Abstract
The stage of brain development at the time of stroke has a major impact on the pathophysiological mechanisms of ischemic damage, including the neuroinflammatory response. Microglial cells have been shown to contribute to acute and sub-chronic injury in adult stroke models, whereas in neonatal rodents we showed that microglial cells serve as endogenous neuroprotectants early following transient middle cerebral artery occlusion (tMCAO), limiting neuroinflammation and injury. In the neonate, microglial depletion or lack of the scavenger receptor CD36 exacerbates injury. In this study we asked if lack of CD36 affects microglial phenotypes after neonatal stroke. Using RT-PCR we characterized the patterns of gene expression in microglia isolated from injured regions following acute tMCAO in postnatal day 10 mice and showed that expression of several pro-inflammatory genes, including Toll-like receptors (TLR), remains largely unaffected in activated microglia in injured regions. Using multiple biochemical assays we demonstrated that lack of CD36 alters several functions of microglia in acutely injured neonatal brain: it further enhances accumulation of the chemokine MCP-1, affects the number of CD11b+/CD45+ cells, along with protein expression of its co-receptor, TLR2, but does not affect accumulation of superoxide in microglia or the cytokines TNFα and IL-1β in injured regions.
Keywords: Middle cerebral artery, Toll-like receptor 2 (TLR2), Gene expression, inflammation, neonate
Introduction
It has become apparent over the last decade that inflammation is not as one-dimensional or necessarily detrimental after brain injury, as was traditionally thought (Iadecola and Anrather, 2011, Vexler and Yenari, 2009). Microglial cells are major contributors to neuroinflammation. In response to injury they can acquire diverse context-dependent phenotypes; they can become classically activated (so called M1 phenotype) and produce cytotoxic effects or acquire a supportive phenotype to promote brain repair (so called M2a phenotype). They can also acquire an immunoregulatory phenotype (so called M2b phenotype) (Schwartz et al., 2006). In adult stroke, microglia traditionally were considered purely toxic, but microglial diversity is being increasingly recognized. Data are emerging that microglia can play both injurious (Monje et al., 2003, Iosif et al., 2006) and beneficial (Faustino et al., 2011) role after stroke.
There is now ample evidence that the mechanisms of ischemic injury differ greatly between immature brain and adult brain (Yager and Ashwal, 2009, Fernandez-Lopez et al., 2014). We recently demonstrated that microglia in fact play a neuroprotective role in the acute and sub-chronic injury phases after neonatal stroke by showing that microglial depletion exacerbates injury and further enhances production of inflammatory cytokines and chemokines induced by stroke (Faustino et al., 2011). It remains largely unknown how microglia protect an injured neonatal brain and whether the entire population or a subpopulation of microglia posses endogenously proprotective functions.
The scavenger receptor CD36 exerts multiple cell type-specific and ligand-specific functions (Silverstein et al., 2010, Abe et al., 2010) and is central to several aspects of microglia/macrophage function, including migratory activity of these cells, engulfment and removal of neuronal debris and production of inflammatory mediators (Helming et al., 2009, Hoosdally et al., 2009). The effects of CD36 are context-dependent in injured adult brain, injurious in the experimental setting of Alzheimer’s disease and adult stroke (Rahaman et al., 2006, Silverstein et al., 2010, Cho et al., 2005) but beneficial following intracerebral hemorrhage, by facilitating hematoma resolution by mediating PPARg-dependent phagocytosis of red blood cells by microglia (Zhao et al., 2007). We showed that genetic deletion of CD36 enhances ischemic injury in neonatal brain, in part by diminishing phagocytotic activity of microglia after acute transient middle cerebral artery occlusion (tMCAO) (Woo et al., 2012). However, the extent of CD36-mediated effects on microglial functions in injured immature brain remains poorly understood. Therefore, we asked if the microglial phenotypes are predominantly cytotoxic or protective in neonatal mice after tMCAO and whether CD36 alters these microglial phenotypes.
Materials and Methods
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco. Every effort was made to minimize animal suffering. Mothers were housed in a temperature- and light-controlled facility and given food and water.
Transient MCAO was performed using the Derugin model in postnatal day 9 (P9)-P10 C57BL/6 wild type (WT; purchased from Charles River) mice and CD36 knockout mice on C57BL/6 background (CD36ko), as we previously described (Woo et al., 2012). Briefly, a midline cervical incision was made under isoflurane anesthesia, the common carotid artery and internal carotid artery (ICA) exposed, single threads from a 7-0 silk suture used to temporary tie a knot below the origin of the ICA to prevent retrograde bleeding from the arteriotomy. A coated 8-0 nylon suture was advanced 4–5mm and removed three hours later. Temperature was maintained.
Microglial cells were isolated from mice perfused with ice-cold Ca2+/Mg2+ free Hanks’ BSS. Injured and matching contralateral regions were dissected on ice, enzymatically digested using Neural Tissue Dissociation Kit, myelin was removed using myelin-conjugated magnetic beads, myelin-free cell fraction centrifuged, resuspended in 10μl CD11b beads/90μl degassed buffer and incubated at 4°C for 15min. Cells bound to CD11b+-conjugated magnetic beads were separated using MS column (Miltenyi Biotec).
RT-PCR
Total RNA (1μg) was extracted (RNase Mini kit, Qiagen) from 2×105 CD11b+ cells. Reverse transcription was performed with HotStart Taq® Plus DNA Polymerase kit (Qiagen) and PCR was carried out using Bio-rad iCycler. PCR products were verified by 2% agarose gel stained with EtBr. Quantitative RT-PCR was performed using a Biosystem 7900HT Sequence Detection System and FastStart universal SYBR Green Master (ROS) kit (Roche Diagnostics). Gene expression was quantified using a modification of the 2−ΔΔCT method (Livak and Schmittgen, 2001). Primer sequences were designed using “Primer 5” software and primer BLAST sequences by NCBI: mCD68 (forward [F]: tagcccaaggaacagaggaa; reverse [R]: ggagctggtgtgaactgtga), mCd36, transcript variant 3 (Gene ID: 12491; F: gagcaactggtggatggttt; R: gcagcagaatcaagggagag), nitric oxide synthase 2, inducible, minos (Gene ID: 18126; F: ctctgacagcccagagttcc; R: aggcaaaggaggagaaggag), arginase 1, mArg1 (F: cgcctttctcaaaaggacag; R: ccagctcttcattggctttc), mCd163 (F: catgtgggtagatcgtgtgc; R: tgtatgcccttcctggagtc), Toll like receptor (TLR) 2, mTlr2 (Gene ID: 24088; F: ctcccacttcaggctctttg; R: acccaaaacacttcctgctg), TLR3, mTlr3 (Gene ID: 142980; F: cgccctcttcgtaacttgac; R: cccgactgggatttcatcta), TLR4, mTlr4 (Gene ID: 21898; F: gccggaaggttattgtggta; R: aggcgatacaattccacctg), soluble3, mLgals3, transcript variant 1 (Gene ID: 16854; F: cctggttgaagctgaccact; R: ttcccactcctaaggcacac), GAPDH (F: acccagaagactgtggatgg; R: acacattgggggtaggaaca).
Flow Cytometry
Single-cell myelin-free suspensions from contralateral and injured regions were plated (5 ×105/96 well), centrifuged, pellet resuspended in 100ul blocking buffer containing CD16/32 (1:70, Biolegend) and incubated in 150ul FACs staining buffer containing 2% FBS.
Cells were fixed (Fixation and Permeabilization kit, BD Bioscience), incubated with antibody mixture on ice for 20min, washed, centrifuged, resuspended in staining buffer and evaluated on BD LSRII flow cytometer (BD Biosciences). The following combinations of antibodies diluted 1:200 in FACS staining buffer were used: anti-CD45-Pacific Blue (Biolegend)/CD11b-APC-Cy7 (Biolegend)/CD36-Alexab647 (Biolegend)/Mac-2(Gal-3)-Alexa 488 (Biolegend). Alternatively, a combination of anti-CD45-Pacific Blue/CD11b-APC-Cy7/TRL2-PE (eBioscience)/Ly6C-APC (eBioscience) was used.
Compensation beads (BD CompBeads) were incubated in Fixation and Permeabilization solution (100ul, 4°C, 20min), incubated with antibody mixture (4°C, 30 min) and resuspended in staining buffer. Gating and data analysis were performed using FlowJo software (Tree Star). Live cell scatter, the percentage of CD45low/CD45medium/CD45high, CD11b+ and TLR2+ were analyzed.
Multiplex cytokine/chemokine assay (IL-1β, TNFα, IL-10, MCP-1, MIP-1α and KC) was performed in lysates from injured/corresponding contralateral regions using LINCOplexTM mouse cytokine kit (LINCO/Millipore), Luminex100 reader (Luminex) and StatLIA® software (Brendan Scientific) with a 5-parameter logistic curve fitting, as described (Faustino et al., 2011). The results were normalized to protein concentration in individual lysates.
Superoxide production was determined by immunofluorescence in perfusion-fixated brains following administration of a cell-permeable dye, dihydroethidium (DHE; 5 mg/kg, i.p. 3 hours before sacrifice). Cell identity was determined by Ox-DHE+ co-localization with Iba1+/Ib4+/Glut-1+, as we described (Woo et al., 2012).
Statistical Analysis
ANOVA with post-hoc Bonferroni multiple comparisons test was used to compare individual variables between contralateral and injured regions within and between WT and CD36ko groups. Differences were considered significant at p<0.05. Results are shown as mean ± SD.
Results
Transient MCAO in neonatal mice affects gene expression of both pro- and anti-inflammatory genes
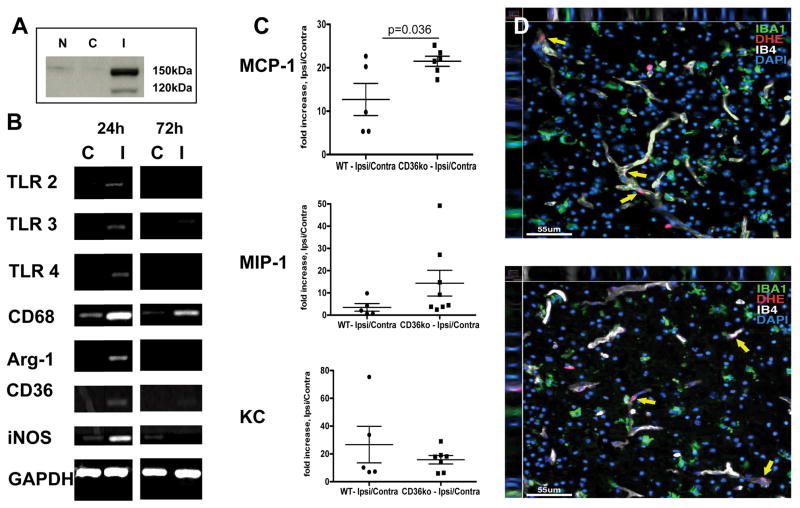
In the neonatal rat tMCAO model we previously showed that microglia contribute to endogenous protection (Faustino et al., 2011). Here, in the mouse tMCAO model, we determined expression of pro-inflammatory and anti-inflammatory genes in whole tissue lysates of WT mice with injury confirmed by the presence of spectrin cleavage (Figure 1A). Qualitatively, gene expression of CD68, CD36, iNOS and arginase-1 was increased but increase of TLR2/3/4 gene expression was subtle in injured region (Figure 1B).
Figure 1. tMCAO affects gene expression of both pro- and anti-inflammatory genes and chemokine accumulation.
A. The presence of spectrin cleavage in injured (I) regions as compared to naïve (N) or contralateral (C) regions was used to select injured mice. B. Representative RT-PCR examples of pro- and anti-inflammatory genes in whole tissue lysates. C. Fold increase in the levels of individual chemokines 24 hours after reperfusion. Significance is shown for CD36ko Vs. WT. N – naïve, C – contralateral, I – injured. C. Representative examples of superoxide accumulation (DHE, Red) in WT (top) and CD36ko (bottom) 24 hours after reperfusion. Yellow arrows point at DHE+ cells. No significant increase in superoxide accumulation is seen in Iba1+ cells (green).
Genetic deletion of CD36 affects chemokine accumulation in injured neonatal brain
We previously demonstrated that lack of CD36 reduces phagocytosis of neurons undergoing caspase-3-dependent death after acute neonatal tMCAO (Woo et al., 2012). Considering that inability to remove apoptotic debris after injury can enhance neuroinflammation, we asked if the levels of inflammatory cytokines and chemokines are affected in injured CD36ko mice.
In WT mice the levels of chemokines MCP-1, MIP-1α and KC were significantly increased in injured regions 24 hours after reperfusion (Figure 1C) whereas levels of TNFα remained below detectible levels in almost all samples in both hemispheres and the levels of IL-1β were unchanged by the injury (not shown). In CD36ko mice, injury induced an additional significant increase in MCP-1 but MIP-1α and KC levels were not significantly increased (Figure 1C). No changes were observed in IL-1β levels. TNFα levels remained below detectible levels. There was no significant increase in superoxide levels in Iba1+-microglia either in WT or CD36ko (Figure 1D), distinct from reported superoxide accumulation in microglia following tMCAO in adult mice (Cho et al., 2005) and in our experiments (not shown).
Injury induces pro- and anti-inflammatory genes in microglia
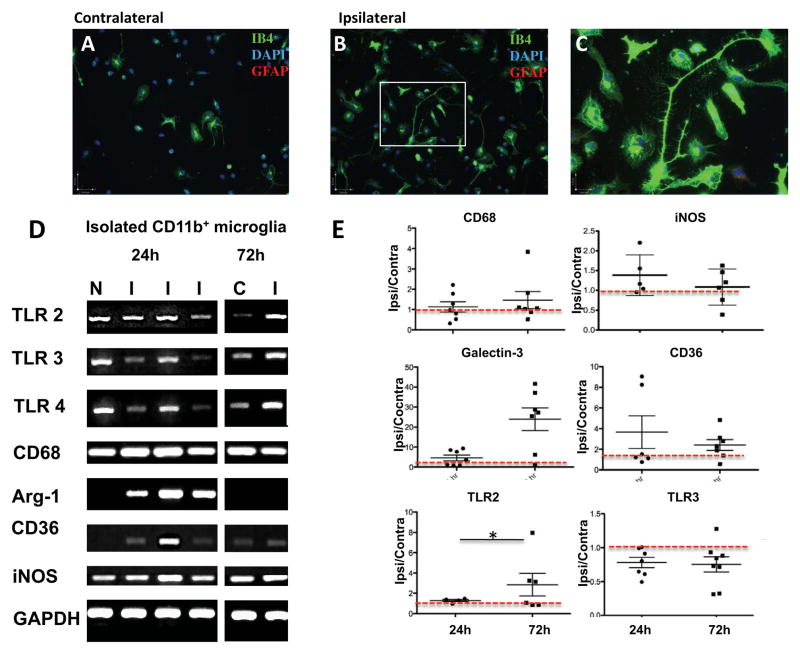
Various cell types can increase cytokine expression following tMCAO in neonates (Faustino et al., 2011). To determine the contribution of activated microglia in production of inflammatory mediators we isolated CD11b+ cells from acutely injured brains. Characterization of the yield and purity of isolated CD11b+ cells in WT mice showed that compared to that in contralateral hemisphere, in injured regions the yield of CD11b+ cells was significantly increased 24 hours (2.2±0.6 fold, n=7) and 72 hours (3.1±2.2 fold, n=8) after reperfusion. We plated isolated CD11b+ cells and established that the majority of plated cells remain viable (live/dead assay) and that based on co-labeling with cell-type specific markers for neurons (NeuN) and astrocytes (GFAP), there are no NeuN+ cells; microglia are >99% pure 24 hours after plating. A range of morphologies was observed in microglia from contralateral and injured regions and, as expected, cells from injured regions were larger, with frequent presence of filopodia and lamelopodia (Figure 2A–C). While plated at the same densities, microglial number was significantly higher in injured than in contralateral regions (1.46±0.45 fold), demonstrating that isolated cells retain features of activation.
Figure 2. tMCAO changes gene expression of pro- and anti-inflammatory mediators in microglia within injured regions.
A–C. Representative examples of microglial cells isolated 24 hours after tMCAO using CD11b-conjugated magnetic beads and plated on coverslips for 24 hours. Ib4+ cells from contralateral (A) and injured region (B). C. Magnified image in B (white squire). Note the differing morphology of cells from injured and uninjured brain, including the presence of cells with lamellipodia and filopodia. D. Representative RT-PCR examples of pro- and anti-inflammatory genes in isolated microglia. E. Quantification of RT-PCR data in microglia isolated 24 and 72 hours after tMCAO. Data shown as mean±SD for fold change in injured compared to matching contralateral regions. Red dotted line outlines the level in contralateral region. N – naïve, C – contralateral, I – ipsilateral. * - p<0.05.
Injury affected expression of pro-inflammatory and anti-inflammatory genes in CD11b+-microglia isolated from WT pups. Compared to contralateral regions, at 24 hours, CD36 signal varied between individual mice, Arg-1 was markedly increased whereas iNOS gene was unchanged in microglia from injured regions (Figure 2D). TLR3/4 signals were either unaffected or decreased in microglia in individual mice while TLR2 signal remained unchanged (Figure 2D). Quantitative RT-PCR 24 hours after reperfusion demonstrated that compared to contralateral hemisphere, there were no significant changes in TLR2, TLR4, arginase-1, iNOS, CD36 and CD163 gene expression while galectin-3 expression was significantly increased. At 72 hours, gene expression of most antigens, including TLR2, significantly increased while expression of TLR3/4 was significantly decreased (Figure 2E).
CD36 deletion affects microglial activation 24 hours after tMCAO
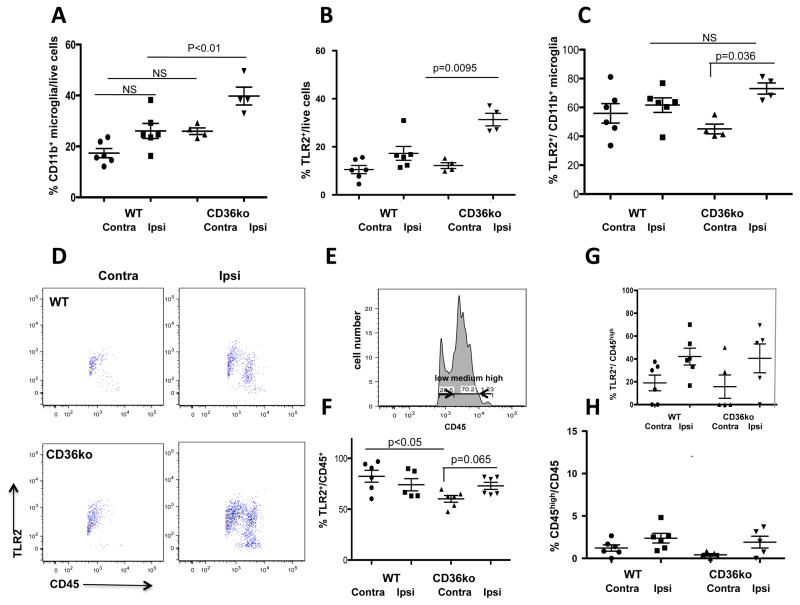
We then determined how lack of CD36 affects the pattern of CD11b+ and CD45+ accumulation after injury. Considering that CD36 acts as a co-receptor for TLR heterodimers (Stewart et al., 2010) and CD36/TLR2 signaling mediates injury in adult stroke (Abe et al., 2010), we tested whether lack of CD36 affects TLR2 expression.
In tissue lysates pre-cleared with myelin magnetic beads, the relative number of CD11b+ cells was significantly higher in injured CD36ko mice (Figure 3A). Lack of CD36 increased the number cells expressing one of its partner receptors, TLR2 (Figure 3B) and the number of CD11b+/TLR2+ cells (Figure 3C). Expression of a microglial antigen with no known direct interactions with CD36, Gal-3, was unaffected by lack of CD36 (not shown). CD11b+ upregulation was associated with a shift toward CD45 acquisition, from CD45low to CD45medium (Figure 3D–E). Percent of CD45+ cells was significantly increased in injured regions of WT mice (8.6±1.1% Vs. 21.1±3.3%, respectively), and even further in CD36ko mice (15.4±1.8%Vs. 37.7±4.6% in injured regions, significantly higher in injured CD36ko mice; n=4–6 per group) but only less than 5% of cells in injured regions were CD45high (Figure 3H). CD45high cells expressed TLR2 but the relative number of CD45high/TLR2+ cells was similar in injured WT and CD36ko mice (Figure 3G).
Figure 3. Genetic depletion of CD36 alters TLR2 expression and microglial phenotypes after tMCAO.
A–C. Lack of CD36 affects acquisition of CD11b+ cells (A) and TLR2+ cells (B) and TLR2+/CD11b+ cells (C). D. Representative dot plots for TLR2+/CD45+ cells from injured and contralateral regions of WT and CD36ko. E. Representative example of identification of CD45low/medium/high. F–H. Quantification of relative numbers of TLR2+/CD45+ (F), TLR2+/CD45high (G) and CD45high/CD45+ cells (H). Ipsi–ipsilateral; Contra–contralateral, NS–non-significant. The levels of significance are indicated on individual graphs.
Discussion
In this very first study focused on how neonatal focal arterial stroke changes microglial phenotypes, we report a largely unchanged expression of several inflammatory genes, but increased gene expression of regulatory molecules in microglial cells isolated from acutely injured neonatal brains, a pattern consistent with a neuroprotective function of activated microglia. We also demonstrate that lack of CD36 affects activation of several individual aspects of microglia in injured regions and dysregulates protein expression of one of CD36 co-partner receptors, the TLR2 receptor.
Historically, in adult stroke, microglia were viewed as purely injurious (reviewed in (Vexler and Yenari, 2009)) but limited tools did not allow discrimination of the effects of microglial cells from those of monocytes. The diversity of microglial phenotypes was not sufficiently understood. It is now clear that the origin of microglia and monocytes is different (Ginhoux et al., 2010) and that the cell origin of cytokine production, microglia or monocytes, may have opposite effects in stroke (Lambertsen et al., 2009). In vitro studies demonstrated that microglia acquire stimuli-specific phenotypes (Chhor et al., 2013). Recent studies have demonstrated that microglial phenotypes undergo marked changes during late embryonic and postnatal brain development (Butovsky et al., 2014) and that microglia have unique features in normally developing brain, playing a key role in controlling synaptic pruning and the formation of precise synaptic circuits (Paolicelli et al., 2011, Derecki et al., 2012). However, microglial phenotypes are insufficiently characterized in the normally developing brain and are unknown after neonatal stroke.
To characterize changes in microglial gene expression after injury we chose several antigens that represent cytotoxic (iNOS), alternatively activated (arginase-1), and phagocytotic and regulatory microglia (CD36, CD163). Knowing that CD36-mediated ligand recognition and binding to multiple TLRs is the initial step in TLR activation (Stewart et al., 2010), that CD36-dependent TLR2 signaling modifies injury in adult stroke (Abe et al., 2010) and that TLRs play a major role in brain injury in neonates following hypoxia-ischemia or infection (Mallard et al., 2009), we examined expression of TLRs. While TLR4 seems purely injurious in stroke (Kilic et al., 2008), the role of TLR2 is more complex. It can elicit context-dependent effects— injurious (Abe et al., 2010) or beneficial (Rolls et al., 2007, Hua et al., 2009)—based on the type(s) of heterodimers that it forms with other TLRs. In a hypoxia-ischemia model in neonates, TLR2 agonist does not affect injury, whereas genetic TLR2 deletion decreases infarct volume (Stridh et al., 2011). TLR2/3/4 gene expression was not increased in microglia isolated from acutely injured regions 24 hours after tMCAO. Quantitative RT-PCR showed no significant changes for TLR2/3/4 and iNOS at that point. A variable extent of increase in gene and protein CD36 expression was evident 24–72 hours after injury. These data are consistent with the notion of the overall non-cytotoxic nature of activated microglia in post-ischemic neonatal brain (Faustino et al., 2011). Increased proliferation of acutely isolated plated microglia also suggests non-cytotoxic microglial phenotypes in our study, as proliferating microglia protect against adult stroke (Lalancette-Hebert et al., 2007).
We previously showed that after neonatal stroke microglia protect through at least two mechanisms, removal of apoptotic debris and controlling neuroinflammation (Faustino et al., 2011). The contribution of apoptotic neuronal death is markedly higher in ischemia-related injury in neonates than in adults (Hu et al., 2000) and an inability to remove debris by microglia enhances inflammation and exacerbates injury (Faustino et al., 2011). CD36 participates in several phagocytotic steps, including recognition, engulfment and processing of apoptotic cells (Stolzing and Grune, 2004, Lucas et al., 2006). Therefore, it is not surprising that phagocytotic activity of microglia is diminished in CD36ko after neonatal tMCAO, contributing to more severe injury in CD36ko (Woo et al., 2012). Another important distinction in the microglial response is that in adult neurodegenerative models CD36 toxicity is mediated via robust superoxide production in microglia/macrophages (Cho et al., 2005, Park et al., 2011, Abe et al., 2010, Rahaman et al.) but superoxide production is not increased nearly to the same extent in activated microglia in neonatal WT mice after acute tMCAO. Consistent with our previous observations that lack of CD36 increases the number and affects the size of Iba1+ microglia in acutely injured neonatal brain (Woo et al., 2012), we demonstrate by flow cytometry that the number of CD11b+ microglia is significantly higher in injured CD36ko. However, the number of CD11b+/CD45high+ cells, monocytes, remains relatively low regardless of CD36 deletion. Importantly, the number of TLR2+ cells is increased in CD36ko mice, indicating enhanced CD36-independent TLR2 signaling, but changes are not due to CD45high+ cells.
The observed CD36-dependent effects may not fully depend on lack of CD36 in microglia because CD36 is expressed in several cell types, including endothelial cells, and signals via multiple ligands and in cooperation with other receptors. Lack of CD36 signaling in other cells may ultimately activate microglia. While effects of CD36 in mediating cell-cell interactions would require further investigation, our results show that altered receptor interactions in microglial cells affect the modes of microglial activation and injury after neonatal stroke.
Acknowledgments
The authors acknowledge Dr. Xiangning Jiang for useful discussions of PCR results. RO1 NS44025 (ZV), RO1 NS76726 (ZV), Leducq foundation (ZV), NSFC 31260242 (FL).
List of abbreviations
- DHE
dihydroethidium
- tMCAO
transient middle cerebral artery occlusion
- TLR2
Toll-like receptor 2
- WT
wild type
- KO
knockout
Footnotes
Disclosures: Dr. Vexler consults Omniox, Inc.
References
- ABE T, SHIMAMURA M, JACKMAN K, KURINAMI H, ANRATHER J, ZHOU P, IADECOLA C. Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke. 2010;41:898–904. doi: 10.1161/STROKEAHA.109.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTOVSKY O, JEDRYCHOWSKI MP, MOORE CS, CIALIC R, LANSER AJ, GABRIELY G, KOEGLSPERGER T, DAKE B, WU PM, DOYKAN CE, FANEK Z, LIU L, CHEN Z, ROTHSTEIN JD, RANSOHOFF RM, GYGI SP, ANTEL JP, WEINER HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–43. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHHOR V, LE CHARPENTIER T, LEBON S, ORE MV, CELADOR IL, JOSSERAND J, DEGOS V, JACOTOT E, HAGBERG H, SAVMAN K, MALLARD C, GRESSENS P, FLEISS B. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHO S, PARK EM, FEBBRAIO M, ANRATHER J, PARK L, RACCHUMI G, SILVERSTEIN RL, IADECOLA C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25:2504–12. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERECKI NC, CRONK JC, LU Z, XU E, ABBOTT SB, GUYENET PG, KIPNIS J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–9. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAUSTINO J, WANG X, JONHSON C, KLIBANOV A, DERUGIN N, WENDLAND M, VEXLER ZS. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-LOPEZ D, NATARAJAN N, ASHWAL S, VEXLER ZS. Mechanisms of perinatal arterial ischemic stroke. J Cereb Blood Flow Metab. 2014;34:921–32. doi: 10.1038/jcbfm.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINHOUX F, GRETER M, LEBOEUF M, NANDI S, SEE P, GOKHAN S, MEHLER MF, CONWAY SJ, NG LG, STANLEY ER, SAMOKHVALOV IM, MERAD M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELMING L, WINTER J, GORDON S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J Cell Sci. 2009;122:453–9. doi: 10.1242/jcs.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOOSDALLY SJ, ANDRESS EJ, WOODING C, MARTIN CA, LINTON KJ. The Human Scavenger Receptor CD36: glycosylation status and its role in trafficking and function. J Biol Chem. 2009;284:16277–88. doi: 10.1074/jbc.M109.007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU BR, LIU CL, OUYANG Y, BLOMGREN K, SIESJO BK. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- HUA F, MA J, HA T, KELLEY JL, KAO RL, SCHWEITZER JB, KALBFLEISCH JH, WILLIAMS DL, LI C. Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. 2009;1262:100–8. doi: 10.1016/j.brainres.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IADECOLA C, ANRATHER J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOSIF RE, EKDAHL CT, AHLENIUS H, PRONK CJ, BONDE S, KOKAIA Z, JACOBSEN SE, LINDVALL O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–12. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILIC U, KILIC E, MATTER CM, BASSETTI CL, HERMANN DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- LALANCETTE-HEBERT M, GOWING G, SIMARD A, WENG YC, KRIZ J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBERTSEN KL, CLAUSEN BH, BABCOCK AA, GREGERSEN R, FENGER C, NIELSEN HH, HAUGAARD LS, WIRENFELDT M, NIELSEN M, DAGNAES-HANSEN F, BLUETHMANN H, FAERGEMAN NJ, MELDGAARD M, DEIERBORG T, FINSEN B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–30. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVAK KJ, SCHMITTGEN TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- LUCAS M, STUART LM, ZHANG A, HODIVALA-DILKE K, FEBBRAIO M, SILVERSTEIN R, SAVILL J, LACY-HULBERT A. Requirements for apoptotic cell contact in regulation of macrophage responses. J Immunol. 2006;177:4047–54. doi: 10.4049/jimmunol.177.6.4047. [DOI] [PubMed] [Google Scholar]
- MALLARD C, WANG X, HAGBERG H. The role of Toll-like receptors in perinatal brain injury. Clin Perinatol. 2009;36:763–72. v–vi. doi: 10.1016/j.clp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- MONJE ML, TODA H, PALMER TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- PAOLICELLI RC, BOLASCO G, PAGANI F, MAGGI L, SCIANNI M, PANZANELLI P, GIUSTETTO M, FERREIRA TA, GUIDUCCI E, DUMAS L, RAGOZZINO D, GROSS CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- PARK L, WANG G, ZHOU P, ZHOU J, PITSTICK R, PREVITI ML, YOUNKIN L, YOUNKIN SG, VAN NOSTRAND WE, CHO S, ANRATHER J, CARLSON GA, IADECOLA C. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci U S A. 2011;108:5063–8. doi: 10.1073/pnas.1015413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAHAMAN SO, LENNON DJ, FEBBRAIO M, PODREZ EA, HAZEN SL, SILVERSTEIN RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–21. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROLLS A, SHECHTER R, LONDON A, ZIV Y, RONEN A, LEVY R, SCHWARTZ M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–8. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M, BUTOVSKY O, BRUCK W, HANISCH UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN RL, LI W, PARK YM, RAHAMAN SO. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc. 2010;121:206–20. [PMC free article] [PubMed] [Google Scholar]
- STEWART CR, STUART LM, WILKINSON K, VAN GILS JM, DENG J, HALLE A, RAYNER KJ, BOYER L, ZHONG R, FRAZIER WA, LACY-HULBERT A, EL KHOURY J, GOLENBOCK DT, MOORE KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLZING A, GRUNE T. Neuronal apoptotic bodies: phagocytosis and degradation by primary microglial cells. Faseb J. 2004;18:743–5. doi: 10.1096/fj.03-0374fje. [DOI] [PubMed] [Google Scholar]
- STRIDH L, SMITH PL, NAYLOR AS, WANG X, MALLARD C. Regulation of Toll-like receptor 1 and -2 in neonatal mice brains after hypoxia-ischemia. J Neuroinflammation. 2011;8:45. doi: 10.1186/1742-2094-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEXLER ZS, YENARI MA. Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci. 2009;31:378–93. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOO MS, WANG X, FAUSTINO J, DERUGIN N, WENDLAND MF, ZHOU P, IADECOLA C, VEXLER ZS. Genetic deletion of CD36 enhances injury after acute neonatal stroke. Ann Neurol. 2012;72:961–970. doi: 10.1002/ana.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAGER JY, ASHWAL S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40:156–67. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- ZHAO X, SUN G, ZHANG J, STRONG R, SONG W, GONZALES N, GROTTA JC, ARONOWSKI J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–62. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]