Abstract
Src family tyrosine kinases (SFKs) are critical players in normal and aberrant biological processes. While phosphorylation importantly-regulates SFKs at two known tyrosines, large-scale phosphoproteomics have revealed four additional tyrosines commonly-phosphorylated in SFKs. We found these novel tyrosines to be autophosphorylation sites. Mimicking phosphorylation at the site C-terminal to the activation loop decreased Fyn activity. Phosphomimetics and direct phosphorylation at the three SH2 domain sites increased Fyn activity while reducing phosphotyrosine-dependent interactions. While 68% of human SH2 domains exhibit conservation of at least one of these tyrosines, few have been found phosphorylated except when found in cis to a kinase domain.
Keywords: Src Family Kinase, Mass Spectrometry, Phosphorylation
1. Introduction
Studies leading to the identification of v-Src as the oncogenic agent behind Peyton Rous' sarcoma virus precipitated more than a century of extraordinary science and critically fueled the birth of the field of signal transduction [1]. Given its oncogenic potential, the mechanism behind v-src's ability to transform cells was investigated with vigor [1]. However, while hyperactive cellular Src's place as a major therapeutic target for cancer therapy has not been front and center [2] it has strong potential as a target in cancer subtypes, particularly when using inhibitor cocktails [3–6]. Importantly, this aggressive research led to the uncovering of many of the critical and wide-ranging normal roles of the Src family of non-receptor tyrosine kinases (SFKs)[7–9], including roles in homeostatic processes [10–12], acute responses to injury and infection [13–15], a variety of developmental processes [12,16–20] and critical evolutionary transitions [21].
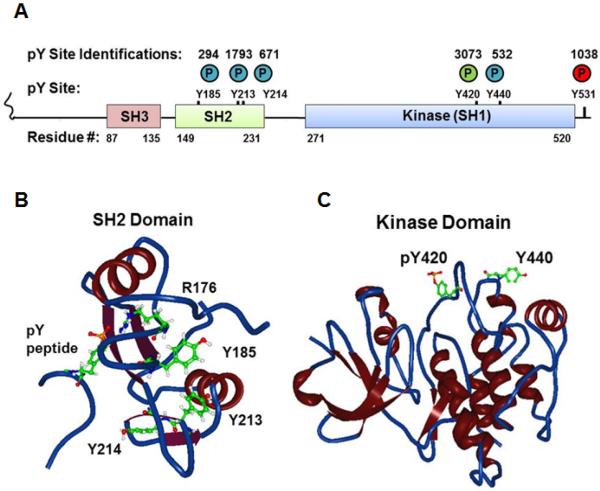
Key to understanding the molecular regulation of SFKs was the identification of two tyrosine phosphorylation sites conserved on all eleven of the extended SFK family members. The first is a product of intermolecular autophosphorylation at the activation loop in the kinase domain and leads to increased catalytic activity. The second, located at the C-terminus, is primarily phosphorylated by Csk (C-terminal Src kinase or c-Src kinase) and leads to intramolecular, autoinhibitory rearrangement. This site is absent in v-Src, and this absence is the major reason for v-Src's transforming activity [8,9,22,23]. However, SFKs are known to be importantly-regulated by other modifications including serine phosphorylation and lipid modification [8,9,22,23]. Our current omics era has delivered mountains of relatively unbiased descriptive data enabling investigators to form targeted hypotheses of molecular function. Tests of such hypotheses are ultimately required if omics data are to be more than correlations or biomarkers. Intriguingly, in hundreds of large-scale phosphoproteomic analyses conducted by our group and several others, four additional tyrosine phosphorylation sites have been identified in SFKs from a wide variety of primary tissues, cell lines and cancer cells. These studies comprise both published studies and many in-house assays compiled and curated at PhosphoSitePlus [24]. Fig. 1A summarizes the number of times these sites have been identified and the positions of these phosphorylation sites relative to the major structural domains of SFKs (using the residue numbers of human Fyn which contains all four of the variably-conserved, novel tyrosine phosphorylation sites). One of the sites is located 20 amino acids C-terminal to the activation loop phosphorylation site and the other three are located in the Src Homology 2 (SH2) domain. In Fig. 1B and C these sites are mapped onto the backbone and ribbon structural depictions of the SH2 and kinase domains respectively. Given the striking number of studies in which they had been identified, and given their proximity to major structural features in SFKs, we hypothesized that their phosphorylation would play important regulatory roles. We therefore sought to understand ways that these sites might become phosphorylated and to characterize their effects on kinase activity and on protein-protein interactions driven by the SH2 domain.
Fig. 1.
Localization of novel tyrosine phosphorylation sites (A) in a cartoon schema of the domain structure of human Fyn and in a backbone and ribbon 3D View rendering of structural data of (B) the Fyn SH2 domain with a phosphotyrosine peptide (PDB#1AOU) or (C) the Fyn kinase domain (PDB#2DQ7). Circled P denotes phosphate with colors corresponding to regulatory roles (green=positive; red=negative, blue=uncharacterized). Numbers above circles in (A) denote the number of independent identifications recorded in PhosphositePlus for each phosphorylation site. For the structural data atoms are portrayed of the phosphorylated tyrosine on the associated peptide; Arg176 and Tyr185, Tyr213, Tyr 214 on the SH2 domain; and the Fyn equivalents of phosphoTyr420 and Tyr440 on the kinase domain.
2. Materials and methods
Detailed materials and methods are provided as Supplementary Information Document 1.
3. Results and discussion
3.1. SFKs autophosphorylate their kinase domain and their SH2 domain at novel sites of tyrosine phosphorylation
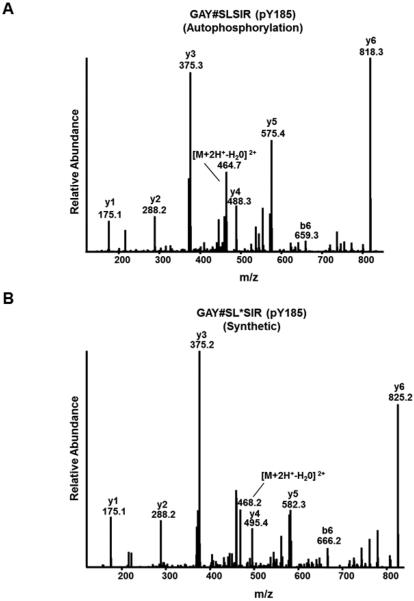
SFKs undergo intermolecular autophosphorylation at their positive regulatory sites within their activation loops [8,23]. Therefore, we asked if any of the novel but poorly characterized SFK tyrosine phosphorylation sites could also be the result of autophosphorylation. To assess autophosphorylation and to avoid ambiguity due to indirect phosphorylation, we expressed eight of the eleven human SFKs in yeast [25], which are devoid of tyrosine-specific kinases [26]. Pioneering studies used this approach a few decades ago and found that a kinase-dead version of Src was not phosphorylated on any tyrosine residue when expressed in yeast [27], indicating that yeast kinases could not directly phosphorylate Src on tyrosine. Furthermore, SFKs expressed in yeast could autophosphorylate at their activation loops, and to a far lesser extent their C-terminal negative regulatory sites [28,29]. Intriguingly, SFKs expressed in yeast that had phenylalanine substitutions at both the known positive and negative regulatory sites showed intermolecular phosphorylation at additional tyrosines [28]. We reasoned, therefore, that if a SFK expressed in yeast were phosphorylated at one or more of the newly identified tyrosine phosphorylation sites in the SH2 and kinase domains, it would be the result of autophosphorylation. Yeast expressing individual SFKs (Blk, Fgr, Frk, Fyn, Hck, Lyn, Srm and Yes), were lysed and individual extracts were digested with trypsin. The desalted peptides were subjected to anti-phosphotyrosine peptide immunoprecipitation [30–32] and peptides retrieved in the immune complexes were subjected to liquid chromatography-tandem mass spectrometry. The tandem mass spectra were searched using a database of all human tyrosine kinases. Yeast expressing a given SFK only yielded phosphotyrosyl peptides matching to the kinase expressed, except in cases where tryptic peptides were identical among SFKs. Strikingly, in addition to detecting autophosphorylation in the activation loop for seven out of eight of the expressed SFKs, we found the majority of the other four sites phosphorylated when they were conserved tyrosine residues (Table 1). In two cases (Fgr and Frk) we also found autophosphorylation of the C-terminal negative regulatory site. The lack of the identification of phosphorylation at the C-terminal site in the other tested SFKs was likely due to their C-termini falling in rather large tryptic peptides (>30 amino acids). Supplementary Table 1 provides a list of all pY peptides identified in these assays. We chose to further validate the mass spectrometry results using Fyn as a representative SFK, as it harbors tyrosines at all of the major SFK tyrosine phosphorylation sites and given we found each was phosphorylated when Fyn was expressed in yeast. We did this by acquiring tandem mass spectra of synthetic peptides harboring phosphotyrosine singly at each of the three SH2 domain sites (Tyr185, Tyr213 and Tyr214), at the activation loop site (Tyr420) and at the novel (Tyr440) kinase domain site (Fig. 2A and Supplementary Fig. 1–5).
Table 1.
Autophosphorylation sites identified following the expression of the indicated human SFKs in yeast. Following cell lysis, extracts were digested with trypsin and tryptic peptides were immunoprecipitated with anti-phosphotyrosine antibodies. Retrieved peptides were subjected to LC-MS/MS. “Yes” indicates the site was found phosphorylated by mass spectrometry. “Yes?” at Tyr214 indicates the phosphorylation site could not unambiguously be distinguished from the Tyr213 equivalent. “No” indicates the site was not identified as phosphorylated, and “Phe”, “Leu”, or “Ser” indicate these non-tyrosine residues were found in the homologous position. Human Fyn numbering is used on the top row for reference. Homologous site in the other kinases are in each column.
| Y185 | Y213 | Y214 | Y420 | Y440 | |
|---|---|---|---|---|---|
| Yes | No | No | Yes | Yes | Yes |
| Fyn | Yes | Yes | Yes | Yes | Yes |
| Fgr | No | Yes | Yes | Yes | Phe |
| Lyn | Phe | Yes | Yes? | Yes | Phe |
| Hck | No | Phe | Yes | Yes | Phe |
| Blk | Phe | Yes | Yes | Yes | Phe |
| Frk | Phe | Phe | Phe | No | Ser |
| Srm | Yes | Leu | Yes | Yes | Yes |
Fig. 2.
Low-energy collision-induced dissociation mass spectra of (A) a tryptic peptide harboring autophosphorylated Tyr185 which was immunoprecipitated from tryptic digests of yeast expressing active Fyn or (B) a synthetic peptide of the same with an added stable isotope-containing mass tag on Leu187 to distinguish it from the otherwise near-identical spectrum in (A). See Supplementary Figs. 1–5 for additional validation spectra.
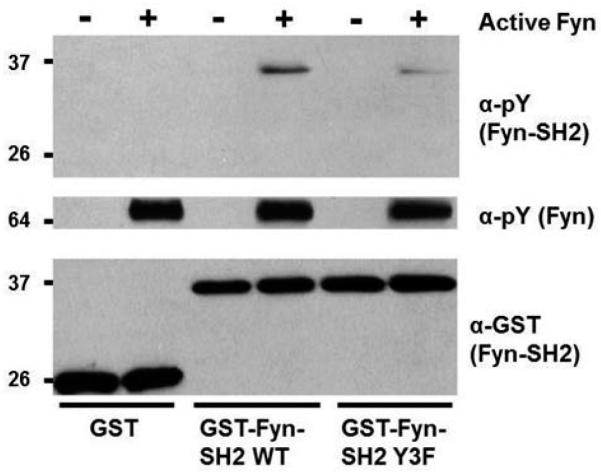
In a separate assay we also found Fyn specifically capable of intermolecular SH2 autophosphorylation when we incubated purified, active, full-length Fyn with a soluble GST-fusion of Fyn's SH2 domain in an in vitro kinase assay (Fig. 3). Furthermore, the majority of this phosphorylation went away when using a GST-Fyn-SH2 domain substrate harboring Tyr-to-Phe substitutions at Tyr185, Tyr213 and Tyr214 (Fyn-SH2-3F). These results suggest Fyn intermolecular SH2 domain autophosphorylation is predominately at Tyr185, TyrY213 and Tyr214. However, they also indicate the presence of an additional site of Fyn SH2 domain autophosphorylation. There are three additional tyrosine residues in Fyn's SH2 domain (Tyr150, Tyr203 and Tyr231), all falling into MS-compatible tryptic peptides. However, only Tyr150 has been curated as phosphosphorylated in PhosphoSitePlus [24], and albeit only four times. While we did not find Tyr150, Tyr203 or Tyr231 tyrosine phosphorylated in the SH2 domain of Fyn from our expression studies in yeast, we did find the equivalent of Tyr150 phosphorylated when expressing Fgr or Srm in yeast (Supplementary Tables 1–2). Taken together, our results and the high number of site identifications reported for SFK equivalents of Fyn Tyr185, Tyr213 and Tyr214 (Fig. 1A; Supplementary Tables 1–2)[24], suggest these three sites are the major sites of SFK SH2 domain autophosphorylation.
Fig. 3.
Fyn intermolecularly autophosphorylates its SH2 domain primarily at Tyr185, Tyr213 and/or Tyr214. Soluble GST, GST-Fyn-SH2 wild type, and GST-Fyn-SH2 Y3F were subjected individually to in vitro kinase reactions with (+) or without purified (−), active Fyn prior to SDS-PAGE and immunoblotting with the indicated antibodies.
3.2. Fyn Tyr440Asp shows decreased activation loop phosphorylation in cultured cells
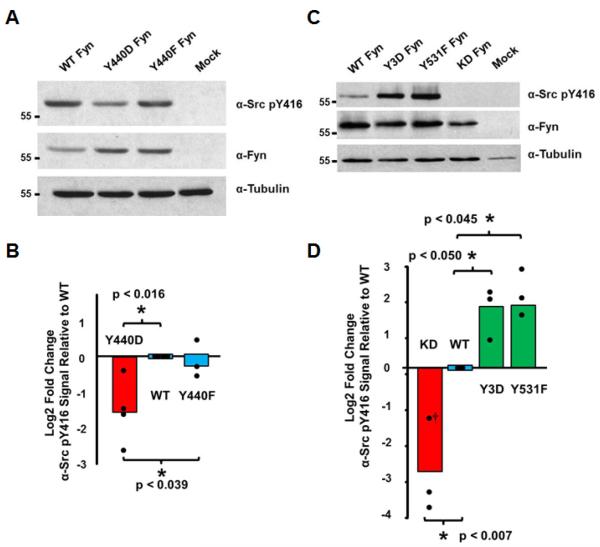
Given the proximity of Tyr440 to the activation loop of Fyn (Fig. 1C), one might predict Tyr440 phosphorylation could either disrupt or enhance kinase activity. To examine this we generated Fyn Tyr440Asp and Tyr440Phe mutants, expressed them in cultured cells and then measured the levels of phosphorylation at their activation loops relative to levels from cells expressing wild type Fyn. Tyr440Asp Fyn consistently showed decreased activation loop phosphorylation while the Tyr440Phe mutant showed little difference relative to wild type (Fig. 4A–B). These results are consistent with Fyn autophosphorylation at Tyr440 as a means of autoinhibition.
Fig. 4.
Charge-based phosphomimetics decrease Fyn activation loop phosphorylation for the Tyr440Asp allele (A–B) and increase activation loop phosphorylation for the Y3D allele (C–D). The indicated Fyn alleles were expressed in HEK 293 cells and whole cell extracts were subjected to SDS-PAGE and immunoblotting with the indicated antibodies. The Log2 fold change in activation loop phosphorylation relative to the expression of individual alleles was determined by densitometry. The means of three experiments normalized to wild type levels are indicated as well as the individual data points and comparisons of statistical significance. †For this KD replicate pY416 levels were not above background. A very conservative noise level of 7.5 fold lower than WT was used for the calculation, giving a value ~1/3 the mean of the other two KD values.
3.3. Fyn Tyr185Asp, Tyr213Asp and Tyr214Asp show increased activation in cultured cells
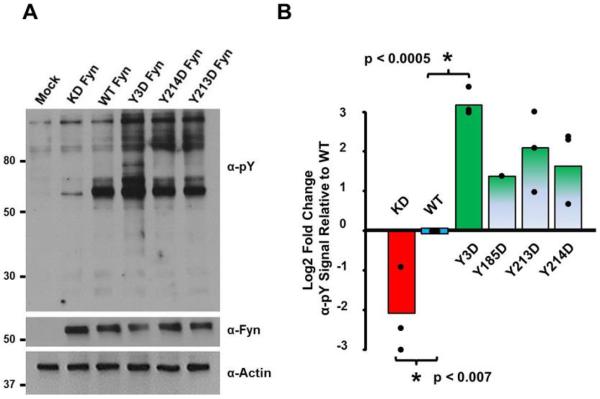
Although Tyr185, Tyr213 and Tyr214 do not appear to be in close interfering-proximity to the phosphotyrosine of a bound substrate (Fig. 1B), it may be possible that when phosphorylated they provide some form of generalized electrostatic repulsion for potential phosphotyrosyl-containing SH2 interactors. Alternatively, phosphorylation at these residues might compromise the shape of the SH2 domain, also potentially reducing binding to interacting partners. If either of these were true, we reasoned this would increase the overall activity of full-length Fyn as it would be less likely to be locked in its autoinhibited conformation with its SH2 domain bound to its own phosphorylated C-terminus [8,22,28]. We tested this hypothesis in two ways. As we did for experiments shown in Fig. 4A, we first used a phospho-specific antibody to examine the phosphorylation state of the activation loop of a Fyn molecule harboring aspartic acid residues at the three major sites of tyrosine phosphorylation within the SH2 domain (Fyn-Y3D). We found that the allele encoding Fyn-Y3D led to elevated activation loop phosphorylation comparable to that encoded by an allele of a Fyn Tyr-to-Phe mutation at the negative regulatory site (Tyr531Phe) that cannot be inhibited by Csk or autophosphorylation (Fig. 4C–D).
We next examined the effect of expressing Fyn-Y3D, or individual Tyr-to-Asp SH2 domain mutants within the context of full-length Fyn on general cellular tyrosine phosphorylation. We used an assay of simple design described by Azam et al. that was instrumental in identifying novel regulatory residues in the Src kinase domain [33]. In this approach, wildtype and mutant SFK constructs are transiently expressed in cultured cells and the activity of the transfected kinase is inferred by the overall change in phosphotyrosine signal on proteins within whole cell extracts. Consistent with its increased activation loop phosphorylation, whole cell extracts from cells expressing Fyn-Y3D showed an increase in tyrosine phosphorylated bands relative to cells expressing wild type Fyn (Fig. 5A). As work from other labs found that the equivalent of Fyn Tyr214 phosphorylation in Src led to increased kinase activity [34,35] we compared in three separate experiments the difference between the individual point mutants Fyn-Tyr213Asp and Fyn-Tyr214Asp with the Fyn-Y3D mutant. We also included the Tyr185Asp mutant in one of these sets of experiments. We observed that each individual Tyr-to-Asp point mutant showed an increase over wild type in the overall tyrosine phosphorylation of cellular substrates. However, the observed increase in cellular tyrosine phosphorylation of the Fyn-Y3D was greater than any individual Tyr-to-Asp mutant (Fig. 5B). Taken together, these data suggest that individual phosphorylation events of Fyn at Tyr185, Tyr213 and Tyr214 contribute in an additive fashion to increase Fyn's kinase activity, likely by preventing the adoption of an autoinhibitory conformation.
Fig. 5.
Charge-based phosphomimetics in Fyn's SH2 domain increase Fyn kinase activity as measured by total tyrosine phosphorylation in whole cell extracts. The indicated Fyn alleles were expressed in HEK 293 cells and whole cell extracts were subjected to SDS-PAGE and immunoblotting with the indicated antibodies. The Log2 fold change in tyrosine phosphorylation of cellular substrates above 70 kDa relative to the expression of individual alleles was determined by densitometry. The means of three experiments (one for Tyr185Asp) normalized to wild type levels are indicated as well as the individual data points and comparisons of statistical significance.
3.4. Phosphorylation of the Fyn SH2 domain reduces its capacity for phosphotyrosine-dependent interactions
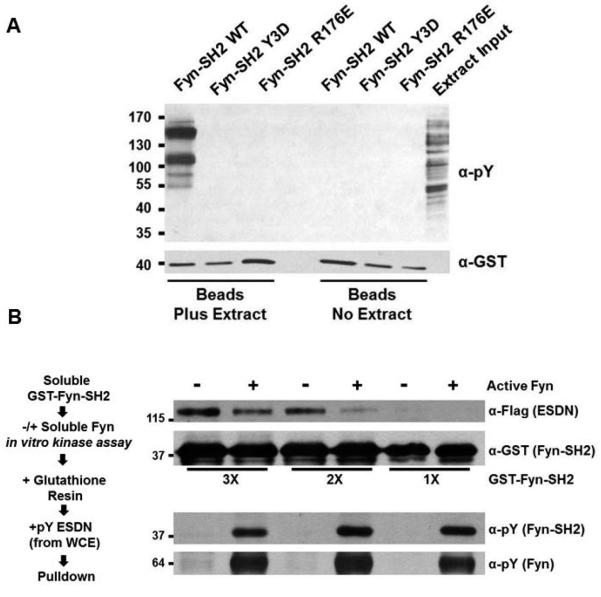
As Fyn harboring charge-based phosphomimetics in its SH2 domain suggested decreased autoinhibition, we hypothesized that autophosphorylation of Fyn's SH2 domain would lead to a reduced affinity for phosphotyrosine-containing binding partners. We focus here on autophosphorylation of all three sites in Fyn and note that previous work has focused on the effect of the equivalent of Tyr214 phosphorylation by a heterologous kinase on Src, Lck and Lyn [34,36,37]. To test this we performed two types of experiments. We first examined the capacity of Fyn-SH2-Y3D to bind tyrosine phosphorylated proteins in a GST fusion protein pulldown assay. As binding partners we used an extract generated from cells treated with hydrogen peroxide to non-specifically boost tyrosine phosphorylation via the inhibition of tyrosine phosphatases [38]. We observed that a subset of tyrosine phosphorylated proteins bound prominently to the wildtype Fyn SH2 domain (Fig. 6A). However, we saw no binding of these same proteins to the Fyn SH2-Y3D domain or to Fyn-SH2-Arg176Glu, a charge-reversed mutant where the known phosphotyrosyl-recognizing arginine [22] was exchanged for glutamate (Fig. 6A). While these results show that charge-based phosphomimetics in the Fyn SH2 domain are sufficient to dramatically reduce the binding of phosphotyrosyl-dependent interactions, they do not directly assess the role of phosphorylation, and more specifically, autophosphorylation. To determine if Fyn intermolecular autophosphorylation of its SH2 domain could directly alter the binding of a known Fyn-SH2-binding partner, we phosphorylated in vitro GST-Fyn-SH2 using purified, active Fyn. We then tested if a phosphotyrosine-dependent SH2-binding partner of Fyn, ESDN/DCBLD2 [39], would show reduced binding in a pulldown assay with the autophosporylated Fyn SH2 domain. Indeed we observed that in vitro, intermolecular Fyn-SH2 autophosphorylation reduced its interaction with ESDN/DCBLD2 (Fig. 6B). In doing this pulldown assay, we reasoned it was critical to keep the levels of GST-SH2 relatively low to increase the stoichiometry of phosphorylation at the three SH2 domain sites and achieve a sufficiently robust signal in spite of comprising enzymatic velocity. We therefore conducted this assay under three dilutions containing approximately 300, 200 and 100 molecules respectively of GST-Fyn-SH2 per one molecule of full-length, active kinase. The results show that Fyn intermolecular autophosphorylation indeed reduces its ability to bind to ESDN/DCBLD2 (Fig. 6B).
Fig. 6.
Charge-based phosphomimetics (A) or in vitro autophosphorylation (B) reduce the binding of the Fyn SH2 domain to tyrosine phosphorylated proteins. (A) GST fusion-based pulldown assays using GST-Fyn-SH2 Y3D and GST-Fyn-SH2 R176E show no binding to phosphorylated proteins in contrast to GST-Fyn-SH2 wild type. Pulldowns were subjected to SDS-PAGE and immunblotting with the indicated antibodies. (B) As indicated in the work flow at left, increasing dilute amounts of soluble GST-Fyn-SH2 wild type were subjected to in vitro kinase assays with or without purified Fyn before being captured on glutathione agarose and used in pulldown assays using phosphotyrosyl-ESDN as a binding partner. Pulldowns were subjected to SDS-PAGE and immunoblotting with the indicated antibodies.
3.5. Homologous residues of Fyn Tyr185, Tyr213, Tyr214 and Tyr440 are variably conserved among SFKs and SH2 domain-containing proteins
Our data showing the Fyn SH2 domain has reduced binding capacity when intermolecularly autophosphorylated, or when in a phosphomimetic state, are consistent with a quantitative proteomics screen recently conducted by Jin et al. that compared the interacting partners of the SH2 domain of the SFK Lyn with or without in vitro phosphorylation with the Ephrin type-A receptor 4 (EphA4) [36]. Lyn harbors the homologous residues to Fyn Tyr213 and Tyr214, but has a phenylalanine at the homologous Tyr185 residue (Supplementary Fig. 6). Jin et al. reported that all of the binding partners to Lyn's EphA4-phosphorylated SH2 domain showed reduced binding [36]. Interestingly, tyrosine residues at the equivalent positions of Fyn tyrosines 185, 213, 214 and 440 are variably conserved among human SFKs and within SFKs across evolutionary space (Supplementary Figs. 6–7). Interestingly, of human SFKs the highly related group containing Fyn, Src, Yes and Fgr has conservation of all three SH2 domain tyrosine sites with the exception of Src which harbors phenylalanine at the equivalent residue of Fyn Tyr213 (Supplementary Fig. 6). Among the next group of four closely related SFKs (Lyn, Hck, Lck and Blk), all have tyrosine at the equivalent position of Tyr214, but feature phenylalanines more prominently at the other two SH2 domain regulatory sites. Additionally, from an evolutionary perspective, vertebrate Fyn proteins have all three sites conserved (Supplementary Fig. 6) while their closest relatives in invertebrate metazoans are more variable and resemble the spectrum of tyrosine conservation for these sites observed in human SFKs. While the three SH2 tyrosines show variable conservation across metazoan taxa, metazoans of more basal derivation have high conservation of tyrosine at the equivalent of Tyr440 in their SFKs, suggesting that the Tyr440 equivalent diverged to other amino acids as SFKs diversified within vertebrates (Supplementary Fig. 6–7).
3.6. Conservation of residues homologous to Fyn Tyr185, Tyr213 and Tyr214 in non-SFK SH2 domains and their identified phosphorylation profiles
The human proteome contains 120 SH2 domains in 110 distinct proteins [40]. Given our results above we hypothesized that we would find a similar signature of phosphorylation at homologous residues in the SH2 domains of other proteins. To assess this, we manually examined a multiple sequence alignment of all 120 human SH2 domains [40] and found that 81 (68%), including 10 of the 11 SFKs, had at least one tyrosine residue at the equivalent sites of Fyn Tyr185, Tyr213 and Tyr214. In total 111 conserved tyrosines were identified on these 81 SH2 domains. We next used PhosphositePlus [24] to determine if these tyrosines had been found phosphorylated in either small- or large-scale analyses. We found this to be true of only 49 of the 111 conserved tyrosines and these 49 came from only 35 discreet SH2 domains and only 25 coming from non-SFKs. While a few additional phosphotyrosine residues, as well as phosphoserine and phosphothreonine residues, within SH2 domains have been identified [24], they are relatively sparse and suggest that SH2 domain regulation by phosphorylation is generally restricted. However, we did find that of the 30 SH2 domains encoded by tyrosine kinase genes, 18 have been found phosphorylated at one or more of the residues homologous to Fyn's SH2 domain autophosphorylation sites [24]. This is striking, as only 17 of 90 SH2 domains encoded by non-kinase genes have similarly been found phosphorylated (Fig. 7; Supplementary Table 3).
Fig. 7.
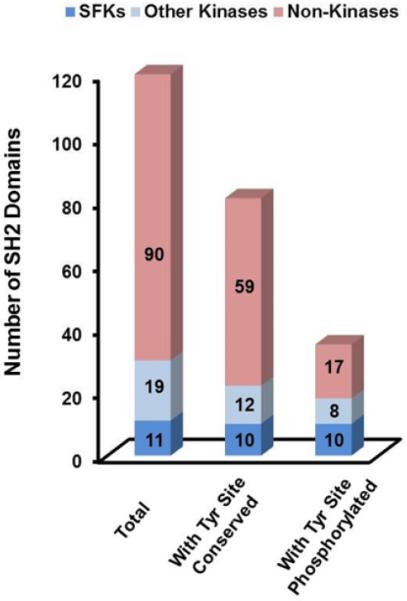
Homologous residues to at least one of Tyr185, Tyr 213 or Tyr214 are found in 81 of the 120 human SH2 domains. Gene products with both a kinase domain and a SH2 domain are 2.8 times more likely to have tyrosines phosphorylated at the equivalents of Tyr185, Tyr213 or Tyr214 than gene products with SH2 domains but without a kinase domain (18/22 (82%) compared with 17/59 (29%)).
3.7. Potential cellular regulation by novel SFK tyrosine phosphorylation sites
To date a few studies have provided clues to the potential cellular consequences of tyrosine phosphorylation of the SH2 domain sites on SFKs, while no study has addressed the site at the C-terminal base of the activation loop. As mentioned previously, non-SFKs have been shown to phosphorylate the equivalent of Fyn Tyr214 to increase SFK activity and reduce SH2 binding capacity [34,36,37] suggesting that mechanisms other than autophosphorylation can regulate SFKs at this site. Furthermore, Tyr214 was shown to be phosphorylated downstream of the EGF receptor family [35,41] and possibly by CD3 in T cells [42]. In these cases Tyr214 phosphorylation correlated with SFK activation but whether Tyr214 phosphorylation specifically was essential for a biological consequence was not clear. Similarly, Fyn Tyr185 phosphorylation correlated with Fyn activity and increased EGFR expression in gliobastomas with sensitivity to SFK inhibitors [43]. A more in-depth, cell-based functional characterization of Fyn Tyr213 was conducted by Kaspar and Jaiswal [44]. They expressed Fyn in HepG2 cells and following immunoprecipitation and mass spectrometry analysis, they found Fyn Tyr213 tyrosine could be phosphorylated. Using biochemical fractionation they found a Tyr213Phe mutant is less associated with the nucleus than wild type Fyn and shows less redox-dependent regulation of nuclear association. The Tyr213Phe mutant also showed reduced binding to and transcriptional regulation of specific proteins. However, it isn't clear from these studies if phosphorylation, per se, of Tyr213 is responsible for these cellular phenotypes.
In summary, we have identified that SFKs autophosphorylate at tyrosines in their SH2 and kinase domains. Using Fyn as a prototypical SFK, we show that phosphomimics at these residues alters kinase activity and SH2-dependent binding interactions and that direct phosphorylation similarly reduces SH2-dependent binding interactions. Finally, bioinformatic analyses suggest that most SH2 domains are not similarly regulated unless they are encoded by genes for tyrosine kinases. The ultimate cellular consequences of these novel tyrosine phosphorylation events and whether heterologous kinases might importantly contribute to their phosphorylation await further characterization. However, we suggest that phosphorylation of these sites might be either a mechanism for the cell to reduce tyrosine kinase activity following prolonged activation, or perhaps a mechanism of shifting away from SFK-dependent phosphorylation of SH2-binding partners and toward phosphorylation of their SH3-binding partners. Future experiments will address these hypotheses.
Supplementary Material
Highlights.
Mass spectrometry identifies novel autophosphorylation sites in 7 Src family kinases.
A phosphomimetic mutant of Fyn at Tyr440 lowers Fyn kinase activity.
Fyn SH2 phosphomimetics increase the phosphorylation of cellular substrates.
Fyn SH2 autophosphorylation reduces its phosphotyrosine-dependent interactions.
Homologous SH2 tyrosines are commonly phosphorylated when in cis with kinase domains.
Acknowledgements
We thank Lionel Arnaud for helpful discussions. This work was supported by U.S. National Science Foundation IOS grant 1021795, the Vermont Genetics Network through U. S. National Institutes of Health Grant 8P20GM103449 from the INBRE program of the NIGMS, and U.S. National Institutes of Health Grants 5P20RR016435 and P20RR021905 from the COBRE program of the NIGMS.
Abbreviations
- SH
Src Homology
- SFK
Src Family Kinase
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
Footnotes
Author Contributions B.A.B and K.L.H. oversaw the work as a whole, designed experiments and created reagents. U.S. oversaw the expression of kinases in yeast and the immunoprecipitation of phosphopeptides there-derived. P.B.D. oversaw the expression of Fyn constructs in mammalian cells to examine changes in the phosphorylation of its cellular substrates. M.E.W. and B.A.B. conducted mass spectrometry analyses. All authors conducted experiments and analyzed data. M.E.W. and B.A.B. wrote the manuscript.
All authors approved the manuscript and all authors declare no conflict of interest, financial or otherwise.
Supporting information: Supplementary Document 1 (Materials and methods) including 12 references.
References
- [1].Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–75. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- [2].Creedon H, Brunton VG. Src kinase inhibitors: promising cancer therapeutics? Crit Rev Oncog. 2012;17:145–59. doi: 10.1615/critrevoncog.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- [3].Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- [4].Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Puls LN, Eadens M, Messersmith W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist. 2011;16:566–78. doi: 10.1634/theoncologist.2010-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gelman IH. Src-family tyrosine kinases as therapeutic targets in advanced cancer. Front Biosci (Elite Ed) 2011;3:801–7. doi: 10.2741/e287. [DOI] [PubMed] [Google Scholar]
- [7].Reynolds AB, Kanner SB, Bouton AH, Schaller MD, Weed SA, Flynn DC, Parsons JT. SRChing for the substrates of Src. Oncogene. 2014;33:4537–47. doi: 10.1038/onc.2013.416. [DOI] [PubMed] [Google Scholar]
- [8].Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–49. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- [9].Bjorge JD, Jakymiw A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–35. doi: 10.1038/sj.onc.1203923. [DOI] [PubMed] [Google Scholar]
- [10].Zambuzzi WF, Milani R, Teti A. Expanding the role of Src and protein-tyrosine phosphatases balance in modulating osteoblast metabolism: lessons from mice. Biochimie. 2010;92:327–32. doi: 10.1016/j.biochi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- [11].Horne WC, Sanjay A, Bruzzaniti A, Baron R. The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol Rev. 2005;208:106–25. doi: 10.1111/j.0105-2896.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- [12].Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- [13].Scapini P, Pereira S, Zhang H, Lowell CA. Multiple roles of Lyn kinase in myeloid cell signaling and function. Immunol Rev. 2009;228:23–40. doi: 10.1111/j.1600-065X.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- [15].Senis YA, Mazharian A, Mori J. Src family kinases: at the forefront of platelet activation. Blood. 2014;124:2013–24. doi: 10.1182/blood-2014-01-453134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gauld SB, Cambier JC. Src-family kinases in B-cell development and signaling. Oncogene. 2004;23:8001–6. doi: 10.1038/sj.onc.1208075. [DOI] [PubMed] [Google Scholar]
- [17].Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- [18].Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–10. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- [19].Kuo G, Arnaud L, Kronstad-O'Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–86. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol. 2004;14:606–10. doi: 10.1016/j.cub.2004.03.038. [DOI] [PubMed] [Google Scholar]
- [21].Miller WT. Tyrosine kinase signaling and the emergence of multicellularity. Biochim Biophys Acta. 2012;1823:1053–7. doi: 10.1016/j.bbamcr.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–27. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- [23].Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- [24].Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–20. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grossmann A, Benlasfer N, Birth P, Hegele A, Wachsmuth F, Apelt L, Stelzl U. Phospho-tyrosine dependent protein-protein interaction network. Mol Syst Biol. 2015;11:794. doi: 10.15252/msb.20145968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Manning G, Plowman GD, Hunter T, Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci. 2002;27:514–20. doi: 10.1016/s0968-0004(02)02179-5. [DOI] [PubMed] [Google Scholar]
- [27].Jove R, Kornbluth S, Hanafusa H. Enzymatically inactive p60c-src mutant with altered ATP-binding site is fully phosphorylated in its carboxy-terminal regulatory region. Cell. 1987;50:937–43. doi: 10.1016/0092-8674(87)90520-4. [DOI] [PubMed] [Google Scholar]
- [28].Cooper JA, MacAuley A. Potential positive and negative autoregulation of p60c-src by intermolecular autophosphorylation. Proc Natl Acad Sci U S A. 1988;85:4232–6. doi: 10.1073/pnas.85.12.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kornbluth S, Jove R, Hanafusa H. Characterization of avian and viral p60src proteins expressed in yeast. Proc Natl Acad Sci U S A. 1987;84:4455–9. doi: 10.1073/pnas.84.13.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res. 2008;7:311–8. doi: 10.1021/pr0701254. [DOI] [PubMed] [Google Scholar]
- [31].Doubleday PF, Ballif BA. Developmentally-Dynamic Murine Brain Proteomes and Phosphoproteomes Revealed by Quantitative Proteomics. Proteomes. 2014;2:197–207. doi: 10.3390/proteomes2020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rush J, et al. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- [33].Azam M, Seeliger MA, Gray NS, Kuriyan J, Daley GQ. Activation of tyrosine kinases by mutation of the gatekeeper threonine. Nat Struct Mol Biol. 2008;15:1109–18. doi: 10.1038/nsmb.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stover DR, Furet P, Lydon NB. Modulation of the SH2 binding specificity and kinase activity of Src by tyrosine phosphorylation within its SH2 domain. J Biol Chem. 1996;271:12481–7. doi: 10.1074/jbc.271.21.12481. [DOI] [PubMed] [Google Scholar]
- [35].Vadlamudi RK, Sahin AA, Adam L, Wang RA, Kumar R. Heregulin and HER2 signaling selectively activates c-Src phosphorylation at tyrosine 215. FEBS Lett. 2003;543:76–80. doi: 10.1016/s0014-5793(03)00404-6. [DOI] [PubMed] [Google Scholar]
- [36].Jin LL, Wybenga-Groot LE, Tong J, Taylor P, Minden MD, Trudel S, McGlade CJ, Moran MF. Tyrosine phosphorylation of the Lyn Src homology 2 (SH2) domain modulates its binding affinity and specificity. Mol Cell Proteomics. 2015;14:695–706. doi: 10.1074/mcp.M114.044404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Couture C, Songyang Z, Jascur T, Williams S, Tailor P, Cantley LC, Mustelin T. Regulation of the Lck SH2 domain by tyrosine phosphorylation. J Biol Chem. 1996;271:24880–4. doi: 10.1074/jbc.271.40.24880. [DOI] [PubMed] [Google Scholar]
- [38].Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–42. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- [39].Aten TM, et al. Tyrosine phosphorylation of the orphan receptor ESDN/DCBLD2 serves as a scaffold for the signaling adaptor CrkL. FEBS Lett. 2013;587:2313–8. doi: 10.1016/j.febslet.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu BA, Jablonowski K, Raina M, Arce M, Pawson T, Nash PD. The human and mouse complement of SH2 domain proteins-establishing the boundaries of phosphotyrosine signaling. Mol Cell. 2006;22:851–68. doi: 10.1016/j.molcel.2006.06.001. [DOI] [PubMed] [Google Scholar]
- [41].Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–35. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Smida M, Posevitz-Fejfar A, Horejsi V, Schraven B, Lindquist JA. A novel negative regulatory function of the phosphoprotein associated with glycosphingolipid-enriched microdomains: blocking Ras activation. Blood. 2007;110:596–615. doi: 10.1182/blood-2006-07-038752. [DOI] [PubMed] [Google Scholar]
- [43].Lu KV, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69:6889–98. doi: 10.1158/0008-5472.CAN-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kaspar JW, Jaiswal AK. Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression. FASEB J. 2011;25:1076–87. doi: 10.1096/fj.10-171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.