Abstract
Acute kidney injury (AKI) is a global public health concern associated with high morbidity, mortality, and healthcare costs. Other than dialysis, no therapeutic interventions reliably improve survival, limit injury, or speed recovery. Despite recognized shortcomings of in vivo animal models, the underlying pathophysiology of AKI and its consequence, chronic kidney disease (CKD), is rich with biological targets. We review recent findings relating to the renal vasculature and cellular stress responses, primarily the intersection of the unfolded protein response, mitochondrial dysfunction, autophagy, and the innate immune response. Maladaptive repair mechanisms that persist following the acute phase promote inflammation and fibrosis in the chronic phase. Here macrophages, growth-arrested tubular epithelial cells, the endothelium, and surrounding pericytes are key players in the progression to chronic disease. Better understanding of these complex interacting pathophysiological mechanisms, their relative importance in humans, and the utility of biomarkers will lead to therapeutic strategies to prevent and treat AKI or impede progression to CKD or end-stage renal disease (ESRD).
Keywords: renal ischemia-reperfusion, nephrotoxicity, chronic kidney disease progression, pathophysiology, biomarkers, maladaptive repair
Introduction
Despite harmonization in clinical definition and staging, identification of novel renal biomarkers for clinical use, and progress in understanding the underlying pathophysiology, acute kidney injury (AKI) remains a major unmet medical need without any pharmacological treatments. It continues to be a global public health concern impacting ∼13.3 million patients per year (1). Moreover, although direct causality between AKI and death has been controversial, AKI associates with high morbidity, increased costs, and mortality—about 1.7 million deaths per year (1).
In lower- and middle-income countries, lack of access to trained healthcare professionals and underutilization of diagnostic tests and dialysis further contribute to the already high human burden and a marked underestimation of the true prevalence of AKI (2), prompting the International Society of Nephrology in 2015 to institute the 0by25 initiative, that is, zero preventable deaths from AKI by 2025 (1). Furthermore, whereas in the developed world AKI tends to manifest in older patients in the Intensive Care Unit, in lower- to middle-income countries young adults and women are particularly prone and at risk of death (3, 4). Among those patients who survive, long-term outcomes of AKI can include the development of chronic kidney disease (CKD) and end-stage renal disease (ESRD), or exacerbation of pre-existing CKD accelerating the progression to ESRD (5, 6). Thus, AKI, previously thought to have a benign course in patients who recovered, can lead to poor quality of life and high long-term costs (5, 7).
For these many reasons, treatments are needed to reduce the high morbidity and mortality and improve recovery of renal function; however, the multifactorial etiology of AKI and the complicated clinical course of this patient population has created challenges in the search for pharmacological agents that work. In the hospital setting, generalized or localized ischemic injury to the kidney due to surgery, sepsis or trauma, and toxic responses to medications (often in combination) account for many cases of AKI. Toxins (radiocontrast agents, nonsteroidal anti-inflammatories, etc.) can directly injure the nephron or cause ischemic injury or both. In community-acquired AKI, dehydration, infection, and toxins, usually in association with acute illness, are frequent causes. This diverse and complex etiology coupled with a heterogeneous patient population provide unique treatment challenges and potential opportunities in the AKI-to-CKD continuum. In some cases, such as surgery, the timing of the insult is known and one might intervene prior to the insult. Alternatively, one might intervene after the patient has been diagnosed with AKI to attempt to enhance recovery and reduce progression to CKD/ESRD in patients at high risk.
In this review, we highlight recent progress in the field, with a particular focus on novel pathophysiological mechanisms found in preclinical animal models that may offer new opportunities for therapeutic intervention in humans.
Pathophysiology
Animal models of AKI, which represent renal ischemia-reperfusion injury and nephrotoxicity (caused by cisplatin, folic acid, aristolochic acid, etc.), have provided important insight into the underlying pathophysiology. We have gleaned a great deal of fundamental information on disease mechanisms (e.g., redistribution of blood flow, proximal tubule injury mechanisms, inflammation, biomarker identification, fibrotic consequences of tubular injury) that have carried over to humans with similar pathological features. To date, however, there has been little successful clinical translation of proposed therapeutic agents found to be protective in these models. Thus, although animal models of AKI are very effective in recapitulating pathological and biomarker features that have been found in humans, the models have not been predictive of clinical effectiveness of potential therapeutic agents, which work to limit injury in the animal but not in humans. Although current in vivo models of AKI in young, healthy rodents continue to provide a great deal of information regarding pathophysiological mechanisms of injury and repair, they do not capture the complex comorbidities of a heterogeneous patient population with varying components of aging, diabetes, hypertension, vascular disease, and pre-existing CKD. There is an urgent need for animal models that more closely mimic the patient population. Rodent models of renal ischemia-reperfusion and nephrotoxicity on a background of aging (8), diabetes (9), and CKD induced by three-quarters nephrectomy (10) have been developed, and others are in progress.
Vasculature
The renal microvasculature plays a key role in the pathophysiology of AKI. The kidney has a high energy demand with a relatively low net oxygen (O2) extraction, yet the oxygenation of the outer medulla is quite marginal and the vascular architecture in this region is very susceptible to further compromise of vascular perfusion and oxygenation (11–13). In steady-state conditions, the O2 supply to the kidney is well regulated. Adequate O2 delivery is necessary for the production of mitochondrial adenosine triphosphate (ATP) as well as nitric oxide (NO) and reactive oxygen species (ROS) necessary for homeostatic control of renal function (14). With injury, the microcirculation is compromised, leading to an imbalance in NO, ROS, and O2 supply and use. Subsequent pathogenic effects include hypoxia and oxidative stress. Injury to the microvascular endothelium and changes in the glycocalyx lead to endothelial cell activation with new expression of cell surface markers that promote the recruitment and adhesion of leukocytes and platelets, leading to further changes in perfusion and O2 delivery and to additional endothelial cell injury and inflammation (12, 13). With damage, there is increased vascular permeability, interstitial edema, and further compromise in blood flow. Moreover, oxidative stress and vasoconstrictive prostaglandin production by damaged tubules further impairs O2 delivery, leading to a local “no-reflow” phenomenon where the occluded microvasculature exacerbates the initial injury (12, 13). The primary long-term consequence of microvascular injury is a reduction in peritubular capillary density, which is in part a response to decreased VEGF (vascular endothelial growth factor) and increased TGF-β (transforming growth factor beta) signaling, which contributes to ongoing hypoxia and the development of renal fibrosis (15).
Endoplasmic Reticulum Stress
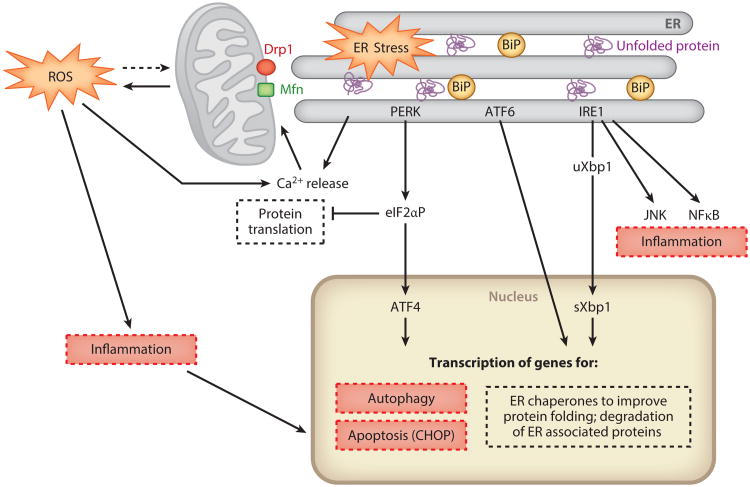
In response to endothelial or epithelial cell stress, unfolded or misfolded proteins accumulate in the endoplasmic reticulum (ER), triggering the unfolded protein response (UPR) (16–19). The UPR is an adaptive mechanism to restore cell and tissue homeostasis through activation of the PERK, IRE1, and ATF6 pathways (Figure 1).1 This provides short-term protection from acute stress by (a) inhibiting global protein translation through phosphorylation of eIF2α, (b) increasing ER protein folding capacity through upregulation of ER chaperones, and (c) increasing ER-associated degradation. Following acute ischemia and nephrotoxicity (16, 20–22), ER stress is induced in kidney epithelial cells both in vivo and in vitro. Overexpression of the ER chaperones BiP/GRP78 (23) and ORP150 (24) or the use of chemical chaperones, such as PBA or TUDCA (25), which enhances the adaptive UPR, dampens the ER stress response by improving protein folding capacity and facilitating the trafficking of unfolded or misfolded proteins, thereby protecting cells and tissues against injury (23–25).
Figure 1.
Schematic illustration of the cross-talk between the unfolded protein response (UPR), mitochondria, autophagy, and inflammatory and cell death pathways. In response to cellular stress, unfolded or misfolded proteins accumulate in the endoplasmic reticulum (ER), triggering the UPR, which consists of three ER stress sensors, PERK, ATF6, and IRE1. PERK phosphorylates eIF2α to decrease global protein translation while selectively increasing translation of ATF4, to direct transcription of autophagy genes, a subset of which is controlled by CHOP (26). If the stress is severe or prolonged, then CHOP induction leads to apoptosis. ATF6 is proteolytically cleaved and translocates to the nucleus, where, in combination with spliced Xbp1 (sXbp1), it leads to the transcription of ER chaperones to improve protein folding. IRE1 also promotes mRNA degradation, decreasing overall protein flux to the ER; induces expression of ER-associated degradation components; and is linked to other stress-induced pathways, such as JNK and NFk-B. The UPR counteracts ER stress to restore homeostasis or proceeds to induce cell death pathways via CHOP if the stress is severe or persists. Calcium release from the ER leads to mitochondrial ROS production and amplification of inflammatory and apoptotic pathways. Drp1 on the outer mitochondrial membrane mediates mitochondrial fission, whereas mitofusins (Mfn) are involved in mitochondrial fusion. Persistent ER stress, inflammation, and cell death that override adaptive responses are key factors in maladaptive repair (see Figure 2). For simplicity, some components of the cross-talk between the cellular stress responses have been omitted, and we refer the reader to recent reviews (18, 19). Definitions: ATF6, activating transcription factor 6; BiP, an ER chaperone; CEBP, CCAAT/enhancer binding protein; CHOP, CEBP-homologous protein; eIF2α, eukaryotic initiation factor 2 alpha; IRE1, inositol-requiring kinase 1; JNK, JUN N-terminal kinase; NF-κB, nuclear factor kappa B; PERK, protein kinase RNA-like endoplasmic reticulum kinase; ROS, reactive oxygen species; uXbp1, unspliced Xbp1.
If the stress is too severe or prolonged, then the maladaptive response is activated by the induction of CHOP (CEBP-homologous protein; CEBP, CCAAT/enhancer binding protein), a transcription factor downstream from the PERK-ATF4 pathway, responsible for apoptosis. CHOP knockout mice are protected from renal ischemia-reperfusion injury (27), and in these mice postischemic recovery of the microcirculation is improved. In contrast, prolonged and overwhelming ER stress has been implicated in the development of renal fibrosis (28). Thus, targeting the UPR may present a possible approach to prevent or treat AKI. However, whether it is a potential therapeutic option for the chronic, progressive phase, when there is tubular atrophy, fibrosis, and inflammation, requires further study.
Mitochondrial Dysfunction
There is structural continuity between the ER and mitochondria at multiple contact sites termed the mitochondrial-ER-associated membrane (MAM) (29). The MAM contains ER-bound and mitochondrial proteins important for maintaining structural communication between the two organelles. This interconnection along with calcium release functionally links ER stress to mitochondrial pathways activated with the cellular stress response. In the pathogenesis of AKI, proximal tubules are especially vulnerable to mitochondrial dysfunction as they rely on aerobic metabolism and their mitochondria are in a more oxidized state than those in distal tubule cells, which can use glycolysis (30). The study of mitochondrial dysfunction has emerged as an exciting new area to identify therapies for AKI (31, 32).
The peptide SS-31, also known as Bendavia, is currently under evaluation in clinical studies targeting mitochondrial dysfunction. It has been found to be protective in a rat model of ischemic renal injury when administered prior to the insult. By binding to cardiolipin on the inner mitochondrial membrane and inhibiting cytochrome c peroxidase activity, which leads to mitochondrial damage (33), the SS-31/cardiolipin complex protects mitochondrial structure and respiration during early reperfusion, accelerating recovery of ATP. Tubular cell death and dysfunction are reduced, as are vascular congestion, oxidative stress, and inflammation (34). Moreover, chronic treatment for four weeks initiated prior to the insult reduces peritubular capillary loss, interstitial inflammation, and fibrosis (35).
Mitochondrial biogenesis is a homeostatic mechanism that under basal conditions replaces damaged mitochondria. It is rapidly induced in response to cellular injury, and improving mitochondrial biogenesis has been reported to facilitate recovery of the damaged kidney. Thus, agents that stimulate mitochondrial biogenesis could serve as viable therapies for AKI. The primary regulator of mitochondrial biogenesis is PGC-1α, which activates the transcription factors Nrf1, Nrf2, ERRα, and PPARα, which, in turn, regulate the transcription of mitochondrial DNA, antioxidants, and biogenesis genes.2 Agents that stimulate the expression and activity of PGC-1α include activators of AMPK, such as metformin and AICAR, as well as activators of SIRT1, which include isoflavones, resveratrol, and the small molecule SIRT1720. All of these increase mitochondrial number and function (36–40). Activation of PGC-1α reduces oxidative injury and cell death and accelerates recovery of renal mitochondria and tubule homeostasis (41). In sepsis-induced AKI without cell death (42), induction of PGC-1α promotes renal recovery by improving mitochondrial respiration and restoring expression of mitochondrial electron transport genes. Formoterol, a β2-adrenergic agonist, restores renal function and protects kidney tubules from injury and death (43) by restoring the expression and function of mitochondrial proteins, including PGC-1α, through the cAMP/PKA/CREB axis. Likewise, activation of the 5-hydroxytryptamine 1F receptor with selective, commercially available small molecules (44) and cGMP-selective phosphodiesterase inhibitors (45) promotes mitochondrial biogenesis and recovery from AKI through increased expression of PGC-1α and mitochondrial electron transport chain genes.
Because mitochondrial fusion and fission control mitochondrial morphology and shape, which are key determinants of mitochondrial function, regulating mitochondrial dynamics is another therapeutic option for the prevention or treatment of AKI. Interestingly, knockout in proximal tubules of mitofusin 2, a GTPase in the outer mitochondrial membrane essential for mitochondrial fusion, accelerates kidney recovery and improves survival after renal ischemia (46) through activation of Ras-ERK1/2 signaling to promote cell proliferation. Pharmacological inhibition of Drp1—a fission protein that constricts and cleaves mitochondria, leading to fragmentation— reduces mitochondrial damage, cytochrome c release, apoptosis, and renal injury (31). Mitochondrial fission leads to heightened Bax/Bak sensitivity, greater expression of apoptotic regulators, and cell death. Recently, SIRT3, a mitochondrial sirtuin, has been demonstrated to improve mitochondrial dynamics by preventing Drp1-dependent fission, loss of mitochondrial membrane potential, and mitophagy (47). Thus, regulation of mitochondrial dynamics may offer new therapeutic opportunities for AKI.
Autophagy
Included in the cross-talk between mitochondria and the UPR is autophagy, a catabolic process in which proteins, organelles, and cytoplasmic components are delivered to lysosomes for degradation and recycling. Activation of the UPR, specifically the PERK-ATF4 pathway, transcriptionally regulates more than a dozen autophagy genes (26).
Autophagy is induced in kidneys in response to AKI and protects against kidney injury (48). It is initiated by encapsulation of cytoplasmic proteins and organelles in autophagosomes, which fuse with lysosomes for degradation. Upon activation, it may also decrease cellular stress by removing ER membranes containing UPR sensors and/or clearing aberrant proteins from the ER. Inhibition of autophagy with chloroquine worsens cisplatin-induced AKI. Lysosomal degradation of autophagosomes is blocked, leading to greater loss in renal function, more severe tissue injury, and greater cell death (48). During recovery following AKI, resolution of autophagy may promote proliferation necessary for tubular regeneration and repair (49). We have recently reported a pathway by which luminal apoptotic debris, taken up by the proximal tubule through KIM-1 (kidney injury molecule-1)–mediated phagocytosis, is subsequently processed through autophagy with antigens presented to MHC (major histocompatibility complex) to downregulate the inflammatory response (50, 51). However, the role of autophagy in the transition from acute to chronic kidney injury warrants further study. In this setting, autophagy may be less effective and hence unable to mediate intracellular degradation of newly synthesized fibrotic collagens.
Inflammation
Changes in protein folding and mitochondrial function influence the innate immune response, contributing to inflammation. During the UPR, activation of IRE-1 stimulates the JNK (JUN N-terminal kinase) pathway, which regulates cell death and survival and downstream proinflammatory cytokines to promote the inflammatory milieu characteristic of AKI (52). Moreover, in response to cell and tissue injury, resident dendritic cells and macrophages contribute to ensuing inflammation (53, 54), which is further amplified by leukocyte infiltration in response to chemotactic signals. Neutrophils and monocytes mediate the acute phase within the first 24 h of injury (55), whereas studies in mouse models support a role for T and B lymphocytes, not present in large numbers (55), in the evolution of renal injury (53). Inhibition of leukocyte infiltration into the kidney by the small molecule plerixafor, an antagonist of the leukocyte CXCR4 chemokine receptor (56), ameliorates the loss in renal function, decreasing renal injury, cell death, and long-term fibrosis. Furthermore, when the cholinergic anti-inflammatory pathway mediated by the α7 nicotinic acetylcholine receptor on splenic CD4+ T cells is activated with ultrasound prior to ischemia-reperfusion injury (57), there is renoprotection. Kidney histology and function are preserved, and kidney inflammation and subsequent fibrosis are reduced.
Maladaptive Repair
With injury, adaptive responses are activated to restore normal cell and tissue homeostasis. These processes involve replacement of lost epithelia by proliferation of epithelial cells that have survived the insult without evidence for a stem cell compartment that plays a significant role (58–60). However, when the injury is severe or sustained, the balance tips to maladaptive responses that lead to cell and tissue malfunction (Figure 2). In a setting of severe injury, or less severe acute injury in the setting of chronic renal injury, especially in the aging kidney (12), inflammation and fibrosis are maladaptive and are central to CKD progression.
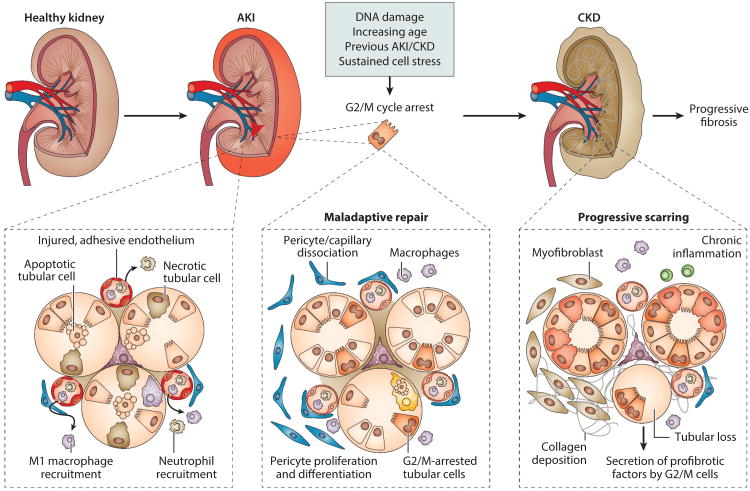
Figure 2.
Maladaptive repair following acute kidney injury (AKI) leads to chronic kidney disease (CKD). In response to acute injury, there is activation of cellular stress responses, cell death pathways, and the innate immune response, which in turn lead to endothelial and epithelial dysfunction. If the injury persists or is severe, maladaptive repair mechanisms promote cell and tissue malfunction. Here, inflammation and fibrosis are central to chronic disease. Profibrotic, inflammatory macrophages are recruited. Epithelial cells that arrest in G2/M release cytokines and growth factors to promote ongoing inflammation and fibrosis. Pericytes dissociate from the endothelium to further promote endothelial dysfunction, which leads to microvascular loss. In chronic disease, extracellular matrix– depositing myofibroblasts proliferate and also form from activated, proliferating pericytes. From Reference 12 with permission.
Macrophages are key regulators of inflammation and fibrosis. Although it does not prove causality, a prospective study of biopsy samples from 215 CKD patients (61) revealed that the number of interstitial macrophages correlated directly with glomerular scarring, interstitial fibrosis, atrophic tubules, reduced capillary density, and decreased renal survival. As macrophages have a heterogeneous phenotype reflective of their plasticity and response to extracellular cues (62), we recently profiled, by whole-genome microarray, macrophage subpopulations in the renal ischemia-reperfusion model at different times postreperfusion in order to assign phenotype and function to the macrophage population associated with the injury, repair, and fibrotic phases (64). Based on differential expression of Ly6C in CD11b+ cells, three populations were identified, each with a unique gene signature. The CD11b+/Ly6Chigh population appeared during the acute injury phase, was linked with monocyte/macrophage immune responses, and when depleted led to renoprotection (64), as reported by others (64, 65). The CD11b+/Ly6Cintermediate population was present during the repair phase, had a gene signature of wound healing, and when depleted, associated with prolonged renal injury (63). The CD11b+/Ly6Clow population had a profibrotic phenotype and dominated during the progressive phase. All populations carried differential inflammatory signatures, signifying persistent parenchymal inflammation, regulating injury, repair, or fibrosis. Interestingly, both the CD11b+/Ly6Cintermediate and CD11b+/Ly6Clow populations expressed M2 macrophage markers, but some markers were differentially expressed. Because macrophage phenotype and function change during the course of disease, these findings underscore the importance of understanding macrophage function in the pathophysiology of human AKI and suggest that targeting macrophage paracrine signaling rather than macrophage subsets may be a better therapeutic approach.
Injured tubular epithelial cells also mediate inflammation and fibrosis (54). Epithelia that express KIM-1 secrete MCP-1 (monocyte chemotactic protein-1) to increase macrophage chemotaxis (66), and perturbations in the cell cycle due to arrest in G2/M mediated at least in part by p53, a cell cycle regulatory protein, lead to the production of the profibrotic cytokines TGFβ-1 and CTGF (connective tissue growth factor) (67). These are also produced in combination with PDGF-B (platelet-derived growth factor B) when the regenerating epithelium fails to differentiate, leading to persistent profibrotic signaling in tubules undergoing atrophy after AKI (30, 68). Elevated p53 has been linked to replicative and stress-induced senescence (69). The senescence-associated secretory phenotype (SASP) has been linked to maladaptive repair (67), although it has been most studied in in vitro models of carcinogenesis (70), where it is responsible for the secretion of growth factors, proteases, chemokines, and cytokines, with proinflammatory cytokines and chemokines most robustly expressed. Cellular senescence is detected following renal ischemia reperfusion injury by the senescence marker β-galactosidase, with greater senescence in aged kidneys (8). It is possible that following AKI, cellular senescence may contribute to a proinflammatory milieu to promote fibrogenesis, as this correlates with increased leukocyte infiltration and enhanced expression of chemokines and cytokines in the chronic phase following acute injury (8, 56, 67).
Profibrotic and proinflammatory signaling factors elaborated by growth-arrested cells and undifferentiated tubular epithelia amplify the fibrotic/inflammatory cascade by disrupting cellular interactions in the interstitial space. As endothelial cells are damaged in response to AKI, endothelial–pericyte interactions are further altered. These changes lead to activation and proliferation of pericytes, the major source of smooth muscle actin-containing myofibroblasts, which drive fibrosis. Expansion of the resident fibroblast population also contributes (71), as do bone marrow–derived precursors (72), hematopoietic stem cells (73), and rare monocytes/macrophages that express α-smooth muscle actin (74). Recent data do not support epithelial–mesenchymal transition as a source of fibroblasts to account for progressive fibrosis (71, 75); moreover, whether myofibroblasts can derive from endothelial–mesenchymal transition remains controversial due to the limited specificity of endothelial markers (76).
Essential to inflammation and tissue homeostasis are pattern recognition receptors (PRRs), which consist of the transmembrane toll-like receptors (TLRs) and the intracellular NOD (nucleotide-binding oligomerization domain)-like receptors (NLRs). PRRs are expressed on renal parenchymal and resident immune cells, as well as on recruited leukocytes upon kidney injury (77, 78). Members of the NLR family can oligomerize to form cytoplasmic, multimeric protein-inflammasome complexes that regulate the maturation and secretion of the inflammatory cytokines interleukin (IL)–1β, IL-18, and IL-33. PRRs respond to danger-associated molecular patterns (DAMPs) through direct binding or indirect mechanisms to promote sterile inflammation. In a setting of AKI, DAMPs include components of necrotic cellular debris, such as histones, heat shock proteins, and HMGB1, as well as components of fragmented extracellular matrix. DAMPs, TLRs, NLRs, and the inflammasome are thought to contribute to kidney regeneration (77) by regulating clearance of cellular debris and by tissue-repair programs. Specifically, certain DAMPs activate TLR2 on renal progenitor cells and accelerate tubular repair (79). TLR4 activation of kidney dendritic cells leads to release of IL-22, which in turn activates its receptor on tubular epithelia to accelerate re-epithelialization (80). When inflammatory pathways are maladaptive, however, renal tissue is damaged and fibrosis ensues. Here activation of TLRs induces the NLRP3 inflammasome needed for SMAD2 phosphorylation, which is critical for TGF-β receptor signaling (81), which, in turn, leads to proliferation and formation of myofibroblasts. Thus, the current interest in PRRs as promising drug targets to modulate inflammation and fibrosis in CKD argues for improved understanding of this complex biology in both preclinical models and patients so the timing and dose of therapeutic modulators can be optimal.
Biomarkers
The search for AKI biomarkers began more than 15 years ago and was advanced in 2006 when the US Food and Drug Administration (FDA), the European Medicines Agency, and the Japanese Pharmaceutical and Medical Devices Agency joined with several pharmaceutical companies to form the Predictive Safety Testing Consortium with the goal of identifying sensitive and specific biomarkers of nephrotoxicity in preclinical animal models. This effort was consistent with the FDA's Critical Path Initiative to identify new biomarkers to predict drug toxicity in preclinical animal studies and thereby improve decision making on whether or not to terminate costly drug development. In 2008, seven biomarkers of nephrotoxicity were qualified by the FDA for use in animals and, on a case-by-case basis, in humans. These included urinary KIM-1, β2-microglobulin, cystatin C, clusterin, trefoil factor-3, albumin, and total protein. These markers—and others, e.g., urinary NGAL (neutrophil gelatinase-associated lipocalin), urinary IL-18, and L-FABP (liver fatty acid binding protein)—have been studied in a variety of conditions. In most cases, they are compared to changes in serum creatinine concentration because more accurate metrics for kidney injury are not readily available. Using creatinine as the gold standard, the markers have generally performed well with respect to their negative predictive power, but their positive predictive power has been less impressive. This is not surprising given the presence of renal reserve and the large amount of injury necessary before an increase in creatinine is measured (82). Studies of these markers in humans by the Predictive Safety Testing Consortium in collaboration with the NIH and FDA are ongoing.
In September 2014, the FDA allowed marketing of the NephroCheck test, which helps to determine if certain critically ill patients are at risk of developing moderate-to-severe AKI within 12 h following testing. NephroCheck measures urinary levels of TIMP-2 (tissue inhibitor of metalloproteinase 2) and IGFBP7 (insulin-like growth factor binding protein 7), implicated in G1 cell cycle arrest (83).
There are few markers for progression of AKI to CKD, other than the validated markers of proteinuria and microalbuminuria, identifying this as an important area for future investigation (84). However, in patients with type I diabetes and proteinuria, serum KIM-1 levels at baseline strongly predicted rate of eGFR (estimated glomerular filtration rate) loss and risk of ESRD during 5–15 years of follow-up (85), identifying serum KIM-1 as a marker for CKD and as a predictor of CKD progression. Furthermore, some of the biomarkers originally identified for AKI, such as urinary and plasma NGAL, have been evaluated in CKD of various etiologies (86). Whether they can be used as markers of CKD progression following AKI, either alone or in combination, remains to be determined; however, in rodent models, markers of renal structural injury persist in the chronic phase (56, 87). In patients, the ASSESS-AKI Study (88), a natural history study, will collect biosamples to address this question as well as to identify potential new biomarkers.
Unique methodologies are being applied for biomarker identification, such as proteomics, metabolomics, and transcriptomics (for review see 89–91). Circulating or urinary microRNAs are being evaluated (92), and kidney-specific DNA methylation patterns are also being analyzed (93). Recent attention has also focused on urinary extracellular vesicles (94), which contain mRNA, microRNA, and proteins reflective of cellular physiology and pathophysiology along the nephron. The constituents of urinary extracellular vesicles may serve as diagnostic and prognostic biomarkers and, because they are a source of information about disease pathogenesis, may enable target identification for drug discovery or provide evidence of biological activity in response to therapeutic agents.
Thus, the biomarkers described for AKI will likely play a role in the diagnosis and prognosis of AKI, and by extension may facilitate clinical trial design by permitting patient stratification and enrichment. Biomarkers can also be used in many other ways in drug development. They can be used to link the patient population to preclinical animal models when the biomarker reflects a mechanism of injury that drives a disease process in animals which is very similar to one that drives the disease in humans. Translational biomarkers can also be used to evaluate drug targets and pathways by demonstrating that they are modulated in both humans and preclinical models with disease. Moreover, biomarkers can be identified as pharmacodynamics markers that measure pharmacological response to a drug for use in dose-optimization studies. Last, biomarkers can demonstrate the expected biological effect of the drug, that is, whether the drug is targeting the pathophysiological pathway for which it was developed and is linked to the clinical endpoint.
The Path Forward
The connections between cellular stress responses and maladaptive repair mechanisms are biologically complex and rich with therapeutic targets, where an impact on one can affect multiple pathways. There is no doubt that pharmacological modulators can be developed or existing ones repurposed to be evaluated in in vivo preclinical models; however, whether the target and pathway are driving disease and are biologically active in the patient population at the time of therapy administration is key to accelerate identification and approval of new treatments for AKI patients. Members of the AKI community—academia, the biotechnology and pharmaceutical industries, and funding agencies—are urged to engage in an active dialogue and collective effort to find and advance therapies for this major unmet medical need. We urge the creation of consortia that can serve as a repository of biosamples from patients with AKI of various etiologies in sufficient quantity to extensively profile therapeutic targets, pathways, and biomarkers. The goal would be to generate a molecular signature for each AKI patient population to speed clinical translation. Patient advocacy groups for those AKI survivors faced with progression to CKD or ESRD or for families impacted by AKI-associated mortality would focus national and international attention on this global public health concern, raising awareness and support. In the words of the International Society of Nephrology's 0by25 initiative (1), “The ability to provide lifesaving treatments for AKI provides a compelling argument to consider therapy for AKI as much of a basic right as it is to give antiretroviral drugs to treat HIV.”
Acknowledgments
Research studies from the laboratory of J.V.B. discussed in this article were supported by the US National Institute of Diabetes and Digestive and Kidney Diseases (DK39773 and DK72381).
Glossary
- AKI
acute kidney injury
- CKD
chronic kidney disease
- ESRD
end-stage renal disease
Footnotes
Definitions in this paragraph: PERK, protein kinase RNA-like endoplasmic reticulum kinase; IRE1, inositol-requiring kinase 1; ATF6, activating transcription factor 6; BiP, binding immunoglobulin protein; GRP78, glucose-regulated protein, 78 kD; ORP150, oxygen-regulated protein, 150 kD; PBA, phenyl butyric acid; TUDCA, tauroursodeoxycholic acid.
Definitions in this paragraph: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; ERRα, estrogen-related receptor alpha; PPARα, peroxisome proliferator-activated receptor alpha; AMPK, adenosine monophosphate–activated protein kinase; AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; SIRT1, sirtuin 1; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; CREB, cAMP response element–binding protein; cGMP, cyclic guanosine monophosphate.
Disclosure Statement: J.V.B. is coinventor on KIM-1 patents assigned to Partners HealthCare. He consults on acute kidney injury for Astellas and Amgen, and holds equity in MediBeacon and Sentien. He derives research support related to acute kidney injury from Roche.
Contributor Information
Anna Zuk, Email: drazukphd@yahoo.com.
Joseph V. Bonventre, Email: joseph_bonventre@hms.harvard.edu.
Literature Cited
- 1.Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:1–28. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Xing G, Wang L, et al. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465–71. doi: 10.1016/S0140-6736(15)00344-X. [DOI] [PubMed] [Google Scholar]
- 3.Cerda J, Bagga A, Kher V, Chakravarthi RM. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4:138–53. doi: 10.1038/ncpneph0722. [DOI] [PubMed] [Google Scholar]
- 4.Jha V, Parameswaran S. Community-acquired acute kidney injury in tropical countries. Nat Rev Nephrol. 2013;9:278–90. doi: 10.1038/nrneph.2013.36. [DOI] [PubMed] [Google Scholar]
- 5.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–28. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–48. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS ONE. 2013;8:e70464. doi: 10.1371/journal.pone.0070464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng J, Li X, Zhang D, et al. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int. 2015;87:137–50. doi: 10.1038/ki.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polichnowski AJ, Lan R, Geng H, et al. Severe renal mass reduction impairs recovery and promotes fibrosis after AKI. J Am Soc Nephrol. 2014;25:1496–507. doi: 10.1681/ASN.2013040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Investig. 2011;121:4210–21. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–76. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Investig. 2014;124:2355–63. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aksu U, Demirci C, Ince C. The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contrib Nephrol. 2011;174:119–28. doi: 10.1159/000329249. [DOI] [PubMed] [Google Scholar]
- 15.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Ren Physiol. 2001;281:F887–99. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 16.Inagi R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Exp Nephrol. 2009;112:e1–e9. doi: 10.1159/000210573. [DOI] [PubMed] [Google Scholar]
- 17.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–62. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40:141–48. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 20.Hodeify R, Megyesi J, Tarcsafalvi A, et al. Gender differences control the susceptibility to ER stress-induced acute kidney injury. Am J Physiol Ren Physiol. 2013;304:F875–82. doi: 10.1152/ajprenal.00590.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pallet N, Fougeray S, Beaune P, et al. Endoplasmic reticulum stress: an unrecognized actor in solid organ transplantation. Transplantation. 2009;88:605–13. doi: 10.1097/TP.0b013e3181b22cec. [DOI] [PubMed] [Google Scholar]
- 22.Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci. 2007;99:346–53. doi: 10.1093/toxsci/kfm152. [DOI] [PubMed] [Google Scholar]
- 23.Lhotak S, Sood S, Brimble E, et al. ER stress contributes to renal proximal tubule injury by increasing SREBP-2-mediated lipid accumulation and apoptotic cell death. Am J Physiol Ren Physiol. 2012;303:F266–78. doi: 10.1152/ajprenal.00482.2011. [DOI] [PubMed] [Google Scholar]
- 24.Bando Y, Tsukamoto Y, Katayama T, et al. ORP150/HSP12A protects renal tubular epithelium from ischemia-induced cell death. FASEB J. 2004;18:1401–3. doi: 10.1096/fj.03-1161fje. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Fu L, Xiao M, et al. The nephroprotective effect of tauroursodeoxycholic acid on ischaemia/reperfusion-induced acute kidney injury by inhibiting endoplasmic reticulum stress. Basic Clin Pharmacol Toxicol. 2012;111:14–23. doi: 10.1111/j.1742-7843.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- 26.B'Chir W, Maurin AC, Carraro V, et al. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41:7683–99. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong B, Zhou H, Han C, et al. Ischemia/reperfusion-induced CHOP expression promotes apoptosis and impairs renal function recovery: the role of acidosis and GPR4. PLoS ONE. 2014;9:e110944. doi: 10.1371/journal.pone.0110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang CK, Hsu SP, Wu CT, et al. Endoplasmic reticulum stress implicated in the development of renal fibrosis. Mol Med. 2011;17:1295–305. doi: 10.2119/molmed.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raturi A, Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM) Biochim Biophys Acta. 2013;1833:213–24. doi: 10.1016/j.bbamcr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Venkatachalam MA, Griffin KA, Lan R, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Ren Physiol. 2010;298:F1078–94. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Investig. 2009;119:1275–85. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhan M, Brooks C, Liu F, et al. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013;83:568–81. doi: 10.1038/ki.2012.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birk AV, Liu S, Soong Y, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24:1250–61. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szeto HH, Liu S, Soong Y, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–52. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Soong Y, Seshan SV, Szeto HH. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Ren Physiol. 2014;306:F970–80. doi: 10.1152/ajprenal.00697.2013. [DOI] [PubMed] [Google Scholar]
- 36.Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injuryinrenal proximal tubule cells. J Pharmacol Exp Ther. 2010;333:593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol. 2013;273:345–54. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasegawa K, Wakino S, Yoshioka K, et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem. 2010;285:13045–56. doi: 10.1074/jbc.M109.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He W, Wang Y, Zhang MZ, et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Investig. 2010;120:1056–68. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasbach KA, Schnellmann RG. Isoflavones promote mitochondrial biogenesis. J Pharmacol Exp Ther. 2008;325:536–43. doi: 10.1124/jpet.107.134882. [DOI] [PubMed] [Google Scholar]
- 41.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Ren Physiol. 2012;302:F853–64. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran M, Tam D, Bardia A, et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Investig. 2011;121:4003–14. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeskiney SR, Funk JA, Stallons LJ, et al. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol. 2015;25:1157–62. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garrett SM, Whitaker RM, Beeson CC, Schnellmann RG. Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J Pharmacol Exp Ther. 2014;350:257–64. doi: 10.1124/jpet.114.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitaker RM, Wills LP, Stallons LJ, Schnellmann RG. cGMP-selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. J Pharmacol Exp Ther. 2013;347:626–34. doi: 10.1124/jpet.113.208017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gall JM, Wang Z, Bonegio RG, et al. Conditional knockout of proximal tubule mitofusin 2 accelerates recovery and improves survival after renal ischemia. J Am Soc Nephrol. 2015;26:1092–102. doi: 10.1681/ASN.2014010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morigi M, Perico L, Rota C, et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Investig. 2015;125:715–26. doi: 10.1172/JCI77632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang M, Wei Q, Dong G, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–83. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He L, Livingston MJ, Dong Z. Autophagy in acute kidney injury and repair. Nephron Clin Pract. 2014;127:56–60. doi: 10.1159/000363677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks CR, Yeung MY, Brooks YS, et al. KIM-1/TIM-1-mediated phagocytosis links ATG5/ULK1-dependent clearance of apoptotic cells to antigen presentation. EMBO J. 2015;34(19):2441–64. doi: 10.15252/embj.201489838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, Brooks CR, Xiao S, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Investig. 2015;125:1620–36. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–85. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 53.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11:88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 54.Kinsey GR, Okusa MD. Role of leukocytes in the pathogenesis of acute kidney injury. Crit Care. 2012;16:214. doi: 10.1186/cc11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ysebaert DK. Identification and kinetics of leukocytes after severe ischemia/reperfusion renal injury. Nephrol Dial Transpl. 2000;15:1562–74. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 56.Zuk A, Gershenovich M, Ivanova Y, et al. CXCR4 anatagonism as a therapeutic approach to prevent acute kidney injury. Am J Physiol Ren Physiol. 2014;307:F783–F97. doi: 10.1152/ajprenal.00685.2013. [DOI] [PubMed] [Google Scholar]
- 57.Gigliotti JC, Huang L, Ye H, et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol. 2013;24:1451–60. doi: 10.1681/ASN.2013010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl. 1):S55–61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 59.Humphreys BD, Czerniak S, DiRocco DP, et al. Repair of injured proximal tubule does not involve specialized progenitors. PNAS. 2011;108:9226–31. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Humphreys BD, Valerius MT, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–91. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 61.Eardley KS, Zehnder D, Quinkler M, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–97. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- 62.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical versus functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clements M, Gershenovich M, Chaber C, et al. Differential Ly6C expression after renal ischemia-reperfusion identifies unique macrophage populations. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111138. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–26. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang MZ, Yao B, Yang S, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Investig. 2012;122:4519–32. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Humphreys BD, Xu F, Sabbisetti V, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Investig. 2013;123:4023–35. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–43. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26:1765–76. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang H, Fogo AB. Cell senescence in the aging kidney. J Am Soc Nephrol. 2010;21:1436–39. doi: 10.1681/ASN.2010020205. [DOI] [PubMed] [Google Scholar]
- 70.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–46. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barnes JL, Glass WF., 2nd Renal interstitial fibrosis: a critical evaluation of the origin of myofibroblasts. Contrib Nephrol. 2011;169:73–93. doi: 10.1159/000313946. [DOI] [PubMed] [Google Scholar]
- 72.Broekema M, Harmsen MC, van Luyn MJ, et al. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol. 2007;18:165–75. doi: 10.1681/ASN.2005070730. [DOI] [PubMed] [Google Scholar]
- 73.Lin F, Cordes K, Li L, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischema-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–99. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 74.Ludin A, Itkin T, Gur-Cohen S, et al. Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol. 2012;13:1072–82. doi: 10.1038/ni.2408. [DOI] [PubMed] [Google Scholar]
- 75.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Investig. 2011;121:468–74. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez IG, Duffield JS. The FOXD1 lineage of kidney perivascular cells and myofibroblasts: functions and responses to injury. Kidney Int Suppl. 2014;4:26–33. doi: 10.1038/kisup.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anders HJ, Schaefer L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol. 2014;25:1387–400. doi: 10.1681/ASN.2014010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leemans JC, Kors L, Anders HJ, Florquin S. Pattern recognition receptors and the inflammasome in kidney disease. Nat Rev Nephrol. 2014;10:398–414. doi: 10.1038/nrneph.2014.91. [DOI] [PubMed] [Google Scholar]
- 79.Sallustio F, Constantino V, Cox SN, et al. Human renal stem/progenitor cells repair tubular epithelial cell injury through TLR-2 driven inhibin-A and microvesicle-shuttled decorin. Kidney Int. 2013;83:392–403. doi: 10.1038/ki.2012.413. [DOI] [PubMed] [Google Scholar]
- 80.Kulkarni OP, Hartter I, Mulay SR, et al. Toll-like receptor 4-induced IL-22 accelerates kidney regeneration. J Am Soc Nephrol. 2014;25:978–89. doi: 10.1681/ASN.2013050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang W, Wang X, Chun J, et al. Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium. J Immunol. 2013;190:1239–49. doi: 10.4049/jimmunol.1201959. [DOI] [PubMed] [Google Scholar]
- 82.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23:905–14. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10:147–55. doi: 10.2215/CJN.12191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kashani K, Kellum JA. Novel biomarkers indicating repair or progression after acute kidney injury. Curr Opin Nephrol Hypertens. 2015;24:21–27. doi: 10.1097/MNH.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 85.Sabbisetti VS, Waikar SS, Antoine DJ, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–86. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fassett RG, Venuthurupalli SK, Gobe GC, et al. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–21. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 87.Ko GJ, Grigoryev DN, Linfert D, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD progression. Am J Physiol Ren Physiol. 2010;298:F1472–F83. doi: 10.1152/ajprenal.00619.2009. [DOI] [PubMed] [Google Scholar]
- 88.Go AS, Parikh CR, Ikizler TA, et al. The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: design and methods. BMC Nephrol. 2010;11:22. doi: 10.1186/1471-2369-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho J, Dart A, Rigatto C. Proteomics in acute kidney injury—current status and future promise. Pediatr Nephrol. 2014;29:163–71. doi: 10.1007/s00467-013-2415-x. [DOI] [PubMed] [Google Scholar]
- 90.Sun J, Shannon M, Ando Y, et al. Serum metabolomic profiles from patients with acute kidney injury: a pilot study. J Chromatogr B Anal Technol Biomed Life Sci. 2012:893–894. 107–13. doi: 10.1016/j.jchromb.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee CG, Kim JG, Kim HJ, et al. Discovery of an integrative network of microRNAs and transcriptomics changes for acute kidney injury. Kidney Int. 2014;86:943–53. doi: 10.1038/ki.2014.117. [DOI] [PubMed] [Google Scholar]
- 92.Ramachandran K, Saikumar J, Bijol V, et al. Human miRNome profiling identifies microRNAs differentially present in the urine after kidney injury. Clin Chem. 2013;59:1742–52. doi: 10.1373/clinchem.2013.210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Endo K, Kito N, Fukushima Y, et al. A novel biomarker for acute kidney injury using TaqMan-based unmethylated DNA-specific polymerase chain reaction. Biomed Res. 2014;35:207–13. doi: 10.2220/biomedres.35.207. [DOI] [PubMed] [Google Scholar]
- 94.Salih M, Zietse R, Hoorn EJ. Urinary extracellular vesicles and the kidney: biomarkers and beyond. Am J Physiol Ren Physiol. 2014;306:F1251–59. doi: 10.1152/ajprenal.00128.2014. [DOI] [PubMed] [Google Scholar]