Abstract
Background
Adipocyte–macrophage communication plays a critical role regulating white adipose tissue (WAT) inflammatory gene expression. Because WAT inflammation contributes to the development of metabolic diseases, there is significant interest in understanding how exogenous compounds regulate the adipocyte–macrophage crosstalk. An aqueous (AQ) extract of North American (NA) ginseng (Panax quinquefolius) was previously shown to have strong inflammo-regulatory properties in adipocytes. This study examined whether different ginseng extracts influence adipocyte–macrophage crosstalk, as well as WAT inflammatory gene expression.
Methods
The effects of AQ and ethanol (EtOH) ginseng extracts (5 μg/mL) on adipocyte and macrophage inflammatory gene expression were studied in 3T3-L1 and RAW264.7 cells, respectively, using real-time reverse transcription polymerase chain reaction. Adipose tissue organ culture was also used to examine the effects of ginseng extracts on epididymal WAT (EWAT) and inguinal subcutaneous WAT (SWAT) inflammatory gene expression.
Results
The AQ extract caused significant increases in the expression of common inflammatory genes (e.g., Mcp1, Ccl5, Tnf-α, Nos2) in both cell types. Culturing adipocytes in media from macrophages treated with the AQ extract, and vice versa, also induced inflammatory gene expression. Adipocyte Ppar-γ expression was reduced with the AQ extract. The AQ extract strongly induced inflammatory gene expression in EWAT, but not in SWAT. The EtOH extract had no effect on inflammatory gene expression in either both cell types or WAT.
Conclusion
These findings provide important new insights into the inflammo-regulatory role of NA ginseng in WAT.
Keywords: adipose tissue, gene expression, inflammation, Panax quinquefolius
1. Introduction
White adipose tissue (WAT) is a metabolically active endocrine organ that plays a central role in whole-body energy homeostasis [1]. The secretion of a wide range of proteins from WAT, collectively termed “adipokines,” is important for communication between adipocytes and other cell types. Of particular relevance is the paracrine dialog that exists between adipocytes and macrophages. Hypertrophic adipocytes secrete specific chemokines [e.g., monocyte chemoattractant protein 1 (MCP1)] that promote macrophage recruitment and activation [2]. These macrophages secrete a myriad of cytokines [e.g., tumor necrosis factor-alpha (TNF-α)] that activate adipocyte inflammatory signaling pathways, leading to recruitment of additional immune cells. Together, this cyclical dialog induces a chronic, low-grade inflammatory state in WAT. Because WAT inflammation is a major contributor to the development of metabolic diseases, there is significant interest in understanding how exogenous compounds can regulate adipocyte–macrophage crosstalk.
Increasing evidence demonstrates that many natural health products (NHPs) are able to regulate WAT inflammation [3]. This is particularly relevant given recent reports highlighting the widespread use of NHPs in the US population [4]. Ginseng, which is the fifth most highly consumed NHP in the United States [4], has been shown to regulate immune function, inflammatory processes, glucose metabolism, and response to stress and fatigue [5], [6], [7], [8]. Ginseng constitutes a family of herbs in which the primary species are Panax ginseng (Korean ginseng) and Panax quinquefolius [North American (NA) ginseng]. Although similar in genus, these species have distinct profiles of bioactive compounds, such as ginsenosides and polysaccharides. Compared with P. ginseng, relatively little is known about the bioactivity of P. quinquefolius.
We have previously reported that different NA ginseng extracts (which vary in ginsenoside and polysaccharide contents) had differential effects on inflammatory signaling pathways in differentiated 3T3-L1 adipocytes [5]. Specifically, an aqueous (AQ) ginseng extract induced adipocyte inflammatory signaling through the Toll-like receptor-4 (TLR4) pathway, whereas an ethanol (EtOH) extract had no effect. Azike et al [8] showed that these same NA ginseng extracts had different effects on cytokine production in macrophages. In their study, the AQ extract showed immune-stimulating effects and the EtOH extract showed immunoinhibitory effects [8]. Although the bioactivity of these extracts has been studied in cultured adipocytes and macrophages individually, it is unknown whether ginseng regulates the paracrine dialog between them. The goal of this study was to investigate how AQ and EtOH ginseng extracts influence the crosstalk between adipocytes and macrophages. We also examined whether the effects of these extracts were observed in different mouse WAT depots. Together, this research provides new insights into the molecular mechanisms by which NA ginseng extracts mediate cellular communication in adipose tissue.
2. Materials and methods
2.1. Ginseng acquisition and extraction
The Ontario Ginseng Growers Association provided 4-yr-old NA ginseng roots. Ginseng roots were pooled from five different Ontario farms and prepared at Naturex (South Hackensack, NJ, USA) for The Ontario Ginseng Research and Innovation Consortium [8]. In brief, dried ginseng root samples were soaked three times during 5 h at 40°C in either 16 L of water or an EtOH/water (75/25, v/v) solution to obtain the AQ and EtOH extracts, respectively [8]. Following extraction, solutions were filtered, solvent removed using a rotary evaporator, and concentrates were lyophilized using a freeze dryer. The contents of ginsenoside and polysaccharide were measured using high-performance liquid chromatography and size exclusion chromatography, as described elsewhere [8], [9]. The EtOH extract contained more than two times the total ginsenoside content than the AQ extract: 28.25% versus 13.87% dry weight of the extract. Rb1 and Re were the two most predominant ginsenosides in both extracts, however, the Rb1/Re ratio was higher in the EtOH extract: 1.8 versus 1.1 [8]. For all cell culture studies, ginseng extracts were dissolved in Hank's balanced salt solution (HBSS) and filtered using 0.2-μm filters (Fisher Scientific, ON, Canada) prior to their use. The concentration of extracts used in cell culture studies was selected following a dose–response pilot study to maximize the biological effects of the extracts while preventing cell toxicity. Toxicity was assessed using the Promega cytotoxicity assay (Madison, WI, USA).
2.2. Cell culture reagents
Murine 3T3-L1 preadipocytes were purchased from American Type Culture Collection (ATCC; Rockville, MD, USA). Murine RAW264.7 macrophages (ATCC) were kindly provided by Dr. Lindsay Robinson (University of Guelph, Guelph, ON, Canada). Dulbecco's modified Eagle's medium (DMEM), penicillin–streptomycin, and 0.25% trypsin–EDTA were purchased from HyClone Laboratories (Logan, UT, USA). Dexamethasone (DEX), 3-isobutyl-1-methylxanthine (IBMX), human insulin, fetal bovine serum (FBS), and HBSS were obtained from Sigma Aldrich (Oakville, ON, Canada).
2.3. Adipocyte cell culture studies
Four separate studies were conducted with murine 3T3-L1 preadipocytes, with each performed in triplicate using different passage numbers (Table 1, Fig. S1 in the supplementary material online). Each study used the same basic protocol for adipocyte differentiation, as previously described [5]. In brief, preadipocytes were seeded at a density of 6.0 × 104 and cultured in DMEM containing 5% FBS and 1% penicillin–streptomycin (i.e., basic media). Differentiation was induced 2 d postconfluence (i.e., Day 0) using a standard differentiation cocktail consisting of basic media supplemented with 1μM DEX, 1mM IBMX, and 5 μg/mL human insulin (i.e., differentiation media). After 2 d (i.e., Day 2), the differentiation media was removed and replaced with the basic media containing only 5 μg/mL human insulin (i.e., maintenance media). Maintenance media was changed every 2 d during the differentiation protocol. On Day 7, FBS was removed and the remainder of the experiments were conducted in serum-free conditions. Treatment conditions and incubation times for the four studies are outlined in Table 1. On Day 10, total RNA was extracted from differentiated adipocytes from all studies (A1–A4).
Table 1.
Overview of various adipocyte and macrophage studies
| Study No. | Study description |
|---|---|
| Adipocyte studies | |
| A1 | Differentiated adipocytes were treated for 48 h with 5 μg/mL of the EtOH and AQ ginseng extracts. The CTRL corresponded to serum-free media. After 48 h, total RNA was extracted and ACMs were collected and used for Study M3. |
| A2 | Differentiated adipocytes were treated for 24 h with 5 μg/mL of the EtOH and AQ ginseng extracts. The CTRL corresponded to serum-free media. After 24 h, media containing ginseng extracts were removed and replaced with ginseng-free, serum-free media for an additional 24 h. ACMs from this second 24-h period were collected and used for Study M4. |
| A3 | Differentiated adipocytes were treated for 48 h with MCMs collected from Study M1 (see below). |
| A4 | Differentiated adipocytes were treated for 48 h with MCMs collected from Study M2 (see below). |
| Macrophage studies | |
| M1 | Macrophages were treated with 5 μg/mL of the EtOH and AQ ginseng extracts for 24 h. The CTRL corresponded to serum-free media. After 24 h, total RNA was extracted and MCMs were collected and used for Study A3. |
| M2 | Macrophages were treated with 5 μg/mL of the EtOH and AQ ginseng extracts for 12 h. The CTRL corresponded to serum-free media. After the 12-h treatment period, media containing ginseng extracts were removed and replaced with ginseng-free, serum-free media for an additional 12 h. MCMs from this second 12-h period were collected and used for Study A4. |
| M3 | Macrophages were treated for 24 h with ACM collected from Study A1 (see above). |
| M4 | Macrophages were treated for 24 h with ACM collected from Study A2 (see above). |
ACMs, adipocyte-conditioned media; AQ, aqueous; CTRL, control; EtOH, ethanol; MCMs, macrophage-conditioned media
2.4. Macrophage cell culture studies
Four separate studies were conducted with murine RAW264.7 macrophages, with each performed in triplicate using different passages (Table 1, Fig. S1 in the supplementary material online). Each study used the same basic protocol for culturing. Macrophages were seeded at a density of 1.5 × 105 cells/well in six-well plates, and cultured in DMEM containing 10% FBS and 1% penicillin–streptomycin at 37°C in 5% CO2. Once confluence was achieved (approximately 2 d after seeding), FBS was removed from the media to terminate proliferation. The remainder of the experiments were conducted in serum-free conditions. Treatment conditions and incubation times for the four studies are outlined in Table 1. After the treatment period, total RNA was extracted from macrophages from all studies (M1–M4).
2.5. Adipose tissue organ culture
All protocols followed Canadian Council on Animal Care guidelines and were approved by the Animal Use and Welfare Committee at the University of Guelph. Adult male C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine, USA) weighing approximately 25 g were housed two/cage with a 12:12-h light–dark cycle. Mice were given access to standard mouse chow and water ad libitum. Animals were anesthetized with pentobarbital sodium (5 mg/100 g body weight), and epididymal WAT (EWAT) and inguinal subcutaneous WAT (SWAT) were excised, weighed, and immediately placed in 50-mL conical tubes containing phosphate-buffered saline (PBS) and 1% penicillin–streptomycin. Subsequently, 100 mg of tissue was placed into a well of a six-well plate containing 3.3 mL of M199 media (Sigma Aldrich) supplemented with 1% penicillin–streptomycin, 50 μU insulin, and 2.5nM DEX. The tissue was then minced into small pieces and placed in an incubator at 37°C in 5% CO2 for 24 h. After 24 h, the media was removed and replaced with fresh media containing 500 μg/mL of the ginseng extracts. This concentration of ginseng was selected following a dose–response pilot study in cultured WAT explants. The control (CTRL) corresponded to fresh M199 media. Each treatment group had four to six animals. After an additional 24-h period, tissue was collected, washed with ice-cold sterile PBS, flash frozen, and stored at −80°C. Total RNA was extracted from tissue, complementary DNA (cDNA) was prepared, and real-time reverse transcription polymerase chain reaction (RT-PCR) was conducted using the protocols outlined in the following sections.
2.6. RNA preparation
Total RNA was extracted using the Qiagen RNeasy Mini Kit, according to the manufacturer's protocol (Qiagen, Mississauga, ON, Canada). RNA was quantified using a NanoDrop 8000 UV–Vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
2.7. Real-time RT-PCR
Single-strand cDNA was synthesized for use in real-time RT-PCR from 1 μg of total RNA using a high-capacity cDNA kit (Applied Biosystems, Concord, ON, Canada). All real-time RT-PCR reagents and plastics were purchased from Bio-Rad (Mississauga, ON, Canada). All primers were designed using the Universal Probe Library Assay Design Center (Roche Applied Sciences, Table S1 in the supplementary material online). Primer specificity was determined using the Basic Local Alignment Search Tool (NCBI, Bethesda, MD, USA) and AutoDimer software [10]. Specific primers and SsoFast EvaGreen master mix were used to amplify 0.025-μg cDNA. A Bio-Rad CFX96 PCR detection system was used for amplification, as previously described [5]. Real-time RT-PCR data were normalized to the housekeeping gene Rplp0 and analyzed using the ΔΔCT method. Data are expressed as mean ± standard error, where each treatment was performed in triplicate.
2.8. Statistical analyses
Statistical analyses were performed using GraphPad Prism 6 software (La Jolla, CA, USA). A one-way analysis of variance (ANOVA) was first used to determine differences within an experiment. If the ANOVA achieved statistical significance (p < 0.05), we subsequently performed post hoc Student t tests. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Ginseng extracts and inflammatory gene expression in adipocytes and macrophages
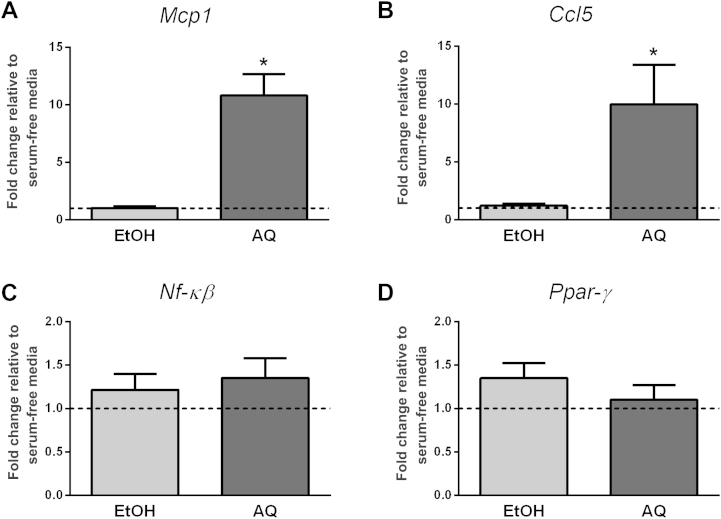
In Study A1, we treated differentiated adipocytes with either the AQ or EtOH ginseng extracts for 48 h and then examined inflammatory gene expression. Neither ginseng extract influenced adipocyte differentiation, as reflected by a lack of change in Ppar-γ expression (Fig. 1). Although the EtOH extract did not influence inflammatory gene expression, the AQ extract caused an approximately 11-fold increase in Mcp1 (p = 0.02) and an approximately 10-fold increase in Ccl5 (p = 0.003) expression (Fig. 1). The ginseng extracts had no effect on Nf-κβ expression (Fig. 1) and we were unable to detect Tnf-α expression (data not shown).
Fig. 1.
Regulation of adipocyte inflammatory gene expression (Study A1). Adipocytes were treated with either ethanol (EtOH) or aqueous (AQ) ginseng extracts (5 μg/mL) and the expression of (A) Mcp1, (B) Ccl5, and (C) Nf-κβ was examined after 48 h. (D) Ppar-γ was used as a marker of adipocyte differentiation. Fold changes are relative to the serum-free media control (indicated by the dotted line at 1.0). Experiments were conducted in triplicate. * Indicates p < 0.05 compared with the serum-free media control.
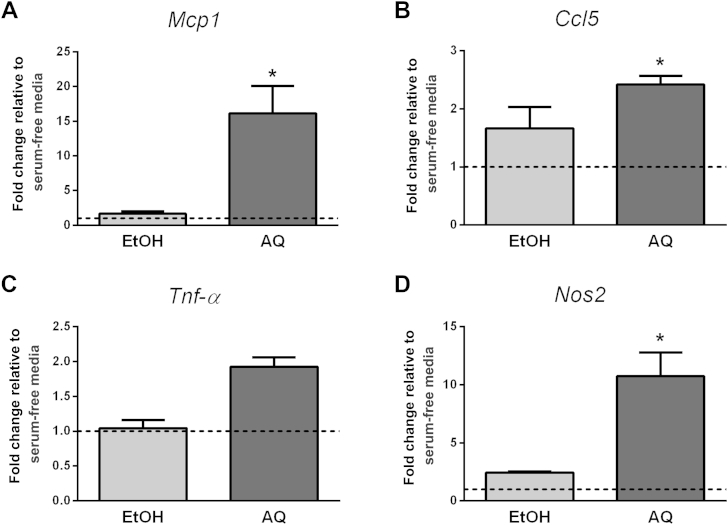
The ginseng extracts caused a similar trend in inflammatory gene expression in macrophages (Study M1). Macrophages treated for 24 h with the AQ extract experienced an approximately 16-fold increase in Mcp1 (p < 0.001) and a 2.4-fold increase in Ccl5 (p = 0.001) expression, whereas the EtOH extract had no effect (Fig. 2). Although no changes were detected in Nf-κβ expression with either ginseng extract (data not shown), a trend for increased Tnf-α expression was observed with the AQ extract (1.9-fold, p = 0.3). We also examined Nos2 and Arg1 gene expression to assess macrophage phenotype. Previous reports indicate that high Nos2/low Arg1 expression is indicative of classically activated macrophages, whereas the opposite expression pattern reflects alternatively activated macrophages [11]. We found that the AQ extract caused an approximately 11-fold increase in Nos2 expression (p = 0.004, Fig. 2), whereas Arg1 was not detected (data not shown). Overall, this suggests that the macrophages have a more classical, proinflammatory phenotype. By contrast, the EtOH extract had no effect on the expression of any of the genes examined.
Fig. 2.
Regulation of macrophage inflammatory gene expression (Study M1). Macrophages were treated with either ethanol (EtOH) or aqueous (AQ) ginseng extracts (5 μg/mL) and the expression of (A) Mcp1, (B) Ccl5, (C) Tnf-α, and (D) Nos2 was examined after 24 h. Fold changes are relative to the serum-free media control (indicated by the dotted line at 1.0). Experiments were conducted in triplicate. * Indicates p < 0.05 compared with the serum-free media control.
3.2. Adipocyte–macrophage crosstalk: role of macrophages
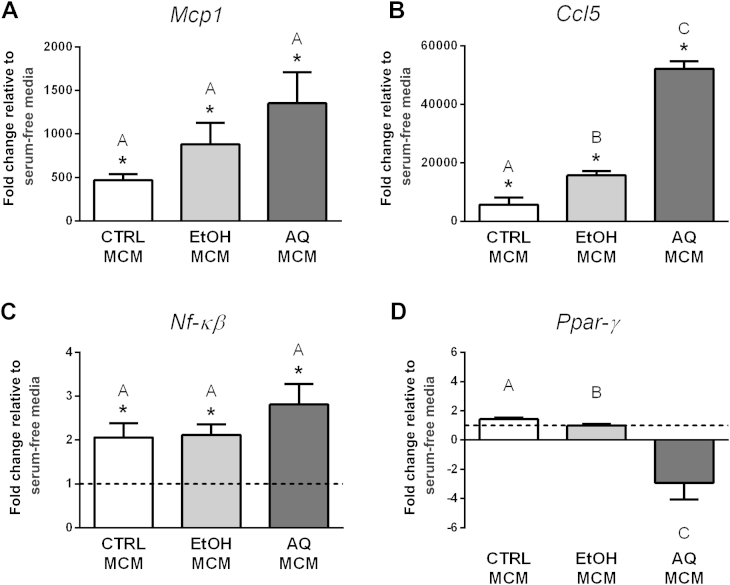
We conducted two studies to examine whether conditioned media from macrophages treated with ginseng extracts could influence adipocyte inflammatory gene expression (Studies A3 and A4). First, in Study A3, we transferred the macrophage-conditioned media (MCMs) from Study M1 onto differentiated adipocytes and compared their effects on inflammatory gene expression to adipocytes cultured in serum-free media. The three MCMs (i.e., CTRL MCM, AQ MCM, and EtOH MCM) all caused significant increases in Mcp1, Ccl5, and Nf-κβ expression compared with adipocytes cultured in serum-free media (Fig. 3). This demonstrates that macrophages, independent of ginseng, are able to activate adipocyte inflammatory gene expression. However, a difference between the three MCM treatments was seen with Ccl5 expression (Fig. 3). Specifically, the AQ MCM treatment caused an approximately 52,000-fold increase in Ccl5 adipocyte gene expression compared with adipocytes cultured in serum-free media, whereas the EtOH MCM treatment caused an approximately 15,000-fold increase. None of the MCM treatments had an effect on Ppar-γ expression compared with adipocytes cultured in serum-free media; however, the AQ MCM treatment decreased Ppar-γ expression compared with the other MCM treatments (Fig. 3). Given possible changes in Ppar-γ, we also examined Fabp4 (another marker of adipocyte differentiation). The Fabp4 expression showed a similar trend with the AQ MCM as that seen with Ppar-γ (data not shown).
Fig. 3.
Regulation of inflammatory gene expression in adipocytes treated with macrophage-conditioned media (MCMs; Study A3). Macrophages were treated with either ethanol (EtOH) or aqueous (AQ) ginseng extracts (5 μg/mL) or control (CTRL) media for 24 h and then MCMs were collected. The expression of (A) Mcp1, (B) Ccl5, and (C) Nf-κβ was examined in adipocytes after 48-h treatment with MCMs. (D) Ppar-γ was used as a marker of adipocyte differentiation. Fold changes are relative to adipocytes cultured in serum-free media (indicated by the dotted line at 1.0, visible when the scale permits). Experiments were conducted in triplicate. * Indicates p < 0.05 compared with adipocytes cultured in serum-free media. Bars not sharing the same letter indicate MCM treatments that were significantly different from each other (p < 0.05), as determined by post hoc tests.
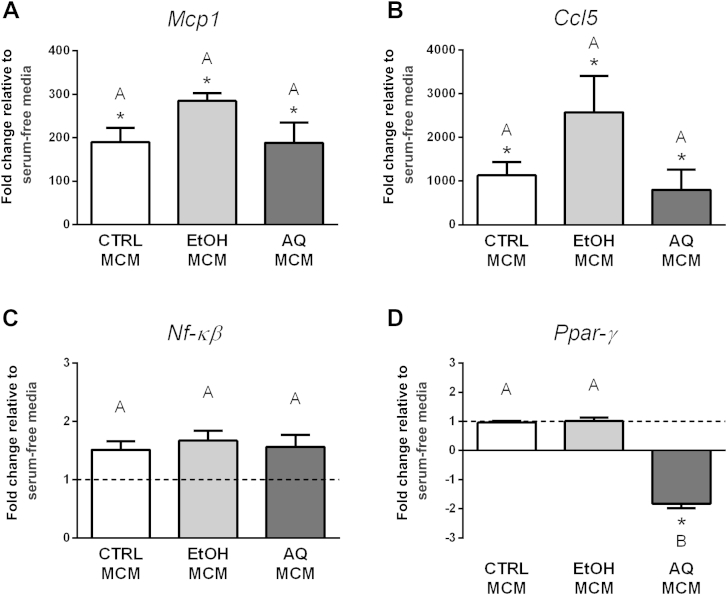
Study A3 suggested differential effects between conditioned media from macrophages treated with either AQ or EtOH extracts; however, a limitation was that the collected MCM still contained the ginseng extracts. To eliminate this confounder, we subsequently performed Study A4. In Study A4, adipocytes were treated with conditioned media from macrophages pretreated with ginseng extracts. Similar to results of Study A3, we found that all three MCMs (i.e., CTRL, AQ, and EtOH) caused a significant increase in adipocyte Mcp1 and Ccl5 gene expression compared with adipocytes cultured in serum-free media (Fig. 4); however, there was no significant difference between the three MCM treatments. No changes were seen in Nf-κβ expression between the MCM treatments and adipocytes cultured in serum-free media. However, the AQ MCM treatment caused a significant 1.8-fold decrease in Ppar-γ expression (p < 0.001, Fig. 4) compared with the CTRL and EtOH MCMs as well as adipocytes cultured in serum-free media. We did not observe changes in Fabp4 expression (data not shown).
Fig. 4.
Regulation of inflammatory gene expression in adipocytes treated with macrophage-conditioned media (MCMs; Study A4). Macrophages were pretreated with either ethanol (EtOH) or aqueous (AQ) ginseng extracts (5 μg/mL) or control (CTRL) media for 12 h and then the MCMs were collected after a second 12-h period. The expression of (A) Mcp1, (B) Ccl5, and (C) Nf-κβ was examined in adipocytes after 48-h treatment with MCMs. (D) Ppar-γ was used as a marker of adipocyte differentiation. Fold changes are relative to adipocytes cultured in serum-free media (indicated by the dotted line at 1.0, visible when the scale permits). Experiments were conducted in triplicate. * Indicates p < 0.05 compared with adipocytes cultured in serum-free media. Bars not sharing the same letter indicate MCM treatments that were significantly different from each other (p < 0.05), as determined by post hoc tests.
3.3. Adipocyte–macrophage crosstalk: role of adipocytes
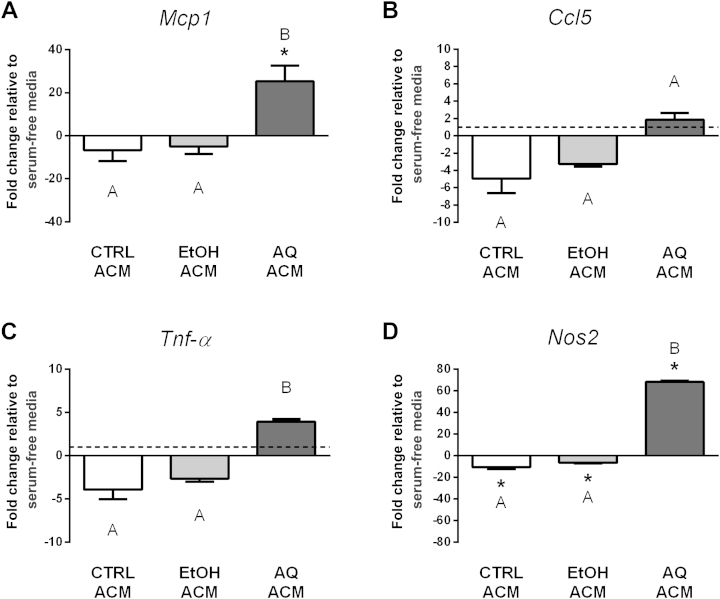
We next examined whether conditioned media from adipocytes treated with ginseng extracts could influence macrophage inflammatory gene expression (Studies M3 and M4). First, we transferred the adipocyte-conditioned media (ACMs) from Study A1 onto macrophages and compared their effects on inflammatory gene expression to macrophages cultured in serum-free media. Overall, the AQ ACM treatment induced macrophage inflammatory gene expression (Fig. 5). Specifically, the AQ ACM caused an approximately 25-fold increase in Mcp1 and an approximately 68-fold increase in Nos2 gene expression, compared with both the other ACM treatments and macrophages cultured in serum-free media (Fig. 5). Although not statistically significant, a trend for increased expression of macrophage Ccl5 and Tnf-α expression was also seen following AQ ACM treatment. The CTRL and EtOH ACMs had no effect on Mcp1, Ccl5, and Tnf-α expression compared with macrophages cultured in serum-free conditions. By contrast, the CTRL and EtOH ACMs acted similarly to reduce macrophage Nos2 gene expression by approximately 10-fold and approximately sevenfold, respectively, compared with macrophages cultured in serum-free media (Fig. 5). Arg1 expression was assessed in all treatment groups, but not detected (data not shown). Together, this suggests that the AQ ACM may induce a more classical, proinflammatory phenotype in macrophages, whereas the EtOH ACM may induce a more alternative, less inflammatory phenotype.
Fig. 5.
Regulation of inflammatory gene expression in macrophages treated with adipocyte-conditioned media (ACMs; Study M3). Adipocytes were treated with either ethanol (EtOH) or aqueous (AQ) ginseng extracts (5 μg/mL) or control (CTRL) media for 48 h and then ACMs were collected. The expression of (A) Mcp1, (B) Ccl5, (C) Tnf-α, and (D) Nos2 was examined in macrophages after 24-h treatment with ACMs. Fold changes are relative to macrophages cultured in serum-free media (indicated by the dotted line at 1.0, visible when the scale permits). Experiments were conducted in triplicate. * Indicates p < 0.05 compared with macrophages cultured in serum-free media. Bars not sharing the same letter indicate ACM treatments that were significantly different from each other (p < 0.05), as determined by post hoc tests.
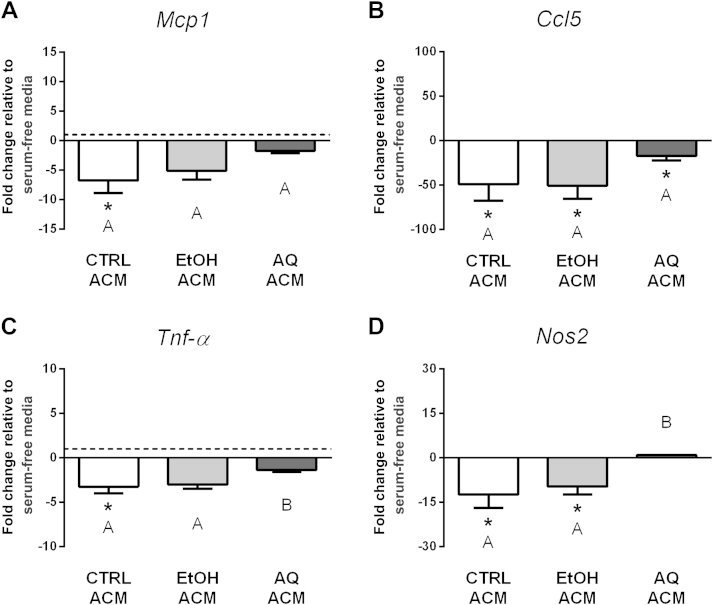
Study M3 suggested that conditioned media from adipocytes treated with the AQ extract may promote an inflammatory phenotype in macrophages. However, similar to Study A3, the ginseng extracts were still present in ACMs and are therefore a confounder. In Study M4, macrophages were treated with ACM from adipocytes pretreated with ginseng extracts, and inflammatory gene expression was compared with macrophages cultured in serum-free media. The EtOH ACM reduced macrophage Mcp1, Ccl5, Tnf-α, and Nos2 gene expression similar to macrophages treated with CTRL ACM; however, the AQ ACM had distinct effects for the most part (Fig. 6). The AQ ACM only caused a significant decrease in Ccl5 expression compared with macrophages cultured in serum-free media, whereas no changes were seen in Mcp1, Tnf-α, and Nos2 expression. Further, Tnf-α and Nos2 expression differed significantly between the AQ ACM and EtOH ACM treatments. This suggests that pretreating adipocytes for 24 h with the AQ extract led to changes in the secretion profile that lasted into the second 24-h period. Similar to Study M3, Arg1 expression was assessed for all treatment groups, but not detected (data not shown).
Fig. 6.
Regulation of inflammatory gene expression in macrophages treated with adipocyte-conditioned media (ACMs; Study M4). Adipocytes were pretreated with either ethanol (EtOH) or aqueous (AQ) ginseng extracts (5 μg/mL) or control (CTRL) media for 24 h and then ACMs were collected after a second 24-h period. The expression of (A) Mcp1, (B) Ccl5, (C) Tnf-α, and (D) Nos2 was examined in macrophages after 24-h treatment with ACMs. Fold changes are relative to macrophages cultured in serum-free media (indicated by the dotted line at 1.0, visible when the scale permits). Experiments were conducted in triplicate. * Indicates p < 0.05 compared with macrophages cultured in serum-free media. Bars not sharing the same letter indicate ACM treatments that were significantly different from each other (p < 0.05), as determined by post hoc tests.
3.4. NA ginseng extracts regulate inflammatory gene expression in a WAT-specific manner
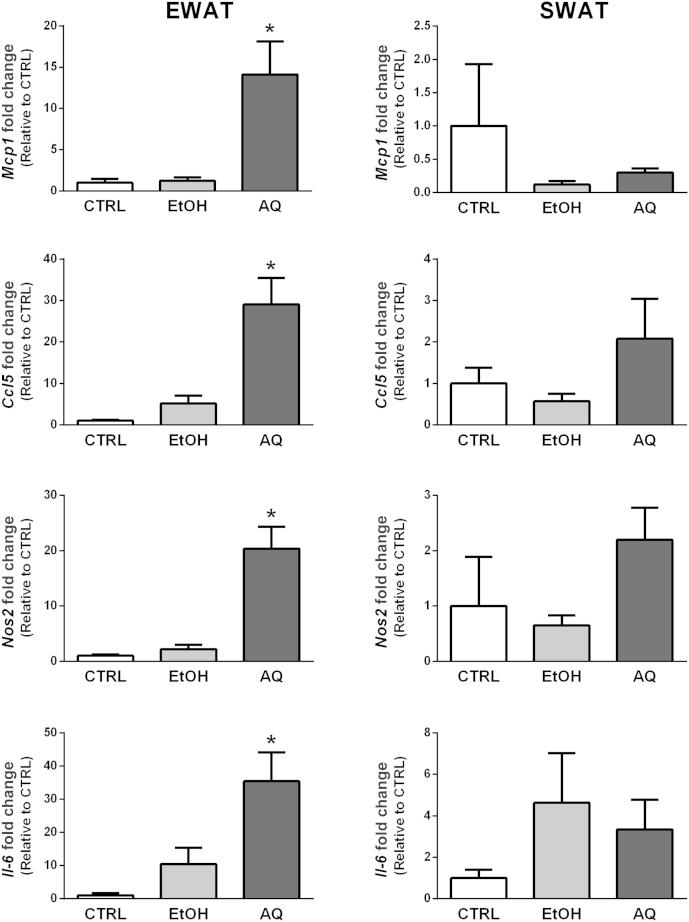
The aforementioned cell culture studies showed that the AQ extract has direct effects on both adipocyte and macrophage inflammatory gene expression. Moreover, it appears that the AQ extract alters the secretion profile of both cell types, thereby regulating the paracrine dialog between adipocytes and macrophages that controls inflammatory gene expression. We next examined whether the inflammatory effects seen in cells are similarly seen in EWAT and SWAT fat pads. We chose to study EWAT and SWAT due to documented differences in the production of proinflammatory adipokines between these two fat depots [12], [13]. As shown in Fig. 7, we found that the AQ extract caused significant increases in all inflammatory genes examined in EWAT compared with the EtOH extract. Specifically, we detected increases of approximately 14-fold, 29-fold, and 20-fold in the EWAT expression of Mcp1, Ccl5, and Nos2, respectively. In addition, we were able to detect a significant 35-fold increase in Il-6 expression in EWAT treated with the AQ extract. These changes were not observed in SWAT. The Tnf-α and Arg1 expressions were also assessed; however, no changes were seen compared with the control condition (data not shown). Similar to observations in cell culture studies, the EtOH extract had no effect on inflammatory gene expression in either adipose depot.
Fig. 7.
Regulation of inflammatory gene expression in different white adipose tissue (WAT) depots. Epididymal WAT (EWAT) and abdominal inguinal WAT (SWAT) were treated with either ethanol (EtOH) or aqueous (AQ) ginseng extracts (500 μg/mL) for 24 h. The expression of Mcp1, Ccl5, Nos2, and Il-6 was examined in each depot. Fold changes are relative to the control (CTRL) condition. Each treatment group contained four to six animals. * Indicates p < 0.05 compared with CTRL.
4. Discussion
Findings from this study indicate that the AQ ginseng extract from NA ginseng appears to induce inflammatory gene expression in adipocytes and macrophages, as well as EWAT. By contrast, the EtOH ginseng extract had negligible effects on inflammatory gene expression in both cellular and tissue models. Crosstalk studies demonstrated an important paracrine dialog between adipocytes and macrophages that seemingly amplifies the proinflammatory potential of the AQ extract. Further, results from the different crosstalk studies suggest that the AQ extract has a lasting effect on adipocyte and macrophage secretion profiles. Along these lines, we showed that the AQ extract may induce a more classical phenotype in macrophages. Taken together, our results demonstrate that the AQ extract of NA ginseng regulates inflammatory gene expression directly in adipocytes and macrophages as well as by altering the paracrine dialog between these two cell types.
Communication between adipocytes and macrophages plays a critical role in the regulation of WAT function, in particular, with regard to inflammation [2], [14]. For example, TNF-α secretion from macrophages can induce adipocyte inflammatory signaling pathways [15], whereas the release of saturated fatty acids from adipocytes triggers an increase in macrophage TNF-α secretion [16]. Furthermore, the secretion of various chemokines such as MCP1 and chemokine (C–C motif) ligand 5 (CCL5) from adipocytes causes immune cell recruitment into WAT [16]. Consequently, increased WAT inflammation promotes insulin resistance, ectopic lipid deposition, and the dysfunction of numerous metabolic processes throughout the body. Therefore, compounds that regulate this cellular crosstalk may have an important influence on tissue function.
We previously showed that treating adipocytes with 50 μg/mL of the AQ extract triggered a proinflammatory gene expression network that was, in part, mediated by TLR4 [5]. However, this higher dose of the AQ extract was found to cause cytotoxicity in macrophages (data not shown); therefore, this study used a lower dose of ginseng extracts (5 μg/mL) for consistency across all cell-based studies. Despite the lower dose, we observed increases in inflammatory gene expression in both adipocytes (e.g., Mcp1 and Ccl5) and macrophages (e.g., Mcp1, Ccl5, Tnf-α, Nos2). Notably, the significant increase in Nos2 expression seen in macrophages treated with the AQ extract, coupled with the inability to detect Arg1, suggests a more classical macrophage phenotype [17]. Future studies examining how NA ginseng regulates macrophage phenotype in vivo will provide additional insights into the immunoregulatory functions of this commonly used NHP.
We next explored whether ginseng extracts regulated adipocyte–macrophage crosstalk. We first examined whether conditioned media from ginseng-treated macrophages influenced adipocyte inflammatory gene expression (Studies A3 and A4). In our first set of experiments (Study A3), adipocytes were treated with MCMs collected from macrophages treated for 24 h with the ginseng extracts. It is noteworthy that in these first experiments, the MCMs still contained the ginseng extracts. Thus, it was not possible to conclude whether changes in adipocyte inflammatory gene expression stemmed directly from the presence of ginseng extracts or indirectly from changes in macrophage secretion profiles or a combination of both. Despite this limitation, this first study was highly informative. First, in comparison with adipocytes treated with serum-free media, all three MCM conditions (CTRL, AQ, and EtOH) caused highly significant increases in adipocyte inflammatory gene expression (Fig. 3). This indicated that macrophages release compounds that induce adipocyte inflammatory gene expression independent of ginseng. Although a trend for increased expression of Mcp1, Ccl5, and Nf-κβ was seen in adipocytes treated with AQ MCM, statistical differences between the three MCM treatments were only detected for CCL5. Incredibly, the AQ MCM treatment led to a massive 52,000-fold increase in adipocyte Ccl5 expression (Fig. 3). The ramifications of such an increase are unclear; however, CCL5 was previously shown to play a role in T-cell recruitment [18].
To clarify whether the aforementioned effects were due to the presence of the AQ extract in the MCM or changes in the macrophage secretion profile, or a combination of both, we next conducted studies using MCM from macrophages pretreated with the ginseng extracts (Study A4). In other words, these macrophages were treated with ginseng extracts for 12 h, the ginseng-containing media were removed, and fresh media without ginseng was added for another 12 h. This approach enabled us to delineate whether ginseng extracts influenced adipocyte inflammation by modifying the macrophage secretion profile. Our results suggested that MCM from macrophages pretreated with the AQ extract did not have lasting effects on adipocyte inflammatory gene expression compared with CTRL or EtOH treatments. However, it is noteworthy to highlight the dramatically lower-fold changes observed in Study A4 (Fig. 4) compared with those in Study A3 (Fig. 3). We suspect that this is due to the fact that macrophage-secreted proteins accumulated in media for two times as long in Study A3 (24 h) compared with Study A4 (12 h).
One intriguing finding from these MCM studies was the effect of the AQ extract on adipocyte Ppar-γ expression. In both Studies A3 and A4, we found that AQ MCM reduced Ppar-γ expression (Fig. 3, Fig. 4). This finding is unique to the AQ treatment group, thereby indicating that this effect is not caused by something that is constitutively secreted by macrophages. Rather, this implies that the AQ extract is causing macrophages to release a protein that downregulates adipocyte Ppar-γ expression. Further, the effect of the AQ extract on the macrophage secretion profile persists for at least 12 h following treatment, because the reduction in Ppar-γ expression was also observed in Study A4. Constituents of P. ginseng were previously reported to suppress Ppar-γ expression in adipocytes [19], [20]. However, whether the reduction in Ppar-γ expression observed in this study is influencing adipocyte differentiation is questionable given that we did not see changes in Fabp4 expression in Study A4, which is another classical marker of differentiation (data not shown). Because peroxisome proliferator-activated receptor-γ (PPAR-γ) is a critical transcription factor in adipocytes that regulates numerous biological processes, subsequent studies are required to understand the consequences of the reduced adipocyte Ppar-γ expression caused by AQ MCM.
We also explored whether conditioned media from ginseng-treated adipocytes influenced macrophage inflammatory gene expression (Studies M3 and M4). Similar to Study A3, it is noteworthy that ginseng extracts were still present in ACMs when applied to macrophages in Study M3; therefore, this study precluded us from determining whether changes in macrophage gene expression stemmed directly from the presence of ginseng extracts or by modifications to the adipocyte secretion profiles or a combination of both. Neither the CTRL nor the EtOH ACMs had any effect on the macrophages expression of Mcp1, Ccl5, or Tnf-α compared with macrophages in serum-free media (Fig. 5). Interestingly, both these conditions caused a significant reduction in macrophage Nos2 expression, suggesting that noninflammatory adipocytes can help maintain a more alternative macrophage phenotype. By contrast, the AQ ACM caused a significant increase in Mcp1 expression and trends for increases in Ccl5 and Tnf-α expression (Fig. 5). Furthermore, the AQ ACM caused a highly significant increase in macrophage Nos2 expression, suggesting a more classical proinflammatory macrophage phenotype. We subsequently examined whether the observed effects were caused directly by the presence of the AQ extract or indirectly by AQ-mediated changes in the adipocyte secretion profile (Study M4). The effects of the CTRL and EtOH treatments in Study M4 resembled those seen in Study M3, that is, that the CTRL and EtOH ACM treatments similarly reduced macrophage inflammatory gene expression compared with macrophages cultured in serum-free media. By contrast, the effects of the AQ ACM differed significantly from those of the CTRL and EtOH treatments and generally did not cause a reduction in macrophage inflammatory gene expression (Fig. 6). This suggests that the effects of the AQ extract on the adipocyte secretion profile lasted 24 h. The notion that experimental treatments may have a lasting effect on secretion profiles after their removal has been previously demonstrated in adipocyte–muscle crosstalk studies [21].
Consistent with our cell studies, we also found that the AQ extract activated inflammatory gene expression in murine WAT, whereas the EtOH extract had no effect. Specifically, we have shown that the AQ extract caused strong increases in the expression of Mcp1, Ccl5, Nos2, and Il-6 in EWAT, but not in SWAT (Fig. 7). This is particularly noteworthy given the strong relationship that exists between visceral adipose tissue inflammation and metabolic dysfunction [13], [22], [23]. Furthermore, EWAT contains more resident macrophages compared with SWAT [24], which is relevant given our findings that the AQ extract influences adipocyte–macrophage crosstalk and may induce a classical macrophage phenotype. Finally, previous reports have demonstrated that visceral fat is more responsive to regulation by exogenous factors, including nutrients [25] and NHPs [26]. Collectively, this suggests that the AQ ginseng extract may have significant effects on metabolic health by differentially regulating inflammatory gene expression in different WAT depots.
In conclusion, we show that the AQ extract of NA ginseng induced inflammatory gene expression in both adipocytes and macrophages, as well as in EWAT. The primary challenge moving forward will be to identify the proteins secreted from adipocytes and macrophages that are changed by the AQ extract. Moreover, we cannot exclude the possibility that cell metabolites may also contribute to the regulation of inflammatory gene expression. Therefore, comprehensive analyses of secretion media are required to further our understanding of the mechanism(s) regulated by the AQ extract and how this influences adipocyte–macrophage crosstalk. Furthermore, our data demonstrate a clear induction of inflammatory gene expression in EWAT; however, conducting future studies in primary cells isolated from the different adipose depots will provide a logical continuum to the cell-based studies presented here. Finally, we have performed short-term treatments in this study. Performing longer studies in which the various ginseng extracts are administered chronically will enable us to determine whether the AQ extract leads to the development of metabolic dysfunction. Such studies are imperative to better understand the inflammo-regulatory role of NA ginseng on cellular communication and, ultimately, adipose tissue function.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research was funded by the Ontario Ginseng Research and Innovation Consortium (DMM and EMKL), NSERC (DCW – #341158 and DMM – #371564), and CFI/ORF (DMM – #24121). DCW is a Tier II Canada Research Chair in lipids, metabolism, and health. JCR is funded by an NSERC doctoral award.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2015.07.001.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1.
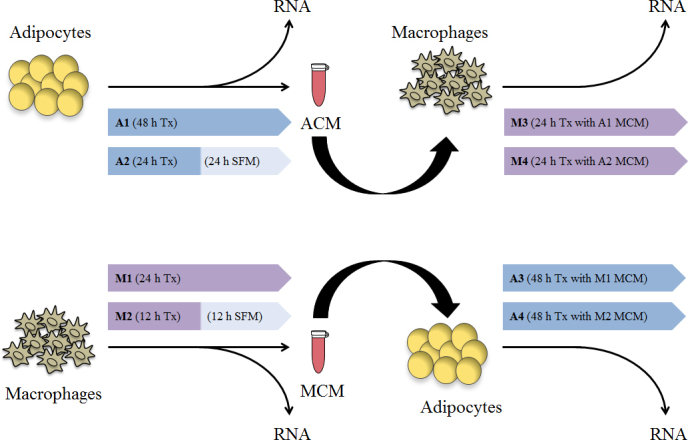
Experimental overview of adipocyte–macrophage crosstalk studies. Details regarding the various studies (A1–A4 and M1–M4) are described in Table 1. SFM, serum-free medium; ACM, adipocyte-conditioned medium; MCM, macrophage-conditioned medium; Tx, treatment.
References
- 1.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisberg S.P., McCann D., Desai M., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siriwardhana N., Kalupahana N.S., Fletcher S., Xin W., Claycombe K.J., Quignard-Boulange A., Zhao L., Saxton A.M., Moustaid-Moussa N. n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-κB-dependent mechanisms. J Nutr Biochem. 2012;23:1661–1667. doi: 10.1016/j.jnutbio.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Barnes P.M., Bloom B., Nahin R.L. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1–23. [PubMed] [Google Scholar]
- 5.Wilson S.A., Wong M.H., Stryjecki C., De Boer A., Lui E.M., Mutch D.M. Unraveling the adipocyte inflammomodulatory pathways activated by North American ginseng. Int J Obes (Lond) 2013;37:350–356. doi: 10.1038/ijo.2012.50. [DOI] [PubMed] [Google Scholar]
- 6.Shergis J.L., Zhang A.L., Zhou W., Xue C.C. Panax ginseng in randomised controlled trials: a systematic review. Phytother Res. 2013;27:949–965. doi: 10.1002/ptr.4832. [DOI] [PubMed] [Google Scholar]
- 7.Nakaya T.A., Kita M., Kuriyama H., Iwakura Y., Imanishi J. Panax ginseng induces production of proinflammatory cytokines via toll-like receptor. J Interferon Cytokine Res. 2004;24:93–100. doi: 10.1089/107999004322813336. [DOI] [PubMed] [Google Scholar]
- 8.Azike C.G., Charpentier P.A., Hou J., Pei H., King Lui E.M. The Yin and Yang actions of North American ginseng root in modulating the immune function of macrophages. Chin Med. 2011;6:21. doi: 10.1186/1749-8546-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azike C.G., Charpentier P.A., Lui E.M. Stimulation and suppression of innate immune function by American ginseng polysaccharides: biological relevance and identification of bioactives. Pharm Res. 2015;32:876–897. doi: 10.1007/s11095-014-1503-3. [DOI] [PubMed] [Google Scholar]
- 10.Vallone P.M., Butler J.M. AutoDimer: a screening tool for primer-dimer and hairpin structures. Biotechniques. 2004;37:226–231. doi: 10.2144/04372ST03. [DOI] [PubMed] [Google Scholar]
- 11.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 12.Arner P. Regional differences in protein production by human adipose tissue. Biochem Soc Trans. 2001;29:72–75. doi: 10.1042/bst0290072. [DOI] [PubMed] [Google Scholar]
- 13.Fain J.N., Madan A.K., Hiler M.L., Cheema P., Bahouth S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 14.Suganami T., Nishida J., Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chawla A., Nguyen K.D., Goh Y.P. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H., Ghosh S., Perrard X.D., Feng L., Garcia G.E., Perrard J.L., Sweeney J.F., Peterson L.E., Chan L., Smith C.W. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 19.Yang J.W., Kim S.S. Ginsenoside Rc promotes anti-adipogenic activity on 3T3-L1 adipocytes by down-regulating C/EBPα and PPARγ. Molecules. 2015;20:1293–1303. doi: 10.3390/molecules20011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Yu L., Cai W., Fan S., Feng L., Ji G., Huang C. Protopanaxatriol, a novel PPARγ antagonist from Panax ginseng, alleviates steatosis in mice. Sci Rep. 2014;4:7375. doi: 10.1038/srep07375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sell H., Eckardt K., Taube A., Tews D., Gurgui M., Van Echten-Deckert G., Eckel J. Skeletal muscle insulin resistance induced by adipocyte-conditioned medium: underlying mechanisms and reversibility. Am J Physiol Endocrinol Metab. 2008;294:E1070–E1077. doi: 10.1152/ajpendo.00529.2007. [DOI] [PubMed] [Google Scholar]
- 22.Fontana L., Eagon J.C., Trujillo M.E., Scherer P.E., Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 23.Samaras K., Botelho N.K., Chisholm D.J., Lord R.V. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–889. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- 24.Cancello R., Tordjman J., Poitou C., Guilhem G., Bouillot J.L., Hugol D., Coussieu C., Basdevant A., Bar Hen A., Bedossa P. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 25.Einstein F.H., Atzmon G., Yang X.M., Ma X.H., Rincon M., Rudin E., Muzumdar R., Barzilai N. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes. 2005;54:672–678. doi: 10.2337/diabetes.54.3.672. [DOI] [PubMed] [Google Scholar]
- 26.Rokling-Andersen M.H., Rustan A.C., Wensaas A.J., Kaalhus O., Wergedahl H., Røst T.H., Jensen J., Graff B.A., Caesar R., Drevon C.A. Marine n-3 fatty acids promote size reduction of visceral adipose depots, without altering body weight and composition, in male Wistar rats fed a high-fat diet. Br J Nutr. 2009;102:995–1006. doi: 10.1017/S0007114509353210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.