Abstract
Separating ethene (C2H4) from ethane (C2H6) is of paramount importance and difficulty. Here we show that C2H4 can be efficiently purified by trapping the inert C2H6 in a judiciously designed metal-organic framework. Under ambient conditions, passing a typical cracked gas mixture (15:1 C2H4/C2H6) through 1 litre of this C2H6 selective adsorbent directly produces 56 litres of C2H4 with 99.95%+ purity (required by the C2H4 polymerization reactor) at the outlet, with a single breakthrough operation, while other C2H6 selective materials can only produce ca. ⩽ litre, and conventional C2H4 selective adsorbents require at least four adsorption–desorption cycles to achieve the same C2H4 purity. Single-crystal X-ray diffraction and computational simulation studies showed that the exceptional C2H6 selectivity arises from the proper positioning of multiple electronegative and electropositive functional groups on the ultramicroporous pore surface, which form multiple C–H···N hydrogen bonds with C2H6 instead of the more polar competitor C2H4.
 The separation of high purity ethene from the mixed gaseous
products of cracking poses significant obstacles. Here, the authors present a
metal-organic framework which, in contrast to most absorbents, selectively binds the
less polar ethane thus allowing the efficient collection of the target
product.
The separation of high purity ethene from the mixed gaseous
products of cracking poses significant obstacles. Here, the authors present a
metal-organic framework which, in contrast to most absorbents, selectively binds the
less polar ethane thus allowing the efficient collection of the target
product.
As the most important chemical product, ethene (C2H4) is generally obtained through steam cracking and thermal decomposition of naphtha or ethane (C2H6) (ref. 1). Besides being obtained as a byproduct of petroleum refining, C2H6 is also isolated on an industrial scale from natural gas (CH4 70∼96%, C2H6 1∼14% and CO2 0∼8%) (ref. 2). As a result of their similar physical properties, it is difficult to separate C2H6, C2H4 and CO2 (refs 3, 4, 5). In industry, C2H6 and C2H4 are separated by cryogenic high-pressure distillation, typically at 7–28 bar and 183–258 K using very high towers consisting of over 150 trays, which is very energy consuming (7 GJ t−1) and constitutes a notable portion of the ethylene cost6,7. To save energy, separation methods effective at the ambient temperature and pressure are highly demanded8,9,10,11. Passing the gas mixture through a fixed-bed adsorber can be a very simple and promising approach to afford low energy consumption and high product purity.
Because unsaturated hydrocarbons like to coordinate with metal ions, C2H4 can be selectively bound and separated from its saturated counterpart C2H6 (refs 12, 13, 14, 15, 16). Compared with other types of porous materials, porous metal-organic frameworks (MOFs) are unique for their diversified/designable framework structures and pore surfaces, including the ease of introducing open metal sites (OMSs), which have shown great potentials for C2H4/C2H6 separation17,18,19,20,21,22. In the fix-bed separation process, the un-adsorbed C2H6 first breakthrough, and C2H4 enriched in the stationary phase is later obtained by heating and/or inert-gas blowing. Because the un-adsorbed C2H6 residing in the mobile phase contaminates the desired product C2H4 during the desorption stage, the highest C2H4 purity produced by a full adsorption–desorption cycle can just reach 99%+ (refs 13, 17, 23, 24), and at least four such cycles are necessary to achieve 99.95%+ (ref. 25), the lower limit required by the C2H4 polymerization reactor26,27,28. Obviously, this problem can be solved by using a C2H6 selective adsorbent, which not only improves the C2H4 purity but also reduces energy consumption. The simple separation operation and device (just one adsorption process in a single breakthrough operation) are also necessary for onsite supply of purified C2H4. However, such an unusual adsorption behaviour has been only reported for a few low-polarity or hydrophobic MOFs29,30,31,32,33,34,35,36, and their C2H4/C2H6 separation performances (that is, C2H6/C2H4 selectivities) are poor, because the polarities of C2H4 and C2H6 are very similar and can be hardly distinguished by hydrophobic adsorbents.
As C2H6 possesses the lowest polarity (quadrupole moment) compared with similar molecules such as C2H4 and CO2 (Supplementary Table 1)37; polar adsorbents are generally selective for the latter gases. However, considering that the electropositive and electronegative portions locate quite differently among these gas molecules, we speculated that by rational utilization of polar functional groups, it is still possible to design a MOF with optimized pore size/shape and surface electrostatic distribution that can bind C2H6 much stronger than for C2H4. Herein, we report the design, structure and gas adsorption/separation properties of such a C2H6-trapping MOF, which is useful for not only direct producing highly pure C2H4 from C2H4/C2H6 mixtures, but also efficient separation of four-component CH4/C2H4/C2H6/CO2 mixtures and extraction of C2H6 from natural gas.
Results
Synthesis, structure and stability
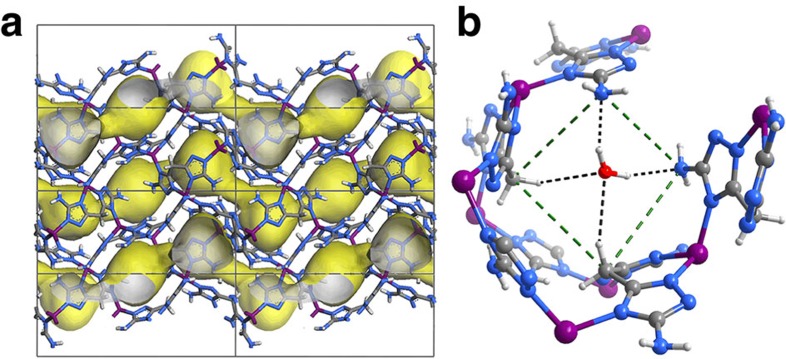
Bis(5-amino-1H-1,2,4-triazol-3-yl)methane (H2batz) with two 3-amino-1,2,4-triazole rings bridged by a methylene group was designed as a new ligand combining multiple nitrogen atoms as hydrogen-bonding acceptors and methylene groups as dipole repulsion groups, as well as short bridging lengths for construction of an ultramicroporous framework. Reaction of H2batz and Zn(OH)2 in dilute aqueous ammonia produced a porous metal-azolate framework [Zn(batz)]·0.5H2O (MAF-49·H2O). Single-crystal X-ray diffraction (SCXRD) analysis of MAF-49·H2O (Supplementary Table 2 and Supplementary Data 1) showed that each Zn(II) is tetrahedrally coordinated by four triazolate nitrogen atoms from three batz2– ligands (Supplementary Fig. 1), and each batz2– ligand coordinates to three Zn(II) ions in a bisimidazolate mode, giving a three-dimensional (3D) coordination framework with narrow 1D zigzag channels (Fig. 1a). Since only four of the eight nitrogen donors of batz2– are utilized according to the coordination requirement of Zn(II), the pore surface of MAF-49 is rich with electronegative nitrogen atoms, although some of them form intra-framework N–H···N hydrogen bonds to reduce their abilities as hydrogen-bonding acceptors. Notably, the narrowest section of the 1D channel (3.3 × 3.0 Å2) is approximately a folded four-membered ring defined by a pair of free amino groups (with their lone electron pairs) and a pair of methylene groups with a cis-configuration, which is occupied by a guest H2O molecule with two O–H···N and two C–H···O hydrogen bonds (Fig. 1b).
Figure 1. X-ray crystal structure of MAF-49·H2O.
(a) Framework (Zn purple, C dark grey, H light grey, N blue) and pore surface (yellow/grey curved surface) structures. Guest molecules are omitted for clarity. (b) Local environment and hydrogen-bonding interactions of the narrowest channel neck (highlighted by green dashed lines).
Thermogravimetry and powder XRD showed that MAF-49·H2O can be readily activated and is stable to 450 °C in nitrogen (Supplementary Fig. 2), in boiling water for at least 1 month and in aqueous acid/base (4≤pH≤12) at room temperature for at least 1 week (Supplementary Fig. 3), which is extraordinary among MOFs and can be partly explained by the strong metal-azolate coordination bonds38. SCXRD showed that complete dehydration leads to a slight framework expansion (0.17% in volume, Supplementary Table 2 and Supplementary Data 2).
Gas adsorption property and mechanism
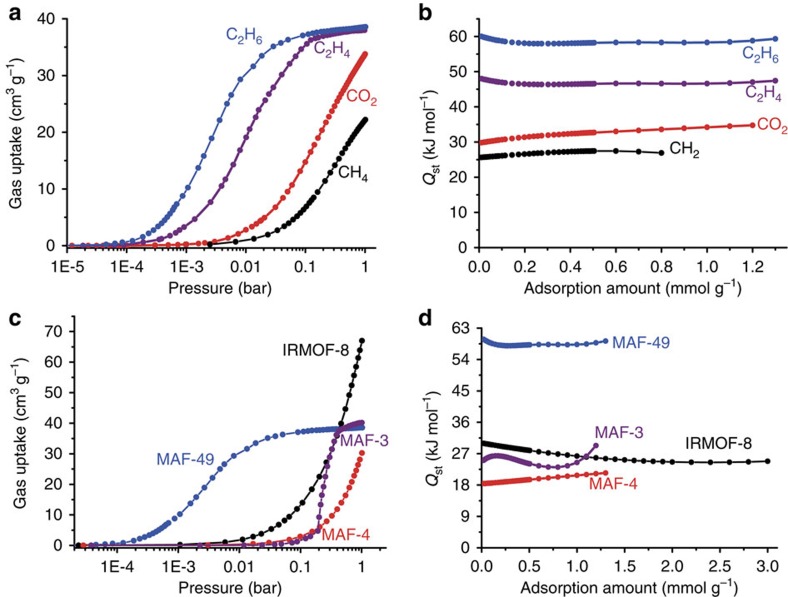
Single-component adsorption isotherms for CH4, C2H6, C2H4 and CO2 were measured for guest-free MAF-49 at 298 K, 307 K and 316 K (Fig. 2a and Supplementary Fig. 4). According to their different isotherm shapes, it can be judged that the host–guest binding follows C2H6>C2H4>CO2>CH4. The gas adsorption enthalpies were calculated quantitatively by Virial analyses (Fig. 2b and Supplementary Fig. 5), which are 60 kJ mol−1, 48 kJ mol−1, 30 kJ mol−1 and 25 kJ mol−1 for C2H6, C2H4, CO2, and CH4, respectively, at zero-coverage. The mixed gas adsorption isotherms for equimolar C2H6/C2H4, C2H6/CO2 and C2H6/CH4 mixtures were simulated by the ideal adsorbed solution theory39, in which the single-component adsorption isotherms were fitted by the Langmuir−Freundlich model (Supplementary Fig. 6). At total pressure of 100 kPa and a temperature of 316 K, the C2H6/C2H4, C2H6/CO2 and C2H6/CH4 selectivities of these mixtures were calculated as ca. 9, 40 and 170, respectively (Supplementary Fig. 7). Notably, the C2H6/C2H4 selectivity of MAF-49 is much higher than the highest value in the literature (2.4 for IRMOF-8 at 318 K) (ref. 31). Except for CH4 with obviously lower molecular weight and boiling point, which interacts weakly with all adsorbents, the binding strength order of MAF-49 for other three heavier gases is unusual. Among a variety of physical properties of the four gases, only the polarizability trend is consistent with the binding trend (Supplementary Table 1). Nevertheless, the small differences of their polarizabilities are not enough to explain the large variation of their adsorption enthalpies, especially for C2H6 and C2H4. Notably, the C2H6 adsorption enthalpy is significantly higher than reported values, while the C2H4 one is moderate18.
Figure 2. Single-component gas adsorption properties.
(a) Gas adsorption isotherms for C2H6, C2H4, CO2 and CH4 in MAF-49 at 316 K. (b) The coverage-dependent C2H6, C2H4, CO2 and CH4 adsorption enthalpy obtained by the Virial method. (c) C2H6 adsorption isotherms of MAF-49, MAF-3, MAF-4 and IRMOF-8 measured at 316 K. (d) Coverage-dependent C2H6 adsorption enthalpy of MAF-49, MAF-3, MAF-4 and IRMOF-8.
To elucidate the very different C2H6, C2H4 and CO2 affinities of MAF-49, their preferential host–guest structures and energy changes were calculated by grand canonical Monte Carlo simulation and further periodic density functional theory optimization. The obtained binding energies of the final host–guest structures are −56.7, −45.5 and −41.3 kJ mol–1 for C2H6, C2H4 and CO2, respectively. However, to adsorb these gas molecules, the host framework undergoes different structural distortions from the guest-free form and consumes energies of +0.2 kJ mol–1, +0.3 kJ mol–1 and +5.6 kJ mol–1, respectively. Taking both the host–guest binding and host-framework distortion into consideration, the total energies or adsorption enthalpies can be calculated as −56.5 kJ mol–1, −45.2 kJ mol–1 and −35.7 kJ mol–1 for C2H6, C2H4, and CO2, respectively, which are consistent with the experimental values (Supplementary Table 3). In the density functional theory optimized host–guest structures, it can be seen that C2H6, C2H4 and CO2 are all adsorbed in or very close to the narrowest channel neck, but they interact very differently with the pore surface.
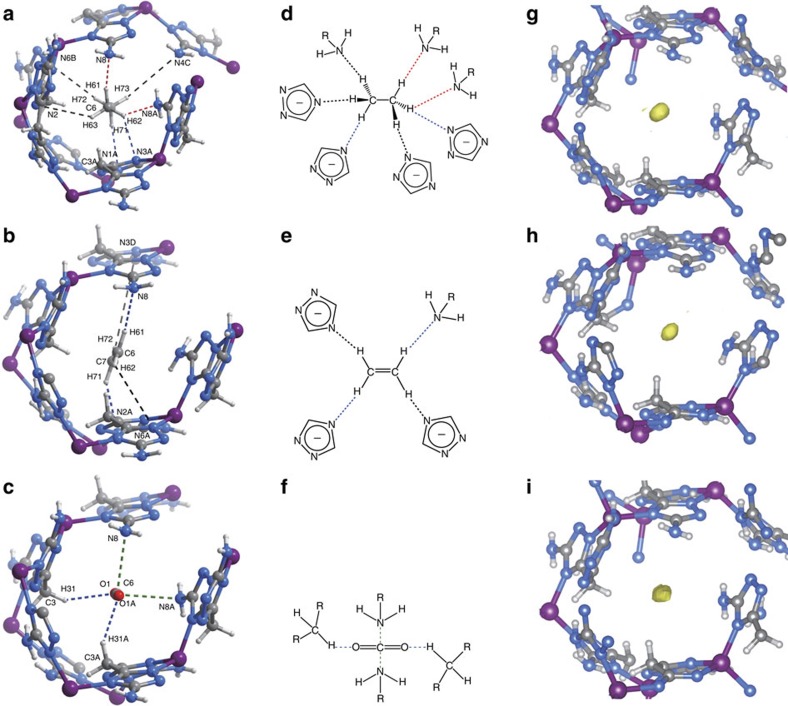
C2H6 forms three strong C–H···N hydrogen bonds and three weak C–H···N electrostatic interactions with MAF-49 (Fig. 3a,d, Supplementary Table 4). Specifically, one methyl group interacts with two amino groups and an coordinated triazolate nitrogen atom of the narrowest channel neck, forming one very short and directional (C6-H61···N8) and one unsymmetrical-bifurcated/three-centred (C6-H62···N8A/N3A) hydrogen bonds, in which the H···N separations (2.15 Å) are much shorter than the sum of van der Waals radii of nitrogen (1.55 Å) and hydrogen (1.20 Å) atoms. The third strong hydrogen bond involves the hydrogen atom (H71) of another methyl group and a coordinated triazolate nitrogen atom (C8-H71···N1A), which is approximately centro-symmetric with the strongest one (C6-H61···N8) about the molecular centre and fits well with the most stable stagger conformation of C2H6. Besides, the less polar part of the pore surface, that is, two methylene groups of the batz2– ligand (C3), fits well with the guest C2H6 molecule in the context of both molecular shape and electrostatic potential.
Figure 3. Host–guest fittings and interactions.
Preferential adsorption sites for (a) C2H6, (b) C2H4 and (c) CO2 in MAF-49 revealed by computational simulations (Zn purple, C dark grey, H light grey, N blue). Schematic representation of the corresponding host–guest interactions for (d) C2H6, (e) C2H4 and (f) CO2. Strong (H···N/O<2.3 Å), weak (2.3 Å<H···N/O<2.8 Å) and almost negligible (H···N/O>2.8 Å) C–H···N interactions are displayed as red, blue and black dashed lines, respectively. 3D electron density maps (Fo–Fc contoured at 0.80 e Å−3 in yellow) of MAF-49 loaded with trace amounts of (g) C2H6, (h) C2H4 and (i) CO2.
For C2H4, two less strong C–H···N hydrogen bonds and two very weak C–H···N electrostatic interactions were observed (Fig. 3b,e and Supplementary Table 4). The strongest one involves one methylene group and one amino group at the narrowest channel neck (C6-H61···N8), while the secondary one involves another methylene group and an uncoordinated triazolate nitrogen atom (C7-H71···N2A), which are also approximately centro-symmetric about the molecular centre. These two C–H···N hydrogen bonds are similar in geometry with the first and third strongest ones for C2H6. However, their H···N separations (2.54–2.65 Å) are obviously longer, albeit C2H4 is more polar (Supplementary Table 4). The cis-configuration of the two electronegative amino groups and two electropositive methylene groups of the narrow channel neck is crucial for the very different host–guest interactions. Obviously, the molecular geometry of C2H4 prevents the two hydrogen atoms of a methylene group to form two strong hydrogen bonds with the narrow channel neck like H2O and C2H6. Furthermore, there is significant steric hindrance and electrostatic repulsion between the two C–H moieties of the two methylene groups from the host channel neck and the guest C2H4 (C3···C6=3.88 Å, Supplementary Fig. 8), which pushes the guest away from the best position for forming a strong C–H···N hydrogen bond with the p-position amino group. Conversely, the methylene group of the host channel neck fits well with the threefold symmetric methyl group of C2H6 (Fig. 3a). For the less strong C–H···N hydrogen bonds and other weak electrostatic attractive interactions, C2H6 also fits much better with the locations of the electronegative nitrogen atoms, as compared with those for C2H4 (Fig. 3 and Supplementary Table 4). These observations indicate that the proper locations of both the electronegative nitrogen atoms and the electropositive methylene groups play critical roles in distinguishing C2H6 and C2H4 with large adsorption enthalpy difference.
In the simulated host–guest structure for CO2, the guest carbon atom locates exactly at the centre of host channel neck, forming short contacts with two free amino groups simultaneously (N···C=2.91 Å), while two oxygen atoms of CO2 interact with two methylene groups, respectively, through weak C–H···O hydrogen bonds (C···O=3.33, H···N=2.45 Å, ∠C–H···N=135°) (Fig. 3c,f, Supplementary Table 4). Although these host–guest interactions seem relatively strong, the channel neck diameter (measured by the separation of the p-position amino and methylene groups, N8···C3 3.60 Å) significantly expanded from the guest-free state (3.13 Å), while it changes little after loading C2H6 (3.18 Å) and C2H4 (3.31 Å), indicating that there is significant steric hindrance and repulsive effect between the CO2 molecule and the host framework, and the very short C···N separation is actually the result enforced by the contraction action of the channel neck (Supplementary Fig. 9). It should be noted that all carbon atoms of C2H6 and C2H4 reside on one side of the quadrangular channel neck, resulting in much smaller steric hindrance effects compared with CO2 (Supplementary Fig. 10).
To confirm the simulation results and directly visualize the host–guest interactions, we carried out SCXRD analyses for MAF-49 loaded with trace amounts of C2H6, C2H4 and CO2 (denoted as MAF-49·C2H6, MAF-49·C2H4 and MAF-49·CO2, respectively, see Supplementary Table 2 and Supplementary Data 3,4,5). Compared with the unit-cell volume of guest-free MAF-49, those of MAF-49·C2H6 and MAF-49·C2H4 showed minor shrinkage (<0.2%), while that of MAF-49·CO2 showed relatively large expansion (1.4%). Further, the N8···C3 separation order of MAF-49, MAF-49·C2H6, MAF-49·C2H4 and MAF-49·CO2 is consistent with that predicted by computational simulations (Supplementary Fig. 10). In all host–guest crystal structures, the residue electron density peaks can be unambiguously found inside the narrow host channel neck (Fig. 3g–i). Furthermore, in the final crystal structures, all guest molecules locate very similar or identical with those predicted by computational simulations (Supplementary Fig. 10).
Mixed gas separation
To investigate the practical separation performance of MAF-49, breakthrough experiments were carried out at 313 K and 1 bar. To evaluate and compare the performances of the materials unambiguously, identical column and flow rate were used, and the parameters of each column were optimized (all columns have similar voidage, Supplementary Table 5). Besides, we used the specific injection amount (mmol g–1) of the mixed gas as the abscissa, meaning that the breakthrough time (s) was not only divided by the adsorbent weight (g) but also multiplied by the flow rate of the injected mixed gas (mmol s–1)40.
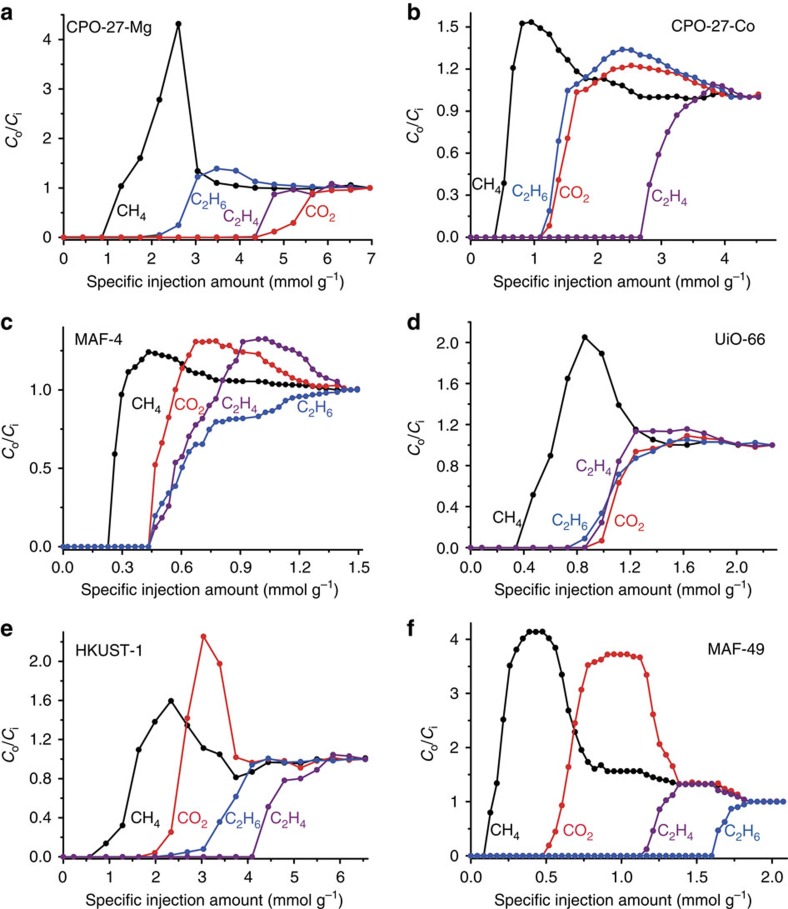
To compare the gas adsorption and separation properties of MAF-49 with other protopytical MOFs, breakthrough experiments using an equimolar C2H6/C2H4/CO2/CH4 mixture injection were carried out (Fig. 4 and Supplementary Figs 11 and 12). For MAF-49, a clean and sharp separation of all four gases was observed, while other MOFs showed much poor separation performances and complicated effluent sequences dependent on their pore surface structures. With transition-metal OMSs, [Cu3(btc)2] (HKUST-1, H3btc=benzene-1,3,5-tricarboxylic acid) and [Co2(dobdc)] (MOF-74-Co/CPO-27-Co, H4dobdc=2,5-dihydroxyl-1,4-benzenedicarboxylic acid) showed binding strength orders C2H4>C2H6>CO2. Because the main-group-metal OMS tends to form strong interaction with the oxygen atom of CO2, [Mg2(dobdc)] (MOF-74-Mg/CPO-27-Mg) showed a binding strength order CO2>C2H4>C2H6. Without pore surface active site, [Zr6O4(OH)4(bdc)12] (UiO-66, H2bdc=1,4-benzenediarboxylic acid) and [Zn(mim)2] (MAF-4 or ZIF-8, Hmim=2-methylimidazole) can barely distinguish the three heavier gases. Nevertheless, the low-polarity adsorbent MAF-4 exhibits slightly better performance compared with UiO-66, and exhibits a separation order similar with that of MAF-49. As expected from the analyses of adsorption isotherms, MAF-49 can also clearly separate two-component C2H4/C2H6, C2H6/CO2 and C2H6/CH4 mixtures (Supplementary Fig. 13). It should be noted that C2H6 could not be detected before its breakthrough points, meaning that C2H6 is efficiently extracted and high-purity C2H4/CO2/CH4 can be obtained directly.
Figure 4. Four-component gas mixture separation.
Breakthrough curves of CH4/CO2/C2H4/C2H6 mixture (1:1:1:1 (vol)) for (a) CPO-27-Mg, (b) CPO-27-Co, (c) MAF-4, (d) UiO-66, (e) HKUST-1 and (f) MAF-49 measured at 313 K and 1 bar. Lines are drawn to guide eyes. Ci and Co are the concentrations of each gas at the inlet and outlet, respectively.
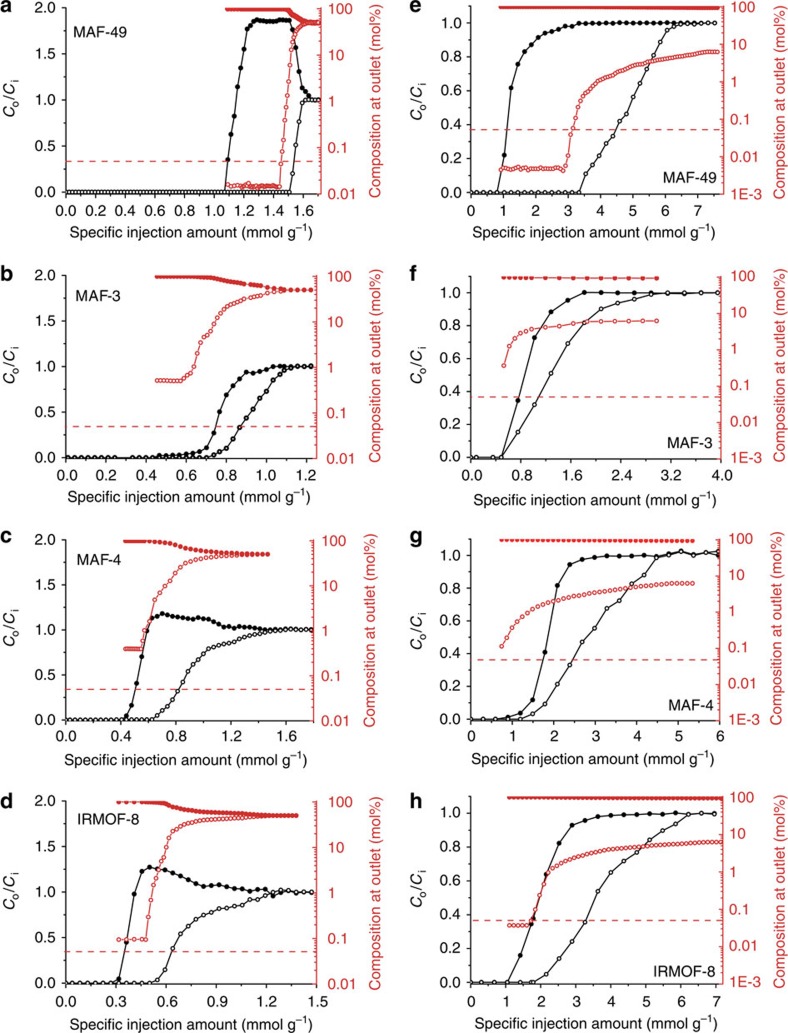
Considering that selective adsorption of C2H6 over C2H4 could be beneficial for purification of C2H4 under fixed-bed adsorption/breakthrough processes, and some hydrophobic MOFs29,30,31,32,33,34,35,36, such as [Zn(bim)2] (MAF-3 or ZIF-7, Hbim=benzimidazole), MAF-4 and [Zn4O(ndc)3] (IRMOF-8, H2ndc=naphthalene-2,6-dicarboxylic acid), were recently reported to exhibit such a property, we compared the C2H4/C2H6 adsorption and separation properties of these MOFs with MAF-49 in detail. Single-component C2H6 adsorption isotherms were measured for MAF-3, MAF-4 and IRMOF-8, which show adsorption enthalpies of 25 kJ mol−1, 18 kJ mol−1 and 30 kJ mol−1, respectively (Fig. 2c and Supplementary Fig. 14), at zero loading, being much lower than that of MAF-49. Although the C2H6 uptake at 1 bar for MAF-49 (38 cm3 g−1) is lower than that of the more porous adsorbents IRMOF-8 (91 cm3 g−1), MAF-4 (48 cm3 g−1) and MAF-3 (41 cm3 g−1), its C2H6 uptake at 0.06 bar (36 cm3 g−1) is ca. 4 times that of IRMOF-8 (9 cm3 g−1), 19 times that of MAF-4 (1.9 cm3 g−1) and 45 times that of MAF-3 (0.8 cm3 g−1) (Fig. 2d). Considering that a purity of 100% is impossible and the C2H6 concentration before its breakthrough point is lower than the detection limit of the conventionally used thermal conductivity detector, the gas stream at the column outlet was analysed with a mass spectrometer (MS). For a 1:1 C2H4/C2H6 mixture injection (Fig. 5a and Supplementary Fig. 15a), the breakthrough points of C2H4 and C2H6 for MAF-49 were observed by thermal conductivity detector at 1.09 and 1.44 mmol g−1, respectively, during which the C2H6 concentration was determined as 0.014–0.016% by MS, corresponding to a C2H4 purity of 99.986–99.984% (Fig. 5a and Supplementary Figs 15a and 16). Under identical conditions, the highest C2H4 purities achieved by MAF-3, MAF-4 and IRMOF-8 are only 99.5%, 99.6% and 99.9% (C2H6 concentrations of 0.5%, 0.4% and 0.1%), respectively, reflecting their much lower C2H6/C2H4 selectivity compared with MAF-49 (Fig. 5b–d, Supplementary Table 6 and Supplementary Figs 15–19). Nevertheless, such C2H4 purities are obviously higher than those reported for C2H4 selective adsorbent materials (99%+)13,17,23,24, which exemplify the feasibility of using C2H6 selective adsorbents for purifying C2H4, because the desired gas can be continuously purified by passing through the column and directly obtained from the first effluents. Indeed, desorbing the MAF-49 column saturated by 1:1 C2H4/C2H6 mixture can give C2H6 with 99%+ purity with a peak value of only 99.7% (Supplementary Fig. 20). A realistic comparison for the C2H4 purification performance of different adsorbents, of relevance to industrial operations, can be obtained by comparing the breakthrough amount of C2H4 (denoted as productivity) with the desired purity in a single breakthrough operation (for the calculation method see Supplementary Methods). For the MAF-49 column, 0.28 mmol g−1 or 0.44 mol l−1 of C2H4 with 99.95%+ purity can be recovered from a 1:1 C2H4/C2H6 mixture injection. For the MAF-3, MAF-4 and IRMOF-8 columns, their productivities are zero because the C2H4 effluents are not pure enough. Even for a C2H4 purity of 99%+, the productivity of the MAF-49 column (0.32 mmol g−1 or 0.47 mol l−1) is still much higher than the others (the largest value is 0.11 mmol g−1 or 0.10 mol l−1) (Supplementary Table 6).
Figure 5. C2H4/C2H6 separation performances.
C2H4/C2H6 (1:1) mixture breakthrough curves of (a) MAF-49, (b) MAF-3, (c) MAF-4 and (d) IRMOF-8, and C2H4/C2H6 (15:1) mixture breakthrough curves of (e) MAF-49, (f) MAF-3, (g) MAF-4 and (h) IRMOF-8 measured at 313 K and 1 bar. Solid symbols: C2H4, Open symbols: C2H6. Lines are drawn to guide eyes. Ci and Co are the concentrations of each gas at the inlet and outlet, respectively. Horizontal red dashed lines highlight C2H6 composition at outlet of 0.05%, that is, C2H4 purity of 99.95%.
Since the C2H6 concentration in C2H4/C2H6 mixtures produced by hydrocarbon cracking is just ca. 5–9% (refs 41, 42, 43), breakthrough experiments using a 15:1 C2H4/C2H6 mixture injection were carried out. The lowest C2H6 impurity or highest C2H4 purities achieved by the MAF-49, MAF-3, MAF-4 and IRMOF-8 columns are decreased to 0.005%, 0.4%, 0.1% and 0.04% or improved to 99.995%, 99.6%, 99.9% and 99.96%, respectively (Fig. 5e–h and Supplementary Figs 21–24). Obviously, using C2H6 selective adsorbents, the C2H4 purity can be increased by lengthening the adsorbent bed (increasing adsorbent amount), which is simpler and more convenient than the C2H4 selective adsorbents13,17,25. For the 15:1 C2H4/C2H6 mixture injection and the C2H4 output purity of 99.95%+, the MAF-49 column gave a C2H4 productivity of 1.68 mmol g−1 or 2.48 mol l−1, which is about 30 or 50 times that of IRMOF-8 (0.06 mmol g−1 or 0.05 mol l−1), in the gravimetric or volumetric point-of-view, respectively (Supplementary Table 7). Note that for C2H4 purification, the adsorbent volume is more practical than its weight because the fixed-bed equipment does not need to move during operation. For lower C2H4 purities such as 99.5%+ and 99%+, the C2H4 productivities of MAF-49 and IRMOF-8 were also increased (Supplementary Tables 6 and 7), because the adsorber needs more time to reach adsorption saturation for the mixture gas containing low-concentration C2H6. Nevertheless, the C2H4 productivity of MAF-49 improved more significantly than for IRMOF-8 at all purity standards (Supplementary Tables 6 and 7), because the former material exhibits much higher C2H6 uptakes at the low pressure region (Fig. 2c). In contrast, the MAF-3 and MAF-4 columns only showed slightly increased C2H4 purities (did not reach 99.95%+) at a C2H4/C2H6 feeding ratio of 15:1 (Fig. 2c), because lengthening the adsorber is not so effective to improve the effluent purity by using adsorbents with weak impurity affinity. For C2H4 purities of 99.5%+ and 99%+, the C2H4 productivities of the MAF-3 and MAF-4 columns obtained by using a 15:1 C2H4/C2H6 input were unexpectedly lower than for the 1:1 C2H4/C2H6 mixture (Supplementary Tables 6 and 7), which can be attributed to the extremely low C2H6 adsorption ability of the adsorbents at the low pressure region. Also, the partial pressures of C2H4 and C2H6 in the 15:1 C2H4/C2H6 mixture are not beneficial for utilizing the differential gate-opening effect of MAF-3 (ref. 44).
Discussion
In summary, we reported a unique adsorbent material showing selective adsorption of C2H6 over more polar analogous molecules such as C2H4 and CO2, which can be useful for extraction of C2H6 from natural gases and particularly valuable for direct production of high-purity C2H4 from C2H4/C2H6 mixtures. The key to this C2H6 selectivity is a combination of multiple hydrogen-bonding acceptors and dipole repulsion groups locating at appropriate positions on the pore surface of a very narrow channel, which not only allows multiple attractive interactions for C2H6 but also enforces C2H4 to adopt a position that can only form fewer and weaker attractive interactions. In short, this work provides not only a new MOF with exceptionally high C2H4 separation/purification performances, but also a new molecular design strategy for developing next-generation adsorbents.
Methods
Materials and general methods
Reagents and solvents were commercially available and were used without further purification, H2batz (ref. 45), MAF-3 (ref. 30), MAF-4 (ref. 46), IRMOF-8 (ref. 47), HKUST-1 (ref. 48), CPO-27-Mg (ref. 49), CPO-27-Co (ref. 49) and UiO-66 (ref. 50) were synthesized according to the literature methods. Elemental analyses (C, H, N) were performed with a Vario EL elemental analyzer. Thermogravimetry analysis was performed under N2 with temperature increased with 5 °C min–1 using a TA-Q50 system. Powder XRD patterns were collected on a Bruker D8 Advance diffractometer (Cu Kα) at room temperature.
Synthesis of [Zn(batz)]·0.5H2O (MAF-49·H2O)
A mixture of Zn(OH)2 (0.100 g, 1.0 mmol), H2batz (0.180 g, 1.0 mmol), aqueous ammonia (25%, 4 ml) and water (4 ml) was stirred for 15 min in air, then transferred and sealed in a 15 ml Teflon reactor, which was heated in an oven at 160 °C for 72 h. The oven was cooled to room temperature at a rate of 5 °C h−1. The resulting colourless block crystals were filtered, washed and dried in air (yield ca. 86%). Anal. Calcd (%) for C5H7N8O0.5Zn: C, 23.77; H, 2.79; N, 44.36. Found: C, 23.97; H, 2.82; N, 44.13. Guest-free MAF-49 was obtained by heating the as-synthesized sample under high vacuum at 150 °C for 12 h.
Single-crystal X-ray crystallography
Diffraction intensities were collected on a Pilatus XtaLAB P300DS diffractometer with graphite-monochromated Mo Kα radiation. Absorption corrections were applied by using the multi-scan programme REQAB. The structures were solved by the direct method and refined with the full-matrix least-squares technique using the SHELXTL programme package. It should be noted that, because the molecular centres of the very short C2H6 and C2H4 molecules do not locate at the centre of the two-fold symmetric host channel neck as predicted by computational simulations, their molecular geometries have to be restricted during refinement of the crystal structures. Also, the positions of their hydrogen atoms were added according to the computational simulation result. Because of disorder and low occupancies of the gas molecules, anisotropic thermal parameters were only applied to all non-hydrogen atoms of the host framework. Crystal data for the compounds were summarized in Supplementary Table 2. Electron density maps were generated using the output from standard SHELXL refinements in a number of ways using WinGX and VESTA 3.0.8.
Gas sorption measurement
The sorption isotherms were measured with an automatic volumetric adsorption apparatus (BELSORP-max). The as-synthesized sample (about 200−300 mg) was placed in the sample tube and dried for 12 h at 320 °C to remove the remnant solvent molecules prior to measurement. CO2 (99.999%), C2H4 (99.95%), CH4 (99.999%) and C2H6 (99.99%) were used for all adsorption isotherm and breakthrough experiments (Supplementary Fig. 25). The temperatures were controlled by a water bath (298, 307 and 316 K).
Additional information
Accession codes: The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1421354-1421358. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
How to cite this article: Liao, P.-Q. et al. Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat. Commun. 6:8697 doi: 10.1038/ncomms9697 (2015).
Supplementary Material
Supplementary Figures 1-25, Supplementary Tables 1-7, Supplementary Methods and Supplementary References
X-ray single-crystal structures of as-synthesized MAF-49 in CIF format
X-ray single-crystal structures of guest-free MAF-49 in CIF format
X-ray single-crystal structures of C2H6 included MAF-49 in CIF format
X-ray single-crystal structures of C2H4 included MAF-49 in CIF format
X-ray single-crystal structures of CO2 included MAF-49 in CIF format
Acknowledgments
This work was supported by the ‘973 Project' (2014CB845602 and 2012CB821706) and NSFC (21225105, 21290173 and 21473260).
Footnotes
Author contributions J.-P.Z. designed the research. P.-Q.L. performed the syntheses and measurements. P.-Q.L. and W.-X.Z. performed the SCXRD analyses. P.-Q.L., J.-P.Z. and X.-M.C. wrote the manuscript.
References
- Matar S. & Hatch L. F. Chemistry of Petrochemical Processes 2nd edn Gulf Publishing Company (2000). [Google Scholar]
- Baker R. W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 41, 1393–1411 (2002). [Google Scholar]
- Horike S. et al. Dense coordination network capable of selective CO2 capture from C1 and C2 hydrocarbons. J. Am. Chem. Soc. 134, 9852–9855 (2012). [DOI] [PubMed] [Google Scholar]
- Zhou J. et al. Removal of C2H4 from a CO2 stream by adsorption: a study in combination of ab initio calculation and experimental approach. Energy Fuels 20, 778–782 (2006). [Google Scholar]
- Liao P.-Q., Zhu A.-X., Zhang W.-X., Zhang J.-P. & Chen X.-M. Self-catalysed aerobic oxidization of organic linker in porous crystal for on-demand regulation of sorption behaviours. Nat. Commun. 6, 6350 (2015). [DOI] [PubMed] [Google Scholar]
- Worrell E., Phylipsen D., Einstein D. & Martin N. Energy Use and Energy Intensity of the U.S. Chemical Industry. Report No. LBNL-44314 (Energy Analysis Department, Environmental Energy Technologies Division, Ernest Orlando Lawrence Berkeley National Laboratory, University of California, Berkeley, California 9472, USA, 2000).
- Ren T., Patel M. & Blok K. Olefins from conventional and heavy feedstocks: energy use in steam cracking and alternative processes. Energy 31, 425–451 (2006). [Google Scholar]
- Safarik D. J. & Eldridge R. B. Olefin/paraffin separations by reactive absorption: a review. Ind. Eng. Chem. Res. 37, 2571–2581 (1998). [Google Scholar]
- Yang R. T. Adsorbents: Fundamentals and Applications 191–230John Wiley & Sons, Inc. (2003). [Google Scholar]
- Tanaka K., Taguchi A., Hao J., Kita H. & Okamoto K. Permeation and separation properties of polyimide membranes to olefins and paraffins. J. Membr. Sci. 121, 197–207 (1996). [Google Scholar]
- Bux H., Chmelik C., Krishna R. & Caro J. Ethene/ethane separation by the MOF membrane ZIF-8: molecular correlation of permeation, adsorption, diffusion. J. Membr. Sci. 369, 284–289 (2011). [Google Scholar]
- Uchida S. et al. Selective sorption of olefins by halogen-substituted macrocation-polyoxometalate porous ionic crystals. Chem. Mater. 24, 325–330 (2012). [Google Scholar]
- Li B. et al. Introduction of π-complexation into porous aromatic framework for highly selective adsorption of ethylene over ethane. J. Am. Chem. Soc. 136, 8654–8660 (2014). [DOI] [PubMed] [Google Scholar]
- Uchida S., Kawamoto R., Tagami H., Nakagawa Y. & Mizuno N. Highly selective sorption of small unsaturated hydrocarbons by nonporous flexible framework with silver ion. J. Am. Chem. Soc. 130, 12370–12376 (2008). [DOI] [PubMed] [Google Scholar]
- Denysenko D., Grzywa M., Jelic J., Reuter K. & Volkmer D. Scorpionate-type coordination in MFU-4l metal–organic frameworks: small-molecule binding and activation upon the thermally activated formation of open metal sites. Angew. Chem. Int. Ed. 53, 5832–5836 (2014). [DOI] [PubMed] [Google Scholar]
- Aguado S., Bergeret G., Daniel C. & Farrusseng D. Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite A. J. Am. Chem. Soc. 134, 14635–14637 (2012). [DOI] [PubMed] [Google Scholar]
- Bloch E. D. et al. Hydrocarbon separations in a metal-organic framework with open iron(ii) coordination sites. Science 335, 1606–1610 (2012). [DOI] [PubMed] [Google Scholar]
- Herm Z. R., Bloch E. D. & Long J. R. Hydrocarbon separations in metal–organic frameworks. Chem. Mater. 26, 323–338 (2014). [Google Scholar]
- Geier S. J. et al. Selective adsorption of ethylene over ethane and propylene over propane in the metal-organic frameworks M2(dobdc) (M=Mg, Mn, Fe, Co, Ni, Zn). Chem. Sci. 4, 2054–2061 (2013). [Google Scholar]
- Zhang Y. et al. Highly selective adsorption of ethylene over ethane in a MOF featuring the combination of open metal site and π-complexation. Chem. Commun. 51, 2714–2717 (2015). [DOI] [PubMed] [Google Scholar]
- Bao Z. et al. Adsorption of ethane, ethylene, propane, and propylene on a magnesium-based metal–organic framework. Langmuir 27, 13554–13562 (2011). [DOI] [PubMed] [Google Scholar]
- Yang S. et al. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal–organic framework. Nat. Chem. 7, 121–129 (2015). [DOI] [PubMed] [Google Scholar]
- He Y., Krishna R. & Chen B. Metal-organic frameworks with potential for energy-efficient adsorptive separation of light hydrocarbons. Energy Environ. Sci. 5, 9107–9120 (2012). [Google Scholar]
- Rege S. U., Padin J. & Yang R. T. Olefin/paraffin separations by adsorption: π-Complexation vs. kinetic separation. AIChE J. 44, 799–809 (1998). [Google Scholar]
- Silva F. A. D. & Rodrigues A. E. Propylene/propane separation by vacuum swing adsorption using 13X zeolite. AIChE J. 47, 341–357 (2001). [Google Scholar]
- Brookhart M., Findlater M., Guironnet D. & Lyons T. W. Synthesis of para-xylene and toluene. US Patent 13/875,610 (2013).
- Vogler D. E. & Sigrist M. W. Near-infrared laser based cavity ringdown spectroscopy for applications in petrochemical industry. Appl. Phys. B 85, 349–354 (2006). [Google Scholar]
- Meyers R. & Meyers R. A. Handbook of Petrochemicals Production Processes McGraw-Hill Prof Med/Tech (2005). [Google Scholar]
- Böhme U. et al. Ethene/ethane and propene/propane separation via the olefin and paraffin selective metal–organic framework adsorbents CPO-27 and ZIF-8. Langmuir 29, 8592–8600 (2013). [DOI] [PubMed] [Google Scholar]
- Gücüyener C., van den Bergh J., Gascon J. & Kapteijn F. Ethane/ethene separation turned on its head: selective ethane adsorption on the metal−organic Framework ZIF-7 through a gate-opening mechanism. J. Am. Chem. Soc. 132, 17704–17706 (2010). [DOI] [PubMed] [Google Scholar]
- Pires J., Pinto M. L. & Saini V. K. Ethane selective IRMOF-8 and its significance in ethane–ethylene separation by adsorption. ACS Appl. Mater. Interfaces 6, 12093–12099 (2014). [DOI] [PubMed] [Google Scholar]
- Cai J. et al. A doubly interpenetrated metal–organic framework with open metal sites and suitable pore sizes for highly selective separation of small hydrocarbons at room temperature. Cryst. Growth Des. 13, 2094–2097 (2013). [Google Scholar]
- He Y. et al. A microporous metal–organic framework for highly selective separation of acetylene, ethylene, and ethane from methane at room temperature. Chem. Eur. J. 18, 613–619 (2012). [DOI] [PubMed] [Google Scholar]
- He Y. et al. A robust doubly interpenetrated metal-organic framework constructed from a novel aromatic tricarboxylate for highly selective separation of small hydrocarbons. Chem. Commun. 48, 6493–6495 (2012). [DOI] [PubMed] [Google Scholar]
- Das M. C. et al. A Zn4O-containing doubly interpenetrated porous metal-organic framework for photocatalytic decomposition of methyl orange. Chem. Commun. 47, 11715–11717 (2011). [DOI] [PubMed] [Google Scholar]
- He Y. et al. A microporous lanthanide-tricarboxylate framework with the potential for purification of natural gas. Chem. Commun. 48, 10856–10858 (2012). [DOI] [PubMed] [Google Scholar]
- Li J.-R., Sculley J. & Zhou H.-C. Metal–organic frameworks for separations. Chem. Rev. 112, 869–932 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang J.-P., Zhang Y.-B., Lin J.-B. & Chen X.-M. Metal azolate frameworks: from crystal engineering to functional materials. Chem. Rev. 112, 1001–1033 (2012). [DOI] [PubMed] [Google Scholar]
- Myers A. L. & Prausnitz J. M. Thermodynamics of mixed-gas adsorption. AIChE. J. 11, 121–126 (1965). [Google Scholar]
- Liao P.-Q. et al. Monodentate hydroxide as a super strong yet reversible active site for CO2 capture from high-humidity flue gas. Energy Environ. Sci. 8, 1011–1016 (2015). [Google Scholar]
- Sedighi M., Keyvanloo K. & Darian T. J. Olefin production from heavy liquid hydrocarbon thermal cracking: kinetics and product distribution. Iran. J. Chem. Chem. Eng. 29, 135–147 (2010). [Google Scholar]
- Marquevich M., Coll R. & Montané D. Steam reforming of sunflower oil for hydrogen production. Ind. Eng. Chem. Res. 39, 2140–2147 (2000). [Google Scholar]
- Guo J., Wu X., Jing S., Zhang Q. & Zheng D. Vapor-liquid equilibrium of ethylene+mesitylene system and process simulation for ethylene recovery. Chin. J. Chem. Eng. 19, 543–548 (2011). [Google Scholar]
- van den Bergh J. et al. Understanding the anomalous alkane selectivity of ZIF-7 in the separation of light alkane/alkene mixtures. Chem. Eur. J. 17, 8832–8840 (2011). [DOI] [PubMed] [Google Scholar]
- Dippold A. A., Feller M. & Klapoetke T. M. 5,5'-dinitrimino-3,3'-methylene-1H-1,2,4-bistriazole - a metal free primary explosive combining excellent thermal stability and high performance. Cent. Eur. J. Energ. Mater. 8, 261–278 (2011). [Google Scholar]
- Huang X.-C., Lin Y.-Y., Zhang J.-P. & Chen X.-M. Ligand-directed strategy for zeolite-type metal–organic frameworks: Zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 45, 1557–1559 (2006). [DOI] [PubMed] [Google Scholar]
- Feldblyum J. I., Wong-Foy A. G. & Matzger A. J. Non-interpenetrated IRMOF-8: synthesis, activation, and gas sorption. Chem. Commun. 48, 9828–9830 (2012). [DOI] [PubMed] [Google Scholar]
- Chui S.S.-Y., Lo S.M.-F., Charmant J. P. H., Orpen A. G. & Williams I. D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283, 1148–1150 (1999). [DOI] [PubMed] [Google Scholar]
- Caskey S. R., Wong-Foy A. G. & Matzger A. J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 130, 10870–10871 (2008). [DOI] [PubMed] [Google Scholar]
- Cavka J. H. et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130, 13850–13851 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-25, Supplementary Tables 1-7, Supplementary Methods and Supplementary References
X-ray single-crystal structures of as-synthesized MAF-49 in CIF format
X-ray single-crystal structures of guest-free MAF-49 in CIF format
X-ray single-crystal structures of C2H6 included MAF-49 in CIF format
X-ray single-crystal structures of C2H4 included MAF-49 in CIF format
X-ray single-crystal structures of CO2 included MAF-49 in CIF format