Abstract
Objectives
Adaptive physiological stress regulation is rarely studied in mild cognitive impairment (MCI). Here we targeted mental fatigability (MF) as a determinant of altered high frequency heart rate variability (HF-HRV) reactivity in individuals with MCI, and examined fronto-basal ganglia circuitry as a neural basis supporting the link between MF and HF-HRV reactivity.
Methods
We measured mental fatigability and HF-HRV during a 60-minute cognitive stress protocol in 19 individuals with MCI. HF-HRV responses were modeled using a quadratic equation. Resting state functional connectivity of intra- and inter-network fronto-basal ganglia circuitry was assessed using blood-oxygen-level dependent MRI among 7 of the participants.
Results
Lower MF was associated with faster and greater rebound in U-shape HF-HRV reactivity, which linked to a stronger connectivity between right middle frontal gyrus and left putamen.
Conclusions
Results suggest that MF may contribute to abnormal physiological stress regulation in MCI, and fronto-basal ganglia circuitry may support the link.
Keywords: Mild cognitive impairment, mental fatigability, cardiovascular reactivity, fronto-basal ganglia circuitry, physiological stress regulation
Introduction
Evidence suggests that the reduced capacity to physiologically adapt to environmental challenges may lead to neurodegeneration; however, there has been little work examining physiological adaptation in individuals beginning to show early signs of cognitive deficits, such as mild cognitive impairment (MCI). 1 Cognitive impairment and maladaptive physiological responses to challenges may be signaling neurobiological alterations that underpin a downward spiral in overall health in MCI and accelerate the progression to dementia. 2 The parasympathetic branch of the autonomic nervous system, often measured by the high frequency (HF) domain of heart rate variability (HRV), sensitively and quickly responds to the changing environment, both withdrawing and activating as needed, which is a critical component of flexible physiological adaptation. Reduced capacity for flexible automatic regulation may reflect pending transition to dementia. 3 Among the small number of studies available, evidence is inconsistent regarding autonomic regulation in MCI, suggesting further study is needed of the factors that may predict HF-HRV alterations in MCI.
Here, we examined mental fatigability (MF, i.e., increased fatigue over acute but constant mental load), one of the most common neurological phenomena associated with aging, as a potential determinant of HF-HRV alteration. MF is associated with the alteration of activity in multiple peripheral homeostatic pathways in response to acute stressors in old age. 4, 5 Importantly, MF and altered HF-HRV are both related to selective regions of prefrontal cortex (PFC), basal ganglia, insula, and anterior cingulate cortex, 6, 7 which together build a functional circuit called the fronto-basal ganglia circuitry. In this pilot study of MCI patients, we aimed to provide proof of concept of a shared central origin of MF and adaptive autonomic regulation. Specifically, we examined whether MF was indeed associated with altered HF-HRV during a cognitive stress task; in a small subgroup for whom resting-state functional connectivity (rsFC) data were collected, we also explored whether alterations in the fronto-basal ganglia circuitry were associated with MF and HF-HRV.
Methods
An exploratory cross-sectional study was conducted of 19 patients with amnestic MCI (mean age = 72.3, 11 males, mean education = 16.63 years, and mean Montreal Cognitive Assessment (MOCA) = 24.5) who were referred by local memory clinics. Participants had no use (73.7%) or stable use (26.3%) of memantine or cholinesterase inhibitors, no treatment with antidepressants or anxiolytics, and no major cardiovascular conditions (e.g., stroke, congestive heart failure, myocardial infarction). Seven (36.8%) reported use of anti-hypertensive medications. Seven of the 19 participants without MRI contraindications attended MRI scans. The study was approved by the institutional research subject review board.
The stress protocol was conducted within a two-hour window in the morning (9:00am –11:00 am). Participants were instructed to refrain from caffeine, exercise, and medications on the morning of testing to limit potential acute effects on outcomes. The stress protocol lasted for 70 minutes, including a 10-minute resting (baseline) period and 60-minute cognitive tasks period. There were five computerized tasks sharing similar visual components and targeting sustained and divided attention, requiring fast reaction time.8 The purpose of using five similar tasks instead of a single task was to balance the familiarity and novelty in stimuli, in order to simultaneously manipulate MF and induce HF-HRV reactivity.
MF: A six-item visual analog scale (items selected from a 18-item scale 9 based on content validity and understandability for individuals with MCI) was used to assess fatigue severity at the end of baseline and immediately after the cognitive tasks; Cronbach’s alpha reliability was .91 and .97, respectively. The ratings were summed for each period (baseline and post-tasks); there was a significant increase in fatigue rating from baseline to post-cognitive tasks (Wilcoxon Signed Ranks test: Z = −2.33, p = .020). MF was calculated using the discrepancy between the two sum scores with higher scores indicating more fatigue.
HF-HRV: Electrocardiography (ECG) was continuously monitored during the stress protocol. Series of intervals between consecutive R waves (every 20 seconds) were analyzed to generate HF-HRV data (0.15 – 0.5 Hz) with log-transformation applied and averaged by minute. Consistent with models of HRV as an index of flexible, adaptive responding to the environment (e.g., 10) our recent work suggests an early decline followed by an increase HF-HRV in response to acute cognitive stress (i.e., a U-shape pattern).11 Therefore, we modeled HF-HRV responses over the last minute baseline and 60-minute cognitive tasks, a total of 61 time points, using the statistical mixed-effects model with YHF-HRV = aXTime2 + bXTime2 + c + ε where parameters (a, b, c) were modeled as random-effects at individual level with AR(1) covariance matrix. All parameters were significant (a: B(SE) = 2.25(0.22), t (df) = 10.24 (20), p < .001; b: B(SE) = −8.03e−3(0.004), t (df) = −2.07 (133), p = .041; c: B(SE) = 1.60e−4 (0.62e−6), t (df) = 2.59 (86), p = .011). We next calculated four indices using parameter coefficient: vertex (i.e., −b/2a, the length of time when HF-HRV starts rebounding), change (i.e., a×602+b×60, change of HF-HRV over the tasks), and bottom (i.e., a×vertex2+b×vertex+c) and initial level (i.e., c) of HF-HRV.
rsFC data was collected using a research dedicated 3T Siemens TrioTIM scanner equipped with a 32-channel receive-only head and body coil transmission (functional images: gradient echo-planar imaging sequence, TR = 2000ms, TE = 30ms, FA = 90, matrix = 64 × 64, resolution 4x4x4 mm3), preprocessed using Data Processing Assistant for Resting-state fMRI, and analyzed using independent component analysis (ICA) to identify resting networks using GIFT v3.0a (icatb.sourceforge.net). Details were described elsewhere. 8
Results
We first determined the covariates that should be included in understanding the relationship between MF and HF-HRV. None of the candidate covariates (i.e., gender, anti-hypertensive use, memantine or cholinesterase inhibitors use, education, or MOCA) influenced MF or HF-HRV (all p > .30). Using Spearman’s correlation (rs), MF was significantly related to vertex (rs = .44, n = 19, p = .030) and change (rs = −.41, n = 19, p = .039), but not the bottom or initial level of HF-HRV model (see Figure 1). We also calculated the average HRV over 60 minutes cognitive stress task; its correlation with MF was not significant (rs = −.15, n = 19, p = .27). In the following imaging-related analyses, we only included the vertex and change as indices for HF-HRV response.
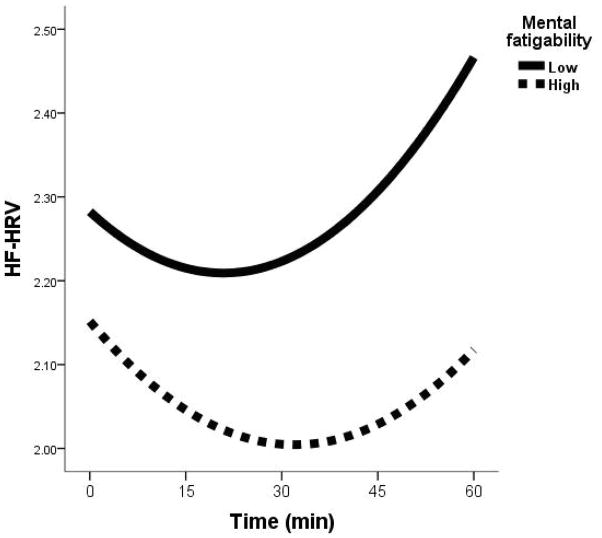
Figure 1.
HF-HRV response over 60 minutes cognitive tasks by dividing MF to high (n = 6) vs. low (n = 13) level using a cut-off score = 12 for display purpose.
ICA generated two networks related to fronto-basal ganglia circuitry: the basal ganglia network (BGN) and the central executive network (CEN) (see Supplemental Figure 1). The level of MF was significantly related to the intra-network connectivity of BGN (rs = −.67, n = 7, p = .049) but not CEN (rs = −.56, n = 7, p = .10) using Spearman’s correlation. Neither intra-network related to HF-HRV indices (|rs|: .39 – .54, p > .10).
We also analyzed the 4 pairs of ROIs as inter-network connectivity using relevant peak coordinates: bilateral putamen from the BGN and bilateral middle frontal gyrus (MFG) from the CEN (see Supplemental Figure 1). The level of MF (rs = −.73, n = 7, p = .031), as well as vertex (rs = −.79, n = 7, p = .018) and change (rs = .79, n = 7, p = .018) of HF-HRV model were significantly correlated to the connectivity of left putamen-right MFG.
Finally, we present two cases with lowest (i.e., 0, ID_L) and highest (i.e., 42, ID_H) MF scores, their fronto-basal ganglia circuitry (taking left putamen as the seed) and HF-HRV response. Qualitatively, ID_L had stronger network connectivity than ID_H (Supplemental Figure 2A), and the HF-HRV response patterns (Supplemental Figure 2B) are similar to those for the high vs. low MF levels at Figure 1.
Discussion
To our knowledge, this is the first study to demonstrate a relationship between MF and HF-HRV reactivity, and does so in a group at high risk for dementia. MF and HF-HRV reactivity were both significantly inducible over the 60-minute cognitive stress task in individuals with MCI. Next, MF was associated with dynamic change in HF-HRV U-shape response. More specifically, individuals with lower fatigability presented a faster (i.e., seen in smaller vertex) and greater (i.e., seen in larger change) rebound of HF-HRV, regardless of the initial or lowest level or average of HF-HRV response. Finally, consistent with our hypothetical model, the inter-network between left putamen and right MFG was linked to both MF and HF-HRV U-shape response.
Consistent with a previous study in healthy adults, 5 the current study confirmed that individuals with lower MF tended to have a faster rebound from HF-HRV suppression over the task to an even higher ending level, reflecting a more flexible autonomic response to environmental challenge. More flexible autonomic responses, indexed by HRV, are associated with enhanced PFC function and better cognition, 12, 13 while insufficient autonomic flexibility predicts incident Alzheimer’s disease. 3 Intriguingly, by examining the functional connectivity of both intra- and inter-networks related to PFC and basal ganglia, the findings here suggest a common neural support explaining the link between MF and HF-HRV reactivity. Although MF, broadly, may be linked to BGN, MF and HF-HRV reactivity seemed to share a more selective inter-network between right MFG at CEN and left putamen at BGN. The results are consistent with a recent meta-analysis suggesting that left putamen may play a selective role in regulating HF-HRV reactivity during acute stressor tasks, particularly when the tasks primarily engage cognitive resources.7
Study limitations include the lack of a cognitively healthy comparison group and the small number of participants that precluded more refined models with multiple covariate adjustment (e.g., specific medication classes, medical conditions, progress in the neurodegenerative process). The lack of relationship between resting HF-HRV and MF may due to the sample size. Further, more complex dynamics of HF-HRV are worth considering in future study, 14, 15 as these dynamics may help clarify links between the autonomic nervous system, fatigability, and neural connectivity. We could not examine the causal relations among fronto-basal ganglia network, MF, and HF-HRV reactivity. Thus, whether the fronto-basal ganglia network mediates the association between MF and HF-HRV reactivity remains to be determined, as does the etiology of altered fronto-basal ganglia network activity in MCI should larger scale studies support the current findings. Despite these limitations, our intriguing pilot findings suggest that MF may index susceptibility to the pernicious effects of stress in individuals at risk for dementia. Thus, MF may be a critically useful clinical target to slow progression to dementia and promote well-being in MCI.
Supplementary Material
Supplemental Figure 1 Spatial maps and ROIs of basal ganglia network (BGN) and central executive network (CEN). Note. The color maps represent the results of one-sample t-test (p < 0.05, FWE corrected). MNI = Montreal Neurological Institute.
Supplemental Figure 2 Inter-network functional connectivity of left putamen in BGN (A) and HF-HRV responses in individual MCI cases with lowest (=0, ID_L) and highest MF (=42, ID_H) from the MRI subsample (B).
Acknowledgments
The present study was funded by the University of Rochester CTSA award number KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health to F. Lin, and the manuscript preparation was also funded by the Alzheimer’s Association New Investigator Grant (NIRG-14-317353) and NIH R01 grant (NR015452) to F. Lin. F. Lin had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Dr. Michael Hasselburg, Dr. Giovanni Schifitto, Dr. Madalina Tiravus for the comments on the initial version of manuscript.
Footnotes
Author Contributions:
F. Lin designed the study, collected and analyzed data, and wrote the manuscript.
P. Ren analyzed data and wrote the manuscript.
K. Cotton collected and analyzed data, and wrote the manuscript.
A. Porsteinsson designed the study and wrote the manuscript.
M. Mapstone designed the study and wrote the manuscript.
K. Heffner designed the study and wrote the manuscript.
Conflicts of Interest: See relevant forms.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Feng Lin, Email: vankee_lin@urmc.rochester.edu.
Ping Ren, Email: Ping_Ren@urmc.rochester.edu.
Kelly Cotton, Email: kcotton@u.rochester.edu.
Anton Porsteinsson, Email: Anton_Porsteinsson@URMC.Rochester.edu.
Mark Mapstone, Email: Mark_Mapstone@URMC.rochester.edu.
Kathi L. Heffner, Email: Kathi_heffner@urmc.rochester.edu.
References
- 1.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 2.Liang P, Wang Z, Yang Y, Jia X, Li K. Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PLoS One. 2011;6:e22153. doi: 10.1371/journal.pone.0022153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zulli R, Nicosia F, Borroni B, et al. QT dispersion and heart rate variability abnormalities in Alzheimer's disease and in mild cognitive impairment. J Am Geriatr Soc. 2005;53:2135–2139. doi: 10.1111/j.1532-5415.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 4.Lin F, Roiland R, Polesskaya O, et al. Fatigability Disrupts Cognitive Processes' Regulation of Inflammatory Reactivity in Old Age. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuno K, Tanaka M, Yamaguti K, Kajimoto O, Kuratsune H, Watanabe Y. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav Brain Funct. 2011;7:17. doi: 10.1186/1744-9081-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leavitt VM, DeLuca J. Central fatigue: issues related to cognition, mood and behavior, and psychiatric diagnoses. PM R. 2011;2:332–337. doi: 10.1016/j.pmrj.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Lin F, Heffner K, Ren P, et al. Cognitive and Neural Effects of Vision-Based Speed of Processing Training in Older Adults with Amnestic Mild Cognitive Impairment: A Pilot Study. J Am Geriatr Soc. doi: 10.1111/jgs.14132. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 10.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 11.Lin F, Heffner K, Ren P, Tadin D. High-Frequency Heart Rate Variability As A Peripheral Marker For Neuroplasticity. under review. [Google Scholar]
- 12.Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin F, Heffner K, Mapstone M, Chen DG, Porsteisson A. Frequency of Mentally Stimulating Activities Modifies the Relationship Between Cardiovascular Reactivity and Executive Function in Old Age. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaves PH, Varadhan R, Lipsitz LA, et al. Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J Am Geriatr Soc. 2008;56:1698–1703. doi: 10.1111/j.1532-5415.2008.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckers F, Verheyden B, Aubert AE. Aging and nonlinear heart rate control in a healthy population. American journal of physiology Heart and circulatory physiology. 2006;290:H2560–2570. doi: 10.1152/ajpheart.00903.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Spatial maps and ROIs of basal ganglia network (BGN) and central executive network (CEN). Note. The color maps represent the results of one-sample t-test (p < 0.05, FWE corrected). MNI = Montreal Neurological Institute.
Supplemental Figure 2 Inter-network functional connectivity of left putamen in BGN (A) and HF-HRV responses in individual MCI cases with lowest (=0, ID_L) and highest MF (=42, ID_H) from the MRI subsample (B).