Abstract
Objective
This study aims to evaluate associations between variations in genes involved in the metabolism of environmental chemicals and steroid hormones and risk of menopause in smokers.
Methods
Survival analysis was performed on 410 eligible participants from the Penn Ovarian Aging study (ongoing for 14 years), a cohort study of late-reproductive-age women. Single nucleotide polymorphisms at the following loci were studied: COMT Val158Met, CYP1B1*4 Asn452Ser, CYP1B1*3 Leu432Val, and CYP3A4*1B.
Results
Significant interactions between smoking and single nucleotide polymorphisms were observed in European-American carriers of CYP3A4*1B and CYP1B1*3, supporting a greater risk of menopause entry compared with those not carrying these alleles. Among CYP1B1*3 carriers, smokers had a greater risk of menopause entry than nonsmokers (adjusted hazard ratio [HR], 2.26; 95% CI, 1.4–3.67; median time to menopause, 10.42 and 11.07 y, respectively). No association between smoking and menopause was identified in CYP1B1 wild types. Among CYP3A4*1B carriers, smokers were at greater risk for menopause entry than nonsmokers (adjusted HR, 15.1; 95% CI, 3.31–69.2; median time to menopause, 11.36 and 13.91 y, respectively). Risk of menopause entry in CYP3A4 wild types who smoked was far lower (adjusted HR, 1.59; 95% CI, 1.03–2.44). Heavily smoking CYP1B1*3 carriers (adjusted HR, 3.0; 95% CI, 1.54–5.84; median time to menopause, 10.41 y) and heavily smoking CYP3A4*1B carriers (adjusted HR, 17.79; 95% CI, 3.21–98.65; median time to menopause, 5.09 y) had the greatest risk of menopause entry.
Conclusions
Our finding that the risk of menopause entry in European-American smokers varies depending on genetic background represents a novel gene-environment interaction in reproductive aging.
Keywords: Menopause, Early menopause, Smoking, Polyaromatic hydrocarbons, Gene-environment interaction
Natural menopause is a sentinel event in the female life course that marks the cessation of reproductive functioning and impacts the risks of several serious conditions in maturing women. The onset of menopause is considered a marker of overall aging and health that is associated with risks of coronary artery disease, osteoporosis, and all-cause mortality.1–3 The reported typical menopause age of approximately 51 years has been highly reproduced in series across time, both domestically and globally.4–7 Those entering menopause earlier than the age of 45 years experience higher-than-average menopause-related disease risks and mortality.8–10
More than 20 million women in the United States currently smoke—a figure that represents nearly 18% of the adult female population.11 Globally, it is estimated that more than 200 million women are smokers.12 Smoking has repeatedly been identified as a risk factor for earlier time to menopause (TTM), hastening its onset by approximately 1 to 2 years.4–7,13–18 Of the more than 4,000 chemicals found in tobacco smoke, a subset contributes to the development of hypoestrogenism through mechanisms that include oxidative metabolism of estrogens and inhibition of aromatase.19–21 In addition, polyaromatic hydrocarbons (PAHs) are a powerful class of carcinogens in cigarettes that are cytotoxic to both murine and human oocytes in vitro.14,22–26 Many PAHs are protoxins that require bioactivation by enzymes in the cytochrome P450 (CYP) superfamily to exert their toxic effects.27,28
A prevailing theory of reproductive senescence is that women are born with a complete complement of oocytes, which can never be replenished,29 and that chemicals in tobacco that hasten the natural trajectory of oocyte atresia increase the risk of menopause entry and hasten TTM. Factors that enhance the toxicity of these chemicals could increase the risk of menopause further. A comprehensive assessment of potential modifiers of the risk of smoking in relation to menopause timing is currently lacking in the literature. Of great importance is that smoking and menopause have overlapping negative health consequences that could worsen health outcomes in aging women.30
We have recently described that the effects of smoking on the prevalence and severity of hot flashes vary according to the presence of specific single nucleotide polymorphisms (SNPs) in genes responsible for the metabolism of sex steroids and/or the bioactivation of PAHs.31 In the current investigation, we hypothesized that genetic variation in these pathways is associated with further acceleration of onset of menopause in smokers. To test this hypothesis, we examined the risk of menopause in smokers from a population-based cohort of reproductive-age women and evaluated whether the risk of menopause entry in smokers was modified by the presence of relevant gene variants. A candidate gene approach was used to select variants that (1) could potentiate the effects of tobacco-related ovotoxins such as PAHs through mechanisms such as increased bioactivation (CYP1B1 and CYP3A4) and/or (2) had a known association with menopause onset or menopausal symptoms (COMT, CYP1B1, and CYP3A4).32–34
METHODS
Study cohort
Participants from the Penn Ovarian Aging study, a longitudinal population-based study of hormonal changes and symptoms in late-reproductive-age women, were studied. Eligibility criteria at enrollment included the following: aged 35 to 47 years, menstrual cycles within the reference range (22–35 d) for 3 months before enrollment, intact uterus, and at least one ovary. Exclusion criteria included use of hormonal or psychotropic medications, hysterectomy, pregnancy or lactation, serious health problems known to compromise ovarian function, and drug or alcohol abuse in the year before enrollment. The study was approved by the institutional review board of the University of Pennsylvania, and written informed consent form was obtained from all participants. The cohort, which has been described in detail elsewhere,35,36 recruited 436 women by random digit dialing and stratified enrollment to achieve balance in the proportions of women by race (218 African Americans and 218 European Americans). Clinical data for this report were collected from 410 participants for whom genetic data were available at baseline.
Data collection
During each study assessment period, two visits were conducted, each occurring between days 2 and 6 of two consecutive menstrual cycles. At each visit, a trained research coordinator conducted a standardized interview to collect data such as self-reported race and smoking behaviors, collected blood samples, and measured height and weight to determine body mass index. Smoking categories included current smoker, nonsmoker, never smoker, former smoker (no smoking in the last 6 mo), light current smoker (less than one pack daily), and heavy current smoker (at least one pack daily).
Clinical measures
The primary outcome variable was TTM, which was measured in years from the first study assessment (when all participants were premenopausal) to the first follow-up assessment when the participants reported no menstrual bleeding for at least 12 months. The point 1 year before 12 months of no menstrual bleeding was defined as menopause.
Laboratory measures
Nonfasting blood samples for hormone assays were collected between days 2 and 6 of the menstrual cycle in two consecutive cycles during each assessment period. The samples were centrifuged and frozen in aliquots at −80°C. Baseline antimüllerian hormone (AMH) was measured as a marker of ovarian reserve. Among participants (n = 290) with detectable baseline AMH and at least one additional measure one or more years later, the decline in AMH during follow-up (AMH slope) was calculated. AMH was measured using enzyme-linked immunosorbent assay kits (Beckman Coulter Inc, Brea, CA). The intra-assay and interassay coefficients of variation were 4.6% and 6.8%, respectively. The lower limit of detection was 0.10 ng/mL.
DNA was extracted from peripheral blood leukocytes using the QIAamp 96 DNA Buccal Swab BioRobot Kit and the 9604 BioRobot (QIAGEN Inc, Valencia, CA) and amplified by polymerase chain reaction. Genotypes were determined using previously described methods.37,38 Four functionally relevant SNPs in three genes were selected for the study. The SNPs included the following: COMT Val158Met (rs4680), CYP1B1*4 Asn452Ser (rs1800440), CYP1B1*3 (Leu432Val, rs1056836), and CYP3A4*1B (rs2740574). To identify potential deviations from the Hardy-Weinberg equilibrium, we calculated exact tests of expected genotype proportions in each racial group.
Statistical analysis
Comparisons of continuous data were analyzed using Mann-Whitney U test or Kruskal-Wallis test, as appropriate. Categorical data were analyzed using χ2 test. Survival analysis was performed using multivariable Cox proportional hazards models to evaluate the risk of menopause during the 14-year follow-up period.
From the proportional hazards analysis, we generated adjusted hazard ratios (HRs) that estimated the risk of reaching menopause based on smoking behavior after controlling for baseline age and educational attainment (proportion with high school education or less); models were further stratified by race. Each SNP was evaluated separately in multivariable models. For each SNP tested, likelihood ratio tests were used to determine whether the association between smoking and menopause onset was modified by genetics. Linear combinations of coefficients from the models with interaction terms permitted assessment of the adjusted HRs for menopause in smokers versus nonsmokers in the strata of each variant (carrier vs noncarrier).
Proportionality of hazards was evaluated by plots of transformed hazard estimates and by calculation of Schoenfeld residuals.39,40 No violations of modeling assumptions were observed. Interactions between smoking and two of the SNPs investigated (CYP1B1*3 and CYP3A4*1B) were significant for menopause onset in European Americans only. The findings from the Cox proportional hazards models with these SNPs are the focus of the reported results.
AMH levels were reported as geometric means. The two hormone values obtained at baseline were averaged for each participant, with the mean of the two hormone measurements for that assessment period used in the analysis. In cases where the two hormone values were not obtained, the single value was used as the mean. The rate of decline in log-transformed AMH was calculated from the initial level to the time to the first observed undetectable level. Linear regression was used to compare AMH slope according to baseline smoking behavior. Associations are reported as a ratio of geometric means (relative risk; exposed to unexposed).
Wilcoxon rank sum tests and nonparametric tests for trend were used to compare AMH slope according to baseline smoking behavior and strata of SNPs in the genes CYP1B1 and CYP3A4. Statistical analyses were performed using STATA 12 software (STATA Corp, College Station, TX).
RESULTS
Baseline demographic and clinical data on the cohort are shown in Table 1. More than 30% of the group reported current smoking; of these, nearly 13% smoked heavily. The smoking behaviors of the cohort did not significantly differ across racial groups. Fifty-one percent of participants (212) in the cohort entered menopause during study follow-up; in those women, the median age of menopause was 51.2 years, and mean age was 50.9 years.
TABLE 1.
Baseline demographics
| Variable | All participants (N = 410) | European Americans (n = 205) | African Americans (n = 205) | P (race comparisons) |
|---|---|---|---|---|
| Smoking history, n (%) | 0.10 | |||
| Current smoker | 157 (32.7) | 70 (34.2) | 87 (42.2) | |
| Former smoker | 119 (29.8) | 68 (33.2) | 51 (42.9) | |
| Never smoker | 134 (38.3) | 67 (32.7) | 67 (32.7) | |
| Heaviness of smoking, n (%) | 0.20 | |||
| Nonsmoker | 253 (61.7) | 135 (65.9) | 118 (57.6) | |
| Light smokera | 104 (25.4) | 44 (21.5) | 60 (29.3) | |
| Heavy smokerb | 53 (12.9) | 26 (12.7) | 27 (13.2) | |
| Age, median (IQR) | 41.7 (38.8–44.7) | 42.1 (39.1–44.7) | 41.3 (38.7–44.6) | 0.28 |
| High school graduate or less, n (%) | 179 (43.9) | 71 (34.6) | 108 (52.7) | 0.001 |
| BMI, median (IQR) | 27.2 (23.6–32.6) | 25.2 (22.4–29.3) | 30.6 (25.3–35) | 0.001 |
IQR, interquartile range; BMI, body mass index.
Smokes less than one pack of cigarettes daily.
Smokes one or more packs of cigarettes daily.
Current smokers were approximately 1 year younger at menopause entry than were nonsmokers (median age, 50.3 and 51 y, respectively, P = 0.05). The results of multivariable Cox regression models demonstrate the independent effects of smoking behaviors on risk of menopause entry after adjusting for race, baseline age, and educational attainment (Table 2). In the analysis that was stratified by race, current heavy smoking was associated with menopause onset in European Americans only.
TABLE 2.
Multivariable Cox regression: associations between smoking behavior and menopause
| Risk factor | All participantsa | P | European Americansb | P | African Americansb | P |
|---|---|---|---|---|---|---|
| Current smoker vs nonsmoker | 1.27 (0.96–1.69) | 0.09 | 1.55 (1.05–2.3) | 0.05 | 1.05 (0.7–1.58) | 0.8 |
| Former smoker vs never smoker | 1.5 (1.06–2.15) | 0.02 | 1.44 (0.88–2.37) | 0.15 | 1.39 (0.8–2.4) | 0.20 |
| Current smoker vs never smoker | 1.53 (1.1–2.12) | 0.01 | 1.87 (1.16–3.00) | 0.01 | 1.19 (0.75–1.89) | 0.46 |
| Light smokerc vs nonsmoker | 1.17 (0.84–1.62) | 0.35 | 1.4 (0.87–2.25) | 0.16 | 0.98 (0.62–1.56) | 0.95 |
| Heavy smokerd vs nonsmoker | 1.52 (1.01–2.27) | 0.04 | 1.84 (1.05–3.23) | 0.03 | 1.25 (0.66–2.34) | 0.50 |
Data are presented as adjusted hazard ratio (95% CI).
Adjusted for race, baseline age, and educational attainment.
Adjusted for baseline age and educational attainment.
Smokes less than one pack of cigarettes daily.
Smokes one or more packs of cigarettes daily.
As expected, the frequency of each SNP varied significantly across racial categories (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/MENO/A85). Deviation from expected Hardy-Weinberg proportions occurred only in African Americans for CYP3A4.
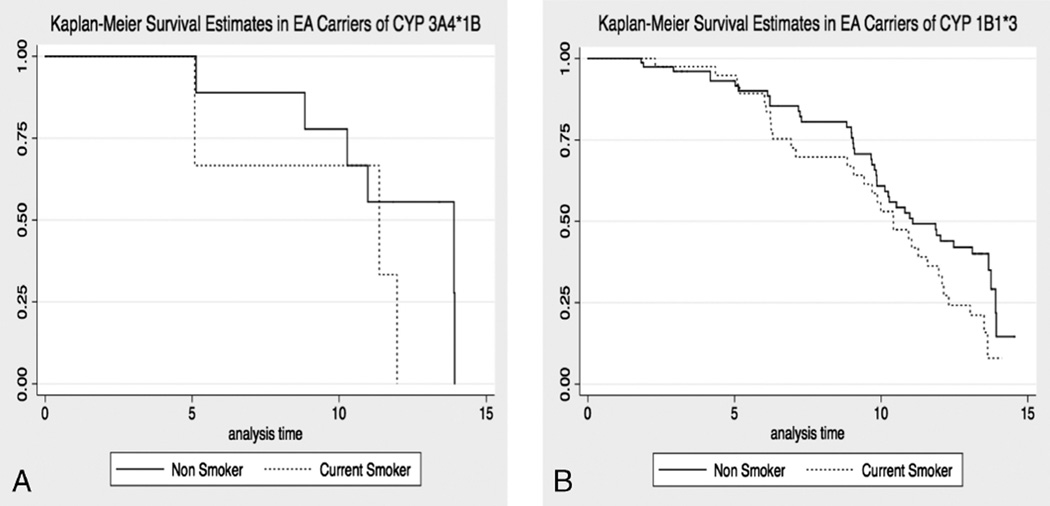
Significant findings of earlier menopause onset were observed in European-American carriers of either CYP1B1*3 or CYP3A4*1B who smoked (Table 3). European-American carriers of CYP1B1*3 who reported current smoking at baseline had a greater-than-twofold increased risk of entering menopause during follow-up (adjusted HR, 2.26; 95% CI, 1.4–3.67; P = 0.01) compared with carriers who did not smoke. The median TTM in CYP1B1*3 smokers and nonsmokers was 10.42 years (25th–75th percentile, 6.93–12.31) and 11.07 years (25th–75th percentile, 9.04–13.91), respectively (Fig. 1; Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/MENO/A86). In contrast, the adjusted HR for menopause in CYP1B1*3 wild types who smoked was not consistent with an increased risk of menopause during follow-up. CYP3A4*1B carriers who reported smoking were more than 16 times as likely to enter menopause (adjusted HR, 15.1; 95% CI, 3.31–69.2; P < 0.001) as were CYP3A4*1B carriers who did not smoke. The median TTM in CYP3A4*1B smokers and nonsmokers was 11.36 years (25th–75th percentile, 5.09–11.97) and 13.91 years (25th–75th percentile, 10.28–13.92), respectively (Fig. 1). Although the adjusted HR for menopause in CYP3A4 wild-type smokers was also significant (adjusted HR, 1.59; 95%CI, 1.03–2.44; P = 0.04), the magnitude of the effects of smoking on the risk of menopause was much lower than that in CYP3A4*1B carriers. The median TTM in CYP3A4 wild-type smokers and nonsmokers was 11.04 years (25th–75th percentile, 9.00–13.51) and 10.27 years (25th–75th percentile, 8.99–13.75), respectively. Table 3 presents the P values (by likelihood ratio test) assessing the interaction between current smoking and genotypes, which were statistically significant.
TABLE 3.
Adjusted hazard ratios (95% CIs) for menopause by baseline smoking behavior, stratified by genotype in European Americans
| SNP | na | Current smokers vs nonsmokersb |
P | na | Current smokers vs never smokersb |
P | na | Former smokers vs never smokersb |
P |
|---|---|---|---|---|---|---|---|---|---|
| CYP1B1*3−/− | 23 | 0.82 (0.4–1.70) | 0.59 | 23 | 1.65 (0.62–4.4) | 0.32 | 25 | 3.2 (1.27–8.06) | 0.01 |
| CYP1B1*3+/− and CYP1B1*3+/+ | 42 | 2.26 (1.4–3.67) | 0.001 (Pint = 0.02c) | 42 | 2.3 (1.32–4.0) | 0.003 (Pint = 0.009c) | 37 | 0.96 (0.5–1.86) | 0.9 (Pint = 0.009c) |
| CYP3A4*1B−/− | 60 | 1.59 (1.03–2.44) | 0.04 | 60 | 2.12 (1.26–3.56) | 0.005 | 58 | 1.78 (1.04–3.06) | 0.04 |
| CYP3A4*1B+/− and CYP3A4*1B+/+ | 4 | 15.1 (3.31–69.2) | <0.0001 (Pint = 0.01c) | 4 | 18.3 (2.75–122.01) | 0.003 (Pint = 0.03c) | 6 | 1.2 (0.2–7.31) | 0.85 (Pint = 0.03c) |
Hazard ratios were adjusted for baseline age and educational attainment.
SNP, single nucleotide polymorphism.
Number of participants who smoked within a particular stratum of the gene variant.
Adjusted hazard ratios for menopause in smokers by genotype status (carrier vs noncarrier).
P value for the interaction of smoking status and genotype status on risk of menopause.
FIG. 1.
Kaplan-Meier curves for time to menopause in single nucleotide polymorphism (SNP) carriers by baseline smoking behavior. A: Median time from study entry to menopause in CYP3A4*1B carriers reporting smoking at baseline, 11.36 years. Median time from study entry to menopause in CYP3A4*1B carriers reporting no smoking at baseline, 13.91 years. Solid line, survival curve in carriers who smoke; dashed line, survival curve in carriers who do not smoke. B: Median time from study entry to menopause in CYP1B1*3 carriers reporting smoking at baseline, 10.42 years. Median time from study entry to menopause in CYP1B1*3 carriers reporting no smoking at baseline, 11.36 years. Solid line, survival curve in carriers who smoke; dashed line, survival curve in carriers who do not smoke. EA, European American.
The risk of menopause entry during follow-up among carriers of either SNP who were current smokers, compared with carriers who never smoked, was similar to that of current versus noncurrent smokers (Table 3). There was no increased risk of menopause entry for either CYP1B1*3 or CYP3A4*1B carriers who were former smokers (compared with never smokers); however, wild types for either gene who were former smokers had an increased HR for menopause compared with never-smoking wild types. The interactions of these additional categories of smoking and SNPs on the risk of menopause were also statistically significant.
Heavily smoking CYP1B1*3 carriers (adjusted HR, 3.0; 95% CI, 1.54–5.84; P = 0.001) and heavily smoking CYP3A4*1B carriers (adjusted HR, 17.79; 95% CI, 3.21–98.65; P = 0.001) were also at higher risk for menopause entry than nonsmoking carriers (Table 4). Conversely, heavy smoking was not a risk factor for menopause entry in wild types. In CYP1B1*3 carriers, the median TTM in heavy smokers, light smokers, and nonsmokers was 10.41 years (25th–75th percentile, 6.23–11.97), 10.42 years (25th–75th percentile, 8.84–13.51), and 11.08 years (25th–75th percentile, 9.04–13.91), respectively. In CYP3A4*1B carriers, the median TTM in heavy smokers, light smokers, and nonsmokers was 5.09 years (25th–75th percentile, 5.09–11.97), 11.36 years (25th–75th percentile, could not be estimated), and 13.91 years (25th–75th percentile, 10.28–13.92), respectively.
TABLE 4.
Adjusted hazard ratios (95% CIs) for menopause by baseline heaviness of smoking, stratified by genotype in European Americans
| SNP | n | Light smokers vs nonsmokersa | P | n | Heavy smokers vs nonsmokersa | P |
|---|---|---|---|---|---|---|
| CYP1B1*3−/− | 15 | 0.81 (0.33–2.01) | 0.66 | 8 | 0.83 (0.28–2.45) | 0.74 |
| CYP1B1*3+/− and CYP1B1*3+/+ | 25 | 1.92 (1.08–3.42) | 0.03 (Pint = 0.04b) | 17 | 3.0 (1.54–5.84) | 0.001 |
| CYP3A4*1B−/− | 39 | 1.61 (0.98–2.66) | 0.06 | 21 | 1.54 (0.82–2.9) | 0.18 |
| CYP3A4*1B+/− and CYP3A4*1B+/+ | 22 | 11.73 (1.27–107.91) | 0.03 (Pint = 0.04b) | 2 | 17.79 (3.21–98.65) | 0.001 |
Hazard ratios were adjusted for baseline age and educational attainment.
SNP, single nucleotide polymorphism.
Adjusted hazard ratios for menopause in smokers by genotype status (carrier vs noncarrier).
P value for the interaction of heaviness of smoking and genotype status on risk of menopause.
Baseline levels of AMH and AMH slope were compared across smoking categories and by smoking status within SNP strata in European Americans (Table 5). Although no significant associations were observed for baseline AMH in carriers of either SNP, a significant increase in the magnitude of AMH decline was noted in smokers compared with nonsmokers and in heavy smokers compared with light smokers and nonsmokers. Specifically, AMH declined 21% faster per year in European-American women reporting smoking at study entry compared with nonsmokers (relative risk, 0.79; 95% CI, 0.69–0.92; P = 0.002). In European-American carriers of CYP1B1*3 and CYP3A4*1B, a significant increase in the magnitude of AMH decline was noted in smokers compared with nonsmokers (P = 0.002 and P = 0.01, respectively; Table 5). A trend of increasing magnitude in AMH decline was also noted as women smoked more heavily (test for trend P = 0.005 in CYP1B1*3 carriers and P = 0.008 in CYP3A4*1B carriers; Table 5). No such associations between smoking and AMH slope were observed in wild types, except for actively smoking CYP3A4 wild types (P value for comparison of AMH slope in smokers vs nonsmokers = 0.048). No relationships between smoking and AMH decline were observed in African Americans.
TABLE 5.
AMH and AMH slope by smoking status in European-American carriers of CYP1B1*3 and CYP3A4 *1F
| Genotype and smoking status |
AMH (ng/mL), geometric mean (95% CI)a |
AMH slope, median (IQR) | Test for trend P |
AMH for menopause in smokers, adjusted HR (95% CI) |
|---|---|---|---|---|
| CYP1B1*3 carrier | 0.005b | 2.18 (1.18–4.04) [P = 0.01] | ||
| Nonsmoker | 0.6 (0.5–0.71) | −0.363 (−0.562 to −0.218) | ||
| Light smoker | 0.51 (0.37–0.68) | −0.454 (−0.586 to −0.364) | ||
| Heavy smoker | 0.58 (0.4–0.84) | −0.797 (−1.62 to −0.391) [P = 0.002c] | ||
| CYP1B1*3 WT | 0.3b | 1.02 (0.43–2.43) [P = 0.9] | ||
| Nonsmoker | 0.47 (0.33–0.67) | −0.369 (−0.684 to −0.286) | ||
| Light smoker | 0.54 (0.32–0.9) | −0.418 (−0.679 to −0.307) | ||
| Heavy smoker | 0.5 (0.19–1.28) | −0.494 (−0.676 to −0.343) [P = 0.7c] | ||
| CYP3A4*1B carrier | 0.008b | 14.56 (3.31–69.22) [P < 0.0001] | ||
| Nonsmoker | 0.59 (0.46–0.76) | −0.333 (−0.363 to −0.216) | ||
| Light smoker | 0.51 (0.34–0.74) | −0.546 (−0.679 to −0.413) | ||
| Heavy smoker | 0.5 (0.29–0.85) | −1.21 (−1.62 to −0.797) [P = 0.01c] | ||
| CYP3A4*1B WT | 0.03c | 1.46 (0.86–2.48) [P = 0.2] | ||
| Nonsmoker | 0.55 (0.45–0.67) | −0.382 (−0.643 to −0.252) | ||
| Light smoker | 0.48 (0.34–0.69) | −0.454 (−0.699 to −0.346) | ||
| Heavy smoker | 0.61 (0.38–0.98) | −0.471 (−0.905 to −0.343) [P = 0.06c] |
International System of Units conversion: AMH (ng/mL) × 7.14.
AMH, antimüllerian hormone; IQR, interquartile range; HR, hazard ratio; WT, wild type.
P values for comparisons of AMH across smoking strata within categories of gene variants were all nonsignificant.
P values for test of trend in AMH slope across smoking strata within categories of gene variants.
P values for comparisons of median AMH slope in smokers versus nonsmokers within categories of gene variants.
When AMH slope was added to the Cox proportional hazards models for risk of menopause, the HRs supporting an association between smoking and menopause in SNP carriers became somewhat attenuated but remained statistically significant (Table 5).
DISCUSSION
We observed that, in European-American carriers of the SNPs CYP1B1*3 and CYP3A4*1B, smoking conferred a much more powerful risk of menopause onset than it did in those who did not carry these variants. In addition to the HRs showing the relative effects of smoking, in combination with genetic variation, on risk of menopause, the median TTM in European-American gene variant carriers who smoked (especially in carriers of CYP3A4*1B) supports the clinical significance of our findings. Particularly notable are the patterns of menopause entry in heavily smoking CYP3A4*1B carriers in whom the median TTM was approximately 5 years after study entry—8 years earlier than in nonsmoking carriers and 6 years earlier than in participants who smoked heavily and were CYP3A4 wild types. Smoking exposes users to a variety of toxic chemicals; although its association with hastened TTM is well known, the effect is menopausal onset just a few years earlier than the mean age of onset (1–2 y).4–7 Our investigation, to our knowledge, is the first to demonstrate that genetic background is significantly associated with an increased risk of menopause in smokers.
CYP3A4 is the most abundant cytochrome P450 enzyme in humans, accounting for 30% to 60% of total liver cytochrome P450 content41 and up to 80% of hepatic estradiol oxidative metabolism.42,43 Although CYP3A4 activity is inducible by smoking,44 the enzyme (compared with other cytochrome P450 enzymes) makes a relatively minor contribution to the activation of chemicals such as PAHs.27
Molecular studies have demonstrated that the CYP3A4*1B variant results in increased enzyme expression41 compared with the wild type. Overall, carriers of this SNP experience greater estrogen degradation and possibly greater activation of environmental toxins such as PAHs compared with wild types. Given that CYP3A4 is the most abundant cytochrome P450 in the liver, even minor differences between wild-type and variant activities could play a clinically significant role in menopause timing.41
CYP1B1 primarily metabolizes estrogens to 4-hydroxy in termediates19,45 and is highly expressed in the ovary.27 Among cytochrome P450 enzymes, CYP1B1 plays a primary role in the bioactivation of PAHs in smokers. Once activated, PAHs become very powerful inducers of CYP1B1 activity through the aryl hydrocarbon receptor; native PAHs are able to up-regulate CYP1B1 but not as efficiently as when they are bioactivated.27,28,45 Activated PAHs have the ability to bind xenobiotic response elements in the promoter region of genes such as Bax, which mediates apoptosis in human germ cells.14,22–24,26 PAHs also generate intraovarian oxidative stress and DNA adducts that could simultaneously damage ovarian germ cells and somatic cells.46,47
Functionally, CYP1B1*3 results in increased enzyme activity and amplification of the initial phase of estrogen metabolism.28 Catalytic activity of CYP1B1*3 is also more inducible by smoking than is the wild type.27,28 As with the CYP3A4*1B variant, carriers of CYP1B1*3 inactivate estrogens more rapidly and bioactivate PAHs to a greater degree than wild types. Although we do not have functional assays associated with the alleles studied, the considerable evidence on the metabolic impact of these variants lends credibility to our findings.
The strengths of this investigation include the use of a racially balanced study population, permitting race-specific analyses unbiased by the differential distribution of gene variant frequencies in African-American and European-American women. The four variants studied were selected a priori based on their roles in the metabolism of sex steroid hormones and environmental chemicals. Collecting outcomes and exposures at study entry and in a prospective fashion eliminated information bias and poor recall of smoking behaviors and timing of menopause. The prospective nature of the study also ensured that the temporal relationship between exposure and outcome was properly characterized.
Despite these strengths, some limitations also exist. Self-report was used to characterize smoking behaviors, which can lead to misclassification caused by social desirability bias. Based on reports from comparable populations, the likelihood of this is expected to be extremely low.48,49 Several of the strata representing the combination of smoking behaviors and gene variant status were small, especially for carriers of CYP3A4*1B. The small strata were byproducts of regressions stratified by race and generation of separate HRs for carriers and wild types. The interactions between smoking and CYP3A4*1B and between smoking and CYP1B1*3 on risk of menopause entry were not uniformly observed across racial groups. It is possible that uniform relationships were not found owing to other factors associated with race that modify the interaction between smoking and genotypes. It is well known that race impacts multiple features of menopause,50 and racially specific effects of SNPs have previously been described for reproductive aging outcomes. In the race-stratified multivariable Cox regression analysis that evaluated the impact of smoking without genotype as covariate (Table 2), risk of menopause was significantly associated with smoking in European Americans but not in African Americans. Although a statistically significant race-smoking interaction on menopause was not observed in these models (data not shown), our findings leave open the possibility that race could modify the relationship of genetics and smoking on menopause.
Baseline AMH assessed as a marker of ovarian reserve in European-American carriers of CYP1B1*3 or CYP3A4*1B was comparable in smokers and nonsmokers. Conversely, AMH decline across time, represented as AMH slope, was most rapid in European-American carriers of CYP1B1*3 or CYP3A4*1B who smoked. When AMH slope was added to Cox regression models for menopause, the HRs for menopause in carriers who smoked became attenuated compared with the HRs in non–AMH-adjusted models. These findings support the biologic plausibility of our findings and suggest that AMH slope may be a marker of reproductive senescence in a putative model involving tobacco smoke and variations in the specific genes studied herein. Additional investigation is required to clarify these pathways further.
CONCLUSIONS
We have presented data supporting gene-environment interactions on menopause onset among European-American smokers who are carriers of CYP1B1*3 and CYP3A4*1B gene variants. Our results identify genotypes at high risk for environmental reproductive toxicity and reinforce the complex interplay of factors associated with reproductive senescence culminating in menopause onset. In the significant body of research dedicated to identifying factors related to the tempo of reproductive aging, many have evaluated the role of genetics or the environment, and most have studied them separately. In general, estimates of risk (ie, odds ratios and HRs) of menopause in smokers, as reported in the literature, have ranged between 1.4 and 1.9.6,15,16 In comparison, the risk of menopause in carriers of CYP1B1*3 and CYP3A4*1B who smoked ranged from 2.26 to 17.79, based on degree of smoking. Wild types who smoked had little increased risk of menopause; if the risk was elevated, the magnitude was far less than in SNP carriers. Smoking cessation alleviates reproductive risks in these individuals but does not protect women exposed to second-hand smoke or environmental chemicals with toxicant profiles similar to tobacco. Further research along these lines should expand on results such as these and contribute to the identification of women at high risk for both early menopause and significant postmenopausal morbidity.
Supplementary Material
Acknowledgments
We acknowledge the staff who contributed to the implementation and management of the Penn Ovarian Aging study. We also wish to acknowledge Carol Winkelman, MA, for her critical review of this manuscript.
Funding/support: This study was supported by National Institutes of Health grant R01-AG-12745 to E.W.F., National Institute of Environmental Health Sciences grant 5P30ES013508-07 to S.F.B., Perelman School of Medicine Center of Excellence for Diversity grant to S.F.B., and Perelman School of Medicine Translational and Clinical Research Center grant RR024134 to E.W.F.
M.D.S. serves as a paid consultant for Swiss Precision Diagnostics. E.W.F. has previously received grant support from Forest and Xenodyne Pharmaceuticals.
Footnotes
This paper was presented as an oral presentation at the 60th Annual Scientific. Meeting of the Society for Gynecologic Investigation, Orlando, FL, March 20 to 23, 2013.
Financial disclosure/conflicts of interest: S.F.B., T.R.R., C.G., and D.W.B. declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.menopause.org).
REFERENCES
- 1.Lobo RA. Cardiovascular disease, menopause, and the influence of hormone replacement therapy. Prog Clin Biol Res. 1989;320:313–332. [PubMed] [Google Scholar]
- 2.Snowdon DA, Kane RL, Beeson WL, et al. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79:709–714. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers MR, La Pietra MT. Menopause: its epidemiology and potential association with chronic diseases. Epidemiol Rev. 1995;17:287–302. doi: 10.1093/oxfordjournals.epirev.a036194. [DOI] [PubMed] [Google Scholar]
- 4.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103–115. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 5.Meschia M, Pansini F, Modena AB, et al. Determinants of age at menopause in Italy: results from a large cross-sectional study. ICARUS Study Group. Italian Climacteric Research Group Study. Maturitas. 2000;34:119–125. doi: 10.1016/s0378-5122(99)00095-x. [DOI] [PubMed] [Google Scholar]
- 6.Cramer DW, Harlow BL, Xu H, Fraer C, Barbieri R. Cross-sectional and case-controlled analyses of the association between smoking and early menopause. Maturitas. 1995;22:79–87. doi: 10.1016/0378-5122(95)00928-e. [DOI] [PubMed] [Google Scholar]
- 7.Cramer DW, Xu H. Predicting age at menopause. Maturitas. 1996;23:319–326. doi: 10.1016/0378-5122(96)00992-9. [DOI] [PubMed] [Google Scholar]
- 8.Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18:199–206. doi: 10.1093/humrep/deg005. [DOI] [PubMed] [Google Scholar]
- 9.Committee on Practice Bulletins-Gynecology, The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin N. 129. Osteoporosis. Obstet Gynecol. 2012;120:718–734. doi: 10.1097/AOG.0b013e31826dc446. [DOI] [PubMed] [Google Scholar]
- 10.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 11.American Lung Association. Trends in tobacco use. [Accessed July 15, 2013];2011 Available at: www.lungusa.org/finding-cures/our-research/trend-reports/Tobacco-Trend-Report.pdf. [Google Scholar]
- 12.World Health Organization. Tobacco. [Accessed July 15, 2013]; Available at: http://www.who.int/topics/tobacco/en.
- 13.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 14.Mattison DR, Shiromizu K, Nightingale MS. Oocyte destruction by polycyclic aromatic hydrocarbons. Am J Ind Med. 1983;4:191–202. [PubMed] [Google Scholar]
- 15.Palmer JR, Rosenberg L, Wise LA, Horton NJ, Adams-Campbell LL. Onset of natural menopause in African American women. Am J Public Health. 2003;93:299–306. doi: 10.2105/ajph.93.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145:124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 17.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westhoff C, Murphy P, Heller D. Predictors of ovarian follicle number. Fertil Steril. 2000;74:624–628. doi: 10.1016/s0015-0282(00)01527-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Barbieri RL, Gochberg J, Ryan KJ. Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. J Clin Invest. 1986;77:1727–1733. doi: 10.1172/JCI112494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986;315:1305–1309. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- 22.Jurisicova A, Taniuchi A, Li H, et al. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J Clin Invest. 2007;117:3971–3978. doi: 10.1172/JCI28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matikainen T, Perez GI, Jurisicova A, et al. Aromatic hydrocarbon receptor–driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 24.Matikainen TM, Moriyama T, Morita Y, et al. Ligand activation of the aromatic hydrocarbon receptor transcription factor drives Bax-dependent apoptosis in developing fetal ovarian germ cells. Endocrinology. 2002;143:615–620. doi: 10.1210/endo.143.2.8624. [DOI] [PubMed] [Google Scholar]
- 25.Matsunawa M, Amano Y, Endo K, et al. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicol Sci. 2009;109:50–58. doi: 10.1093/toxsci/kfp044. [DOI] [PubMed] [Google Scholar]
- 26.Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- 27.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada T, Watanabe J, Kawajiri K, et al. Catalytic properties of polymorphic human cytochrome P450 1B1 variants. Carcinogenesis. 1999;20:1607–1613. doi: 10.1093/carcin/20.8.1607. [DOI] [PubMed] [Google Scholar]
- 29.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McVay MA, Copeland AL. Smoking cessation in peri- and postmenopausal women: a review. Exp Clin Psychopharmacol. 2011;19:192–202. doi: 10.1037/a0023119. [DOI] [PubMed] [Google Scholar]
- 31.Butts SF, Freeman EW, Sammel MD, Queen K, Lin H, Rebbeck TR. Joint effects of smoking and gene variants involved in sex steroid metabolism on hot flashes in late reproductive-age women. J Clin Endocrinol Metab. 2012;97:E1032–E1042. doi: 10.1210/jc.2011-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long JR, Shu XO, Cai Q, et al. Polymorphisms of the CYP1B1 gene may be associated with the onset of natural menopause in Chinese women. Maturitas. 2006;55:238–246. doi: 10.1016/j.maturitas.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 33.He C, Kraft P, Chasman DI, et al. A large-scale candidate gene association study of age at menarche and age at natural menopause. Hum Genet. 2010;128:515–527. doi: 10.1007/s00439-010-0878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hefler LA, Grimm C, Heinze G, et al. Estrogen-metabolizing gene polymorphisms and age at natural menopause in Caucasian women. Hum Reprod. 2005;20:1422–1427. doi: 10.1093/humrep/deh848. [DOI] [PubMed] [Google Scholar]
- 35.Freeman EW, Grisso JA, Berlin J, Sammel M, Garcia-Espana B, Hollander L. Symptom reports from a cohort of African American and white women in the late reproductive years. Menopause. 2001;8:33–42. doi: 10.1097/00042192-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Manson JM, Sammel MD, Freeman EW, Grisso JA. Racial differences in sex hormone levels in women approaching the transition to menopause. Fertil Steril. 2001;75:297–304. doi: 10.1016/s0015-0282(00)01723-4. [DOI] [PubMed] [Google Scholar]
- 37.Shatalova EG, Walther SE, Favorova OO, Rebbeck TR, Blanchard RL. Genetic polymorphisms in human SULT1A1 and UGT1A1 genes associate with breast tumor characteristics: a case-series study. Breast Cancer Res. 2005;7:R909–R921. doi: 10.1186/bcr1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rebbeck TR, Troxel AB, Wang Y, et al. Estrogen sulfation genes, hormone replacement therapy, and endometrial cancer risk. J Natl Cancer Inst. 2006;98:1311–1320. doi: 10.1093/jnci/djj360. [DOI] [PubMed] [Google Scholar]
- 39.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 40.Kleinman D, Klein M. Survival Analysis: A Self-Learning Text. New York, NY: Springer-Verlag; 2005. [Google Scholar]
- 41.Amirimani B, Ning B, Deitz AC, Weber BL, Kadlubar FF, Rebbeck TR. Increased transcriptional activity of the CYP3A4*1B promoter variant. Environ Mol Mutagen. 2003;42:299–305. doi: 10.1002/em.10199. [DOI] [PubMed] [Google Scholar]
- 42.Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993;57:237–257. doi: 10.1016/0163-7258(93)90057-k. [DOI] [PubMed] [Google Scholar]
- 43.Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 44.Thum T, Erpenbeck VJ, Moeller J, Hohlfeld JM, Krug N, Borlak J. Expression of xenobiotic metabolizing enzymes in different lung compartments of smokers and nonsmokers. Environ Health Perspect. 2006;114:1655–1661. doi: 10.1289/ehp.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450–mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Zenzes MT, Puy LA, Bielecki R. Immunodetection of benzo[a]pyrene adducts in ovarian cells of women exposed to cigarette smoke. Mol Hum Reprod. 1998;4:159–165. doi: 10.1093/molehr/4.2.159. [DOI] [PubMed] [Google Scholar]
- 47.Uno S, Makishima M. Benzo[a]pyrene toxicity and inflammatory disease. Curr Rheumatol Rev. 2009;5:266–271. [Google Scholar]
- 48.Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23:47–53. [PubMed] [Google Scholar]
- 49.Wagenknecht LE, Burke GL, Perkins LL, Haley NJ, Friedman GD. Misclassification of smoking status in the CARDIA study: a comparison of self-report with serum cotinine levels. Am J Public Health. 1992;82:33–36. doi: 10.2105/ajph.82.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butts SF, Seifer DB. Racial and ethnic differences in reproductive potential across the life cycle. Fertil Steril. 2010;93:681–690. doi: 10.1016/j.fertnstert.2009.10.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.