Abstract
Adipokines may be potential mediators of the association between excess adiposity and vascular dysfunction. We assessed the cross-sectional associations of circulating adipokines with vascular stiffness in a community-based cohort of younger adults. We related circulating concentrations of leptin and leptin receptor, adiponectin, retinol binding protein 4, and fatty acid binding protein 4 to vascular stiffness measured by arterial tonometry in 3505 Framingham Third Generation cohort participants free of cardiovascular disease (mean age 40 years, 53% women). Separate regression models estimated the relations of each adipokine to mean arterial pressure and aortic stiffness, as carotid femoral pulse wave velocity, adjusting for age, sex, smoking, heart rate, height, antihypertensive treatment, total and high-density lipoprotein cholesterol, diabetes, alcohol consumption, estimated glomerular filtration rate, glucose and C-reactive protein. Models evaluating aortic stiffness also were adjusted for mean arterial pressure. Mean arterial pressure was positively associated with blood retinol binding protein 4, fatty acid binding protein 4, and leptin concentrations (all P<0.001) and inversely with adiponectin (p=0.002). In fully adjusted models, mean arterial pressure was positively associated with retinol binding protein 4 and leptin receptor levels (p<0.002 both). In fully adjusted models, aortic stiffness was positively associated with fatty acid binding protein 4 concentrations (p=0.02), but inversely with leptin and leptin receptor levels (p≤0.03 both). In our large community-based sample, circulating concentrations of select adipokines were associated with vascular stiffness measures, consistent with the hypothesis that adipokines may influence vascular function and may contribute to the relation between obesity and hypertension.
Keywords: adipokines, biomarkers, epidemiology, obesity, vascular function
Excess adiposity is associated with an increased risk of cardiovascular disease (CVD) events. On a parallel note, aortic stiffness also has been linked to an increased risk of CVD.1, 2 Other studies, including from our group, have underscored the association of obesity and related comorbidities such as hypertension, dyslipidemia, and diabetes with increased vascular stiffness in community-dwelling individuals.3, 4 These observations raise the possibility that adiposity may increase CVD risk in part through effects on vascular stiffness. Yet, mechanisms linking adiposity to vascular function are incompletely elucidated.
Toward identifying possible mechanisms, adipose tissue is biologically active, elaborating multiple compounds (often termed as adipokines) such as leptin, leptin receptor (LEP-R), adiponectin, fatty acid binding protein 4 (A-FABP), and retinol binding protein (RBP4). Adipokines may affect arterial structure and function, by multiple mechanisms including hyperglycemia, hyperinsulinemia, inflammation, activation of sympathetic activity, and vascular smooth muscle cell proliferation..5, 6 The anatomical distribution of various adipose depots may also modulate the metabolic activity of adipokines.7, 8 Previous work has suggested relations between individual adipokines and arterial stiffness.9-11 Prior data also indicate adipokines may be altered in patients with hypertension.12-15
We hypothesized that higher concentrations of leptin, A-FABP, and RBP4, and lower concentrations of LEP-R and adiponectin would be associated with increased aortic stiffness, as reflected by CFPWV, and with elevated blood pressure, as assessed by mean arterial pressure (MAP). To test this hypothesis, we related circulating concentrations of a panel of adipokines to vascular stiffness in a large community-based sample of young to middle-aged adults.
METHODS
The longitudinal community-based Framingham Third Generation cohort, comprised of the grandchildren of the Original Framingham Cohort, was recruited between 2002 to 2005 to undergo their first examination cycle.,16 During the examination visit, a targeted physical examination that included measurement of resting blood pressure and anthropometry was performed and a brief medical history that focused on cardiometabolic disease was obtained. Standardized questionnaires were used to assess diet, current smoking, alcohol use, and physical activity. Arterial tonometry was performed during the examination visit (see below). Laboratory evaluation of standard CVD risk factors was performed on venous blood sample obtained after an overnight fast. At the first examination cycle of this cohort, a panel of novel adipokines was measured on biosamples stored at −80° C without prior freeze thaw cycles. Of the 4095 attendes who were eligible for the present investigation, we excluded 167 for missing tonometry data, 196 for missing adipokine levels, 177 for missing covariate data, and 50 individuals with prevalent CVD, yielding a final sample of 3505 participants for the present analyses, 86% of the eligible Third Generation cohort. All attendees gave written informed consent and the study protocol was approved by the Boston University Medical Center Institutional Review Board.
Adipokine Measurement
Commercially available kits were used to assay blood concentrations of adiponectin, leptin, LEP-R and RBP4 (R&D Systems Inc., Minneapolis, MN) and A-FABP (Biovendor Inc., Candler, NC). Mean interassay coefficients of variation for all adipokines were less than 10% as reported previously.17
Applanation Tonometry
After 5 minutes of rest, participants underwent applanation tonometry in a supine position.2 The timing of the cardiac cycle was determined by electrocardiography, and measurements of systolic and diastolic blood pressures were obtained on the right arm of the supine participant using a semi-automated auscultatory method (Cardiovascular Engineering, Inc. Norwood MA). Arterial tonometry on right-sided carotid, brachial, radial, and femoral arteries was performed using a hand-held customized tonometer. Distances were measured from the suprasternal notch to each pulse measurement site using a caliper for the femoral site and a fiberglass tape measure for other sites. Arterial waveform signals were digitized (1000Hz), transferred to the core laboratory (Cardiovascular Engineering Inc, Norwood MA) and then analyzed blinded to all clinical data.
As previously described, the ECG-derived R wave was used as the reference point for signal averaging and timing waveforms.3 Tonometry-derived signal averaged brachial waveform was calibrated to the brachial cuff systolic and diastolic blood pressure. The calibrated brachial artery waveform was then integrated to calculate MAP. The carotid waveform was used as a surrogate for central arterial pressure. The carotid waveform was calibrated to brachial waveform by assuming identical diastolic and MAP. Carotid femoral pulse wave velocity (CFPWV) was calculated by dividing the transit distance between carotid and femoral sites by the transit time difference between those sites. Carotid femoral transit distance was adjusted for parallel transmission by using the suprasternal notch as a fiducial point. CFPWV was inverse transformed in order to eliminate skewness and heteroscedasticity. Inverse CFPWV was multipled by −1000 to convert units to ms/m and restore directionality (high value corresponds to higher aortic stiffness).
Assessment of Covariates
Prevalent CVD was defined by the presence of cerebrovascular disease (stroke or transient ischemic attack), coronary heart disease (angina, or acute coronary syndrome, including myocardial infarction), congestive heart failure, or intermittent claudication, as adjudicated by the Framingham endpoints reveiw committee. Hypertension was defined as arm blood pressure ≥140/90 mm Hg or current use of anti-hypertensive medications. Diabetes was defined as fasting glucose concentration ≥126 mg/dl or the use of hypoglycemic medications. Alcohol use was self-reported and quantified in fluid ounces per month. Obesity was defined as body mass index (BMI) ≥30 kg/m2. Estimated glomerular filtration rate(eGFR) was calculated by the Chronic Kidney Disease-Epidemiology Collaboration equation.18 High-sensitivity C reactive protein was measured using the nephelometric method (Dade-Behring BN 100 nephelometer).
Statistical Analyses
The primary exposures of interest (independent variables) were circulating sex-standardized concentrations of the five adipokines. CFPWV, a measure of large artery stiffness, and MAP, a measure of steady-flow arterial pressure, were the primary dependent variables (separate models for each). The cross-sectional associations of adipokines with CFPWV and MAP were examined using sex-pooled multivariable generalized linear regression models, adjusting for age, sex, heart rate, height, diabetes, lipid levels (total cholesterol and HDL), anti-hypertensive treatment, smoking status, and eGFR. CFPWV models were additionally adjusted for MAP in order to account for potential effects of distending pressure on aortic stiffness. In additional analyses, we also adjusted for weight or waist circumference in order to evaluate whether adjustment for adiposity altered any of the observed associations of adipokines with vascular measures. Finally we added adjustment for alcohol intake, high sensitivity C-reactive protein as an index of inflammation, and fasting blood glucose as a continuous measure. Primary analyses treated adipokines as continuous variables. We also conducted analyses using sex-specific quartiles for each adipokine as predictors, with the first quartile serving as the referent and conducting a statistical test for trend across the quartiles. General estimating equations were used to account for the relatedness of individuals in the Third Generation cohort.19 Statistical significance was indicated by a two-sided P-value of <0.05. SAS 9.1 (Cary, North Carolina) was used for all analyses.
RESULTS
The clinical and vascular stiffness characteristics of our young-to-middle aged adult sample (mean age 40±9 years, range 20 to 72 years, 53% women) are shown in Table 1. While age was similar for men and women, BMI, blood pressure, total cholesterol, smoking prevalence, and waist circumference tended to be higher in men, while HDL-C was higher in women. Leptin and adiponectin concentrations were susbtantially higher in women than in men. CFPWV and MAP were higher in men (p<0.001).
Table 1.
Characteristics of the study sample by sex (N=3505)
| Characteristic | Men (n=1644) | Women (n=1861) | P value |
|---|---|---|---|
| Clinical | |||
| Age, years | 40 ± 9 | 40 ± 9 | 0.39 |
| Body mass index, kg/m2 | 27.6±4.4 | 25.4±5.3 | <0.0001 |
| Height (meters) | 1.78±0.07 | 1.64±0.06 | <0.0001 |
| Heart rate, bpm | 61±10 | 63±10 | <0.0001 |
| Systolic blood pressure, mm Hg | 120±12 | 113 ± 14 | <0.0001 |
| Diastolic blood pressure, mm Hg | 78±9 | 72±9 | <0.0001 |
| Hypertension, % | 20 (n=325) | 11 (n=199) | <0.0001 |
| Total cholesterol, mmol/L | 4.99± 0.96 | 4.77± 0.86 | <0.0001 |
| HDL-C, mmol/L | 1.22±0.32 | 1.59±0.41 | <0.0001 |
| Glucose | 98±16 | 92±18 | <0.0001 |
| Diabetes, % | 3 (n=49) | 2 (n=38) | 0.07 |
| Current Smoking, % | 16 (n=267) | 14 (n=259) | 0.05 |
| Alcohol (ounces per month) | 13.9±19.0 | 5.9±7.9 | <0.0001 |
| Waist circumference, cm | 98±12 | 87±14 | <0.0001 |
| eGFR, mL/min per 1.73 m2 | 99±17 | 99±19 | 0.82 |
| C reactive protein() | 2.01±4.24 | 2.85±4.98 | <0.0001 |
| Adipokines | |||
| RBP4, ng/ml | 43.8±10.0 | 38.0±10.5 | <0.0001 |
| A-FABP, ng/ml | 16.4±8.0 | 20.8±11.9 | <0.0001 |
| Leptin, ng/ml | 5.7±5.4 | 16.7±15.6 | <0.0001 |
| LEP-R, ng/ml | 19.0±8.1 | 20.1±8.9 | <0.0001 |
| Adiponectin, ug/ml | 6.1±3.7 | 11.1±5.7 | <0.0001 |
| Tonometry | |||
| CFPWV, m/s | 7.4±1.4 | 6.6±1.2 | <0.0001 |
| Mean arterial pressure, mm Hg | 92±10 | 87±11 | <0.0001 |
Values are % (n), or mean± SD
Abbreviations: kg/m2- kilograms per meters squared, bpm- beats per minute, mmol/L- millimoles per liter, mm Hg- millimeters of mercury, cm- centimeters, eGFR- estimated glomerular filtration rate, ml/min per 1.73 m2- milliliters per minute-1.73 meters squared body surface area, ng/mL- nanograms per milliliter, LEP-R- leptin receptor, ug/mL- micrograms per milliliter, A-FABP- fatty acid binding protein 4, RBP4- retinol binding protein 4, CFPWV- carotid femoral pulse wave velocity, m/s- meters per second
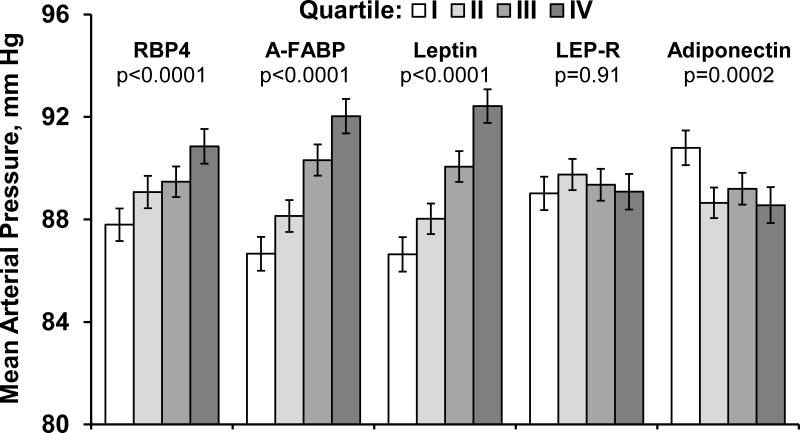
After adjustment for age, sex, anti-hypertensive treatment, total and HDL cholesterol, smoking, and diabetes, a one standard deviation higher value for leptin, RBP4, or A-FABP concentration was associated with approximately a 1-2 mm Hg higher MAP (P<0.0001 for all; Table 2), while a similar increment in adiponectin was associated with a 0.6 mm Hg lower MAP (p=0.002). MAP was higher across quartiles of, RBP4, A-FABP, and leptin but was lower across quartiles of adiponectin (P for trend<0.0001 for all, Figure 1). Upon adjustment for weight (or alternatively waist circumference; results not shown for latter), RBP4 remained positively associated with MAP while LEP-R emerged as positively related to MAP, (P<0.001 for both). After final adjustment including alcohol intake, C-reactive protein and blood glucose levels, the associations of RBP4 and LEP-R were essentially unchanged.
Table 2.
Associations of each adipokine with mean arterial pressure
| Model 1* | Model 2† | Model 3§ | ||||
|---|---|---|---|---|---|---|
| Adipokine | Regression coefficient per SD (95%CI) | P-value | Regression coefficient per SD (95%CI) | P-value | Regression coefficient per SD (95%CI) | P value |
| RBP4 | 1.31 (0.94,1.68) | <.0001 | 1.28 (0.93,1.51) | <0.001 | 1.15 (0.78, 1.51) | <0.001 |
| A-FABP | 1.77 (1.34,2.20) | <.0001 | 0.35 (−0.08,0.79) | 0.11 | 0.29 (−0.15, 0.73) | 0.20 |
| Leptin | 2.09 (1.70,2.48) | <.0001 | 0.16 (−0.32,0.65) | 0.50 | 0.17 (−0.31, 0.65) | 0.48 |
| Lep-R | −0.10 (−0.44,0.25) | 0.58 | 0.53 (0.20,0.87) | 0.002 | 0.52 (0.19, 0.85) | 0.002 |
| Adiponectin | −0.64 (−1.05,−0.23) | 0.0023 | −0.09 (−0.49,0.30) | 0.64 | 0.00 (−0.40, 0.39) | 0.99 |
Beta coefficient per standard deviation(SD) of sex-standardized biomarkers as in Table 1.
Model 1: adjusted for age, sex, heart rate, height, anti-hypertensive treatment, total and HDL cholesterol, smoking, and diabetes.
Model 2: adjusted for Model 1 covariates and body weight.
Model 3: adjusted for Model 2 covariates plus estimated glomerular filtration, alcohol, glucose, log transformed c reactive protein.
Abbreviations: Lep-R- leptin receptor, A-FABP- fatty acid binding protein 4, RBP4- retinol binding protein 4
Figure 1.
Shows average mean arterial pressure levels in groups defined according to sex-specific quartiles of each adipokine.
Adjustment covariates included age, sex, heart rate, height, anti-hypertensive treatment, total and HDL cholesterol, smoking, diabetes. Error bars represent 95% confidence intervals.
Abbreviations: MAP- mean arterial pressure, LEP-R- leptin receptor, ug/mL-micrograms per milliliter, A-FABP- fatty acid binding protein 4, RBP4- retinol binding protein 4.
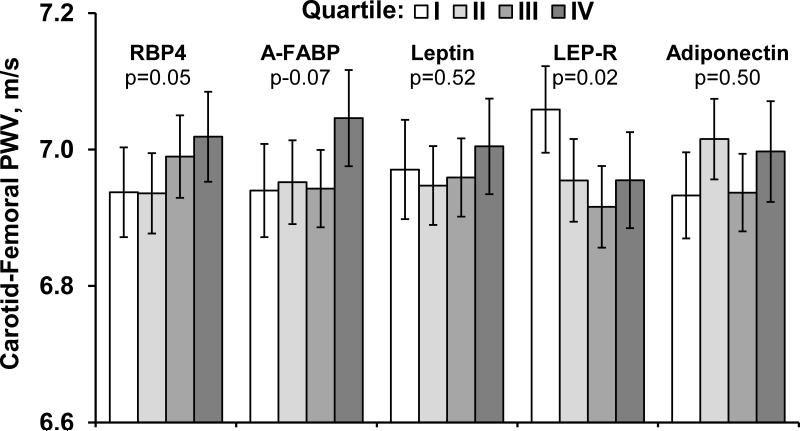
Blood leptin and LEP-R were inversely related (p<0.03 for both) to CFPWV whereas A-FABP was positively related (p=0.008;Table 3). In comparison by quartile-based models, CFPWV decreased across LEP-R quartiles but rose across RBP4 quartiles (P for trend= 0.02 and 0.05, respectively, Figure 2). Upon additional adjustment for weight (or waist), the relations of LEP-R and A-FABP with CFPWV were maintained in terms of directionality and statistical signficance. However leptin emerged also being inversely related to CFPWV (p=0.02;Table 3). These associations for FABP, LEP-R, and leptin were largely unchanged by further adjustment for inflammation, alcohol consumption, and blood glucose levels.
Table 3.
Associations of each adipokine with transformed CFPWV (−1000/CFPWV)
| Model 1* | Model 2† | Model 3§ | ||||
|---|---|---|---|---|---|---|
| Adipokine | Regression coefficient. per SD (95%CI) | P Value | Regression coefficient. per SD (95%CI) | P Value | Regression coefficient. per SD (95%CI) | P Value |
| RBP4 | 0.58 (−0.08,1.23) | 0.09 | 0.60 (−0.06,1.25) | 0.08 | 0.58 (−0.08,1.25) | 0.09 |
| A-FABP | 1.14 (0.54,1.73) | 0.0002 | 0.96 (0.25,1.67) | 0.008 | 0.88 (0.16,1.60) | 0.02 |
| Leptin | −0.03 (−0.70,0.64) | 0.93 | −1.06 (−1.95,−0.18) | 0.02 | −1.22 (−2.12,−0.32) | 0.008 |
| LEP-R | −0.79 (−1.35,−0.24) | 0.005 | −0.65 (−1.21,−0.08) | 0.02 | −0.65 (−1.21,−0.08) | 0.03 |
| Adiponectin | 0.22 (−0.54,0.97) | 0.57 | 0.38 (−0.40,1.15) | 0.34 | 0.47 (−0.31,1.25) | 0.24 |
Beta coefficient per standard deviation (SD) of sex standardized biomarkers as in Table 1.
Model 1: adjusted for age, sex, heart rate, height, anti-hypertensive treatment, total and HDL cholesterol, smoking, diabetes and mean arterial pressure.
Model 2: adjusted for Model 1 covariates and body weight.
Model 3: adjusted for Model 2 covariates plus estimated glomerular filtration, alcohol, glucose, log transformed c reactive protein.
Abbreviations: LEP-R- leptin receptor, A-FABP- fatty acid binding protein 4, RBP4- retinol binding protein 4, CFPWVcarotid femoral pulse wave velocity, m/s- meters per second
Figure 2.
Shows the average carotid-femoral pulse wave velocity levels in groups defined according to sex-specific quartiles of each adipokine.
Adjustment covariates included adjusted for age, sex, heart rate, height, anti-hypertensive treatment, total and HDL cholesterol, smoking, diabetes and mean arterial pressure. Error bars represent 95% confidence intervals.
Abbreviations: CFPWV- carotid femoral pulse wave velocity, LEP-R- leptin receptor, ug/mL- micrograms per milliliter, A-FABP- fatty acid binding protein 4, RBP4- retinol binding protein 4.
DISCUSSION
Principal findings
In this comprehensive cross-sectional analysis of a large, community-based sample of young-to-middle-aged adults, we related circulating concentrations of a panel of five key adipokines to arterial stiffness measures. After multivariable adjustment, higher RBP4, A-FABP, leptin levels and lower adiponectin levels were associated with higher MAP. Upon additional adjustment for weight, the positive association of RBP4 and MAP remained robust, and an additional new positive association of LEP-R and MAP emerged with little change after additional adjustment for inflammation (C-reactive protein), alcohol use, or blood glucose concentrations. Higher A-FABP and lower LEP-R were associated with higher CFPWV. These 2 relations were only modestly attenuated after adjustment for weight and intriguingly an inverse relation of leptin concentrations and CFPWV also emerged, with modest change after additional adjustment for alcohol intake, blood glucose, and inflammation. The observed association patterns support the hypothesis that adipokines are associated with altered small and large artery function, and also suggest that several of the observed relations of adipokines persist after accounting for weight, inflammation, alcohol use, and other confounders.
Circulating adipokines could mediate the well-described relation between obesity and increased vascular stiffness. Underscoring the relevance of vascular stiffness, metaanalysis indicates that 1 SD higher CFPWV is associated with a hazard ratio of 2.04 (1.70-2.44) for major CVD events in younger adults (<61 years of age).1, 2, 20 Adipokines influence glucose metabolism and insulin resistance, and these relations can vary according to fat quantity and regional location.21, 22 Therefore studying adipokines also may provide mechanistic insights into the link between glucose intolerance, insulin resistance, and arterial dysfunction.23 Adipokines can directly modulate endothelial cell and vascular smooth muscle cell activation, proliferation and migration.24 Indeed, as noted above, prior work has shown that obesity is associated with endothelial dysfunction and increased vascular stiffness.21, 24, 25 Overall, our findings expand upon prior reports of the relations between adipokines and vascular function. Prior studies relating circulating levels of adipokines and measures of vascular function have been largely focused on a single biomarker and evaluated limited measures of vascular function.
Leptin is a key long-term energy homeostasis signal.26, 27 Circulating LEP-R binds to leptin and reduces its bioavailability.26, 28 Both markers have been linked to BMI and fat distribution.29 In obesity, paradoxical elevation of leptin occurs due to end-organ resistance.7, 26 Relevant to the present hypothesis, leptin receptors are present on vascular cells and in atherosclerotic lesions, suggesting a role for leptin in vascular physiology.24 Studies also have shown that leptin increases renal sympathetic activation, which increases arterial pressure.30 Leptin has been described in youth, young adults, and older adults to be associated with aortic stiffness.9, 31 These previous investigations also demonstrate the relation between obesity and vascular stiffness is attenuated with the inclusion of leptin, suggesting leptin may mediate the association between adiposity and vascular stiffness. Consistent with these prior studies, our investigation found higher LEP-R, which would lead to lower free leptin signalling, was associated with lower CFPWV and higher MAP. Further adjustment for weight influenced the relations observed for leptin. Paradoxically, after adjustment for weight both higher leptin and LEP-R were associated with lower CFPWV. The associations for leptin and LEP-R may parallel so-called ‘leptin resistance‘ where leptin levels are counterintuitively elevated in obese persons. Despite replete energy stores, altered cell bound leptin receptor signal transduction inadequately senses energy repletion status, while circulating LEP-R activity is largely unaffected. 13, 26 The association of LEP-R with lower CFPWV and higher MAP is complex to reconcile given our cross-sectional analysis. It may be that LEP-R has different effects on small muscular arteries versus larger conduit arteries. For example, normal arterial aging may affect one arterial system (conduit arteries) and spare others (muscular arteries), perhaps due to different tissue constituents.32, 33 It is unlikely that the LEP-R vascular associations are causally interrelated as the counterregulatory response to lower CFPWV would not engender higher MAP. While our results are generally consistent with a protective role for free leptin in promoting lower arterial stiffness and lower MAP, caution should be exercised in drawing causal inferences from our cross-sectional data.
Adiponectin exerts favorable metabolic and cardiovascular effects by promoting insulin sensitivity and fatty acid oxidization and inhibiting gluconeogenesis.17, 34, 35 Adiponectin increases nitric oxide availability and suppresses vascular smooth muscle cell proliferation and migration.36, 37 Endothelial function is diminished in adiponectin knock-out mice.5, 6 Hypoadiponectinaemia has been associated with impaired endothelial function in patients with hypertension and diabetes.5, 38 Higher adiponectin levels were associated with lower arterial stiffness and better endothelial function in healthy and clinical samples;11 however, we were not able to detect a relation between adiponectin level and CFPWV. Data are conflicting regarding the relation between adiponectin and hypertension.12-14 In metaanalysis, circulating adiponectin concentrations appear to be lower in hypertensive persons.39 Our data are consistent with these metaanalytic findings as lower adiponectin was associated with higher MAP. Our data are also consistent with the hypothesis that adiponectin may mediate the vascular consequences of excess weight as inclusion of weight in the model attenuated the association of adiponectin with MAP.
RBP4 and A-FABP have been implicated as mediators of insulin sensitivity and lipid metabolism.40-42 Cross-sectional data from the Framingham Study demonstrated that circulating levels of RBP4 were associated with insulin resistance and elevated blood pressure but, intriguingly, were not associated with BMI17 Other investigators also found inconsistent relations between RBP4 and obesity.28, 43, 44 Despite the inconsistent relation with excess weight, small, patient-based studies support a relation between RBP4 and vascular function as higher circulating and urinary RBP4 concentrations have been associated with carotid intima media thickness and arterial stiffness.10, 45, 46 Our results demonstrating positive associations of RBP4 and MAP with little attenuation after weight adjustment, are consistent with prior results. Blood A-FABP levels are elevated in persons with insulin resistance and hypertension but lower in treated hypertension.15, 47 Therefore, our observed positive association of A-FABP with MAP (in models not adjusted for weight) and with CFPWV (models without and with adjustment for weight) are consistent with previous literature.
Our investigation has several limitations including its cross-sectional observational study design, which precludes causal or temporal inferences. Our observations have uncertain generalizability to younger or elderly people of non-white ethnicity due to its constitution with predominantly young and middle-aged adults of European ancestry. While we focused on 5 key adipokines, other adiposity biomarkers may mediate relations of obesity to vascular dysfunction. We used a simple measure of adiposity, body weight, rather than quantitative measures of visceral fat depots, which are known to be more strongly related to adipokine levels.7, 48, 49 As these analyses are hypothesis-generating, we did not correct for multiple statistical testing. Nonetheless, if we use an overly conservative Bonferroni correction (p of 0.005, i.e., 0.05/10 for relating 5 biomarkers to 2 primary vascular stiffness measures), several observed associations still remain statistically significant.
PERSPECTIVES
Leveraging contemporaneous tonometry and adipokine assays measured in a large well-characterized community-based sample, we observed novel relations of several circulating adipokines with vascular stiffness and MAP. The study findings are consistent with the notion that adipokines may be important mediators of the association of excess adiposity with vascular dysfunction. Additional studies are warranted to examine temporal relation between adipokines, adiposity, and vascular dysfunction.
NOVELTY AND SIGNIFICANCE.
-
1)What is new?
- In a large group of younger adults, we studied the relations between five blood proteins and hormones derived from fat tissue and the stiffness of large and small arteries.
-
2)What is relevant?
- Large and small blood vessel stiffness explains current and predicts future high blood pressure.
-
3)Summary
- Key fat tissue-produced blood proteins are related to large and small artery stiffness even after accounting for participants‘ weight
- Therefore these fat-tissue related blood proteins may have a role in the presence and/or development of high blood pressure
ACKNOWLEDGEMENTS
None
Sources of Funding
Research supported, in part, by NHLBI contract N01-HC-25195 and HHSN268201500001I (RSV), R01-DK-080739 (RSV) and R01-HL-107385 R01-HL-126136 (RSV, GFM), 1RO1-HL-70100 (EJB), and HL111335 (JPZ)
Disclosures
Dr. Mitchell is owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives honoraria from Novartis, Merck and Servier, and was funded by research grants HL094898, DK082447, HL107385 and HL104184 from the National Institutes of Health.
References
- 1.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Tao L, Yuan Y, Jiao X, Lau WB, Wang Y, Christopher T, Lopez B, Chan L, Goldstein B, Ma XL. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. Journal of molecular and cellular cardiology. 2009;46:413–419. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata R, Skurk C, Ouchi N, Galasso G, Kondo K, Ohashi T, Shimano M, Kihara S, Murohara T, Walsh K. Adiponectin promotes endothelial progenitor cell number and function. FEBS letters. 2008;582:1607–1612. doi: 10.1016/j.febslet.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giusti V, Suter M, Verdumo C, Gaillard RC, Burckhardt P, Pralong FP. Molecular determinants of human adipose tissue: differences between visceral and subcutaneous compartments in obese women. The Journal of clinical endocrinology and metabolism. 2004;89:1379–1384. doi: 10.1210/jc.2003-031507. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scuteri A, Orru M, Morrell C, Piras MG, Taub D, Schlessinger D, Uda M, Lakatta EG. Independent and additive effects of cytokine patterns and the metabolic syndrome on arterial aging in the SardiNIA Study. Atherosclerosis. 2011;215:459–464. doi: 10.1016/j.atherosclerosis.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SE, Lee NS, Park JW, Rhee EJ, Lee WY, Oh KW, Park SW, Park CY, Youn BS. Association of urinary RBP4 with insulin resistance, inflammation, and microalbuminuria. European journal of endocrinology / European Federation of Endocrine Societies. 2014;171:443–449. doi: 10.1530/EJE-14-0247. [DOI] [PubMed] [Google Scholar]
- 11.Johansen NB, Vistisen D, Brunner EJ, Tabak AG, Shipley MJ, Wilkinson IB, McEniery CM, Roden M, Herder C, Kivimaki M, Witte DR. Determinants of aortic stiffness: 16-year follow-up of the Whitehall II study. PloS one. 2012;7:e37165. doi: 10.1371/journal.pone.0037165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aso Y, Wakabayashi S, Terasawa T, Naruse R, Hara K, Takebayashi K, Inukai T. Elevation of serum high molecular weight adiponectin in patients with Type 2 diabetes and orthostatic hypotension: association with arterial stiffness and hypercoagulability. Diabetic medicine : a journal of the British Diabetic Association. 2012;29:80–87. doi: 10.1111/j.1464-5491.2011.03364.x. [DOI] [PubMed] [Google Scholar]
- 13.Seven E, Husemoen LL, Wachtell K, Ibsen H, Linneberg A, Jeppesen JL. Overweight, adipocytokines and hypertension: a prospective population-based study. Journal of hypertension. 2014;32:1488–1494. doi: 10.1097/HJH.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 14.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 15.Ota H, Furuhashi M, Ishimura S, Koyama M, Okazaki Y, Mita T, Fuseya T, Yamashita T, Tanaka M, Yoshida H, Shimamoto K, Miura T. Elevation of fatty acid-binding protein 4 is predisposed by family history of hypertension and contributes to blood pressure elevation. American journal of hypertension. 2012;25:1124–1130. doi: 10.1038/ajh.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr., Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. American journal of epidemiology. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 17.Kaess BM, Enserro DM, McManus DD, Xanthakis V, Chen MH, Sullivan LM, Ingram C, O'Donnell CJ, Keaney JF, Vasan RS, Glazer NL. Cardiometabolic correlates and heritability of fetuin-A, retinol-binding protein 4, and fatty-acid binding protein 4 in the Framingham Heart Study. The Journal of clinical endocrinology and metabolism. 2012;97:E1943–1947. doi: 10.1210/jc.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD EPI A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang KY, Zeger SL. Regression analysis for correlated data. Annual review of public health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. doi: 10.1161/01.hyp.38.3.429. [DOI] [PubMed] [Google Scholar]
- 22.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42:468–473. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 23.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 24.Singhal A. Endothelial dysfunction: role in obesity-related disorders and the early origins of CVD. The Proceedings of the Nutrition Society. 2005;64:15–22. doi: 10.1079/pns2004404. [DOI] [PubMed] [Google Scholar]
- 25.Wildman RP, Farhat GN, Patel AS, Mackey RH, Brockwell S, Thompson T, Sutton-Tyrrell K. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45:187–192. doi: 10.1161/01.HYP.0000152200.10578.5d. [DOI] [PubMed] [Google Scholar]
- 26.Attele AS, Shi ZQ, Yuan CS. Leptin, gut, and food intake. Biochemical pharmacology. 2002;63:1579–1583. doi: 10.1016/s0006-2952(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 27.Gale SM, Castracane VD, Mantzoros CS. Energy homeostasis, obesity and eating disorders: recent advances in endocrinology. The Journal of nutrition. 2004;134:295–298. doi: 10.1093/jn/134.2.295. [DOI] [PubMed] [Google Scholar]
- 28.Ingelsson E, Larson MG, Yin X, Wang TJ, Meigs JB, Lipinska I, Benjamin EJ, Keaney JF, Jr., Vasan RS. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. The Journal of clinical endocrinology and metabolism. 2008;93:3149–3157. doi: 10.1210/jc.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prior LJ, Davern PJ, Burke SL, Lim K, Armitage JA, Head GA. Exposure to a high-fat diet during development alters leptin and ghrelin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2014;63:338–345. doi: 10.1161/HYPERTENSIONAHA.113.02498. [DOI] [PubMed] [Google Scholar]
- 30.da Silva AA, do Carmo JM, Freeman JN, Tallam LS, Hall JE. A functional melanocortin system may be required for chronic CNS-mediated antidiabetic and cardiovascular actions of leptin. Diabetes. 2009;58:1749–1756. doi: 10.2337/db08-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Racial (black-white) divergence in the association between adiponectin and arterial stiffness in asymptomatic young adults: the Bogalusa heart study. American journal of hypertension. 2008;21:553–557. doi: 10.1038/ajh.2008.14. [DOI] [PubMed] [Google Scholar]
- 32.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009;93:583–604. doi: 10.1016/j.mcna.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart Fail Rev. 2002;7:51–62. doi: 10.1023/a:1013749806227. [DOI] [PubMed] [Google Scholar]
- 34.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends in endocrinology and metabolism: TEM. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 35.Dzielinska Z, Januszewicz A, Wiecek A, Demkow M, Makowiecka-Ciesla M, Prejbisz A, Kadziela J, Mielniczuk R, Florczak E, Janas J, Januszewicz M, Ruzyllo W. Decreased plasma concentration of a novel anti-inflammatory protein--adiponectin--in hypertensive men with coronary artery disease. Thrombosis research. 2003;110:365–369. doi: 10.1016/j.thromres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. The Journal of biological chemistry. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda M, Shimomura I, Sata M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. The Journal of biological chemistry. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 39.Kim DH, Kim C, Ding EL, Townsend MK, Lipsitz LA. Adiponectin levels and the risk of hypertension: a systematic review and meta-analysis. Hypertension. 2013;62:27–32. doi: 10.1161/HYPERTENSIONAHA.113.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. The New England journal of medicine. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 41.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 42.Xu A, Tso AW, Cheung BM, Wang Y, Wat NM, Fong CH, Yeung DC, Janus ED, Sham PC, Lam KS. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007;115:1537–1543. doi: 10.1161/CIRCULATIONAHA.106.647503. [DOI] [PubMed] [Google Scholar]
- 43.Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clinical chemistry. 2006;52:405–413. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- 44.Gomez-Ambrosi J, Rodriguez A, Catalan V, Ramirez B, Silva C, Rotellar F, Gil MJ, Salvador J, Fruhbeck G. Serum retinol-binding protein 4 is not increased in obesity or obesity-associated type 2 diabetes mellitus, but is reduced after relevant reductions in body fat following gastric bypass. Clinical endocrinology. 2008;69:208–215. doi: 10.1111/j.1365-2265.2007.03156.x. [DOI] [PubMed] [Google Scholar]
- 45.Vlachopoulos C, Manesis E, Baou K, Papatheodoridis G, Koskinas J, Tiniakos D, Aznaouridis K, Archimandritis A, Stefanadis C. Increased arterial stiffness and impaired endothelial function in nonalcoholic Fatty liver disease: a pilot study. American journal of hypertension. 2010;23:1183–1189. doi: 10.1038/ajh.2010.144. [DOI] [PubMed] [Google Scholar]
- 46.Bobbert T, Raila J, Schwarz F, Mai K, Henze A, Pfeiffer AF, Schweigert FJ, Spranger J. Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis. 2010;213:549–551. doi: 10.1016/j.atherosclerosis.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 47.Miyoshi T, Doi M, Hirohata S, Kamikawa S, Usui S, Ogawa H, Sakane K, Izumi R, Ninomiya Y, Kusachi S. Olmesartan reduces arterial stiffness and serum adipocyte fatty acid-binding protein in hypertensive patients. Heart and vessels. 2011;26:408–413. doi: 10.1007/s00380-010-0060-x. [DOI] [PubMed] [Google Scholar]
- 48.Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–2810. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 49.Stefan N, Hennige AM, Staiger H, Schleicher E, Fritsche A, Haring HU. Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects: response to Broch et al. Diabetes care. 2007;30:e91. doi: 10.2337/dc07-0767. [DOI] [PubMed] [Google Scholar]