-efficient way can be a crucial asset under environmental stress conditions, when energy is required to set up an appropriate defense response (Gechev et al., 2006; Das and Roychoudhury, 2014).
Reactive oxygen species are able to transmit a signal by oxidizing a target molecule, for example a transcription factor (Mittler et al., 2004). The relatively long-living H2O2 can travel a cellular distance up to 1 μm and brings the signal close to its target, thereby acting as primary messenger. However, the cellular distance traveled by more short-living ROS ranges from a mere nm (•OH) up to 30 nm (1O2 and ). These will therefore react with a cellular compound close to their production site, with the oxidation product acting as second messenger (Møller et al., 2007). However, both routes lead to the same net signaling result for ROS with different physicochemical properties. In the following paragraphs, the production and scavenging of H2O2 is discussed in the light of metal stress. Furthermore, results from priming experiments and screenings of metal tolerant vs. sensitive genotypes/ecotypes have revealed a strong relationship between H2O2 and metal tolerance in plants.
Production and scavenging of H2O2 in metal-exposed plants
In plants, H2O2 and other ROS are continuously produced in different subcellular compartments as byproducts of various metabolic reactions. While most ROS in plant cells originate from chloroplasts and peroxisomes, mitochondria are the most important ROS producers under dark conditions and in non-photosynthetic tissues (Navrot et al., 2007; Das and Roychoudhury, 2014). In chloroplasts and mitochondria, leakage of electrons to O2 as a consequence of electron transport chain over reduction can generate radicals, which can subsequently be converted to H2O2. In peroxisomes, H2O2 can be directly produced by oxidation reactions of fatty acids and glycolate formed during photorespiration (Petrov and Van Breusegem, 2012).
On the other hand, ROS can also be enzymatically generated in the apoplast. At the plasma membrane, is generated by NADPH oxidases. These enzymes are homologs of the mammalian respiratory burst oxidase gp91phox and are therefore referred to as respiratory burst oxidase homologs (RBOHs) (O'Brien et al., 2012). Using NADPH as a cytosolic electron donor, they catalyze the reduction of apoplastic O2 to , which can then be dismutated to H2O2 either non-enzymatically or by the action of SOD. Furthermore, apoplastic ROS can also be produced by cell wall-anchored class III peroxidases. Although these enzymes are also involved in H2O2 scavenging, they are able to generate H2O2 in the presence of a strong reductant. Reactive oxygen species produced by the action of these peroxidases play an important role in several developmental processes including cell wall cross-linking and loosening (O'Brien et al., 2012; Kärkönen and Kuchitsu, 2015).
While ROS production in organelles and the apoplast continuously occurs under physiological growth conditions, it can be greatly enhanced by biotic and abiotic stress factors (Gechev et al., 2006; Petrov and Van Breusegem, 2012). As demonstrated in Table 1, exposure to even environmentally relevant metal concentrations increased the production of H2O2 in a wide variety of plant species. As discussed before, the mechanisms underlying metal-induced ROS production in plants are dependent on the chemical properties of the metal. Indirect metal-induced ROS production can be achieved by several mechanisms (Cuypers et al., 2012). Metals can for example inhibit the activity of various enzymes by binding to their functional groups or by displacement of essential cations in specific binding sites (Gupta et al., 2009; Cuypers et al., 2011). In this way, they can disturb the action of enzymes involved in antioxidative defense and physiological processes such as respiration and photosynthesis, thereby increasing ROS production. Furthermore, metals are able to deplete the pool of the important antioxidant glutathione (GSH), thereby also disturbing the ROS balance (Lee et al., 2003). In addition, several metals were shown to increase ROS production by plasma membrane-bound NADPH oxidases (Figure 1; Romero-Puertas et al., 2004; Hao et al., 2006; Remans et al., 2010).
Table 1.
Metal-induced H2O2 production and scavenging in plants.
| Metal | Species | H2O2 scavenging | References | |||||
|---|---|---|---|---|---|---|---|---|
| APX | CAT | |||||||
| H2O2 production | Activity | Gene expression | Activity | Gene expression | ||||
| Essential | Cu | Arabidopsis thaliana | x | x | x | x | x | Cuypers et al., 2011 |
| x | Liu et al., 2015 | |||||||
| x | Martínez-Peñalver et al., 2012 | |||||||
| x | x | x | Opdenakker et al., 2012a | |||||
| x | Yuan et al., 2013 | |||||||
| Cucumis sativus | x | x | İşeri et al., 2011 | |||||
| Hordeum vulgare | x | x | x | Hu et al., 2015 | ||||
| Ipomoea batatas | x | Kim et al., 2010 | ||||||
| Matricaria chamomilla | x | Kováčik et al., 2010b | ||||||
| x | x | Kováčik et al., 2010a | ||||||
| Medicago truncatula | x | Macovei et al., 2010 | ||||||
| Nicotiana tabacum | x | x | Xia et al., 2012 | |||||
| Oryza sativa | x | x | x | Mostofa et al., 2015a | ||||
| x | x | Thounaojam et al., 2012 | ||||||
| Pauwlonia fortunei | x | x | x | Wang J. et al., 2010 | ||||
| Silene dioica | x | x | Kováčik et al., 2010b | |||||
| Silene vulgaris | x | x | Kováčik et al., 2010b | |||||
| Solanum lycopersicuma | x | x | İşeri et al., 2011 | |||||
| Spirodela polyrhiza | x | x | x | Upadhyay and Panda, 2010 | ||||
| Ni | Brassica juncea | x | x | Khan and Khan, 2014 | ||||
| Brassica napus | x | x | x | Kazemi et al., 2010 | ||||
| Vicia sativa | x | x | Ivanishchev and Abramova, 2015 | |||||
| Zn | Arabidopsis thaliana | x | x | x | x | x | Remans et al., 2012a | |
| Brassica juncea | x | x | Feigl et al., 2015 | |||||
| x | x | Khan and Khan, 2014 | ||||||
| Gossypium hirsutum | x | x | x | Anwaar et al., 2015 | ||||
| Ipomoea batatas | x | Kim et al., 2010 | ||||||
| Lactuca sativa | x | Barrameda-Medina et al., 2014 | ||||||
| Myracrodruon urundeuva | x | x | x | Gomes et al., 2013 | ||||
| Pauwlonia fortunei | x | x | x | Wang J. et al., 2010 | ||||
| Phaseolus vulgaris | x | Michael and Krishnaswamy, 2011 | ||||||
| Populus × canescens | x | x | x | Shi et al., 2015 | ||||
| Solanum melongena | x | x | Wu et al., 2015 | |||||
| Solanum nigrum | x | x | x | x | x | Xu Q. S. et al., 2010 | ||
| Spirodela polyrhiza | x | x | x | Upadhyay and Panda, 2010 | ||||
| Verbacum thapsus | x | x | Morina et al., 2010 | |||||
| Non-essential | Al | Cucumis sativus | x | x | x | Pereira et al., 2010 | ||
| Nicotiana tabacum | x | x | Yin et al., 2010 | |||||
| Non-essential | Cd | Arabidopsis thaliana | x x |
x | x | x | Cuypers et al., 2011 Martínez-Peñalver et al., 2012 |
|
| x | x | Tao et al., 2013 | ||||||
| Boehmeria nivea | x | x | Tang et al., 2015 | |||||
| Brassica campestris | x | Anjum et al., 2014 | ||||||
| Brassica juncea | x | Masood et al., 2012 | ||||||
| Brassica napus | x | x | x | Ali et al., 2013 | ||||
| Citrus paradisi × Poncirus trifoliata | x | x | Podazza et al., 2012 | |||||
| Dittrichia viscosa | x | x | x | Fernández et al., 2013 | ||||
| Glycine max | x | x | x | Pérez-Chaca et al., 2014 | ||||
| Helianthus annuus | x | x | x | Saidi et al., 2014 | ||||
| Ipomoea batatas | x | Kim et al., 2010 | ||||||
| Kosteletzkya virginica | x | x | x | Han et al., 2013 | ||||
| Lactuca sativa | x | x | x | Monteiro et al., 2012 | ||||
| Lepidium sativum | x | x | x | Gill et al., 2012 | ||||
| Lupinus luteus | x | Arasimowicz-Jelonek et al., 2012 | ||||||
| Nicotiana tabacum | x | x | x | Iannone et al., 2010 | ||||
| Oryza sativa | x | x | x | Chou et al., 2011 | ||||
| x | x | x | Mostofa et al., 2015b | |||||
| x | x | Singh and Shah, 2014 | ||||||
| x | x | Srivastava et al., 2014 | ||||||
| x | x | Srivastava et al., 2015 | ||||||
| x | Wang et al., 2014 | |||||||
| x | Yu et al., 2015 | |||||||
| Populus cathayana | x | x | He et al., 2013 | |||||
| Populus nigra | x | x | x | He et al., 2013 | ||||
| Populus popularis | x | x | He et al., 2013 | |||||
| Populus × canadensis | x | Di Baccio et al., 2014 | ||||||
| Populus × canescens | x | x | x | He et al., 2011 | ||||
| Sedum alfredii | x | x | Tian et al., 2011 | |||||
| Solanum lycopersicum | x | x | x | Ahammad et al., 2013 | ||||
| x | x | x | Monteiro et al., 2011 | |||||
| Solanum nigrum | x | x | x | Deng et al., 2010 | ||||
| x | x | x | Liu et al., 2013 | |||||
| Trigonella foenum-graecum | x | x | x | Zayneb et al., 2015 | ||||
| Triticum aestivum | x | Moussa and El-Gamal, 2010 | ||||||
| Vigna radiata | x | Anjum et al., 2014 | ||||||
| Zea mays | x | Wahid and Khaliq, 2015 | ||||||
| Zygophyllum fabago | x | x | x | Yildiztugay and Ozfidan-Konakci, 2015 | ||||
| Hg | Juncus maritimus | x | x | x | Anjum et al., 2015 | |||
| Medicago sativa | x | Montero-Palmero et al., 2014 | ||||||
| Non-essential | Pb | Arabidopsis thaliana | x x |
x | Tao et al., 2013 Yu et al., 2012 |
|||
| Atractylodes macrocephala | x | x | x | Wang et al., 2013 | ||||
| Brassica napus | x | x | x | Ali et al., 2014 | ||||
| Hordeum vulgare | x | Legocka et al., 2015 | ||||||
| Lemna trisulca | x | Samardakiewicz et al., 2015 | ||||||
| Nymphoides peltatum | x | x | Qiao et al., 2013 | |||||
| Oryza sativa | x | x | Srivastava et al., 2014 | |||||
| Pauwlonia fortunei | x | x | Wang J. et al., 2010 | |||||
| Talinum triangulare | x | x | x | Kumar et al., 2013 | ||||
| Triticum aestivum | x | x | x | Kaur et al., 2013 | ||||
| x | x | x | Kaur et al., 2015 | |||||
| Vicia faba | x | Shahid et al., 2012 | ||||||
| Zygophyllum fabago | x | x | x | López-Orenes et al., 2014 | ||||
In article as Lycopersicum esculentum.
Metals have the capacity to induce oxidative stress in plants. An increase in H2O2 levels is an indicator of the disturbed redox balance. Plant cells have defense mechanisms to scavenge excess ROS, such as the antioxidative enzymes ascorbate peroxidase (APX) and catalase (CAT). The following table catalogs recent research articles (published since 2010) that reported metal-induced H2O2 production. The effects on APX and CAT, at the level of both gene expression and enzymatic activity, are indexed according to essential (Cu, Ni, and Zn) and non-essential metals (Al, Cd, Hg, and Pb) and plant species.
In order to prevent cellular damage as a result of increased ROS production, plants possess an extensive antioxidative defense system consisting of both non-enzymatic and enzymatic compounds (Figure 1). Two important non-enzymatic antioxidants are the water-soluble metabolites ascorbate (AsA) and GSH. Ascorbate can directly scavenge , H2O2, and •OH radicals and is involved in the regeneration of other antioxidants such as α-tocopherol (Das and Roychoudhury, 2014). Furthermore, it plays an important role in the AsA-GSH cycle. In the first step of this cycle, ascorbate peroxidase (APX) detoxifies H2O2 to H2O using AsA as the reducing agent. Subsequently, the reconversion of AsA to its reduced form is coupled to the oxidation of GSH, which is again reduced by the action of glutathione reductase (GR) (Cuypers et al., 2012). In addition to its involvement in the AsA-GSH cycle, GSH can also directly detoxify ROS and is the substrate of glutathione-S-transferase (GST) enzymes, catalyzing the conjugation of GSH with electrophilic compounds. Plant GSTs are subdivided into several classes and are involved in a wide range of functions including the detoxification of xenobiotics (e.g. herbicides) and products of oxidative DNA and lipid damage (Marrs, 1996; Gill and Tuteja, 2010). Furthermore, GSH plays a role in the scavenging of metals via its sulfhydryl group and is also the precursor of metal-chelating phytochelatins (PCs) (Jozefczak et al., 2012; Noctor et al., 2012). In addition to PCs, also metallothioneins (MTs) are able to bind metals such as Cu, Cd and Zn through the thiol groups of their cysteine residues. Furthermore, several studies suggest that MTs are directly involved in ROS scavenging (Hassinen et al., 2011).
In contrast to the water-soluble AsA and GSH, α-tocopherol and carotenoids are important lipid-soluble antioxidative metabolites. They are involved in protecting membranes against lipid peroxidation and preventing damage to the photosynthetic machinery, respectively (Das and Roychoudhury, 2014). In addition, the amino acid proline accumulates in plants under abiotic stress conditions including metal exposure (Sharma and Dietz, 2006). Proline is able to quench 1O2 and scavenge •OH radicals in vitro, and several studies have attributed an antioxidant function to proline under metal stress in vivo as well (Sharma and Dietz, 2006). For example, pretreatment of Oryza sativa plants with proline decreased the accumulation of H2O2 and lipid peroxidation after Hg exposure (Wang et al., 2009). These observations might be related to the fact that proline is able to protect and stabilize ROS scavenging enzymes such as CAT and peroxidases (Sharma and Dietz, 2006; Szabados and Savouré, 2009).
Among the antioxidative enzymes, SODs are responsible for the conversion of to O2 and H2O2. Based on the metal present in the active center, these enzymes are classified as Cu/Zn-SOD (localized in the apoplast, cytosol, chloroplasts and peroxisomes), Mn-SOD (localized in mitochondria) or Fe-SOD (localized in chloroplasts) (Alscher et al., 2002; Das and Roychoudhury, 2014). Scavenging of H2O2 is performed by CAT, ascorbate peroxidase (APX), glutathione peroxidase (GPX), guaiacol peroxidase, class III peroxidases and peroxiredoxins. In general, peroxidases oxidize a wide range of substrates, thereby reducing peroxides including H2O2 (Mathé et al., 2010). While APX reduces H2O2 to H2O using the reducing power of AsA, GPX uses thioredoxin and GSH as electron donors (Das and Roychoudhury, 2014; Bela et al., 2015; Passaia and Margis-Pinheiro, 2015). On the other hand, guaiacol peroxidase prefers aromatic compounds such as guaiacol and pyrogallol as electron donors to reduce H2O2 (Das and Roychoudhury, 2014). As mentioned before, class III peroxidases can both scavenge and produce ROS. In their regular peroxidative cycle, they catalyze the reduction of H2O2 using a variety of electron donors including phenolic compounds, lignin precursors, secondary metabolites and auxins (Mathé et al., 2010; Zipor and Oren-Shamir, 2013). In contrast to the above-mentioned peroxidases, peroxiredoxins detoxify H2O2 by oxidizing their own thiol groups, which are back-reduced by the action of thioredoxin, glutaredoxin, cyclophilin or GSH (Tripathi et al., 2009). While GPX, guaiacol peroxidase, class III peroxidases and peroxiredoxins are also involved in other cellular processes, CAT and APX are specifically dedicated to H2O2 scavenging and the regulation of redox homeostasis. Therefore, both enzymes are discussed in more detail in this review (Table 1). Catalase is a tetrameric heme-containing enzyme catalyzing the detoxification of H2O2 to H2O and O2, which is mainly present in peroxisomes. The APX enzyme is localized in the cytosol, mitochondria, chloroplasts and peroxisomes and converts H2O2 into H2O during the first step of the AsA-GSH cycle (Das and Roychoudhury, 2014). While APX uses AsA as a reducing agent for H2O2 detoxification, the action of CAT does not require any reducing equivalents. This provides plants with an energy-efficient way of H2O2 removal, which can be of particular interest under stress conditions (Gechev et al., 2006). However, it is important to note that the affinity of APX for H2O2 is much higher than that of CAT (micromolar vs. millimolar range). Therefore, APX is assumed to be mainly involved in the fine-tuning of H2O2 detoxification important for its signaling function, while CAT is responsible for the bulk removal of excess H2O2 generated during stress conditions (Figure 2; Mittler, 2002). As shown in Table 1, both H2O2 scavenging enzymes are affected at transcriptional and activity level in metal-exposed plants. For example, Cuypers et al. (2011) demonstrated differential effects of Cd and Cu on CAT and APX gene expression in Arabidopsis thaliana plants. Dependent on the metal concentration and isoform considered, expression levels were specifically affected in roots or leaves. Furthermore, expression changes were not always mirrored by the enzyme activities, suggesting that CAT and APX regulation also occurs at the post-transcriptional level under metal stress (Cuypers et al., 2011).
The link between H2O2 and metal tolerance in plants
In recent years, multiple studies have focused on the role of H2O2 in plant tolerance to a diverse array of abiotic stress conditions. Research has shown that pretreatment of plants with H2O2 can decrease the extent of adverse effects induced by subsequent exposure to abiotic stress factors including salinity, drought, heat, chilling and metals, a phenomenon which is generally referred to as H2O2 priming (Hossain et al., 2015). Exposure of plants to low concentrations of H2O2 (ranging from 100 to 500 μM) prior to metal treatment was shown to minimize metal-induced growth reduction, lipid peroxidation, chlorophyll degradation and programmed cell death in different plant species (Chao et al., 2009; Hu et al., 2009; Bai et al., 2011; Xu et al., 2011; Guzel and Terzi, 2013; Yildiz et al., 2013). Heat shock, known to increase H2O2 levels, can also induce metal tolerance in plants (Chao et al., 2009; Chou et al., 2012). Even though the mechanisms underlying these observations are not fully elucidated yet, available data so far point to the involvement of metal chelation, antioxidative defense and osmotic regulation in increased metal tolerance.
One of the key players in H2O2-induced metal tolerance is GSH. Indeed, many studies demonstrate an elevated GSH level in metal-exposed plants pretreated with H2O2 as compared to non-primed plants (Hu et al., 2009; Bai et al., 2011; Xu et al., 2011). As GSH is an important component of the AsA-GSH cycle, the elevated GSH level induced by H2O2 pretreatment of metal-exposed plants can contribute to an enhanced H2O2 detoxification, thereby reducing the negative effects of metal-induced oxidative stress (Apel and Hirt, 2004). Furthermore, GSH can directly chelate metals, which have a high affinity toward its sulfhydryl group. In addition, GSH is the main constituent of metal-chelating PCs. Metals sequestered by GSH and PCs are transported to the vacuole, decreasing the concentrations of free metal ions in the cytosol and thereby preventing metal-induced damage to cellular macromolecules such as DNA, proteins and membrane lipids. Moreover, vacuolar compartmentalization can also affect the transport of metals from roots to aerial plant parts (Liu W. J. et al., 2010; Jozefczak et al., 2012; Najmanova et al., 2012; Noctor et al., 2012). Indeed, Hu et al. (2009) and Bai et al. (2011) demonstrated a reduced root-to-shoot translocation of Cd in O. sativa plants pretreated with H2O2. In contrast, Yildiz et al. (2013) showed that H2O2 priming increased root-to-shoot translocation of Cr(VI) in Brassica napus plants. In these experiments however, H2O2 was able to counteract the decrease in fresh weight and the induction of lipid peroxidation caused by subsequent metal exposure. These data suggest that the mechanisms underlying H2O2-induced metal tolerance strongly depend on the metal and the plant species under study.
In addition to GSH, other antioxidants also seem to be involved in H2O2-induced metal tolerance. Xu et al. (2011) have shown that H2O2 priming enhanced the Al-induced increase in AsA levels in root tips of an Al-sensitive Triticum aestivum genotype. However, this was not observed in an Al-tolerant genotype, indicating that the inherent plant metal tolerance can influence the effect of exogenous H2O2 on the responses to subsequent metal exposure. Besides their levels, also the redox state of GSH and AsA can be affected, as indicated by increases in reduced vs. oxidized metabolite ratios by H2O2 priming in root tips of both T. aestivum genotypes after Al exposure (Xu et al., 2011).
Besides metabolic antioxidants such as GSH and AsA, also antioxidative enzymes could be involved in H2O2 priming. Indeed, several studies demonstrated differences in the activities of antioxidative enzymes such as SOD, CAT and APX between metal-exposed plants that were either primed with H2O2 or not (Chao et al., 2009; Hu et al., 2009; Xu et al., 2011; Yildiz et al., 2013). This is either related to the fact that H2O2 priming (1) counteracts a metal-induced reduction in antioxidative enzyme activities, probably due to binding of the metal to the protein's cysteine residues or (2) increases basal antioxidative enzyme activities to protect plants from metal-induced oxidative damage. Furthermore, it has been shown that H2O2 pretreatment can further stimulate metal-induced increases in the activity of GST (Hu et al., 2009; Bai et al., 2011). Together, these data suggest that H2O2 priming reduces the negative consequences of metal exposure, while stimulating the plant's defense mechanisms. This H2O2-induced enhancement of antioxidative defense, combined with an increase in metal scavenging, can possibly explain the fact that H2O2 priming often diminished metal-induced increases in ROS levels (Hu et al., 2009; Xu et al., 2011; Guzel and Terzi, 2013).
In addition to its effects on metal scavenging and antioxidative defense, other processes were also affected by H2O2 priming in metal-exposed plants. A study by Guzel and Terzi (2013) showed that H2O2 pretreatment counteracted the reductions in dry matter production, relative water content and water potential in leaves of Cu-exposed Zea mays. In addition, H2O2 priming reduced the negative effects of Cu on the levels of soluble proteins, sugars, and mineral ions and enhanced the Cu-mediated increase in proline content. These results suggest that the water balance may be a target of H2O2 priming in metal-exposed plants (Guzel and Terzi, 2013). Interestingly, proline levels are constitutively enhanced in different metal-tolerant plant species (Sharma and Dietz, 2006). While this may be related to its role in osmoregulation, proline might also confer metal tolerance through its function as metal chelator and ROS scavenger as discussed before (reviewed by Sharma and Dietz, 2006).
It is interesting to note that whereas H2O2 priming affects plant responses to metal stress, H2O2 alone (without subsequent metal exposure) does not always influence the parameters studied. As mentioned, metal-induced increases in antioxidative enzyme activities are often enhanced by H2O2 pretreatment. This does not always imply, however, that the activities of these enzymes are also increased in H2O2-primed plants that are not subsequently exposed to metal stress. In a recent review on this topic, Hossain et al. (2015) propose that pretreatment with H2O2 induces a mild oxidative challenge activating a ROS-dependent signaling network which results in the accumulation of latent defense proteins including antioxidative enzymes and transcription factors. As a consequence, plants enter a primed state that enables enhanced defense responses upon exposure to subsequent abiotic stressors such as metals.
It has been demonstrated that metal-induced oxidative stress is more powerful in sensitive genotypes or ecotypes (reviewed by Sharma and Dietz, 2009). Among the flowering plants, the metal hyperaccumulating plants A. halleri, Noccaea caerulescens, and Alyssum bertolonii exhibit a greater antioxidative capacity than their sensitive relatives (Sharma and Dietz, 2009). For example, activities of APX and class III peroxidases were highly increased in the Cd and Zn hyperaccumulator A. halleri as opposed to its sensitive counterpart A. thaliana (Chiang et al., 2006). In addition, CAT activity was more than 500 times higher in roots of the Ni hyperaccumulator A. bertolonii as compared to the non-hyperaccumulator Nicotiana tabacum, explaining the higher increase in H2O2 levels in the latter after Ni exposure (Boominathan and Doran, 2002). Interestingly, results of different studies on contrasting ecotypes or species indicate that H2O2 in particular is a crucial mediator of metal phytotoxicity. Indeed, tolerant and hyperaccumulating plant species often display a constitutively increased level of H2O2 scavenging enzymes (Sharma and Dietz, 2009). For example, Cho and Seo (2005) observed a higher survival rate and less lipid peroxidation in Cd-resistant A. thaliana mutants as compared to wild-type (WT) plants exposed to 300 or 500 μM Cd, even though the Cd content in the mutants was higher. The decreased Cd sensitivity of the mutants was mainly related to increased activities of several antioxidative enzymes such as APX and GR. Interestingly, the authors did not observe a relation between CAT activity and Cd tolerance. Nevertheless, Cd-resistant mutants had lower H2O2 levels as compared to WT plants (Cho and Seo, 2005), again supporting a role for H2O2 in plant metal tolerance. Furthermore, ROS production under metal stress could also mediate cross-tolerance to pathogens as reviewed by Poschenrieder et al. (2006). Underlying mechanisms could be the induction of antioxidants and the synthesis of secondary metabolites involved in mechanical defense against pathogen attack (Poschenrieder et al., 2006).
Hydrogen peroxide mediates damage and/or signaling in metal-stressed plants
The balance between the generation and removal of ROS affects which reactive oxygen compound is present and at which level. This ultimately determines the extent of oxidative damage and/or signaling (Møller et al., 2007). Indeed, antioxidants function to limit the levels of ROS, thereby enabling them to execute beneficial cellular functions without causing too much damage (Halliwell, 2006). Based mainly on its concentration, but also on its production site and the plant's developmental stage, H2O2 affects plant stress responses in two ways (Petrov and Van Breusegem, 2012). In general, high levels of H2O2 induce cell death (Gechev and Hille, 2004; Petrov and Van Breusegem, 2012; Petrov et al., 2015). This process is critical during leaf senescence and the hypersensitive response, which are both known to occur in response to different developmental as well as environmental cues (Gechev et al., 2006; Quan et al., 2008; Petrov and Van Breusegem, 2012). At low concentrations, H2O2 acts as a signaling molecule by (1) directly affecting the activity of a target molecule involved in signaling or transcription, (2) oxidizing a biological molecule that in its turn acts as second messenger or (3) shifting the cellular redox balance to a more oxidized state (Apel and Hirt, 2004; Petrov and Van Breusegem, 2012). The essential role of H2O2 in cellular signaling is underlined by the global transcriptomic analysis of Desikan and coworkers, who demonstrated a H2O2-induced change in expression for approximately 1% of all Arabidopsis genes represented on the microarray (Desikan et al., 2001). In addition, H2O2 is a crucial mediator of plant responses to metal stress as discussed in the following sections.
Ample studies have demonstrated the occurrence of ROS-induced oxidative damage at the molecular level in plants exposed to various metals (Table 2). Lipids [especially polyunsaturated fatty acids (PUFAs)], DNA and proteins can be oxidatively damaged by ROS, depending on the reactivity of the latter. Hydrogen peroxide is moderately reactive as compared to other ROS and therefore only directly targets sulfur-containing residues in proteins (Møller et al., 2007). However, H2O2 can indirectly contribute to oxidative damage when it—together with —is converted to highly toxic •OH radicals in the Fenton and Haber-Weiss reactions (Figure 1). Hydroxyl radicals are able to abstract a hydrogen atom from PUFA residues in a membrane, thereby initiating lipid peroxidation. The resulting carbon-centered radical quickly reacts with O2 to produce peroxyl radicals, attacking neighboring PUFA side chains and generating lipid hydroperoxides. These can freely decompose into different reactive species such as aldehydes (e.g. malondialdehyde) and lipid epoxides. Overall, lipid peroxidation leads to increased membrane leakiness and damage to receptors, enzymes and ion channels (Halliwell, 2006). Lipid peroxidation—concomitantly with a rise in H2O2/ROS levels—was shown to occur in different plant species exposed to Al (Pereira et al., 2010), Cd (Masood et al., 2012), Cu (Opdenakker et al., 2012a), Hg (Montero-Palmero et al., 2014), Ni (Khan and Khan, 2014), Pb (Kaur et al., 2015), and Zn (Khan and Khan, 2014; Table 2). It must be noted that redox-active metals accelerate lipid peroxidation by catalyzing the Fenton and Haber-Weiss reactions and splitting up lipid hydroperoxides into alkoxyl and new •OH radicals to feed the chain reaction (Halliwell, 2006). This was clearly demonstrated by the results of Opdenakker et al. (2012a), comparing H2O2 levels and lipid peroxidation in A. thaliana plants exposed to either Cu or Cd in a similar setup. Both parameters were more rapidly increased and higher after exposure to the redox-active Cu as opposed to Cd, pointing toward a greater and quicker disturbance of the cellular redox state by the former metal (Opdenakker et al., 2012a). However, plant responses to specific metals must always be interpreted with the applied metal concentration, the duration of exposure, the cultivation system and the considered tissue(s) in mind. Interestingly, oxygenation of PUFAs leads to the production of oxylipins in an enzymatic or non-enzymatic manner (see Section “A Relationship between H2O2 and Oxylipins in Metal-Exposed Plants”). As oxylipins mediate plant responses to different stressors (Mithöfer et al., 2004; Dave and Graham, 2012), ROS-induced oxidation of lipids causes the emergence of new signaling molecules (Chmielowska-Bąk et al., 2015).
Table 2.
Oxidative damage in plants related to an elevated H2O2 content induced by metal exposure.
| Metal | Species | Damage | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Molecular | Cellular | ||||||||
| Lipid peroxidation | DNA damage | Protein oxidation | Hallmark genes | Chloroplast | Cell death | ||||
| Essential | Cu | Arabidopsis thaliana | x | Cuypers et al., 2011 | |||||
| x | Opdenakker et al., 2012a | ||||||||
| x | x | Martínez-Peñalver et al., 2012 | |||||||
| Cucumis sativus | x | İşeri et al., 2011 | |||||||
| Hordeum vulgare | x | x | Hu et al., 2015 | ||||||
| Matricaria chamomilla | x | Kováčik et al., 2010a,b | |||||||
| Medicago truncatula | x | x | x | Macovei et al., 2010 | |||||
| Nicotiana tabacum | x | Xia et al., 2012 | |||||||
| Oryza sativa | x | x | Mostofa et al., 2015a | ||||||
| x | Thounaojam et al., 2012 | ||||||||
| Paulownia fortunei | x | x | Wang J. et al., 2010 | ||||||
| Solanum lycopersicuma | x | İşeri et al., 2011 | |||||||
| Spirodela polyrhiza | x | x | Upadhyay and Panda, 2010 | ||||||
| Ni | Brassica juncea | x | x | Khan and Khan, 2014 | |||||
| Brassica napus | x | x | Kazemi et al., 2010 | ||||||
| Chlamydomonas reinhardtii | x | x | x | Zheng et al., 2013 | |||||
| Vicia sativa | x | Ivanishchev and Abramova, 2015 | |||||||
| Zn | Brassica juncea | x | x | Khan and Khan, 2014 | |||||
| Brassica napus | x | x | Feigl et al., 2015 | ||||||
| Brassica oleracea | x | Barrameda-Medina et al., 2014 | |||||||
| Lactuca sativa | x | Barrameda-Medina et al., 2014 | |||||||
| Myracrodruon urundeuva | x | Gomes et al., 2013 | |||||||
| Oryza sativa | x | Thounaojam et al., 2012 | |||||||
| Paulownia fortunei | x | x | Wang J. et al., 2010 | ||||||
| Phaseolus vulgaris | x | Michael and Krishnaswamy, 2011 | |||||||
| Populus × canescens | x | Shi et al., 2015 | |||||||
| Solanum melongena | x | Wu et al., 2015 | |||||||
| Solanum nigrum | x | x | Xu J. et al., 2010 | ||||||
| Spirodela polyrhiza | x | Upadhyay and Panda, 2010 | |||||||
| Non-essential | Al | Cucumis sativus | x | x | x | Pereira et al., 2010 | |||
| Nicotiana tabacum | x | x | Yin et al., 2010 | ||||||
| Triticum aestivum | x | x | Sun et al., 2015 | ||||||
| Cd | Arabidopsis thaliana | x | Cuypers et al., 2011 | ||||||
| x | Keunen et al., 2015 | ||||||||
| x | x | Martínez-Peñalver et al., 2012 | |||||||
| x | x | x | Tao et al., 2013 | ||||||
| Non-essential | Cd | Boehmeria nivea | x | x | Tang et al., 2015 | ||||
| Brassica campestris | x | Anjum et al., 2014 | |||||||
| Brassica juncea | x | x | Masood et al., 2012 | ||||||
| Brassica napus | x | x | Ali et al., 2013 | ||||||
| Citrus paradisi × Poncirus trifoliata | x | Podazza et al., 2012 | |||||||
| Dittrichia viscosa | x | x | Fernández et al., 2013 | ||||||
| Glycine max | x | x | Pérez-Chaca et al., 2014 | ||||||
| Helianthus annuus | x | Saidi et al., 2014 | |||||||
| Kosteletzkya virginica | x | x | x | Han et al., 2013 | |||||
| Lactuca sativa | x | x | x | Monteiro et al., 2012 | |||||
| Lepidium sativum | x | x | x | Gill et al., 2012 | |||||
| Lupinus luteus | x | x | Arasimowicz-Jelonek et al., 2012 | ||||||
| Nicotiana tabacum | x | Iannone et al., 2010 | |||||||
| Oryza sativa | x | x | Chou et al., 2011 | ||||||
| x | x | Mostofa et al., 2015b | |||||||
| x | x | Singh and Shah, 2014 | |||||||
| x | x | x | Srivastava et al., 2014 | ||||||
| x | x | x | Srivastava et al., 2015 | ||||||
| x | x | Yu et al., 2015 | |||||||
| Paulownia fortunei | x | x | Wang J. et al., 2010 | ||||||
| Populus cathayana | x | He et al., 2013 | |||||||
| Populus deltoides | x | He et al., 2013 | |||||||
| Populus × euramericana | x | He et al., 2013 | |||||||
| P. alba × P. glandulosa | x | He et al., 2013 | |||||||
| Sedum alfredii | x | Tian et al., 2011 | |||||||
| Solanum lycopersicum | x | x | Ahammad et al., 2013 | ||||||
| x | x | Monteiro et al., 2011 | |||||||
| Solanum nigrum | x | Deng et al., 2010 | |||||||
| x | Liu et al., 2013 | ||||||||
| Trigonella foenum-graecum | x | x | Zayneb et al., 2015 | ||||||
| Triticum aestivum | x | x | Moussa and El-Gamal, 2010 | ||||||
| Vigna radiata | x | Anjum et al., 2014 | |||||||
| Zea mays | x | Wahid and Khaliq, 2015 | |||||||
| Zygophyllum fabago | x | x | Yildiztugay and Ozfidan-Konakci, 2015 | ||||||
| Hg | Juncus maritimus | x | x | Anjum et al., 2015 | |||||
| Medicago sativa | x | Montero-Palmero et al., 2014 | |||||||
| Pb | Arabidopsis thaliana | x | x | x | Tao et al., 2013 | ||||
| Atractylodes macrocephala | x | x | Wang et al., 2013 | ||||||
| Non-essential | Pb | Brassica napus | x | Ali et al., 2014 | |||||
| Hordeum vulgare | x | x | Legocka et al., 2015 | ||||||
| Nymphoides peltatum | x | x | Qiao et al., 2013 | ||||||
| Oryza sativa | x | x | x | Srivastava et al., 2014 | |||||
| Paulownia fortunei | x | x | Wang J. et al., 2010 | ||||||
| Talinum triangulare | x | x | x | x | Kumar et al., 2013 | ||||
| Triticum aestivum | x | x | Kaur et al., 2013 | ||||||
| x | Kaur et al., 2015 | ||||||||
| Vicia faba | x | x | Shahid et al., 2012 | ||||||
| Zygophyllum fabago | x | x | López-Orenes et al., 2014 | ||||||
In article as Lycopersicum esculentum.
Exposure to excess metals affects H2O2 production and causes molecular and cellular damage in plants. At the molecular level, lipids, DNA and proteins can be oxidized by H2O2. Expression of genes that are commonly induced by oxidative stress (Gadjev et al., 2006) can be assessed as marker of metal-induced oxidative damage. Furthermore, damage at the level of the chloroplast and even cell death are often observed under metal stress conditions. The effects of excess essential metals (Cu, Ni, and Zn) as well as non-essential metals (Al, Cd, Hg, and Pb) are shown and categorized based upon the metal and plant species studied. Only recently published papers (starting from 2010) demonstrating a metal-induced rise in H2O2 content and damage at molecular and/or cellular level were included in this overview.
Although H2O2 itself is poorly reactive, different studies have demonstrated oxidative DNA damage and protein oxidation accompanied by an increased H2O2 level in various plant species under metal stress (Table 2). Oxidative DNA damage is often assessed by the amount of 8-hydroxyguanosine, the most commonly observed ROS-induced modification (Møller et al., 2007). Its levels were increased in Al-exposed N. tabacum (Yin et al., 2010) and Cu-treated Medicago truncatula plants (Macovei et al., 2010). Moreover, the alkaline comet assay revealed DNA damage in roots of Al-exposed Allium cepa (Achary et al., 2008), Cd-treated Lactuca sativa (Monteiro et al., 2012) and Pb-exposed Talinum triangulare plants (Kumar et al., 2013). Although many studies concentrated on DNA oxidation, it is now postulated that RNA is more susceptible to this process. Therefore, targeted RNA oxidation by ROS might be a novel mechanism to post-transcriptionally regulate expression of defense genes (Chmielowska-Bąk et al., 2015).
High intracellular levels of H2O2 oxidize both cysteine (-SH) and methionine (-SCH3) residues present in various proteins such as Cu/Zn- and Fe-SOD (Das and Roychoudhury, 2014). Although this may disrupt their enzymatic function and thereby lead to irreversible cell damage, it has been recently postulated to be a way to perceive and further relay a H2O2 signal in plant cells (Hardin et al., 2009; Petrov and Van Breusegem, 2012). In addition, protein carbonylation is commonly observed under metal stress (Table 2). For example, Al increased the carbonyl protein content in A. cepa roots (Achary et al., 2008) and Cucumis sativus seedlings (Pereira et al., 2010). Protein carbonyls were significantly enhanced in roots and leaves of L. sativa plants after Cd exposure (Monteiro et al., 2012), while similar results were observed in roots and shoots of O. sativa seedlings exposed to Pb (Srivastava et al., 2014). Not all proteins are equally sensitive to oxidation (Møller et al., 2007). For example, it has been demonstrated that mainly mitochondrial proteins are oxidized under well-irrigated and drought stress conditions in T. aestivum leaves (Bartoli et al., 2004). Moreover, Kristensen et al. (2004) have revealed specific subpopulations of O. sativa leaf mitochondrial matrix proteins that were carbonylated after in vitro treatment with H2O2 or Cu. Again, the possibility exists that ROS-mediated protein oxidation in plant mitochondria (and other compartments) functions as stress indicator, provoking an alarm signal to induce plant responses to developmental as well as environmental changes (Møller and Kristensen, 2004; Møller and Sweetlove, 2010; Chmielowska-Bąk et al., 2015). In conclusion, various oxidatively modified molecules serve as signaling compounds, supporting the view that oxidative damage and signaling are two sides of the same coin (Møller et al., 2007). Providing experimental evidence for this hypothesis during metal stress is an intriguing research challenge for the future.
In addition to damage at the molecular level, metal-exposed plants also suffer from (sub)cellular damage. This is often visible at the chloroplast level, leading to inhibition of photosynthesis (Table 2; Cuypers et al., 2009). Chlorophyll content was decreased in various plant species exposed to Al (Pereira et al., 2010), Cd (Zawoznik et al., 2007), Cu (Hu et al., 2015), Ni (Kazemi et al., 2010), Pb (Legocka et al., 2015), and Zn (Khan and Khan, 2014). In addition, different photosynthetic parameters (e.g. net photosynthesis rate) were reduced in A. thaliana plants exposed to Cd or Pb (Tao et al., 2013). Levels of H2O2 were significantly increased after metal exposure in all of the above-mentioned studies, pointing toward a correlation between H2O2 and the observed effects at the chlorophyll/photosynthesis level. In addition to chloroplast function and morphology, Cd exposure disturbed the distribution and mobility of mitochondria in A. thaliana protoplasts (Bi et al., 2009). Finally, it is important to note that metals are able to initiate H2O2-induced programmed cell death (Table 2). In Cd-exposed N. tabacum cells, NADPH oxidase was activated by a rise in cytosolic free Ca2+ concentrations, leading to H2O2 production and cell death (Garnier et al., 2006). Cadmium was also shown to increase the production of H2O2, which preceded cell death in A. thaliana cell suspension cultures (De Michele et al., 2009). Similarly, other studies indicate a relationship between metal exposure, oxidative stress and cell death using roots, root tips or leaf disks and different techniques to assess cell viability (Table 2; Pan et al., 2001; Achary et al., 2008; Iannone et al., 2010; Arasimowicz-Jelonek et al., 2012; Kumar et al., 2013; Feigl et al., 2015). Reactive oxygen species and H2O2 in particular are considered as crucial signals that modulate (programmed) cell death in plants (Gechev and Hille, 2004; Gadjev et al., 2008; Petrov et al., 2015), again highlighting the intimate relationship between ROS-mediated damage and signaling (Figure 2).
Hydrogen peroxide directly mediates metal-induced oxidative signaling
The use of ROS as signaling molecules offers various potential advantages as discussed by Mittler et al. (2011). Their levels can rapidly change by shifting the balance between production and scavenging, which are both tightly controlled in space because of the presence of pro- and anti-oxidative enzymes at different subcellular locations (Mittler et al., 2004). The different molecular properties of various ROS offer the potential to transmit specific signals, also with regard to second messenger products formed after oxidative modification. Signaling is possible both within and across cells, generating a so-called ROS “wave” (Mittler et al., 2011; Baxter et al., 2014). Finally, ROS signaling integrates with several other signaling molecules and mechanisms such as Ca2+ and protein phosphorylation. In addition, ROS are directly linked to the plant's cellular homeostasis and metabolism. Therefore, they are perfectly suited to signal any metabolic change occurring during developmental and environmental stimuli (Mittler et al., 2011; Baxter et al., 2014).
Foyer and Noctor (2005) have described ROS-induced signaling through a “ripple” or domino effect over space and/or time, starting with a localized and/or transient oxidative burst affecting the expression of defense and regulatory genes in a transient or more sustained manner. Indeed, ROS are shown to activate various signaling compounds such as kinases/phosphatases, metabolites and hormones, which in their turn affect the expression of different target genes. This finally triggers acclimation to the altered developmental or environmental conditions a plant is experiencing (Mittler et al., 2004; Bienert and Chaumont, 2014). Particularly with regard to H2O2, it is interesting to note that it is produced in response to a wide variety of internal and external stimuli and therefore potentially contributes to cross-tolerance toward various stressors (Neill S. J. et al., 2002; Perez and Brown, 2014). Although oxidative stress commonly occurs in various stress conditions, the underlying signaling mechanisms may be highly stress-specific. This is underlined by the identification of marker transcripts specifically regulated by 1O2, or H2O2 after exposure to different oxidative stress-causing agents. However, several transcripts were classified as general oxidative stress response markers because they responded to most of the applied treatments (Gadjev et al., 2006) and were also induced by Cd stress (Keunen et al., 2015; Table 2). Interestingly, Sewelam et al. (2014) have shown that H2O2 originating specifically from either chloroplasts or peroxisomes did have a differential impact on the A. thaliana transcriptome. Specificity of ROS-induced signaling might be related to the ROS type, amount, source and subcellular location of production, as well as their perception by different sensors (Miller et al., 2008; Cuypers et al., 2012).
Perception of H2O2 during metal stress
Researchers have long been puzzled by the mechanism(s) used by plants to perceive stress-induced increases in H2O2 production and to relay this signal. A minimum of three potential mechanisms has been described: (1) H2O2 receptors that remain unidentified to date, (2) redox-sensitive transcription factors and (3) ROS-mediated inhibition of phosphatases (Mittler et al., 2004; Miller et al., 2008). Currently, it is still assumed that redox-sensitive transcription factors are oxidized by H2O2 and directly activate downstream signaling cascades (Neill S. et al., 2002; Miller and Mittler, 2006; Dietz, 2014). For example, class A heat shock factors (HSFs) are known to respond to oxidative stress in animals and plants (Petrov and Van Breusegem, 2012). The potential involvement of HSFs in perceiving H2O2 during metal stress (Miller and Mittler, 2006) is supported by the observed production of heat shock proteins in various metal-exposed plants (di Toppi and Gabbrielli, 1999; Cuypers et al., 2009). Miller et al. (2008) have proposed a model for ROS signaling using plants that lack the cytosolic APX1 isoform. In this model, different HSFs function as H2O2 sensors upstream of other transcription factors of the zinc finger protein ZAT (ZAT7, 10 and 12) and WRKY family (e.g. WRKY25) (Miller et al., 2008). Interestingly, expression levels of ZAT12 and WRKY25 genes were induced in A. thaliana plants exposed to Cd or Cu (Opdenakker et al., 2012a). Both genes were more rapidly induced upon exposure to Cu than to Cd in the roots, corresponding with the observed differences in H2O2 levels and potentially related to the contrasting redox properties of both metals (Opdenakker et al., 2012a).
A central protein involved in ROS sensing is the serine/threonine protein kinase oxidative signal-inducible 1 (OXI1). This enzyme is directly induced by H2O2 and forms an essential part of the signal transduction pathway linking ROS production to diverse downstream responses (Rentel et al., 2004). It also connects redox to lipid signaling via phosphatidic acid in a phosphoinositide-dependent kinase (PDK1)-related manner (Anthony et al., 2004, 2006). Interestingly, Opdenakker et al. (2012a) demonstrated highly increased OXI1 transcription in Cd- or Cu-exposed A. thaliana plants. Again, its upregulation was higher and earlier induced after exposure to Cu, potentially related to its redox-active properties. Results by Smeets et al. (2013) underscore the key role of OXI1 in cellular signaling responses to Cu stress using oxi1 knockout A. thaliana mutants. As compared to WT plants, plants lacking OXI1 responded differently to redox-induced changes (Smeets et al., 2013). Downstream of OXI1, mitogen-activated protein kinases (MAPKs) control the activation of multiple defense mechanisms in response to oxidative stress as discussed in the following section.
Hydrogen peroxide signal transduction by MAPKs and transcription factors
One of the typical downstream signaling events associated with H2O2 sensing is the activation of MAPK pathways (Table 3; Mittler et al., 2004; Colcombet and Hirt, 2008). These signaling modules are found in all eukaryotic cells and consist of at least three kinases (MAP3K, MAP2K and MAPK) specifically phosphorylating and thereby activating each other (Colcombet and Hirt, 2008; Opdenakker et al., 2012b). Several authors have reported the involvement of MAPK signaling during exposure to Cd, Cu, Hg, Pb and Zn in different plant species (Opdenakker et al., 2012b and references therein). Upstream of MAPKs, the OXI1 kinase is considered to be a central player in metal-induced oxidative stress responses. Rentel et al. (2004) have shown that the activation of the MAPK isoforms MPK3 and MPK6 by H2O2 is reduced in A. thaliana plants lacking OXI1. Concurrently with OXI1, expression levels of its targets MPK3 and MPK6 were enhanced in Cd- or Cu-exposed A. thaliana plants (Opdenakker et al., 2012a). Jonak et al. (2004) studied the kinetics of different MAPK activities after exposure to either Cd or Cu in M. sativa seedlings. Similar to the results at the transcript level (Opdenakker et al., 2012a), Cu ions rapidly activated these enzymes while Cd exposure led to a delayed stimulation (Jonak et al., 2004). Since GSH effectively inhibited MPK3 and MPK6 activation in Cd-exposed A. thaliana plants, H2O2/ROS were shown to play a crucial role in this process (Liu X. M. et al., 2010).
Table 3.
Signaling responses related to an elevated H2O2 content induced by metal exposure.
| Metal | Species | TFs | MAPKs | Phytohormones | References | |
|---|---|---|---|---|---|---|
| Essential | Cu | Arabidopsis thaliana | WRKY, ZAT | MPK3/6 | Opdenakker et al., 2012a | |
| Aux | Yuan et al., 2013 | |||||
| Oryza sativa | JAa | Mostofa et al., 2015a | ||||
| Spirodela polyrhiza | JAa | Upadhyay and Panda, 2010 | ||||
| Ni | Brassica juncea | Eth | Khan and Khan, 2014 | |||
| Zn | Brassica juncea | Eth | Khan and Khan, 2014 | |||
| Brassica oleracea | JAa | Barrameda-Medina et al., 2014 | ||||
| Lactuca sativa | JAa | Barrameda-Medina et al., 2014 | ||||
| Populus × canescens | ABA, SA | Shi et al., 2015 | ||||
| Solanum melongena | ABA, Aux, CK | Wu et al., 2015 | ||||
| Non-essential | Cd | Arabidopsis thaliana | MPK3/6 | Liu X. M. et al., 2010 | ||
| WRKY, ZAT | MPK3/6 | Opdenakker et al., 2012a | ||||
| JAa | Remans et al., 2010 | |||||
| JA | Keunen et al., 2013 | |||||
| SA | Tao et al., 2013 | |||||
| Brassica juncea | Eth | Masood et al., 2012 | ||||
| Citrus paradisi × Poncirus trifoliata | JAa | Podazza et al., 2012 | ||||
| Kosteletzkya virginica | Aux, CK, Eth, SA | Han et al., 2013 | ||||
| Lupinus luteus | SA | Arasimowicz-Jelonek et al., 2012 | ||||
| Oryza sativa | JAa | Mostofa et al., 2015b | ||||
| Aux | Yu et al., 2015 | |||||
| Triticum aestivum | ABA | Moussa and El-Gamal, 2010 | ||||
| Hg | Medicago sativa | Eth | Montero-Palmero et al., 2014 | |||
| Pb | Arabidopsis thaliana | SA | Tao et al., 2013 | |||
| Zygophyllum fabago | SA | López-Orenes et al., 2014 | ||||
Solely reported as an effect on LOX gene expression or LOX activity in article.
During metal stress, several signaling responses are induced by increased H2O2 levels. Several transcription factors (TFs) and MAPKs and are activated by H2O2. In addition, multiple phytohormone signaling pathways are affected by different metals. The effects of excess essential metals (Cu, Ni, and Zn) as well as non-essential metals (Al, Cd, Hg, and Pb) are shown and categorized based upon the metal and plant species studied. Only recently published papers (starting from 2010) demonstrating a metal-induced rise in H2O2 content and signaling were included in this overview. Abbreviations: ABA, abscisic acid; Aux, auxins; CK, cytokinin; Eth, ethylene; JA, jasmonic acid; SA, salicylic acid.
In addition to OXI1, also the MAP3K Arabidopsis NPK1-like protein kinase 1 (ANP1) is directly activated by H2O2 and initiates a phosphorylation cascade via MPK3 and MPK6 (Kovtun et al., 2000). Expression levels of ANP1 were increased in roots of Cu-exposed A. thaliana plants after 6 and 24 h (Opdenakker et al., 2012a). Although MAPKs can be activated by H2O2, they also trigger an H2O2-mediated oxidative burst themselves (Mittler et al., 2004; Petrov and Van Breusegem, 2012). Indeed, MEK2 (the Nicotiana ortholog of Arabidopsis MKK4/5) was implicated in ROS production upon fungal infection in N. benthamiana by acting upstream of RBOH genes known to evoke H2O2 production (Yoshioka et al., 2003). Similarly, expression of constitutively active MKK4/5 led to H2O2 generation and cell death in A. thaliana (Ren et al., 2002). As MAPK cascades function both up- and downstream of H2O2 (Mittler et al., 2004; Pitzschke and Hirt, 2006; Pitzschke et al., 2009; Petrov and Van Breusegem, 2012), the existence of positive feedback loops between H2O2 and MAPKs such as MKK4/5 deserves further attention under metal stress conditions.
Activated MAPK cascades are able to regulate downstream gene expression by activating or repressing transcription factors (Colcombet and Hirt, 2008). Transcription factors of the ZAT, WRKY, NAC, DREB, bZIP and MYB family therefore constitute the final link in the signaling chain induced by H2O2 (Petrov and Van Breusegem, 2012). Results by Pitzschke et al. (2009) have demonstrated the involvement of a complete MAPK cascade consisting of MEKK1, MKK1/MKK2, and MPK4 in regulating ROS-induced stress signaling. Indeed, the majority of transcription factors responsive to multiple ROS-producing conditions are controlled by this pathway (Pitzschke et al., 2009). Furthermore, MEKK1 is able to directly interact with and phosphorylate the transcription factor WRKY53 (Miao et al., 2007), which could be involved in metal-induced senescence (see Section “Metal-Induced Responses at the Cellular Level: is H2O2 Involved in Root Growth Inhibition and Senescence?”).
Different members of the ZAT family of zinc finger transcription factors were strongly induced by ROS at the transcript level (Gadjev et al., 2006). In particular, isoforms 7, 10 and 12 have been put forward to be involved in ROS signaling during abiotic stress (Davletova et al., 2005a; Miller et al., 2008). In addition, WRKY transcription factors could function up- or downstream of ZAT proteins (Miller et al., 2008). The WRKY proteins, belonging to one of the largest transcription factor families in plants (Eulgem and Somssich, 2007), all contain the invariable WRKY amino acid signature and recognize W-box cis elements in target gene promoter regions. The induction of WRKY25 during oxidative stress was shown to be ZAT12-dependent (Rizhsky et al., 2004). As mentioned before, both ZAT12 and WRKY25 expression was induced in Cd- or Cu-exposed A. thaliana plants (Opdenakker et al., 2012a), further supporting their involvement in metal-induced ROS signaling. For members of the NAC, DREB, bZIP and MYB family associated with H2O2 signaling, their relation to metal stress is to our knowledge generally unexplored to date. Nevertheless, several NAC transcription factors were shown to be H2O2-responsive (Balazadeh et al., 2010) and govern leaf senescence in A. thaliana (Balazadeh et al., 2008). As discussed in the Section “Metal-Induced Responses at the Cellular Level: Is H2O2 Involved in Root Growth Inhibition and Senescence?,” metal exposure might induce a hastening of this naturally occurring process and the role of NAC transcription factors herein might be an interesting topic for future research. This is further supported by promising results of Fang and coworkers, who recently demonstrated the stress-responsive SNAC3 transcription factor to confer tolerance to heat and drought stress in O. sativa plants by modulating ROS (Fang et al., 2015).
Although OXI1, MPK3 and MPK6 were shown to be activated in metal-exposed plants, information on upstream signaling pathways as well as downstream targets under metal stress conditions is rather scarce. Nevertheless, defined end points of specific MAPK signaling pathways are critical to activate the plant's antioxidative defense during metal-induced oxidative stress (Cuypers et al., 2012). In response to H2O2, MAPK regulation of ZAT12 led to enhanced expression of the APX1 gene in A. thaliana (Rizhsky et al., 2004). This gene, encoding a cytosolic H2O2 scavenging enzyme, was shown to protect the chloroplast redox state during light stress (Davletova et al., 2005b). Interestingly, also the CAT1 gene was shown to be regulated by MAPK signaling in A. thaliana (Xing et al., 2007, 2008). Both APX1 and CAT1 are critical in scavenging metal-induced H2O2 and were induced in A. thaliana plants exposed to Cd, Cu, or Zn (Table 1; Cuypers et al., 2011; Remans et al., 2012a). Interestingly, Davletova et al. (2005b) have postulated the involvement of MAPK-regulated RBOHD expression in ROS signal amplification during light stress, and further studies confirmed its role in abiotic stress-induced systemic signaling (Miller et al., 2009). Expression of RBOHD was also induced upon Cd, Cu and Zn exposure in A. thaliana (Remans et al., 2010, 2012a; Cuypers et al., 2011). Although all of the above-mentioned components have been separately assessed under metal stress conditions, further efforts should be made to reveal the sequence of events from stress perception to response in metal-exposed plants.
Metal-induced MAPK signaling pathways show extensive crosstalk with phytohormone signaling. Upon activation, both MPK3 and MPK6 can phosphorylate 1-aminocyclopropane-1-carboxylate synthase (ACS) isoforms 2 and 6, increasing their half-life and the production of ethylene by these enzymes (Liu and Zhang, 2004; Joo et al., 2008; Han et al., 2010). Transcription of both ACS isoforms can also be enhanced by MPK3/6 via the WRKY33 transcription factor (Li et al., 2012). In addition, Yoo et al. (2008) have shown that a MKK9-MPK3/6 cascade promotes ethylene signaling by phosphorylating the nuclear transcription factor ethylene-insensitive 3 (EIN3) in A. thaliana. Increasing evidence supports a role for ethylene in regulating metal stress responses in plants (reviewed by Thao et al., 2015; Keunen et al., 2016). It has been demonstrated that the increase in ethylene levels was mainly related to upregulated ACS2 and ACS6 expression in Cd-exposed A. thaliana plants (Schellingen et al., 2014). Furthermore, MPK3 and MPK6 were proposed to connect ROS production to ethylene signaling in A. thaliana leaves under Cd exposure. Cadmium activates NADPH oxidases that produce ROS, which are sensed by OXI1. This kinase then activates MPK3 and MPK6, both affecting ACS2 and ACS6 enzymes at various levels (Schellingen et al., 2015). In conclusion, ethylene shows extensive crosstalk with signaling by ROS or H2O2 under metal stress (Thao et al., 2015; Keunen et al., 2016), which should definitely be explored in more detail in future studies. Also the production of other phytohormones such as abscisic acid (ABA), auxins, cytokinins, jasmonic acid (JA) and salicylic acid (SA) is affected by metal exposure in different plant species (Table 3). Compelling evidence for a role of endogenous SA in Pb and Cd tolerance of A. thaliana was provided by Tao et al. (2013). Metal-induced phytotoxicity was potentiated by elevating endogenous SA levels, while plants with lower SA levels performed better when exposed to Pb or Cd. One of the underlying mechanisms of SA-mediated toxicity is related to plant redox homeostasis, with SA-accumulating plants showing higher metal-induced H2O2 concentrations as compared to SA-deficient plants (Tao et al., 2013). As discussed by Petrov and Van Breusegem (2012), interactions between H2O2 and SA can range from cooperation to inhibition depending on the used experimental conditions. Therefore, much work remains to be done to fully unravel the interaction between H2O2 and phytohormones such as ethylene and SA during metal stress in plants. In addition, a link between H2O2 and JA in metal-exposed plants is evident and discussed in the Section “A Relationship between H2O2 and Oxylipins in Metal-Exposed Plants”.
Hydrogen peroxide interacts with other signaling pathways and regulating mechanisms
As mentioned before, H2O2 is connected to a variety of signaling molecules (e.g. MAPK) and plant hormones (e.g. ethylene). In this section, we discuss its relation to Ca2+, nitric oxide (NO•), oxylipins and microRNAs in general and demonstrate evidence for their involvement during the metal-induced oxidative challenge in plants (Figure 2).
Interaction between H2O2 and Ca2+ in metal-exposed plants
Compelling evidence indicates a reciprocal relationship between H2O2 and Ca2+, two crucial messengers involved in plant responses to multiple stress conditions (Tuteja and Mahajan, 2007; Quan et al., 2008; Mazars et al., 2010; Petrov and Van Breusegem, 2012). Rentel and Knight (2004) observed a biphasic increase in cytosolic Ca2+ levels of Arabidopsis seedlings upon treatment with H2O2. Enhancing or reducing the height of the Ca2+ peaks had a corresponding effect on the expression of the H2O2-responsive GST1 gene, indicating crosstalk between H2O2 and Ca2+ signaling in plants (Rentel and Knight, 2004). Whereas ROS modulate cytosolic Ca2+ levels through the activation of Ca2+ channels in the plasma membrane, H2O2 production by NADPH oxidases reversely depends on Ca2+ (reviewed by Mazars et al., 2010). In Cd-exposed bright yellow-2 N. tabacum cells, H2O2 production was preceded by an enhanced cytosolic Ca2+ level essential to activate NADPH oxidases (Garnier et al., 2006). Indeed, Ca2+ directly binds EF-hand motifs in the cytosolic N-terminal domain of the NADPH oxidase enzyme and leads to phosphorylation of the N-terminus by activating a calcium-dependent protein kinase (CDPK) (Sagi and Fluhr, 2006; Kobayashi et al., 2007; Ogasawara et al., 2008). The potential involvement of CDPK in metal stress responses is supported by the transcriptional induction of the CDPK1 gene in roots of Cd-exposed A. thaliana plants (Smeets et al., 2013). Furthermore, several CDPK isoforms in T. aestivum were responsive to H2O2 treatment, indicating a role for these enzymes in oxidative signaling in plants (Li et al., 2008; Schulz et al., 2013). Interestingly, an increased Ca2+ concentration in peroxisomes caused by elevated cytosolic Ca2+ levels was shown to stimulate CAT3 activity in vivo. The resulting rise in peroxisomal H2O2 scavenging potential (Costa et al., 2010) could also be important during metal-induced oxidative stress. In this regard, the cellular response of Pisum sativum plants to long-term Cd exposure was shown to involve extensive crosstalk between Ca2+, ROS and NO• (Rodríguez-Serrano et al., 2009) as discussed in the following section. Finally, Baliardini et al. (2015) recently reported a positive correlation between the expression of a gene encoding a Ca2+/H+ exchanger (CAX1) and Cd tolerance in Arabidopsis. Indeed, its expression was higher in the Cd-tolerant A. halleri as compared to its Cd-sensitive relative species A. lyrata and A. thaliana. Plants without functional CAX1 also show increased accumulation of H2O2 when exposed to Cd, suggesting a role for CAX1 in maintaining cytosolic Ca2+ levels and thereby avoid uncontrolled ROS accumulation during oxidative stress conditions (Baliardini et al., 2015).
Nitric oxide and H2O2: friends or foes during metal exposure?
Nitric oxide (NO•) production is often induced by abiotic stress in plants, for example during exposure to different metals (reviewed by Xiong et al., 2010). In contrast, P. sativum plants showed reduced NO• levels under long-term (14 days) Cd exposure (Rodríguez-Serrano et al., 2009). The authors hypothesized, since NO• is able to react with , that these lower NO• levels could result in accumulation under Cd stress. This was further supported by decreased levels when NO• production was restored in Cd-exposed plants by application of additional Ca (Rodríguez-Serrano et al., 2009). Different authors have reported the potential of exogenous NO• to alleviate metal toxicity in plants (Xiong et al., 2010). For example, it has been proposed that NO•-induced Cu tolerance in Lycopersicon esculentum plants was mediated by H2O2 detoxification and the accumulation of Cu-scavenging metallothioneins (Wang L. et al., 2010). Although external application of NO• activated the antioxidative defense system, endogenous NO• could also contribute to metal phytotoxicity (reviewed by Arasimowicz-Jelonek et al., 2011). For example, NO• is known to promote the upregulation of genes involved in Fe uptake under Cd stress, thereby also contributing to increased Cd uptake in A. thaliana (Besson-Bard and Wendehenne, 2009; Besson-Bard et al., 2009). On the other hand, it is proposed that NO• produced by plants challenged with low Cd concentrations could mediate signaling responses leading toward metal tolerance (Arasimowicz-Jelonek et al., 2011). It is clear that further research is required to fully unravel the role of NO• and its interaction with H2O2 and oxidative stress (Petrov and Van Breusegem, 2012) during metal exposure in plants.
A relationship between H2O2 and oxylipins in metal-exposed plants
Various stress stimuli, such as exposure to different metals, activate biosynthetic enzymes responsible for the accumulation of oxylipins. These are derived from the oxidation of PUFAs by lipoxygenase (LOX) enzymes, with the phytohormone JA and its volatile derivative methyl jasmonate (MeJA) often considered to be the most important in signaling (Browse, 2009; Dave and Graham, 2012; Santino et al., 2013; Wasternack and Hause, 2013). In addition, a non-enzymatic route triggered by ROS is responsible for the synthesis of phytoprostane oxylipins that are also involved in plant stress responses (Dave and Graham, 2012). Evidence for a role of oxylipins during metal stress is provided by the observed induction of LOX at the transcript and activity level in various plant species (Table 3; Skórzyńska-Polit et al., 2006; Tamás et al., 2009; Remans et al., 2010; Keunen et al., 2013; Barrameda-Medina et al., 2014). Furthermore, JA levels increased in A. thaliana and Phaseolus coccineus plants exposed to Cd or Cu (Maksymiec et al., 2005), supporting a role for JA signaling in mediating stress responses in metal-exposed plants (Maksymiec, 2007). For example, MeJA was shown to upregulate the same set of genes involved in GSH biosynthesis that were also induced in Cd- or Cu-exposed A. thaliana plants (Xiang and Oliver, 1998). Interestingly, exogenously applied MeJA induced H2O2 production, lipid peroxidation and LOX activity in Taxus chinensis cells (Wang and Wu, 2005). Similarly, application of MeJA to A. thaliana roots strongly increased H2O2 concentrations in the leaves (Maksymiec and Krupa, 2002). This points toward a link between both JA and H2O2, suggesting that JA may contribute to metal-induced oxidative stress responses in plants (Rodríguez-Serrano et al., 2009).
MicroRNAs and redox signaling in metal-exposed plants
Together with small interfering RNAs (siRNAs), microRNAs (miRNAs) are endogenous non-coding small RNAs involved in the regulation of plant development and stress responses (Vazquez et al., 2010). MicroRNAs negatively regulate their target genes by (1) mRNA cleavage or inhibition of translation or (2) DNA methylation. Expression of different miRNAs is affected by metal stress in different plant species (reviewed by Gielen et al., 2012; Gupta et al., 2014). In general, miRNA-mediated responses are related to metal complexation, antioxidative defense and stress signaling. For example, miR395 regulates sulfate assimilation and was induced in Cd-exposed B. napus seedlings (Huang et al., 2010). Sulfate assimilation into cysteine is ultimately required to synthesize GSH and PCs able to chelate free Cd ions, suggesting a role for miR395 in regulating Cd complexation in plants (Gielen et al., 2012). In Arabidopsis, miR398 expression is downregulated by excess Cu, resulting in transcriptional induction of its target genes Cu/Zn-SOD 1 and 2 (CSD1/2). As compared to Cu, Cd exposure oppositely affected both miRNA398 and CSD1/2 expression levels, indicating metal-specific regulation potentially related to the redox-active vs. non-redox-active nature of Cu vs. Cd (Cuypers et al., 2011). Interestingly, Cu exposure did not reduce miR398 expression in leaves of A. thaliana plants lacking functional OXI1 as it did in WT plants, pointing toward an interaction between miR398 and MAPK signaling during metal stress (Smeets et al., 2013). Finally, various target genes of metal-induced miRNAs are involved in phytohormone biosynthesis and signaling, often by affecting transcription factors (Gielen et al., 2012; Gupta et al., 2014). Panda and Sunkar (2015) have recently discussed the potential role of redox signaling and/or ROS in inducing stress-responsive miRNAs in plants. This is further supported by a genome-wide study in O. sativa, showing seven miRNA families to be induced or downregulated by H2O2 treatment (Li et al., 2011). One of the miRNAs upregulated by H2O2 is miR397, targeting laccase enzymes involved in lignin biosynthesis. Interestingly, metal exposure was also shown to induce miR397 (reviewed by Gielen et al., 2012; Gupta et al., 2014), suggesting a potential role for H2O2 in mediating this induction under metal stress conditions. Future studies should aim to unravel the interplay between metal-induced production of ROS/H2O2 and its effects on the induction or downregulation of specific miRNAs targeting downstream response genes.
Metal-induced responses at the cellular level: is H2O2 involved in root growth inhibition and senescence?
As indicated in Tables 1–3, metal exposure increases H2O2 levels in a variety of plant species, thereby inducing both oxidative damage and signaling responses. At the cellular level, this might underlie metal-induced responses observed in roots (e.g. growth inhibition) and leaves (e.g. premature senescence). For example, Cd-induced oxidative stress could be related to the inhibition of root initiation and elongation (Lux et al., 2011). However, also plant hormones might regulate root growth of metal-exposed plants (Remans et al., 2012b; De Smet et al., 2015). As ROS are shown to interact with phytohormones such as ethylene, future research efforts should be made to dissect their role as potential modulators of root development under metal stress conditions.
Many of the parameters listed in Table 2 (e.g. lipid peroxidation) can also be regarded as indicators of plant senescence. Indeed, it is known that plants exposed to metals such as Cu and Cd show an accelerated appearance of senescence symptoms (Maksymiec, 2007). During the senescence process, leaves are degraded in a highly regulated fashion in order to remobilize nutrients to developing plant tissues. Leaf senescence comprises the final stage of leaf development and its onset is determined by the developmental age of leaves (Lim et al., 2007). It has been shown, however, that this process can be prematurely induced by several biotic and abiotic stress factors such as pathogen attack, wounding, darkness, drought, salinity, UV-B irradiation and ozone (Miller et al., 1999; John et al., 2001; Espinoza et al., 2007; Zhou et al., 2011; Guo and Gan, 2012; Allu et al., 2014; Zhou et al., 2014).
An important characteristic of senescence is the degradation of cellular macromolecules such as chlorophyll, lipids, proteins and nucleic acids. During the end stage of senescence, cells undergo programmed cell death (Lim et al., 2007). As shown in Table 2, many of these features are also affected by metal exposure in plants. In addition, it is known that several components of metal-induced signaling responses are also key players in the initiation and regulation of the senescence process. For example, changes in phytohormone levels are known to affect the onset of leaf senescence. While cytokinins, gibberellins and auxins delay the appearance of senescence symptoms, increases in the levels of other phytohormones such as ethylene, ABA, JA and SA have been shown to accelerate the process (Lim et al., 2007; Fischer, 2012).
Furthermore, transcriptional regulation mechanisms also play an important role in leaf senescence. In A. thaliana leaves, for example, more than 800 genes are differentially expressed during senescence (Buchanan-Wollaston et al., 2005). While certain genes such as those encoding photosynthetic proteins are transcriptionally downregulated, the expression of many other genes significantly increases when leaves enter the senescent stage. The latter genes are generally termed “senescence-associated genes” or SAGs and encode proteins involved in the breakdown of cellular compounds (e.g. nucleases, proteases and cell wall hydrolases) and the remobilization of nutrients to developing plant tissues. Also numerous transcription factors, many of which belong to the NAC and WRKY transcription factor families, are considered as SAGs (Miao et al., 2004; Fischer, 2012). For example, overexpression of the NAC transcription factor ORESARA1 SISTER1 (ORS1) accelerates senescence in A. thaliana, whereas the appearance of senescence symptoms is delayed in plants lacking functional ORS1. Furthermore, 42 genes were shown to be induced by ORS1, many of which are known to be involved in age-dependent senescence and in the response to long-term salinity (Balazadeh et al., 2011). Of the WRKY transcription factors, WRKY53 is one of the most studied genes with regard to senescence. It can affect the expression of several other transcription factors including other WRKYs, indicating that it might be a key player in a transcription factor signaling cascade (Miao et al., 2004). In addition, the MAP3K MEKK1 can directly phosphorylate the WRKY53 protein thereby increasing its DNA-binding activity, suggesting that MAPK signaling is also involved in the regulation of senescence (Miao et al., 2007). This idea is supported by the fact that plants overexpressing or lacking MKK9 and MPK6 show an accelerated or delayed onset of senescence, respectively (Zhou et al., 2009).
As mentioned above, metal exposure induces many effects associated with senescence in a broad range of plant species (Table 2). McCarthy et al. (2001) demonstrated Cd-induced increases in lipid peroxidation and protease activity in P. sativum leaves. Furthermore, they reported a decreased leaf chlorophyll content and a disorganization of chloroplast structure in leaves of Cd-exposed plants. Similar results were obtained by Rodríguez-Serrano et al. (2006), showing Cd-induced lipid peroxidation in P. sativum roots. In addition, levels of the senescence-promoting phytohormones SA, JA and ethylene were significantly elevated in roots of Cd-exposed plants as compared to those of control plants. Interestingly, these changes were accompanied by increases in and H2O2 levels, suggesting a role for ROS in Cd-induced accelerated senescence. In addition to Cd, other metals were shown to induce senescence-associated processes as well. Upadhyay and Panda (2010) demonstrated lipid peroxidation and decreased chlorophyll content associated with increased ROS levels in Spirodela polyrhiza. Furthermore, lipid peroxidation and negative effects on chlorophyll content or chloroplast structure were reported in Pb-exposed Ceratophyllum demersum (Mishra et al., 2006) and Zn-exposed Hydrilla verticillata (Xu et al., 2013).
Taken together, these data strongly suggest that metal exposure induces accelerated senescence in plants. However, little or no data are available on the effect of metal exposure on SAG expression levels. It is known, however, that transcription of many SAGs is increased in plants treated with H2O2 (Miao et al., 2004; Yan et al., 2007; Zhou et al., 2013, Zhou et al., 2014). Interestingly, ORS1 and WRKY53 expression was also induced by H2O2, suggesting that both transcription factors play a key role in the H2O2-induced senescence response in plants (Miao et al., 2004; Balazadeh et al., 2011).
A role for ROS in regulating senescence is further supported by the observed increased concentrations of and H2O2 in senescing tissues (Fischer, 2012). This can be caused by lipid peroxidation, which is known to occur during senescence (Zimmermann and Zentgraf, 2005). However, it could also be due to a decrease in the plant's antioxidative defense as reported by several authors (Jiménez et al., 1998; Prochazkova et al., 2001; Procházková and Wilhelmová, 2007). This hypothesis is further supported by the fact that the Arabidopsis vtc1-1 mutant, which is deficient in the antioxidative metabolite AsA, has a higher expression of certain SAGs and an earlier appearance of senescence symptoms as compared to WT plants (Barth et al., 2004). In addition to AsA, also the antioxidative enzyme CAT could be involved in regulating senescence. Indeed, Zimmermann et al. (2006) proposed that a downregulation of the CAT2 isoform contributes to the senescence-associated H2O2 peak, subsequently causing an increase in the expression levels of the stress-responsive CAT3 gene. Interestingly, Cuypers et al. (2011) reported a downregulation of CAT2 and an upregulation of CAT3 in Cd-exposed A. thaliana plants, possibly pointing to a Cd-induced acceleration of senescence.
As metals are known to increase ROS production, thereby inducing an oxidative challenge, we hypothesize a role for H2O2 in the damage and signaling events ultimately leading to premature leaf senescence under metal stress. In order to gain more insight into the effect of metal exposure on leaf senescence, future research should aim to identify the influence of different metals on the expression levels of SAGs including transcription factors such as ORS1 and WRKY53.
Conclusions and a look forward
By compiling the gathered evidence, the role of ROS and particularly H2O2 in regulating metal stress responses in plants is unequivocally demonstrated. Furthermore, it is becoming increasingly clear that oxidative damage and signaling are two sides of the same coin, potentially cooperating to establish plant acclimation and tolerance to metal exposure. Different studies highlight the interaction between ROS/H2O2 and signaling components such as MAPKs, phytohormones, Ca2+, NO•, oxylipins and regulating systems like miRNAs (Figure 2). Nevertheless, our current knowledge only represents the tip of the iceberg, encouraging further research efforts in the field of H2O2 perception, signal transduction and its role in plant acclimation to and growth under metal stress conditions.
Author contributions
All authors participated in the conception of the topic. AC, SH and EK wrote the manuscript. Figures and Tables were designed by RAR, SDS, JD, HG, MJ, CL and HV. All authors read and approved the final manuscript after critically revising it for important intellectual content.
Funding
This work was supported by the Research Foundation Flanders (FWO) by a postdoctoral grant for EK and projects [G0D3414] and [G0D1114]. Additional funding came from Hasselt University (BOF12NI28, BOF14DOC04) and PhD grants from the Agency for Innovation by Science and Technology (IWT-Flanders).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Achary V. M. M., Jena S., Panda K. K., Panda B. B. (2008). Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol. Environ. Saf. 70, 300–310. 10.1016/j.ecoenv.2007.10.022 [DOI] [PubMed] [Google Scholar]
- Ahammad G. J., Choudhary S. P., Chen S., Xia X., Shi K., Zhou Y., et al. (2013). Role of brassinosteroids in alleviation of phenanthrene-cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 64, 199–213. 10.1093/jxb/ers323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B., Huang C. R., Qi Z. Y., Ali S., Daud M. K., Geng X. X., et al. (2013). 5-Aminolevulinic acid ameliorates cadmium-induced morphological, biochemical, and ultrastructural changes in seedlings of oilseed rape. Environ. Sci. Pollut. Res. 20, 7256–7267. 10.1007/s11356-013-1735-5 [DOI] [PubMed] [Google Scholar]
- Ali B., Xu X., Gill R. A., Yang S., Ali S., Tahir M., et al. (2014). Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind. Crops Prod. 52, 617–626. 10.1016/j.indcrop.2013.11.033 [DOI] [Google Scholar]
- Allu A. D., Soja A. M., Wu A., Szymanski J., Balazadeh S. (2014). Salt stress and senescence: identification of cross-talk regulatory components. J. Exp. Bot. 65, 3993–4008. 10.1093/jxb/eru173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alscher R. G., Erturk N., Heath L. S. (2002). Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 53, 1331–1341. 10.1093/jexbot/53.372.1331 [DOI] [PubMed] [Google Scholar]
- Anjum N. A., Duarte A. C., Pereira E., Ahmad I. (2015). Juncus maritimus root biochemical assessment for its mercury stabilization potential in Ria de Aveiro coastal lagoon (Portugal). Environ. Sci. Pollut. Res. 22, 2231–2238. 10.1007/s11356-014-3455-x [DOI] [PubMed] [Google Scholar]
- Anjum N. A., Umar S., Iqbal M. (2014). Assessment of cadmium accumulation, toxicity, and tolerance in Brassicaceae and Fabaceae plants-implications for phytoremediation. Environ. Sci. Pollut. Res. 21, 10286–10293. 10.1007/s11356-014-2889-5 [DOI] [PubMed] [Google Scholar]
- Anthony R. G., Henriques R., Helfer A., Mészáros T., Rios G., Testerink C., et al. (2004). A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in Arabidopsis. EMBO J. 23, 572–581. 10.1038/sj.emboj.7600068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R. G., Khan S., Costa J., Pais M. S., Bögre L. (2006). The Arabidopsis protein kinase PTI1-2 is activated by convergent phosphatidic acid and oxidative stress signaling pathways downstream of PDK1 and OXI1. J. Biol. Chem. 281, 37536–37546. 10.1074/jbc.M607341200 [DOI] [PubMed] [Google Scholar]
- Anwaar S. A., Ali S., Ali S., Ishaque W., Farid M., Farooq M. A., et al. (2015). Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ. Sci. Pollut. Res. 22, 3441–3450. 10.1007/s11356-014-3938-9 [DOI] [PubMed] [Google Scholar]
- Apel K., Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- Arasimowicz-Jelonek M., Floryszak-Wieczorek J., Deckert J., Rucińska-Sobkowiak R., Gzyl J., Pawlak-Sprada S., et al. (2012). Nitric oxide implication in cadmium-induced programmed cell death in roots and signaling response of yellow lupine plants. Plant Physiol. Biochem. 58, 124–134. 10.1016/j.plaphy.2012.06.018 [DOI] [PubMed] [Google Scholar]
- Arasimowicz-Jelonek M., Floryszak-Wieczorek J., Gwóźdź E. A. (2011). The message of nitric oxide in cadmium challenged plants. Plant Sci. 181, 612–620. 10.1016/j.plantsci.2011.03.019 [DOI] [PubMed] [Google Scholar]
- Bai X.-J., Liu L.-J., Zhang C.-H., Ge Y., Cheng W.-D. (2011). Effect of H2O2 pretreatment on Cd tolerance of different rice cultivars. Rice Sci. 18, 29–35. 10.1016/S1672-6308(11)60005-X [DOI] [Google Scholar]
- Balazadeh S., Kwasniewski M., Caldana C., Mehrnia M., Zanor M. I., Xue G.-P., et al. (2011). ORS1, an H2O2-responsive NAC transcription factor, controls senescence in Arabidopsis thaliana. Mol. Plant 4, 346–360. 10.1093/mp/ssq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazadeh S., Riaño-Pachón D. M., Mueller-Roeber B. (2008). Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant Biol. 10(Suppl. 1), 63–75. 10.1111/j.1438-8677.2008.00088.x [DOI] [PubMed] [Google Scholar]
- Balazadeh S., Wu A., Mueller-Roeber B. (2010). Salt-triggered expression of the ANAC092-dependent senescence regulon in Arabidopsis thaliana. Plant Signal. Behav. 5, 733–735. 10.1111/j.1365-313X.2010.04151.x.he [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliardini C., Meyer C.-L., Salis P., Saumitou-Laprade P., Verbruggen N. (2015). CATION EXCHANGER1 cosegregates with cadmium tolerance in the metal hyperaccumulator Arabidopsis halleri and plays a role in limiting oxidative stress in Arabidopsis Spp. Plant Physiol. 169, 549–559. 10.1104/pp.15.01037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrameda-Medina Y., Montesinos-Pereira D., Romero L., Blasco B., Ruiz J. M. (2014). Role of GSH homeostasis under Zn toxicity in plants with different Zn tolerance. Plant Sci. 227, 110–121. 10.1016/j.plantsci.2014.07.010 [DOI] [PubMed] [Google Scholar]
- Barth C., Moeder W., Klessig D. F., Conklin P. L. (2004). The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol. 134, 1784–1792. 10.1104/pp.103.032185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli C. G., Gómez F., Martínez D. E., Guiamet J. J. (2004). Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J. Exp. Bot. 55, 1663–1669. 10.1093/jxb/erh199 [DOI] [PubMed] [Google Scholar]
- Baxter A., Mittler R., Suzuki N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65, 1229–1240. 10.1093/jxb/ert375 [DOI] [PubMed] [Google Scholar]
- Bela K., Horváth E., Gallé Á., Szabados L., Tari I., Csiszár J. (2015). Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol. 176, 192–201. 10.1016/j.jplph.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Besson-Bard A., Gravot A., Richaud P., Auroy P., Duc C., Gaymard F., et al. (2009). Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol. 149, 1302–1315. 10.1104/pp.108.133348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson-Bard A., Wendehenne D. (2009). NO contributes to cadmium toxicity in Arabidopsis thaliana by mediating an iron deprivation response. Plant Signal. Behav. 4, 252–254. 10.4161/psb.4.3.8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y. H., Chen W. L., Zhang W. N., Zhou Q., Yun L. J., Xing D. (2009). Production of reactive oxygen species, impairment of photosynthetic function and dynamic changes in mitochondria are early events in cadmium-induced cell death in Arabidopsis thaliana. Biol. Cell 101, 629–643. 10.1042/BC20090015 [DOI] [PubMed] [Google Scholar]
- Bienert G. P., Chaumont F. (2014). Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 1840, 1596–1604. 10.1016/j.bbagen.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Bienert G. P., Møller A. L. B., Kristiansen K. A., Schulz A., Møller I. M., Schjoerring J. K., et al. (2007). Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192. 10.1074/jbc.M603761200 [DOI] [PubMed] [Google Scholar]
- Bienert G. P., Schjoerring J. K., Jahn T. P. (2006). Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 1758, 994–1003. 10.1016/j.bbamem.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Boominathan R., Doran P. M. (2002). Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol. 156, 205–215. 10.1046/j.1469-8137.2002.00506.x [DOI] [PubMed] [Google Scholar]
- Browse J. (2009). Jasmonate passes muster: a receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 60, 183–205. 10.1146/annurev.arplant.043008.092007 [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Page T., Harrison E., Breeze E., Lim P. O., Nam H. G., et al. (2005). Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 42, 567–585. 10.1111/j.1365-313X.2005.02399.x [DOI] [PubMed] [Google Scholar]
- Chao Y. Y., Hsu Y. T., Kao C. H. (2009). Involvement of glutathione in heat shock- and hydrogen peroxide-induced cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Soil 318, 37–45. 10.1007/s11104-008-9815-x [DOI] [Google Scholar]
- Cheeseman J. M. (2006). Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 57, 2435–2444. 10.1093/jxb/erl004 [DOI] [PubMed] [Google Scholar]
- Chiang H.-C., Lo J.-C., Yeh K.-C. (2006). Genes associated with heavy metal tolerance and accumulation in Zn/Cd hyperaccumulator Arabidopsis halleri: a genomic survey with cDNA microarray. Environ. Sci. Technol. 40, 6792–6798. 10.1021/es061432y [DOI] [PubMed] [Google Scholar]
- Chmielowska-Bąk J., Izbiańska K., Deckert J. (2015). Products of lipid, protein and RNA oxidation as signals and regulators of gene expression in plants. Front. Plant Sci. 6:405. 10.3389/fpls.2015.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho U., Seo N. (2005). Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci. 168, 113–120. 10.1016/j.plantsci.2004.07.021 [DOI] [Google Scholar]
- Chou T.-S., Chao Y.-Y., Huang W.-D., Hong C.-Y., Kao C. H. (2011). Effect of magnesium deficiency on antioxidant status and cadmium toxicity in rice seedlings. J. Plant Physiol. 168, 1021–1030. 10.1016/j.jplph.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Chou T. S., Chao Y. Y., Kao C. H. (2012). Involvement of hydrogen peroxide in heat shock- and cadmium-induced expression of ascorbate peroxidase and glutathione reductase in leaves of rice seedlings. J. Plant Physiol. 169, 478–486. 10.1016/j.jplph.2011.11.012 [DOI] [PubMed] [Google Scholar]
- Colcombet J., Hirt H. (2008). Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem. J. 413, 217–226. 10.1042/BJ20080625 [DOI] [PubMed] [Google Scholar]
- Costa A., Drago I., Behera S., Zottini M., Pizzo P., Schroeder J. I., et al. (2010). H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J. 62, 760–772. 10.1111/j.1365-313X.2010.04190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers A., Keunen E., Bohler S., Jozefczak M., Opdenakker K., Gielen H., et al. (2012). Cadmium and copper stress induce a cellular oxidative challenge leading to damage versus signalling, in Metal Toxicity in Plants: Perception, Signaling and Remediation, eds Gupta D. K., Sandalio L. M. (Berlin; Heidelberg: Springer-Verlag GmbH; ), 65–90. [Google Scholar]
- Cuypers A., Smeets K., Ruytinx J., Opdenakker K., Keunen E., Remans T., et al. (2011). The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J. Plant Physiol. 168, 309–316. 10.1016/j.jplph.2010.07.010 [DOI] [PubMed] [Google Scholar]
- Cuypers A., Smeets K., Vangronsveld J. (2009). Heavy metal stress in plants, in Plant Stress Biology. From Genomics to Systems Biology, ed Hirt H. (Weinheim: Wiley-VCH Verlagsgesellschaft GmbH & Co. KGaA; ), 161–178. [Google Scholar]
- Das K., Roychoudhury A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2:53 10.3389/fenvs.2014.00053 [DOI] [Google Scholar]
- Dat J., Vandenabeele S., Vranová E., Van Montagu M., Inzé D., Van Breusegem F. (2000). Dual action of the active oxygen species during plant stress responses. Cell Mol. Life Sci. 57, 779–795. 10.1007/s000180050041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave A., Graham I. A. (2012). Oxylipin signaling: a distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA). Front. Plant Sci. 3:42. 10.3389/fpls.2012.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S., Rizhsky L., Liang H. J., Zhong S. Q., Oliver D. J., Coutu J., et al. (2005b). Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17, 268–281. 10.1105/tpc.104.026971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S., Schlauch K., Coutu J., Mittler R. (2005a). The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 139, 847–856. 10.1104/pp.105.068254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michele R., Vurro E., Rigo C., Costa A., Elviri L., Di Valentin M., et al. (2009). Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol. 150, 217–228. 10.1104/pp.108.133397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Xia Y., Hu W., Zhang H., Shen Z. (2010). Cadmium-induced oxidative damage and protective effects of N-acetyl-L-cysteine against cadmium toxicity in Solanum nigrum L. J. Hazard. Mater. 180, 722–729. 10.1016/j.jhazmat.2010.04.099 [DOI] [PubMed] [Google Scholar]
- Desikan R., A.-H-Mackerness S., Hancock J. T., Neill S. J. (2001). Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 127, 159–172. 10.1104/pp.127.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet S., Cuypers A., Vangronsveld J., Remans T. (2015). Gene networks involved in hormonal control of root development in Arabidopsis thaliana: a framework for studying its disturbance by metal stress. Int. J. Mol. Sci. 16, 19195–19224. 10.3390/ijms160819195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Baccio D., Castagna A., Tognetti R., Ranieri A., Sebastiani L. (2014). Early responses to cadmium of two poplar clones that differ in stress tolerance. J. Plant Physiol. 171, 1693–1705. 10.1016/j.jplph.2014.08.007 [DOI] [PubMed] [Google Scholar]
- Dietz K.-J. (2014). Redox regulation of transcription factors in plant stress acclimation and development. Antioxid. Redox Signal. 21, 1356–1372. 10.1089/ars.2013.5672 [DOI] [PubMed] [Google Scholar]
- di Toppi L. S., Gabbrielli R. (1999). Response to cadmium in higher plants. Environ. Exp. Bot. 41, 105–130. 10.1016/S0098-8472(98)00058-6 [DOI] [Google Scholar]
- Espinoza C., Medina C., Somerville S., Arce-Johnson P. (2007). Senescence-associated genes induced during compatible viral interactions with grapevine and Arabidopsis. J. Exp. Bot. 58, 3197–3212. 10.1093/jxb/erm165 [DOI] [PubMed] [Google Scholar]
- Eulgem T., Somssich I. E. (2007). Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 10, 366–371. 10.1016/j.pbi.2007.04.020 [DOI] [PubMed] [Google Scholar]
- Faller P., Kienzler K., Krieger-Liszkay A. (2005). Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site. Biochim. Biophys. Acta 1706, 158–164. 10.1016/j.bbabio.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Fang Y., Liao K., Du H., Xu Y., Song H., Li X., et al. (2015). A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 66, 6803–6817. 10.1093/jxb/erv386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigl G., Lehotai N., Molnár Á., Ördög A., Rodríguez-Ruiz M., Palma J. M., et al. (2015). Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 116, 613–625. 10.1093/aob/mcu246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández R., Bertrand A., Reis R., Mourato M. P., Martins L. L., González A. (2013). Growth and physiological responses to cadmium stress of two populations of Dittrichia viscosa (L.) Greuter. J. Hazard. Mater. 244–245, 555–562. 10.1016/j.jhazmat.2012.10.044 [DOI] [PubMed] [Google Scholar]
- Fischer A. M. (2012). The complex regulation of senescence. Crit. Rev. Plant Sci. 31, 124–147. 10.1080/07352689.2011.616065 [DOI] [Google Scholar]
- Foyer C. H., Noctor G. (2005). Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 28, 1056–1071. 10.1111/j.1365-3040.2005.01327.x [DOI] [Google Scholar]
- Gadjev I., Stone J. M., Gechev T. S. (2008). Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int. Rev. Cell Mol. Biol. 270, 87–144. 10.1016/S1937-6448(08)01403-2 [DOI] [PubMed] [Google Scholar]
- Gadjev I., Vanderauwera S., Gechev T. S., Laloi C., Minkov I. N., Shulaev V., et al. (2006). Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 141, 436–445. 10.1104/pp.106.078717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier L., Simon-Plas F., Thuleau P., Agnel J. P., Blein J. P., Ranjeva R., et al. (2006). Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ. 29, 1956–1969. 10.1111/j.1365-3040.2006.01571.x [DOI] [PubMed] [Google Scholar]
- Gechev T. S., Hille J. (2004). Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 168, 17–20. 10.1083/jcb.200409170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev T. S., Van Breusegem F., Stone J. M., Denev I., Laloi C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28, 1091–1101. 10.1002/bies.20493 [DOI] [PubMed] [Google Scholar]
- Gielen H., Remans T., Vangronsveld J., Cuypers A. (2012). MicroRNAs in metal stress: specific roles or secondary responses? Int. J. Mol. Sci. 13, 15826–15847. 10.3390/ijms131215826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. S., Khan N. A., Tuteja N. (2012). Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci. 182, 112–120. 10.1016/j.plantsci.2011.04.018 [DOI] [PubMed] [Google Scholar]
- Gill S. S., Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Gomes M. P., Duarte D. M., Carneiro M. M. L. C., Barreto L. C., Carvalho M., Soares A. M., et al. (2013). Zinc tolerance modulation in Myracrodruon urundeuva plants. Plant Physiol. Biochem. 67, 1–6. 10.1016/j.plaphy.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Guo Y., Gan S. S. (2012). Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ. 35, 644–655. 10.1111/j.1365-3040.2011.02442.x [DOI] [PubMed] [Google Scholar]
- Gupta D. K., Nicoloso F. T., Schetinger M. R., Rossato L. V., Pereira L. B., Castro G. Y., et al. (2009). Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J. Hazard. Mater. 172, 479–484. 10.1016/j.jhazmat.2009.06.141 [DOI] [PubMed] [Google Scholar]
- Gupta O. P., Sharma P., Gupta R. K., Sharma I. (2014). MicroRNA mediated regulation of metal toxicity in plants: present status and future perspectives. Plant Mol. Biol. 84, 1–18. 10.1007/s11103-013-0120-6 [DOI] [PubMed] [Google Scholar]
- Guzel S., Terzi R. (2013). Exogenous hydrogen peroxide increases dry matter production, mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot. Stud. 54, 10 10.1186/1999-3110-54-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. (2006). Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 141, 312–322. 10.1104/pp.106.077073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Li G.-J., Yang K.-Y., Mao G., Wang R., Liu Y., et al. (2010). Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 64, 114–127. 10.1111/j.1365-313X.2010.04318.x [DOI] [PubMed] [Google Scholar]
- Han R. M., Lefèvre I., Albacete A., Pérez-Alfocea F., Barba-Espín G., Díaz-Vivancos P., et al. (2013). Antioxidant enzyme activities and hormonal status inresponse to Cd stress in the wetland halophyte Kosteletzkya virginica under saline conditions. Physiol. Plant. 147, 352–368. 10.1111/j.1399-3054.2012.01667.x [DOI] [PubMed] [Google Scholar]
- Hänsch R., Mendel R. R. (2009). Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 12, 259–266. 10.1016/j.pbi.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Hao F., Wang X., Chen J. (2006). Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci. 170, 151–158. 10.1016/j.plantsci.2005.08.014 [DOI] [Google Scholar]
- Hardin S. C., Larue C. T., Oh M.-H., Jain V., Huber S. C. (2009). Coupling oxidative signals to protein phosphorylation via methionine oxidation in Arabidopsis. Biochem. J. 422, 305–312. 10.1042/BJ20090764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinen V. H., Tervahauta A. I., Schat H., Ka S. O. (2011). Plant metallothioneins – metal chelators with ROS scavenging activity? Plant Biol. 13, 225–232. 10.1111/j.1438-8677.2010.00398.x [DOI] [PubMed] [Google Scholar]
- He J., Ma C., Ma Y., Li H., Kang J., Liu T., et al. (2013). Cadmium tolerance in six poplar species. Environ. Sci. Pollut. Res. 20, 163–174. 10.1007/s11356-012-1008-8 [DOI] [PubMed] [Google Scholar]
- He J., Qin J., Long L., Ma Y., Li H., Li K., et al. (2011). Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus x canescens. Physiol. Plant. 143, 50–63. 10.1111/j.1399-3054.2011.01487.x [DOI] [PubMed] [Google Scholar]
- Hossain M. A., Bhattacharjee S., Armin S.-M., Qian P., Xin W., Li H.-Y., et al. (2015). Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front. Plant Sci. 6:420. 10.3389/fpls.2015.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. L., Ge Y., Zhang C. H., Ju T., Cheng W. D. (2009). Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Regul. 59, 51–61. 10.1007/s10725-009-9387-7 [DOI] [Google Scholar]
- Hu Y., You J., Liang X. (2015). Nitrate reductase-mediated nitric oxide production is involved in copper tolerance in shoots of hulless barley. Plant Cell Rep. 34, 367–379. 10.1007/s00299-014-1715-3 [DOI] [PubMed] [Google Scholar]
- Huang S. Q., Xiang A. L., Che L. L., Chen S., Li H., Song J. B., et al. (2010). A set of miRNAs from Brassica napus in response to sulphate deficiency and cadmium stress. Plant Biotechnol. J. 8, 887–899. 10.1111/j.1467-7652.2010.00517.x [DOI] [PubMed] [Google Scholar]
- Iannone M. F., Rosales E. P., Groppa M. D., Benavides M. P. (2010). Reactive oxygen species formation and cell death in catalase-deficient tobacco leaf disks exposed to cadmium. Protoplasma 245, 15–27. 10.1007/s00709-009-0097-9 [DOI] [PubMed] [Google Scholar]
- İşeri Ö. D., Körpe D. A., Yurtcu E., Sahin F. I., Haberal M. (2011). Copper-induced oxidative damage, antioxidant response and genotoxicity in Lycopersicum esculentum Mill. and Cucumis sativus L. Plant Cell Rep. 30, 1713–1721. 10.1007/s00299-011-1079-x [DOI] [PubMed] [Google Scholar]
- Ivanishchev V. V., Abramova E. A. (2015). Accumulation of nickel ions in seedlings of Vicia sativa L. and manifestations of oxidative stress. Environ. Sci. Pollut. Res. Int. 22, 7897–7905. 10.1007/s11356-015-4173-8 [DOI] [PubMed] [Google Scholar]
- Järup L. (2003). Hazards of heavy metal contamination. Br. Med. Bull. 68, 167–182. 10.1093/bmb/ldg032 [DOI] [PubMed] [Google Scholar]
- Jiménez A., Hernández J. A., Pastori G., del Río L. A., Sevilla F. (1998). Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 118, 1327–1335. 10.1104/pp.118.4.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John C. F., Morris K., Jordan B. R., Thomas B., A.-H.-Mackerness S. (2001). Ultraviolet-B exposure leads to up-regulation of senescence-associated genes in Arabidopsis thaliana. J. Exp. Bot. 52, 1367–1373. 10.1093/jexbot/52.359.1367 [DOI] [PubMed] [Google Scholar]
- Jonak C., Nakagami H., Hirt H. (2004). Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 136, 3276–3283. 10.1104/pp.104.045724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S., Liu Y., Lueth A., Zhang S. (2008). MAPK phosphorylation-induced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. Plant J. 54, 129–140. 10.1111/j.1365-313X.2008.03404.x [DOI] [PubMed] [Google Scholar]
- Jozefczak M., Remans T., Vangronsveld J., Cuypers A. (2012). Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 13, 3145–3175. 10.3390/ijms13033145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärkönen A., Kuchitsu K. (2015). Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112, 22–32. 10.1016/j.phytochem.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Kaur G., Singh H. P., Batish D. R., Kohli R. K. (2013). Lead (Pb)-induced biochemical and ultrastructural changes in wheat (Triticum aestivum) roots. Protoplasma 250, 53–62. 10.1007/s00709-011-0372-4 [DOI] [PubMed] [Google Scholar]
- Kaur G., Singh H. P., Batish D. R., Mahajan P., Kohli R. K., Rishi V. (2015). Exogenous nitric oxide (NO) interferes with lead (Pb)-induced toxicity by detoxifying reactive oxygen species in hydroponically grown wheat (Triticum aestivum) roots. PLoS ONE 10:e0138713. 10.1371/journal.pone.0138713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi N., Khavari-Nejad R. A., Fahimi H., Saadatmand S., Nejad-Sattari T. (2010). Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci. Hortic. 126, 402–407. 10.1016/j.scienta.2010.07.037 [DOI] [Google Scholar]
- Keunen E., Remans T., Opdenakker K., Jozefczak M., Gielen H., Guisez Y., et al. (2013). A mutant of the Arabidopsis thaliana LIPOXYGENASE1 gene shows altered signalling and oxidative stress related responses after cadmium exposure. Plant Physiol. Biochem. 63, 272–280. 10.1016/j.plaphy.2012.12.005 [DOI] [PubMed] [Google Scholar]
- Keunen E., Schellingen K., Van Der Straeten D., Remans T., Colpaert J., Vangronsveld J., et al. (2015). ALTERNATIVE OXIDASE1a modulates the oxidative challenge during moderate Cd exposure in Arabidopsis thaliana leaves. J. Exp. Bot. 66, 2967–2977. 10.1093/jxb/erv035 [DOI] [PubMed] [Google Scholar]
- Keunen E., Schellingen K., Vangronsveld J., Cuypers A. (2016). Ethylene and metal stress: small molecule, big impact. Front. Plant Sci. 7:23. 10.3389/fpls.2016.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. I. R., Khan N. A. (2014). Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 251, 1007–1019. 10.1007/s00709-014-0610-7 [DOI] [PubMed] [Google Scholar]
- Kim Y. H., Lee H. S., Kwak S. S. (2010). Differential responses of sweetpotato peroxidases to heavy metals. Chemosphere 81, 79–85. 10.1016/j.chemosphere.2010.06.063 [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Ohura I., Kawakita K., Yokota N., Fujiwara M., Shimamoto K., et al. (2007). Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19, 1065–1080. 10.1105/tpc.106.048884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kováčik J., Grúz J., Klejdus B., Štork F., Marchiosi R., Ferrarese-Filho O. (2010a). Lignification and related parameters in copper-exposed Matricaria chamomilla roots: role of H2O2 and NO in this process. Plant Sci. 179, 383–389. 10.1016/j.plantsci.2010.06.014 [DOI] [Google Scholar]
- Kováčik J., Klejdus B., Hedbavny J., Zoń J. (2010b). Copper uptake is differentially modulated by phenylalanine ammonia-lyase inhibition in diploid and tetraploid chamomile. J. Agric. Food Chem. 58, 10270–10276. 10.1021/jf101977v [DOI] [PubMed] [Google Scholar]
- Kovtun Y., Chiu W. L., Tena G., Sheen J. (2000). Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. U.S.A. 97, 2940–2945. 10.1073/pnas.97.6.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen B. K., Askerlund P., Bykova N. V., Egsgaard H., Møller I. M. (2004). Identification of oxidised proteins in the matrix of rice leaf mitochondria by immunoprecipitation and two-dimensional liquid chromatography-tandem mass spectrometry. Phytochemistry 65, 1839–1851. 10.1016/j.phytochem.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Kumar A., Prasad M. N. V., Mohan Murali Achary V., Panda B. B. (2013). Elucidation of lead-induced oxidative stress in Talinum triangulare roots by analysis of antioxidant responses and DNA damage at cellular level. Environ. Sci. Pollut. Res. Int. 20, 4551–4561. 10.1007/s11356-012-1354-6 [DOI] [PubMed] [Google Scholar]
- Lee S., Moon J. S., Ko T. S., Petros D., Goldsbrough P. B., Korban S. S. (2003). Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol. 131, 656–663. 10.1104/pp.014118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocka J., Sobieszczuk-Nowicka E., Wojtyla Ł., Samardakiewicz S. (2015). Lead-stress induced changes in the content of free, thylakoid- and chromatin-bound polyamines, photosynthetic parameters and ultrastructure in greening barley leaves. J. Plant Physiol. 186-187, 15–24. 10.1016/j.jplph.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Li A., Wang X., Leseberg C. H., Jia J., Mao L. (2008). Biotic and abiotic stress responses through calcium-dependent protein kinase (CDPK) signaling in wheat (Triticum aestivum L.). 3, 654–656. 10.1007/s11103-007-9281-5.654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Meng X., Wang R., Mao G., Han L., Liu Y., et al. (2012). Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 8:e1002767. 10.1371/journal.pgen.1002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Li H., Zhang Y.-X., Liu J.-Y. (2011). Identification and analysis of seven H2O2-responsive miRNAs and 32 new miRNAs in the seedlings of rice (Oryza sativa L. ssp. indica). Nucleic Acids Res. 39, 2821–2833. 10.1093/nar/gkq1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P. O., Kim H. J., Nam H. G. (2007). Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136. 10.1146/annurev.arplant.57.032905.105316 [DOI] [PubMed] [Google Scholar]
- Lin Y.-F., Aarts M. G. M. (2012). The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 69, 3187–3206. 10.1007/s00018-012-1089-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Shi X., Qian M., Zheng L., Lian C., Xia Y., et al. (2015). Copper-induced hydrogen peroxide upregulation of a metallothionein gene, OsMT2c, from Oryza sativa L. confers copper tolerance in Arabidopsis thaliana. J. Hazard. Mater. 294, 99–108. 10.1016/j.jhazmat.2015.03.060 [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang H., Zhang Y., Chai T. (2013). Silicon attenuates cadmium toxicity in Solanum nigrum L. by reducing cadmium uptake and oxidative stress. Plant Physiol. Biochem. 68, 1–7. 10.1016/j.plaphy.2013.03.018 [DOI] [PubMed] [Google Scholar]
- Liu W. J., Wood B. A., Raab A., McGrath S. P., Zhao F. J., Feldmann J. (2010). Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol. 152, 2211–2221. 10.1104/pp.109.150862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. M., Kim K. E., Kim K. C., Nguyen X. C., Han H. J., Jung M. S., et al. (2010). Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry 71, 614–618. 10.1016/j.phytochem.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang S. (2004). Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16, 3386–3399. 10.1105/tpc.104.026609.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Orenes A., Martínez-Pérez A., Calderón A. A., Ferrer M. A. (2014). Pb-induced responses in Zygophyllum fabago plants are organ-dependent and modulated by salicylic acid. Plant Physiol. Biochem. 84, 57–66. 10.1016/j.plaphy.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Lux A., Martinka M., Vaculík M., White P. J. (2011). Root responses to cadmium in the rhizosphere: a review. J. Exp. Bot. 62, 21–37. 10.1093/jxb/erq281 [DOI] [PubMed] [Google Scholar]
- Macovei A., Balestrazzi A., Confalonieri M., Carbonera D. (2010). The tyrosyl-DNA phosphodiesterase gene family in Medicago truncatula Gaertn.: bioinformatic investigation and expression profiles in response to copper- and PEG-mediated stress. Planta 232, 393–407. 10.1007/s00425-010-1179-9 [DOI] [PubMed] [Google Scholar]
- Maksymiec W. (2007). Signaling responses in plants to heavy metal stress. Acta Physiol. Plant. 29, 177–187. 10.1007/s11738-007-0036-3 [DOI] [Google Scholar]
- Maksymiec W., Krupa Z. (2002). The in vivo and in vitro influence of methyl jasmonate on oxidative processes in Arabidopsis thaliana leaves. Acta Physiol. Plant. 24, 351–357. 10.1007/s11738-002-0029-1 [DOI] [Google Scholar]
- Maksymiec W., Wianowska D., Dawidowicz A. L., Radkiewicz S., Mardarowicz M., Krupa Z. (2005). The level of jasmonic acid in Arabidopsis thaliana and Phaseolus coccineus plants under heavy metal stress. J. Plant Physiol. 162, 1338–1346. 10.1016/j.jplph.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Marrs K. A. (1996). The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 127–158. 10.1146/annurev.arplant.47.1.127 [DOI] [PubMed] [Google Scholar]
- Martínez-Peñalver A., Graña E., Reigosa M. J., Sánchez-Moreiras A. M. (2012). The early response of Arabidopsis thaliana to cadmium- and copper-induced stress. Environ. Exp. Bot. 78, 1–9. 10.1016/j.envexpbot.2011.12.017 [DOI] [Google Scholar]
- Masood A., Iqbal N., Khan N. A. (2012). Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant. Cell Environ. 35, 524–533. 10.1111/j.1365-3040.2011.02432.x [DOI] [PubMed] [Google Scholar]
- Mathé C., Barre A., Jourda C., Dunand C. (2010). Evolution and expression of class III peroxidases. Arch. Biochem. Biophys. 500, 58–65. 10.1016/j.abb.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Mazars C., Thuleau P., Lamotte O., Bourque S. (2010). Cross-talk between ROS and calcium in regulation of nuclear activities. Mol. Plant 3, 706–718. 10.1093/mp/ssq024 [DOI] [PubMed] [Google Scholar]
- McCarthy I., Romero-Puertas M. C., Palma J. M., Sandalio L. M., Corpas F. J., Gómez M., et al. (2001). Cadmium induces senescence symptoms in leaf peroxisomes of pea plants. Plant Cell Environ. 24, 1065–1073. 10.1046/j.1365-3040.2001.00750.x [DOI] [Google Scholar]
- Miao Y., Laun T. M., Smykowski A., Zentgraf U. (2007). Arabidopsis MEKK1 can take a short cut: it can directly interact with senescence-related WRKY53 transcription factor on the protein level and can bind to its promoter. Plant Mol. Biol. 65, 63–76. 10.1007/s11103-007-9198-z [DOI] [PubMed] [Google Scholar]
- Miao Y., Laun T., Zimmermann P., Zentgraf U. (2004). Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol. Biol. 55, 853–867. 10.1007/s11103-004-2142-6 [DOI] [PubMed] [Google Scholar]
- Michael P. I., Krishnaswamy M. (2011). The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings. Environ. Exp. Bot. 74, 171–177. 10.1016/j.envexpbot.2011.05.016 [DOI] [Google Scholar]
- Miller G., Mittler R. (2006). Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 98, 279–288. 10.1093/aob/mcl107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Schlauch K., Tam R., Cortes D., Torres M. A., Shulaev V., et al. (2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2:ra45. 10.1126/scisignal.2000448 [DOI] [PubMed] [Google Scholar]
- Miller G., Shulaev V., Mittler R. (2008). Reactive oxygen signaling and abiotic stress. Physiol. Plant. 133, 481–489. 10.1111/j.1399-3054.2008.01090.x [DOI] [PubMed] [Google Scholar]
- Miller J. D., Arteca R. N., Pell E. J. (1999). Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiol. 120, 1015–1023. 10.1104/pp.120.4.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S., Srivastava S., Tripathi R. D., Kumar R., Seth C. S., Gupta D. K. (2006). Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 65, 1027–1039. 10.1016/j.chemosphere.2006.03.033 [DOI] [PubMed] [Google Scholar]
- Mithöfer A., Schulze B., Boland W. (2004). Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett. 566, 1–5. 10.1016/j.febslet.2004.04.011 [DOI] [PubMed] [Google Scholar]
- Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. 10.1016/S1360-1385(02)02312-9 [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. 10.1016/j.tplants.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V. B., Vandepoele K., et al. (2011). ROS signaling: the new wave? Trends Plant Sci. 16, 300–309. 10.1016/j.tplants.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Møller I. M., Jensen P. E., Hansson A. (2007). Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 58, 459–481. 10.1146/annurev.arplant.58.032806.103946 [DOI] [PubMed] [Google Scholar]
- Møller I. M., Kristensen B. K. (2004). Protein oxidation in plant mitochondria as a stress indicator. Photochem. Photobiol. Sci. 3, 730–735. 10.1039/b315561g [DOI] [PubMed] [Google Scholar]
- Møller I. M., Sweetlove L. J. (2010). ROS signalling - specificity is required. Trends Plant Sci. 15, 370–374. 10.1016/j.tplants.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Monteiro C. C., Carvalho R. F., Gratão P. L., Carvalho G., Tezotto T., Medici L. O., et al. (2011). Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environ. Exp. Bot. 71, 306–320. 10.1016/j.envexpbot.2010.12.020 [DOI] [Google Scholar]
- Monteiro C., Santos C., Pinho S., Oliveira H., Pedrosa T., Dias M. C. (2012). Cadmium-induced cyto- and genotoxicity are organ-dependent in lettuce. Chem. Res. Toxicol. 25, 1423–1434. 10.1021/tx300039t [DOI] [PubMed] [Google Scholar]
- Montero-Palmero M. B., Martín-Barranco A., Escobar C., Hernández L. E. (2014). Early transcriptional responses to mercury: a role for ethylene in mercury-induced stress. New Phytol. 201, 116–130. 10.1111/nph.12486 [DOI] [PubMed] [Google Scholar]
- Morina F., Jovanovic L., Mojovic M., Vidovic M., Pankovic D., Veljovic Jovanovic S. (2010). Zinc-induced oxidative stress in Verbascum thapsus L. is caused by an accumulation of reactive oxygen species and quinhydrone in the cell wall. Physiol. Plant. 140, 209–225. 10.1111/j.1399-3054.2010.01399.x [DOI] [PubMed] [Google Scholar]
- Mostofa M. G., Hossain M. A., Fujita M., Tran L.-S. P. (2015a). Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci. Rep. 5, 1–16. 10.1038/srep11433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofa M. G., Rahman A., Ansary M. U., Watanabe A., Fujita M., Tran L. S. (2015b). Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 5:14078. 10.1038/srep14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa H. R., El-Gamal S. M. (2010). Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol. Plant. 54, 315–320. 10.1007/s10535-010-0054-7 [DOI] [Google Scholar]
- Nair A., DeGheselle O., Smeets K., Van Kerkhove E., Cuypers A. (2013). Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int. J. Mol. Sci. 14, 6116–6143. 10.3390/ijms14036116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmanova J., Neumannova E., Leonhardt T., Zitka O., Kizek R., Macek T., et al. (2012). Cadmium-induced production of phytochelatins and speciation of intracellular cadmium in organs of Linum usitatissimum seedlings. Ind. Crop. Prod. 36, 536–542. 10.1016/j.indcrop.2011.11.008 [DOI] [Google Scholar]
- Navrot N., Rouhier N., Gelhaye E., Jacquot J.-P. (2007). Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol. Plant. 129, 185–195. 10.1111/j.1399-3054.2006.00777.x [DOI] [Google Scholar]
- Neill S., Desikan R., Hancock J. (2002). Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 5, 388–395. 10.1016/S1369-5266(02)00282-0 [DOI] [PubMed] [Google Scholar]
- Neill S. J., Desikan R., Clarke A., Hurst R. D., Hancock J. T. (2002). Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 53, 1237–1247. 10.1093/jexbot/53.372.1237 [DOI] [PubMed] [Google Scholar]
- Noctor G., Mhamdi A., Chaouch S., Han Y., Neukermans J., Marquez-Garcia B., et al. (2012). Glutathione in plants: an integrated overview. Plant Cell Environ. 35, 454–484. 10.1111/j.1365-3040.2011.02400.x [DOI] [PubMed] [Google Scholar]
- O'Brien J. A., Daudi A., Butt V. S., Bolwell G. P. (2012). Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236, 765–779. 10.1007/s00425-012-1696-9 [DOI] [PubMed] [Google Scholar]
- Ogasawara Y., Kaya H., Hiraoka G., Yumoto F., Kimura S., Kadota Y., et al. (2008). Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 283, 8885–8892. 10.1074/jbc.M708106200 [DOI] [PubMed] [Google Scholar]
- Opdenakker K., Remans T., Keunen E., Vangronsveld J., Cuypers A. (2012a). Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAPKinase transcript levels. Environ. Exp. Bot. 83, 53–61. 10.1016/j.envexpbot.2012.04.003 [DOI] [Google Scholar]
- Opdenakker K., Remans T., Vangronsveld J., Cuypers A. (2012b). Mitogen-activated protein (MAP) kinases in plant metal stress: regulation and responses in comparison to other biotic and abiotic stresses. Int. J. Mol. Sci. 13, 7828–7853. 10.3390/ijms13067828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Zhu M., Chen H. (2001). Aluminum-induced cell death in root-tip cells of barley. Environ. Exp. Bot. 46, 71–79. 10.1016/S0098-8472(01)00083-1 [DOI] [PubMed] [Google Scholar]
- Panda S. K., Sunkar R. (2015). Nutrient- and other stress-responsive microRNAs in plants: Role for thiol-based redox signaling. Plant Signal. Behav. 10:e1010916. 10.1080/15592324.2015.1010916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaia G., Margis-Pinheiro M. (2015). Glutathione peroxidases as redox sensor proteins in plant cells. Plant Sci. 234, 22–26. 10.1016/j.plantsci.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Pereira L. B., Mazzanti C. M. D. A., Gonçalves J. F., Cargnelutti D., Tabaldi L. A., Becker A. G., et al. (2010). Aluminum-induced oxidative stress in cucumber. Plant Physiol. Biochem. 48, 683–689. 10.1016/j.plaphy.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Pérez-Chaca M. V., Rodríguez-Serrano M., Molina A. S., Pedranzani H. E., Zirulnik F., Sandalio L. M., et al. (2014). Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant. Cell Environ. 37, 1672–1687. 10.1111/pce.12280 [DOI] [PubMed] [Google Scholar]
- Perez I. B., Brown P. J. (2014). The role of ROS signaling in cross-tolerance: from model to crop. Front. Plant Sci. 5:754. 10.3389/fpls.2014.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov V. D., Van Breusegem F. (2012). Hydrogen peroxide - a central hub for information flow in plant cells. AoB Plants 2012:pls014. 10.1093/aobpla/pls014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov V., Hille J., Mueller-Roeber B., Gechev T. S. (2015). ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 6:69. 10.3389/fpls.2015.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon M., Cohu C. M., Ravet K., Abdel-Ghany S. E., Gaymard F. (2009). Essential transition metal homeostasis in plants. Curr. Opin. Plant Biol. 12, 347–357. 10.1016/j.pbi.2009.04.011 [DOI] [PubMed] [Google Scholar]
- Pitzschke A., Djamei A., Bitton F., Hirt H. (2009). A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol. Plant 2, 120–137. 10.1093/mp/ssn079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzschke A., Hirt H. (2006). Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiol. 141, 351–356. 10.1104/pp.106.079160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podazza G., Arias M., Prado F. E. (2012). Cadmium accumulation and strategies to avoid its toxicity in roots of the citrus rootstock Citrumelo. J. Hazard. Mater. 215-216, 83–89. 10.1016/j.jhazmat.2012.02.031 [DOI] [PubMed] [Google Scholar]
- Poschenrieder C., Tolrá R., Barceló J. (2006). Can metals defend plants against biotic stress? Trends Plant Sci. 11, 288–295. 10.1016/j.tplants.2006.04.007 [DOI] [PubMed] [Google Scholar]
- Prochazkova D., Sairam R. K., Srivastava G. C., Singh D. V. (2001). Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 161, 765–771. 10.1016/s0168-9452(01)00462-9 [DOI] [Google Scholar]
- Procházková D., Wilhelmová N. (2007). Leaf senescence and activities of the antioxidant enzymes. Biol. Plant. 51, 401–406. 10.1007/s10535-007-0088-7 [DOI] [Google Scholar]
- Qiao X., Shi G., Chen L., Tian X., Xu X. (2013). Lead-induced oxidative damage in steriled seedlings of Nymphoides peltatum. Environ. Sci. Pollut. Res. 20, 5047–5055. 10.1007/s11356-013-1475-6 [DOI] [PubMed] [Google Scholar]
- Quan L.-J., Zhang B., Shi W.-W., Li H.-Y. (2008). Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 50, 2–18. 10.1111/j.1744-7909.2007.00599.x [DOI] [PubMed] [Google Scholar]
- Remans T., Opdenakker K., Guisez Y., Carleer R., Schat H., Vangronsveld J., et al. (2012a). Exposure of Arabidopsis thaliana to excess Zn reveals a Zn-specific oxidative stress signature. Environ. Exp. Bot. 84, 61–71. 10.1016/j.envexpbot.2012.05.005 [DOI] [Google Scholar]
- Remans T., Opdenakker K., Smeets K., Mathijsen D., Vangronsveld J., Cuypers A. (2010). Metal-specific and NADPH oxidase dependent changes in lipoxygenase and NADPH oxidase gene expression in Arabidopsis thaliana exposed to cadmium or excess copper. Funct. Plant Biol. 37, 532–544. 10.1071/FP0919426974871 [DOI] [Google Scholar]
- Remans T., Thijs S., Truyens S., Weyens N., Schellingen K., Keunen E., et al. (2012b). Understanding the development of roots exposed to contaminants and the potential of plant-associated bacteria for optimization of growth. Ann. Bot. 110, 239–252. 10.1093/aob/mcs105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D., Yang H., Zhang S. (2002). Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J. Biol. Chem. 277, 559–565. 10.1074/jbc.M109495200 [DOI] [PubMed] [Google Scholar]
- Rentel M. C., Knight M. R. (2004). Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol. 135, 1471–1479. 10.1104/pp.104.042663.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentel M. C., Lecourieux D., Ouaked F., Usher S. L., Petersen L., Okamoto H., et al. (2004). OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427, 858–861. 10.1038/nature02353 [DOI] [PubMed] [Google Scholar]
- Rizhsky L., Davletova S., Liang H., Mittler R. (2004). The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J. Biol. Chem. 279, 11736–11743. 10.1074/jbc.M313350200 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Serrano M., Romero-Puertas M. C., Pazmiño D. M., Testillano P. S., Risueno M. C., del Río L. A., et al. (2009). Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 150, 229–243. 10.1104/pp.108.131524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Serrano M., Romero-Puertas M. C., Zabalza A., Corpas F. J., Gómez M., del Río L. A., et al. (2006). Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ. 29, 1532–1544. 10.1111/j.1365-3040.2006.01531.x [DOI] [PubMed] [Google Scholar]
- Romero-Puertas M. C., Rodríguez-Serrano M., Corpas F. J., Gomez M., del Río L. A., Sandalio L. M. (2004). Cadmium-induced subcellular accumulation of and H2O2 in pea leaves. Plant Cell Env. 27, 1122–1134. 10.1111/j.1365-3040.2004.01217.x [DOI] [Google Scholar]
- Sagi M., Fluhr R. (2006). Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 141, 336–340. 10.1104/pp.106.078089.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi I., Chtourou Y., Djebali W. (2014). Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 171, 85–91. 10.1016/j.jplph.2013.09.024 [DOI] [PubMed] [Google Scholar]
- Samardakiewicz S., Krzeszowiec-Jeleń W., Bednarski W., Jankowski A., Suski S., Gabryś H., et al. (2015). Pb-induced avoidance-like chloroplast movements in fronds of Lemna trisulca L. PLoS ONE 10:e0116757. 10.1371/journal.pone.0116757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santino A., Taurino M., De Domenico S., Bonsegna S., Poltronieri P., Pastor V., et al. (2013). Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 32, 1085–1098. 10.1007/s00299-013-1441-2 [DOI] [PubMed] [Google Scholar]
- Schellingen K., Van Der Straeten D., Remans T., Vangronsveld J., Keunen E., Cuypers A. (2015). Ethylene signalling is mediating the early cadmium-induced oxidative challenge in Arabidopsis thaliana. Plant Sci. 239, 137–146. 10.1016/j.plantsci.2015.07.015 [DOI] [PubMed] [Google Scholar]
- Schellingen K., Van Der Straeten D., Vandenbussche F., Prinsen E., Remans T., Vangronsveld J., et al. (2014). Cadmium-induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biol. 14:214. 10.1186/s12870-014-0214-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz P., Herde M., Romeis T. (2013). Calcium-dependent protein kinases: hubs in plant stress signaling and development. Plant Physiol. 163, 523–530. 10.1104/pp.113.222539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schützendübel A., Polle A. (2002). Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53, 1351–1365. 10.1093/jexbot/53.372.1351 [DOI] [PubMed] [Google Scholar]
- Sewelam N., Jaspert N., Van Der Kelen K., Tognetti V. B., Schmitz J., Frerigmann H., et al. (2014). Spatial H2O2 signaling specificity: H2O2 from chloroplasts and peroxisomes modulates the plant transcriptome differentially. Mol. Plant 7, 1191–1210. 10.1093/mp/ssu070 [DOI] [PubMed] [Google Scholar]
- Shahid M., Dumat C., Silvestre J., Pinelli E. (2012). Effect of fulvic acids on lead-induced oxidative stress to metal sensitive Vicia faba L. Plant Biol. Fertil. Soils 48, 689–697. 10.1007/s00374-012-0662-9 [DOI] [Google Scholar]
- Sharma S. S., Dietz K.-J. (2006). The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 57, 711–726. 10.1093/jxb/erj073 [DOI] [PubMed] [Google Scholar]
- Sharma S. S., Dietz K.-J. (2009). The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 14, 43–50. 10.1016/j.tplants.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Shi W.-G., Li H., Liu T.-X., Polle A., Peng C.-H., Luo Z.-B. (2015). Exogenous abscisic acid alleviates zinc uptake and accumulation in Populus × canescens exposed to excess zinc. Plant. Cell Environ. 38, 207–223. 10.1111/pce.12434 [DOI] [PubMed] [Google Scholar]
- Singh P., Shah K. (2014). Evidences for reduced metal-uptake and membrane injury upon application of nitric oxide donor in cadmium stressed rice seedlings. Plant Physiol. Biochem. 83, 180–184. 10.1016/j.plaphy.2014.07.018 [DOI] [PubMed] [Google Scholar]
- Skórzyńska-Polit E., Pawlikowska-Pawlega B., Szczuka E., Drażkiewicz M., Krupa Z. (2006). The activity and localization of lipoxygenases in Arabidopsis thaliana under cadmium and copper stresses. Plant Growth Regul. 48, 29–39. 10.1007/s10725-005-4745-6 [DOI] [Google Scholar]
- Smeets K., Opdenakker K., Remans T., Forzani C., Hirt H., Vangronsveld J., et al. (2013). The role of the kinase OXI1 in cadmium- and copper-induced molecular responses in Arabidopsis thaliana. Plant. Cell Environ. 36, 1228–1238. 10.1111/pce.12056 [DOI] [PubMed] [Google Scholar]
- Srivastava R. K., Pandey P., Rajpoot R., Rani A., Dubey R. S. (2014). Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251, 1047–1065. 10.1007/s00709-014-0614-3 [DOI] [PubMed] [Google Scholar]
- Srivastava R. K., Pandey P., Rajpoot R., Rani A., Gautam A., Dubey R. S. (2015). Exogenous application of calcium and silica alleviates cadmium toxicity by suppressing oxidative damage in rice seedlings. Protoplasma 252, 959–975. 10.1007/s00709-014-0731-z [DOI] [PubMed] [Google Scholar]
- Sun C., Liu L., Yu Y., Liu W., Lu L., Jin C., et al. (2015). Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J. Integr. Plant Biol. 57, 550–561. 10.1111/jipb.12298 [DOI] [PubMed] [Google Scholar]
- Szabados L., Savouré A. (2009). Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97. 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Tamás L., Dudíková J., Durceková K., Halusková L., Huttová J., Mistrík I. (2009). Effect of cadmium and temperature on the lipoxygenase activity in barley root tip. Protoplasma 235, 17–25. 10.1007/s00709-008-0027-2 [DOI] [PubMed] [Google Scholar]
- Tang H., Liu Y., Gong X., Zeng G., Zheng B., Wang D., et al. (2015). Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ. Sci. Pollut. Res. 22, 9999–10008. 10.1007/s11356-015-4187-2 [DOI] [PubMed] [Google Scholar]
- Tao S., Sun L., Ma C., Li L., Li G., Hao L. (2013). Reducing basal salicylic acid enhances Arabidopsis tolerance to lead or cadmium. Plant Soil 372, 309–318. 10.2307/4295306527078211 [DOI] [Google Scholar]
- Thao N. P., Khan M. I. R., Binh N., Thu N. B. A., Hoang X. L. T., Asgher M., et al. (2015). Role of ethylene and its cross talk with other signaling molecules in plant responses to heavy metal stress. Plant Cell Environ. 169, 73–84. 10.1104/pp.15.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thounaojam T. C., Panda P., Mazumdar P., Kumar D., Sharma G. D., Sahoo L., et al. (2012). Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol. Biochem. 53, 33–39. 10.1016/j.plaphy.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Tian S., Lu L., Zhang J., Wang K., Brown P., He Z., et al. (2011). Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere 84, 63–69. 10.1016/j.chemosphere.2011.02.054 [DOI] [PubMed] [Google Scholar]
- Tripathi B. N., Bhatt I., Dietz K. J. (2009). Peroxiredoxins: a less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma 235, 3–15. 10.1007/s00709-009-0032-0 [DOI] [PubMed] [Google Scholar]
- Tuteja N., Mahajan S. (2007). Calcium signaling network in plants: an overview. Plant Signal. Behav. 2, 79–85. 10.4161/psb.2.2.4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay R., Panda S. K. (2010). Zinc reduces copper toxicity induced oxidative stress by promoting antioxidant defense in freshly grown aquatic duckweed Spirodela polyrhiza L. J. Hazard. Mater. 175, 1081–1084. 10.1016/j.jhazmat.2009.10.016 [DOI] [PubMed] [Google Scholar]
- Vazquez F., Legrand S., Windels D. (2010). The biosynthetic pathways and biological scopes of plant small RNAs. Trends Plant Sci. 15, 337–345. 10.1016/j.tplants.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Wahid A., Khaliq S. (2015). Architectural and biochemical changes in embryonic tissues of maize under cadmium toxicity. Plant Biol 17, 1005–1012. 10.1111/plb.12326 [DOI] [PubMed] [Google Scholar]
- Wang C.-R., Wang Q.-Y., Tian Y., Zhang J.-F., Li Z.-X., Cao P., et al. (2014). Lanthanum ions intervened in enzymatic production and elimination of reactive oxygen species in leaves of rice seedlings under cadmium stress. Environ. Toxicol. Chem. 33, 1656–1664. 10.1002/etc.2610 [DOI] [PubMed] [Google Scholar]
- Wang F., Zeng B., Sun Z., Zhu C. (2009). Relationship between proline and Hg2+-induced oxidative stress in a tolerant rice mutant. Arch. Environ. Contam. Toxicol. 56, 723–731. 10.1007/s00244-008-9226-2 [DOI] [PubMed] [Google Scholar]
- Wang J., Chen J., Pan K. (2013). Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environ. Sci. Pollut. Res. 20, 1441–1449. 10.1007/s11356-012-1048-0 [DOI] [PubMed] [Google Scholar]
- Wang J., Li W., Zhang C., Ke S. (2010). Physiological responses and detoxific mechanisms to Pb, Zn, Cu and Cd in young seedlings of Paulownia fortunei. J. Environ. Sci. (China) 22, 1916–1922. 10.1016/S1001-0742(09)60339-9 [DOI] [PubMed] [Google Scholar]
- Wang J. W., Wu J. Y. (2005). Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol. 46, 923–930. 10.1093/pcp/pci098 [DOI] [PubMed] [Google Scholar]
- Wang L., Yang L., Yang F., Li X., Song Y., Wang X., et al. (2010). Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to copper toxicity. J. Plant Physiol. 167, 1298–1306. 10.1016/j.jplph.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Wasternack C., Hause B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 111, 1021–1058. 10.1093/aob/mct067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., He J., Ding H., Zhu Z., Chen J., Xu S., et al. (2015). Modulation of zinc-induced oxidative damage in Solanum melongena by 6-benzylaminopurine involves ascorbate – glutathione cycle metabolism. Environ. Exp. Bot. 116, 1–11. 10.1016/j.envexpbot.2015.03.004 [DOI] [Google Scholar]
- Xia Y., Qi Y., Yuan Y., Wang G., Cui J., Chen Y., et al. (2012). Overexpression of Elsholtzia haichowensis metallothionein 1 (EhMT1) in tobacco plants enhances copper tolerance and accumulation in root cytoplasm and decreases hydrogen peroxide production. J. Hazard. Mater. 233-234, 65–71. 10.1016/j.jhazmat.2012.06.047 [DOI] [PubMed] [Google Scholar]
- Xiang C., Oliver D. J. (1998). Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10, 1539–1550. 10.1105/tpc.10.9.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Jia W., Zhang J. (2007). AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis. J. Exp. Bot. 58, 2969–2981. 10.1093/jxb/erm144 [DOI] [PubMed] [Google Scholar]
- Xing Y., Jia W. S., Zhang J. H. (2008). AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 54, 440–451. 10.1111/j.1365-313X.2008.03433.x [DOI] [PubMed] [Google Scholar]
- Xiong J., Fu G., Tao L., Zhu C. (2010). Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch. Biochem. Biophys. 497, 13–20. 10.1016/j.abb.2010.02.014 [DOI] [PubMed] [Google Scholar]
- Xu F. J., Jin C. W., Liu W. J., Zhang Y. S., Lin X. Y. (2011). Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. J. Integr. Plant Biol. 53, 44–53. 10.1111/j.1744-7909.2010.01008.x [DOI] [PubMed] [Google Scholar]
- Xu J., Yin H., Li Y., Liu X. (2010). Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum. Plant Physiol. 154, 1319–1334. 10.1104/pp.110.162982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Chu W., Qiu H., Fu Y., Cai S., Sha S. (2013). Responses of Hydrilla verticillata (L.f.) Royle to zinc: in situ localization, subcellular distribution and physiological and ultrastructural modifications. Plant Physiol. Biochem. 69, 43–48. 10.1016/j.plaphy.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Xu Q. S., Hu J. Z., Xie K. B., Yang H. Y., Du K. H., Shi G. X. (2010). Accumulation and acute toxicity of silver in Potamogeton crispus L. J. Hazard. Mater. 173, 186–193. 10.1016/j.jhazmat.2009.08.067 [DOI] [PubMed] [Google Scholar]
- Yan H., Saika H., Maekawa M., Takamure I., Tsutsumi N., Kyozuka J., et al. (2007). Rice tillering dwarf mutant dwarf3 has increased leaf longevity during darkness-induced senescence or hydrogen peroxide-induced cell death. Genes Genet. Syst. 82, 361–366. 10.1266/ggs.82.361 [DOI] [PubMed] [Google Scholar]
- Yildiz M., Terzi H., Bingül N. (2013). Protective role of hydrogen peroxide pretreatment on defense systems and BnMP1 gene expression in Cr(VI)-stressed canola seedlings. Ecotoxicology 22, 1303–1312. 10.1007/s10646-013-1117-2 [DOI] [PubMed] [Google Scholar]
- Yildiztugay E., Ozfidan-Konakci C. (2015). Profiling of rutin-mediated alleviation of cadmium-induced oxidative stress in Zygophyllum fabago. Environ. Toxicol. 30, 816–835. 10.1002/tox [DOI] [PubMed] [Google Scholar]
- Yin L., Wang S., Eltayeb A. E., Uddin M. I., Yamamoto Y., Tsuji W., et al. (2010). Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231, 609–621. 10.1007/s00425-009-1075-3 [DOI] [PubMed] [Google Scholar]
- Yoo S.-D., Cho Y.-H., Tena G., Xiong Y., Sheen J. (2008). Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature 451, 789–795. 10.1038/nature06543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H., Numata N., Nakajima K., Katou S., Kawakita K., Rowland O., et al. (2003). Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15, 706–718. 10.1105/tpc.008680.a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Bian X., Qian J., Chen X., Wang R., Cao S. (2012). Arabidopsis desaturase 2 gene is involved in the regulation of cadmium and lead resistance. Plant Soil 358, 289–300. 10.1007/s11104-012-1186-7 [DOI] [Google Scholar]
- Yu C., Sun C., Shen C., Wang S., Liu F., Liu Y., et al. (2015). The auxin transporter, OsAUX1, is involved in primary root and root hair elongation and in Cd stress responses in rice (Oryza sativa L.). Plant J. 83, 818–830. 10.1111/tpj.12929 [DOI] [PubMed] [Google Scholar]
- Yuan H.-M., Xu H.-H., Liu W.-C., Lu Y.-T. (2013). Copper regulates primary root elongation through PIN1-mediated auxin redistribution. Plant Cell Physiol. 54, 766–778. 10.1093/pcp/pct030 [DOI] [PubMed] [Google Scholar]
- Zawoznik M. S., Groppa M. D., Tomaro M. L., Benavides M. P. (2007). Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana. Plant Sci. 173, 190–197. 10.1016/j.plantsci.2007.05.004 [DOI] [Google Scholar]
- Zayneb C., Bassem K., Zeineb K., Grubb C. D., Noureddine D., Hafedh M., et al. (2015). Physiological responses of fenugreek seedlings and plants treated with cadmium. Environ. Sci. Pollut. Res. Int. 22, 10679–10689. 10.1007/s11356-015-4270-8 [DOI] [PubMed] [Google Scholar]
- Zheng Q., Cheng Z. Z., Yang Z. M. (2013). HISN3 mediates adaptive response of chlamydomonas reinhardtii to excess nickel. Plant Cell Physiol. 54, 1951–1962. 10.1093/pcp/pct130 [DOI] [PubMed] [Google Scholar]
- Zhou C., Cai Z., Guo Y., Gan S. (2009). An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol. 150, 167–177. 10.1104/pp.108.133439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Yu Q., Wang Z., Pan Y., Lv W., Zhu L., et al. (2013). Knockdown of GDCH gene reveals reactive oxygen species-induced leaf senescence in rice. Plant Cell Environ. 36, 1476–1489. 10.1111/pce.12078 [DOI] [PubMed] [Google Scholar]
- Zhou T., Yang X., Wang L., Xu J., Zhang X. (2014). GhTZF1 regulates drought stress responses and delays leaf senescence by inhibiting reactive oxygen species accumulation in transgenic Arabidopsis. Plant Mol. Biol. 85, 163–177. 10.1007/s11103-014-0175-z [DOI] [PubMed] [Google Scholar]
- Zhou X., Jiang Y., Yu D. (2011). WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Mol. Cells 31, 303–313. 10.1007/s10059-011-0047-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Heinlein C., Orendi G., Zentgraf U. (2006). Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 29, 1049–1060. 10.1111/j.1365-3040.2005.01459.x [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Zentgraf U. (2005). The correlation between oxidative stress and leaf senescence during plant development. Cell Mol. Biol. Lett. 10, 515–534. [PubMed] [Google Scholar]
- Zipor G., Oren-Shamir M. (2013). Do vacuolar peroxidases act as plant caretakers? Plant Sci. 199–200, 41–47. 10.1016/j.plantsci.2012.09.018 [DOI] [PubMed] [Google Scholar]