Abstract
A conjugate addition/asymmetric protonation/aza-Prins cascade reaction has been developed for the enantioselective synthesis of fused polycyclic indolines. A catalyst system generated from ZrCl4 and 3,3’-dibromo-BINOL enables the synthesis of a range of polycyclic indolines in good yields and high enantioselectivity. A key finding is the use of TMSCl and 2,6-dibromophenol as a stoichiometric source of HCl to facilitate catalyst turnover. This transformation is the first in which a ZrCl4•BINOL complex serves as a chiral Lewis acid-assisted Brønsted acid.
Keywords: Lewis acid-assisted Brønsted acid, catalysis, cascade, indoline, enantioselective
Cascade reactions, in which a single substrate undergoes a series of chemical transformations, provide rapid access to molecular complexity.1 Many beautiful examples of cascade reactions have emerged from total synthesis endeavors, both in the context of biomimetic and de novo synthetic strategies.2 More recently, attention has turned to developing cascade reactions involving asymmetric catalysis, which can convert simple, achiral starting materials to complex products with control over both the absolute and relative stereochemistry.3 These transformations powerfully merge the attributes of asymmetric catalysis and cascade reactions for the synthesis of stereochemically rich small molecules.
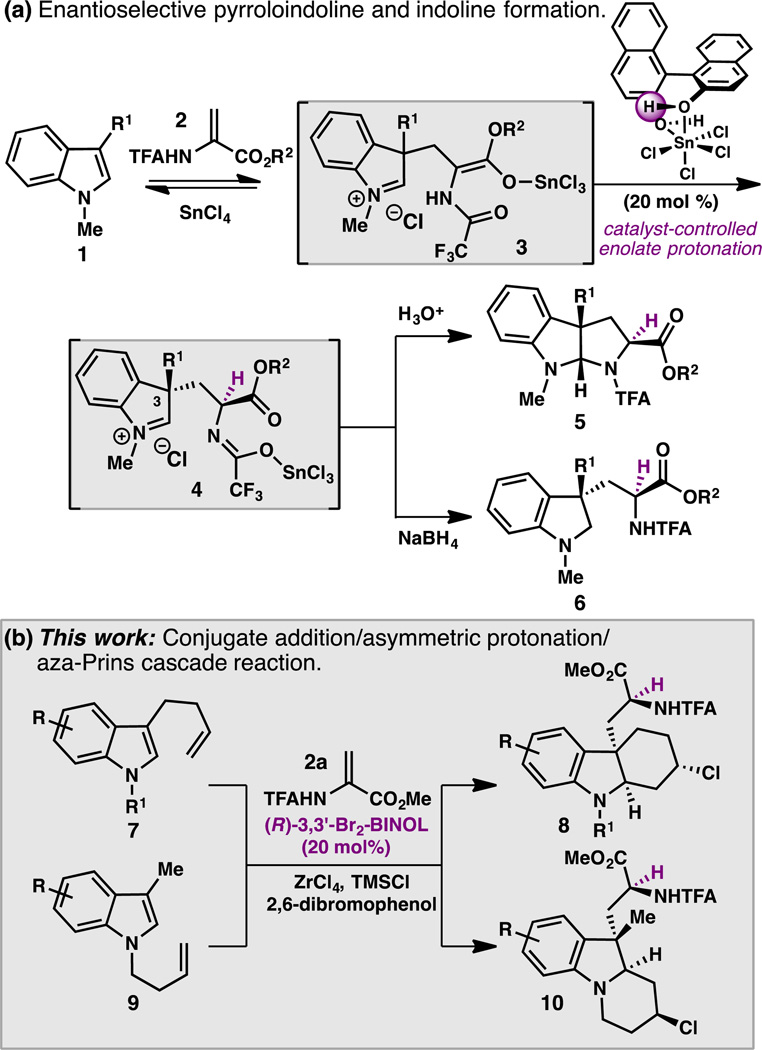
In 2010, we reported a new method to prepare enantioenriched pyrroloindolines (5) from C3-substituted indoles (1) and 2-amidoacrylates (2) using SnCl4 and catalytic (R)-BINOL (L1) (Scheme 1, a).4,5 Mechanistic investigations suggest that this reaction proceeds by a reversible conjugate addition of 1 to 2, followed by a highly selective, catalyst-controlled protonation of enolate 3, in which a SnCl4·L1 complex serves as a chiral Lewis acid-assisted Brønsted acid (LBA).6 Subsequent studies in our group determined that persistent indolinium ion 4 can be trapped with hydride to provide direct access to the corresponding indoline 6.7 Recognizing that iminium ions such as 4 can serve as electrophiles for C–C bond formation, we sought to develop an enantioselective cascade reaction that would terminate with an aza-Prins cyclization.8,9 Here we report the successful realization of this concept, which has resulted in a direct synthesis of complex polycyclic indolines in a single step from simple indole derivatives (Scheme 1, b).
Scheme 1.
Tandem conjugate addition/asymmetric protonation reactions of indoles.
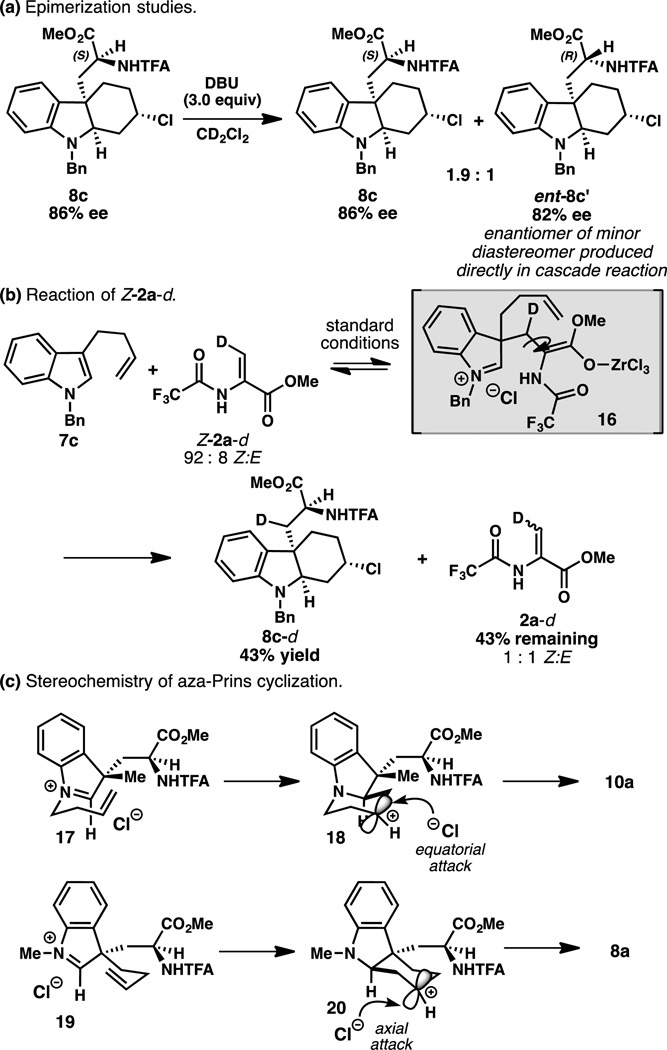
We began our studies with 3-(but-3-en-1-yl)-1-methylindole (7a), a substrate in which the pendant alkene is poised to undergo intramolecular attack to form a six-membered ring (Table 1). Exposure of a mixture of indole 7a and amidoacrylate 2a to SnCl4 (1.2 equiv) and (R)-BINOL (L1, 20 mol %) – the standard conditions developed for the previously reported pyrroloindoline formation4 – provided hexahydrocarbazole 8 in low yield but with promising levels of diastereo- and enantioselection (Table 1, entry 1). Presumably 8 arises from a conjugate addition/asymmetric protonation/aza-Prins cyclization cascade, with chloride trapping of the resulting carbocation. A screen of additional Lewis acids revealed that ZrCl4 provides slightly higher yield of 8, although the major diastereomer (8a) was produced with lower ee. Notably, Zr(OtBu)4 and related metal alkoxides failed to catalyze the reaction.10 A further survey of alternate chiral diols in combination with ZrCl4 determined that (R)-3,3’-dibromo-BINOL (L3) delivers hexahydrocarbazole 8 in 40% yield and 7:1 dr; 8a was formed with 76% ee (entry 7).
Table 1.
Reaction optimization: Lewis Acid and chiral diol screen.[a]
 | |||||
|---|---|---|---|---|---|
| Entry | Lewis Acid |
Diol | Yield 8 (%)[b] |
dr | ee 8a (%)[c] |
| 1 | SnCl4 | (R)-BINOL (L1) | 19 | 10:1 | 88 |
| 2 | TiCl4 | (R)-BINOL | 27 | 6:1 | 0 |
| 3 | SbCl5 | (R)-BINOL | 0 | -- | -- |
| 4 | ZrCl4 | (R)-BINOL | 30 | 9:1 | 40 |
| 5 | Zr(OtBu)4 | (R)-BINOL | 0 | -- | -- |
| 6 | ZrCl4 | (R)-6,6’-dibromo-BINOL (L2) | 38 | 9:1 | 30 |
| 7 | ZrCl4 | (R)-3,3’-dibromo-BINOL (L3) | 40 | 7:1 | 76 |
| 8 | ZrCl4 | (S)-VANOL (L4) | 33 | 6:1 | 74 |
| 9 | ZrCl4 | (4R, 5R)-Ph-TADDOL (L5) | 37 | >10:1 | 66 |
Reactions conducted with 0.20 mmol 7a and 0.24 mmol 2a.
Combined isolated yield of two diastereomers.
The ee of the major diastereomer was determined by SFC using a chiral stationary phase.
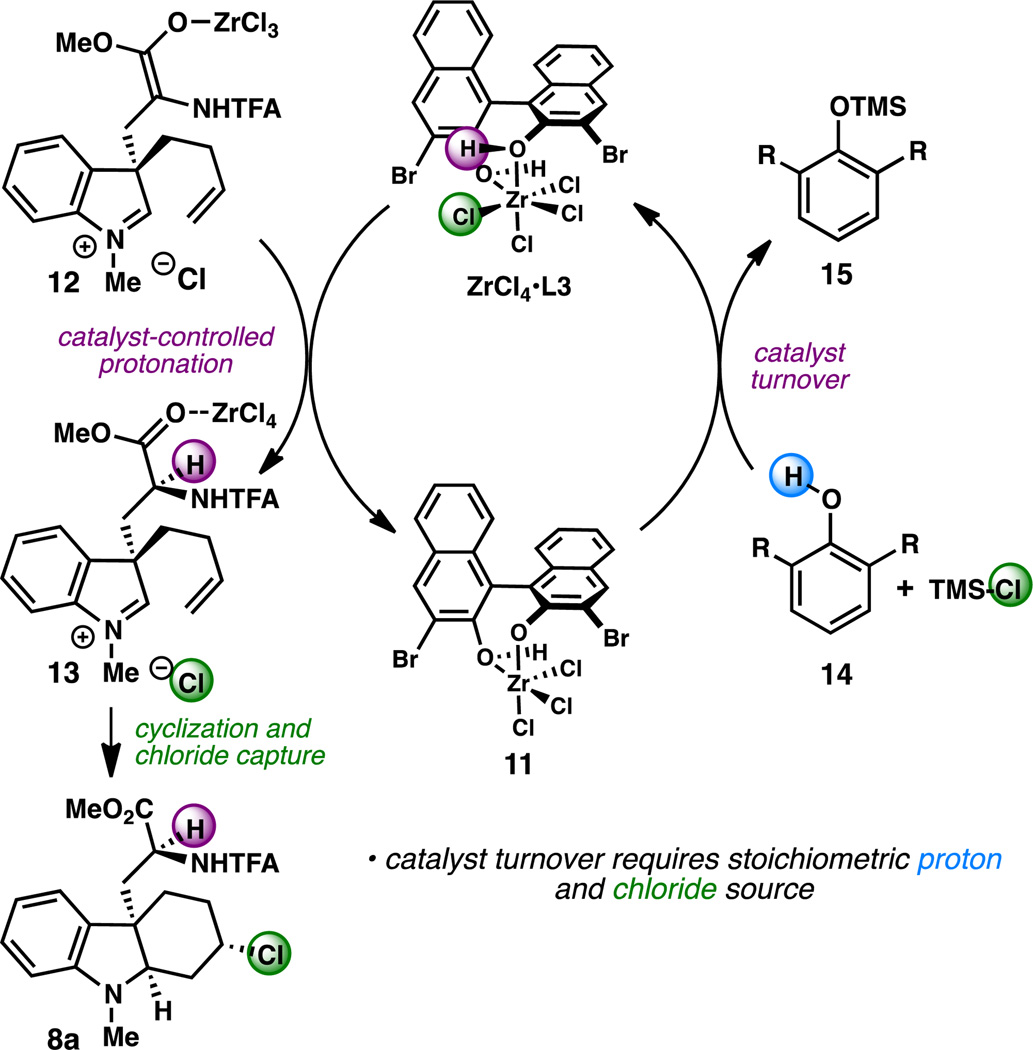
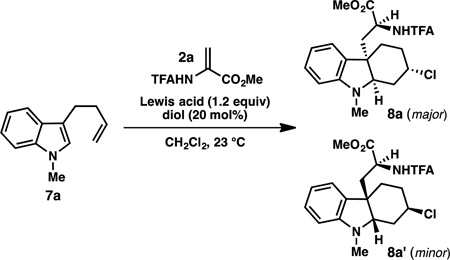
We hypothesized that the modest yields of 8 might result from poor catalyst turnover due to incorporation of chloride into the product. As illustrated by the simplified catalytic cycle in Figure 1 (conjugate addition step is not shown), upon protonation of Zr-enolate 12 with ZrCl4·L3, indolinium ion 13 and complex 11 are formed. In order to regenerate the active catalyst, ZrCl4·L3, an equivalent of HCl must be added to 11. Since chloride from the catalyst is captured in the aza-Prins step, we suspected that this might inhibit the regeneration of ZrCl4·L3. Guided by the pioneering studies of Yamamoto and co-workers on the chiral LBA-catalyzed protonation of silyl enol ethers,6a,b we hypothesized that the combination of a silyl chloride and achiral phenol could serve as our stoichiometric source of “HCl”. In order to maintain high enantio- and diastereoselectivity in the reaction, it would be necessary to identify a phenol that would not promote a non-selective, background protonation of the enolate.
Figure 1.
Proposed catalytic cycle (conjugate addition is omitted for simplicity).
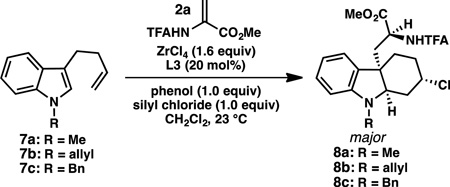
To test this hypothesis, a variety of phenols and silyl chlorides were investigated for their ability to improve the yield and selectivity of the reaction. The addition of simple, achiral phenols – in the absence of silyl chloride – improved the yields in some cases, but did not increase the enantioselectivity of the transformation (Table 2, entry 3). However, we were pleased to find that the combination of 2,6-disubstituted phenols and TMSCl improved both the yield and enantioselectivity of the reaction (entry 4). In general, 2,6-dihalogenated phenols provided the highest combination of yield and selectivity. For N-methyl substrate 7a, the most synthetically useful results were obtained with 2,6-dichloro-4-methylphenol, which provided 8a in 84% yield and 87% ee (major diastereomer, entry 6). However, for substrates with slightly larger protecting groups on the indole nitrogen, superior results were obtained with 2,6-dibromophenol (entries 8–11). For example, with N-Bn substrate 7c, hexahydrocarbazole 8c is obtained in 82% yield, 5:1 dr and 86% ee (major diastereomer) when 2,6-dibromophenol is used as the stoichiometric proton source (entry 11). To the best of our knowledge, this is the first example of a chiral diol·ZrCl4 complex acting as a chiral Lewis acid-assisted Brønsted acid catalyst for a highly enantioselective reaction.10
Table 2.
Reaction optimization: achiral phenol and silyl chloride screen.[a]
 | |||||
|---|---|---|---|---|---|
| Entry | R | Phenol | Silyl Chloride |
Yield (%)[b] |
ee 8 (%)[c] |
| 1 | Me | – | – | 30 | 40 |
| 2 | Me | phenol | – | 33 | 35 |
| 3 | Me | 2,6-dimethylphenol | – | 66 | 19 |
| 4 | Me | 2,6-dimethylphenol | TMSCl | 72 | 50 |
| 5 | Me | 2,6-dimethylphenol | TBSCl | 68 | 44 |
| 6 | Me | 2,6-dichloro-4-methylphenol | TMSCl | 84 | 87 |
| 7 | Me | 2,6-dibromophenol | TMSCl | 56 | 86 |
| 8 | Allyl | 2,6-dichloro-4-methylphenol | TMSCl | 75 | 86 |
| 9 | Allyl | 2,6-dibromophenol | TMSCl | 70 | 90 |
| 10 | Bn | 2,6-dichloro-4-methylphenol | TMSCl | 74 | 85 |
| 11 | Bn | 2,6-dibromophenol | TMSCl | 82[d] | 86 |
Reactions conducted with 0.20 mmol 7a and 0.24 mmol 2a.
Combined isolated yield of two diastereomers.
The ee of major diastereomer was determined by SFC using chiral stationary phase.
Product formed as 5:1 mixture of 8c:8c’.
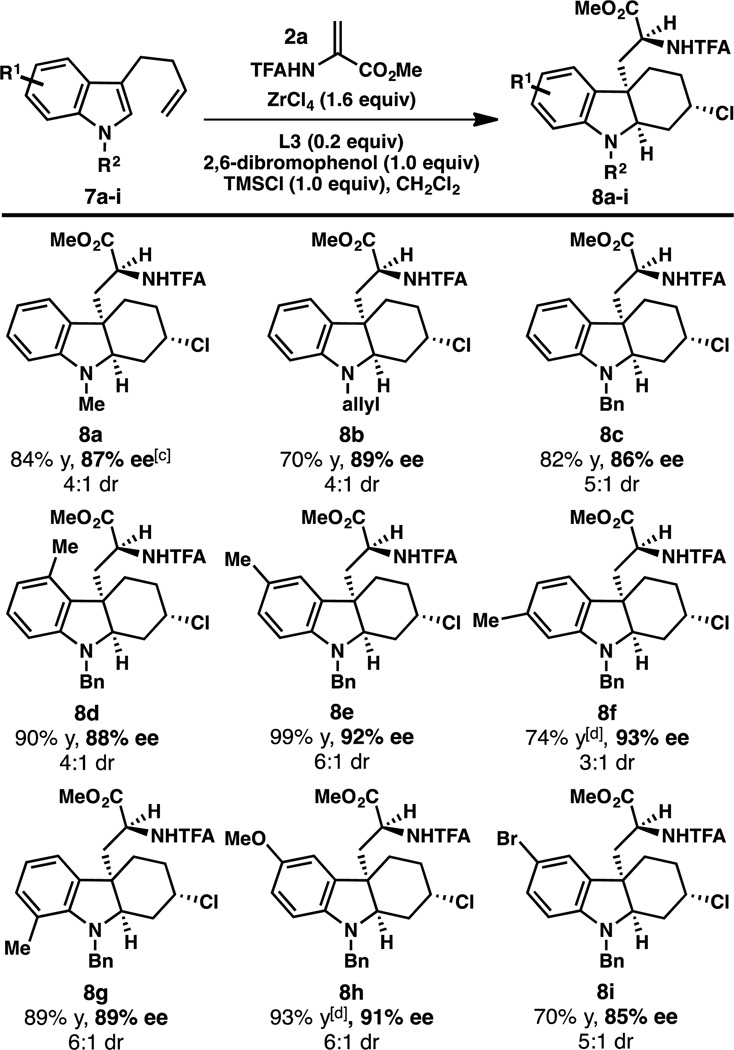
A series of 3-(but-3-en-1-yl)-indoles were prepared and evaluated in the conjugate addition/asymmetric protonation/aza-Prins cascade reaction (Table 3). This transformation tolerates a range of indole substitution, including substituents at C4, C5, C6, and C7. Although the reaction is compatible with halogenated indoles (e.g. 8i), substrates bearing more electron-withdrawing functional groups are less reactive, providing products in lower yield.11
Table 3.
Reactions conducted with 0.20 mmol 7 and 0.24 mmol 2a.
Combined isolated yield of two diastereomers. The dr was determined by integration of the α-amino ester peaks in the 1H NMR spectrum of the unpurified reaction mixture. The ee of the major diastereomer is reported, and was determined by SFC using a chiral stationary phase.
2,6-dichloro-4-methylphenol was used instead of 2,6-dibromophenol.
1.1 equiv of ZrCl4 was used.
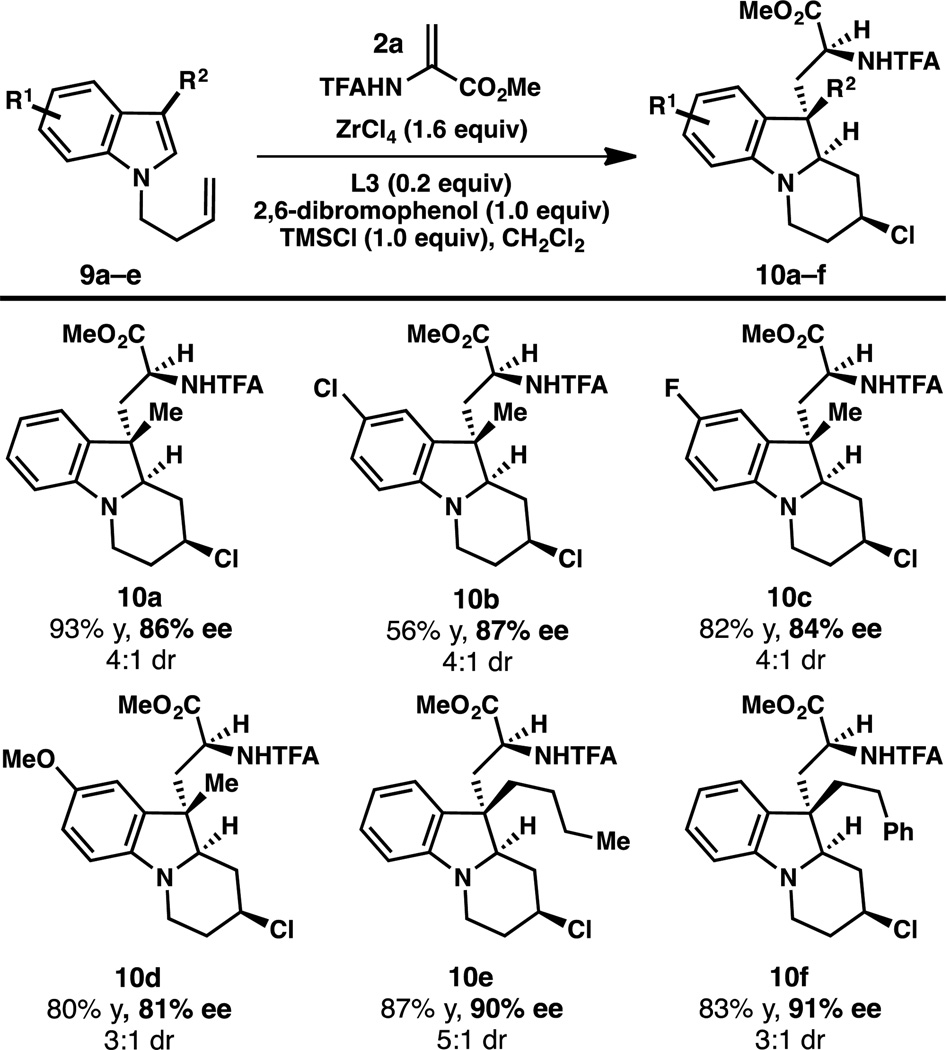
Substrates in which the pendant alkene is linked through the indole nitrogen also undergo the cascade reaction (Table 4). Notably, a substrate bearing a tethered aryl nucleophile at C3 undergoes cyclization exclusively by the alkene; no product resulting from attack of the arene at the iminium ion can be detected.
Table 4.
Reactions conducted with 0.20 mmol 9 and 0.24 mmol 2a.
Combined isolated yield of two diastereomers. The dr was determined by integration of the α-amino ester peaks in the 1H NMR spectrum of the unpurified reaction mixture. The ee of major diastereomer is reported, and was determined by SFC using chiral stationary phase.
The stereochemical assignments of 8c and 10b were confirmed by single crystal X-ray diffraction.12 Treatment of diastereomerically pure 8c with DBU in CD2Cl2 at room temperature results in equilibration to a 1.9:1 mixture of diastereomers 8c and ent-8c’ (Figure 2). Analysis by SFC using a chiral stationary phase confirmed that diastereomer ent-8c’, resulting from epimerization, is the enantiomer of the minor diastereomer produced directly in the conjugate addition/asymmetric protonation/aza-Prins cyclization. This finding is consistent with the catalyst-controlled protonation mechanism proposed in Figure 1.
Figure 2.
Mechanistic investigations of the conjugate addition/asymmetric protonation/aza-Prins cyclization. Standard conditions: 7c (1.0 equiv), Z-2a-d (1.0 equiv), (R)-3,3’-dibromo-BINOL (20 mol %), ZrCl4 (1.6 equiv), 2,6-dibromophenol (1.0 equiv), TMSCl (1.0 equiv), CH2Cl2, 23 °C.
To probe the reversibility of the initial conjugate addition step, deuterium labelled acrylate Z-2a-d was prepared. The reaction between 7c and Z-2a-d was conducted under standard conditions, and stopped after 30 minutes. The recovered acrylate was a 1:1 mixture of Z- and E-isomers. A control experiment determined that Z-2a-d does not isomerize in the absence of indole under otherwise identical conditions. These findings support a mechanism involving a reversible conjugate addition, which enables the isomerization of Z-2a-d via rotation around the single C–C bond of intermediate 16.
It is also interesting to note the divergence in the stereochemical outcome of the aza-Prins cyclizations of the C3- and N-linked substrates (8 and 10, respectively). For polycyclic indoline 10, the configuration at the chlorine-bearing carbon results from equatorial trapping of the carbocation 18, and is consistent with a process that is driven by orbital alignment.13,14 In contrast, the configuration at the chlorine-bearing carbon of 8 results from cyclization through an anti-periplanar arrangement between the alkene and iminium ion (19), followed by axial trapping of 20 by chloride. This diastereoselection is opposite to that observed by Hanessian and coworkers in their studies of aza-Prins reactions of structurally similar N-acyloxy iminium ions.14 However, Rychnovsky and coworkers have reported that TMSBr-mediated oxonia-Prins reactions of α-bromoethers result in axial trapping.15 In the present case, the origin of the axial-selective trapping is unclear.16
In conclusion, a conjugate addition/asymmetric protonation/aza-Prins cyclization reaction to prepare enantioenriched indolines has been developed. This reaction provides direct access to functionalized heterocyclic products in a single step from simple indole derivatives, and is the first example of a transformation in which a ZrCl4•BINOL complex serves as a chiral LBA. This transformation demonstrates the utility of asymmetric cascade catalysis for rapidly generating molecules of complexity.
Experimental Section
General procedure for the conjugate addition/asymmetric protonation/Prins cyclization cascade: To a flame-dried flask were added indole (0.20 mmol, 1.00 equiv), acrylate (0.24 mmol, 1.20 equiv), (R)-3,3’-dibromo-BINOL (0.04 mmol, 0.20 equiv), and 2,6-dibromophenol (0.20 mmol, 1.00 equiv) under an N2 atmosphere. The flask was charged with CH2Cl2 (1.5 mL), followed by addition of TMSCl (0.2 mmol, 1.00 equiv) and ZrCl4 (0.32 mmol, 1.60 equiv unless specifically indicated). The mixture was stirred at room temperature for 24 h. The reaction was quenched by diluting with 1 mL MeCN and 1 mL 1 M HCl, followed by addition of 5 mL H2O. The aqueous layer was extracted with ethyl acetate (3 × 5 mL) and the combined organic layers were washed with saturated NaHCO3(aq) (10 mL). The aqueous layer was back extracted with EtOAc (10 mL) and the combined organic layers were dried (Na2SO4), filtered, and concentrated. The crude residue was purified by silica gel chromatography.
Supplementary Material
Acknowledgments
We thank Prof. Brian Stoltz, Dr. Scott Virgil, and the Caltech Center for Catalysis and Chemical Synthesis for access to analytical equipment. Victor W. Mak is gratefully acknowledged for checking of the experimental procedure. We also thank Dr. Michael Takase and Larry Henling for assistance with X-ray structure analysis. NMR spectra were obtained on a spectrometer funded by the National Institutes of Health (NIH) (RR027690). J.N. is grateful for a pre-doctoral fellowship from NSERC. S.E.R. is an American Cancer Society Research Scholar. Financial support from the NIH (NIGMS RGM097582A) and the donors of the American Chemical Society PRF is gratefully acknowledged.
References
- 1.Tietze LF. Chem. Rev. 1996;96:115. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]
- 2.(a) Grondal C, Jeanty M, Enders D. Nat. Chem. 2010;2:167. doi: 10.1038/nchem.539. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. Int. Ed. 2006;45:7136. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]
- 3.(a) Volla CMR, Atodiresei I, Rueping M. Chem. Rev. 2014;114:2390. doi: 10.1021/cr400215u. [DOI] [PubMed] [Google Scholar]; (b) Wasilke J-C, Obrey SJ, Baker RT, Bazan GC. Chem. Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]; (c) Fogg DE, Dos Santos EN. Coord. Chem. Rev. 2004;248:2365. [Google Scholar]
- 4.Repka LM, Ni J, Reisman SE. J. Am. Chem. Soc. 2010;132:14418. doi: 10.1021/ja107328g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For a review of catalytic asymmetric dearomatization reactions, including indole dearomatization, see: Zhou C-X, Zhang W, You S-L. Angew. Chem. Int. Ed. 2012;51:12662. doi: 10.1002/anie.201204822.
- 6.Seminal reports: Nakamura S, Kaneeda M, Ishihara K, Yamamoto H. J. Am. Chem. Soc. 2000;122:8120. Ishihara K, Nakamura S, Kaneeda M, Yamamoto H. J. Am. Chem. Soc. 1996;118:12854. Ishihara K, Kaneeda M, Yamamoto H. J. Am. Chem. Soc. 1994;116:11179. For a review, see: Yamamoto H, Futatsugi K. Angew. Chem., Int. Ed. 2005;44:1924. doi: 10.1002/anie.200460394.
- 7.Ni J, Wang, H H, Reisman SE. Tetrahedron. 2013;69:5622. doi: 10.1016/j.tet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Kijkink J, Speckamp WN. Tetrahedron Lett. 1975:4047. [Google Scholar]; (b) Schoemaker HE, Speckamp WN. Tetrahedron Lett. 1978:1515. [Google Scholar]; (c) Evans DA, Thomas EW. Tetrahedron Lett. 1979;20:411. [Google Scholar]; (e) Demailly G, Solladie G. J. Org. Chem. 1981:3102. [Google Scholar]
- 9.For selected examples of cascade reactions involving aza-Prins cyclizations, see: Chio FKI, Guesné SJJ, Hassall L, McGuire T, Dobbs AP. J. Org. Chem. 2015;80:9868. doi: 10.1021/acs.joc.5b01301. Sun Y, Chen P, Zhang D, Baunach M, Hertweck C, Li A. Angew. Chem. Int. Ed. 2014;53:9012. doi: 10.1002/anie.201404191. Altman RA, Nilsson BL, Overman LE, de Alaniz JR, Rohde JM, Taupin V. J. Org. Chem. 2010;75:7519. doi: 10.1021/jo101619d. Knowles RR, Lin S, Jacobsen EN. J. Am. Chem. Soc. 2010;132:5030. doi: 10.1021/ja101256v. Yadav JS, Borkar P, Chakravvarthy P, Reddy BVS, Sarma AVS, Basha SJ, Sridhar B, Grée R. J. Org. Chem. 2010;75:2081. doi: 10.1021/jo902683p. Frank KE, Aubé J. Tetrahedron Lett. 1998;39:7239. Ruggeri RRB, Hansen MM, Heathcock CH. J. Am. Chem. Soc. 1988;110:8734. Dijkink J, Speckamp WN. Tetrahedron Lett. 1977;18:935.
- 10.Highly enantioselective reactions catalyzed by Lewis-acidic Zr-BINOLate complexes have been reported; for a review, see: Yamasaki S, Kanai M, Shibasaki M. Chem. Eur. J. 2001;7:4066. doi: 10.1002/1521-3765(20011001)7:19<4066::aid-chem4066>3.0.co;2-k.
- 11.For example, 3-(but-3-en-1-yl)-1-methyl-5-trifluoromethylindole provided less than 5% yield of the cascade product under standard conditions.
- 12.See Supporting Information.
- 13.Alder RW, Harvey JN, Oakley MT. J. Am. Chem. Soc. 2002;124:4960. doi: 10.1021/ja025902+. [DOI] [PubMed] [Google Scholar]
- 14.(a) Hanessian S, Tremblay M, Marzi M, Del Valle JR. J. Org. Chem. 2005;70:5070. doi: 10.1021/jo050326w. [DOI] [PubMed] [Google Scholar]; (b) Hanessian S, Tremblay M, Petersen JFW. J. Am. Chem. Soc. 2004;126:6064. doi: 10.1021/ja030669g. [DOI] [PubMed] [Google Scholar]
- 15.(a) Jasti R, Anderson CD, Rychnovsky SD, D S. J. Am. Chem. Soc. 2005;127:9939. doi: 10.1021/ja0518326. [DOI] [PubMed] [Google Scholar]; (b) Jasti R, Vitale J, Rychnovsky SD. J. Am. Chem. Soc. 2004;126:9904. doi: 10.1021/ja046972e. [DOI] [PubMed] [Google Scholar]
- 16.It is possible that in the cyclization of 8, the chloride trapping is directed by the pendant amide through coordination to zirconium; however, this requires a transition structure with an unusually large ring size.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.