Abstract
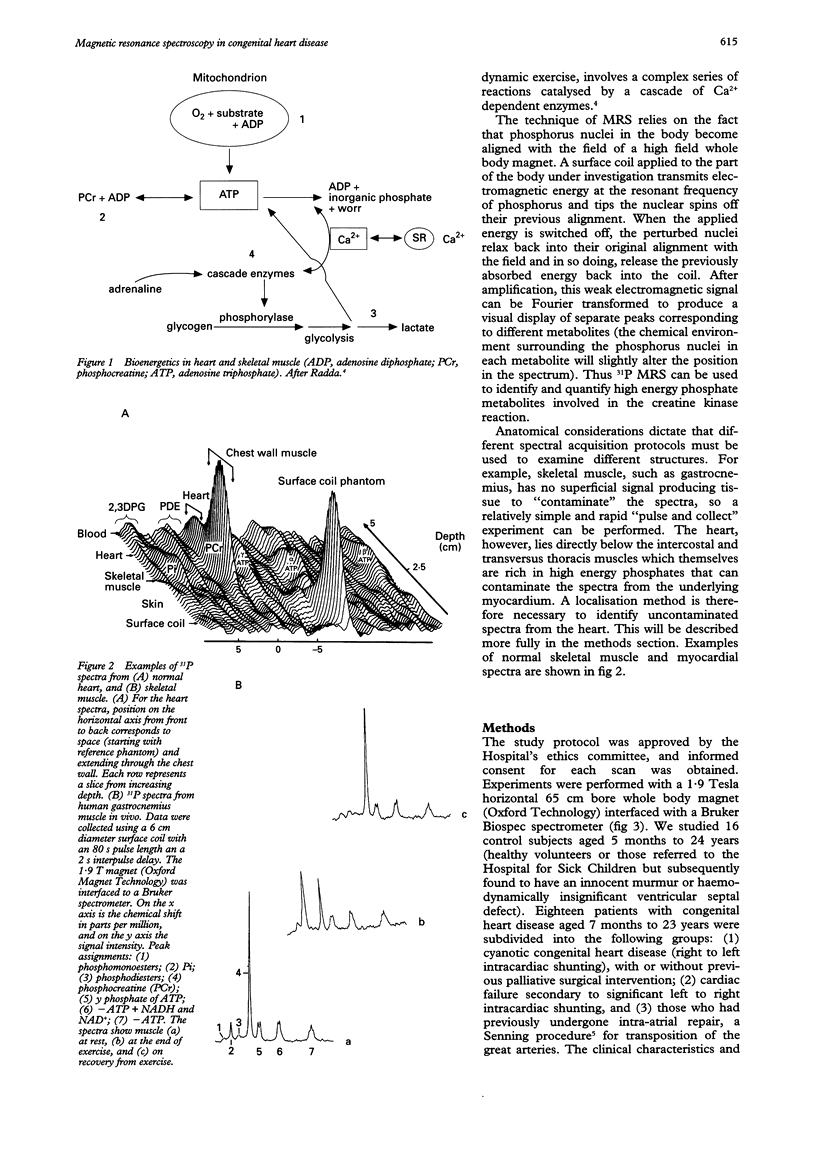
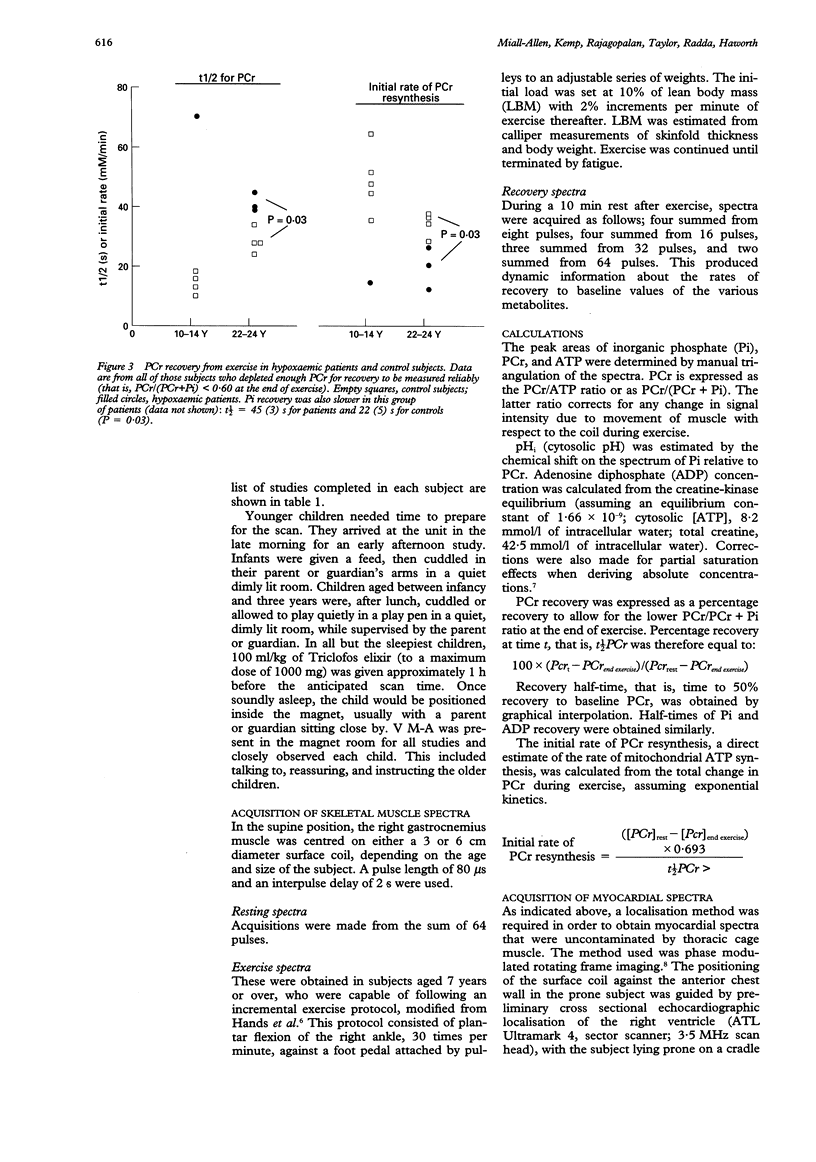
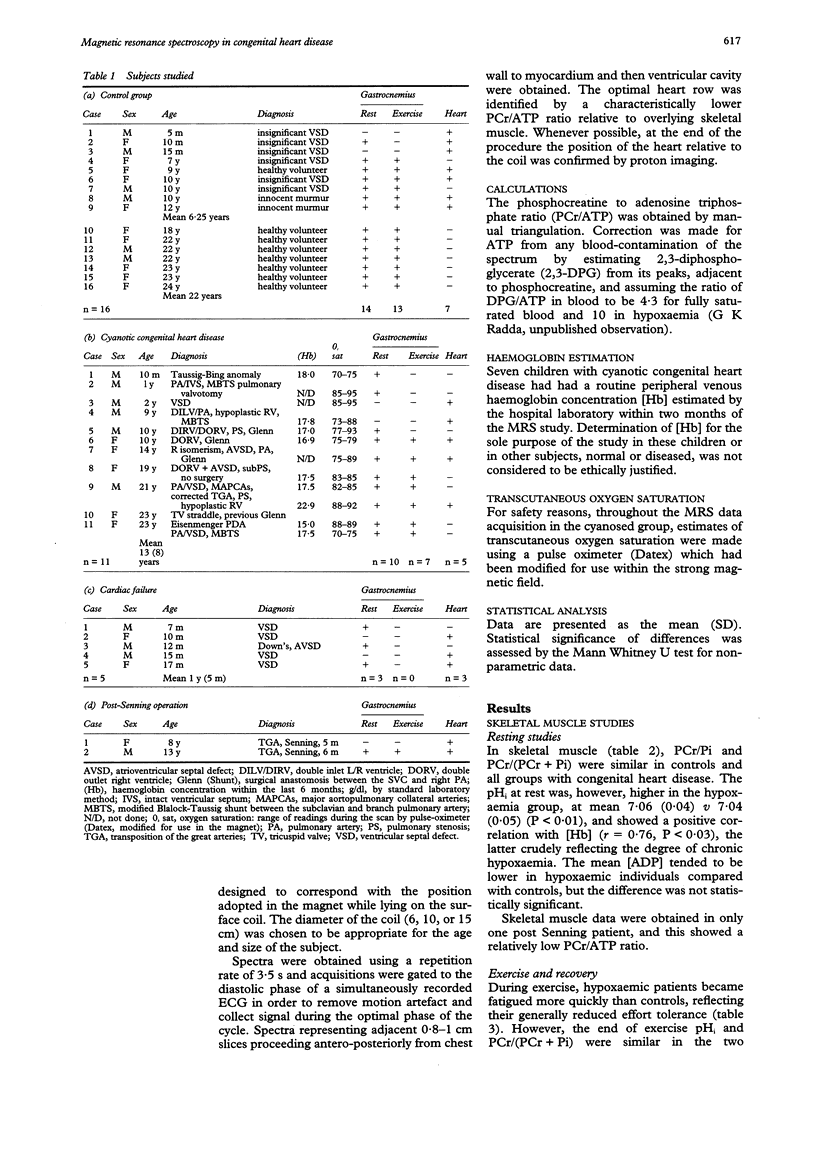
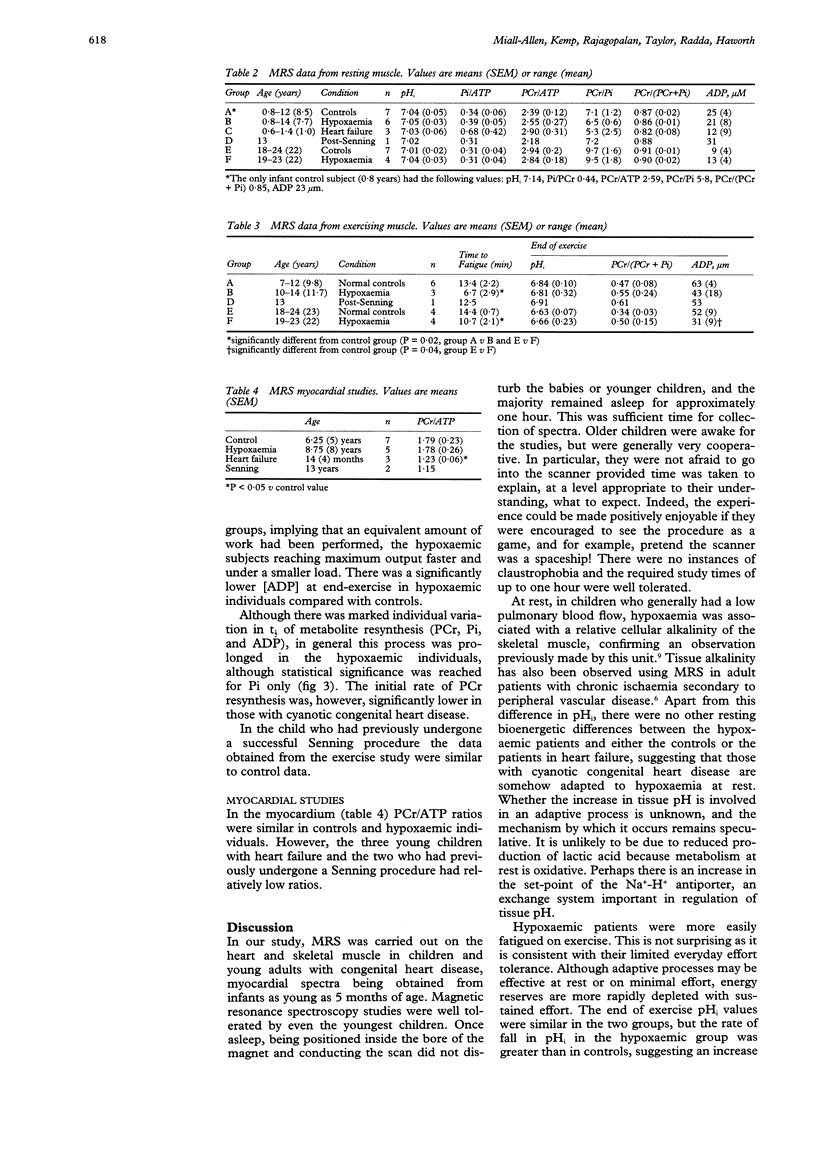
OBJECTIVE: To determine the feasibility of studying myocardial and skeletal muscle bioenergetics using 31P magnetic resonance spectroscopy (MRS) in babies and young children with congenital heart disease. SUBJECTS: 16 control subjects aged 5 months to 24 years and 18 patients with CHD, aged 7 months to 23 years, of whom 11 had cyanotic CHD, five had cardiac failure, and two had had a Senning procedure. DESIGN: 31P MRS was carried out using a 1.9 Tesla horizontal 65 cm bore whole body magnet to study the myocardium in 10 patients and skeletal muscle (gastrocnemius) in 14 patients, eight of whom were exercised, together with appropriate controls. RESULTS: In hypoxaemic patients, in skeletal muscle at rest intracellular pH (pHi) was abnormally high [7.06 (SEM 0.04) v 7.04 (0.05), P < 0.01] and showed a positive correlation with haemoglobin (P < 0.03). On exercise, hypoxaemic patients fatigued more quickly but end-exercise pHi and phosphocreatine recovery were normal, implying that an equivalent but smaller amount of work had been performed. End-exercise ADP concentration was lower. On recovery, the initial rate of phosphocreatine resynthesis was low. Skeletal muscle bioenergetics were within normal limits in those in heart failure. In the myocardium, the phosphocreatine/ATP ratio was similar in controls and hypoxaemic subjects, but low in those in heart failure. CONCLUSIONS: In heart failure, the myocardial phosphocreatine/ATP ratio was reduced, as in adults, while resting skeletal muscle studies were normal. By contrast, hypoxaemic children had normal myocardial bioenergetics, but showed skeletal muscle alkalinity, and energy reserves were more readily depleted on exercise. On recovery, the initially slow phosphocreatine resynthesis rate reflects a low rate of mitochondrial ATP synthesis, probably due to an inadequate oxygen supply. 31P MRS offers a safe, non-invasive method of studying myocardial and skeletal muscle bioenergetics in children as young as 5 months.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adatia I., Kemp G. J., Taylor D. J., Radda G. K., Rajagopalan B., Haworth S. G. Abnormalities in skeletal muscle metabolism in cyanotic patients with congenital heart disease: a 31P nuclear magnetic resonance spectroscopy study. Clin Sci (Lond) 1993 Jul;85(1):105–109. doi: 10.1042/cs0850105. [DOI] [PubMed] [Google Scholar]
- Arnold D. L., Taylor D. J., Radda G. K. Investigation of human mitochondrial myopathies by phosphorus magnetic resonance spectroscopy. Ann Neurol. 1985 Aug;18(2):189–196. doi: 10.1002/ana.410180205. [DOI] [PubMed] [Google Scholar]
- Arnolda L., Conway M., Dolecki M., Sharif H., Rajagopalan B., Ledingham J. G., Sleight P., Radda G. K. Skeletal muscle metabolism in heart failure: a 31P nuclear magnetic resonance spectroscopy study of leg muscle. Clin Sci (Lond) 1990 Dec;79(6):583–589. doi: 10.1042/cs0790583. [DOI] [PubMed] [Google Scholar]
- Blackledge M. J., Rajagopalan B., Oberhaensli R. D., Bolas N. M., Styles P., Radda G. K. Quantitative studies of human cardiac metabolism by 31P rotating-frame NMR. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4283–4287. doi: 10.1073/pnas.84.12.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway M. A., Allis J., Ouwerkerk R., Niioka T., Rajagopalan B., Radda G. K. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet. 1991 Oct 19;338(8773):973–976. doi: 10.1016/0140-6736(91)91838-l. [DOI] [PubMed] [Google Scholar]
- Dudley G. A., Tullson P. C., Terjung R. L. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem. 1987 Jul 5;262(19):9109–9114. [PubMed] [Google Scholar]
- Hands L. J., Bore P. J., Galloway G., Morris P. J., Radda G. K. Muscle metabolism in patients with peripheral vascular disease investigated by 31P nuclear magnetic resonance spectroscopy. Clin Sci (Lond) 1986 Sep;71(3):283–290. doi: 10.1042/cs0710283. [DOI] [PubMed] [Google Scholar]
- Radda G. K. Potential and limitations of nuclear magnetic resonance for the cardiologist. Br Heart J. 1983 Sep;50(3):197–201. doi: 10.1136/hrt.50.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Radda G. K., Gadian D. G., Rocker G., Esiri M., Falconer-Smith J. Examination of a case of suspected McArdle's syndrome by 31P nuclear magnetic resonance. N Engl J Med. 1981 May 28;304(22):1338–1342. doi: 10.1056/NEJM198105283042206. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Fink L., Maris J., Ferraro N., Power-Vanwart J., Eleff S., Chance B. Evaluation of energy metabolism in skeletal muscle of patients with heart failure with gated phosphorus-31 nuclear magnetic resonance. Circulation. 1985 Jan;71(1):57–62. doi: 10.1161/01.cir.71.1.57. [DOI] [PubMed] [Google Scholar]


