Abstract
The development of therapeutic and prophylactic HIV vaccines for African countries is urgently needed, but the question of what immunogens to use needs to be answered. One approach is to include HIV envelope immunogens derived from HIV-positive individuals from a geographically concentrated epidemic with more limited viral genetic diversity for a region-based vaccine. To address if there is a basis for a regional selected antibody vaccine, we have screened two regionally separate cohorts from Guinea-Bissau and Denmark for neutralizing antibody activity and antibody-dependent cellular cytotoxicity (ADCC) against local and nonlocal circulating HIV-1 strains. The neutralizing activity did not demonstrate higher potential against local circulating strains according to geography and subtype determination, but the plasma from Danish individuals demonstrated significantly higher inhibitory activity than that from Guinea-Bissau individuals against both local and nonlocal virus strains. Interestingly, an opposite pattern was observed with ADCC activity, where Guinea-Bissau individual plasma demonstrated higher activity than Danish plasma and was specifically against the local circulating subtype. Thus, on basis of samples from these two cohorts, no local-specific neutralizing activity was detected, but a local ADCC response was identified in the Guinea-Bissau samples, suggesting potential use of regional immunogens for an ADCC-inducing vaccine.
Introduction
An effective prophylactic HIV-1 vaccine will preferably induce antibodies with broad neutralizing activity and antibodies that mediate efficient antibody-dependent cellular cytotoxicity (ADCC). Passive administration of monoclonal neutralizing antibodies to macaques and subsequent protection against SHIV challenge are strong evidence for the protective effect of vaccine-elicited neutralizing antibodies.1,2 The neutralizing antibodies have the ability to inhibit the viral transmission if present at the time of infection. These antibodies have been studied extensively in in vitro studies, and common characteristics, such as long HCDR3s, have been identified.3 However, eliciting broadly neutralizing antibodies seems very difficult since only 10%–30% of infected individuals develop such antibodies4–7 after long maturation and somatic hypermutation processes.8,9 The ADCC-mediating antibodies have gained more attention since the Thai RV144 vaccine efficacy trial demonstrated that the observed protection was correlated with low plasma levels of IgA envelope (Env) antibodies in association with a high level of nonneutralizing IgG antibodies with ADCC activity,10,11 which highlights the importance of also nonneutralizing antibodies. These types of antibodies have also been revealed to occur in elite controllers,12 and it has been suggested that control of viremia is associated with a broader ADCC response.13 The two different functions of antibodies, ADCC and neutralization, have been demonstrated to coincide with some characterized monoclonal antibodies.14,15
Whether the vaccine-induced antibodies are neutralizing or nonneutralizing, they should most likely be targeting the HIV-1 envelope (Env). Specific regions of the Env trimer are known as the targets for broadly neutralizing anti-HIV antibodies, and extensive attempts have been undertaken to construct immunogens to direct antibodies to these areas.16 However, the growing knowledge of neutralizing epitope structures on the HIV-1 Env has not automatically translated into the generation of improved immunogens,16 emphasizing the importance of continuing all approaches in the search for HIV-1 vaccine immunogens. The main challenge of the tremendous genetic diversity of globally circulating HIV-1 strains17,18 remains unsolved, and it is still unclear which vaccine antigen to use to address this hurdle.
The two different approaches when designing new immunogens are to elicit region-specific and broadly targeting immune responses. The RV144 trial used the region-specific approach when the immunogens used matched the local circulating strains.19 It is unlikely that the developed response would have a protection against other subtypes found elsewhere in the world.20 No vaccine candidate tested to date has demonstrated a sufficient, potent, and broad immune response. Another promising attempt tested immunization with several different Env subtypes, which indeed induced broad multisubtype anti-Env–binding antibodies in a phase IIa clinical trial.21 However, a following phase IIb trial, HVTN 505, with the same immunization regime was halted prematurely due to lack of efficacy.22 Future vaccine candidates may benefit from the recent development of stabilized soluble Env trimers,23,24 which mimic the native envelope spike and could be useful both in DNA25 and antigen vaccines. Despite the recent discovery of a large number of broadly neutralizing antibodies, it is unknown how to elicit such antibodies. To determine if there is any basis for a local vaccine, we have tested the antiviral activity in two different patient cohorts from two different geographical regions. As a model for this study, Guinea-Bissau and Denmark were chosen as these two relatively small and distinct regions harbor different circulating HIV-1 strains with subtypes A and CRF02_AG dominating in Guinea-Bissau26,27 and subtype B dominating in Denmark.28,29 Neutralization and ADCC activities against circulating HIV-1 subtypes in Guinea-Bissau and Denmark and against a subtype not found in these regions were evaluated in these two cohorts. A distinct pattern of crossclade neutralization was particularly apparent in the plasma of Danish HIV-1–positive individuals, whereas neutralization in the Guinea-Bissau plasma was less potent. However, the ADCC activity demonstrated a different pattern, with the Guinea-Bissau plasma having a higher potency than the Danish plasma and was specifically against Env of the local subtype A origin.
Materials and Methods
Study subjects
Plasma samples from Guinea-Bissau were obtained from HIV-1–infected individuals participating in the Bissau HIV Cohort30,31 based at the Hospital Nacional Simão Mendes, Bissau. The recruited individuals were enrolled for a clinical phase 1 study of a therapeutic HIV-1 vaccine (www.ClinicalTrials.gov id: NCT01141205).32 The HIV status of the enrolled individuals was confirmed at the National Public Health Laboratory, Bissau. Preimmunization plasma samples from 13 ART-naive individuals were selected for this study (Table 1), none of them coinfected with HIV-2. The plasma samples from Denmark were selected from a group of HIV-1–infected individuals enrolled for a clinical phase 1 therapeutic HIV-1 vaccine study (www.ClinicalTrials.gov id: NCT01009762).33 Preimmunization plasma samples from 10 ART-naive individuals were selected (Table 1). The studies were approved by the National Committee for Health Research Ethics of the Danish Ministry of Health (H-D-2008-063), the Danish Medicines Agency (Journal no. 2612–3785), and UCEPS, the National Ethics Committee of Guinea-Bissau (Parecer NCP/No.15/2007). All the patients provided written and informed consent.
Table 1.
Clinical Data for Study Individuals at the Time of Sampling
| Country | Study individuals | Sex | HIV-1 subtype | HIV-1 plasma viral load (copies/ml) | CD4+ T-cell count (cells/μl) |
|---|---|---|---|---|---|
| Guinea-Bissau | ViFU1 111 | F | A3/02 | n.a. | 496 |
| ViFU1 207 | M | A3/02 | 16,500 | 284 | |
| ViFU1 235 | M | A3 | n.a. | 456 | |
| ViFU1 238 | M | A3 | 720 | 609 | |
| ViFU1 250 | F | CRF02_AG | 251,000 | 354 | |
| ViFU1 261 | F | A3 | n.a. | 529 | |
| ViFU1 282 | F | CRF02_AG | 10,338 | 541 | |
| ViFU1 284 | M | CRF02_AG | 642,000 | 281 | |
| ViFU1 285 | F | A3/02 | 733,000 | 266 | |
| ViFU1 288 | F | A3/02 | 135,280 | 701 | |
| ViFU1 290 | F | CRF02_AG | 6,782 | 851 | |
| ViFU1 295 | F | CRF02_AG | 12,746 | 530 | |
| ViFU1 299 | F | A3/02 | 45,386 | 506 | |
| Denmark | PV01 | M | B | 2,060 | 542 |
| PV03 | M | B | 29,518 | 430 | |
| PV04 | M | B | 128,543 | 320 | |
| PV05 | M | B | 21,180 | 660 | |
| PV06 | M | B | 16,468 | 530 | |
| PV07 | M | B | 70,898 | 380 | |
| PV08 | M | B | 27,862 | 570 | |
| PV09 | M | B | 458,300 | 520 | |
| PV10 | M | B | 28,123 | 570 | |
| PV16 | M | B | 3,200 | 457 |
M, male; F, female; n.a., not available.
Genomic sequencing and subtype determination
Subtype determination of Guinea-Bissau material was based on sequencing of the envelope C2-V3 region, as described by Palm et al.26 In brief, viral RNA was extracted from plasma samples and amplified by reverse transcription–polymerase chain reaction (PCR), and the env C2-V3 region was sequenced. The sequences were aligned in Mega 5,34 with A3 and CRF02_AG used as reference sequences, and A1 sequences were used as the outgroup sequences (a detailed description of the reference sequences is described by Palm et al.26). Maximum likelihood phylogenetic trees were generated, and the subtypes were identified by the BootScan analysis using SimPlot v3.5.35 The sequences determined to be A3/CRF02_AG recombinants, as described by Palm et al.,26 will be referred to as A3/02 recombinants. The subtype determination of Danish material was conducted by an analysis of partial pol sequences of 1,200 base pairs containing the protease and partial reverse transcriptase; the sequences were generated through population-based sequencing using ViroSeq HIV-1 genotyping System v. 2 (Abbott Diagnostics, Foster City, CA).
Cloning of the HIV-1 envelope
To include viral variants from Guinea-Bissau in the neutralization assay, two cloned gp160 genes from two different HIV-1–infected individuals, ViFU288 and ViFU262, were used for pseudovirus production. The extracted viral RNA (MagNa Pure 96 DNA and Viral NA Small Volume Kit; Roche Diagnostics, Mannheim, Germany) from plasma samples was reverse transcribed with SuperScript III (Invitrogen, Carlsbad, CA), followed by env amplification with the Expand Long Template PCR System (Roche Applied Science, Basel, Switzerland) and gel purification. The env regions were cloned into the pcDNA™3.3-TOPO® vector (Invitrogen) and were transformed into JM109 (Stratagene, La Jolla, CA). The bacterial colonies were screened by PCR for the correct insert, and the selected plasmids were recovered using an S.N.A.P. MiniPrep Kit (Invitrogen) and were sequenced for confirmation.
Pseudoviruses
The virus panel used in the TZM-bl neutralization assay consisted of HIV-1 pseudovirus from subtypes A (ITM1-4, Q461), B (SF162, Bx08, BaL, QH0692, AC10.0.29), C (92Br025), CRF02_AG (VI1090, ViFU262), and A3/02 (ViFU288), and the panel included both neutralization-sensitive36 and more resistant variants. Pseudoviruses were produced by cotransfecting HEK293T cells37 with a plasmid containing the viral gp160 gene and the pNL4.3LucR-E- plasmid containing a luciferase reporter gene. The pNL4.3LucR-E- plasmid was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: pNL4.3LucR-E- from Dr. Nathaniel Landau.38,39 Forty-eight hours after transfection, the pseudovirus stocks were harvested and tested for infectivity using TZM-bl target cells.40 The pseudoviruses were diluted to titers optimal for use in neutralization assays, which were ∼105 relative light units.
Neutralization assay
Plasma neutralizing activities were measured by the luciferase-based assay in TZM-bl cells, as previously described.41,42 Briefly, heat-inactivated plasma, which was threefold diluted in six steps starting at 1:20, was mixed with pseudovirus and incubated for 1 h at 37°C before adding TZM-bl cells. The ability of plasma to neutralize virus infection was assessed by measuring luciferase activity 48 h after virus inoculation and comparing the results to a control infection without any plasma. TriMab, a mix of three mAbs (b12, 2F5, and 2G12) (obtained from the Centre for AIDS Reagents, NIBSC, United Kingdom), was used in parallel in every neutralization assay as a strongly neutralizing control. The neutralization titers are expressed as the reciprocal of the plasma dilution that inhibited virus infection by 50% (ID50). Plasma samples that did not reach the ID50 at the 1:20 dilution were assigned a value of 20 for the geometric mean titer calculations.
Anti-Env antibody enzyme-linked immunosorbent assay
Envelope-specific IgG titers were determined by enzyme-linked immunosorbent assay (ELISA) according to a previously described method,25 which uses recombinant gp120BaL protein (Immune Technology, New York, NY) as the coating antigen. Total Env-specific IgG was determined with conjugate rabbit anti-human IgG (cat. no. P214; Dako A/S, Glostrup, Denmark), and the four different IgG subclasses were determined with mouse anti-human IgG1, IgG2, IgG3, and IgG4 (clone HP6069, HP6014, HP6047, and HP6025; Life Technologies, Carlsbad, CA, respectively).
ADCC assay
ADCC activity was detected using PanToxiLux (OncoImmunin, Gaithersburg, MD) according to the previously described ADCC-GranToxiLux procedure.43,44 Env-coated CEM.NKRCCR5 cells45 were used as target cells and coated 90 min at 4°C with recombinant Env. Env-coated target cells have previously been demonstrated to be equally effective in the ADCC assay as infected target cells,44 which we confirmed in a separate experiment (data not shown). Recombinant Env was derived from envelopes of subtype B, BaL (Immune Technology), and subtype A, UG37 (Polymun Scientific, Klosterneuburg, Austria). Targets cells were labeled with TFL4 and NFL1 (target cell marker and viability marker, respectively). The PBMC from two HIV-1–seronegative donors were pooled and used as the source of NK effector cells. Targets cells and effector cells were counted for viability, adjusted to the effector-to-target cell (E:T) ratio of 30:1, and incubated with granzyme B (GzB) substrate in 96-well plate for 5 min at room temperature before antibodies were added. The plasma samples were tested as fivefold serial dilutions starting at 1:300 and incubated at room temperature for 15 min, followed by 60-min incubation at 37°C. Samples were acquired using BD LSRII instrument and analyzed using FlowJo (Tree Star version 8.8.7, Ashland, OR), see gating strategy in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/aid). A pool of 17 in-house HIV-negative plasma was used as negative control and a standard antibody HIVIG (obtained from NIH AIDS Research and Reagent Program) as positive control. A threshold for positive ADCC activity was set to the mean percent GzB activity mediated by HIV-negative plasma samples plus three standard deviations, which corresponded to >9.53% of effector cells positive for active GzB for rgp120BaL and >6.77% for rgp140UG37. The final results are reported as the endpoint dilution, that is, the highest reciprocal of the plasma dilution that was positive for ADCC activity.10 The results are also demonstrated in Supplementary Figure S2 as percent GzB activity, that is, percentage of cells positive for proteolytically active GzB out of the total viable target cell population.
Statistical analysis
The differences between Guinea-Bissau and Denmark plasma samples in neutralization and ADCC and IgG titers were evaluated for statistical significance by the nonparametric Mann–Whitney U test. When comparing neutralization and ADCC titers by the same samples but against different subtypes, Wilcoxon's matched pairs test was used. The data were analyzed with Prism v.5.0 (GraphPad Software, Inc., San Diego, CA).
Results
Crossclade neutralizing antibodies differ in HIV-1–infected individuals from Guinea-Bissau and Denmark
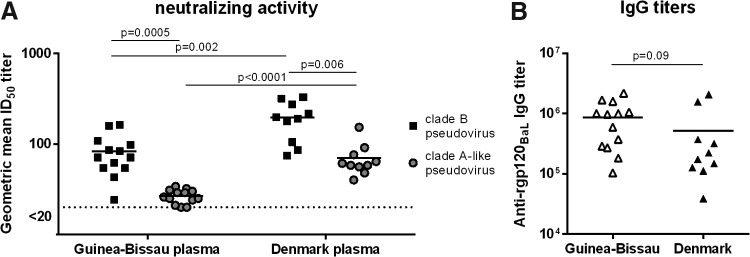
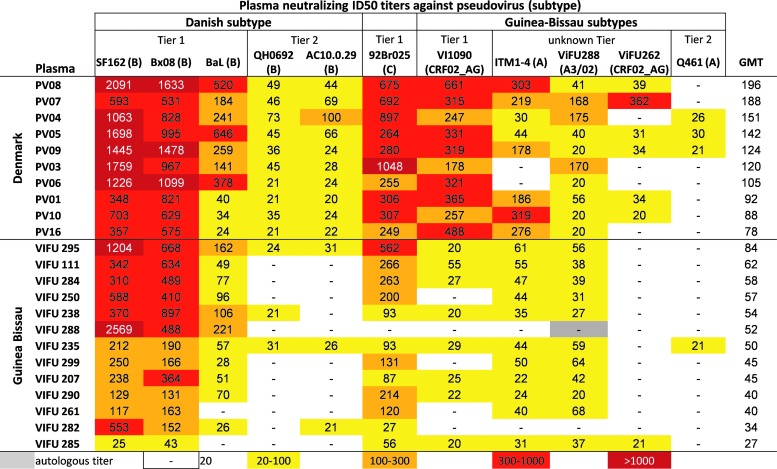
We studied plasma from 13 and 10 HIV-1–infected individuals living in Guinea-Bissau and Denmark, respectively, to determine intra- and crossclade HIV-1 neutralizing activity. The subtype/CRF distribution in the two groups was similar to previous estimates,26–29 including A3, CRF02_AG, and A3/02 subtypes in the Guinea-Bissau group and only subtype B in the Danish group (Table 1). The median CD4+ T-cell count was similar in the two groups, 506 cells/μl blood [interquartile range (IQR) 319–575] in Guinea-Bissau and 525 cells/μl blood (IQR 417–570) in Denmark, and the median plasma viral load was comparable at 30,943 (IQR 9,449–348,750) and 27,993 (IQR 13,151–85,309) copies/ml, respectively. Plasma samples were screened for neutralizing activity using the TZM-bl assay against a pseudotyped virus panel that represented several different subtypes, including Danish subtype B and the Guinea-Bissau subtypes A, CRF02_AG, and A3/02. To compare the intra- and crossclade neutralization between the two different geographical groups, a geometric mean ID50 neutralizing antibody titer was calculated for both groups against the regional local subtypes. Interestingly, the Danish samples displayed significantly higher neutralizing antibody titers against both subtype B and subtypes A, CRF02_AG, and A3/02 pseudoviruses than the Guinea-Bissau plasma samples (Fig. 1A). To determine if the lower neutralizing activity in the Guinea-Bissau samples reflected lower HIV-1 Env–specific IgG, the levels of anti-rgp120BaL antibody titers in the plasma samples were measured in ELISA. The levels of Env-specific IgG were similar in Guinea-Bissau and Danish individuals (Fig. 1B) despite the fact that the rgp120BaL used in the ELISA is a subtype B envelope, which could favor the antibody titers in the Danish samples. The individual neutralizing titers for each individual plasma–virus combination are shown in a heat map (Fig. 2). All viruses in the panel could be neutralized by some of the plasma samples. In addition to the regional subtypes, a Tier 1 subtype C virus, 92Br025, was included in the neutralization assay to test a nonregional subtype for both cohorts. Plasma from both groups showed neutralizing activity against the subtype C virus, but the Danish samples had significantly higher neutralizing titers (Mann–Whitney U test, p = .0009). The subtype B pseudoviruses were overall more sensitive to neutralization; however, this result may be expected as the three Tier 1 subtype B viruses used have previously been demonstrated to be highly sensitive to neutralization, whereas the subtypes A, AG, and A3/02 have been shown to be generally more resistant.36 To summarize, there appears to be no enhancement of regional intraclade neutralization in our study subjects. Instead, the plasma samples from Denmark with a subtype B background seem to be more potent and neutralize to higher titers compared to those from Guinea-Bissau with subtypes of A3, CRF02_AG, and A3/02 origin. On average, the Danish plasma samples had a threefold higher geometric mean neutralizing titer against each pseudovirus as the plasma from Guinea-Bissau. This was especially seen for VI1090 (Tier 1 subtype CRF02_AG), with a 14-fold higher titer for the Danish samples than Guinea-Bissau.
FIG. 1.
Heterologous neutralizing activities in plasma from Guinea-Bissau (n = 13) and Denmark (n = 10). (A) The potency of neutralizing activity was determined by the geometric mean ID50 titer for each individual across the five subtype B pseudoviruses and the five subtype A-like pseudoviruses. Individual plasma titers of <20 were assigned a value of 20 for the geometric mean titer (GMT) calculations. (B) The gp120BaL-specific antibody response was determined by ELISA. The values are mean ± standard error of the mean. ELISA, enzyme-linked immunosorbent assay.
FIG. 2.
HIV-1 inhibitory activity in plasma from Denmark and Guinea-Bissau. The plasma neutralization titer ID50 is shown as the reciprocal of the plasma dilution at which 50% inhibition of virus infection was achieved. The highest titers are shown in dark red and the lowest in yellow, as depicted in the legend. The titers below the detection limit (<1:20) have been omitted. The plasma samples were ranked based on the GMT across the seven viruses. Plasma titers <20 were assigned a value of 20 for the GMT calculations.
ADCC-mediated activity is higher against regional virus variants
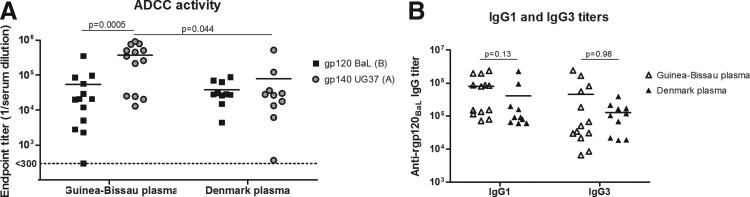
The presence of ADCC-inducing Env antibodies in plasma from HIV-1–infected individuals in Guinea-Bissau and Denmark was investigated. Recombinant gp120 and gp140 proteins from two viral strains, HIV-1BaL (subtype B) and HIV-1UG37 (subtype A), respectively, were used as the target antigen, representing viral subtypes from each of the two different geographical regions. The use of gp120 protein in in vitro assays has previously demonstrated a generally higher ADCC response than that of gp140 protein.46 Despite this finding, ADCC activity against both the gp120 and gp140 proteins was detected in nearly all Guinea-Bissau and all Danish samples (Fig. 3A). In addition, a significantly higher plasma ADCC activity was detected against subtype A gp140 in the Guinea-Bissau samples compared with activity against subtype B gp120. This subtype-specific activity was not detected in the Danish samples. In addition, the Guinea-Bissau samples demonstrated higher ADCC activity against subtype A Env than the Danish samples. The higher ADCC activity in the Guinea-Bissau samples could not be related to Env-specific IgG of the IgG1 and IgG3 subclasses, which are the two IgG subclasses with the strongest affinity for the NK-cell Fc receptor CD1647 because ELISA levels of rgp120BaL-specific IgG1 and IgG3 were similar in plasma from both regions (Fig. 3B). Rgp120BaL-specific IgG2 and IgG4 also demonstrated similar levels between the two groups (data not shown). Thus, we detected ADCC activity, including crossclade, in nearly all samples. However, the intraclade activity was higher in the plasma samples from Guinea-Bissau compared to crossclade activity (p = .0005) and was higher than the ADCC activity observed in the Danish samples (p = .044).
FIG. 3.
ADCC activity in Guinea-Bissau and Denmark plasma against subtypes B (gp120BaL) and A (gp140UG37) Env. (A) ADCC activity expressed as the endpoint titer for each individual from Guinea-Bissau and Denmark against gp120BaL clade B and gp140UG37 clade A. The values represent the reciprocal plasma dilution at which the threshold of ADCC positivity is reached. (B) IgG type 1- and 3–specific antibody response against gp120BaL, determined by ELISA. The values are mean ± standard error of the mean. ADCC, antibody-dependent cellular cytotoxicity.
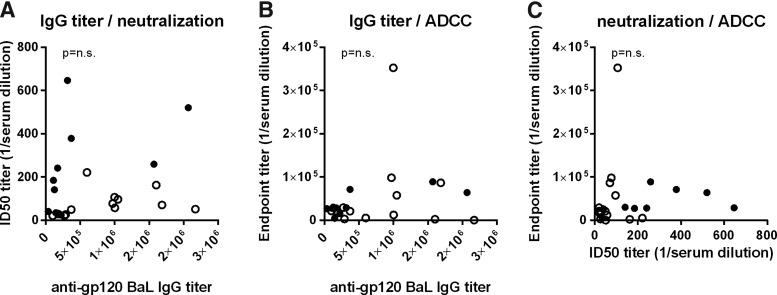
Finally, no correlation between the ability of plasma to neutralize the subtype B virus BaL to mediate ADCC activity against the same virus or the presence of EnvBaL-specific IgG ELISA was observed (Fig. 4).
FIG. 4.
ADCC activity of plasma does not correlate with neutralization or IgG binding. The gp120BaL-specific antibody response for each individual was compared to the neutralizing titer of BaL pseudovirus (A) and the ADCC activity against target cells coated with EnvBaL (B). The neutralizing titer of BaL pseudovirus was also compared to ADCC activity against target cells coated with EnvBaL (C). Open circles, Guinea-Bissau plasma; filled circles, Danish plasma.
Discussion
The primary goal of this study was to evaluate the basis for the construction of a regionally tailored HIV-1 antibody–based vaccine. Given the high diversity of HIV-1 (Env), we hypothesized that an antibody-based vaccine component may have to be based on regional immunogens of the same subtype. This approach could facilitate a more precisely focused immunological response to a more limited pool of similar circulating strains. We have characterized intra- and crossclade neutralizing and ADCC activities in plasma from HIV-1–infected individuals from Guinea-Bissau and Denmark harboring circulating viral strains of different subtypes. The two cohorts were matched for viral load and CD4 cell count to eliminate factors that may influence the neutralizing and ADCC activities.48–51 Interestingly, we found that plasma from Danish individuals was more potent in neutralizing activity regardless of the virus subtype than that from Guinea-Bissau individuals. The greater neutralizing titers of the Danish plasma were seen for all 11 pseudoviruses tested and surprisingly most pronounced for a Tier 1 CRF02_AG virus (VI1090). However, the ADCC activity in the same plasma samples demonstrated a different pattern. The Guinea-Bissau samples revealed a higher intraclade than crossclade ADCC activity, which was a pattern that was not observed in the Danish samples. In addition, the ADCC activity against the subtype A Env was higher in the Guinea-Bissau samples than in the Danish samples. These differences in inhibitory activity were not due to simply higher titers of anti–HIV-1 Env IgG as the levels were similar in the two cohorts. Moreover, a concern that the samples from the two regions have been treated differently, which may influence the antibody quality, is ruled out because the Guinea-Bissau plasma, which had lower neutralizing activity, demonstrated high ADCC activity compared with the Danish plasma, and the ELISA antibody titers were the same in both groups. Rather, the antibody specificity may differ perhaps by exposure of crossreactive neutralizing antibody epitopes or ADCC antibody epitopes. The neutralizing and ADCC activities of the two groups could not be correlated with gender (data not shown), which is in agreement with Mata et al.52 The Guinea-Bissau–specific subtypes A3, CRF02_AG, and A3/02 have previously been demonstrated to differ in disease progression,26 but this did not reflect any variations in neutralization or ADCC activity in our assays (data not shown).
The observed higher neutralizing titers and broadness of plasma samples from subtype B–infected individuals were unexpected but agree with Seaman et al.,36 who demonstrated that pools of the subtype B plasma in general demonstrated higher levels of neutralizing potency than other plasma pools, such as A or CRF02_AG. However, the observed superiority of the subtype B plasma to additionally neutralize A, CRF02_AG, and A3/02 pseudoviruses over the Guinea-Bissau plasma samples is in contrast with Jacob et al.,53 who reported high intraclade neutralization of CRF02_AG plasma samples. An explanation for the broader neutralization by the Danish samples could be that an infection with subtype B viruses elicits broader antibody responses due to the more sensitive phenotype of the subtype B viruses, as observed in Figure 1. Brown et al.54 support the theory that neutralization-sensitive viruses can have a greater exposure of neutralizing epitopes and thus elicit a broader and more potent immune response in individuals infected with such viruses. Indeed, the large classification of 107 viruses conducted by Seaman et al.36 ordered many of the subtype B viruses into more sensitive categories and ordered the CRF02_AG subtype viruses to relatively higher neutralization-resistant categories. The idea of using a viral strain for immunization, such as the subtype B isolate Bx08, which exposes common epitopes for neutralizing antibodies and is known to be commonly recognized by immune sera from a variety of HIV-1–infected individuals,55 has previously been tested, although with a limited neutralizing humoral response against primary virus.56–58
We have not examined if there is an overlap between the antibodies responsible for the neutralizing and ADCC activities in this study, but ADCC-mediating antibodies have previously been shown to be both neutralizing and nonneutralizing.14,59,60 Nevertheless, we found no association between ADCC and neutralizing activity when comparing the response from both assays and while using the same viral strain in the assays, the subtype B HIVBaL. However, such a correlation was not expected, and the broad neutralizing capacity of plasma has previously been demonstrated to not necessarily translate into ADCC activity and vice versa.12,61
We found ADCC activity against both subtypes B and A Env in nearly all plasma samples, which is consistent with previous reports of crossclade ADCC activity in HIV-infected individuals.46,52,61 The Guinea-Bissau plasma samples had increased ADCC activity against the subtype A Env, and we denote this as intraclade activity, as the subtypes A3, CRF02_AG, and A3/02 contain mainly the subtype A sequence in the env region.62 Augmented intraclade-specific ADCC activity has been previously noted for the subtype B plasma,46,52 but surprisingly in our study, the subtype B Danish samples did not show any enhanced ADCC activity against the subtype B Env. The higher ADCC activity in samples from Guinea-Bissau was not due to the concentrations of the primary IgG1 and IgG3 ADCC-mediating antibodies47 because a similar IgG antibody subclass distribution was observed in both groups. Rather, a difference in antibody specificities to ADCC epitopes, which differ from the broadly neutralizing epitopes, is a possibility. There are several epitopes within gp120 that can bind monoclonal antibodies to both neutralizing and ADCC activities; however, there are also epitopes that only bind nonneutralizing antibodies.14 These epitopes are both CD4 inducible and present on the surface of gp120. Alterations of these gp120 epitopes for different clade viruses, resulting in nonneutralizing antibodies, could explain the ADCC activity variation we see in the Guinea-Bissau and Danish plasma.
The data presented in this study may contribute to the selection of empiric candidate vaccines in limited test of concept clinical studies in Denmark and/or West Africa. Although broad neutralizing and ADCC responses may additionally depend on individual factors, it is still the particular virus Env to which they were exposed that induced the antibody specificity in that individual. We believe that the elicited response stems from viral variants with specific envelope properties, such as harboring neutralizing and nonneutralizing epitopes. Hopefully, these responses can be elicited again in human or animal models if formulated into an appropriate vaccine immunogen, or cocktails of immunogens, and with the right delivery strategy.25,63
Supplementary Material
Acknowledgments
We are grateful to the individuals who participated in this study. We thank Betty Willems and Randi Thøgersen for their excellent technical assistance at the Institute of Tropical Medicine and Statens Serum Institut, respectively. The members of the Bissau HIV Cohort Study Group are Amabelia Rodrigues, David da Silva, Zacarias da Silva, Candida Medina, Ines Oliviera-Souto, Lars Østergaard, Alex Laursen, Morten Sodemann, Peter Aaby, Anders Fomsgaard, Christian Erikstrup, Jesper Eugen-Olsen, and Christian Wejse (chair).
Sequence Data
Env sequences of ViFU288 and ViFU262 were deposited into GenBank under accession numbers KR709173 and KR709172.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mascola JR, Stiegler G, VanCott TC, et al. : Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 2000;6:207–210 [DOI] [PubMed] [Google Scholar]
- 2.Baba TW, Liska V, Hofmann-Lehmann R, et al. : Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 2000;6:200–206 [DOI] [PubMed] [Google Scholar]
- 3.Julien JP, Sok D, Khayat R, et al. : Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans. PLoS Pathog 2013;9:e1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binley JM, Lybarger EA, Crooks ET, et al. : Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol 2008;82:11651–11668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binley JM, Wrin T, Korber B, et al. : Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol 2004;78:13232–13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simek MD, Rida W, Priddy FH, et al. : Human immunodeficiency virus type 1 elite neutralizers: Individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol 2009;83:7337–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gils MJ, Euler Z, Schweighardt B, Wrin T, Schuitemaker H: Prevalence of cross-reactive HIV-1-neutralizing activity in HIV-1-infected patients with rapid or slow disease progression. AIDS 2009;23:2405–2414 [DOI] [PubMed] [Google Scholar]
- 8.Mascola JR, Haynes BF: HIV-1 neutralizing antibodies: Understanding nature's pathways. Immunol Rev 2013;254:225–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong PD, Mascola JR: Human antibodies that neutralize HIV-1: Identification, structures, and B cell ontogenies. Immunity 2012;37:412–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonsignori M, Pollara J, Moody MA, et al. : Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 2012;86:11521–11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes BF, Gilbert PB, McElrath MJ, et al. : Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012;366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambotte O, Ferrari G, Moog C, et al. : Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 2009;23:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wren LH, Chung AW, Isitman G, et al. : Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology 2013;138:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollara J, Bonsignori M, Moody MA, Pazgier M, Haynes BF, Ferrari G: Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses. Curr HIV Res 2013;11:378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollara J, Bonsignori M, Moody MA, et al. : HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol 2014;88:7715–7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Gils MJ, Sanders RW: Broadly neutralizing antibodies against HIV-1: Templates for a vaccine. Virology 2013;435:46–56 [DOI] [PubMed] [Google Scholar]
- 17.Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM: The challenge of HIV-1 subtype diversity. N Engl J Med 2008;358:1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndung'u T, Weiss RA: On HIV diversity. AIDS 2012;26:1255–1260 [DOI] [PubMed] [Google Scholar]
- 19.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. : Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 20.Stephenson KE, Barouch DH: A global approach to HIV-1 vaccine development. Immunol Rev 2013;254:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Churchyard GJ, Morgan C, Adams E, et al. : A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204). PLoS One 2011;6:e21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammer SM, Sobieszczyk ME, Janes H, et al. : Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013;369:2083–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julien JP, Cupo A, Sok D, et al. : Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 2013;342:1477–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders RW, Derking R, Cupo A, et al. : A next-generation cleaved, soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 2013;9:e1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borggren M, Vinner L, Andresen BS, et al. : Optimization of HIV-1 envelope DNA vaccine candidates within three different animal models, Guinea pigs, rabbits and cynomolgus macaques. Vaccines 2013;1:305–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palm AA, Esbjornsson J, Mansson F, et al. : Faster progression to AIDS and AIDS-related death among seroincident individuals infected with recombinant HIV-1 A3/CRF02_AG compared with sub-subtype A3. J Infect Dis 2013. [Epub ahead of print]; DOI: 10.1093/infdis/jit416 [DOI] [PubMed] [Google Scholar]
- 27.Esbjornsson J, Mild M, Mansson F, Norrgren H, Medstrand P: HIV-1 molecular epidemiology in Guinea-Bissau, West Africa: Origin, demography and migrations. PLoS One 2011;6:e17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abecasis AB, Wensing AM, Paraskevis D, et al. : HIV-1 subtype distribution and its demographic determinants in newly diagnosed patients in Europe suggest highly compartmentalized epidemics. Retrovirology 2013;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Audelin AM, Cowan SA, Obel N, Nielsen C, Jorgensen LB, Gerstoft J: Phylogenetics of the Danish HIV epidemic: The role of very late presenters in sustaining the epidemic. J Acquir Immune Defic Syndr 2013;62:102–108 [DOI] [PubMed] [Google Scholar]
- 30.Oliveira I, Andersen A, Furtado A, et al. : Assessment of simple risk markers for early mortality among HIV-infected patients in Guinea-Bissau: A cohort study. BMJ Open 2012;2:e001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jespersen S, Honge BL, Oliveira I, et al. : Cohort profile: The Bissau HIV Cohort-a cohort of HIV-1, HIV-2 and co-infected patients. Int J Epidemiol 2015;44:756–763 [DOI] [PubMed] [Google Scholar]
- 32.Roman VR, Jensen KJ, Jensen SS, et al. : Therapeutic vaccination using cationic liposome-adjuvanted HIV type 1 peptides representing HLA-supertype-restricted subdominant T cell epitopes: Safety, immunogenicity, and feasibility in Guinea-Bissau. AIDS Res Hum Retroviruses 2013;29:1504–1512 [DOI] [PubMed] [Google Scholar]
- 33.Karlsson I, Brandt L, Vinner L, et al. : Adjuvanted HLA-supertype restricted subdominant peptides induce new T-cell immunity during untreated HIV-1-infection. Clin Immunol 2013;146:120–130 [DOI] [PubMed] [Google Scholar]
- 34.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S: MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lole KS, Bollinger RC, Paranjape RS, et al. : Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 1999;73:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaman MS, Janes H, Hawkins N, et al. : Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 2010;84:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham FL, Smiley J, Russell WC, Nairn R: Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 1977;36:59–74 [DOI] [PubMed] [Google Scholar]
- 38.Connor RI, Chen BK, Choe S, Landau NR: Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 1995;206:935–944 [DOI] [PubMed] [Google Scholar]
- 39.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR: Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol 1995;69:6705–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei X, Decker JM, Liu H, et al. : Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 2002;46:1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 2009;485:395–405 [DOI] [PubMed] [Google Scholar]
- 42.Heyndrickx L, Heath A, Sheik-Khalil E, et al. : International network for comparison of HIV neutralization assays: The NeutNet report II. PLoS One 2012;7:e36438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen SS, Hartling HJ, Tingstedt JL, et al. : HIV-specific ADCC improves after antiretroviral therapy and correlates with normalization of the NK cell phenotype. J Acquir Immune Defic Syndr 2015;68:103–111 [DOI] [PubMed] [Google Scholar]
- 44.Pollara J, Hart L, Brewer F, et al. : High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 2011;79:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP: A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J Virol 1999;73:8966–8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madhavi V, Wren LH, Center RJ, et al. : Breadth of HIV-1 Env-specific antibody-dependent cellular cytotoxicity: Relevance to global HIV vaccine design. AIDS 2014;28:1859–1870 [DOI] [PubMed] [Google Scholar]
- 47.Nimmerjahn F, Ravetch JV: Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008;8:34–47 [DOI] [PubMed] [Google Scholar]
- 48.Johansson SE, Rollman E, Chung AW, et al. : NK cell function and antibodies mediating ADCC in HIV-1-infected viremic and controller patients. Viral Immunol 2011;24:359–368 [DOI] [PubMed] [Google Scholar]
- 49.Moore PL, Williamson C, Morris L: Virological features associated with the development of broadly neutralizing antibodies to HIV-1. Trends Microbiol 2015;23:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray ES, Madiga MC, Hermanus T, et al. : The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol 2011;85:4828–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sather DN, Armann J, Ching LK, et al. : Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 2009;83:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mata MM, Iwema JR, Dell S, et al. : Comparison of antibodies that mediate HIV type 1 gp120 antibody-dependent cell-mediated cytotoxicity in asymptomatic HIV type 1-positive men and women. AIDS Res Hum Retroviruses 2014;30:50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacob RA, Abrahams F, Tongo M, et al. : Refined identification of neutralization-resistant HIV-1 CRF02_AG viruses. J Virol 2012;86:7699–7703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown BK, Wieczorek L, Sanders-Buell E, et al. : Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology 2008;375:529–538 [DOI] [PubMed] [Google Scholar]
- 55.Moog C, Fleury HJ, Pellegrin I, Kirn A, Aubertin AM: Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol 1997;71:3734–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corbet S, Vinner L, Hougaard DM, et al. : Construction, biological activity, and immunogenicity of synthetic envelope DNA vaccines based on a primary, CCR5-tropic, early HIV type 1 isolate (BX08) with human codons. AIDS Res Hum Retroviruses 2000;16:1997–2008 [DOI] [PubMed] [Google Scholar]
- 57.Vinner L, Therrien D, Wee E, et al. : Immune response in rhesus macaques after mixed modality immunisations with DNA, recombinant adenovirus and recombinant gp120 from human immunodeficiency virus type 1. APMIS 2006;114:690–699 [DOI] [PubMed] [Google Scholar]
- 58.Vinner L, Wee EG, Patel S, et al. : Immunogenicity in Mamu-A*01 rhesus macaques of a CCR5-tropic human immunodeficiency virus type 1 envelope from the primary isolate (Bx08) after synthetic DNA prime and recombinant adenovirus 5 boost. J Gen Virol 2003;84(Pt 1):203–213 [DOI] [PubMed] [Google Scholar]
- 59.Hessell AJ, Hangartner L, Hunter M, et al. : Fc receptor but not complement binding is important in antibody protection against HIV. Nature 2007;449:101–104 [DOI] [PubMed] [Google Scholar]
- 60.Chung AW, Isitman G, Navis M, et al. : Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A 2011;108:7505–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smalls-Mantey A, Doria-Rose N, Klein R, et al. : Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. J Virol 2012;86:8672–8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carr JK, Salminen MO, Albert J, et al. : Full genome sequences of human immunodeficiency virus type 1 subtypes G and A/G intersubtype recombinants. Virology 1998;247:22–31 [DOI] [PubMed] [Google Scholar]
- 63.Heyndrickx L, Stewart-Jones G, Jansson M, et al. : Selected HIV-1 Env trimeric formulations act as potent immunogens in a rabbit vaccination model. PLoS One 2013;8:e74552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.