Abstract
Head and neck malignancies have the propensity to invade nerves. Perineural tumor invasion is common, with some series reporting rates of 30 to 100%. Squamous cell carcinoma and adenoid cystic carcinoma are the most commonly involved tumors. The most commonly involved nerves are the trigeminal (cranial nerve [CN] V) and facial (CN VII) and their branches. Neural spread away from a tumor is encountered less often and usually causes specific symptoms such as pain, muscle weakness, and atrophy, depending on the involved nerves. While clinical symptoms and physical examination may suggest the presence of neural invasion, specific imaging modalities such as fat-suppressed T1-weighted magnetic resonance images, should be utilized to identify perineural tumor spread in its early phases. Perineural tumor spread should be considered and addressed in the treatment planning of patients with head and neck or skull base cancers as it can influence the extent of surgery, and the dosage and fields of radiation therapy. In the current review, we discuss the clinical course of perineural tumor spread and its therapeutic implications.
Keywords: adenoid cystic carcinoma, squamous cell carcinoma, survival, neural, base of skull, head and neck cancer
Introduction
Many head and neck malignancies have the propensity to invade nerves and use them as a conduit of direct spread. While nerves are important routes of tumor spread in head and neck malignancies, the mechanism and impact on survival of perineural spread (PNS) remain to be defined. PNS is a direct form of cancer spread, defined as the presence of gross tumor spread along a nerve, which is at least in part distinct from the main tumor mass. PNS should be distinguished from perineural invasion (PNI), which is a microscopic finding of tumor cells infiltrating nerves.1 2 The presence of PNI does not necessarily imply PNS.
The finding of neural involvement has important implications for patient management. In cases in which surgical margin areas include nerve involved with a burden smaller than microscopic disease, and coincide with a distal-end PNS, a false-negative margin status could be determined. Although neural involvement does not necessarily reflect the potential local cancer aggressiveness or kinetics of disease progression, it is generally accepted that PNI is associated with poorer prognosis and increased risk for locoregional recurrence.3 4 5 6
The aim of this review is to provide an overview of the literature addressing PNS in noncutaneous head and neck malignancies. The diagnosis and surgical considerations in the management of PNS will be presented. We will also briefly present principles of radiotherapy management of PNS that account for anatomical region of treatment, volume, and dose fractionation. The biological mechanism of PNS pathogenesis is beyond the scope of this review and is presented elsewhere in this issue.
Epidemiology
The overall rates of PNS are difficult to estimate as PNS is not always reported as a separate entity from PNI. Also, the incidence of perineural involvement in noncutaneous head and neck cancer varies according to the histopathology and the anatomical location of the primary cancer site. Reported incidence rates of PNI are in the range of 30 to 50% depending on the pathological diagnosis. High incidence rates of PNS are found in patients with squamous cell carcinoma (SCC), adenoid cystic carcinoma (ACC), and salivary duct carcinoma.3 4 7 8 9 Other salivary gland carcinomas (e.g., mucoepidermoid carcinoma and acinic cell carcinoma) have lower rates of perineural involvement.10 11 Melanoma and rhabdomyosarcoma rarely invade nerves. Tumor origin also plays a role in PNS. High rates of PNS are found in tumors of the skull base, skin, and paranasal sinuses, while the oral cavity, tonsil, larynx, and pharynx are less likely to present PNS.1
Protocols of the American College of Pathologists mandate inclusion of PNI status for head and neck malignancies.12 However, while PNI is often clinically silent and may be underreported due to inadequate imaging and limitations of computed tomographic (CT) scan or magnetic resonance imaging (MRI), PNS is almost always symptomatic. This often leads to considerable variation in the rates of neural involvement.13
Patterns of Perineural Spread in Head and Neck Cancer
Tumors can propagate both antegrade and retrograde along nerves. Retrograde PNS toward the brain stem occurs more often, with antegrade spread along branching and interconnecting nerves.4 14 This pattern of progression results in pathologically continuous abnormal regions of the nerve where there is a low-tumor burden.14 15 Radiologically, such pattern of progression along the nerve may appear discontinuous on imaging (Fig. 1).2 The nerves most commonly involved in PNS of the head and neck are the facial and trigeminal nerves.13
Fig. 1.
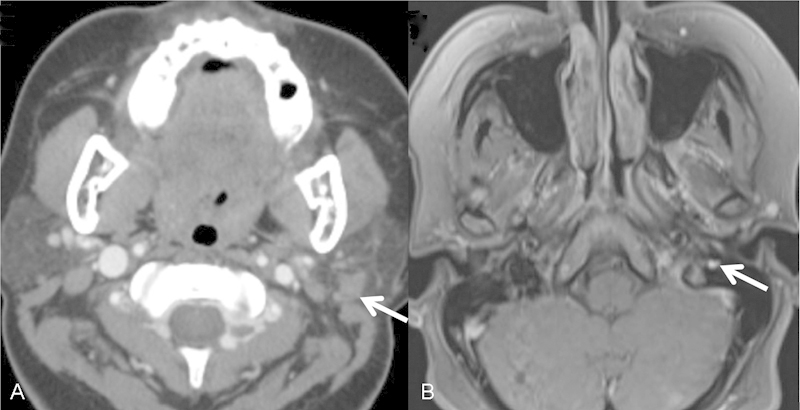
Basaloid salivary neoplasm (A, arrow) with PNS along the mastoidal segment of the facial nerve (B, arrow). Note a tumor in the parotid with an enlarged facial nerve lateral to the jugular bulb demonstrating the discontinuity between the lesion and the nerve. PNS, perineural spread.
Nerve Invasion Occurs within the Perineural and Intraneural Space
The traditional classification of tumors with neural invasion (NI) is based on the involvement of a named or unnamed nerve.16 An alternative classification is based on nerve diameter.17 Histopathological features of NI include PNI, defined as the presence of viable tumor cells in the perineural space; intraneural invasion, defined as the presence of PNI with tumor cells invading into and/or with irregular destruction of the axon of the nerve bundles, as reported previously; and the presence of perineural inflammation (invasion of cancer cells into the perineural space with the presence of lymphocytic infiltration).18 19
Facial Nerve (Cranial Nerve VII)
PNS along the facial nerve occurs most commonly in the parotid cancer (Fig. 1), either primary salivary gland tumors or metastatic lesions. Invasion occurs immediately after its emergence from the stylomastoid foramen where it divides into two main trunks, and then into the five peripheral branches. PNS spread along cranial nerve (CN) VII results in metastasis inside the internal auditory canal20 21, and to a lesser extent, a retrograde spread along CN VII may result in PNS to the geniculate ganglion, the greater superior petrosal nerve, and vidian nerve, which then travels through the pterygoid (vidian) canal to the pterygopalatine fossa (PPF).22
Maxillary Nerve (Cranial Nerve V2)
Maxillary nerve involvement occurs mainly in SCC or ACC of the paranasal sinuses or the oral cavity through invasion to the palatine nerves. Tumor cells then spread in a retrograde fashion to the main trunk of V2 in the PPF (Fig. 2). The maxillary nerve passes through the foramen rotundum on its way to the cavernous sinus (Fig. 3).
Fig. 2.
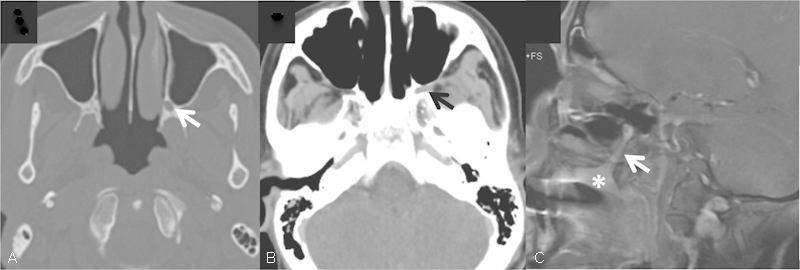
Adenoid cystic carcinoma of the hard palate (C, asterisk) with PNS along the greater palatine nerve to the pterygopalatine fossa (A–C, arrows). Note enlargement of the greater palatine foramen on CT (A, arrow). CT, computed tomography; PNS, perineural spread.
Fig. 3.
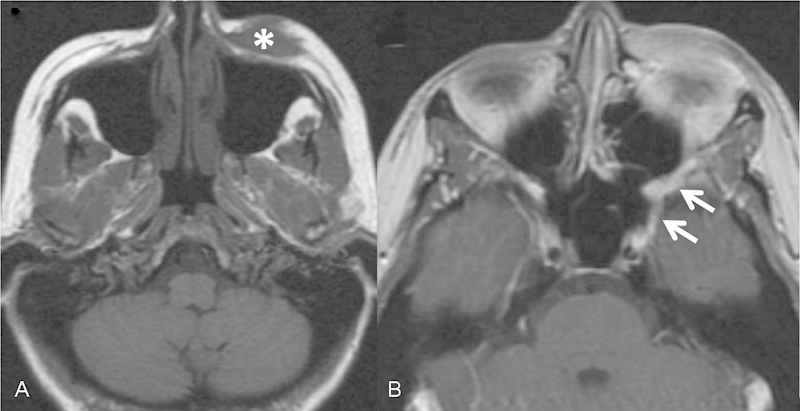
Periorbital melanoma (A, asterisk) with PNS along the infraorbital nerve to the pterygopalatine fossa and foramen rotundum (B, arrows). PNS, perineural spread.
Mandibular Division (Cranial Nerve V3)
Any tumor involving the mandible or the masticator space may spread via the mandibular nerve through the foramen ovale (Fig. 4). In these cases, it is crucial to assess the full extent of PNS radiologically, along V3, from the inferior alveolar nerve back to the Meckel cave (Fig. 4). As well diffuse enhancement of the pterygoid and masseter muscles is consistent with denervation myositic changes from V3 injury.
Fig. 4.
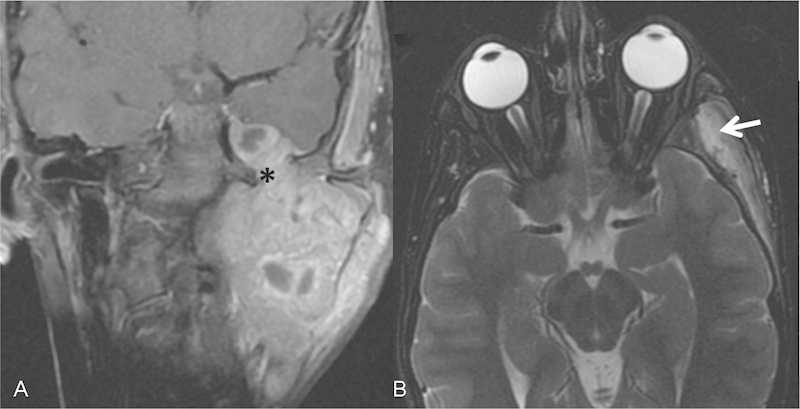
Rhabdomyosarcoma of the masticator space on the left spreading along V3 and the foramen ovale (A, asterisk) to Meckel cave. Note denervation of the temporalis (B, arrow) and masseter muscles visualized with increased T2 signal and enhancement consistent with denervation myositic changes from V3 injury.
The Gasserian ganglion in the Meckel cave is where the three divisions of the trigeminal nerve are united. Involvement of all three divisions of CN V is suggestive of a lesion posterior to the cavernous sinus in the Meckel cave. Nasopharyngeal carcinoma may extend directly into the cavernous sinus and invade the CNs via this route.
Cavernous Sinus
Within the cavernous sinus, V2 and CN VI are affected more commonly than are CN V1, III, and IV.9 Spread into the PPF and masticator space involves V2, and V3, respectively.9 23 Posterolateral spread into the parapharyngeal space may result in involvement of the lower CNs—IX, X, XI, and XII.10 Patients with cavernous sinus involvement usually present with ophtalmoplegia and masticatory muscle wasting (V3), most prominent at the temporal fossa.
The spread between nerves might occur via anastomosing branches. The most common interconnection is between the CN V2 from the pterygopalatine ganglion via the vidian nerve in a retrograde manner to the geniculate ganglion and CN VII. Alternatively, parasympathetic contributories to the auriculotemporal nerve, originating from CN VII, connect CN V to the facial nerve in the region of the stylomastoid foramen.11
Signs and Symptoms
Most cases of PNS are initially asymptomatic.15 Symptoms reflect the involved nerves. Since the most common CNs involved are the trigeminal and facial nerves, the symptoms most noticed by patients are sensory including pain, paresthesia, numbness, and formication. Painless sensory neuropathy is suggestive of PNS.24 Motor weakness of the muscles of mastication or facial expression may occur with more prolonged or extensive PNS. In contrast to other causes of facial weakness, involvement of selective branches of the nerve, and progressive or relapsing course are indicative of PNS.
Involvement of the lower CNs can also cause motor weakness with palatal deviation, vocal cord paralysis, shoulder weakness, or tongue deviation. Muscle atrophy with loss of masseter muscle bulk, or glossal atrophy is apparent in longstanding cases.
Diagnostic Imaging
Better soft tissue contrast and reduced artifacts from dental hardware make MRI superior to CT scanning for detecting PNS.13 14 25 MRI has a sensitivity of 95 to 100% for detection of PNS, as well, its accuracy in predicting the entire extent of disease was reported up to 83%.15 26 27 Some authors stressed the importance of radiological technique and diligence in searching for PNS especially in asymptomatic patients.15 28 29 Some areas are best depicted on a particular plane; for example, foramen ovale and V3, and also involvement of the Meckel cave, may be best visualized on coronal images.14
Characteristic features of PNS on CT scan include: (1) widening of foramina, (2) convexity of the cavernous sinus wall, and (3) the presence of soft tissue or (4) enhancement within the Meckel cave.1 13 14 In some cases, nerve might appear thickened on CT scan but CT is less sensitive than MRI (Fig. 2). Masticator muscle atrophy occurring with chronic mandibular division (V3) denervation can be appreciated on both MRI and CT scan. In such cases, increased intensity on T2-weighted images and abnormal enhancement in the muscle after acute or subacute denervation is present (Fig. 4).25
Some conditions can mimic PNS such as chronic infections (fungal and viral neuritis), sarcoidosis involving the skull base and meningiomas extending into, and widening adjacent foramina.14 25 Also, normal enhancement of venous plexus along the nerve might be difficult to differentiate from PNS.
Perineural Spread and Survival
Neural involvement in the forms of PNI and PNS has prognostic implications, and thus therapeutic significance. While PNS can be detected preoperatively and guide surgical planning, PNI, as a pathological feature, is often appreciated only postoperatively. Hence, the association between PNI and survival was extensively studied as a prognostic factor. PNI is increasingly recognized in several human tumors as an adverse prognostic factor, associated with increased risk of locoregional recurrence, especially in the presence of intraneural invasion. Furthermore, in some tumor sites, PNI has been found to be associated with an increased risk of distant metastasis.4 Still, the evidence for this is conflicting.4 30 31
Squamous Cell Carcinoma
In head and neck SCC, PNI is generally considered as an adverse feature and has been associated with an increased risk of locoregional recurrence. Table 1 summarizes the data regarding the prognostic significance of PNI in head and neck SCC.6 7 32 33 34 35 36 37 38 The evidence is conflicting regarding the influence of PNI on disease control and survival.37 39 40 41
Table 1. Outcome measurements reported in the studies of squamous cell carcinoma with PNI included in the review.
| Author | Year | n | Site | PNI (%) | OS | DSS | DM | LRR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period (y) | % PNI + /%PNI− | p Value | Period (y) | % PNI + /%PNI− | p Value | Period (y) | % PNI + /%PNI− | p Value | Period (y) | % PNI + /%PNI− | p Value | |||||
| O'Brien et al | 1986 | 167 | T | 5 (6) | 10 | 40/77 | 0.002 | 5 | 80/30 | 0.003 | ||||||
| Fagan et al | 1998 | 142 | OC,OP, HP, L | 74 (52) | 54/25a | 0.001 | NS | 23/9 | 0.02 | |||||||
| Sutton et al | 2003 | 200 | OC | 78 (39) | NS | |||||||||||
| Rahima et al | 2004 | 101 | OC, OP | 26 (26) | 57/95 | < 0.0001 | n/a | 19/3 | 0.021 | n/a | 23/3 | 0.005 | ||||
| Kurtz et al | 2005 | 40 | OC | 33 (82) | NS | |||||||||||
| Brandwein-Gensler et al | 2005 | 289 | OC | 72 (25) | 0.002b/0.039c | 0.005c | ||||||||||
| González-García et al | 2009 | 300 | OC | 79 (26) | 16/15 | NS | ||||||||||
| Tai et al | 2012 | 190 | T | 59(31) | 5 | 72/89 | < 0.001 | 5 | 76/92 | 0.003 | ||||||
Abbreviations: DFS, disease free survival; DM, distant metastasis; HP, hypopharynx; L, larynx; LRR, locoregional recurrence; n/a, not available; NS, not significant; OC, oral cavity; OP, oropharynx; OS, overall survival; PNI, perineural invasion; PS, paranasal sinuses; T, tongue.
Disease-specific mortality.
Nerve diameter < 1 mm.
Nerve diameter > 1 mm.
Various implications for PNS as a prognostic factor have been suggested, according to different subsites within the head and neck. For instance, a significant prognostic effect was found for regional recurrence in early-oral tongue SCC.38 Nerve size was also found to correlate with outcome. Patients with PNI of nerves > 1 mm in the oral cavity had significantly increased local recurrence and decreased overall survival rates.6 While PNS along minor nerves was reported not to be associated with outcome, PNI of small nerves was reported to be associated with an increased risk of local recurrence and decreased disease-specific survival.33 Although most series do not match cohorts according to adjuvant treatment regimens, most head and neck oncologists consider the presence of PNI in the absence of other adverse tumor features to be a relative indication for adjuvant radiotherapy.42 43
There is conflicting evidence regarding the role of concurrent chemoradiation treatment for PNS. Some authors reported improved 5-year locoregional control in patients with PNI treated with concurrent chemoradiation treatment, compared with those without PNI.44 However, other sentinel studies (e.g., European Organisation for Research and Treatment of Cancer [EORTC] 22931 and the Radiation Therapy Oncology Group [RTOG] 9501) showed that the beneficial effect of concurrent cisplatin was for patients with positive margins or nodal extracapsular extension and not PNI.45 46
There is currently no level-I evidence to support the addition of systemic therapy based on the presence of PNI. PNI is identified as a factor associated with an “intermediate” recurrence risk in an ongoing phase III trial (RTOG 0920) that examines the benefit of the addition of concurrent cetuximab to postoperative radiotherapy in patients at intermediate risk (∼30% or less) for postoperative recurrence.47 48
Salivary Gland Cancers
Controversy exists regarding the role of PNI as a prognostic factor in ACC and other salivary gland tumors. The evaluation of patients with salivary gland malignancies is hampered by their rarity and indolent course. Most studies are limited to single institutes and most series are small.3 49 Table 2 summarizes the data regarding the prognostic significance of PNI in head and neck ACC.9 16 50 51 52 53 54 55 56 57 58 59 60 61 Spiro showed that PNI did not correlate with distant metastases or survival rates.62 However, other studies have suggested that specific PNI features might play a role in either locoregional recurrence or overall survival; for instance, some authors reported that PNS in a major or named nerve is significant for locoregional recurrence.16 Still, these conclusions are limited by small sample size, short follow-up, mixed salivary gland pathologies, and variable histological sampling and PNI reporting. Furthermore, in many of these retrospective trials, preoperative MRI is missing and limits the ability to identify the presence of macroscopic PNS. This factor limits the interpretation of these studies.
Table 2. Outcome measures reported in the studies of adenoid cystic carcinoma included in the review.
| Author | Year | n | Site | PNI (%) | OS | DFS | DM | LRR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period (y) | % PNI + /%PNI− | p Value | Period (y) | % PNI + /%PNI− | p Value | Period (y) | % PNI + /%PNI− | p Value | Period (y) | % PNI + /%PNI− | p Value | |||||
| Vrielinck et al63 | 1988 | 37 | Mix | 19 (51) | 10 | 37/94 | < 0.001 | 50/0 | 0.0007 | 57.9/22.2 | n/a | |||||
| Garden et al | 1995 | 198 | Mix | 136 (69) | 10 | 45/73 | 0.002 | 60/39 | < 0.001 | 10 | 89/81 | 0.28 | ||||
| Huang et al | 1997 | 91 | Mix | 57 (63) | 10 | 29/60 | < 0.01 | |||||||||
| Fordice et al | 1999 | 160 | Mix | 79 (49) | 0.04a | |||||||||||
| Kim et al | 1999 | 19 | Paranasal | 5 (29) | 10 | 20/55 | 0.01 | |||||||||
| Chummun et al | 2001 | 45 | Mix | 23 (51) | 5 | 68/93.5 | < 0.05 | |||||||||
| Khan et al | 2001 | 68 | Mix | 3 (32) | NS | |||||||||||
| Kokemueller et al | 2004 | 74 | Mix | 32 (43) | 10 | 77/23 | < 0.001 | |||||||||
| Mendenhall et al | 2004 | 101 | Mix | 32 (32) | 0.0011 | 5 | 61/92 | 0.0015 | 5 | 0.0055 | ||||||
| Rapidis et al | 2005 | 22 | Major and paranasal | 18 (81) | 0.0044 | NS | ||||||||||
| Chen et al | 2006 | 140 | Mix | 82 (59) | 10 | 87/69 | 0.04 | |||||||||
| Agarwal et al | 2008 | 76 | Minor–oral | 24 (32) | 5 | 23/80 | 0.053 | 5 | 29/97 | 0.003 | ||||||
| Gomez et al | 2008 | 59 | Mix | 42 (71) | NS | NS | NS | NS | ||||||||
| Amit et al | 2014 | 495 | Mix | 239 (48) | 5 | 4.8b | 0.0006 | |||||||||
Abbreviations: DFS, disease free survival; DM, distant metastasis; LRR, loco-regional recurrence; n/a, not available; NS, not significant; OS, overall survival; PNI, perineural invasion.
Note: Mix—major salivary glands and Minor salivary glands (oral cavity and paranasal sinuses).
Only named nerves.
Hazard ratio of intraneural invasion—cancer cells in endoneurium.
Some tumors such as polymorphous low-grade adenocarcinomas demonstrate a mild propensity for PNS. These tumors have an indolent natural history with a low risk of late-local recurrence after surgical resection with adequate margins.64 65 66 However, high-grade salivary gland tumors, such as salivary duct carcinomas and adenocarcinoma not otherwise specified, have higher risk of PNS and recur at the skull base.67 Thus, specific histopathology and tumor grade should be considered before adjuvant therapy administration.
Although PNI is considered by most authors not to be associated with outcome, we previously showed that one-third of patients with ACC have invasion within the neural bundles; and that neither PNI by itself or PNI were predictors of outcome; however, intraneural evidence of cancer invasion was identified as an independent histological sign of poor survival.61
For staging purposes, PNS is not a specified staging entity for clinical or pathological primary tumor T-classification. Still, PNS is relevant for anatomical extent (e.g., skull base) or functional impairment (e.g., facial nerve palsy).68 While the pathological finding of PNI should be considered as a relative indication for postoperative radiotherapy of some salivary gland tumors, the presence of PNS should not preclude consideration of curative therapies.
Perineural Spread and Treatment Considerations
The surgical management of PNS requires careful preoperative assessment and planning to achieve clear surgical margins. Surgical resection must follow the path of the nerve. Considerations regarding nerve preservation are discussed elsewhere in this issue. As a general rule, if oncological resection can be achieved safely, functioning nerves should be preserved; and nerves with functional involvement should be sacrificed and resected with the tumor. Skull base involvement is often seen with PNS and requires a multidisciplinary approach. Most patients require either preoperative or postoperative adjuvant radiotherapy with or without chemotherapy. In one series, 60% of the patients required facial nerve resection. Still, the low overall complication rate (6%, stroke, 3% cerebrospinal fluid leak, and 1.5% hematoma formation) and acceptable 2- and 5-year disease-free intervals of 68 and 50%, respectively, emphasize the curability of these patients.69 PNS almost always presents as a nonfunctioning nerve amenable for sacrifice; however, when PNS is unresectable, targeted radiation treatment of the nerve should be considered.
The radiation planning target volume should consider macroscopic PNS seen on imaging with the same dose as the primary epicenter, that is, 66 to 70 Gy.54 60 For cases with high risk of microscopic PNI and no macroscopic disease on imaging, adjuvant treatment with a dose range of 50 to 66 Gy in 1.8 to 2 Gy fractions is appropriate.70 Accordingly, inclusion of the skull base in the irradiated volumes resulted in a trend for lower recurrence compared with treatment in which it was not included.71 Some authors suggested that in cases with ACC involvement of a named nerve, clinical target volume should include the course of the nerve back to the skull base.16 62 Inclusion of the brainstem should possibly be required in the presence of macroscopic disease or positive surgical margins in the skull base and neural foramens. Also, antegrade CN involvement should be considered in clinical target volume planning, to reduce risks of recurrence and failure.
Modern radiotherapy techniques, such as intensity-modulated radiotherapy (IMRT) are highly accepted and broadly available.72 73 A comprehensive review of radiation treatment for PNS is beyond the scope of this article and described elsewhere in this issue. Such techniques enable delivery of therapeutic dose to particular CN pathways in a conformal manner, allowing for very complex volume irradiation while sparing adjacent vital structures (Fig. 5). While good prospective data comparing IMRT and conformal three-dimensional (3D) radiotherapy is lacking, the wide use of IMRT in clinics has produced ample data, and clinicians are urged to use it in most of the cases. The role of systemic therapies in the management of PNS remains unclear.
Fig. 5.
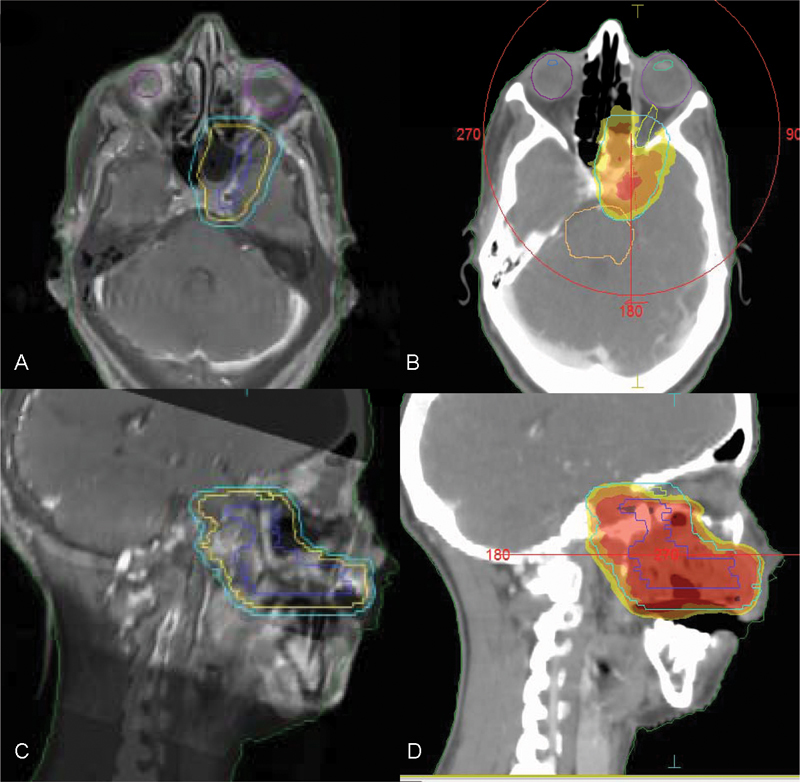
IMRT planning according to preoperative magnetic resonance image (A, axial and C, sagittal) and postoperative simulation computed tomography (B, axial and D, sagittal). Irradiation fields include the hard palate and the course of V2 along the greater palatine canal to the medial cranial fossa, including the foramen rotundum and the vidian canal. Isodose line distribution of 60 Gy (100% of the prescription dose, red), 57 Gy (95% of the prescription dose, orange), and 55 Gy (the tolerance of the optic nerves and optic chiasma). IMRT, intensity-modulated radiotherapy.
Conclusion
The presence of PNS in head and neck cancer poses diagnostic, prognostic, and management challenges for the multidisciplinary team. The existence of PNS should be evaluated. MRI plays a pivotal role in its radiological identification. While cure and long-term disease control should be desired, adverse tumor features, patient comorbidities and performance status alongside potential treatment toxicities should be considered for the recommendation of adjuvant therapy for PNS. Goals of therapy should include minimizing treatment-related toxicities and maintaining patient quality of life. These are usually achieved by optimal oncological surgery and advanced radiation therapy. Clinically, there is no targeted therapy indicated for PNS or PNI in head and neck cancer. While radiotherapy planning for skull base PNS is complex, 3D radiation delivery enables accurate treatment of complex targets in close proximity to nearby critical structures. However, patterns of failure, quality of life, and long-term treatment-related morbidity remain to be assessed.
Acknowledgment
The authors would like to thank Cindy Cohen for her editorial assistance.
References
- 1.Ginsberg L E. Imaging of perineural tumor spread in head and neck cancer. Semin Ultrasound CT MR. 1999;20(3):175–186. doi: 10.1016/s0887-2171(99)90018-5. [DOI] [PubMed] [Google Scholar]
- 2.Nemec S F, Herneth A M, Czerny C. Perineural tumor spread in malignant head and neck tumors. Top Magn Reson Imaging. 2007;18(6):467–471. doi: 10.1097/rmr.0b013e3181645a0d. [DOI] [PubMed] [Google Scholar]
- 3.Barrett A W, Speight P M. Perineural invasion in adenoid cystic carcinoma of the salivary glands: a valid prognostic indicator? Oral Oncol. 2009;45(11):936–940. doi: 10.1016/j.oraloncology.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Liebig C, Ayala G, Wilks J A, Berger D H, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 5.Soo K C, Carter R L, O'Brien C J, Barr L, Bliss J M, Shaw H J. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96(10):1145–1148. doi: 10.1288/00005537-198610000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Brandwein-Gensler M, Teixeira M S, Lewis C M. et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 7.Carter R L, Foster C S, Dinsdale E A, Pittam M R. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36(3):269–275. doi: 10.1136/jcp.36.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabili V, Tan J W, Bhuta S, Sercarz J A, Head C S. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29(10):907–912. doi: 10.1002/hed.20614. [DOI] [PubMed] [Google Scholar]
- 9.Chong V F, Fan Y F, Khoo J B. Nasopharyngeal carcinoma with intracranial spread: CT and MR characteristics. J Comput Assist Tomogr. 1996;20(4):563–569. doi: 10.1097/00004728-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Chong V F, Fan Y F. Hypoglossal nerve palsy in nasopharyngeal carcinoma. Eur Radiol. 1998;8(6):939–945. doi: 10.1007/s003300050492. [DOI] [PubMed] [Google Scholar]
- 11.Schmalfuss I M, Tart R P, Mukherji S, Mancuso A A. Perineural tumor spread along the auriculotemporal nerve. AJNR Am J Neuroradiol. 2002;23(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- 12.Kang H P, Devine L J, Piccoli A L, Seethala R R, Amin W, Parwani A V. Usefulness of a synoptic data tool for reporting of head and neck neoplasms based on the College of American Pathologists cancer checklists. Am J Clin Pathol. 2009;132(4):521–530. doi: 10.1309/AJCPQZXR1NMF2VDX. [DOI] [PubMed] [Google Scholar]
- 13.Caldemeyer K S Mathews V P Righi P D Smith R R Imaging features and clinical significance of perineural spread or extension of head and neck tumors Radiographics 199818197–110., quiz 147 [DOI] [PubMed] [Google Scholar]
- 14.Parker G D, Harnsberger H R. Clinical-radiologic issues in perineural tumor spread of malignant diseases of the extracranial head and neck. Radiographics. 1991;11(3):383–399. doi: 10.1148/radiographics.11.3.1852933. [DOI] [PubMed] [Google Scholar]
- 15.Nemzek W R, Hecht S, Gandour-Edwards R, Donald P, McKennan K. Perineural spread of head and neck tumors: how accurate is MR imaging? AJNR Am J Neuroradiol. 1998;19(4):701–706. [PMC free article] [PubMed] [Google Scholar]
- 16.Garden A S, Weber R S, Morrison W H, Ang K K, Peters L J. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32(3):619–626. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 17.Gil Z, Carlson D L, Gupta A. et al. Patterns and incidence of neural invasion in patients with cancers of the paranasal sinuses. Arch Otolaryngol Head Neck Surg. 2009;135(2):173–179. doi: 10.1001/archoto.2008.525. [DOI] [PubMed] [Google Scholar]
- 18.Mitsunaga S, Hasebe T, Kinoshita T. et al. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol. 2007;31(11):1636–1644. doi: 10.1097/PAS.0b013e318065bfe6. [DOI] [PubMed] [Google Scholar]
- 19.de Matos F R, Lima Ed, Queiroz L M, da Silveira E J. Analysis of inflammatory infiltrate, perineural invasion, and risk score can indicate concurrent metastasis in squamous cell carcinoma of the tongue. J Oral Maxillofac Surg. 2012;70(7):1703–1710. doi: 10.1016/j.joms.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Yildiz O, Buyuktas D, Ekiz E, Selcukbiricik F, Papila I, Papila C. Facial nerve palsy: an unusual presenting feature of small cell lung cancer. Case Rep Oncol. 2011;4(1):35–38. doi: 10.1159/000324182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutsumi T, Nakajima N, Hirose T, Watanabe K. Total-length invasion of the facial nerve by parotid carcinoma ex pleomorphic adenoma. Auris Nasus Larynx. 2009;36(5):618–622. doi: 10.1016/j.anl.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Ojiri H. Perineural spread in head and neck malignancies. Radiat Med. 2006;24(1):1–8. doi: 10.1007/BF02489982. [DOI] [PubMed] [Google Scholar]
- 23.Yu E, O'Sullivan B, Kim J, Siu L, Bartlett E. Magnetic resonance imaging of nasopharyngeal carcinoma. Expert Rev Anticancer Ther. 2010;10(3):365–375. doi: 10.1586/era.10.9. [DOI] [PubMed] [Google Scholar]
- 24.Catalano P J, Sen C, Biller H F. Cranial neuropathy secondary to perineural spread of cutaneous malignancies. Am J Otol. 1995;16(6):772–777. [PubMed] [Google Scholar]
- 25.Gandhi D, Gujar S, Mukherji S K. Magnetic resonance imaging of perineural spread of head and neck malignancies. Top Magn Reson Imaging. 2004;15(2):79–85. doi: 10.1097/01.rmr.0000130601.57619.bd. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi M R, Panizza B, Kennedy D. Detecting and defining the anatomic extent of large nerve perineural spread of malignancy: comparing “targeted” MRI with the histologic findings following surgery. Head Neck. 2011;33(4):469–475. doi: 10.1002/hed.21470. [DOI] [PubMed] [Google Scholar]
- 27.Baulch J, Gandhi M, Sommerville J, Panizza B. 3T MRI evaluation of large nerve perineural spread of head and neck cancers. J Med Imaging Radiat Oncol. 2015;59(5):578–585. doi: 10.1111/1754-9485.12338. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen B D, Roarke M C. Salivary duct carcinoma with perineural spread to facial canal: F-18 FDG PET/CT detection. Clin Nucl Med. 2008;33(12):925–928. doi: 10.1097/RLU.0b013e31818c4e38. [DOI] [PubMed] [Google Scholar]
- 29.Curtin H D. Detection of perineural spread: fat suppression versus no fat suppression. AJNR Am J Neuroradiol. 2004;25(1):1–3. [PMC free article] [PubMed] [Google Scholar]
- 30.Binmadi N O, Basile J R. Perineural invasion in oral squamous cell carcinoma: a discussion of significance and review of the literature. Oral Oncol. 2011;47(11):1005–1010. doi: 10.1016/j.oraloncology.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Woolgar J A. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2006;42(3):229–239. doi: 10.1016/j.oraloncology.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien C J, Lahr C J, Soong S J. et al. Surgical treatment of early-stage carcinoma of the oral tongue—wound adjuvant treatment be beneficial? Head Neck Surg. 1986;8(6):401–408. doi: 10.1002/hed.2890080603. [DOI] [PubMed] [Google Scholar]
- 33.Fagan J J, Collins B, Barnes L, D'Amico F, Myers E N, Johnson J T. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124(6):637–640. doi: 10.1001/archotol.124.6.637. [DOI] [PubMed] [Google Scholar]
- 34.Sutton D N, Brown J S, Rogers S N, Vaughan E D, Woolgar J A. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2003;32(1):30–34. doi: 10.1054/ijom.2002.0313. [DOI] [PubMed] [Google Scholar]
- 35.Rahima B, Shingaki S, Nagata M, Saito C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(4):423–431. doi: 10.1016/j.tripleo.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Kurtz K A, Hoffman H T, Zimmerman M B, Robinson R A. Perineural and vascular invasion in oral cavity squamous carcinoma: increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med. 2005;129(3):354–359. doi: 10.5858/2005-129-354-PAVIIO. [DOI] [PubMed] [Google Scholar]
- 37.González-García R, Naval-Gías L, Román-Romero L, Sastre-Pérez J, Rodríguez-Campo F J. Local recurrences and second primary tumors from squamous cell carcinoma of the oral cavity: a retrospective analytic study of 500 patients. Head Neck. 2009;31(9):1168–1180. doi: 10.1002/hed.21088. [DOI] [PubMed] [Google Scholar]
- 38.Tai S K, Li W Y, Chu P Y. et al. Risks and clinical implications of perineural invasion in T1-2 oral tongue squamous cell carcinoma. Head Neck. 2012;34(7):994–1001. doi: 10.1002/hed.21846. [DOI] [PubMed] [Google Scholar]
- 39.Hinerman R W, Mendenhall W M, Morris C G, Amdur R J, Werning J W, Villaret D B. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35-year experience. Head Neck. 2004;26(11):984–994. doi: 10.1002/hed.20091. [DOI] [PubMed] [Google Scholar]
- 40.Parsons J T, Mendenhall W M, Stringer S P, Cassisi N J, Million R R. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys. 1997;39(1):137–148. doi: 10.1016/s0360-3016(97)00152-1. [DOI] [PubMed] [Google Scholar]
- 41.Liao C T, Chang J T, Wang H M. et al. Does adjuvant radiation therapy improve outcomes in pT1-3N0 oral cavity cancer with tumor-free margins and perineural invasion? Int J Radiat Oncol Biol Phys. 2008;71(2):371–376. doi: 10.1016/j.ijrobp.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Pfister D G, Ang K K, Brizel D M. et al. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11(8):917–923. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- 43.Pfister D G Ang K Brockstein B et al. NCCN practice guidelines for head and neck cancers Oncology (Williston Park) 200014(11A):163–194. [PubMed] [Google Scholar]
- 44.Langendijk J A, Slotman B J, van der Waal I, Doornaert P, Berkof J, Leemans C R. Risk-group definition by recursive partitioning analysis of patients with squamous cell head and neck carcinoma treated with surgery and postoperative radiotherapy. Cancer. 2005;104(7):1408–1417. doi: 10.1002/cncr.21340. [DOI] [PubMed] [Google Scholar]
- 45.Bernier J, Cooper J S, Pajak T F. et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 46.Cooper J S, Pajak T F, Forastiere A A. et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 47.Ang K K, Trotti A, Brown B W. et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal D I, Liu L, Lee J H. et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24(2):115–126. doi: 10.1002/hed.10038. [DOI] [PubMed] [Google Scholar]
- 49.Gomez D R, Katabi N, Zhung J. et al. Clinical and pathologic prognostic features in acinic cell carcinoma of the parotid gland. Cancer. 2009;115(10):2128–2137. doi: 10.1002/cncr.24259. [DOI] [PubMed] [Google Scholar]
- 50.Huang M, Ma D, Sun K, Yu G, Guo C, Gao F. Factors influencing survival rate in adenoid cystic carcinoma of the salivary glands. Int J Oral Maxillofac Surg. 1997;26(6):435–439. doi: 10.1016/s0901-5027(97)80008-2. [DOI] [PubMed] [Google Scholar]
- 51.Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125(2):149–152. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 52.Kim G E, Park H C, Keum K C. et al. Adenoid cystic carcinoma of the maxillary antrum. Am J Otolaryngol. 1999;20(2):77–84. doi: 10.1016/s0196-0709(99)90015-7. [DOI] [PubMed] [Google Scholar]
- 53.Chummun S, McLean N R, Kelly C G. et al. Adenoid cystic carcinoma of the head and neck. Br J Plast Surg. 2001;54(6):476–480. doi: 10.1054/bjps.2001.3636. [DOI] [PubMed] [Google Scholar]
- 54.Khan A J, DiGiovanna M P, Ross D A. et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96(3):149–158. doi: 10.1002/ijc.1013. [DOI] [PubMed] [Google Scholar]
- 55.Kokemueller H, Eckardt A, Brachvogel P, Hausamen J E. Adenoid cystic carcinoma of the head and neck—a 20 years experience. Int J Oral Maxillofac Surg. 2004;33(1):25–31. doi: 10.1054/ijom.2003.0448. [DOI] [PubMed] [Google Scholar]
- 56.Mendenhall W M, Morris C G, Amdur R J, Werning J W, Hinerman R W, Villaret D B. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26(2):154–162. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 57.Rapidis A D, Givalos N, Gakiopoulou H. et al. Adenoid cystic carcinoma of the head and neck. Clinicopathological analysis of 23 patients and review of the literature. Oral Oncol. 2005;41(3):328–335. doi: 10.1016/j.oraloncology.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Chen A M, Bucci M K, Weinberg V. et al. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: prognostic features of recurrence. Int J Radiat Oncol Biol Phys. 2006;66(1):152–159. doi: 10.1016/j.ijrobp.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 59.Agarwal J P, Jain S, Gupta T. et al. Intraoral adenoid cystic carcinoma: prognostic factors and outcome. Oral Oncol. 2008;44(10):986–993. doi: 10.1016/j.oraloncology.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 60.Gomez D R, Hoppe B S, Wolden S L. et al. Outcomes and prognostic variables in adenoid cystic carcinoma of the head and neck: a recent experience. Int J Radiat Oncol Biol Phys. 2008;70(5):1365–1372. doi: 10.1016/j.ijrobp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 61.Amit M, Binenbaum Y, Trejo-Leider L. et al. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck. 2015;37(7):1038–1045. doi: 10.1002/hed.23710. [DOI] [PubMed] [Google Scholar]
- 62.Spiro R H. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174(5):495–498. doi: 10.1016/s0002-9610(97)00153-0. [DOI] [PubMed] [Google Scholar]
- 63.Vrielinck L JG, Ostyn F, Van Damme B. et al. The significance of perineural spread in adenoid cystic carcinoma of the major and minor salivary glands. Int J Oral Maxillofac Surg. 1988;17:190–193. doi: 10.1016/s0901-5027(88)80030-4. [DOI] [PubMed] [Google Scholar]
- 64.Pogodzinski M S, Sabri A N, Lewis J E, Olsen K D. Retrospective study and review of polymorphous low-grade adenocarcinoma. Laryngoscope. 2006;116(12):2145–2149. doi: 10.1097/01.mlg.0000243200.35033.2b. [DOI] [PubMed] [Google Scholar]
- 65.Schwarz S, Müller M, Ettl T, Stockmann P, Zenk J, Agaimy A. Morphological heterogeneity of oral salivary gland carcinomas: a clinicopathologic study of 41 cases with long term follow-up emphasizing the overlapping spectrum of adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma. Int J Clin Exp Pathol. 2011;4(4):336–348. [PMC free article] [PubMed] [Google Scholar]
- 66.Seethala R R, Johnson J T, Barnes E L, Myers E N. Polymorphous low-grade adenocarcinoma: the University of Pittsburgh experience. Arch Otolaryngol Head Neck Surg. 2010;136(4):385–392. doi: 10.1001/archoto.2010.39. [DOI] [PubMed] [Google Scholar]
- 67.Johnston M L Huang S H Waldron J N et al. Salivary duct carcinoma: Treatment, outcomes, and patterns of failure Head Neck 2015(e-pub ahead of print). doi: 10.1002/hed.24107 [DOI] [PubMed] [Google Scholar]
- 68.Edge S B Compton C C The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM Ann Surg Oncol 20101761471–1474. PubMed [DOI] [PubMed] [Google Scholar]
- 69.Dean N R, White H N, Carter D S. et al. Outcomes following temporal bone resection. Laryngoscope. 2010;120(8):1516–1522. doi: 10.1002/lary.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohan R, Wu Q, Wang X, Stein J. Intensity modulation optimization, lateral transport of radiation, and margins. Med Phys. 1996;23(12):2011–2021. doi: 10.1118/1.597848. [DOI] [PubMed] [Google Scholar]
- 71.Chen A M, Garcia J, Granchi P, Bucci M K, Lee N Y. Base of skull recurrences after treatment of salivary gland cancer with perineural invasion reduced by postoperative radiotherapy. Clin Otolaryngol. 2009;34(6):539–545. doi: 10.1111/j.1749-4486.2009.02036.x. [DOI] [PubMed] [Google Scholar]
- 72.Schoenfeld J D, Sher D J, Norris C M Jr. et al. Salivary gland tumors treated with adjuvant intensity-modulated radiotherapy with or without concurrent chemotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):308–314. doi: 10.1016/j.ijrobp.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 73.Münter M W, Schulz-Ertner D, Hof H. et al. Inverse planned stereotactic intensity modulated radiotherapy (IMRT) in the treatment of incompletely and completely resected adenoid cystic carcinomas of the head and neck: initial clinical results and toxicity of treatment. Radiat Oncol. 2006;1:17. doi: 10.1186/1748-717X-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


