Abstract
Background
Individuals with autism spectrum disorder (ASD) show atypical brain activity, perhaps due to delayed maturation. Previous studies examining the maturation of auditory electrophysiological activity have been limited due to their use of cross-sectional designs. The present study took a first step in examining magnetoencephalography (MEG) evidence of abnormal auditory response maturation in ASD via the use of a longitudinal design.
Methods
Initially recruited for a previous study, 27 children with ASD and nine typically developing (TD) children, aged 6- to 11-years-old, were re-recruited two to five years later. At both timepoints, MEG data were obtained while participants passively listened to sinusoidal pure-tones. Bilateral primary/secondary auditory cortex time domain (100 ms evoked response latency (M100)) and spectrotemporal measures (gamma-band power and inter-trial coherence (ITC)) were examined. MEG measures were also qualitatively examined for five children who exhibited “optimal outcome”, participants who were initially on spectrum, but no longer met diagnostic criteria at follow-up.
Results
M100 latencies were delayed in ASD versus TD at the initial exam (~ 19 ms) and at follow-up (~ 18 ms). At both exams, M100 latencies were associated with clinical ASD severity. In addition, gamma-band evoked power and ITC were reduced in ASD versus TD. M100 latency and gamma-band maturation rates did not differ between ASD and TD. Of note, the cohort of five children that demonstrated “optimal outcome” additionally exhibited M100 latency and gamma-band activity mean values in-between TD and ASD at both timepoints. Though justifying only qualitative interpretation, these “optimal outcome” related data are presented here to motivate future studies.
Conclusions
Children with ASD showed perturbed auditory cortex neural activity, as evidenced by M100 latency delays as well as reduced transient gamma-band activity. Despite evidence for maturation of these responses in ASD, the neural abnormalities in ASD persisted across time. Of note, data from the five children whom demonstrated “optimal outcome” qualitatively suggest that such clinical improvements may be associated with auditory brain responses intermediate between TD and ASD. These “optimal outcome” related results are not statistically significant though, likely due to the low sample size of this cohort, and to be expected as a result of the relatively low proportion of “optimal outcome” in the ASD population. Thus, further investigations with larger cohorts are needed to determine if the above auditory response phenotypes have prognostic utility, predictive of clinical outcome.
Keywords: MEG, ASD, Gamma, M100, Maturation
Highlights
-
•
The first longitudinal study of auditory MEG biomarkers in ASD
-
•
ASD demonstrates prolonged M100 latencies and reduced transient gamma-band activity.
-
•
Maturation rates are not different between TD and ASD for M100 latencies.
-
•
Intermediate M100 latencies and gamma-band activity suggested for “optimal outcome”
-
•
M100 latencies correlate to current sociability and also change in language ability.
1. Introduction
Autism spectrum disorder (ASD) describes a group of disorders characterized by social/communication impairments and restricted/repetitive behaviors (American Psychiatric Association, 2013). Recent prevalence estimates report that 1 in 68 children have ASD (Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators and Centers for Disease Control and Prevention (CDC), 2014). Given that interventions to treat symptoms association with ASD show variable success (Dawson et al., 2010, Erickson et al., 2014, Van Hecke et al., 2013), and given that treatment outcome is difficult to predict at treatment onset, there is a need for early response indicators. Preclinical studies of novel treatment efficacy are also hampered by the lack of directly translatable clinical/preclinical metrics such as brain activity profiles; as a result preclinical studies primarily rely on behavioral assays. Although preclinical behavioral assays have successfully modeled isolated features of ASD (Silverman et al., 2010), no behavioral assay fully characterizes the full complexity of ASD. Furthermore, the degree to which this is possible has been questioned (Crawley, 2007). “Biomarkers” offer a biological target for therapeutics, a bridge between preclinical and clinical studies, and may serve as early response indictors of treatment success/failure (Port et al., 2014, Port et al., 2015).
Two prospective brain biomarkers, superior temporal gyrus (STG) M100 auditory latency (Edgar et al., 2014b, Gage et al., 2003b, Roberts et al., 2010) and STG post-stimulus auditory gamma-band activity (Edgar et al., 2015b, Edgar et al., 2016, Gandal et al., 2010, Wilson et al., 2007), have been identified as abnormal in ASD as well as in animal models that recapitulate key aspects of ASD (Engineer et al., 2014, Gandal et al., 2010). The auditory M100 response and it's electrical counterpart (N1) are electrophysiological responses seen in auditory event-related fields/potentials (ERF/P) approximately 100 ms after a stimulus (Hari et al., 1980). M100 responses become stronger and observed at earlier latencies as a function of typical development (Edgar et al., 2014b, Paetau et al., 1995). First described by Gage and colleagues in our group (Gage et al., 2003a), right-hemisphere auditory M100 latencies were observed to be delayed by ~ 10% in children with ASD versus age-matched typically developing controls (TD). This ~ 10 ms right-hemisphere M100 latency prolongation in children with ASD versus TD was observed in later studies, with group differences in M100 latency remaining even after co-varying cognitive and language ability (Edgar et al., 2014b, Edgar et al., 2015b, Roberts et al., 2010). Notably, in several reports, the M100 latency delay in ASD exhibited hemispheric specificity. Hemispheric specificity is germane to, and elucidated by, the large body of studies regarding hemispheric differences in speech processing (Hickok and Poeppel, 2015). Studies examining the hemispheric asymmetry of speech processing demonstrate face validity for the M100 ASD latency findings given the social communication deficits observed in ASD. For example, Poeppel (2003) hypothesized that hemisphere difference in processing speech arises from a left-hemisphere preference for processing auditory information over short integration windows and a right-hemisphere preference for processing auditory information over longer windows. Similarly, Zatorre and Belin (2001) suggested that the left-hemisphere specializes in rapid temporal processing and the right-hemisphere processes spectral signals. Both hypotheses converge on the idea that the left-hemisphere is involved in processing short duration speech-specific stimuli, such as phonemes, whereas the right-hemisphere is involved in processing long duration information such as prosody, or alternatively, the melody of music (Tervaniemi and Hugdahl, 2003). The processing of non-speech sounds may be fundamentally homologous to speech sounds if the non-speech sound is of sufficient complexity (for review see Zatorre and Gandour, 2008). In contrast to sampling-rate based hypotheses, McGettigan and Scott (2012) suggested a mixed model of speech processing, where the left-hemisphere utilizes domain-specific functioning based on experience-driven plasticity and the right-hemisphere utilizes domain general processing. Regardless, the right-hemisphere M100 delay in ASD may be associated with downstream issues in processing percepts such as prosody and emotional intonation, rather than basic phonemes.
Later studies that recapitulated M100 latency delays in individuals with ASD versus TD also observed that M100 latency maturation rates did not differ between TD and ASD (Roberts et al., 2010). This suggested that the persistent delay in auditory M100 latencies in children as well as adolescents with ASD was due to an early initial M100 latency ‘offset’.
As demanded of any biomarker, auditory M100 latencies delays have plausible biological bases, with either disruptions to neural signal conduction and/or synaptic transduction leading to latency prolongation (Port et al., 2015). For instance, ERP/F component latencies, including auditory M100 latencies, have been associated with underlying white-matter microstructure (Dockstader et al., 2012, Roberts et al., 2009, Stufflebeam et al., 2008). While thalamocortical white-matter microstructure may be intact in ASD, the developmental trajectory of white-matter microstructure, as well as the association of white-matter microstructure with ERF component latency, are not (Roberts et al., 2013). Additionally, individuals with genetic copy number variations related to ASD, specifically deletions in the 16p11.2 locus, demonstrated a similar uncoupling of white-matter microstructure and ERF component latency (Berman et al., 2016).
In addition to alterations in signal conduction, multiple laboratories have found alterations with respect to signal transduction in ASD. In particular, perturbations related to synaptic transmission have been observed clinically in in-vivo (Brown et al., 2013, Gaetz et al., 2014, Harada et al., 2011; for review see Rojas et al., 2014, Rojas et al., 2015), in postmortem studies (Fatemi et al., 2002, Fatemi et al., 2009b, Fatemi et al., 2014, Oblak et al., 2010), as well as in preclinical reports (Gogolla et al., 2009, Gogolla et al., 2014; K. Zhang et al., 2014, Gandal et al., 2012, Banerjee et al., 2013, Cellot and Cherubini, 2014, Han et al., 2012). As such, M100 latency deficits may be a non-invasive probe of local neural circuit functioning in ASD.
Preclinical studies have suggested that middle latency responses analogous to the M100 are associated with sociability in murine models relevant to ASD (Billingslea et al., 2014, Saunders et al., 2013). In addition, behavioral interventions for ASD targeting social or language functioning have been observed to partially normalize the latency of cortical responses (Dawson et al., 2012, Russo et al., 2010). An association between M100 latency and social functioning has yet to be observed in clinical populations. Relationships of middle latency responses latency to language functioning are also not clear. M50/M100 response latencies have been observed to predict oral language ability (Oram Cardy et al., 2008), but such an association was not replicated in later studies (Roberts et al., 2010). Of note, these later studies suggested M100 delays are specific to ASD, as compared to specific language impairment (Roberts et al., 2012). It is likely that sample size considerations in the context of ASD heterogeneity (including variability in language impairment in ASD) may account for study differences. Furthermore, it is possible that M100 latency delays reflect system dysfunction common to both ASD and language impairment.
In addition to M100 latency, STG auditory post-stimulus gamma-band activity (typically > 30 Hz) has received considerable attention as a candidate biomarker for ASD. Though not specific to ASD (Edgar et al., 2014a, Krishnan et al., 2009, Maharajh et al., 2007), altered post-stimulus gamma-band activity has been repeatedly demonstrated in different sensory systems with multiple stimulus complexity paradigms in children and adults with ASD (Gandal et al., 2010, Grice et al., 2001, Rojas et al., 2008, Sun et al., 2012, Wilson et al., 2007). Within the auditory system, post-stimulus phase-locked gamma-band measures (i.e. evoked power as well as inter-trial coherence) have been found to be reduced in ASD (Edgar et al., 2015b, Edgar et al., 2016, Gandal et al., 2010, Rojas et al., 2008, Wilson et al., 2007). Auditory gamma-band alterations may represent heritable endophenotypes, as they are observed in first-degree relatives of individuals with ASD (McFadden et al., 2012, Rojas et al., 2008, Rojas et al., 2011), with some evidence that gamma-band activity in relatives is associated with social functioning (Rojas et al., 2011). Although the existence of post-stimulus gamma-band alterations in first-degree relatives may call into question the appropriateness of this measure as a diagnostic or response biomarker, a subclinical expression of social impairments is thought to exist in the relatives of individuals with ASD, often referred to as the Broader Autism Phenotype (Piven et al., 1997). As such, gamma-band biomarkers may support a basis for a clinical discrimination (Port et al., 2015). Along this line, several recent studies have demonstrated the ability of gamma-band metrics to distinguish between infants at low and high risk for ASD, where risk is typically operationalized as the presence of an older sibling with ASD (Elsabbagh et al., 2009, Tierney et al., 2012). For example, Elsabbagh et al. (2009) recruited 10-months-old infants, who either had no sibling (low-risk) or one older sibling (high-risk) diagnosed with ASD. The infants' response to face stimuli with either a direct or adverted gaze was recorded using EEG. In low-risk infants, gamma-band responses were significantly modulated by whether the gaze was direct or adverted. In contrast, high-risk individuals demonstrated delayed and less persistent gamma-band differences to the two stimuli (Elsabbagh et al., 2009). Similarly, Tierney and colleagues (2102) found gamma-band activity alterations in infants at high-risk versus low-risk for autism. Of note, Tierney and colleagues (2102) included age groups ranging from 6 to 24 months, allowing them to characterize developmental trajectories. Tierney and colleagues found that infants at high-risk for ASD exhibited less frontal resting-state gamma-band activity versus the low-risk infants at all timepoints. Moreover, there was a trend towards different developmental trajectories in frontal resting-state gamma-band activity between the low- and high-risk groups (Tierney et al., 2012). Such findings, however, may not be specific to ASD, as other studies have shown that gamma-band activity relates to current (Benasich et al., 2008) as well as future (Gou et al., 2011) cognitive and language abilities. Indeed, Benasich et al. (2008) demonstrated that frontal resting-state gamma-band activity, but not lower frequency activity, correlated with concurrent cognitive and language functioning in infants 24 months of age. Using a similar paradigm, Gou et al. (2011) demonstrated that frontal resting-state gamma-band activity at multiple timepoints before 3 years of age predicted cognitive and language abilities at both 4 and 5 years of age.
Gamma-band activity also has a putative biological basis, with gamma-band activity hypothesized to arise from local circuit interactions between excitatory pyramidal cells and inhibitory interneurons (for review see Buzsáki and Wang, 2012). A key feature in multiple models of gamma-band activity generation is the key role of inhibitory signaling (Whittington et al., 2000), including the time-constant of gamma-aminobutyric acid (GABA)A receptors (Traub et al., 1996). As such, alterations to gamma-band activity in ASD suggest alterations in local circuit function, particularly the balance of local neural circuit excitation and inhibition. The role of excitatory/inhibitory imbalance in ASD has been hypothesized for over a decade (Rubenstein and Merzenich, 2003), with several lines of evidence supporting this hypothesis: the high prevalence of co-morbid epilepsy in ASD (Danielsson et al., 2005, Yasuhara, 2010), alterations to proteins related to neurotransmitter systems (Fatemi et al., 2002, Fatemi et al., 2009a, Fatemi et al., 2009b, Fatemi et al., 2014, Oblak et al., 2010, Purcell et al., 2001) as well as cell-type specific counts detected ex vivo (Lawrence et al., 2010, Zikopoulos and Barbas, 2013), and in vivo alterations to neurometabolites related to excitation and inhibition in ASD (for review see Rojas et al., 2015).
The aforementioned cross-sectional studies of M100 latency prolongation and post-stimulus gamma-band alterations in ASD, although informative, have limitations. In addition to the limits inherent to cross-sectional studies, for example arising from inter-subject “biological” variability, cross-sectional designs do not allow for developmental changes over time. This is a particular limitation as some children initially diagnosed with ASD may show improvement over time, with improvement even resulting in “optimal outcome” (Granpeesheh et al., 2009, Helt et al., 2008, Mukaddes et al., 2014, Zappella, 2002). Though much remains unknown about “optimal outcome”, research suggests a small cohort of individuals originally diagnosed with ASD no longer meet diagnostic criteria at follow-up. Indeed, domain-specific measures suggest some individuals with SAD may later function well within the normal range with respect to cognitive and social abilities (Helt et al., 2008). The role, impact, and specificity of interventions associated with “optimal outcome”, however, are currently unknown. Although it has been suggested that “optimal outcome” individuals demonstrate better social skills and higher intelligence relative to the general ASD population, some of these measures (i.e., higher IQ) do not strongly predict “optimal outcome”. In addition, initial autism severity is not associated with “optimal outcome” (Helt et al., 2008). In addition, it is important to note that individuals showing “optimal outcome” often show subtle residual impairments even after “losing” the ASD diagnosis (Kelley et al., 2006). With regard to the above, an objective prognostic biomarker and/or real-time monitor of intervention efficacy would be of use.
In an attempt to address the aforementioned limitations of cross-sectional studies, the present study utilized a longitudinal design to examine auditory M100 latencies, auditory gamma-band responses, and their maturation in TD children and children with ASD. The present study tested the hypotheses that children with ASD would demonstrate prolonged auditory M100 latencies as well as reduced post stimulus phase-locked gamma-band metrics. Longitudinal analyses allowed direct assessment of whether M100 latencies in ASD show within-subject evidence of abnormal maturation. Given that cross-sectional findings show similar rates of M100 latency maturation in TD and ASD, it was hypothesized that the children with ASD would demonstrate a persistent prolongation in M100 latency delay against the background of a similar rate of maturation. Thus, it was hypothesized that M100 latency in older individuals with ASD does not decrease at a rate fast enough to ‘catch up’ to age-matched TD latencies.
Use of a longitudinal design additionally allowed examination of associations between MEG measures and current as well as future clinical/behavioral status. It was hypothesized that M100 latency would be associated with current autism severity based on the aforementioned preclinical observations of correlations between middle latency responses and sociability (Billingslea et al., 2014, Saunders et al., 2013). Such an association has yet to be demonstrated in a clinical population. Moreover, an association between M100 latency and current language ability was hypothesized to be absent (Roberts et al., 2010), building upon previous studies indicating M100 latency is not related to language ability. Finally, it was hypothesized that gamma-band activity would be associated with follow-up language functioning (Gou et al., 2011) as well as autism severity (Elsabbagh et al., 2009, Tierney et al., 2012). We hypothesized that although the clinical significance of perturbed gamma-band activity in ASD may not be immediately evident at earlier ages, early gamma-band abnormalities would have an downstream effect (Cardin et al., 2009, Sohal et al., 2009).
2. Materials and methods
2.1. Participants
Initial timepoint 1 data were obtained from previous magnetoencephalography (MEG) studies (Edgar et al., 2015b, Roberts et al., 2010). A subsample of participants (TD = 9, ASD = 27) were re-recruited two to five years later (mean age = 12.1 years). The longitudinal cohort was smaller than our previously published studies because of a restricted intake age-range (to allow for subsequent follow-up during the adolescent period) as well as difficulties re-contacting and re-recruiting participants over a long time interval, and additional exclusion criteria that arose across time (e.g., dental work in the intervening period, or later medication use).
Of note, a subset of the re-recruited children from the ASD cohort (N = 5) showed considerable improvement at follow-up, and were sub-threshold for a diagnosis of ASD at this later time. These individuals are referred to here as “had ASD”. All 36 participants (9 TD [3 males], 22 ASD [22 males], 5 “had ASD” [4 males]) had evaluable M100 data. Table 1 reports diagnostic scores and demographics for these three groups (TD, ASD, “had ASD”).
Table 1.
Demographics of M100 study population. No significant differences in age (4th block from left) or Wechsler Intelligence Scale for Children-IV General Ability Index (WISC-IV GAI) (5th block from left) were observed between TD (top), and ASD (middle). Children with ASD exhibited significantly higher Social Responsiveness Scale (SRS) and Autism Diagnostic Observation Schedule Calibrated Severity Scores (ADOS CSS) at both initial and follow-up exams (1st and 2nd block from the right). Wechsler Intelligence Scale for Children-IV Verbal Comprehension Index (WISC-IV VCI) scores were significantly lower in children with ASD (middle right) at initial exam. ASD children also demonstrated significantly lower scores on the Clinical Evaluation of Language Fundamentals—fourth edition (CELF-4 Core Language Index). A subgroup of children who had an initial diagnosis of ASD no longer met diagnosis criteria at the follow-up exam (“had ASD” (bottom)). These children exhibited SRS and ADOS CSS scores similar to children with ASD at the initial exam, and then intermediate corresponding scores at follow-up. These children had similar age and GAI to children with ASD, though intermediate WISC-IV VCI scores. Values are counts or mean (standard deviation). Bold indicates significant p values and their associated Cohen's d.
| Age (yrs) |
WISC-IV GAI |
WISC-IV VCI |
CELF-4 CLI |
SRS (raw) |
ADOS CSS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Male | Initial | Follow-up | Initial | Follow-up | Initial | Follow-up | Initial | Follow-up | Initial | Follow-up | Initial | Follow-up | |
| Control | 9 | 3 | 8.4 (1.3) | 11.9 (1.5) | 116.3 (17.3) | 113.8 (20.4) | 113.2 (19.0) | 110.3 (21.5) | 108 (14.6) | 105.8 (15.5) | 46.3 (7.1) | 41.3 (4.4) | 1.2 (0.4) | 1.3 (0.8) |
| ASD | 22 | 22 | 8.4 (1.1) | 12.1 (1.3) | 104.9 (15.5) | 102.2 (15.0) | 94.0 (15.8) | 97.3 (12.9) | 85.3 (17.1) | 89.5 (18.0) | 75.1 (9.4) | 70.5 (12.5) | 8.3 (1.8) | 7.0 (1.6) |
| “had ASD” | 5 | 4 | 8.7 (0.7) | 11.8 (0.4) | 102.6 (9.0) | 105.6 (13.6) | 104.2 (10.1) | 109.6 (9.7) | 90.8 (12.7) | 96.4 (9.7) | 75.0 (18.7) | 54.4 (12.1) | 7.8 (2.7) | 2.5 (1.0) |
| p(TD [top] vs. ASD [middle]) | 0.99 | 0.77 | 0.11 | 0.18 | 0.02 | 0.15 | < 0.001 | 0.02 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| Cohen's d | 1.1 | 1.43 | 0.97 | − 3.46 | − 3.12 | − 5.45 | − 4.51 | |||||||
The gamma-band measures were more sensitive than M100 latency to artifacts (e.g. motion), which arose primarily from the gamma-band analysis pipeline relying on subject's MRIs. This is in contrast to the M100 latency measures which can be extracted from raw sensor data, if needed, negating the issue of movement within the anatomic MRI scan. As such, eight children (1 TD, 7 ASD) were excluded from gamma-band activity analyses due to MRI-related artifacts. This left 28 participants (8 TD [3 male], 15 ASD [15 male], 5 “had ASD” [4 male]) for the gamma-band analyses. Table 2 reports population diagnostic scores and demographics for the three groups (TD, ASD, “had ASD”) included in gamma-band analyses. As the “had ASD” group was considered too small for statistical analyses (parametric or non-parametric), the “had ASD” individuals were not included in statistical analyses, with findings from this group only descriptively reported.
Table 2.
Demographics of gamma-band study population. Identical to the M100 study population: no significant differences in age (4th block from left) or Wechsler Intelligence Scale for Children-IV General Ability Index (WISC-IV GAI) (5th block from left) were observed between TD (top), and ASD (middle). Children with ASD exhibited significantly higher Social Responsiveness Scale (SRS) and Autism Diagnostic Observation Schedule Calibrated Severity Scores (ADOS CSS) at both initial and follow-up exams (1st and 2nd block from the right). Wechsler Intelligence Scale for Children-IV Verbal Comprehension Index (WISC-IV VCI) scores were significantly lower in children with ASD (middle right) at initial exam. ASD children also demonstrated significantly lower scores on the Clinical Evaluation of Language Fundamentals—fourth edition (CELF-4) Core Language Index. A subgroup of children who had an initial diagnosis of ASD no longer met diagnosis criteria at the follow-up exam (“had ASD” (bottom)). These children exhibited SRS and ADOS CSS scores similar to children with ASD at the initial exam, and then intermediate corresponding scores at follow-up. These children had similar age and GAI to children with ASD, though intermediate Wechsler Intelligence Scale for Children-IV Verbal Comprehension Index WISC-IV VCI scores. Values are counts or mean (standard deviation). Bold indicates significant p values and their associated Cohen's d.
| Age (yrs) |
WISC-IV GAI |
WISC-IV VCI |
CELF-4 CLI |
SRS (raw) |
ADOS CSS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Male | Initial | Follow-up | Initial | Follow-up | Initial | Follow-up | Initial | Follow-up | Initial | Follow-up | Initial | Follow-up | |
| Control | 8 | 2 | 8.4 (1.4) | 12.1 (1.5) | 118.3 (17.5) | 116.0 (21.0) | 114.4 (20.0) | 112.3 (22.4) | 110.3 (13.8) | 108.9 (13.3) | 46.4 (7.6) | 40.1 (3.3) | 1.2 (0.4) | 1.3 (0.8) |
| ASD | 15 | 15 | 8.6 (1.1) | 12.2 (1.3) | 104.9 (13.6) | 105.2 (15.0) | 95.1 (14.3) | 101.0 (12.2) | 88.9 (12) | 93.2 (11.8) | 74.6 (10.5) | 71.5 (14.5) | 8.1 (1.7) | 7.4 (1.6) |
| “had ASD” | 5 | 4 | 8.7 (0.7) | 11.8 (0.4) | 102.6 (9.0) | 105.6 (13.6) | 104.2 (10.1) | 109.6 (9.7) | 90.8 (12.7) | 96.4 (9.7) | 75.0 (18.7) | 54.4 (12.1) | 7.8 (2.7) | 2.5 (1.0) |
| p(TD [top] vs. ASD [middle]) | 0.80 | 0.89 | 0.09 | 0.25 | 0.03 | 0.25 | < 0.001 | 0.01 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| Cohen's d | 1.11 | 1.65 | 1.25 | − 3.08 | − 2.99 | − 5.59 | − 4.82 | |||||||
2.2. Recruitment and inclusion/exclusion criteria
Akin to procedures described in Edgar et al. (2015b), subjects with ASD were originally recruited from the Regional Autism Center of The Children's Hospital of Philadelphia (CHOP), the Neuropsychiatry program of the Department of Psychiatry of the University of Pennsylvania School of Medicine, and from local and regional parent support groups such as ASCEND (Asperger Syndrome Information Alliance for Southeastern Pennsylvania) and local chapters of Autism Speaks. All children screened for inclusion in the ASD sample had a prior ASD diagnosis made by an expert clinician, typically a developmental pediatrician in the Regional Autism Center at the Children's Hospital of Philadelphia. The original diagnosis was made after an extensive clinical interview, documentation of DSM-IV criteria for ASD, and use of various ASD diagnostic tools, such as the Childhood Autism Rating Scale and, in many cases, the ADOS. Subjects with typical development (TD) were recruited through local newspaper advertisements and from pediatric practices of the CHOP primary care network.
Research participants made two visits to CHOP in addition to any prior clinical visits. During the first visit (2–3 weeks prior to the MEG exam), clinical and diagnostic testing was performed to confirm the referral ASD diagnosis, to administer neuropsychological tests, and to ensure that the TD children met study inclusion/exclusion criteria. Assessments were performed by licensed child psychologists with expertise in autism (L.B., E.S.K.). Given the extensive clinical evaluations upon which original ASD diagnosis was made, an abbreviated diagnostic battery confirmed the original diagnosis. Specifically, the ASD diagnosis was confirmed with standard diagnostic tools, including direct observation with the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000)) and parent report on the Social Communication Questionnaire (SCQ; Rutter et al., 2003). Dimensional symptom severity ratings were also obtained by parent report on the Social Responsiveness Scale (SRS; Constantino and Gruber, 2012). The Autism Diagnostic Interview-Revised (ADI-R), a parent interview about current and prior ASD symptoms, was utilized to resolve diagnostic discordances between the ADOS and parent rating scales in the rare instances in which such discordances occurred. At the time of their original study visit, children were required to exceed established cut-offs on both the ADOS and SCQ, or, in the event of a discordance between those measures, on both the ADOS and ADI-R. Children 1 point below ADOS cut-offs were included if they also exceeded cut-offs on at least two parent questionnaires or on the ADI-R. For children for whom original diagnosis was not made by an expert clinician according to DSM criteria (e.g., diagnoses made by a school), more rigorous standards were applied, and the child was required to exceed cut-offs on both the ADOS and ADI-R for inclusion in the ASD group. For final inclusion in the ASD group, children had to meet the above criteria at the time of their original study participation and also had to exceed diagnostic cut-offs on the ADOS at their two to five -year follow-up (parent rating scale corroboration was not required at follow-up). To rule out global cognitive delay, all subjects were required to score at or above the 2nd percentile (SS > 70) on the Perceptual Reasoning Index (PRI) of the Wechsler Intelligence Scale for Children-IV (WISC-IV; Wechsler, 2003). In all subjects, the WISC-IV Verbal Comprehension Index (VCI) was also obtained.
Inclusion criteria for the TD children included scoring below the cut-off for ASD on all domains of the ADOS as well as parent questionnaires, and performance above the 16th percentile on the Clinical Evaluation of Language Fundamentals—4th edition (CELF-4; Semel and Wiig, 2003). In addition to the above inclusion/exclusion criteria, all subjects and families were native English speakers and had no known genetic syndromes or neurological (e.g., cerebral palsy, epilepsy), or sensory (hearing, visual) impairments.
TD children were free of medications at both exams, except for one participant who was prescribed Naltrexone at follow-up. In the children with ASD, 12 were prescribed medications/took dietary supplements at the first scan. At follow-up, 14 took medications/supplements. The “had ASD” cohort did not report taking medications at the initial exam. At follow-up 3 “had ASD” participants were taking medication. Supplemental Table 1 provides medication information.
The study was approved by the CHOP Institutional Review Board and all participants' families gave written informed consent. As indicated by institutional policy, where competent to do so, children over the age of seven gave verbal assent.
2.3. Electrophysiological data collection
MEG data were obtained using a whole-cortex 275-channel system (VSM MedTech Inc., Coquitlam, BC) in a magnetically shielded room. Prior to data acquisition, three head-position indicator coils were attached to the participant's scalp at the nasion and left- and right-preauricular points. These head coils provided continuous specification of head position and orientation in relation to the MEG sensors. To minimize fatigue during the task, participants viewed (but did not listen to) a movie projected onto a screen positioned at a comfortable viewing distance. Electrodes were attached to the left and right clavicles for electrocardiogram recordings (ECG) and to the bipolar oblique (upper and lower left sites) for electro-oculogram recordings (EOG). A band-pass filter (0.03–300 Hz) was applied to the EOG, ECG, and MEG signals, with signals digitized at 1200 Hz, and with third-order gradiometer environmental noise reduction of the MEG data.
After the MEG session, structural magnetic resonance imaging (sMRI) including T1-weighted, 3D MP-RAGE anatomical images for source localization was acquired on a 3 T Siemens Verio™ scanner (Siemens Healthcare, Erlangen, Germany) with voxel size 0.8 × 0.8 × 0.9 mm3.
2.4. Stimuli
Stimuli consisted of sinusoidal tones presented using Eprime v1.1. Tones were presented via a sound pressure transducer and sound conduction tubing to the participant's peripheral auditory canal via ear-tip inserts (ER3A, Etymotic Research, IL). Prior to data acquisition, 1000 Hz tones (300 ms duration, 10 ms rise time) were presented binaurally and loudness monotonically decreased until reaching auditory threshold for each ear. Stimulus tones were then presented at 45 dB sensation level above threshold. During the task participants passively listened to binaurally presented interleaved 200, 300, 500 and 1000 Hz sinusoidal tones (tones of 300 ms duration; 10 ms ramps) with a 1000 ms (± 100) inter-trial interval. Participants heard a total of 130 tones/frequency.
2.5. Data preprocessing
MEG responses were analyzed using the MatLab (Mathworks, Natick, MA) open-source toolbox, Fieldtrip (Oostenveld et al., 2011). Using the continuous data and the procedures outlined in Fieldtrip (FieldtripWiki, 2015a, FieldtripWiki, 2015b), independent component analysis identified heartbeat and eye-movement artifacts (blinks and saccades), and then these artifact components were removed from the stimulus-locked epoched data (± 500 ms around trigger). Trials with jump and muscle artifact were also rejected (using Fieldtrip's Z-score based artifact rejection). Lastly, to account for differences in head motion during the MEG scan, if any fiducial moved > 10 mm from the average head position during a trial, that trial was rejected.
Subject-specific single-shell head models were created from each participant's MP-RAGE MRI. To coregister MEG and sMRI data, the three anatomical landmarks (nasion and right and left preauriculars points) as well as an additional 200 + points on the scalp and face were digitized for each participant using the Probe Position Identification (PPI) System (Polhemus, Colchester, VT), and a transformation matrix that involved rotation/translation between the MEG and sMRI coordinate systems was obtained via a least-squares match of the PPI points to the surface of the scalp and face. This head model was then fitted to the mean head position (in MEG coordinate space) as determined by the fiducials (Fig. 1A). Separately, the participant's MRI was normalized to an age-matched average brain template (Fonov et al., 2011) using non-linear warping. A left and right Heschl's Gyrus location was identified and then reverse transformed to subject space. A linearly-constrained minimum variance (LCMV) beamformer was computed for each participant's left and right Heschl's Gyrus, discarding the contralateral hemisphere's sensors to reduce inter-hemispheric signal cancelation due to correlated activity (Herdman et al., 2003). Heschl's Gyrus virtual electrodes (VE) were then computed using a dipole orientation optimized for post-stimulus gamma-band activity (i.e. orientation determined via principal component analysis on the 0–270 ms post-stimulus window filtered 30–58 Hz).
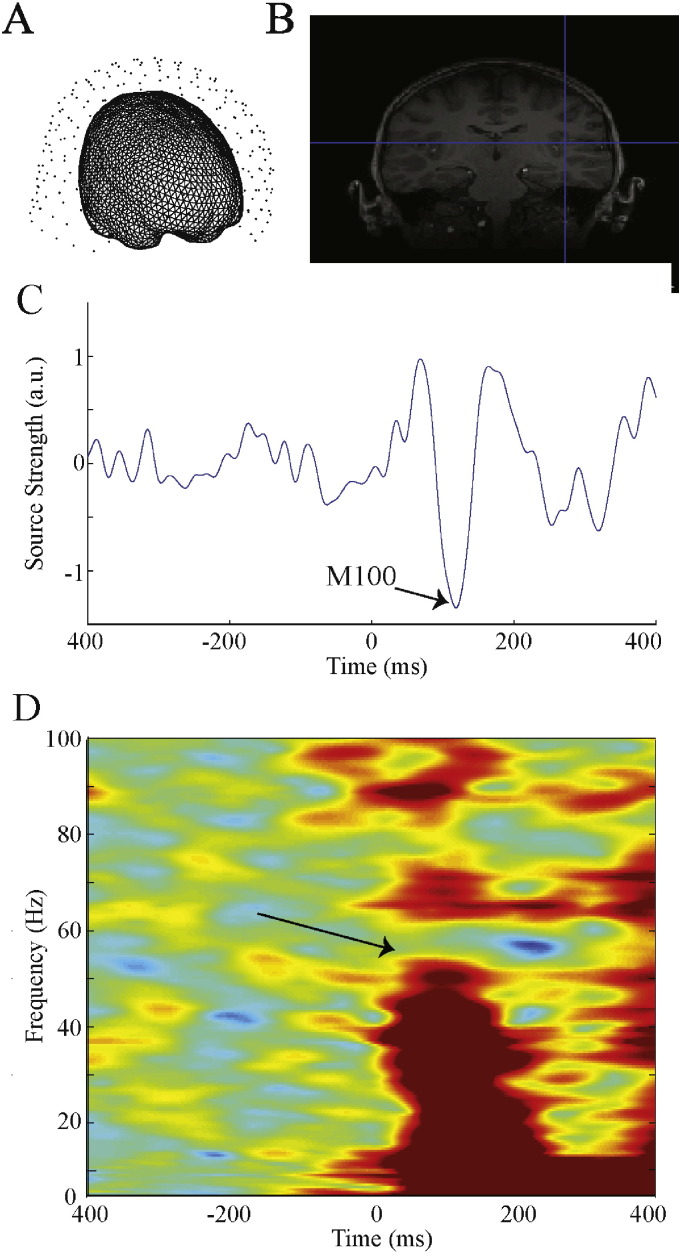
Fig. 1.
Gamma-band activity analyses. (A) Head models were generated from each subject's structural MRIs and centered at the average head position. Trials where any fiducial moved in any direction > 10 mm from this average position were rejected. (B) After normalizing individual MRIs to an age-matched template, Heschl's Gyrus was non-linear reverse source interpolated. (C) A LCMV beamformer (with only ipsilateral sensors included) at subject space Heschl's Gyrus was used to generate virtual electrode time courses. (D) Left and right STG time courses were used to obtain time frequency measures (evoked, total power, ITC) using in-house scripts. Arrow points to gamma-band activity response.
2.6. M100 data analysis
For each group, over 92% of trials remained after motion and artifact rejection (TD = 95.3 ± 1.29%, ASD = 92.7 ± 0.82%). A LMM assessed differences in the number of trials remaining between diagnostic groups. In this LMM, as well as all subsequent LMMs, data were originally modeled with the relevant fixed factors along with random intercepts for Subjects and random slopes for Timepoint. If the random factors proved redundant, the random slopes of Timepoint were removed and the model reran. LMM of the trials remaining for M100 latency analysis utilized random intercepts for Subjects and random slopes of Timepoint. This analysis showed a trend towards a main effect of Diagnosis (F(1,29.02) = 3.02, p < 0.10), and a marginally significant main effect of Timepoint (F(1,28.9) = 4.01 initial = 95.3 ± 0.98, follow-up = 92.7 ± 1.00, p = 0.052). The interaction of Diagnosis × Timepoint was not significant, nor was any term involving stimulus frequency. Although a group difference in number of evaluable trials was suggested, rejection rates across groups were considered low, and given generally similar mean values between the groups, this difference was deemed unlikely to affect any group difference M100 latency finding.
Determination of the latency of M100 sources in the left and right Heschl's Gyrus was accomplished using the data from the above analyses pipeline. In each participant, a 3–40 Hz band-pass filter was applied and then the left- and right-hemisphere M100 peaks identified as the largest point in the M100 scoring window (90–190 ms) using the sensor butterfly plot of the band-passed ipsilateral channel ERFs. To confirm that the M100 was accurately identified, magnetic field topographic plots over all ipsilateral sensors at the selected latency were examined to ensure a topography reflecting a M100 dipolar source.
Given a M100 latency dependence on stimulus frequency (Roberts and Poeppel, 1996), and given that M100 responses are occasionally missing/unidentifiable for individual stimulus tone frequencies, M100 analyses were performed using LMM with both random intercepts for Subjects and random slopes for Timepoint, with pairwise comparisons on the marginal means for Diagnosis, Timepoint, Hemisphere, Condition, as well as the Diagnosis × Hemisphere and Diagnosis × Timepoint, and Diagnosis × Timepoint × Hemisphere interactions. To investigate M100 latency maturation, LMMs were used to create a tone-frequency independent “effective” M100 latency for each hemisphere. The effective M100 latencies were then used to compute a rate of maturation: (follow-up M100 effective latency − initial M100 effective latency) / change in years. A LMM with random intercepts for Subjects investigated statistical differences in rate of maturation, with pairwise comparison for Diagnosis, Hemisphere and Diagnosis × Hemisphere.
2.7. Gamma-band data analysis
For each group, over 93% of trials remained (TD = 95.2 ± 0.83%, ASD = 93.1 ± 0.61%). A LMM analogous to that used for testing of group differences in number of artifact-free M100 trials showed a marginally significant main effect of Diagnosis (p = 0.052), and a main effect of Timepoint (F(1,21) = 5.28; initial = 95.0 ± 0.62, follow-up = 93.3 ± 0.64, p < 0.05). The interaction of Diagnosis × Timepoint was not significant, although ASD, but not TD, demonstrated a gain in the number of trials between initial and follow-up exams. Although a group difference in the number of evaluable trials was suggested, rejection rates across groups were considered low, and given generally similar mean trial values between the groups, this difference was deemed unlikely to affect any group difference gamma-band finding.
Broadband resultant VE timecourses were time-frequency transformed via Hilbert transforms using in-house MatLab scripts. For each participant, evoked power, total power (evoked and induced power) and inter-trial coherence (ITC) were calculated. Mean power/coherence was then derived for the spectrotemporal regions containing a gamma-band response. To avoid erroneously quantifying gamma-band activity by either a) not correctly accounting for gamma-band response maturation, or b) including low-level non-stimulus related signal, gamma-band responses were quantified based on a time-frequency region capturing both TD and ASD group-level activity (initial scan — evoked power: 10–180 ms, 30–100 Hz, ITC: 10–170 ms 30–65 Hz, total power: 20–180 ms, 30–58 Hz; follow-up scan — evoked power: 10–170 ms, 30–100 Hz, ITC: 10–150 ms, 30–75 Hz, total power: 20–160 ms, 30–57 Hz). Gamma-band analyses were performed using LMMs, with pairwise comparisons on Diagnosis, Hemisphere, Timepoint, Diagnosis × Hemisphere, Diagnosis × Timepoint, and Diagnosis × Time-point × Hemisphere. To test maturation group differences, a gamma-band activity maturation measure was computed: (follow-up gamma-band activity metric − initial gamma-band activity metric) / change in years.
2.8. Associations with behavior
To test hypotheses regarding the relationship of MEG measures to clinical measures as well as to examine the specificity of such associations, LMMs with random intercepts for Subjects examined associations between MEG measures and scores on a measure of ASD symptom severity (Social Responsiveness Scale, SRS; Constantino et al., 2003)), a measure of language functioning (CELF-4 core language index), a measure of global cognitive function (WISC-IV GAI), and a measure of verbal comprehension (WISC-IV VCI). With MEG measures as the dependent variable, in separate runs, LMMs examined associations with each of the above behavioral measures, co-varying for Timepoint and Age. As such, the association between the MEG measure and behavioral metric were obtained while concurrently removing variance due to Timepoint and Age.
Hierarchical regressions tested the hypotheses that initial MEG activity explained additional variance in the follow-up behavioral metrics (SRS, CELF-4 CLI, WISC-IV GAI & VCI) beyond the variance accounted for by initial behavioral measure and age. As such, the Dependent Variable of Behavioral Metric at follow-up was modeled with Age and Initial Behavioral Metric as Independent Variables entered into model first and then MEG measure entered second.
3. Results
As shown in Table 1, TD versus ASD did not differ on age at initial exam or at follow-up. As expected, children with ASD had higher SRS and ADOS Calibrated Severity Scores (CSS) (Gotham et al., 2009, Hus and Lord, 2014) than TD at both exams. No group differences were observed in global functioning (WISC-IV GAI) at either time point. Group differences in verbal functioning (WISC-IV VCI) were present at the initial exam but not at follow-up. Children with ASD scored significantly lower on the CELF-4 Core Language Index than TD at both exams. The population included in gamma-band activity analyses exhibited a profile similar to the above.
3.1. M100 latency
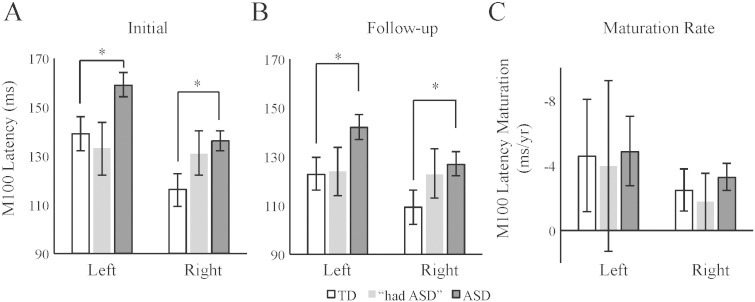
A main effect of Condition (stimulus tone), F(3,114.62) = 17.53 p < 0.001, showed the expected earlier M100 latencies to higher versus lower frequency tones (Roberts and Poeppel, 1996); a main effect of Hemisphere, F(1,154.53) = 111.76, p < 0.001, showed the expected earlier right than left M100 latencies (Roberts et al., 2000); and a main effect of Timepoint, F(1,21.77) = 8.52, p < 0.01, showed the excepted maturational change with earlier latencies at follow-up versus initial exam. The main effect of Diagnosis (ASD versus TD) was significant, F(1,23.27) = 7.43, p < 0.05, confirming the a priori hypothesis of delayed M100 latencies in ASD versus TD (TD = 122 ± 6.0 ms; ASD = 141 ± 4.0 ms). Simple effect analyses of a Diagnosis × Hemisphere × Timepoint interaction, F(2,152.94) = 3.30, p < 0.05, showed earlier M100 latencies at follow-up versus the initial exam in both groups and both hemispheres except for no significant right-hemisphere changes in TD. Of note, however, this interaction is potentially confounded due to inter-subject differences in duration of the inter-exam interval, which ranged from two to five years. As such the three-way interaction cannot be simply conceived as reflecting a simple maturation rate. None of the other interaction terms involving Diagnosis were significant. M100 latency values for all groups at both exams (marginal means after collapsing across condition) are shown in Fig. 2.
Fig. 2.
M100 latencies predict diagnosis. (A) t-Tests showed that children with ASD versus TD had prolonged left and right-hemisphere M100 latencies at the initial exam. (B) At the follow-up exam, children with ASD again showed prolonged M100 latencies. The “had ASD” exhibited non-significant intermediate M100 latencies at both timepoints. (C) No group differences between TD and ASD were present for maturation rates. Intermediate M100 latency maturation (as compared to TD and ASD) rates are exhibited by the “had ASD” cohort. Although analyses were conducted for only ASD and TD, mean and SE values are also shown for “the had” ASD group. #p < 0.10, *p < 0.05.
Analyses also examined group differences in the M100 latency maturation rates (i.e., (follow-up M100 effective latency − initial M100 effective latency) / change in years). Both TD and ASD demonstrated a ~ 3–5 ms/year M100 latency maturation, with no significant differences for Diagnosis, Hemisphere or their interaction (p > 0.05). Thus both TD and ASD showed similar maturational shortening of M100 latency.
3.2. Gamma-band activity
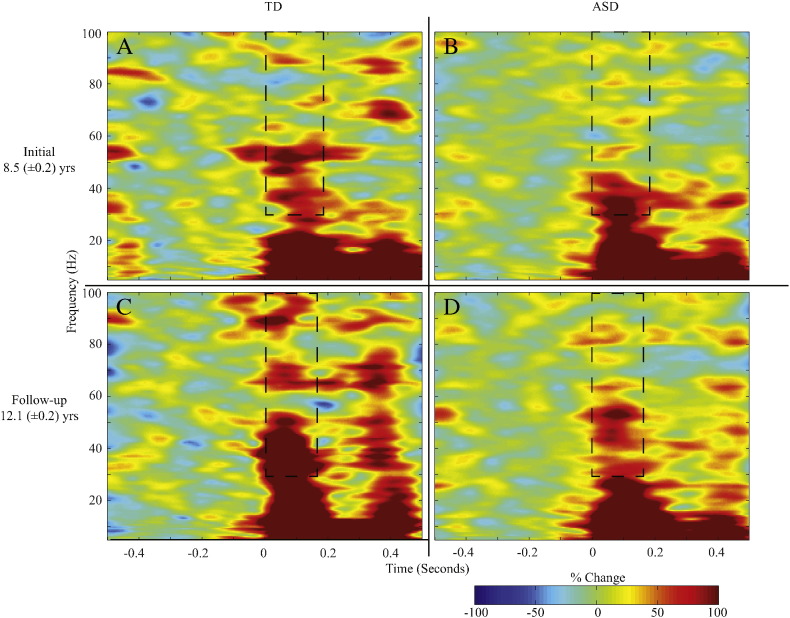
To increase signal-to noise, responses were averaged across condition (shown effective in (Gandal et al., 2010)). As shown in Fig. 3, at both exams, gamma-band activity was visible in the grand average time-frequency response for each group.
Fig. 3.
Children with ASD exhibit reduced gamma-band evoked power. (A) Group average evoked power plots for TD children (left) at initial exam (upper, A) and follow-up exam (lower, C) show the auditory gamma-band post-stimulus response. At both time points, children with ASD (right) showed reduced gamma-band responses (initial exam — upper, B; follow-up exam — lower, D). Dashed box shows gamma-band ROI used.
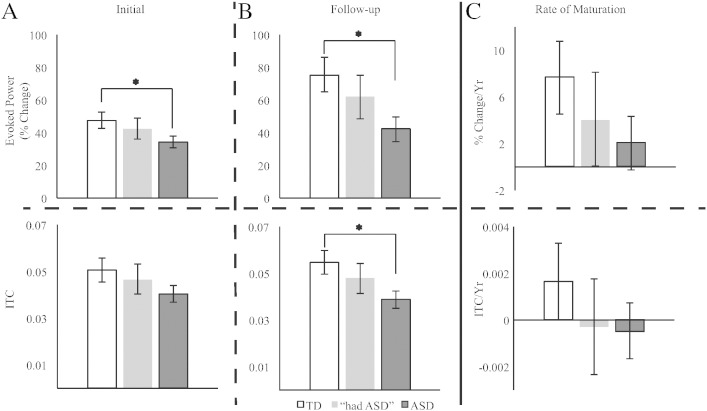
An LMM (with Subjects as a random intercept) tested for group differences in total power. Gamma-band total power did not differ between TD and ASD at either exam (p > 0.10; data not shown). For evoked power (tested via LMM with random intercept for Subject), a main effect of Diagnosis, F(1,41.6) = 8.68, p < 0.01, confirmed the a priori hypotheses of reduced gamma-band evoked activity in ASD (38.4 ± 4.7% change from baseline) versus TD (61.5 ± 6.4% change from baseline; Fig. 4A & B). The main effect of Timepoint was also significant, F(1,43.07) = 8.04, p < 0.01, demonstrating maturation of evoked response power over time (initial = 41.1 ± 3.1% change from baseline, follow-up = 58.8 ± 6.4% change from baseline; Fig. 4). The main effect of Hemisphere and Hemisphere interaction terms were not significant (F(1,43.1) or F(2,43.1) < 0.50, p > 0.1). As such, responses are collapsed across hemispheres for Fig. 4. Additionally the Diagnosis × Timepoint interaction term was not significant (F(1,43.1) = 2.56, p > 0.1). The rate of change in evoked gamma-band activity for TD (7.7 ± 3.0% change from baseline/year) was not significantly different from ASD (2.1 ± 2.2% change from baseline /year; F(1,38.44) = 2.30, p > 0.1, Fig. 4). Again, the main effect of hemisphere and it's interaction term were not significant (F(1,38.44) < 0.98, p > 0.1), and so data were collapsed across hemisphere in Fig. 4.
Fig. 4.
Children with ASD exhibit reduced gamma-band evoked power and inter-trial coherence. Evoked power (top row) responses were reduced in children with ASD at both initial (A) and follow-up exam (B). The “had ASD” group exhibited qualitatively intermediate responses at both time-points. Maturation of the evoked gamma-band response (upper C) was reduced four-fold in ASD versus TD. ITC (bottom row) demonstrated a similar pattern, though not significant at initial exam. #p < 0.10, *p < 0.05.
For gamma-band ITC (tested via LMM with random intercepts for Subjects and Timepoint as a random slope), a main effect of Diagnosis, F(1,22.24) = 6.30, p < 0.05, indicated greater ITC in TD versus ASD (ASD = 0.040 ± 0.003 ITC, TD = 0.053 ± 0.004 ITC). Both the main effect of Timepoint and its interaction with Diagnosis were not significant (F(1,19.9) < 0.55, p > 0.1). Additionally, similar to evoked power, the main effect of Hemisphere and Hemisphere interaction terms were not significant (F(1,42.02) = 0.41, F(2,42.02) = 0.98, p > 0.1). As such, responses were again collapsed across hemispheres. As shown in Fig. 4, analyses examining ITC maturation (LMM with random intercepts for Subjects) showed no significant group differences. As with evoked power maturation, the main effect of Hemisphere and the interaction terms were not significant (F(1,21) = 1.3, p > 0.1). As such, data were collapsed across hemisphere in Fig. 4.
3.3. Correlation of MEG auditory biomarkers and behavioral metrics
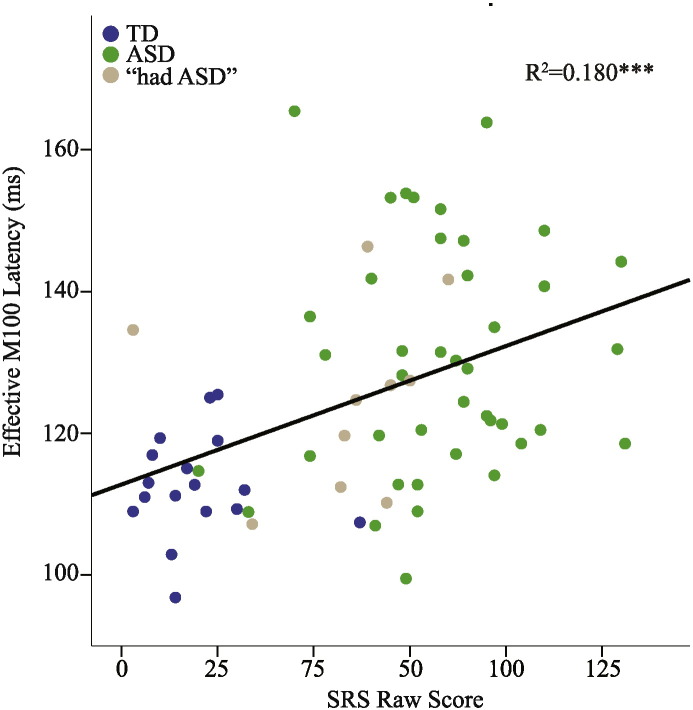
Data from all participants (including “had ASD”) were included in the correlational analyses. To ensure specificity of any associations and to provide negative controls, all comparisons between behavioral metrics and MEG-derived measures (excluding rates of maturation) were examined. Of note, the behavioral measures (SRS, CELF-4 CLI, WISC-IV GAI, WISC-IV VCI) shared considerable variance, especially the CELF-4 CLI, WISC-IV GAI and WISC-IV VCI, with up to 80% variance shared; see Supplemental Table 2 for bivariate correlations among behavioral measures. For all subsequent analyses, gamma-band activity, but not M100 latencies, were averaged across hemisphere due to the lack of significant effects of Hemisphere. Examining all participants at both timepoints, both left- and right-hemisphere M100 latencies were associated with SRS after removing variance associated with Age and Time (LH: SRS F(1,54.30) = 6.48 p < 0.05, estimate = 0.2 ms/SRS point, RH: SRS F(1,62.38) = 15.20 p < 0.001, estimate = 0.2 ms/SRS point; Fig. 5). No associations were observed for CELF-4 Core Language Index or WISC-IV metrics (GAI/VCI) in either hemisphere (p > 0.1), or for any gamma-band metric.
Fig. 5.
M100 latencies and SRS scores. Effective right-hemisphere M100 latencies (removing effect of condition) were associated with social responsiveness scores across the study population. ***p < 0.001.
Although M100 latency did not correlate with current language scores, right-hemisphere initial M100 latency predicted additional variance in the follow-up CELF-4 Core Language Index metric (R2 change = 0.03, p < 0.05). In addition, a trend was observed for an association between initial evoked gamma-band power and follow-up WISC-IV VCI (R2 change = 0.033, p < 0.1). Furthermore, initial gamma-band ITC predicted additional variance in follow-up WISC-IV VCI after removing the effect of age and initial WISC-IV VCI score (R2 change = 0.05, p < 0.05). Thus, these MEG measure may offer a prognostic indication of language outcome, perhaps signifying “capacity for improvement”.
3.4. Qualitative description of “had ASD”
As previously mentioned, a third group of participants emerged over the course of the study — the “had ASD” group. Although too small for statistical assessment, data from these participants are included as descriptive preliminary findings. These participants showed a diagnostic profile similar to the primary ASD group at the initial exam (Table 1, Table 2, SRS = 75.0, ADOS CSS = 7.8). At follow-up, however, these participants had intermediate SRS and ADOS scores (Table 1, Table 2, SRS = 54.4 ADOS CSS = 2.5). Such “optimal outcomes” have been demonstrated before, with such children tending to have initial language/communication scores that predict outcome (Helt et al., 2008). Consistent with this, these five children scored between TD and ASD on the WISC-IV VCI at both time points (initial = 104.2, follow-up = 109.6).
With regard to M100 latencies, as shown in Fig. 2A, M100 latencies in the “had ASD” group at the initial exam were either similar to TD (left hemisphere) or intermediate between TD and ASD (right hemisphere). As shown in 2B and 2C, this profile was also observed at follow-up as well as for the M100 latency maturation rate measure. In addition, “had ASD” showed gamma-band evoked power and ITC values between TD and ASD at the initial and follow-up exams. Follow-up studies are needed to confirm if this represents an electrophysiological signature of “capacity for improvement”.
4. Discussion
M100 latencies and phase-locked gamma-band evoked power matured from initial to follow-up exam. This finding supports findings from cross-sectional studies (Paetau et al., 1995, Rojas et al., 2006). In addition, similar to previous studies (Edgar et al., 2015a, Edgar et al., 2015b, Edgar et al., 2016, Gage et al., 2003b, Roberts et al., 2010, Roberts et al., 2013, Wilson et al., 2007), delayed right-hemisphere M100 latencies and reduced bilateral gamma-band evoked power and ITC were observed in ASD versus TD. Of note, contrary to previous studies, left-hemisphere M100 group differences were also observed in ASD, with resolution of bilateral M100 latency findings in the present study perhaps attributable to greater statistical power in a longitudinal study. As such, the observation of bilateral M100 latency delays questions the hemispheric specificity of prosody versus phoneme processing in ASD alluded to in the introduction, at least at ~ 100 ms. Rather than local processing of speech related sounds, M100 latency deficits may be driven by hemispherically non-specific white-matter microstructural integrity alterations leading to reduced conduction velocity (Dockstader et al., 2012, Roberts et al., 2009, Stufflebeam et al., 2008). Such a statement is supported by the bilateral phase-locked gamma-band activity findings, which did not show any hemisphere effect, suggesting that local circuitry in both auditory cortices are perturbed in ASD rather than a hemisphere-selective deficit.
Interestingly, in the present study M100 latency delays were greater than those reported in previous studies (Edgar et al., 2015b, Gage et al., 2003b, Roberts et al., 2010, Roberts et al., 2013), an effect perhaps due to the use of cross-sectional designs in previous studies. In particular, whereas cross-sectional studies likely include some children that later exhibit “optimal outcome”, in the present study, diagnostic change information was available and the ASD subjects showing significant improvement were excluded from the primary analyses. Indeed, reanalysis of the data for timepoint 1 including the “had ASD” individuals in the ASD group (as would have happened in a cross-sectional design) decreased the effect size by several milliseconds (although significant group differences were still exhibited).
Maturation rates for M100 latency as well as the gamma-band metrics did not differ between TD and ASD, a finding that confirms previous cross-sectional maturation rate estimates (Edgar et al., 2014b, Roberts et al., 2013) As such, present findings support findings from previous studies suggesting a perturbed developmental trajectory, despite a similar maturation rate, that is due to a very early in development latency offset.
Analyses examined the relationship of the two auditory biomarkers to current and future behavior. Four separate, though related, behavioral metrics were tested in order to examine the specificity of associations. Right- and left-hemisphere M100 latencies were associated with greater clinical impairment, here quantified as higher SRS scores. To the authors' knowledge, this is the first time such associations have been demonstrated.
Although analogous relationships were not observed for the gamma-band metrics, initial gamma-band ITC predicted variance in follow-up WISC-IV VCI scores. Gamma-band evoked power exhibited a similar, though non-significant, relationship to WISC-IV VCI scores. Somewhat unexpectedly, right M100 latencies also predicted change in CELF-4 Core Language Index scores. Although in previous studies gamma-band metrics predicted future cognitive and language abilities (Gou et al., 2011), such associations have not been previously reported for M100 latencies. Although the finding of current right-hemisphere M100 associations with subsequent CELF-4 Core Language Index score may appear contradictory given the lack of association with contemporaneous behavior, a potential explanation may be that language-related issues arising from M100 latency prolongations are cumulative. As such, the later language-related measures are taken the greater the observed language deficit for some subjects. Thus, it is hypothesized that a M100 latency delay relates to diminished capacity for language improvement and also that an earlier M100 latency predicts the availability of capacity for improvement. Given “capacity” for improvement, the degree of improvement will likely depend on interventions in the follow-up interval, and thus the M100 latency measure is suggested as a prognostic/predictive biomarker for future intervention studies. Previous cross-sectional studies examining associations between M100 latency and general language ability are inconsistent (Oram Cardy et al., 2008, Roberts et al., 2010, Roberts et al., 2013), perhaps due to the prevalence, as well as variety, of language impairments in ASD or the fact that the language measure was taken concurrently with the M100 measurement. Of note, the M100 latency delay in children appears to be specific to ASD, at least in comparison to children with specific language impairment (SLI) (Roberts et al., 2012).
A few study limitations are of note. First, a potential confound is the gender bias (more females in the control group). In a separate analysis, previously collected and published datasets were investigated for gender effects for M100 latency and gamma-band evoked power and ITC (Edgar et al., 2015b, Roberts et al., 2010). The main effect of Gender was not significant for any analysis. Gender therefore likely does not confound present findings.
A second limitation is that children were only scanned twice with a two to five year inter-scan interval. Multiple time points are required to determine if the findings observed here extend to other developmental periods, for example before or after the age range sampled in the present study. Longer follow-up periods would also provide resolution of statistical tendencies in maturation rates that did not reach significance in the present study. Multiple timepoints and an extended study age-range would also permit the consideration of non-linear dependencies of observed variables on age.
Finally, given the small sample, statistical analyses could not be performed on the “has ASD” cohort. In these children, M100 latency and gamma-band findings were qualitatively observed to be intermediate between TD and the remaining ASD group, suggesting that these auditory neural measures may serve as prognostic biomarkers. These findings are only suggestive though, and a larger cohort is needed to confirm and establish the precision/sensitivity of the above observations. As noted in the Introduction, the endogenous, environmental or therapeutic factors that contribute to clinical change are currently unknown. The literature on “optimal outcomes”, however, does indicate that in children who exhibit “optimal outcome” subtle impairments persist (Orinstein et al., 2015), a finding supported in the present study via the observation in “had ASD” of auditory neural measures at follow-up with values between TD and ASD. In future studies with larger samples, quantitative assessments of the intervention/treatments the children with ASD receive are needed to help identify the basis and/or mechanism for improvement.
To conclude, the present longitudinal study demonstrated altered auditory M100 and gamma-band neural activity in children with ASD. These abnormalities were persistent over the maturation period observed (several years). Electrophysiological measures correlated with and predicted subsequent change in behavioral measures. The children with ASD who showed clinical improvement appeared to have somewhat more intermediate electrophysiological ‘abnormalities’ at first and follow-up exams. As such, present findings suggest that the auditory neural measures investigated in this study may serve as prognostic biomarkers, with further study needed to validate such findings.
Financial disclosures
This work was supported in part by grants from the NIH (R01DC008871-TR) and the Nancy Lurie Marks Family Foundation (NLMFF-TR), and a pre-doctoral fellowship from the Autism Science Foundation (ASF- RGP), and a NIH IDDRC grant to CHOP (U54086984). Dr. Roberts thanks the Oberkircher family for the Oberkircher Family Chair in Pediatric Radiology at CHOP. Dr. Roberts discloses consulting arrangements with Prism Clinical Imaging, Siemens Medical Solutions, Elekta Oy, Guerbet and Johnson and Johnson (Janssen division). Dr Port, Dr Edgar, Mr Ku, Dr Bloy, Ms Murray, Dr Blaskey and Dr Levy declare no financial conflicts.
Acknowledgements
The Authors would like to thank John Dell, Peter Lam and Rachel Golembski for technical assistance. This data was previously presented in part at IMFAR 2015, and a subset of the total data was reported in Roberts et al. (2010) and Edgar et al. (2015b).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.03.021.
Appendix A. Supplementary data
Supplementary tables.
References
- American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders. fifth ed. American Psychiatric Publishing, Inc.; Washington, DC: 2013. [Google Scholar]
- Banerjee A., García-Oscos F., Roychowdhury S., Galindo L.C., Hall S., Kilgard M.P., Atzori M. Impairment of cortical GABAergic synaptic transmission in an environmental rat model of autism. Int. J. Neuropsychopharmacol. 2013;16:1309–1318. doi: 10.1017/S1461145712001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich A.A., Gou Z., Choudhury N., Harris K.D. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav. Brain Res. 2008;195:215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J.I., Chudnovskaya D., Blaskey L., Kuschner E., Mukherjee P., Buckner R., Nagarajan S., Chung W.K., Sherr E.H., Roberts T.P.L. Relationship between M100 auditory evoked response and auditory radiation microstructure in 16p11.2 deletion and duplication carriers. Am. J. Neuroradiol. 2016:1–7. doi: 10.3174/ajnr.A4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingslea E.N., Tatard-Leitman V.M., Anguiano J., Jutzeler C.R., Suh J., Saunders J.A., Morita S., Featherstone R.E., Ortinski P.I., Gandal M.J., Lin R., Liang Y., Gur R.E., Carlson G.C., Hahn C., Siegel S.J. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39:1603–1613. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.S., Singel D., Hepburn S., Rojas D.C. Increased glutamate concentration in the auditory cortex of persons with autism and first-degree relatives: a (1)H-MRS study. Autism Res. 2013;6:1–10. doi: 10.1002/aur.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Wang X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin J.A., Carlén M., Meletis K., Knoblich U., Zhang F., Deisseroth K., Tsai L.-H., Moore C.I. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellot G., Cherubini E. Reduced inhibitory gate in the barrel cortex of Neuroligin3R451C knock-in mice, an animal model of autism spectrum disorders. Physiol. Rep. 2014;2:e12077. doi: 10.14814/phy2.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J., Gruber C.P. 2nd Editio. ed. Western Psychological Services; Los Angeles, CA: 2012. Social Responsiveness Scale. [Google Scholar]
- Constantino J.N., Davis S.A., Todd R.D., Schindler M.K., Gross M.M., Brophy S.L., Metzger L.M., Shoushtari C.S., Splinter R., Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the Autism Diagnostic Interview-Revised. J. Autism Dev. Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Crawley J.N. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2007. What's Wrong With My Mouse? [Google Scholar]
- Danielsson S., Gillberg I.C., Billstedt E., Gillberg C., Olsson I. Epilepsy in young adults with autism: a prospective population-based follow-up study of 120 individuals diagnosed in childhood. Epilepsia. 2005;46:918–923. doi: 10.1111/j.1528-1167.2005.57504.x. [DOI] [PubMed] [Google Scholar]
- Dawson G., Jones E.J.H., Merkle K., Venema K., Lowy R., Faja S., Kamara D., Murias M., Greenson J., Winter J., Smith M., Rogers S.J., Webb S.J. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Rogers S., Munson J., Smith M., Winter J., Greenson J., Donaldson A., Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators, Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- Dockstader C., Gaetz W., Rockel C., Mabbott D.J. White matter maturation in visual and motor areas predicts the latency of visual activation in children. Hum. Brain Mapp. 2012;33:179–191. doi: 10.1002/hbm.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J.C., Chen Y.-H., Lanza M., Howell B., Chow V.Y., Heiken K., Liu S., Wootton C., Hunter M.A., Huang M., Miller G.A., Cañive J.M. Cortical thickness as a contributor to abnormal oscillations in schizophrenia? NeuroImage Clin. 2014;4:122–129. doi: 10.1016/j.nicl.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J.C., Lanza M.R., Daina A.B., Monroe J.F., Khan S.Y., Blaskey L., Cannon K.M., Jenkins J., Qasmieh S., Levy S.E., Roberts T.P.L. Missing and delayed auditory responses in young and older children with autism spectrum disorders. Front. Hum. Neurosci. 2014;8:417. doi: 10.3389/fnhum.2014.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J.C., Fisk Iv C.L., Berman J.I., Chudnovskaya D., Liu S., Pandey J., Herrington J.D., Port R.G., Schultz R.T., Roberts T.P.L. Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Mol. Autism. 2015;6 doi: 10.1186/s13229-015-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J.C., Khan S.Y., Blaskey L., Chow V.Y., Rey M., Gaetz W., Cannon K.M., Monroe J.F., Cornew L., Qasmieh S., Liu S., Welsh J.P., Levy S.E., Roberts T.P.L. Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in autism spectrum disorders. J. Autism Dev. Disord. 2015;45:395–405. doi: 10.1007/s10803-013-1904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J.C., Fisk C.L., IV, Liu S., Pandey J., Herrington J.D., Schultz R.T., Roberts T.P.L. Translating adult electrophysiology findings to younger patient populations: difficulty measuring 40-Hz auditory steady-state responses in typically developing children and children with autism spectrum disorder. Dev. Neurosci. 2016 doi: 10.1159/000441943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M., Volein A., Csibra G., Holmboe K., Garwood H., Tucker L., Krljes S., Baron-Cohen S., Bolton P., Charman T., Baird G., Johnson M.H. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biol. Psychiatry. 2009;65:31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Engineer C.T., Centanni T.M., Im K.W., Borland M.S., Moreno N.A., Carraway R.S., Wilson L.G., Kilgard M.P. Degraded auditory processing in a rat model of autism limits the speech representation in non-primary auditory cortex. Dev. Neurobiol. 2014;74:972–986. doi: 10.1002/dneu.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C.A., Veenstra-Vanderweele J.M., Melmed R.D., McCracken J.T., Ginsberg L.D., Sikich L., Scahill L., Cherubini M., Zarevics P., Walton-Bowen K., Carpenter R.L., Bear M.F., Wang P.P., King B.H. STX209 (arbaclofen) for autism spectrum disorders: an 8-week open-label study. J. Autism Dev. Disord. 2014;44:958–964. doi: 10.1007/s10803-013-1963-z. [DOI] [PubMed] [Google Scholar]
- Fatemi S.H., Folsom T.D., Reutiman T.J., Thuras P.D. Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum. 2009;8:64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.H., Reutiman T.J., Folsom T.D., Thuras P.D. GABA(A) receptor downregulation in brains of subjects with autism. J. Autism Dev. Disord. 2009;39:223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.H., Halt A.R., Stary J.M., Kanodia R., Schulz S.C., Realmuto G.R. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol. Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi S.H., Reutiman T.J., Folsom T.D., Rustan O.G., Rooney R.J., Thuras P.D. Downregulation of GABAA receptor protein subunits α6, β2, δ, ε, γ2, θ, and ρ2 in superior frontal cortex of subjects with autism. J. Autism Dev. Disord. 2014;44:1833–1845. doi: 10.1007/s10803-014-2078-x. [DOI] [PubMed] [Google Scholar]
- FieldtripWiki Use independent component analysis (ICA) to remove ECG artifacts [WWW Document] 2015. http://www.fieldtriptoolbox.org/example/use_independent_component_analysis_ica_to_remove_ecg_artifacts?s[]=artifact&s[]=removal URL. (accessed 1.1.15)
- FieldtripWiki Use independent component analysis (ICA) to remove EOG artifacts [WWW Document] 2015. http://www.fieldtriptoolbox.org/example/use_independent_component_analysis_ica_to_remove_eog_artifacts?s[]=artifact&s[]=removal URL. (accessed 1.1.15)
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W., Bloy L., Wang D.J., Port R.G., Blaskey L., Levy S.E., Roberts T.P.L. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. NeuroImage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage N.M., Siegel B., Callen M., Roberts T.P.L. Cortical sound processing in children with autism disorder: an MEG investigation. Neuroreport. 2003;14:2047–2051. doi: 10.1097/00001756-200311140-00008. [DOI] [PubMed] [Google Scholar]
- Gage N.M., Siegel B., Roberts T.P. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Dev. Brain Res. 2003;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- Gandal M.J., Edgar J.C., Ehrlichman R.S., Mehta M., Roberts T.P.L., Siegel S.J. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol. Psychiatry. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal M.J., Sisti J., Klook K., Ortinski P.I., Leitman V., Liang Y., Thieu T., Anderson R., Pierce R.C., Jonak G., Gur R.E., Carlson G., Siegel S.J. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl. Psychiatry. 2012;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N., LeBlanc J.J., Quast K.K.B., Südhof T.C., Fagiolini M., Hensch T.K. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J. Neurodev. Disord. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N., Takesian A.E.E., Feng G., Fagiolini M., Hensch T.K.K. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K., Pickles A., Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J. Autism Dev. Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Z., Choudhury N., Benasich A.A. Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behav. Brain Res. 2011;220:263–270. doi: 10.1016/j.bbr.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granpeesheh D., Tarbox J., Dixon D.R., Carr E., Herbert M. Retrospective analysis of clinical records in 38 cases of recovery from autism. Ann. Clin. Psychiatry. 2009;21:195–204. (doi:19917210) [PubMed] [Google Scholar]
- Grice S.J., Spratling M.W., Karmiloff-Smith A., Halit H., Csibra G., de Haan M., Johnson M.H. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. Neuroreport. 2001;12:2697–2700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- Han S., Tai C., Westenbroek R.E., Yu F.H., Cheah C.S., Potter G.B., Rubenstein J.L., Scheuer T., de la Iglesia H.O., Catterall W.A. Autistic-like behaviour in Scn1a +/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M., Taki M.M., Nose A., Kubo H., Mori K., Nishitani H., Matsuda T. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J. Autism Dev. Disord. 2011;41:447–454. doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- Hari R., Aittoniemi K., Järvinen M.L., Katila T., Varpula T. Auditory evoked transient and sustained magnetic fields of the human brain. Localization of neural generators. Exp. Brain Res. 1980;40:237–240. doi: 10.1007/BF00237543. [DOI] [PubMed] [Google Scholar]
- Helt M., Kelley E., Kinsbourne M., Pandey J., Boorstein H., Herbert M., Fein D. Can children with autism recover? If so, how? Neuropsychol. Rev. 2008;18:339–366. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- Herdman A.T., Wollbrink A., Chau W., Ishii R., Ross B., Pantev C. Determination of activation areas in the human auditory cortex by means of synthetic aperture magnetometry. NeuroImage. 2003;20:995–1005. doi: 10.1016/S1053-8119(03)00403-8. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Handbook of Clinical Neurology. first ed. Elsevier B.V.; 2015. Neural basis of speech perception. [DOI] [PubMed] [Google Scholar]
- Hus V., Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J. Autism Dev. Disord. 2014;44:1996–2012. doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley E., Paul J.J., Fein D., Naigles L.R. Residual language deficits in optimal outcome children with a history of autism. J. Autism Dev. Disord. 2006;36:807–828. doi: 10.1007/s10803-006-0111-4. [DOI] [PubMed] [Google Scholar]
- Krishnan G.P., Hetrick W.P., Brenner C.A., Shekhar A., Steffen A.N., O'Donnell B.F. Steady state and induced auditory gamma deficits in schizophrenia. NeuroImage. 2009;47:1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence Y.A., Kemper T.L., Bauman M.L., Blatt G.J. Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurol. Scand. 2010;121:99–108. doi: 10.1111/j.1600-0404.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Leventhal B.L., Dilavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Maharajh K., Abrams D., Rojas D.C., Teale P., Reite M.L. Auditory steady state and transient gamma band activity in bipolar disorder. Int. Congr. Ser. 2007;1300:707–710. [Google Scholar]
- McFadden K.L., Hepburn S., Winterrowd E., Schmidt G.L., Rojas D.C. Abnormalities in gamma-band responses to language stimuli in first-degree relatives of children with autism spectrum disorder: an MEG study. BMC Psychiatry. 2012;12:213. doi: 10.1186/1471-244X-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettigan C., Scott S.K. Cortical asymmetries in speech perception: what's wrong, what's right and what's left? Trends Cogn. Sci. 2012;16:269–276. doi: 10.1016/j.tics.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaddes N.M., Tutkunkardas M.D., Sari O., Aydin A., Kozanoglu P. Characteristics of children who lost the diagnosis of autism: a sample from Istanbul, Turkey. Autism Res. Treat. 2014;2014:1–10. doi: 10.1155/2014/472120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak A.L., Gibbs T.T., Blatt G.J. Decreased GABA(B) receptors in the cingulate cortex and fusiform gyrus in autism. J. Neurochem. 2010;114:1414–1423. doi: 10.1111/j.1471-4159.2010.06858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:1–9. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram Cardy J.E., Flagg E.J., Roberts W., Roberts T.P.L. Auditory evoked fields predict language ability and impairment in children. Int. J. Psychophysiol. 2008;68:170–175. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Orinstein A.J., Suh J., Porter K., De Yoe K.A., Tyson K.E., Troyb E., Barton M.L., Eigsti I.-M., Stevens M.C., Fein D.A. Social function and communication in optimal outcome children and adolescents with an autism history on structured test measures. J. Autism Dev. Disord. 2015 doi: 10.1007/s10803-015-2409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetau R., Ahonen A., Salonen O., Sams M. Auditory evoked magnetic fields to tones and pseudowords in healthy children and adults. J. Clin. Neurophysiol. 1995;12:177–185. doi: 10.1097/00004691-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Piven J., Palmer P., Landa R., Santangelo S., Jacobi D., Childress D. Personality and language characteristics in parents from multiple-incidence autism families. Am. J. Med. Genet. 1997;74:398–411. [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as “asymmetric sampling in time.”. Speech Comm. 2003;41:245–255. [Google Scholar]
- Port R.G., Anwar A.R., Ku M., Carlson G.C., Siegel S.J., Roberts T.P.L. Prospective MEG biomarkers in ASD: pre-clinical evidence and clinical promise of electrophysiological signatures. Yale J. Biol. Med. 2015;88:25–36. [PMC free article] [PubMed] [Google Scholar]
- Port R.G., Gandal M.J., Roberts T.P.L., Siegel S.J., Carlson G.C. Convergence of circuit dysfunction in ASD: a common bridge between diverse genetic and environmental risk factors and common clinical electrophysiology. Front. Cell. Neurosci. 2014;8:1–14. doi: 10.3389/fncel.2014.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell A.E., Jeon O.H., Zimmerman A.W., Blue M.E., Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Roberts T.P., Poeppel D. Latency of auditory evoked M100 as a function of tone frequency. Neuroreport. 1996;7:1138–1140. doi: 10.1097/00001756-199604260-00007. [DOI] [PubMed] [Google Scholar]
- Roberts T.P., Ferrari P., Stufflebeam S.M., Poeppel D. Latency of the auditory evoked neuromagnetic field components: stimulus dependence and insights toward perception. J. Clin. Neurophysiol. 2000;17:114–129. doi: 10.1097/00004691-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Roberts T.P.L., Heiken K., Kahn S.Y., Qasmieh S., Blaskey L., Solot C., Parker W.A., Verma R., Edgar J.C. Delayed magnetic mismatch negativity field, but not auditory M100 response, in specific language impairment. Neuroreport. 2012;23:463–468. doi: 10.1097/WNR.0b013e32835202b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T.P.L., Khan S.Y., Blaskey L., Dell J., Levy S.E., Zarnow D.M., Edgar J.C. Developmental correlation of diffusion anisotropy with auditory-evoked response. Neuroreport. 2009;20:1586–1591. doi: 10.1097/WNR.0b013e3283306854. [DOI] [PubMed] [Google Scholar]
- Roberts T.P.L., Khan S.Y., Rey M., Monroe J.F., Cannon K., Blaskey L., Woldoff S., Qasmieh S., Gandal M., Schmidt G.L., Zarnow D.M., Levy S.E., Edgar J.C. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 2010;3:8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T.P.L., Lanza M.R., Dell J., Qasmieh S., Hines K., Blaskey L., Zarnow D.M., Levy S.E., Edgar J.C., Berman J.I. Maturational differences in thalamocortical white matter microstructure and auditory evoked response latencies in autism spectrum disorders. Brain Res. 2013;1537:79–85. doi: 10.1016/j.brainres.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D.C., Becker K.M., Wilson L.B. Magnetic resonance spectroscopy studies of glutamate and GABA in autism: implications for excitation-inhibition imbalance theory. Curr. Dev. Disord. Rep. 2015;2:46–57. [Google Scholar]
- Rojas D.C., Maharajh K., Teale P.D., Kleman M.R., Benkers T.L., Carlson J.P., Reite M.L. Development of the 40 Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin. Neurophysiol. 2006;117:110–117. doi: 10.1016/j.clinph.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Rojas D.C., Maharajh K., Teale P., Rogers S.J. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8:66. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D.C., Singel D., Steinmetz S., Hepburn S., Brown M.S. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. NeuroImage. 2014;86:28–34. doi: 10.1016/j.neuroimage.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D.C., Teale P.D., Maharajh K., Kronberg E., Youngpeter K., Wilson L.B., Wallace A., Hepburn S. Transient and steady-state auditory gamma-band responses in first-degree relatives of people with autism spectrum disorder. Mol. Autism. 2011;2:11. doi: 10.1186/2040-2392-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J.L.R., Merzenich M.M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N.M., Hornickel J., Nicol T., Zecker S., Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behav. Brain Funct. 2010;6:60. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lloyd C. Western Psychological Services; Los Angeles, CA: 2003. SCQ: Social Communication Questionnaire. [Google Scholar]
- Saunders J.A., Tatard-Leitman V.M., Suh J., Billingslea E.N., Roberts T.P., Siegel S.J. Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Res. 2013;6:69–77. doi: 10.1002/aur.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E.M., Wiig E.H. The Psychological Corporation; San Antonio, TX: 2003. Clinical Evaluation Of Language Fundamentals (CELF-4) [Google Scholar]
- Silverman J.L., Yang M., Lord C., Crawley J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal V.S., Zhang F., Yizhar O., Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stufflebeam S.M., Witzel T., Mikulski S., Hämäläinen M.S., Temereanca S., Barton J.J.S., Tuch D.S., Manoach D.S. A non-invasive method to relate the timing of neural activity to white matter microstructural integrity. NeuroImage. 2008;42:710–716. doi: 10.1016/j.neuroimage.2008.04.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Grützner C., Bölte S., Wibral M., Tozman T., Schlitt S., Poustka F., Singer W., Freitag C.M., Uhlhaas P.J. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. J. Neurosci. 2012;32:9563–9573. doi: 10.1523/JNEUROSCI.1073-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M., Hugdahl K. Lateralization of auditory-cortex functions. Brain Res. Rev. 2003;43:231–246. doi: 10.1016/j.brainresrev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Tierney A.L., Gabard-Durnam L., Vogel-Farley V., Tager-Flusberg H., Nelson C.A. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLoS One. 2012;7:e39127. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R.D., Whittington M.A., Colling S.B., Buzsáki G., Jefferys J.G. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J. Physiol. 1996;493(Pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecke A.V., Stevens S., Carson A.M., Karst J.S., Dolan B., Schohl K., McKindles R.J., Remmel R., Brockman S. Measuring the plasticity of social approach: a randomized controlled trial of the effects of the PEERS intervention on EEG asymmetry in adolescents with autism spectrum disorders. J. Autism Dev. Disord. 2013 doi: 10.1007/s10803-013-1883-y. [DOI] [PubMed] [Google Scholar]
- Wechsler D. third ed. The Psychological Corporation; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Whittington M.A., Traub R.D., Kopell N., Ermentrout B., Buhl E.H. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int. J. Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Wilson T.W., Rojas D.C., Reite M.L., Teale P.D., Rogers S.J. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol. Psychiatry. 2007;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara A. Correlation between EEG abnormalities and symptoms of autism spectrum disorder (ASD) Brain Dev. 2010;32:791–798. doi: 10.1016/j.braindev.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Zappella M. Early-onset Tourette syndrome with reversible autistic behaviour: a dysmaturational disorder. Eur. Child Adolesc. Psychiatry. 2002;11:18–23. doi: 10.1007/s007870200003. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Belin P. Spectral and temporal processing in human auditory cortex. Cereb. Cortex. 2001;11:946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Gandour J.T. Neural specializations for speech and pitch: moving beyond the dichotomies. Philos. Trans. R. Soc. Lond. 2008;363:1087–1104. doi: 10.1098/rstb.2007.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Hill K., Labak S., Blatt G.J., Soghomonian J.-J. Loss of glutamic acid decarboxylase (Gad67) in Gpr88-expressing neurons induces learning and social behavior deficits in mice. Neuroscience. 2014;275:238–247. doi: 10.1016/j.neuroscience.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B., Barbas H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front. Hum. Neurosci. 2013;7:609. doi: 10.3389/fnhum.2013.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.