Abstract
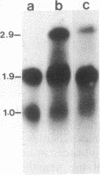
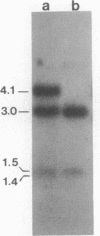
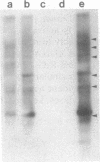
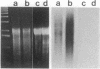
In Drosophila miranda, females have two X1 and two evolving X2 chromosomes, and males have one of each of these two X chromosomes and a Y chromosome. In males, the homologue of the X2 chromosome, the neo-Y chromosome, is attached to the Y chromosome and is under the process of degenerative evolution. We have examined a developmentally regulated X2/neo-Y chromosome-linked gene, 549mr, of D. miranda and found that the neo-Y chromosome-linked copy of this gene (549mr-NY) contains an insertional DNA. We discovered that sequences similar to those in the insertional DNA are present in multiple copies in the genome of both sexes of D. miranda but are more abundant in the males. The insertional DNA also identified a 1.1-kilobase BamHI repeat that is present in at least 6-fold excess in the male genome as compared to the female. This BamHI repeat and similar DNA sequences are predominantly concentrated on the evolving neo-Y chromosome, but very few are found on the homologous X2 and other chromosomes. The BamHI repeat also hybridizes with 2.0- and 1.8-kb RNAs and many other RNA species, which together are also approximately 6-fold greater in males. No sequences similar to the BamHI repeat are found in Drosophila melanogaster. Moreover, the BamHI repeat is not homologous to P, copia, or other D. melanogaster transposable elements. This repeat, named the NY element, may be involved in gene disruption and the process of degenerative evolution of the neo-Y chromosome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charlesworth B. The evolution of sex chromosomes. Science. 1991 Mar 1;251(4997):1030–1033. doi: 10.1126/science.1998119. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., Wilson S. H. Enzymes for modifying and labeling DNA and RNA. Methods Enzymol. 1987;152:94–110. doi: 10.1016/0076-6879(87)52013-4. [DOI] [PubMed] [Google Scholar]
- Isobe T., Ichimura T., Sunaya T., Okuyama T., Takahashi N., Kuwano R., Takahashi Y. Distinct forms of the protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. J Mol Biol. 1991 Jan 5;217(1):125–132. doi: 10.1016/0022-2836(91)90616-e. [DOI] [PubMed] [Google Scholar]
- Krishnan R., Swanson K. D., Ganguly R. Dosage compensation of a retina-specific gene in Drosophila miranda. Chromosoma. 1991 Feb;100(2):125–133. doi: 10.1007/BF00418246. [DOI] [PubMed] [Google Scholar]
- Levy L. S., Ganguly R., Ganguly N., Manning J. E. The selection, expression, and organization of a set of head-specific genes in Drosophila. Dev Biol. 1982 Dec;94(2):451–464. doi: 10.1016/0012-1606(82)90362-1. [DOI] [PubMed] [Google Scholar]
- Levy L. S., Manning J. E. Expression of a set of head-specific genes during Drosophila development. Dev Biol. 1982 Dec;94(2):465–476. doi: 10.1016/0012-1606(82)90363-3. [DOI] [PubMed] [Google Scholar]
- Lucchesi J. C. Gene dosage compensation and the evolution of sex chromosomes. Science. 1978 Nov 17;202(4369):711–716. doi: 10.1126/science.715437. [DOI] [PubMed] [Google Scholar]
- Lucchesi J. C., Manning J. E. Gene dosage compensation in Drosophila melanogaster. Adv Genet. 1987;24:371–429. doi: 10.1016/s0065-2660(08)60013-9. [DOI] [PubMed] [Google Scholar]
- Rice W. R. Genetic hitchhiking and the evolution of reduced genetic activity of the Y sex chromosome. Genetics. 1987 May;116(1):161–167. doi: 10.1093/genetics/116.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Brorein W. J., Jr, Dunsmuir P., Flavell A. J., Levis R., Strobel E., Toole J. J., Young E. Copia-like transposable elements in the Drosophila genome. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):619–628. doi: 10.1101/sqb.1981.045.01.080. [DOI] [PubMed] [Google Scholar]
- Steinemann M. Multiple sex chromosomes in Drosophila miranda: a system to study the degeneration of a chromosome. Chromosoma. 1982;86(1):59–76. doi: 10.1007/BF00330730. [DOI] [PubMed] [Google Scholar]
- Strobel E., Dunsmuir P., Rubin G. M. Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):429–439. doi: 10.1016/0092-8674(79)90169-7. [DOI] [PubMed] [Google Scholar]
- Strobel E., Pelling C., Arnheim N. Incomplete dosage compensation in an evolving Drosophila sex chromosome. Proc Natl Acad Sci U S A. 1978 Feb;75(2):931–935. doi: 10.1073/pnas.75.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. W., Schwartz H. E. Nomadic gene families in Drosophila. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):629–640. doi: 10.1101/sqb.1981.045.01.081. [DOI] [PubMed] [Google Scholar]