ABSTRACT
The establishment of proper kinetochore-microtubule attachments facilitates faithful chromosome segregation. Incorrect attachments activate the spindle assembly checkpoint (SAC), which blocks anaphase onset via recruitment of a cohort of SAC components (Mph1/MPS1, Mad1, Mad2, Mad3/BubR1, Bub1 and Bub3) to kinetochores. KNL1, a component of the outer kinetochore KMN network (KNL1/Mis12 complex/Ndc80 complex), acts as a platform for Bub1 and Bub3 localization upon its phosphorylation by Mph1/MPS1. The Ndc80 protein, a major microtubule-binding site, is critical for MPS1 localization to the kinetochores in mammalian cells. Here we characterized the newly isolated mutant ndc80-AK01 in fission yeast, which contains a single point mutation within the hairpin region. This hairpin connects the preceding calponin-homology domain with the coiled-coil region. ndc80-AK01 was hypersensitive to microtubule depolymerizing reagents with no apparent growth defects without drugs. Subsequent analyses indicated that ndc80-AK01 is defective in SAC signaling, as mutant cells proceeded into lethal cell division in the absence of microtubules. Under mitotic arrest conditions, all SAC components (Ark1/Aurora B, Mph1, Bub1, Bub3, Mad3, Mad2 and Mad1) did not localize to the kinetochore. Further genetic analyses indicated that the Ndc80 hairpin region might act as a platform for the kinetochore recruitment of Mph1, which is one of the most upstream SAC components in the hierarchy. Intriguingly, artificial tethering of Mph1 to the kinetochore fully restored checkpoint signaling in ndc80-AK01 cells, further substantiating the notion that Ndc80 is a kinetochore platform for Mph1. The hairpin region of Ndc80, therefore, plays a critical role in kinetochore recruitment of Mph1.
KEYWORDS: fission yeast, kinetochore, Mph1/MPS1, Ndc80, spindle assembly checkpoint
Introduction
Proper kinetochore-microtubule attachment lies at the heart of faithful segregation of chromosomes during mitosis. The outer kinetochore KMN network (KNL1, Mis12 complex and Ndc80 complex) plays a central role in ensuring proper microtubule attachment.1-4 Numerous studies have shown that the Ndc80 complex binds to microtubules in a tripartite manner – through the unstructured N-tail, the CH (calponin homology) domain and the internal loop region of the Ndc80 protein.5-11 While the N-tail and the CH domain are responsible for lateral attachment of microtubules by directly interacting with microtubule lattice,12-14 the loop, which interrupts the medial coiled-coil domain, helps establish end-on attachment by indirectly interacting with the microtubule plus end through recruiting microtubule-associated proteins.11,15,16 Incorrectly attached or unattached kinetochores are recognized by the spindle assembly checkpoint (SAC). Upon SAC activation, the ensemble of the SAC components (Mph1/MPS1, Mad1, Mad2, Mad3/BubR1, Bub1 and Bub3) is recruited to the kinetochores, generating a ‘wait-anaphase’ signal to halt mitotic progression.17,18
Intriguingly, aside from its microtubule-binding activity, the Ndc80 complex has been implicated to play a role in SAC activation.2,19-22 In humans, MPS1 binds to the CH domain of Ndc80 through its N-terminal extension (NTE) and tetratricopeptide repeat (TPR) in vitro,23 competing for the microtubule binding sites.24,25 This interaction appears to be regulated by Aurora B phosphorylation of the Ndc80 and MPS1 proteins.23,26,27 In addition, the CH domain of Nuf2 binds to the middle region (MR) of MPS1 in vitro.24,26 However, it remains unclear as to whether the CH domains are necessary and sufficient for MPS1 recruitment to unattached kinetochores in vivo. It is also not known how MPS1 is recruited to the kinetochore in simple model organisms such as yeasts. In this study, through analyses of a newly isolated ndc80 mutant (ndc80-AK01) in fission yeast, we show that the hairpin region, situated between the CH domain and the coiled-coil domain, plays a critical role in SAC signaling through recruitment of Mph1 to the unattached kinetochore.
Results
The ndc80-AK01 mutant is hypersensitive to microtubule drugs and contains a point mutation in the internal hairpin region
By isolating temperature sensitive ndc80 mutants specifically defective in kinetochore-microtubule attachment, we previously identified the Ndc80 internal loop as an important platform for regulating microtubule attachment and timely mitotic progression.7,9,11 In this study, we adopted a similar screening method (Fig. 1A) to isolate mutants that are sensitive to the microtubule depolymerising drug thiabendazole (TBZ), rather than high temperature. Subsequently, we isolated the ndc80-AK01 mutant that is TBZ-sensitive to an extent similar to mad1Δ (Fig. 1B). ndc80-AK01 contains a single amino acid change (L246P) in the hairpin region that resides between the CH domain and the coiled-coil region (Figs. 1C and S1). We tested the structural integrity of the Ndc80 complex in the ndc80-AK01 mutant by visualizing other components of the complex. As in wild type, Nuf2 and Spc25 co-localize as discrete dots in the ndc80-AK01 mutant (Fig. 1D and E), indicating that the defective phenotypes of the ndc80-AK01 mutant are not a result of disrupting overall architecture of the Ndc80 complex.
Figure 1.

Isolation and initial characterization of the ndc80-AK01 mutant. (A) Scheme of ndc80 mutant isolation. Randomly mutagenized N-terminal fragments (corresponding to 1st to 280th amino acid residues) of the ndc80 gene were fused with a C-terminal construct (238th to 624th amino acid) containing a kanamycin selection marker. The fusion ndc80 constructs were then transformed into a wild type fission yeast strain, by which the endogenous ndc80+ gene is replaced by the mutated ndc80-kanR gene through homologous recombination. Asterisks represent introduced mutations. Transformants were plated on YE5S plates at 27°C, and replica-plated onto kanamycin (G418) plates after 24 h. Upon 4 d incubation, cells were again replica-plated to YE5S with 10 μg/ml TBZ (thiabendazole). TBZ sensitive mutants cannot grow on TBZ plates. (B) TBZ sensitivity. Ten-fold serial dilutions of individual cells were spotted onto YE5S containing indicated concentrations of TBZ for 3 d at 27°C (5 × 104 cells in the first spot). (C) Schematic presentation of Ndc80 protein. The ndc80-AK01 mutant contains a mutation in the hairpin region of Ndc80 (L246P). (D) The Ndc80 complex in ndc80-AK01 remains intact. Spc25-YFP and Nuf2-mCherry were visualized in wild type and ndc80-AK01 after 120 minutes in YE5S with 50 μg/ml TBZ and 60 μg/ml of CBZ at 27°C. n > 200 cells. (E) Quantification of Spc25-YFP and Nuf2-mCherry signal intensities. Statistical significance was determined by student's t-test (n > 20 cells). Scale bar, 10 μm.
The ndc80-AK01 mutant shows defects in SAC activation
Next, we examined the phenotypic responses of ndc80-AK01 in the presence of microtubule drugs. We found that upon addition of TBZ and CBZ (carbendazim),28 ndc80-AK01 cells displayed an increased septation index and reduced viability, compared to wild type cells (Fig. 2A–C). These responses were very similar, if not identical, to those of mad2Δ.
Figure 2.
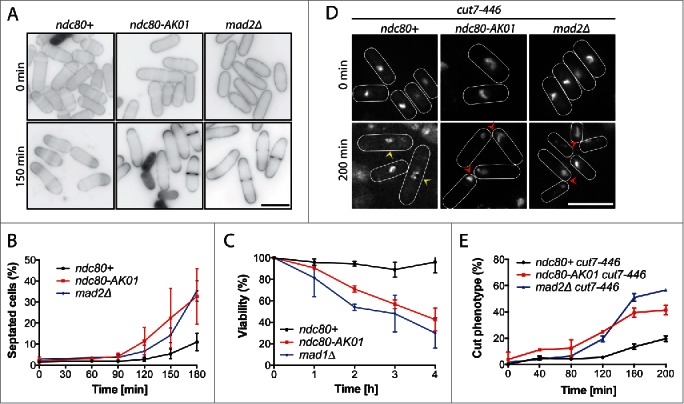
SAC signaling is defective in the ndc80-AK01 mutant. A. Exponentially growing cells were synchronized with 12.5 mM hydroxyurea (HU), washed out and placed in YE5S medium in the presence of 50 μg/ml TBZ and 60 μg/ml of CBZ at 27°C. Samples were stained with Calcofluor. B. Quantification of septated cells. Values are averages from 3 repeats. n > 150 cells for each time point. C. Viability test. Cells were grown in YE5S containing 50 μg/ml TBZ and 60 μg/ml of CBZ at 27°C and 200–500 cells were plated on YE5S plates. After 3 d incubation, the number of viable colonies was counted. D. The ndc80-AK01 and mad2Δ mutant cells display the “cut” phenotype in combination with the temperature-sensitive kinesin-5 mutant. Exponentially growing cells at 25°C were shifted up to 36°C. DAPI was used to stain DNA. Over-condensed chromosomes (yellow arrowheads) and the “cut” phenotype (red arrowheads) are marked. E. Quantification of cells showing the “cut” phenotype. Cells displaying “cut” phenotype as shown in D were quantified every 40 minutes for 200 minutes. Values are averages from 3 repeats. n > 150 cells for each time point. Error bars in B, C and E represent standard deviations. Scale bars, 10 μm.
We also examined the phenotypes of ndc80-AK01 under mitotic arrest conditions. For this purpose, we constructed double mutants between ndc80-AK01 and the temperature sensitive cut7-446 mutant (kinesin-5),29 or the nda3-1828 mutant (β-tubulin).30 As reported previously, 29 cut7-446 mutants displayed over-condensed chromosomes after incubating at 36°C for 200 minutes (Fig. 2D). In sharp contrast, cut7-446 ndc80-AK01 cells, like cut7-446 mad2Δ cells, rapidly exited mitosis and exhibited the characteristic “cut” cell phenotype - lethal cell division in the absence of chromosome segregation (Fig. 2D and E). Likewise, we observed a reduced percentage of cells showing over-condensed chromosomes in nda3-1828 ndc80-AK01 cells, as in nda3-1828 mad2Δ cells (Fig. S2A and B), suggesting that ndc80-AK01 is defective in SAC activation. Consistent with this proposition, double mutants of ndc80-AK01 and deletions of SAC components exhibited no additive adverse effects on growth properties and hypersensitivity to TBZ (Figs. 1B and S2C). Collectively, these results consistently indicate that the ndc80-AK01 mutant is specifically defective in SAC signaling.
The ndc80-AK01 mutant fails to recruit all SAC components to the unattached kinetochore
To determine the underlying reason for the checkpoint defects seen in the ndc80-AK01 mutant, we next observed the localization of individual GFP-tagged SAC components in the presence of TBZ/CBZ (absence of microtubules), using Plo1-mCherry as a mitotic marker (Polo kinase).31 Mad2 is known to be the very last component in the SAC signaling pathway that is recruited to unattached kinetochores.18 In the presence of TBZ/CBZ, wild type cells displayed a long mitotic delay with prolonged Mad2-GFP localization to the kinetochores (Fig. 3A–D). In contrast, we did not observe analogous Mad2-GFP kinetochore signals or mitotic delay in the ndc80-AK01 mutant (Fig. 3A–D).
Figure 3.
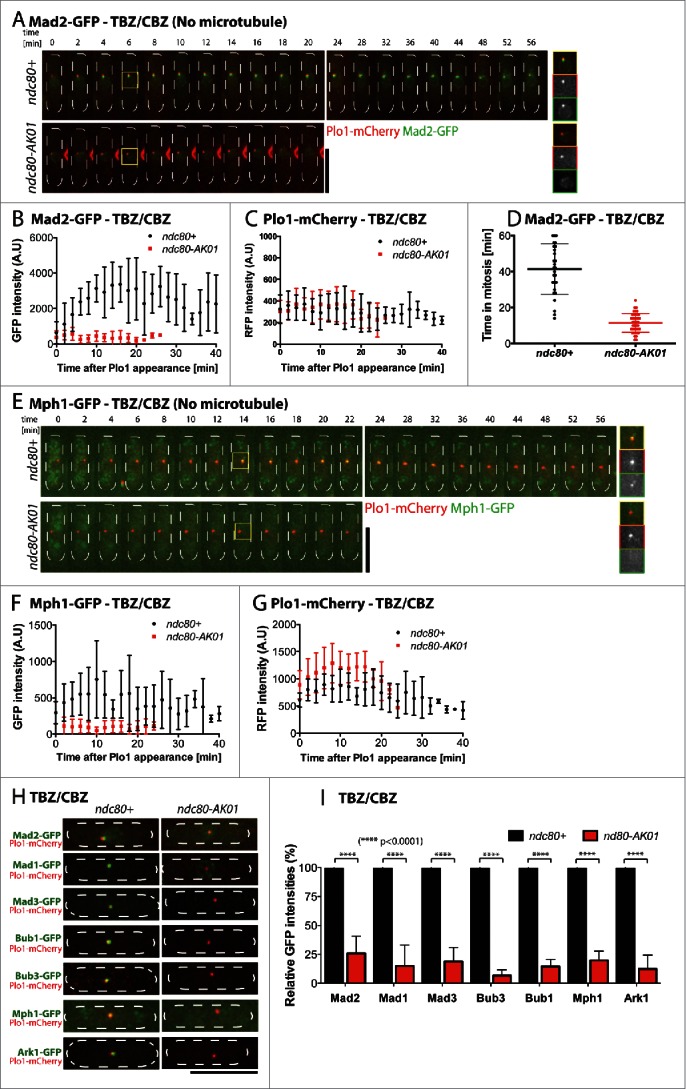
The ndc80-AK01 mutant fails to recruit SAC components to the kinetochore under mitotic arrest condition. A.-H. Exponentially growing cells (4 × 106 cells/ml) were cultured in YE5S with 50 μg/ml TBZ and 60 μg/ml of CBZ. After 30 min, live samples were placed on lectin-coated dishes and imaged for further 60 min. Imaging started after 30 minutes. Representative images of ndc80+ and ndc80-AK01 with Mad2-GFP and Plo1-mCherry (A), Mph1 and Plo1-mCherry (E) and other SAC components (H) are shown. Quantification of signal intensities derived from Mad2-GFP (B), Mph1-GFP (F), Plo1-mCherry (C and G) and other GFP-tagged SAC components (I) are also indicated. The duration in mitosis (judged by localization of Plo1-mCherry at SPBs) was measured and quantified in D. n > 10 cells for B, C, F, and G and n > 30 cells for D. Scale bars, 10 µm.
Mph1 kinase, on the other hand, is one of the most upstream SAC components.31 As in the case of Mad2-GFP, recruitment of Mph1-GFP to the kinetochores was also impaired in the ndc80-AK01 mutant (Fig. 3E–G). Consistent with this notion, systematic analyses of the other SAC components, including Bub1, Bub3, Mad3 and Mad1, revealed that these proteins are also mis-localized from the kinetochores in ndc80-AK01 under mitotic arrest conditions (Fig. 3H, I and S3A–H).
In fission yeast, as in human cells, Ark1/Aurora B is assigned as the most upstream component.31 However, its kinetochore/centromere localization substantially depends on Bub1 through positive feedback regulation; Bub1 phosphorylates histone H2A, thereby recruiting Sgo2/Shugoshin that in turn promotes Ark1/Aurora B localization.28,32,33 In line with this model, we found reduced localization of Ark1-GFP to the kinetochores/centromeres in ndc80-AK01, to a similar extent as in bub1Δ cells (Fig. S4). Together, we concluded that the primary defects observed in the ndc80-AK01 mutant can be attributed to impaired Mph1 recruitment to kinetochores, which leads to failure in recruitment of the other SAC components and abortive mitotic arrest.
Artificial tethering of Mph1 to the kinetochore arrests ndc80-AK01 cells in mitosis
If compromised Mph1 recruitment to unattached kinetochores were the main defect in ndc80-AK01, it would lead to a critical prediction that artificial tethering of Mph1 to the kinetochore should restore SAC signaling. In fission yeast, as in other species,23,34 Mph1 tethering to the kinetochore in wild type cells, but not in mad2Δ, results in constitutive checkpoint activation with a high mitotic index.28,31,35 Accordingly, we constructed strains that contained Mph1 fused to Mis12 under the thiamine-repressible nmt81 promoter (provided by Silke Hauf) in the ndc80-AK01 background. Intriguingly, Mis12-Mph1 cells, irrespective of ndc80+ or ndc80-AK01, showed robust growth inhibition, which depended upon the lack of thiamine (Fig. 4A). Observation of liquid cultures indicated that growth retardation was due to prolonged mitotic arrest (Fig. 4B and C). Consistent with SAC activation under this condition, harmful effects - both poor growth on solid plates and a high mitotic index - were reversed by the deletion of the SAC component Mad2 (Fig. 4A–C). These results were entirely consistent with the idea that the primary, if not sole, defect of ndc80-AK01 is ascribable to its inability to recruit Mph1 to the unattached kinetochore. Hence, we propose that the hairpin region situated between the CH domain and the coiled-coil domain plays a crucial role in SAC signaling through recruitment of Mph1 when proper attachment of the kinetochore to spindle microtubules fails to be established.
Figure 4.

Tethering of Mph1 to the kinetochore rescues the SAC signaling defects in the ndc80-AK01 mutant. A. Rescue of the SAC signaling defects in ndc80-AK01 by kinetochore-tethered Mis12-Mph1. Spot tests of the indicated strains were performed as in Fig. 1B. B. Confirmation of Mis12-Mph1-GFP localization to the kinetochores. Representative images of cells grown in the absence of thiamine are shown. Mitotic cells were identified by the presence of Plo1-mCherry at SPBs. C. Quantification of mitotic cells in the indicated strains. The percentage of mitotic cells was counted using the presence of Plo1-mCherry at SPBs as a mitotic marker. n > 200 cells. D. Mph1 is recruited to the Ndc80 complex at unattached kinetochores. Mph1 potentially localizes to the CH domain (i), the hairpin region (ii) or both (iii). (iv) Scheme of SAC components recruitment to unattached kinetochores in fission yeast. Ark1/Aurora B is recruited to the centromere/kinetochore (in the figure, Bub1-mediated phosphorylated histone H2A is denoted by purple oval adjacent to Sgo2) and Mph1 localizes to the Ndc80 complex. Once at the kinetochore, Mph1 phosphorylates Spc7 leading to the recruitment of Bub1, Bub3 and Mad3, which is critical to recruit the Mad1-Mad2 heterotetramer complex to the kinetochore. The sequential conformation changes of Mad2 result in the formation of the MCC (C-Mad2-Mad3-Slp1/Cdc20) and SAC activation. Scale bar, 10 μm.
Discussion
Our data firmly establish that a single point mutation within the hairpin region of Ndc80 inhibits recruitment of Mph1 to the kinetochore in fission yeast. Interestingly, an earlier report in budding yeast suggested physical interaction between Ndc80 and Mps1,36 and more recent work in human cultured cells show that the CH domains of Ndc80 and Nuf2 directly bind MPS1/Mph1, thereby being responsible for MPS1 localization to the unattached kinetochore.23-26 Given these results, we envision the following 3 scenarios to account for our findings. The first possibility is that, as in humans and possibly budding yeast,34,36 the CH domain of Ndc80 is a direct binding site for Mph1 in fission yeast. However, the hairpin region might be important for structural integrity of the adjacent CH domain (Fig. 4Di). The ndc80-AK01 mutant contains a substitution from leucine to proline at the 246th amino acid situated within the αH helix. This mutation may create an abnormal kink in the structure, thereby shielding the Mph1 binding site within the CH domains of Ndc80 or Nuf2 (Fig. 4Di).
The second scenario is that fission yeast has undergone an evolutionary diversification, by which the hairpin region instead of the CH domain is solely responsible for Mph1 recruitment to the kinetochore (Fig. 4Dii). We tried immunoprecipitation between Ndc80 and Mph1, but so far we could not obtain any positive data; even using mitotically arrested wild type cell lysates, we have been unable to show interaction between Ndc80 and Mph1. It is of note that in human cell lysates, physical interaction between MPS1 and Ndc80 has also not been shown.23-26 We surmise that binding of these 2 proteins upon SAC activation would be transient and/or unstable in vivo. The third possibility is that both the CH domains and the hairpin region are required for Mph1 recruitment to the kinetochore in vivo (Fig. 4Diii). Multiple functional motifs have been identified in the N-terminal domain of Mph1/MPS1; the NTE and MR regions of Mph1/MPS1, respectively, have been shown to interact with each CH domain within Ndc80 and Nuf2.24 Therefore, it is possible that the Mph1/MPS1 N-terminal domain also interacts with the Ndc80 hairpin that is required for Mph1/MPS1 recruitment. It is of note that, despite not mutually exclusive with any possibility mentioned above, the L246P replacement may render the Ndc80-AK01 protein unable to sense a lack of tension/attachment at the kinetochore.
Regardless of several reports on the requirement of the CH domains for MPS1 recruitment to the kinetochore,23-26 the precise roles of the adjacent hairpin region remain to be determined in any organisms, though the hairpin seems conserved across eukaryotic organisms. Interestingly, in budding yeast, the hairpin is proposed to be important for protein-protein interactions by securing overall structures of Ndc80, although the involvement of the hairpin region in SAC signaling has not been appreciated.37 We postulate that the hairpin region directly or indirectly interacts with the nearby CH domains, thus creating an efficient binding pocket for MPS1/Mph1 when microtubules do not interact with the Ndc80 complex.
Although a detailed mode of interaction between Mph1 and Ndc80 has not been solidified, our current study complements the idea that Ndc80 is indeed a crucial platform for Mph1 recruitment as in other organisms. We propose a model to summarize the current understanding of SAC component recruitment to the kinetochore (Fig. 4Div). Ark1/Aurora B localizes to the centromeric region, followed by Mph1/MPS1 recruitment to the Ndc80 complex.31 Once there, Mph1/MPS1 phosphorylates MELT motifs in Spc7/KNL1, where the Bub1-Bub3 complex, as well as Mad3/BubR1, would dock.28,38 The localization of these upstream components leads to recruitment of the Mad1-Mad2 complex to the kinetochore and formation of the mitotic checkpoint complex, which inhibits mitotic progression.
Materials and methods
Fission yeast culture and genetics
All yeast strains used in the study are listed in Table S1. Cells were grown and maintained in standard conditions using rich YE5S medium.39 The experiments using nmt promoters were carried out in Edinburgh Minimal Media (EMM) supplemented with the required amino acids and 2 mM thiamine for cell growth. Upon washout of the reagent using a filtration system, the cells were grown in the absence of thiamine for 18-24 h.
Cells were grown at 27°C, unless otherwise stated. Spot tests were performed after adjusting cell concentration to 2 × 107 cells/ml. Subsequent 10-fold dilutions were spotted on the appropriate plates containing rich YE5S medium in the presence or absence of various concentrations of TBZ or EMM with supplements or with or without 2 mM thiamine.
Yeast strain construction
Genomic DNA from the ndc80+-kanR strain7,11 was extracted as described previously.7,11 The N-terminal fragment (corresponding to 1st to 280th amino acid residues) of ndc80+-kanR was randomly mutagenized using “error prone PCR” with unbalanced dNTP (10x dGTP excess compared to other dNTPs) using Vent DNA polymerase (New England Biolabs). The C-terminus (amino acid 238 to 624) of ndc80+-kanR was amplified by PCR using PrimeSTAR polymerase (TaKaRa). The N- and C- terminal fragments were gel purified and fused together in an equimolar ratio using PrimeSTAR polymerase. Then the amplified fusion fragments were concentrated by ethanol precipitation and transformed into a wild type strain. Kanamycin (G418)-resistant clones were selected and then replica-plated onto YE5S containing 10 μg/ml thiabendazole (TBZ). We screened approximately 10,000 colonies and isolated 5 mutants judged as TBZ-sensitive. These isolates were backcrossed to check for cosegregation of the drug marker with TBZ sensitivity. Nucleotide sequencing was performed to confirm the position of the mutation within the ndc80 gene.
Mitotic arrest conditions
To accumulate cells in mitosis, cultures were synchronized in S phase using 12.5 mM of hydroxyurea (HU) at 25°C for 4 h. Cultures were filtered and released into HU-free YE5S media with 50 μg/ml TBZ and 60 μg/ml CBZ.28 Alternatively, strains were crossed with a cut7-446 conditional mutant29 or an nda3-1828 strain.30 Cells were grown at 25°C overnight until cultures reached mid-log phase and then shifted to 36°C. Cells were observed either upon fixation or by live imaging.
Fluorescence microscopy
Samples were fixed with 1.6% paraformadehyde at 27°C, unless otherwise specified. Live cells were imaged on lectin-coated, glass dishes (MatTek, Ashland, MA). Images were acquired using an Olympus IX70 PlanoApo 100x, NA 1.4, oil immersion objective on an Olympus IX70 wide-field inverted epifluorescence microscope. The DeltaVision-softWoRx system (softWoRx 3.3.0; Applied Precision Co.) with a Coolsnap HQ (Roper Scientific) camera was used for acquisition of all fluorescence microscopy data. Images were taken at 14 positions along the z-axis at 0.3 μm intervals. Images obtained were deconvolved, compressed into a projection using the DeltaVision (DeltaVision-SoftWoRx; Applied Precision Ltd) maximum intensity algorithm. The maximum signal intensities, after subtracting background signals in close proximity to the fluorescent spot, were quantified. Subsequent image processing was performed in Adobe Photoshop CS5 and Adobe Illustrator CS5.1.
Statistical data analysis
All data represent the mean of multiple experiments +/− SD. Experiment sample numbers and the number of replicates used for statistical testing have been described in the corresponding figure legends. All p-values were calculated using 2-tailed unpaired student t-tests. We followed this key for asterisk placeholders for p-values in the figures: ****p < 0.0001.
Supplementary Material
Abbreviations
- SAC
spindle assembly checkpoint
- TBZ
thiabendazole
- CBZ
carbendazim
- HU
hydroxyurea
- CH
calponin homology
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Kevin Hardwick, Tomohiro Matsumoto and Mitsuhiro Yanagida for strains and Silke Hauf for strains and thoughtful discussion.
Author contributions
A.E.C., N.H.T. and T.T. designed experiments and A.E.C. performed all the experiments. A.E.C., N.H.T. and T.T. analyzed the data and wrote the paper. All authors read and approved the final version of the manuscript.
Funding
This work was supported by by Cancer Research UK—by Cancer Research UK and the Francis Crick Institute.
References
- [1].Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 2006; 127:983-997; PMID:17129783; http://dx.doi.org/ 10.1016/j.cell.2006.09.039 [DOI] [PubMed] [Google Scholar]
- [2].Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 2002; 297:2267-70; PMID:12351790; http://dx.doi.org/ 10.1126/science.1075596 [DOI] [PubMed] [Google Scholar]
- [3].Liu X, McLeod I, Anderson S, Yates JR 3rd, He X. Molecular analysis of kinetochore architecture in fission yeast. EMBO J 2005; 24:2919-30; PMID:16079914; http://dx.doi.org/ 10.1038/sj.emboj.7600762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cheeseman IM. The kinetochore. Cold Spring Harbor perspectives in biology 2014; 6:a015826; PMID:24984773; http://dx.doi.org/ 10.1101/cshperspect.a015826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, et al.. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 2008; 133:427-39; PMID:18455984; http://dx.doi.org/ 10.1016/j.cell.2008.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zaytsev AV, Mick JE, Maslennikov E, Nikashin B, DeLuca JG, Grishchuk EL. Multisite phosphorylation of the NDC80 complex gradually tunes its microtubule-binding affinity. Mol Biol Cell 2015; 26:1829-44; PMID:25808492; http://dx.doi.org/ 10.1091/mbc.E14-11-1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hsu KS, Toda T. Ndc80 internal loop interacts with Dis1/TOG to ensure proper kinetochore-spindle attachment in fission yeast. Curr Biol 2011; 21:214-20; PMID:21256022; http://dx.doi.org/ 10.1016/j.cub.2010.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maure JF, Komoto S, Oku Y, Mino A, Pasqualato S, Natsume K, Clayton L, Musacchio A, Tanaka TU. The Ndc80 loop region facilitates formation of kinetochore attachment to the dynamic microtubule plus end. Curr Biol 2011; 21:207-13; PMID:21256019; http://dx.doi.org/ 10.1016/j.cub.2010.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tang NH, Toda T. Alp7/TACC recruits kinesin-8-PP1 to the Ndc80 kinetochore protein for timely mitotic progression and chromosome movement. J Cell Sci 2015; 128:354-63; PMID:25472718; http://dx.doi.org/ 10.1242/jcs.160036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang G, Kelstrup CD, Hu XW, Kaas Hansen MJ, Singleton MR, Olsen JV, Nilsson J. The Ndc80 internal loop is required for recruitment of the Ska complex to establish end-on microtubule attachment to kinetochores. J Cell Sci 2012; 125:3243-53; PMID:22454517; http://dx.doi.org/ 10.1242/jcs.104208 [DOI] [PubMed] [Google Scholar]
- [11].Tang NH, Takada H, Hsu KS, Toda T. The internal loop of fission yeast Ndc80 binds Alp7/TACC-Alp14/TOG and ensures proper chromosome attachment. Mol Biol Cell 2013; 24:1122-33; PMID:23427262; http://dx.doi.org/ 10.1091/mbc.E12-11-0817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tooley J, Stukenberg PT. The Ndc80 complex: integrating the kinetochore's many movements. Chromosome Res 2011; 19:377-91; PMID:21311965; http://dx.doi.org/ 10.1007/s10577-010-9180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Varma D, Salmon ED. The KMN protein network - chief conductors of the kinetochore orchestra. J Cell Sci 2012; 125:5927-36; PMID:23418356; http://dx.doi.org/ 10.1242/jcs.093724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alushin G, Nogales E. Visualizing kinetochore architecture. Curr Opin Struct Biol 2011; 21:661-9; PMID:21862320; http://dx.doi.org/ 10.1016/j.sbi.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tang NH, Toda T. MAPping the Ndc80 loop in cancer: A possible link between Ndc80/Hec1 overproduction and cancer formation. BioEssays 2015; 37:248-56; PMID:25557589; http://dx.doi.org/ 10.1002/bies.201400175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nilsson J. Looping in on Ndc80 - how does a protein loop at the kinetochore control chromosome segregation? BioEssays 2012; 34:1070-7; PMID:23154893; http://dx.doi.org/ 10.1002/bies.201200096 [DOI] [PubMed] [Google Scholar]
- [17].Musacchio A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol 2015; 25:R1002-18; PMID:26485365; http://dx.doi.org/ 10.1016/j.cub.2015.08.051 [DOI] [PubMed] [Google Scholar]
- [18].Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol 2012; 22:R966-80; PMID:23174302; http://dx.doi.org/ 10.1016/j.cub.2012.10.006 [DOI] [PubMed] [Google Scholar]
- [19].Guimaraes GJ, Dong Y, McEwen BF, Deluca JG. Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol 2008; 18:1778-84; PMID:19026543; http://dx.doi.org/ 10.1016/j.cub.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miller SA, Johnson ML, Stukenberg PT. Kinetochore attachments require an interaction between unstructured tails on microtubules and Ndc80(Hec1). Curr Biol 2008; 18:1785-91; PMID:19026542; http://dx.doi.org/ 10.1016/j.cub.2008.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, Musacchio A, Green S, Taylor SS. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J Cell Biol 2010; 190:25-34; PMID:20624899; http://dx.doi.org/ 10.1083/jcb.201002133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Santaguida S, Vernieri C, Villa F, Ciliberto A, Musacchio A. Evidence that Aurora B is implicated in spindle checkpoint signalling independently of error correction. EMBO J 2011; 30:1508-19; PMID:21407176; http://dx.doi.org/ 10.1038/emboj.2011.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nijenhuis W, von Castelmur E, Littler D, De Marco V, Tromer E, Vleugel M, van Osch MH, Snel B, Perrakis A, Kops GJ. A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J Cell Biol 2013; 201:217-31; PMID:23569217; http://dx.doi.org/ 10.1083/jcb.201210033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ji Z, Gao H, Yu H. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science 2015; 348:1260-4; PMID:26068854; http://dx.doi.org/ 10.1126/science.aaa4029 [DOI] [PubMed] [Google Scholar]
- [25].Hiruma Y, Sacristan C, Pachis ST, Adamopoulos A, Kuijt T, Ubbink M, von Castelmur E, Perrakis A, Kops GJ. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science 2015; 348:1264-7; PMID:26068855; http://dx.doi.org/ 10.1126/science.aaa4055 [DOI] [PubMed] [Google Scholar]
- [26].Dou Z, Liu X, Wang W, Zhu T, Wang X, Xu L, Abrieu A, Fu C, Hill DL, Yao X. Dynamic localization of Mps1 kinase to kinetochores is essential for accurate spindle microtubule attachment. Proc Natl Acad Sci U S A 2015; 112:E4546-55; PMID:26240331; http://dx.doi.org/ 10.1073/pnas.1508791112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhu T, Dou Z, Qin B, Jin C, Wang X, Xu L, Wang Z, Zhu L, Liu F, Gao X, et al.. Phosphorylation of microtubule-binding protein Hec1 by mitotic kinase Aurora B specifies spindle checkpoint kinase Mps1 signaling at the kinetochore. J Biol Chem 2013; 288:36149-59; PMID:24187132; http://dx.doi.org/ 10.1074/jbc.M113.507970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat Cell Biol 2012; 14:746-52; PMID:22660415; http://dx.doi.org/ 10.1038/ncb2515 [DOI] [PubMed] [Google Scholar]
- [29].Hagan I, Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature 1990; 347:563-6; PMID:2145514; http://dx.doi.org/ 10.1038/347563a0 [DOI] [PubMed] [Google Scholar]
- [30].Radcliffe P, Hirata D, Childs D, Vardy L, Toda T. Identification of novel temperature-sensitive lethal alleles in essential β-tubulin and nonessential a2-tubulin genes as fission yeast polarity mutants. Mol Biol Cell 1998; 9:1757-71; PMID:9658169; http://dx.doi.org/ 10.1091/mbc.9.7.1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heinrich S, Windecker H, Hustedt N, Hauf S. Mph1 kinetochore localization is crucial and upstream in the hierarchy of spindle assembly checkpoint protein recruitment to kinetochores. J Cell Sci 2012; 125:4720-7; PMID:22825872; http://dx.doi.org/ 10.1242/jcs.110387 [DOI] [PubMed] [Google Scholar]
- [32].Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 2010; 327:172-7; PMID:19965387; http://dx.doi.org/ 10.1126/science.1180189 [DOI] [PubMed] [Google Scholar]
- [33].Caldas GV, DeLuca KF, DeLuca JG. KNL1 facilitates phosphorylation of outer kinetochore proteins by promoting Aurora B kinase activity. J Cell Biol 2013; 203:957-69; PMID:24344188; http://dx.doi.org/ 10.1083/jcb.201306054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aravamudhan P, Goldfarb AA, Joglekar AP. The kinetochore encodes a mechanical switch to disrupt spindle assembly checkpoint signalling. Nat Cell Biol 2015; 17:868-79; PMID:26053220; http://dx.doi.org/ 10.1038/ncb3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ito D, Saito Y, Matsumoto T. Centromere-tethered Mps1 pombe homolog (Mph1) kinase is a sufficient marker for recruitment of the spindle checkpoint protein Bub1, but not Mad1. Proc Natl Acad Sci U S A 2012; 109:209-14; PMID:22184248; http://dx.doi.org/ 10.1073/pnas.1114647109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kemmler S, Stach M, Knapp M, Ortiz J, Pfannstiel J, Ruppert T, Lechner J. Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J 2009; 28:1099-110; PMID:19300438; http://dx.doi.org/ 10.1038/emboj.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tien JF, Fong KK, Umbreit NT, Payen C, Zelter A, Asbury CL, Dunham MJ, Davis TN. Coupling unbiased mutagenesis to high-throughput DNA sequencing uncovers functional domains in the Ndc80 kinetochore protein of Saccharomyces cerevisiae. Genetics 2013; 195:159-70; PMID:23833183; http://dx.doi.org/ 10.1534/genetics.113.152728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JB. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol 2012; 10:891-9; PMID:22521786; http://dx.doi.org/ 10.1016/j.cub.2012.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 1991; 194:795-823; PMID:2005825; http://dx.doi.org/ 10.1016/0076-6879(91)94059-L [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


