Abstract
G protein-coupled receptors (or GPCRs) represent the largest family of membrane proteins in the human genome and are the target of approximately half of all therapeutic drugs. GPCRs contain a conserved structure of seven transmembrane domains. Their amino terminus is located extracellularly, whereas the carboxy terminus extends into the cytoplasm. Accumulating evidence suggests that GPCRs exist and function as monomeric entities. Nevertheless, more recent findings indicate that GPCRs can also form dimers or even higher order oligomers. The differential pharmacological and signaling properties of GPCR heteromeric complexes hint that their physiological effects may be different as compared to those obtained in tissue cultures that express a particular GPCR. In this chapter, we review current data on the role of GPCR heteromerization in receptor signaling, as well as its potential implication in neuropsychiatric disorders such as schizophrenia, depression, and Parkinson’s disease.
1. INTRODUCTION
Among the different proteins found in cell membranes responsible for transmitting information from the extracellular environment into the cytoplasm, there are those ones belonging to the superfamily ofGprotein-coupled receptors (GPCRs).1–4 It has been described in the human genome about 800 members of GPCRs,5,6 and they are the target of nearly half of the drugs currently in use for the treatment of different human diseases.3,7 GPCRs are single polypeptides embedded in the membrane with the amino terminus remaining in the extracellular side and the carboxy terminus in the cytoplasm.8–11 Until recently, GPCRs have been assumed to function as monomers that, upon agonist binding, initiate the activation of heterotrimeric G proteins and consequently modulate the function of intracellular signaling cascades downstream. This is supported by findings that demonstrate functional G protein coupling of a single purified family A GPCR reconstituted into a phospholipid bilayer.12,13 During the past two decades, however, there has been much evidence to suggest that GPCRs exist as dimers and higher order oligomers.14–19 Of particular interest is the demonstration of heteromerization between two GPCRs belonging to different families. These protein complexes have been shown to affect diverse aspects of receptor function.20–24 This chapter reviews some of the latest advances in our understanding of structure and function of GPCR heteromers, and their role in neuropsychiatric disorders such as schizophrenia, depression, and Parkinson’s disease.
2. STRUCTURE OF GPCR HETEROMERS
The three major families of GPCRs include family A (rhodopsin-like), family B (glucagon-related receptors), and family C (metabotropic glutamate (mGlu)-related receptors).1,5,6 Much evidence indicates that family C GPCRs, including GABAB25–31 and mGlu receptors30,32–36, exist and function as constitutive dimers. The GABAB receptor has been shown to be a heterodimer of GABAB-R1 and GABAB-R2, each of which is unable to form a functional GABAB receptor. The domains responsible for GABAB heterodimeric receptor formation are located in the C-termini of both GABAB-R1 and GABAB-R2 receptors. Recent findings also suggest that mGlu receptors are expressed at the plasma membrane as strict dimers, and not higher order oligomers, that are covalently linked through a disulfide bond. In marked contrast, however, family A GPCRs are assembled as homomers and heteromers through structural mechanisms that involve noncovalent interactions between amino acid residues located in transmem-brane (TM) regions. As an example, it has been suggested using biochemical and biophysical approaches such as coimmunoprecipitation of epitope-tagged receptors and fluorescence resonance energy transfer (FRET) that α1b-adrenergic receptors are assembled into oligomeric structures with symmetric contact points involving residues located in TM domains 1 and 414,37–39 (see also Refs. 40 and 41 for reviews about FRET and other techniques based on energy transfer). Expression of α1b-adrenergic receptors as higher order oligomers was suggested based on the use of three-color FRET (3-FRET). Similar findings were obtained with the dopamine D2 receptor. Thus, the combination of bioluminescence/fluorescence complementation and energy transfer provided evidence that dopamine D2 receptors are expressed as higher order oligomers in the plasma membrane.15,38,39 The use of cysteine cross-linking experiments also pointed toward the location of TM1 and TM4 at the symmetrical interfaces of the dopamine D2 receptor oligomers (i.e., TM1–TM1 interface and TM4–TM4 inter-face).15,38,39 The critical role of TM4 in the formation of GPCR complexes is further supported by recent findings with the serotonin 5-HT2A and the metabotropic glutamate 2 (mGlu2) receptors.20,23,24 It was demonstrated that 5-HT2A and mGlu2, but not mGlu3, form a GPCR heteromer in tissue culture. The differences between mGlu2 and mGlu3 receptors to be assembled as a GPCR heteromer with the 5-HT2A receptor provided the rational for the design of mGlu2/mGlu3 chimeras that showed differences in their capacity to be expressed in close proximity with coexpressed 5-HT2A receptors. The authors suggested that three residues located at the intracellular end of TM4 of mGlu2 are necessary to form a GPCR heteromer with the 5-HT2A in HEK293 cells.24 Together, these findings suggest that TM4 and TM1 contribute to the formation of GPCR homo- and heterocomplexes.
Another important question related to structure and function of GPCRs concerns the stability of dimeric/oligomeric states.42 The use of fluorescence recovery after photobleaching (FRAP) has demonstrated transient interactions on a timescale of seconds between the components of the β1-adrenergic receptor homomer. On the contrary, the β2-adrenergic receptor has been shown to be expressed as a stable oligomer in HEK293 cells.43 A dynamic equilibrium between monomers and homodimers/olig-omers has also been recently suggested using FRAP in HEK293 cells expressing dopamine D2 receptors.44 Similarly, the use of total internal reflection fluorescence microscopy in living cells point toward a dynamic nature of muscarinic M1 receptor dimer formation. Thus, muscarinic M1 receptors are expressed as monomers or dimers, but not oligomers. It was also shown that muscarinic M1 receptors do not form constitutive dimers, with ∼30% of the receptor molecules in close proximity at any given time.17
3. ROLE OF GPCR HETEROCOMPLEXES IN NEUROPSYCHIATRIC DISORDERS
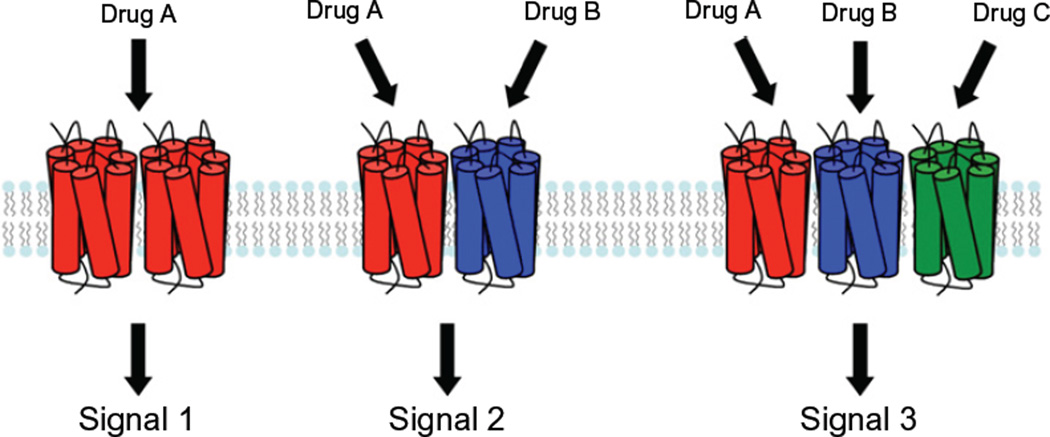
Although is has been clearly established in reconstituted systems that single GPCR molecules are able to couple with and activate heterotrimeric G proteins, recent evidence also demonstrates close molecular proximity between different GPCR molecules that affects functional outcomes such as ligand binding profiles, patterns of G protein coupling, and subcellular location (Fig. 8.1). This has opened in recent years a new field of research that adds complexity to the mechanisms underlying the physiological and behavioral responses induced by signaling pathways downstream GPCRs (Table 8.1). As certain receptor subtypes, such as dopamine, serotonin, glutamate, and adenosine receptors, have been shown to form GPCR heteromers in vitro in tissue culture and in vivo in animal models, a better understanding of their structure, neuroanatomical location, and physiological function may represent a new target for the design of drugs for the treatment of neuropsychiatric disorders such as schizophrenia, depression, suicidal behavior, and drug abuse.45–48 Here, we will review recent findings related to GPCR heteromers and their role in central nervous system (CNS) function.
Figure 8.1.
Schematic model of functional responses induced by GPCR homomers as compared to GPCR heteromers. Combinations of drugs modulate different patterns of signaling responses, and a cross talk between the components of the GPCR heteromeric complex leads to unique signaling properties.
Table 8.1.
Examples of GPCR Heterocomplexes with potential roles in neuropsychiatric disorders
| GPCR heterocomplex |
Experimental approaches | Neuropsychiatric relevance |
|---|---|---|
| A1–A2A | Cell lines, striatum (IP, BRET, FRET) |
Drug tolerance |
| A1–D1 | Cell lines, striatum (IP, BRET, BP) |
Involved in PD and cocaine addiction |
| A1–mGlu1 | Cell lines (IP) | Involved in schizophrenia |
| A1–5HT2A | Cortex (BP) | Involved in schizophrenia |
| A2A–D2 | Cell lines, striatum (IP, BRET, FRET, Phar, BP) |
Reversal of PD deficits, putative antipsychotic, and anticocaine properties |
| A2A–mGlu5 | Cell lines, striatum (IP) | Possible PD, schizophrenia, and cocaine treatment |
| A2A–D2– mGlu5 |
Cell lines, striatum (IP, SRET) |
Possible PD, schizophrenia, and cocaine treatment |
| A2A–CB1 | Cell lines, striatum (IP, BRET, BP) |
|
| A2A–CB1–D2 | Cell lines (BP, SRET) | Involved in schizophrenia |
| α2A–MOR | Cell lines, striatum (IP, BRET, FRET) |
Involved in addiction |
| CB1–D2 | Cell lines, striatum (IP, FRET, Phar, BP) |
Possible PD, addiction, and schizophrenia treatment |
| CRH1–V1b | Cell lines (IP, BRET, FRET) |
Involved in mood disorder, depression |
| D1–D2 | Cell lines, striatum (IP, FRET) |
Possible PD, schizophrenia, and treatment |
| D1–D3 | Cell lines (BRET, FRET, Phar, BP) |
Cocaine addiction |
| D2–D3 | Cell lines, striatum (IP, BP) | Involved in PD and schizophrenia |
| D2–H3 | Cell lines, striatum (BRET, Phar, BP) |
Involved in PD |
| D4–MOR | Striatum (Phar) | Acquisition during drug addiction and risks for drug use initiation |
| mGlu2–5HT2A | Cell lines, cortex (IP, BRET, FRET, Phar, BP) |
Possible schizophrenia treatment |
IP, coimmunoprecipitation; BRET, bioluminescence resonance energy transfer; FRET, fluorescence resonance energy transfer; SRET, sequential resonance energy transfer; Phar, pharmacological allosteric effects in radioligand binding assays; PD, Parkinson’s disease; BP, behavioral paradigms.
4. ADENOSINE AND DOPAMINE RECEPTORS
4.1. Adenosine A1 and dopamine D1 receptors
Expression of adenosine A1 receptor and dopamine D1 receptor as a GPCR heteromer has been demonstrated using approaches such as coimmunoprecipitation, bioluminescence resonance energy transfer (BRET), and FRET.49–53 It has also been shown that adenosine A1 receptor and dopamine D1 receptor are colocalized in soma and dendritic regions of cortical neurons. Selective adenosine A1 receptor antagonists reverse the hyperlocomotor behavioral effects induced by dopamine D1 receptor agonists, and opposite effects were shown after administration of adenosine A1 receptor agonists. These findings suggest adenosine A1 receptor as a potential target to modulate dopamine D1 receptor-dependent signaling. Importantly, suboptimal doses of adenosine A1 receptor antagonists potentiate the therapeutic-like effects of dopamine D1 antagonists in rodent models of Parkinson’s disease.
4.2. Adenosine A2A and dopamine D2 receptors
Kjell Fuxe at the Karolinska Institute and collaborators provided the first evidence of a ligand binding interaction between adenosine A2A and dopamine D2 receptors in rat striatum,54,55 and they suggested intramembrane receptor/receptor interaction as a potential mechanism involved in this pharmacological cross talk. Based on these findings, they demonstrated the existence of adenosine A2A–dopamine D2 receptor heteromers using a variety of approaches such as coimmunoprecipitation, BRET, and FRET in vitro and in tissue culture.56–60 An electrostatic interaction between the intracellular loop 3 of dopamine D2 receptor and the C-terminal tail of adenosine A2A receptor has been proposed as necessary for this protein–protein interaction.60 Behavioral and microdialysis assays in whole animal models suggest the functional role of the adenosine A2A–dopamine D2 receptor heteromer in whole animal models through a mechanism that involves their coexpression in striatopallidal GABAergic neurons and nucleus accumbens.61–72 Thus, adenosine A2A receptor antagonists reduce dopamine D2 receptor-dependent signaling and pharmacological properties,54,73–75 and enhance therapeutic effects in rodent models of Parkinson’s disease.65,76–81 The adenosine A2A receptor antagonist istradefylline (KW-6002) has been used in animal models of Parkinson’s disease.77 Importantly, clinical studies with istradefylline showed a symptomatic improvement in Parkinson’s disease patients.82,83 Bivalent ligands that bind both dopamine D2 and adenosine A2A receptors have also been proposed as a new approach to treat Parkinson’s disease.84
The potential role of the adenosine A2–dopamine D2 receptor heteromer as a target of antipsychotic drugs has been suggested using the hyperlocomotor activity induced by amphetamine and the noncompetitive NMDA receptor antagonist phencyclidine as rodent model of schizophrenia and psychosis.85,86 The adenosine A2A receptor agonist CGS21680 reduces the hyperlocomotor activity induced by amphetamine or phencyclidine.87 The authors provide evidence suggesting that this antipsychotic-like behavioral effect may be mediated by inhibition of dopamine D2 receptor-dependent signaling through the adenosine A2A–dopamine D2 receptor heteromer in striatopallidal GABAergic neurons.88
4.3. Adenosine A2A, dopamine D2, and mGlu5 receptors
In addition to adenosine and dopamine transmission, glutamate transmission plays an important role in the function of striatal GABAergic efferent neurons originating in the nucleus accumbens. In 1984, l-glutamate was found to reduce the affinity of dopamine D2 receptor agonist binding sites in rat striatal membrane preparations, providing the first evidence of the existence of glutamate receptor–dopamine D2 receptor heteromeric complexes.89 It was also shown that adenosine A2A receptor agonists and group I mGlu receptor agonists synergistically reduce the affinity of dopamine D2 receptor agonists binding in striatal membrane preparations.90 In line with these results, adenosine A2A receptor and mGlu5 receptor colocalize in primary cultures of rat striatal neurons91 and in striatal glutamate nerve terminals.92,93 Similarly, adenosine A2A receptors and dopamine D2 receptors colocalize with mGlu5 receptors in rat striatum.90 Additional data showed that adenosine A2A receptor and mGlu5 receptor agonists act synergistically to increase extracellular levels of GABA in the nucleus accumbens, which also potentiate the inhibitory effects of the dopamine D2 receptor.94 In Parkinson’s disease, glutamate transmission is overactive mostly due to the reduced inhibitory effect of the dopamine D2 receptor,95 and anti-parkinsonian drugs regulate functional responses that require adenosine A2A and mGlu5 receptors.96,97
Moreover, recent data suggest that adenosine A2A antagonists and mGlu5 antagonists induce antiparkinsonian-like effects in animal models acting through the adenosine A2A–dopamine D2–mGlu5 receptor oligomer.98 This is further supported by findings showing that the antiparkinsonian-like effects of mGlu5 receptor antagonists need coexpression of adenosine A2A and dopamine D2 receptors.96 Since the intramembrane adenosine A2A–dopamine D2 receptor functional interaction is positively regulated by mGlu5 receptor agonists,90,93,99,100 together, these results suggest that adenosine A2A receptor antagonists and mGlu5 receptor antagonists induce antiparkinsonian-like effects through a mechanism that requires expression of adenosine A2A, mGlu5, and dopamine D2 as a GPCR oligomeric functional unit.98 Based on these findings, the authors proposed the combination of adenosine A2A and mGlu5 receptor antagonists to enhance their antiparkinsonian-like effects in rodent models of motor deficits, and suggested that this nondopaminergic therapy may avoid the adverse effects of dopaminergic drugs such as dyskinesia and cognitive dysfunction.97,101,102
4.4. Adenosine A2A, dopamine D2, and cannabinoid CB1 receptors
The cannabinoid system represents an important inhibitory neuroregulator acting in the CNS.103–107 Cannabinoid CB1 receptors are coexpressed with the dopamine D2 receptor ventral striatopallidal GABAergic neurons, and with the adenosine A2A receptor in corticostriatal glutamatergic terminals.108–114 Activation of cannabinoid CB1 receptors by WIN55,212-2 leads to motor suppression in rodent models.108 Importantly, coimmunoprecipitation and BRET experiments showed that CB1 and adenosine A2A receptors interact together in close molecular proximity in living cells and in rat striatum.115 It was also suggested that this GPCR heteromer is functional since some of the CB1 receptor-dependent locomotor effects were affected in adenosine A2A receptor knockout mice.116 Biochemical and cellular signaling assays in SH-SY5Y neuroglioblastoma cells together with behavioral tests in mice indicated that some of the effects induced by activation of the cannabinoid CB1 receptor in striatum depend upon adenosine A2A receptor-dependent signaling.108,115 Several studies have reported antagonistic interactions between cannabinoid CB1 and dopamine D2 receptors.117–119 Together with coimmunoprecipitation and FRET experiments,46 the reduction of the affinity of dopamine D2 receptor agonists in the presence of cannabinoid CB1 receptor agonists point toward expression of these two receptors as a GPCR heteromer.45 It has also been suggested that the antagonistic interactions between cannabinoid CB1 and dopamine D2 receptors as a GPCR heteromer may also involve the adenosine A2A receptor.120–122 This is supported by the demonstration that adenosine A2A receptors directly interact with both dopamine D256 and cannabinoid CB1115 receptors. The importance of this functional cross talk has been further suggested by recent findings using a method that combined bio-molecular fluorescence complementation (BiFC) and BRET techniques and showed expression of dopamine D2, cannabinoid CB1, and adenosine A2A receptors as a higher order GPCR oligomeric complex in living cells.123–125
In postmortem human brain of schizophrenic subjects, radioligand binding assays showed alterations in expression of adenosine A2A,126 dopamine D2,127 and cannabinoid CB1128 receptors. In rodent models, chronic anti-psychotic treatment with the atypical antipsychotic drug clozapine induces downregulation of cannabinoid CB1 receptor expression in nucleus accumbens,129,130 which has been suggested to be a consequence of a compensatory mechanism that reduces the endocannabinoid-mediated suppression of GABA release.130 Together, these findings suggest that the adenosine A2A–cannabinoid CB1–dopamine D2 receptor heteromer may represent a potential target for new antipsychotic compounds.
4.5. Serotonin and glutamate receptors
Serotonin (5-HT) is one of the most ancient signaling molecules in evolution, and is involved in biological processes such as learning and memory, mood, food intake, sleep, reproduction, circadian rhythm, thermoregulation, pain perception, and social behavior.131,132 Serotonin receptors also play an important role in cardiovascular, gastrointestinal, and endocrine function.132 Glutamate is the primary excitatory neurotransmitter in the CNS.133 Pyramidal neurons represent approximately 80% of the neurons of the cortex, and glutamate serves as the neurotransmitter of the cortical pyramidal cells.134,135 Glutamate receptors are classified either as ion channel receptors (ionotropic) or metabotropic GPCRs.136–138 mGlu receptors have an important function in synaptic modulation throughout the CNS.135
Recent findings demonstrate that 5-HT2A and mGlu2 receptors colocalize at a subcellular level in mouse cortical pyramidal neurons.20,23,24 It has also been shown that these two receptors form a GPCR heteromer in HEK293 cells with consequences on pharmacology, signaling, and behavioral effects of drugs that bind to either 5-HT2A or mGlu2 receptors.20,24 Radioligand binding assays showed that drugs that activate the mGlu2 receptor increase the affinity of hallucinogenic drugs for the 5-HT2A receptor, and that drugs that activate the 5-HT2A receptor decrease the affinity of agonists for the mGlu2 receptor.20 Using ion channels in Xenopus oocytes as markers of Gq/11-dependent and Gi/o-dependent G protein signaling, recent findings demonstrate that both serotonergic and glutamatergic ligands balance the pattern of G protein signaling downstream of the 5-HT2A–mGlu2 receptor heteromer in a way that predicts their anti-psychotic or propsychotic potential, which may provide the basis for the rationale design of new antipsychotic drugs that affect the function of this serotonin–glutamate receptor heteromer.23
The behavioral effects of 5-HT2A and mGlu2 as a GPCR heteromer have been recently demonstrated using hallucinogenic 5-HT2A receptor agonists as a rodent model of psychosis. Hallucinogenic 5-HT2A agonists, such as lysergic acid diethylamide (LSD) and mescaline, induce head-twitch behavior in mice, and this behavior is absent in 5-HT2A receptor knockout mice.139,140 It has been shown that the head-twitch behavioral response is significantly reduced in mGlu2 knockout mice.141 More importantly, viral-mediated overexpression of wild-type mGlu2 receptor in frontal cortex rescues the effects of LSD-like drugs in mGlu2 knockout mice,24,141 and this does not occur by overexpression of mGlu2DTM4N, a construct that is unable to form a GPCR heteromer with the 5-HT2A receptor.24 These observations suggest that it is 5-HT2A–mGlu2 receptor complex, and not the 5-HT2A alone, which is the molecular target responsible for psychoactive-like behavioral effects of LSD-like hallucinogenic drugs in mouse models of pychosis.23,24,141
Expression of the components of the 5-HT2A–mGlu2 receptor complex has been shown to be dysregulated (i.e., increased 5-HT2A receptor and decreased mGlu2 receptor) in postmortem human brain of antipsychotic-free schizophrenic subjects.20 More recent findings provide evidence that the allosteric binding cross talk between 5-HT2A receptor and mGlu2 receptor as a GPCR heterocomplex is upregulated in postmortem schizophrenia brain.24,142 It is then possible that 5-HT2A–mGlu2 receptor complex-dependent signaling effects may integrate serotonin and glutamate signaling, and therefore contribute to the abnormalities of thought and behavior in schizophrenia patients.
5. CONCLUSION
Understanding the structure and function of GPCR heteromers is essential for the discovery of mechanisms that define the signaling cross talk between the components of these protein complexes. Given that a large number of GPCRs are expressed in individual cells at any given time, further work is needed to better unravel the molecular rules that govern GPCR heteromeric formation, as well as the stability and lifetime of such structural units. Here, we have focused our attention on the potential role of GPCR heteromers in the treatment of neuropsychiatric disorders such as schizophrenia and Parkinson’s disease. Despite these advances, further research efforts will focus on a better understanding of the structure, pharmacology, and behavioral function of GPCR heteromers in vitro and in animal models, with the final goal of developing new therapeutic drugs that specifically affect their function.
Acknowledgments
We thank the National Institutes of Health for grant support of our GPCR research.
REFERENCES
- 1.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 2.Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and muta-genesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Perez DM. The evolutionarily triumphant G-protein-coupled receptor. Mol Pharmacol. 2003;63:1202–1205. doi: 10.1124/mol.63.6.1202. [DOI] [PubMed] [Google Scholar]
- 5.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families Phylogenetic analysis, para-logon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 6.Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, et al. International Union of Pharmacology XLVI G protein-coupled receptor list. Pharmacol Rev. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- 7.Kim Ka, von Zastrow M. Old drugs learn new tricks: insights from mammalian trace amine receptors. Mol Pharmacol. 2001;60:1165–1167. doi: 10.1124/mol.60.6.1165. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, et al. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469:236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whorton MR, Jastrzebska B, Park PS, Fotiadis D, Engel A, Palczewski K, et al. Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem. 2008;283:4387–4394. doi: 10.1074/jbc.M703346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Gimenez JF, Canals M, Pediani JD, Milligan G. The alpha1b–adrenoceptor exists as a higher-order oligomer: effective oligomerization is required for receptor maturation, surface delivery, and function. Mol Pharmacol. 2007;71:1015–1029. doi: 10.1124/mol.106.033035. [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008;27:2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung JJ, Deupi X, Pardo L, Yao XJ, Velez-Ruiz GA, Devree BT, et al. Ligand-regulated oligomerization of beta(2)-adrenoceptors in a model lipid bilayer. EMBO J. 2009;28:3315–3328. doi: 10.1038/emboj.2009.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JE, Lazareno S, et al. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc Natl Acad Sci USA. 2010;107:2693–2698. doi: 10.1073/pnas.0907915107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prezeau L, Rives ML, Comps-Agrar L, Maurel D, Kniazeff J, Pin JP. Functional crosstalk between GPCRs: with or without oligomerization. Curr Opin Pharmacol. 2010;10:6–13. doi: 10.1016/j.coph.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha2A–adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 22.Urizar E, Yano H, Kolster R, Gales C, Lambert N, Javitch JA. CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol. 2011;7:624–630. doi: 10.1038/nchembio.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, et al. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuellar F, et al. Identification of three residues essential for 5-HT2A–mGlu2 receptor heteromerization and its psychoactive behavioral function. J Biol Chem. 2012;287:44301–44319. doi: 10.1074/jbc.M112.413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comps-Agrar L, Kniazeff J, Norskov-Lauritsen L, Maurel D, Gassmann M, Gregor N, et al. The oligomeric state sets GABA(B) receptor signalling efficacy. EMBO J. 2011;30:2336–2349. doi: 10.1038/emboj.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, et al. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 27.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 28.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 29.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA (B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 30.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 31.Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, et al. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuji Y, Shimada Y, Takeshita T, Kajimura N, Nomura S, Sekiyama N, et al. Cryptic dimer interface and domain organization of the extracellular region of metabotropic glutamate receptor subtype 1. J Biol Chem. 2000;275:28144–28151. doi: 10.1074/jbc.M003226200. [DOI] [PubMed] [Google Scholar]
- 33.El Moustaine D, Granier S, Doumazane E, Scholler P, Rahmeh R, Bron P, et al. Distinct roles of metabotropic glutamate receptor dimerization in agonist activation and G-protein coupling. Proc Natl Acad Sci USA. 2012;109:16342–16347. doi: 10.1073/pnas.1205838109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, et al. Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 2009;28:2195–2208. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods. 2008;5:561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol. 2004;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- 37.Carrillo JJ, Lopez-Gimenez JF, Milligan G. Multiple interactions between transmembrane helices generate the oligomeric alpha1b–adrenoceptor. Mol Pharmacol. 2004;66:1123–1137. doi: 10.1124/mol.104.001586. [DOI] [PubMed] [Google Scholar]
- 38.Guo W, Shi L, Filizola M, Weinstein H, Javitch JA. Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci USA. 2005;102:17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- 40.James JR, Oliveira MI, Carmo AM, Iaboni A, Davis SJ. A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat Methods. 2006;3:1001–1006. doi: 10.1038/nmeth978. [DOI] [PubMed] [Google Scholar]
- 41.Johnsson K. Visualizing biochemical activities in living cells. Nat Chem Biol. 2009;5:63–65. doi: 10.1038/nchembio0209-63. [DOI] [PubMed] [Google Scholar]
- 42.Vilardaga JP, Agnati LF, Fuxe K, Ciruela F. G-protein-coupled receptor heteromer dynamics. J Cell Sci. 2010;123:4215–4220. doi: 10.1242/jcs.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorsch S, Klotz KN, Engelhardt S, Lohse MJ, Bunemann M. Analysis of receptor oligomerization by FRAP microscopy. Nat Methods. 2009;6:225–230. doi: 10.1038/nmeth.1304. [DOI] [PubMed] [Google Scholar]
- 44.Fonseca JM, Lambert NA. Instability of a class a G protein-coupled receptor oligomer interface. Mol Pharmacol. 2009;75:1296–1299. doi: 10.1124/mol.108.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albizu L, Moreno JL, Gonzalez-Maeso J, Sealfon SC. Heteromerization of G protein-coupled receptors: relevance to neurological disorders and neurotherapeutics. CNS Neurol Disord Drug Targets. 2010;9:636–650. doi: 10.2174/187152710793361586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuxe K, Marcellino D, Rivera A, Diaz-Cabiale Z, Filip M, Gago B, et al. Receptor-receptor interactions within receptor mosaics Impact on neuropsychopharmacology. Brain Res Rev. 2008;58:415–452. doi: 10.1016/j.brainresrev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Fuxe K, Borroto-Escuela DO, Marcellino D, Romero-Fernandez W, Frankowska M, Guidolin D, et al. GPCR heteromers and their allosteric receptor-receptor interactions. Curr Med Chem. 2012;19:356–363. doi: 10.2174/092986712803414259. [DOI] [PubMed] [Google Scholar]
- 48.Moreno JL, Sealfon SC, Gonzalez-Maeso J. Group II metabotropic glutamate receptors and schizophrenia. Cell Mol Life Sci. 2009;66:3777–3785. doi: 10.1007/s00018-009-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gines S, Hillion J, Torvinen M, Le Crom S, Casado V, Canela EI, et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferre S, Ciruela F, Quiroz C, Lujan R, Popoli P, Cunha RA, et al. Adenosine receptor heteromers and their integrative role in striatal function. Scientific World Journal. 2007;7:74–85. doi: 10.1100/tsw.2007.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franco R, Casado V, Cortes A, Mallol J, Ciruela F, Ferre S, et al. G-protein-coupled receptor heteromers: function and ligand pharmacology. Br J Pharmacol. 2008;153(Suppl. 1):S90–S98. doi: 10.1038/sj.bjp.0707571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salamone JD, Farrar AM, Font L, Patel V, Schlar DE, Nunes EJ, et al. Differential actions of adenosine A1 and A2A antagonists on the effort-related effects of dopamine D2 antagonism. Behav Brain Res. 2009;201:216–222. doi: 10.1016/j.bbr.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schindler M, Harris CA, Hayes B, Papotti M, Humphrey PP. Immunohistochemical localization of adenosine A1 receptors in human brain regions. Neurosci Lett. 2001;297:211–215. doi: 10.1016/s0304-3940(00)01643-8. [DOI] [PubMed] [Google Scholar]
- 54.Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA. 1991;88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferre S, Popoli P, Gimenez-Llort L, Finnman UB, Martinez E, Scotti de Carolis A, et al. Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport. 1994;6:73–76. doi: 10.1097/00001756-199412300-00020. [DOI] [PubMed] [Google Scholar]
- 56.Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, et al. Adenosine A2A–dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- 57.Torvinen M, Kozell LB, Neve KA, Agnati LF, Fuxe K. Biochemical identification of the dopamine D2 receptor domains interacting with the adenosine A2A receptor. J Mol Neurosci. 2004;24:173–180. doi: 10.1385/JMN:24:2:173. [DOI] [PubMed] [Google Scholar]
- 58.Navarro G, Aymerich MS, Marcellino D, Cortes A, Casado V, Mallol J, et al. Interactions between calmodulin, adenosine A2A, and dopamine D2 receptors. J Biol Chem. 2009;284:28058–28068. doi: 10.1074/jbc.M109.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- 60.Ciruela F, Burgueno J, Casado V, Canals M, Marcellino D, Goldberg SR, et al. Combining mass spectrometry pull-down techniques for the study of receptor heteromerization Direct epitope-epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal Chem. 2004;76:5354–5363. doi: 10.1021/ac049295f. [DOI] [PubMed] [Google Scholar]
- 61.Gluck MR, Santana LA, Granson H, Yahr MD. Novel dopamine releasing response of an anti-convulsant agent with possible anti-Parkinson’s activity. J Neural Transm. 2004;111:713–724. doi: 10.1007/s00702-004-0107-1. [DOI] [PubMed] [Google Scholar]
- 62.Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 63.Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- 64.Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, et al. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci. 2008;28:2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varty GB, Hodgson RA, Pond AJ, Grzelak ME, Parker EM, Hunter JC. The effects of adenosine A2A receptor antagonists on haloperidol-induced movement disorders in primates. Psychopharmacology (Berl) 2008;200:393–401. doi: 10.1007/s00213-008-1214-8. [DOI] [PubMed] [Google Scholar]
- 67.El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. Adenosine A2A receptor knockout mice are partially protected against drug-induced catalepsy. Neuroreport. 2001;12:983–986. doi: 10.1097/00001756-200104170-00024. [DOI] [PubMed] [Google Scholar]
- 68.Li W, Dai S, An J, Xiong R, Li P, Chen X, et al. Genetic inactivation of adenosine A2A receptors attenuates acute traumatic brain injury in the mouse cortical impact model. Exp Neurol. 2009;215:69–76. doi: 10.1016/j.expneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Matsuya T, Takuma K, Sato K, Asai M, Murakami Y, Miyoshi S, et al. Synergistic effects of adenosine A2A antagonist and L-DOPA on rotational behaviors in 6-hydroxydopamine-induced hemi-Parkinsonian mouse model. J Pharmacol Sci. 2007;103:329–332. doi: 10.1254/jphs.scz070058. [DOI] [PubMed] [Google Scholar]
- 70.Mori A, Shindou T. Modulation of GABAergic transmission in the striatopallidal system by adenosine A2A receptors: a potential mechanism for the antiparkinsonian effects of A2A antagonists. Neurology. 2003;61:S44–S48. doi: 10.1212/01.wnl.0000095211.71092.a0. [DOI] [PubMed] [Google Scholar]
- 71.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perier C, Marin C, Bonastre M, Tolosa E, Hirsch EC. AMPA receptor antagonist LY293558 reverses preproenkephalin mRNA overexpression in the striatum of 6-OHDA-lesioned-rats treated with L-dopa. Eur J Neurosci. 2002;16:2236–2240. doi: 10.1046/j.1460-9568.2002.02275.x. [DOI] [PubMed] [Google Scholar]
- 73.Dasgupta S, Ferre S, Kull B, Hedlund PB, Finnman UB, Ahlberg S, et al. Adenosine A2A receptors modulate the binding characteristics of dopamine D2 receptors in stably cotransfected fibroblast cells. Eur J Pharmacol. 1996;316:325–331. doi: 10.1016/s0014-2999(96)00665-6. [DOI] [PubMed] [Google Scholar]
- 74.Salim H, Ferre S, Dalal A, Peterfreund RA, Fuxe K, Vincent JD, et al. Activation of adenosine A1 and A2A receptors modulates dopamine D2 receptor-induced responses in stably transfected human neuroblastoma cells. J Neurochem. 2000;74:432–439. doi: 10.1046/j.1471-4159.2000.0740432.x. [DOI] [PubMed] [Google Scholar]
- 75.Kull B, Ferre S, Arslan G, Svenningsson P, Fuxe K, Owman C, et al. Reciprocal interactions between adenosine A2A and dopamine D2 receptors in Chinese hamster ovary cells co-transfected with the two receptors. Biochem Pharmacol. 1999;58:1035–1045. doi: 10.1016/s0006-2952(99)00184-7. [DOI] [PubMed] [Google Scholar]
- 76.Kim DS, Palmiter RD. Adenosine receptor blockade reverses hypophagia and enhances locomotor activity of dopamine-deficient mice. Proc Natl Acad Sci USA. 2003;100:1346–1351. doi: 10.1073/pnas.252753799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chase TN, Bibbiani F, Bara-Jimenez W, Dimitrova T, Oh-Lee JD. Translating A2A antagonist KW6002 from animal models to parkinsonian patients. Neurology. 2003;61:S107–S111. doi: 10.1212/01.wnl.0000095223.08711.48. [DOI] [PubMed] [Google Scholar]
- 78.Fuxe K, Marcellino D, Genedani S, Agnati L. Adenosine A(2A) receptors, dopamine D(2) receptors and their interactions in Parkinson’s disease. Mov Disord. 2007;22:1990–2017. doi: 10.1002/mds.21440. [DOI] [PubMed] [Google Scholar]
- 79.Jenner P. Istradefylline, a novel adenosine A2A receptor antagonist, for the treatment of Parkinson’s disease. Expert Opin Investig Drugs. 2005;14:729–738. doi: 10.1517/13543784.14.6.729. [DOI] [PubMed] [Google Scholar]
- 80.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Shiozaki S, Ichikawa S, Nakamura J, Kitamura S, Yamada K, Kuwana Y. Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hyp-okinesia caused by reserpine or MPTP. Psychopharmacology (Berl) 1999;147:90–95. doi: 10.1007/s002130051146. [DOI] [PubMed] [Google Scholar]
- 82.Bara-Jimenez W, Sherzai A, Dimitrova T, Favit A, Bibbiani F, Gillespie M, et al. Adenosine A(2A) receptor antagonist treatment of Parkinson’s disease. Neurology. 2003;61:293–296. doi: 10.1212/01.wnl.0000073136.00548.d4. [DOI] [PubMed] [Google Scholar]
- 83.Hauser RA, Hubble JP, Truong DD. Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology. 2003;61:297–303. doi: 10.1212/01.wnl.0000081227.84197.0b. [DOI] [PubMed] [Google Scholar]
- 84.Soriano A, Ventura R, Molero A, Hoen R, Casado V, Cortes A, et al. Adenosine A2A receptor-antagonist/dopamine D2 receptor-agonist bivalent ligands as pharmacological tools to detect A2A–D2 receptor heteromers. J Med Chem. 2009;52:5590–5602. doi: 10.1021/jm900298c. [DOI] [PubMed] [Google Scholar]
- 85.Rimondini R, Ferre S, Ogren SO, Fuxe K. Adenosine A2A agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacology. 1997;17:82–91. doi: 10.1016/S0893-133X(97)00033-X. [DOI] [PubMed] [Google Scholar]
- 86.Malec D, Poleszak E. Involvement of adenosine receptors in dizocilpine-induced motor activity in mice. Pharmacol Rep. 2006;58:101–106. [PubMed] [Google Scholar]
- 87.Sills TL, Azampanah A, Fletcher PJ. The adenosine A2A agonist CGS 21680 reverses the reduction in prepulse inhibition of the acoustic startle response induced by phencyclidine, but not by apomorphine and amphetamine. Psychopharmacology (Berl) 2001;156:187–193. doi: 10.1007/s002130100777. [DOI] [PubMed] [Google Scholar]
- 88.Ferre S. Adenosine-dopamine interactions in the ventral striatum Implications for the treatment of schizophrenia. Psychopharmacology (Berl) 1997;133:107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- 89.Fuxe K, Celani MF, Martire M, Zini I, Zoli M, Agnati LF. L-Glutamate reduces the affinity of [3H]N-propylnorapomorphine binding sites in striatal membranes. Eur J Pharmacol. 1984;100:127–130. doi: 10.1016/0014-2999(84)90326-1. [DOI] [PubMed] [Google Scholar]
- 90.Ferre S, Popoli P, Rimondini R, Reggio R, Kehr J, Fuxe K. Adenosine A2A and group I metabotropic glutamate receptors synergistically modulate the binding characteristics of dopamine D2 receptors in the rat striatum. Neuropharmacology. 1999;38:129–140. doi: 10.1016/s0028-3908(98)00154-3. [DOI] [PubMed] [Google Scholar]
- 91.Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, et al. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61:S19–S23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- 92.Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA. Co-localization and functional interaction between adenosine A(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem. 2005;92:433–441. doi: 10.1111/j.1471-4159.2004.02887.x. [DOI] [PubMed] [Google Scholar]
- 93.Ferre S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci USA. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diaz-Cabiale Z, Vivo M, Del Arco A, O’Connor WT, Harte MK, Muller CE, et al. Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci Lett. 2002;324:154–158. doi: 10.1016/s0304-3940(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 95.Lindsley CW, Hopkins CR. Metabotropic glutamate receptor 4 (mGlu4)-positive allosteric modulators for the treatment of Parkinson’s disease: historical perspective and review of the patent literature. Expert Opin Ther Pat. 2012;22:461–481. doi: 10.1517/13543776.2012.679437. [DOI] [PubMed] [Google Scholar]
- 96.Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25:10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coccurello R, Breysse N, Amalric M. Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing Parkinso-nian deficits in rats. Neuropsychopharmacology. 2004;29:1451–1461. doi: 10.1038/sj.npp.1300444. [DOI] [PubMed] [Google Scholar]
- 98.Cabello N, Gandia J, Bertarelli DC, Watanabe M, Lluis C, Franco R, et al. Meta-botropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J Neurochem. 2009;109:1497–1507. doi: 10.1111/j.1471-4159.2009.06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Popoli P, Pezzola A, Torvinen M, Reggio R, Pintor A, Scarchilli L, et al. The selective mGlu(5) receptor agonist CHPG inhibits quinpirole-induced turning in 6-hydroxydopamine-lesioned rats and modulates the binding characteristics of dopamine D(2) receptors in the rat striatum: interactions with adenosine A(2a) receptors. Neuropsychopharmacology. 2001;25:505–513. doi: 10.1016/S0893-133X(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 100.Nishi A, Liu F, Matsuyama S, Hamada M, Higashi H, Nairn AC, et al. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci USA. 2003;100:1322–1327. doi: 10.1073/pnas.0237126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marino MJ, Valenti O, Conn PJ. Glutamate receptors and Parkinson’s disease: opportunities for intervention. Drugs Aging. 2003;20:377–397. doi: 10.2165/00002512-200320050-00006. [DOI] [PubMed] [Google Scholar]
- 102.Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA. Heterodimerization of alpha 2A- and beta 1-adrenergic receptors. J Biol Chem. 2003;278:10770–10777. doi: 10.1074/jbc.M207968200. [DOI] [PubMed] [Google Scholar]
- 103.Ashton JC, Appleton I, Darlington CL, Smith PF. Immunohistochemical localization of cerebrovascular cannabinoid CB1 receptor protein. J Cardiovasc Pharmacol. 2004;44:517–519. doi: 10.1097/00005344-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, et al. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci. 2005;25:2530–2536. doi: 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 106.Smith TH, Sim-Selley LJ, Selley DE. Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery? Br J Pharmacol. 2010;160:454–466. doi: 10.1111/j.1476-5381.2010.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aguado T, Romero E, Monory K, Palazuelos J, Sendtner M, Marsicano G, et al. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J Biol Chem. 2007;282:23892–23898. doi: 10.1074/jbc.M700678200. [DOI] [PubMed] [Google Scholar]
- 108.Tebano MT, Martire A, Chiodi V, Pepponi R, Ferrante A, Domenici MR, et al. Adenosine A2A receptors enable the synaptic effects of cannabinoid CB1 receptors in the rodent striatum. J Neurochem. 2009;110:1921–1930. doi: 10.1111/j.1471-4159.2009.06282.x. [DOI] [PubMed] [Google Scholar]
- 109.Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci USA. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, et al. Neuroanatomical relationship between type 1 cannabinoid receptors and dopa-minergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–318. doi: 10.1016/s0306-4522(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 112.Fusco FR, Martorana A, Giampa C, De March Z, Farini D, D’Angelo V, et al. Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse. 2004;53:159–167. doi: 10.1002/syn.20047. [DOI] [PubMed] [Google Scholar]
- 113.Kofalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, et al. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 115.Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, et al. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- 116.Soria G, Castane A, Berrendero F, Ledent C, Parmentier M, Maldonado R, et al. Adenosine A2A receptors are involved in physical dependence and place conditioning induced by THC. Eur J Neurosci. 2004;20:2203–2213. doi: 10.1111/j.1460-9568.2004.03682.x. [DOI] [PubMed] [Google Scholar]
- 117.Meschler JP, Howlett AC. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology. 2001;40:918–926. doi: 10.1016/s0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 118.Jarrahian A, Watts VJ, Barker EL. D2 dopamine receptors modulate Galpha-subunit coupling of the CB1 cannabinoid receptor. J Pharmacol Exp Ther. 2004;308:880–886. doi: 10.1124/jpet.103.057620. [DOI] [PubMed] [Google Scholar]
- 119.Martin AB, Fernandez-Espejo E, Ferrer B, Gorriti MA, Bilbao A, Navarro M, et al. Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology. 2008;33:1667–1679. doi: 10.1038/sj.npp.1301558. [DOI] [PubMed] [Google Scholar]
- 120.Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- 121.Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 122.Ferre S, Goldberg SR, Lluis C, Franco R. Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology. 2009;56(Suppl. 1):226–234. doi: 10.1016/j.neuropharm.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Navarro G, Carriba P, Gandia J, Ciruela F, Casado V, Cortes A, et al. Detection of heteromers formed by cannabinoid CB1, dopamine D2, and adenosine A2A G-protein-coupled receptors by combining bimolecular fluorescence complementation and bioluminescence energy transfer. Scientific World Journal. 2008;8:1088–1097. doi: 10.1100/tsw.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carriba P, Navarro G, Ciruela F, Ferre S, Casado V, Agnati L, et al. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods. 2008;5:727–733. doi: 10.1038/nmeth.1229. [DOI] [PubMed] [Google Scholar]
- 125.Kerppola TK. Bimolecular fluorescence complementation: visualization of molecular interactions in living cells. Methods Cell Biol. 2008;85:431–470. doi: 10.1016/S0091-679X(08)85019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deckert J, Brenner M, Durany N, Zochling R, Paulus W, Ransmayr G, et al. Up-regulation of striatal adenosine A(2A) receptors in schizophrenia. Neuroreport. 2003;14:313–316. doi: 10.1097/00001756-200303030-00003. [DOI] [PubMed] [Google Scholar]
- 127.Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- 128.Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 129.Sundram S, Copolov D, Dean B. Clozapine decreases [3H] CP 55940 binding to the cannabinoid 1 receptor in the rat nucleus accumbens. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:428–433. doi: 10.1007/s00210-005-1074-2. [DOI] [PubMed] [Google Scholar]
- 130.Uriguen L, Garcia-Fuster MJ, Callado LF, Morentin B, La Harpe R, Casado V, et al. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology (Berl) 2009;206:313–324. doi: 10.1007/s00213-009-1608-2. [DOI] [PubMed] [Google Scholar]
- 131.Kriegebaum C, Gutknecht L, Schmitt A, Lesch KP, Reif A. Serotonin now: part 1 Neurobiology and developmental genetics. Fortschr Neurol Psychiatr. 2010;78:319–331. doi: 10.1055/s-0029-1245240. [DOI] [PubMed] [Google Scholar]
- 132.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 133.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 134.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 135.Benarroch EE. Metabotropic glutamate receptors: synaptic modulators and therapeutic targets for neurologic disease. Neurology. 2008;70:964–968. doi: 10.1212/01.wnl.0000306315.03021.2a. [DOI] [PubMed] [Google Scholar]
- 136.Schoepp DD, Conn PJ. Metabotropic glutamate receptors. Pharmacol Biochem Behav. 2002;74:255–256. doi: 10.1016/s0091-3057(02)00953-x. [DOI] [PubMed] [Google Scholar]
- 137.Jingami H, Nakanishi S, Morikawa K. Structure of the metabotropic glutamate receptor. Curr Opin Neurobiol. 2003;13:271–278. doi: 10.1016/s0959-4388(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 138.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 139.Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 141.Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493:76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Muguruza C, Moreno JL, Umali A, Callado LF, Meana JJ, Gonzalez-Maeso J. Dysregulated 5-HT2A receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.10.006. http://dx.doi.org/10.1016/j.euroneuro.2012.10.006 [Epub ahead of print] [DOI] [PMC free article] [PubMed]