Abstract
Background aims
Cord blood (CB) transplantation slows neurodegeneration during certain inherited metabolic diseases. However, the number of donor cells in the brain of patients does not appear to be sufficient to provide benefit until several months after transplant. We developed the cell product DUOC-01 to provide therapeutic effects in the early post-transplant period.
Methods
DUOC-01 cultures initiated from banked CB units were characterized by use of time-lapse photomicroscopy during the 21-day manufacturing process. Antigen expression was measured by means of flow cytometry and immunocytochemistry; transcripts for cytokines and enzymes by quantitative real-time polymerase chain reaction; activities of lysosomal enzymes by direct biochemical analysis; alloreactivity of DUOC-01 and of peripheral blood (PB) mononuclear cells (MNC) to DUOC-01 by mixed lymphocyte culture methods; and cytokine secretion by Bioplex assays.
Results
DUOC-01 cultures contained highly active, attached, motile, slowly proliferating cells that expressed common (cluster of differentiation [CD]11b, CD14 and Iba1), M1 type (CD16, inducible nitric oxide synthase), and M2-type (CD163, CD206) macrophage or microglia markers. Activities of 11 disease-relevant lysosomal enzymes in DUOC-01 products were similar to those of normal PB cells. All DUOC-01 products secreted interleukin (IL)-6 and IL-10. Accumulation of transforming growth factor-β, IL-1β, interferon-γ and TNF-α in supernatants was variable. IL-12, IL-2, IL-4, IL-5 and IL-13 were not detected at significant concentrations. Galactocerebrosidase, transforming growth factor-β and IL-10 transcripts were specifically enriched in DUOC-01 relative to CB cells. PB MNCs proliferated and released cytokines in response to DUOC-01. DUOC-01 did not proliferate in response to mismatched MNC.
Conclusions
DUOC-01 has potential as an adjunctive cell therapy to myeloablative CB transplant for treatment of inherited metabolic diseases.
Keywords: cord blood, cytokines, lysosomal enzymes, macrophage, microglia, transplantation
Introduction
Allogeneic hematopoietic stem cell transplantation with bone marrow or banked unrelated umbilical cord blood (CB) [1,2] slows the progression of central nervous system (CNS) damage associated with several inherited metabolic diseases (IMDs). In this treatment setting, cells derived from the donor eventually provide functional enzyme missing to the transplant recipients to cross-correct inherited enzyme deficiency [3,4]. Neurological symptoms usually stabilize 3 to 6 months after transplant, which suggests that brain engraftment reaches a therapeutic threshold during this period. Until this occurs, ongoing disease-associated CNS damage progresses [5]. To address this barrier to success of transplantation therapy, we developed DUOC-01, an adjunctive cell therapy product derived and manufactured ex vivo from donor CB. DUOC-01 is intended to be delivered intrathecally after systemic transplantation and after engraftment of donor cells as a bridging therapy to provide functional enzyme and perhaps other beneficial products to the brain in the early post-transplant period. DUOC-01 is a more than minimally manipulated cell product manufactured under current Good Manufacturing Practice (cGMP) from the 20% compartment of the systemically transplanted, 80% fraction of the same donor CB unit. In this report, we present data related to the identity, composition, immunogenicity and inflammatory potential of DUOC-01 cell products. Information concerning survival and biodistribution of DUOC-01 after intrathecal administration and the activity of DUOC-01 in treating demyelination in immune incompetent mice will be presented elsewhere. An investigational new drug application including this information has been reviewed by the US Food and Drug Administration, and a phase 1 trial (NCT02254863) exploring the safety and feasibility of the use of DUOC-01 in the clinical setting of allogeneic CB transplantation for IMD is currently enrolling subjects.
Methods
Material from human donors
All CB units (CBUs) and peripheral blood (PB) mononuclear cell fractions (MNC) were obtained from donors who had given informed consent for samples to be used for research purposes under local institutional review board–approved protocols.
Manufacturing DUOC-01
Cell products were manufactured in the Robertson Cell and Translational Cell Therapy cGMP facility from volume-reduced, red cell–depleted, and MNC-enriched cryopreserved CBUs (Carolinas Cord Blood Bank) through the use of validated standard operating procedures. All reagents were qualified for clinical use on the basis of manufacturing history files, certificates of analysis and testing for viruses, endotoxin and sterility. The 20% volume fraction detached from a single CBU was used to produce a single DUOC-01 cell product; we refer to a manufacturing lot as a cell product manufactured from a single CBU throughout this report. A flow chart outlining the manufacturing process is presented in Supplementary Figure 1. Cells were cultured by use of the method of Tracy et al. [6] with two modifications. First, because contaminating red cells interfere with the development of DUOC-01 cells in culture [6], additional red cell depletion was carried out with the use of immunomagnetic beads coated with anti-glycophorin A antibody as previously described [7]. Second, during cultivation, the concentrations of platelet-derived growth factor (5 ng/mL), neurotrophin-3 (1 ng/mL) and vascular endothelial growth factor (10 ng/mL) used were lower and of triiodothyronine (30 ng/mL) was higher than in the published protocol. On day 21, adherent cells were harvested with the use of trypsin and ethylenediaminetetra-acetic acid followed by scraping and were washed in appropriate excipients for further studies.
Imaging of cells in culture during DUOC-01 manufacturing process
Glass-bottomed, 35-mm culture dishes (MatTek Corporation) were seeded with 4.75 × 106 cells in 3 mL of growth medium through the use of standard manufacturing protocols and incubated for 4 days. On culture day 4, the dishes were fitted into the incubator of an Olympus VivaView FL incubator microscope. Transmitted differential interference contrast images of nine positions in each dish were captured with the use of a ×20 objective every 10 or 30 min during the 21-day manufacturing cycle. Images were analyzed and video clips were created with the use of MetaMorph Automation and Image Analysis Software.
Antibodies and isotype controls
Unless noted, all reagents were derived from mice. For flow cytometry, anti–cluster of differentiation (CD)3 Alexa Fluor 488, anti-CD14 Alexa Fluor 488, anti-CD11b-PE, anti-CD45-PECy7, anti-CD45-allophycocyanin (APC) and isotype controls immunoglobulin (Ig)G1 Alexa Fluor 488, IgG2a Alexa Fluor 488, and IgG2a-PE and IgG1 PECy7 were purchased from Becton Dickenson; mouse anti-human CD45 Alexa Fluor 488 and mouse anti-human CD45 Alexa Fluor 647 from AbDSerotec; anti-A2B5-APC and isotype control IgM APC from Miltenyi; and anti-O1 Alexa Fluor 647 and isotype control IgM Alexa Fluor 660 from eBioscience.
Antibodies used for immunocytochemistry were purchased from eBioscience (anti-CD11b, anti-Ki67-Alexa Fluor 488, IgG1 Alexa Fluor 488), Abcam (rabbit anti–human leukocyte antigen [HLA]-DR, anti-CD14, rabbit monoclonal anti-CD16, rabbit anti-CD206, rabbit anti–inducible nitric oxide synthase (iNOS), goat anti-Iba1, rabbit IgG isotype and IgG2bk isotype), Pierce (anti-CD163) and Molecular Probes (donkey anti-mouse IgG and donkey anti-rabbit IgG Alexa Fluor 488 or 568).
Flow cytometric analysis
DUOC-01 cell products were incubated for 15 min at 4°C in appropriate combinations of directly conjugated monoclonal antibodies and viability dyes, washed with phosphate-buffered saline (PBS)/1% human serum albumen and analyzed by use of multiparameter flow cytometry. Side- and forward-scatter gates were set to exclude small debris, and viability dyes were used to exclude dead cells.
Immunocytochemistry
Cells (900,000 in 700-μL medium/well) were seeded in four-well chamber slides (Lab-Tek II CC2) and cultured according to standard protocol. On culture day 21, medium was removed by aspiration, the cells briefly washed in 1 mL of PBS, fixed by addition of 4% paraformaldehyde in PBS for 20 min, washed twice with PBS and stored in PBS at 4°C until staining. Cells were then permeabilized and blocked with 2% bovine serum albumin (BSA)–0.1% Triton-X100 in PBS and incubated with primary antibody in 2% BSA in PBS. Both steps were carried out overnight at 4C°. The cells were then washed three times in PBS for 5 min, stained with fluorochrome-labeled secondary antibody in 2% BSA in PBS for 2 h at room temperature, washed once in PBS for 5 min, incubated in 4′, 6-diamidino-2-phenylindole (DAPI) at 1 μg/mL in PBS for 5 min to label cell nuclei, washed two more times with PBS and coverslip-mounted with Vectashield mounting medium.
For staining of cell proliferation, 5-bromo-2′-deoxyuridine (BrdU) was added to the cell culture at 10 μmol/L for 21 h and the cells were fixed and stained with the use of the anti-BrdU immunohistochemistry kit (Abcam).
Apoptosis was measured with the use of the DeadEnd Fluorometric TUNEL System (Promega). Positive controls were prepared by incubating slides with benzoase (1 μL/mL PBS) for 10 min at room temperature.
Fluorescent and bright-field images were obtained with the use of a Zeiss Axio Imager microscope and analyzed with the use of MetaMorph software.
Secretion of lysosomal enzymes
Five lots of DUOC-01 product were manufactured at Duke. On day 21, flasks containing the cell product were filled with culture medium, placed on ice packs and shipped overnight to the Lysosomal Diseases Testing Laboratory at Thomas Jefferson University in Philadelphia, Pennsylvania. At Jefferson, cells were removed from plates with the use of trypsin, and the specific activity of 11 lysosomal enzymes was measured as described by Wenger and Williams [4].
Accumulation of cytokines in culture medium
Cytokine concentrations in culture supernatants from DUOC-01 products manufactured under cGMP conditions and from DUOC-01 cells that were exposed to inflammatory stimuli in vitro were measured by means of antibody capture immunoassay with fluorescence reporters with the use of the Bio Rad Bioplex 200 instrument. Assays for 10 human pro- and anti-inflammatory cytokines [interleukin (IL)-1b, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13, tumor necrosis factor (TNF)-α and interferon (IFN)-γ] were multiplexes in each well of a Bio Rad Precision Pro plate (No. 171-A1001P). TGF-β content of supernatants was measured with the use of the Bio-Plex Pro TGF-β1 Set (Biorad No. 171-V4001M). Cytokine standards provided by the manufacturer were diluted in uninoculated tissue culture medium to construct standard curves, and the concentration of each cytokine in the supernatants was calculated.
Quantitative real-time polymerase chain reaction (QRT-PCR) was used to measure levels of transcripts for transforming growth factor (TGF)-β, galactocerebrosidase (GALC), IL-10, TNF-α and IFN-γ in cells on days 1 and 21 of the manufacturing process. RNA was isolated from DUOC-01 cells with the use of a PureLink RNA Mini Kit (Life Technologies), followed by DNase treatment (Life Technologies). Complementary DNA (cDNA) was synthesized from 2 μg of total RNA with the use of the SuperScript Vilo cDNA Synthesis Kit (Life Technologies). QRT-PCR was performed with the use of Taqman primer and probe sets run on a Biorad CFX96 Real-Time System. All values are normalized to glyceraldehyde 3-phosphate dehydrogenase expression.
Reactivity in mixed lymphocyte cultures
PB was drawn from healthy donors into heparinized tubes and centrifuged over Ficoll to yield MNC fractions. Freshly prepared PB MNCs from individual donors or frozen pooled PB MNCs from multiple donors were used in different experiments. Standard methods were used for one-way mixed lymphocyte culture (MLC) reactions in which stimulator cells were treated with mitomycin c before responder cells were added [8]. Cells were washed in PBS and cultured in Roswell Park Memorial Institute 1640 tissue culture medium containing 20% fetal calf serum. To assay cytokines released from cells cultured in MLC conditions, supernatants were removed from MLC on day 6 just before pulsing with titrated thymidine, stored at −80°C and then were assayed for the presence of human IL-2, IL-4, IL-10, TNF-α and IFN-γ with the use of BioPlex Product No. M50000005V, Human Cytokine Group 1, 5-plex custom assay. Standards containing known concentrations of each cytokine were diluted in culture medium to construct standard curves and calculate cytokine concentrations.
Results
General aspects of manufacturing process
Cultures initiated with the 20% fraction of frozen, banked CBUs, generally containing a heterogeneous populations of approximately 2 × 108 predominantly mature blood and hematopoietic cells, yielded 3.05 × 106 ± 1.63 × 106 (mean ± standard deviation [SD], range = 0.52–7.2 × 106, n = 14 total DUOC-01 cells after 21 days of manufacturing. Viability of DUOC-01 cells after harvest measured by trypan blue was 83% 9.9% (mean ± SD, n = 14).
Morphology of cells in DUOC-01 cultures
Figure 1 shows the range of morphologies present in the DUOC-01 cell product. Cultures included long, bipolar, phase-dense cells with radial processes (single broad arrow in Figure 1) resembling the “oligodendrocyte-like cells” described by Tracy et al. [6,9]. Very large, flat, vesiculated cells (double broad arrows in Figure 1) as large as 285 μm in diameter were common. Finally, highly refractive, small cells (thin arrow in Figure 1) were abundant. All cells adhered tightly to the plastic flasks. All three types of cells can be seen in the images shown by Tracy et al. [6,9].
Figure 1.
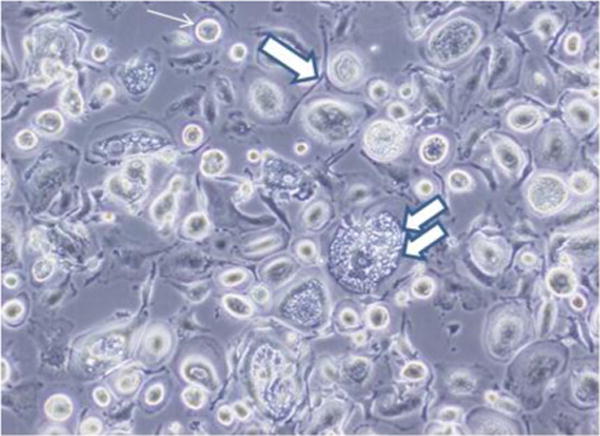
Range of cell morphology in DUOC-01 cell product at time of harvest. Cultures included long, bipolar, phase-dense cells with radial processes (single broad arrow). Very large, flat, vesiculated cells (indicated by double broad arrows) as large as 285 μm in diameter were common. Highly refractive, small cells (thin arrow) were abundant. See text for description of designated cell types.
We used immunocytochemistry of cells cultured on glass slides to study cell proliferation and apoptosis with the use of BrdU, Ki67 and the TUNEL assay. Cells were fixed in situ and not exposed to trypsin. The growth properties and morphologies of cells in slide cultures were similar to those grown under standard conditions in plastic flasks. BrdU and Ki67 staining of cells revealed that all three types of cells could proliferate during the period just before harvesting and confirmed that the large, flat cells were often multinucleated (Figure 2A–C). Apoptotic cells were not detected in slide cultures at the time of harvest (not shown).
Figure 2.
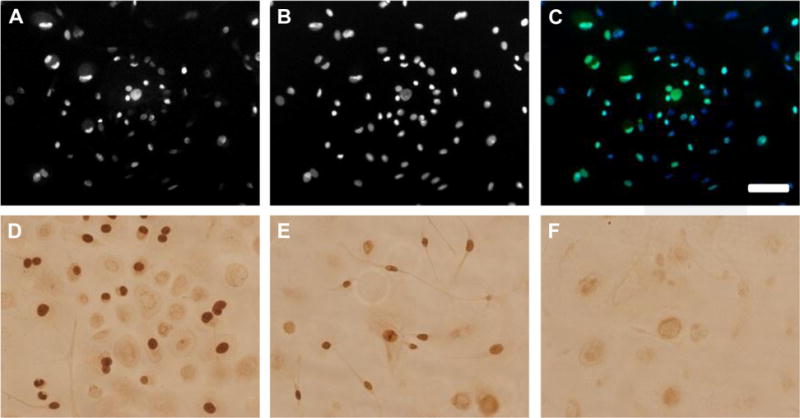
DUOC products include proliferating cells. (A–C) Fluorescent images of DUOC stained with (A) Ki67 and (B) DAPI; (C) is an overlay of green Ki67 and blue DAPI signals. (D–F) Peroxidase/diaminobenzidase-linked BrdU staining of DUOC. (D and E) Dark brown nuclei that have incorporated BrdU. (F) Control cells not exposed to BrdU. Scale bar = 50 mm.
To analyze relationships among the morphological types, we established glass slide cultures in a photomicroscope chamber and photographed defined fields of the cultures every 5, 10 or 30 min in two experiments. Figure 3 illustrates some of the morphological changes that occur in the cultures. The most important finding that emerged from analysis of these videos was that the morphological variants in DUOC-01 interconvert into one another. We observed large vesicular cells detaching from the surface, rounding up and becoming actively motile refractile cells. We also saw the actively motile, round refractile cells adhering to the surface and spreading out to become large cells or small, very motile cells of 27 to 54 μm in size that extended and retracted small, stellate processes. Those cells migrated up to 2 μm/min, frequently rounded up and appeared to divide.
Figure 3.
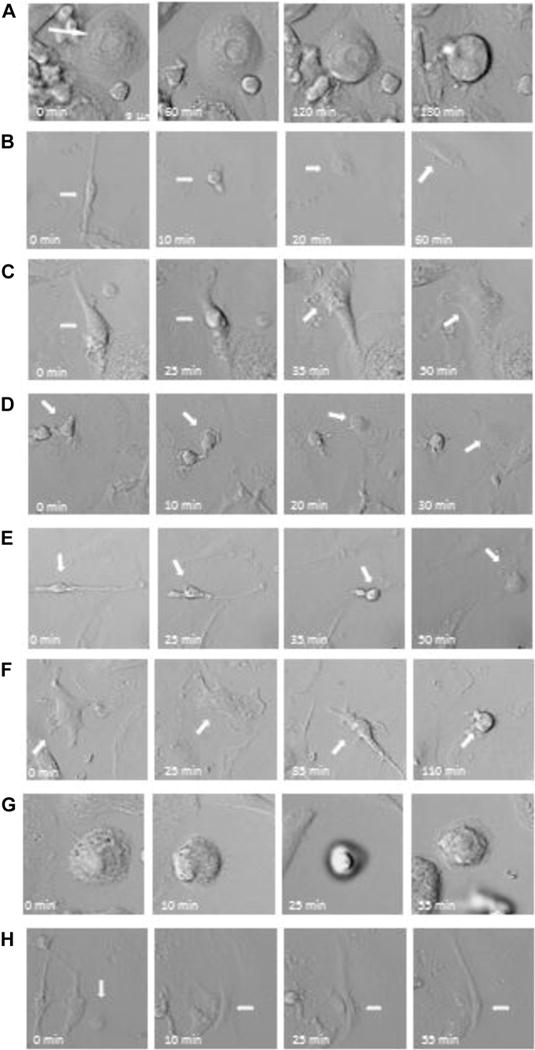
Morphological changes of DUOC-01 cells during manufacturing. In each row, elapsed time from the image on the right is indicated and an arrow shows a single cell in different frames. (A, F, G) Spread-out cells rounding up to become a motile cell. (B, C) Cell shape changing from elongated to round. (D, E) Motile refractile cells attaching to become flat, spread-out cells. (H) A flat cell becoming an elongated, bipolar, motile cell.
Composition of DUOC-01 products
We used flow cytometry and immunocytochemistry to examine the lineage phenotype and characterize the composition of DUOC-01. Figure 4 shows representative flow analysis, and Table I shows the results for the surface antigens evaluated by flow cytometry. We were particularly concerned with removing potentially alloreactive T cells during manufacturing, and flow analysis showed that CD3+ cells were not detected in cell products. Almost all DUOC-01 cells expressed CD45, and most cells expressed CD11b and/or CD14 characteristic of macrophage and microglia.
Figure 4.
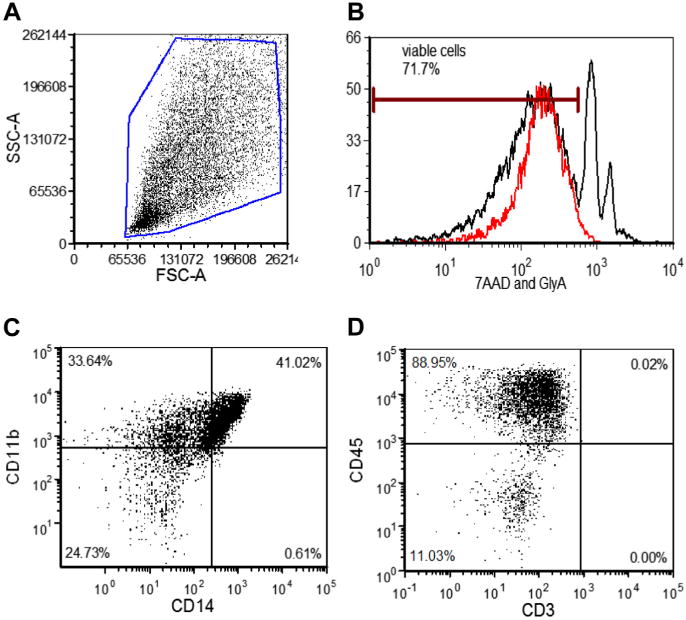
Flow cytometric analysis of DUOC-01. (A) Typical flow forward and side-scatter (FSC, SSC) and viability staining cytograms used to identify viable cells for analysis of marker expression. (B) Flow histogram of gated population in (A) stained with 7-amino-actinmycin (7AAD) (red line) and anti–glycophorin A (GlyA) to identify viable cells and erythrocytes, respectively. (C) Expression of CD11b and CD14 and (D) of CD3 and CD45 by cells in DUOC-01 product. Cells analyzed were gated as shown in (A) and (B). In all cases, fluorescence gates for antibody staining were set by use of cells exposed to appropriate isotype controls.
Table I.
Percentage of cells expressing various antigens in multiple lots of DUOC-01.
| CD3+ | CD11b+ | CD45+ | CD14+ | |
|---|---|---|---|---|
| Average | 0.00 | 69.9 | 86.8 | 40.0 |
| SD | 0.00 | 15.7 | 11.6 | 19.2 |
Samples of 17 DUOC-01 manufacturing runs were analyzed by means of multiparameter flow cytometry as described in Figure 2; n = 17.
We also used immunochemistry to explore expression of other markers in this lineage. Figure 5 shows that all the morphological types in 21-day DUOC-01 cultures grown on slides expressed macrophage/monocyte markers CD11b, CD14, HLA-DR, CD16, CD206, CD163 and iNOS.
Figure 5.
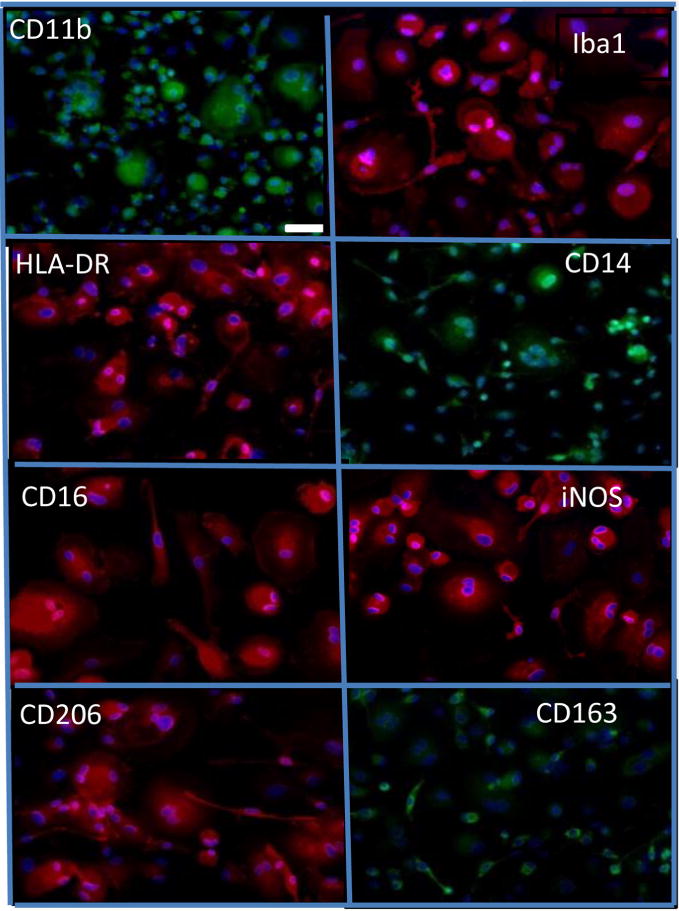
Cells in DUOC-01 product express a variety of macrophage/microglial antigens. All images are prepared for immunocytochemistry with antibody to the antigen indicated and counterstained with DAPI. Immunostaining is red or green; DAPI, blue. All images are at same magnification. White scale bar in CD11b image is 50 μm.
Lysosomal enzyme activity
To determine if DUOC-01 could be a potential source of enzyme to cross-correct secreted enzyme deficiencies in patients with IMDs, activities of 11 lysosomal enzymes were measured in sonicates of cell products. Significant and normal levels of activities of all 11 lysosomal enzymes were readily detected in all five lots of DUOC-01 analyzed (Table II). For each enzyme, the specific activity varied over a range similar to the range characteristically observed in blood cells derived from individuals without enzyme deficiencies.
Table II.
Activity of lysosomal enzymes in DUOC-01 cell products.
| Activity (nmol/h/mg protein) of 11 lysosomal enzymes in 5 cGMP lots of DUOC-01 | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Enzyme | UCB No.
|
Mean | SD | ||||
| 600661 | 601558 | 601217 | 220058 | 220115 | |||
| β-Galactosidase | 914.4 | 902 | 281.2 | 1184.2 | 974 | 851.2 | 302.5 |
| β-Mannosidase | 72.8 | 75.8 | 54.9 | 98.7 | 66.2 | 73.7 | 14.4 |
| α-L-fucosidase | 1819 | 1661 | 5452 | 2171 | 3409 | 2902.4 | 1414.7 |
| α-Mannosidase | 503.8 | 574 | 377.5 | 636 | 757.6 | 569.8 | 127.3 |
| β-Glucuronidase | 1493 | 1620 | 943.7 | 1754 | 1429 | 1447.9 | 275.7 |
| β-Hexosaminidase A | 2351 | 2173 | 1647 | 3246 | 2684 | 2420.2 | 532.0 |
| Arylsulfatase A | 119.6 | 52.2 | 200.1 | 115 | 79.3 | 113.2 | 49.9 |
| Galactocerebrosidase | 1.5 | 6.9 | 14.3 | 9.46 | 6.51 | 7.7 | 4.2 |
| Sphingomyelinase | 16.4 | 22.6 | 30.1 | 27.1 | 20.6 | 23.4 | 4.8 |
| Glucocerebrosidase | 127 | 255.9 | 428.9 | 307 | 373.9 | 298.5 | 103.9 |
| α-L-iduronidase | 48.6 | 65.5 | 43.3 | 28.3 | 36.4 | 44.4 | 12.5 |
Analysis performed by Lysosomal Diseases Testing Laboratory, Thomas Jefferson University.
Cytokine secretion by DUOC-01
The concentration of cytokines in the culture medium of eight lots of DUOC-01 at the time the cells were harvested was measured with the use of Bioplex analysis. IL-4, IL-5 and IL-13 were not detected in any lots. Table III shows the concentrations of the other measured cytokines in each supernatant; these values are normalized to account for the number of cells present in each batch at the time of harvest. IL-1b and IL-12p70 were each only detected in one batch, both at very low levels. Only two cytokines in the panel, IL-6 and IL-10, were detected at appreciable concentrations in all eight batches. TGF-β was detected in harvest supernatants from seven of eight manufacturing lots. Three of eight lots produced relatively high concentrations of INF-γ, four lots produced low levels and no IFN-γ was detected in the medium of one lot. TNF-α and IL-2 were detected at very low levels.
Table III.
Concentration of cytokines in the supernatants of DUOC-01 cell products at the time of harvest.
| Lot ID | TNF-α | IL-2 | IL-10 | IFN-γ | IL-6 | TGF-β |
|---|---|---|---|---|---|---|
| 220055 | 0.023 | 0.805 | 29.6 | 244.5 | 980 | 3522.52 |
| 900181 | 0.225 | 0.388 | 106.4 | 1.2 | 776 | 28.28 |
| 491255 | 0.296 | 0.406 | 95.6 | 2.1 | 1261 | 211.77 |
| 220066 | 0.274 | 3.077 | 40.9 | 115 | 2548 | 1391.29 |
| 491350 | 0.063 | 0.09 | 49.2 | 1.3 | 1212 | 169.41 |
| 220798 | 0.581 | 0.877 | 247.2 | 4.1 | 1914 | 0 |
| 700016 | <OOR | 0.35 | 18.5 | 263.8 | 1775 | 1156.47 |
| 210132 | <OOR | <OOR | 35 | <OOR | 292 | 1508.72 |
| Mean (SD) | 0.183 (0.203) | 0.749 (0.989) | 77.8 (75.3) | 79 (115.1) | 1345 (712) | 998.6 (1198.5) |
Values shown for each lot are means of duplicate analyses (pg/106 cells/mL).<OOR, out-of-range low.
Changes in cytokine secretion by DUOC-01 in response to inflammatory stimuli
Table IV shows the concentration of cytokines that accumulated in culture supernatants from replated DUOC-01 cells maintained in culture for 72 h without any added inflammatory stimulus. We did not obtain consistent evidence for TGF-β secretion with the use of this protocol. IL-6 and IL-10 were the only cytokines detected in unstimulated cultures. Thus, these cells continued to secrete these two cytokines after replating. Stimulation with TNF-α induced accumulation of all the cytokines in the panel except Il-13, but the levels of all cytokines besides IL-6 and IL-10 remained low after treatment with either 0.5 or 1.0 μg of TNF-α. IL-6 accumulation was increased 22.1- and 41.5-fold by 0.5 and 1.00 μg of TNF-α, respectively. IL-10 accumulation was enhanced 70.0- and 57.6-fold by 0.5 and 1.00 μg of TNF-α, respectively. Thus, IL-10 accumulation was stimulated more than was IL-6 at the concentrations of TNF-α used. Stimulation with 10 μg/mL of IFN-γ induced significant increases in accumulation of all the other nine cytokines in the panel. IFN-γ stimulated a 19.8-fold increase in IL-6 accumulation and a 28.3-fold increase in IL-10 accumulation. Phorbol 12-myristate 13-acetate (PMA) treatment induced a slight increase in IL-1β accumulation, downregulated IL-6 accumulation 0.49-fold and increased IL-10 accumulation 2.6-fold. Thus, all inflammatory stimuli stimulated IL-10 accumulation more strongly than IL-6 accumulation. We did not obtain consistent changes of TGF-β accumulation with these lots of DUOC-01 in this protocol.
Table IV.
Concentration of cytokines in medium of DUOC-01 cultures 24 hours after inflammatory stimulation.
| Additions | IL-1b | IL-2 | IL-4 | IL-5 | IL-6 | IL-10 | IL12-p70 | IL-13 | IFN-γ | TNF-α |
|---|---|---|---|---|---|---|---|---|---|---|
| None | OOR< | OOR< | OOR< | OOR< | 181.9 | 27.6 | OOR< | OOR< | OOR< | OOR< |
| SD | 230.7 | 14.6 | ||||||||
| 0.5 mg TNF-α | 14.7 | 43.2 | 7.4 | 19.9 | 4022.5 | 1930.9 | 2.0 | OOR< | 369.2 | NA |
| SD | 2.6 | 2.1 | 0.4 | 1.3 | 414.0 | 356.1 | 0.1 | 15.0 | ||
| 1 μg TNF-α | 16.5 | 47.1 | 8.5 | 20.8 | 7562.6 | 1589.0 | 2.4 | OOR< | 391.2 | NA |
| SD | 3.2 | 4.5 | 0.8 | 1.7 | 1393.1 | 197.4 | 0.3 | 17.9 | ||
| 10 μg IFN-γ | 91.6 | 720.1 | 113.1 | 355.5 | 3596.0 | 780.2 | 344.9 | 103.5 | NA | 169.4 |
| SD | 23.9 | 171.5 | 27.7 | 93.2 | 1000.8 | 172.8 | 94.6 | 27.6 | NA | 30.8 |
| 5 ng PMA | <1.15 | OOR< | OOR< | OOR< | 89.0 | 72.3 | OOR< | OOR< | OOR< | 5.0 |
| SD | 46.8 | 59.8 | 4.8 | |||||||
| Lower limit | 1.1 | 2.8 | 0.5 | 2.6 | 7.3 | 3.5 | 0.6 | 0.9 | 2.7 | 0.1 |
Mean values (expressed in pg/mL) calculated from Bioplex assay immunocapture fluorescence assays from culture supernatants were derived from DUOC cells (manufacturing lots 220055, 900181 and 491255) replated in the presence of inflammatory stimuli for 24 h. Cytokine concentrations were measured in duplicate wells from each lot, and means were calculated from pooled data derived from different lots treated under similar conditions. Lot 220055 was not tested after IFN-γ stimulation.NA, not applicable; this is the cytokine added to stimulate the culture. OOR< means cytokine concentration was below the lower limit of detection is shown in the bottom row.
Changes in messenger RNA levels for selected genes during manufacturing
We analyzed eight DUOC-01 cell products harvested at the end of the 21-day manufacturing period for expression of transcripts for selected gene products. The relative levels of expression (mean amplification cycle), from most to least abundant, of these gene products were TGF-β (24.8), GALC (25.3), IL-10 (25.3), TNF-α (29.1), and IFN-γ (35.0). We also compared expression of these transcripts by DUOC-01 cells harvested at 21 days with expression by cells on the first day of culture. Expression of IL-10 (22.5 ± 13.8-fold, P < 0.01) and GALC (4.4 ± 2.8-fold, P < 0.05) messenger RNA by DUOC-01 increased significantly (unpaired t-test with Welch’s correction) from day zero to harvest. Changes in abundance of the TNF-α (0.9 ± 1.1-fold), TGF-β (1.2 ± 0.8-fold) and IFN-γ (0.02 ± 0.05-fold) transcripts during the culture period were not statistically significant.
Alloreactions to and by DUOC-01
MLC experiments were performed to determine whether cells in DUOC-01 proliferate in response to allogeneic, pooled third-party MNCs and also whether DUOC-01 can stimulate proliferation of allogeneic MNCs in culture. DUOC-01 proliferated very poorly (average CPM of 190; n = 15) in response to autologous or allogeneic cells.
We refer to experiments in which DUOC-01 and MNCs were prepared from different bag compartments of the same CBUs as autologous experiments. The autologous experiments mirrored the clinical protocol in which DUOC-01 is manufactured from the same CBUs that the patient receives as a transplant donor and established background proliferation rates when CB MNCs and DUOC-01 products are matched. Column A in Table V shows, as expected, that titrated thymidine incorporation was low under these conditions.
Table V.
Proliferation of mononuclear cells in response to stimulation by mitomycin-treated DUOC-01 cell products in mixed lymphocyte cultures.
| Cord No. | Responder
|
Fold increase
|
||||
|---|---|---|---|---|---|---|
| [A] Autologous CB MNCs | [B] Random matched PB MNCs | [C] 4/6 HLA-matched CB MNCs | B/A | C/A | B/C | |
| 9442386 | 17,181 ± 1414 | 169,725 ± 21,668 | 9.9 | |||
| 6230351 | 4400 ± 407 | 230,930 ± 28,592 | 52.5 | |||
| 10600790 | 280 ± 40 | 6426 ± 3426 | 23 | |||
| 9491682 | 1968 ± 796 | 40,302 ± 790 | 2004 | 20.5 | 1 | 20.1 |
| 8499116 | 1189 ± 238 | 25,053 ± 1615 | 2551 | 21.1 | 2.1 | 9.8 |
Cord No. unit shows the CBU unit used to manufacture each batch of DUOC-01. In all experiments shown, stimulator cells were mitomycin-treated DUOC-01. In autologous combinations (column A), DUOC-01 and responding CB MNC were derived from the same cord. In random-match PB MNC combinations (column B), MNCs were prepared from randomly selected normal PB donors. In 4/6 HLA-matched CB-MNC combinations (column C), DUOC-01 and CB MNC responders were made from CBUs that were matched for both HLA-B alleles and both HLA-DR alleles but mismatched at both HLA-A alleles. Values are mean ± SD counts per minute of titrated thymidine incorporation. In last 3 columns, values are fold increase in proliferation for donor/responder combinations indicated.
We refer to experiments in which PB or CB MNC respond to DUOC-01 products made from a different CBU as allogeneic experiments. In five experiments, randomly mismatched peripheral blood MNC from individual donors proliferated between 9.9- and 52.5-fold more strongly than autologous CB MNCs derived from the same CBU used to make the DUOC cell product (Table V, Column B). Thus, DUOC-01 can be recognized by alloreactive cells in mismatched PB MNC populations. We also measured proliferation of CB MNC in response to 4/6 HLA-mismatched DUOC-01; this mimics the typical worst-case clinical mismatch for CB transplants. In two experiments, proliferation of 4/6 mismatched CB MNCs was approximately twice as high as the proliferation of autologous CB MNCs (Table V, column C), 10- to 20-fold lower than the response of randomly matched PB MNCs [Table V].
We next explored whether allo-recognition of DUOC-01 by normal MNCs causes release of cytokines or if DUOC-01 releases cytokines in response to MNCs by measuring accumulation cytokines in MLC supernatants. Results of experiments with three manufacturing lots of DUOC-01 and various effector cell populations showed lot-to-lot variation, as discussed in detail in Supplementary Table I. However, consistent with the results of cytokine accumulation during the manufacture of DUOC-01 presented above, IL-4 was not detected in any of the supernatants, and IL-10 accumulated in all cultures containing DUOC-01 whether or not other cell types were present.
Discussion
In this report, we describe a novel cell product, DUOC-01, manufactured from banked umbilical CB for use as an intrathecally delivered adjuvant cell therapy to treat the CNS in patients undergoing myeloablative unrelated donor CB transplantation for selected IMDs. Morphological, flow cytometric and immunohistochemical analyses of DUOC-01 all suggest that the cell product is composed predominantly of monocytic/macrophage lineage cells. As such, more than 95% of DUOC-01 cells express the hematopoietic lineage marker CD45, and >50% of the cells in DUOC-01 express the αM integrin CD11b. In addition, most or all of the cells expressed macrophage markers CD14, HLA-DR, CD16, CD206, CD163, Iba1 and iNOS. All the morphological types that we observed in cultures during manufacturing are similar to the variety of morphologies that have been described in preparations of human macrophage, including brain macrophage and microglia [10–12]. DUOC-01 may also be related to donor microglial cells present in the brains of patients months after transplantation [3]. Ki67 and BrdU staining revealed that DUOC-01 cultures include many proliferative cells, which suggests that expansion of some subpopulation of CB cells occurs during manufacturing. The most straightforward hypotheses for the origin of the cells in DUOC-01 cultures are that they arise from hematopoietic myeloid progenitors or monocytes in the CB unit and differentiate into macrophage-like cells in culture. We are currently exploring this issue further. Because DUOC-01 cells are cultivated for 21 days in the presence of a variety of cytokines and are exposed to products of cells that die and are cleared during the manufacturing process, it is unlikely that cells in DUOC-01 are identical to any discrete population in CB.
Macrophage and microglia can become activated in different ways to express primarily pro- or anti-inflammatory activities. M1-type pro-inflammatory and M2-type anti-inflammatory cells are extremes of a functional continuum with many cells showing mixed expression of inflammatory and anti-inflammatory and regenerative activities [13,14]. The activities of such cells are particularly important in dealing with CNS inflammation [15–21]. Because neuro-inflammation characteristically contributes to the pathology of the IMDs that we are treating with DUOC-01, we explored whether DUOC-01 cells expressed markers characteristic of highly polarized M1 and M2 cells. We did not find a clear polarization in that the cells expressed iNOS and CD16 typically associated with M1 cells and CD163 and CD206, often associated with M2 cells.
We also measured production of cytokines by DUOC-01 cells as a potential indication of potential pro- or anti-inflammatory activities. The concentrations of the cytokines that accumulated in DUOC-01 supernatants during manufacturing reflect several parameters: synthesis and secretion, degradation in the culture medium and/or after re-uptake by cells, the number of cells in culture at the time of harvest and culture feeding schedules. Despite this complexity, IL-6 and IL-10 accumulated in physiologically significant amounts (7.4–144 pmol/L and 0.48–21.2 pmol/L, respectively) in the supernatants of all lots of DUOC-01 during the manufacturing process. TGF-β was detected at physiologically relevant concentrations (mean of approximately 40 pmol/L) in seven of eight batches. IL-6 and IL-10 could also be detected in culture supernatants after DUOC-01 cells were harvested, washed and passaged into fresh tissue culture medium for 3 additional days. IL-10 also accumulated in MLC supernatants from cultures containing DUOC-01 cells. The production of both IL-10 and IL-6 by DUOC-01 could potentially provide beneficial anti-inflammatory and pro-angiogenic and pro-neurogenic effects in vivo.
Cells from DUOC-01 products responded to PMA and TNF-α by increasing secretion of cytokines. Secretion of IL-6 and of IL-10 was increased by both agents. IL-10 secretion was increased more strongly than IL-6. Both agents also stimulated accumulation of IL-1β. Secretion of other cytokines was weakly increased by these agents. IFN-γ strongly increased secretion of all the other cytokines in the panel, including both pro- and anti-inflammatory cytokines. Thus, DUOC-01 cells can respond differentially to inflammatory stimuli, but, again, IL-6 and IL-10 are primary products.
Like the immunocytochemistry analyses, these cytokine data all suggest an intermediate type of polarization between M1 and M2 activities. Expression of IL-10 in all lots of DUOC-01 and of TGF-β in all but one lot is consistent with M2 type macrophage/microglial activities. Both of these cytokines can have anti-inflammatory properties and are capable of dampening pathological reactions caused by IL-1, TNF-α, IFN-γ and other pro-inflammatory molecules [22,23]. The increase in accumulation of these cytokines after stimulation with TNF-α and INF-γ or with allogeneic MNCs is consistent with the idea that these cells could dampen an on-going inflammatory reaction. We found that the manufacturing process for DUOC-01 results in a cell population that has significantly more IL-10 transcripts than the CB cells used to initiate the cultures. Transcript levels for TNF-α, TGF-β and INF-γ transcripts did not change, again suggesting an important role for anti-inflammatory IL-10 in DUOC-01 cells. However, DUOC-01 could respond to IFN-γ by producing inflammatory cytokines.
Expression of IL-6 by these cells also indicates a potentially more complex participation by DUOC-01 in on-going brain inflammatory states. IL-6 is a well-known pro-inflammatory mediator, and IL-6 has been cited as a potential contributor to the pathology of Krabbe disease and other IMDs [24–26]. However, IL-6 also has anti-inflammatory properties, with the pro- and anti-inflammatory effects of this molecule depending in part on whether it signals through soluble or membrane bound receptors [27]. The role of IL-6 in the brain is particularly diversified, and this cytokine can promote repair of brain damage in several different contexts [27–30]. In particular, it helps mediate remyelination of curpizone-induced lesions of the corpus collosum in mice [31]. We will present evidence elsewhere that DUOC-01 cells can promote remyelination in this model [32].
These results support the concept of using intrathecally administered DUOC-01 to deliver factors to the brain in the period before progenitors derived from the systemic CB transplant engraft the brain in sufficient numbers to exert therapeutic effects. As noted above, IL-10, TGF-β and possibly IL-6 could have additional beneficial effects in this clinical context. In addition, we found that DUOC-01, manufactured from normal donors, expresses normal levels of all 11 secreted lysosomal enzymes that we assayed. Transcript abundance for one of these, GALC, was increased more than 4-fold during the manufacture of DUOC-01. Thus, DUOC-01 could also provide wild-type enzyme to cross-correct IMD in the CNS shortly after administration. Biodistribution studies after intrathecal administration of DUOC-01 in newborn, immune-incompetent mice show that DUOC-01 is generally well tolerated and that some DUOC cells persist in the brain and spinal cord for at least 56 days [33]. Thus, these cells could in principle continue to produce therapeutic products for an extended period until permanent engraftment is established.
On the other hand, DUOC-01 cells express HLA-DR, and our MLC studies indicate that DUOC-01 can be recognized by alloreactive MNCs. The magnitude of the proliferative response of MNCs to DUOC-01 in MLCs depended on the degree of HLA mismatch between the cell product and the MNCs. MNCs that were 4/6 matches to DUOC-01 proliferated very weakly if at all in MLC reactions, but randomly mismatched MNCs proliferated strongly.
We have designed our clinical protocol to minimize the likelihood that either host-versus-graft reactions such as those detected in the MLC studies or graft-versus-host disease (GVHD) could mitigate the potential benefits of DUOC-01. First, our flow cytometry and MLC studies demonstrate that alloreactive T cells die during manufacturing of DUOC-01. The DUOC-01 cell product will not be released for clinical use if it contains more than 5 × 103 CD3+ T cells/kg of patient body weight. Second, we will administer DUOC-01 28 days after myeloablative conditioning and conventional CB transplant and only after donor cell engraftment without significant acute graft versus host disease is documented. At this time, the recipient immune system will still be ablated by pre-transplant chemotherapy, and no patient T cells will remain in circulation. Any donor T cells will be autologous to the DUOC-01 cells and therefore recognized as self. Thus, allo-rejection of DUOC-01 is unlikely to occur or to abrogate the potential beneficial effects of the product. When the patient’s immune system regains function many months later, T cells arising in the recipient from the donor CBU will be matched to DUOC-01 because DUOC-01 and the transplant donor are derived from the same CBU. Myeloablation and 9 to 12 months of post-transplant anti-GVHD immunosuppressive therapy will also mitigate against neuro-inflammation and rejection. Finally, DUOC-01 will not be administered if acute grade III or IV GVHD is present in the patient to minimize the chance that dysregulated immune response caused by GVHD could influence interactions between DUOC-01 and the recipient. This protocol will reduce the likelihood of HLA-mediated allogeneic reactions between DUOC-01 and the recipient’s newly formed immune system. Consequently, we believe that intrathecally administered DUOC-01 will be able to persist in the CNS compartment of myelo-ablated CB transplant patients and have the potential to deliver beneficial cytokines and enzyme as a bridging therapy before establishment of full, permanent engraftment in the brains of patients with IMD. A clinical trial (NCT02254863) that embodies the design outlined here has been initiated and is enrolling patients.
Supplementary Material
Acknowledgments
The authors are grateful to the staff at the Carolinas Cord Blood Bank for providing cord blood units for the experiments described, to Ms Roberta Parrott and Dr Rebecca Buckley for providing and preparing donor PB samples and to Dr Yasheng Gao at the Light Microscopy Core Facility at Duke University for assistance with video imaging of live DUOC-01 cultures. This work was supported by the Julian Robertson Foundation and the Legacy of Angels Foundation. A. Wollish was supported by the National Cancer Institute of the National Institutes of Health Award No. 5T32CA074736, Research Training in Neuro-Oncology.
Footnotes
Disclosure of interests: The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jcyt.2015.02.006.
References
- 1.Prasad VK, Kurtzberg J. Cord blood and bone marrow transplantation in inherited metabolic diseases: scientific basis, current status and future directions. Br J Haematol. 2010;148:356–72. doi: 10.1111/j.1365-2141.2009.07974.x. [DOI] [PubMed] [Google Scholar]
- 2.Orchard PJ, Tolar J. Transplant outcomes in leukodystrophies. Semin Hematol. 2010;47:70–8. doi: 10.1053/j.seminhematol.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Krivit W, Sung JH, Shapiro EG, Lockman LA. Microglia: the effector cell for reconstitution of the central nervous system following bone marrow transplantation for lysosomal and peroxisomal storage diseases. Cell Transplant. 1995;4:385–92. doi: 10.1177/096368979500400409. [DOI] [PubMed] [Google Scholar]
- 4.Wenger DA, Williams C. Screening for lysosomal disorders. In: FA H, editor. Techniques in Diagnostic Human Biochemical Genetics A Laboratory Manual. New York: Wiley-Liss; 1991. pp. 587–617. [Google Scholar]
- 5.Escolar ML, Poe MD, Smith JK, Gilmore JH, Kurtzberg J, Lin W, et al. Diffusion tensor imaging detects abnormalities in the corticospinal tracts of neonates with infantile Krabbe disease. AJNR Am J Neuroradiol. 2009;30:1017–21. doi: 10.3174/ajnr.A1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracy ET, Zhang CY, Gentry T, Shoulars KW, Kurtzberg J. Isolation and expansion of oligodendrocyte progenitor cells from cryopreserved human umbilical cord blood. Cytotherapy. 2011;13:722–9. doi: 10.3109/14653249.2011.553592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentry T, Deibert E, Foster SJ, Haley R, Kurtzberg J, Balber AE. Isolation of early hematopoietic cells, including megakaryocyte progenitors, in the ALDH-bright cell population of cryopreserved, banked UC blood. Cytotherapy. 2007;9:569–76. doi: 10.1080/14653240701466347. [DOI] [PubMed] [Google Scholar]
- 8.Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function. In: Coligan JE, editor. Current protocols in immunology. New York: Greene Publishing Associates and Wiley-Interscience; 2004. Chapter 3:Unit 3.12. [DOI] [PubMed] [Google Scholar]
- 9.Tracy E, Aldrink J, Panosian J, Beam D, Thacker J, Reese M, et al. Isolation of oligodendrocyte-like cells from human umbilical cord blood. Cytotherapy. 2008;10:518–25. doi: 10.1080/14653240802154586. [DOI] [PubMed] [Google Scholar]
- 10.Madore C, Joffre C, Delpech JC, De Smedt-Peyrusse V, Aubert A, Coste L, et al. Early morphofunctional plasticity of microglia in response to acute lipopolysaccharide. Brain Behav Immun. 2013;34:151–8. doi: 10.1016/j.bbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Nayak D, Roth TL, McGavern DB. Microglia Development and Function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labonte AC, Tosello-Trampont AC, Hahn YS. The role of macrophage polarization in infectious and inflammatory diseases. Mol Cells. 2014;37:275–85. doi: 10.14348/molcells.2014.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–7. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Giunti D, Parodi B, Cordano C, Uccelli A, Kerlero de Rosbo N. Can we switch microglia’s phenotype to foster neuroprotection? Focus on multiple sclerosis. Immunology. 2013;141:328–39. doi: 10.1111/imm.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greter M, Merad M. Regulation of microglia development and homeostasis. Glia. 2013;61:121–7. doi: 10.1002/glia.22408. [DOI] [PubMed] [Google Scholar]
- 17.Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–69. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–8. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherry JD, Olschowka JA, O’Banion MK. Neuro-inflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 24.LeVine SM, Brown DC. IL-6 and TNFalpha expression in brains of twitcher, quaking and normal mice. J Neuroimmunol. 1997;73:47–56. doi: 10.1016/s0165-5728(96)00166-x. [DOI] [PubMed] [Google Scholar]
- 25.Luzi P, Abraham RM, Rafi MA, Curtis M, Hooper DC, Wenger DA. Effects of treatments on inflammatory and apoptotic markers in the CNS of mice with globoid cell leukodystrophy. Brain Res. 2009;1300:146–58. doi: 10.1016/j.brainres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedchenko TV, LeVine SM. IL-6 deficiency causes enhanced pathology in Twitcher (globoid cell leukodystrophy) mice. Exp Neurol. 1999;158:459–68. doi: 10.1006/exnr.1999.7125. [DOI] [PubMed] [Google Scholar]
- 27.Galun E, Rose-John S. The regenerative activity of interleukin-6. Methods Mol Biol. 2013;982:59–77. doi: 10.1007/978-1-62703-308-4_4. [DOI] [PubMed] [Google Scholar]
- 28.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–66. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perigolo-Vicente R, Ritt K, Pereira MR, Torres PM, Paes-de-Carvalho R, Giestal-de-Araujo E. IL-6 treatment increases the survival of retinal ganglion cells in vitro: the role of adenosine A1 receptor. Biochem Biophys Res Commun. 2013;430:512–8. doi: 10.1016/j.bbrc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Brunssen SH, Moy SS, Toews AD, McPherson CA, Harry GJ. Interleukin-6 (IL-6) receptor/IL-6 fusion protein (Hyper IL-6) effects on the neonatal mouse brain: possible role for IL-6 trans-signaling in brain development and functional neurobehavioral outcomes. Brain Behav Immun. 2013;27:42–53. doi: 10.1016/j.bbi.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tezuka T, Tamura M, Kondo MA, Sakaue M, Okada K, Takemoto K, et al. Cuprizone short-term exposure: Astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiol Dis. 2013;59:63–8. doi: 10.1016/j.nbd.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha A, Buntz S, Patel S, Matsushima GK, Wollish A, Kurtzberg J, et al. DUOC-01, a candidate cell therapy product derived from banked cord blood, accelerates brain remyelination in NSG mice following cuprizone feeding. Cytotherapy. 2014;16:S61–2. [Google Scholar]
- 33.Storms R, Liu C, Gentry T, Zhou J, Ozamiz A, Rusche B, et al. Tissue distribution of a cord blood-derived cell product following intrathecal injection. Cytotherapy. 2014;16:S63–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


