Abstract
These studies examined the influence of 2,5-hexanedione (2,5-HD) intoxication on expression of neuronal nitric oxide synthase (nNOS) in the brainstem nuclei in Zucker Diabetic Fatty (ZDF) vs. lean control (LC) rats. Functional neuropathic changes were also investigated following axonal damage and impaired axonal transport induced by the treatment. Animals were intoxicated by i.p. injection of 2,5-HD plus unilateral administration of 2,5-HD over the sciatic nerve. The mechanical thresholds and withdrawal latencies to heat and cold stimuli on the foot were measured at baseline and after intoxication. The medulla sections were examined by nNOS immunohistochemistry and NADPH-diaphorase histochemistry at the end of the treatments. The mechanical thresholds and withdrawal latencies were significantly decreased while nNOS immunostained neurons and NADPH-diaphorase positive cells were selectively reduced in the gracile nucleus at baseline in ZDF vs. LC rats. NADPH-diaphorase reactivity and nNOS positive neurons were increased in the ipsilateral gracile nucleus in LC rats following 2,5-HD intoxication, but its up-regulation was attenuated in ZDF rats. These results suggest that diabetic and chemical intoxication-induced nNOS expression is selectively reduced in the gracile nucleus in ZDF rats. Impaired axonal damage-induced nNOS expression in the gracile nucleus is involved in neuropathic pathophysiology in type II diabetic rats.
Keywords: Nitric oxide synthase; dorsal medulla; diabetic neuropathy; sensory functions; 2,5-HD intoxication; immunohistochemistry
1. Introduction
Unremitting pain and reduced temperature and vibration thresholds are observed in patients with diabetic neuropathy (Dyck, et al., 1993), and in rats with streptozotocin-induced type I diabetes (Wuarin-Bierman, et al., 1987). Recent studies showed that pain and pressure thresholds are decreased in Zucker Diabetic Fatty (ZDF) rats, a type II diabetic model (Rong, Ma, 2011; Russell, et al., 2008; Zhuang, et al., 1997). Several studies demonstrated that systemic administration of 2,5-hexanedione (2,5-HD) causes neurofilamentous axonal swellings and damages, which prevent proximal-to-distal transport of neurofilaments and other substances, resulting in profound neuropathic changes including hyperalgesia and limb paralysis (Anthony, et al., 1983; LoPachin, et al., 1994). However, the neural pathways and neurotransmissions responsible for the increased susceptibility of the sensory neurons to non-noxious and noxious stimuli in diabetic and chemical neuropathies are poorly understood.
Nitric oxide (NO) is one of the most important messenger molecules produced in many cell types, including neurons in the brain (Bredt, Snyder, 1992; Moncada, Higgs, 1991). Several studies have shown that the nitric oxide (NO)-cGMP pathway plays an inhibitory role in nociceptive modulation, which contributes to analgesic mechanisms (Duarte, Lorenzetti, Ferreira, 1990; Kumar, et al., 1993). Recent studies show that impaired NO production is involved in human diabetic neuropathy (Kilo, et al., 2000) and is associated with hyperalgesia in diabetic rats (Sessa, et al., 1993). nNOS catalyses the transformation of arginine to NO in neurons, and nNOS is also a highly regulated enzyme (Dinerman, Lowenstein, Snyder, 1990; Sessa, et al., 1993). Recent studies have demonstrated that nNOS is an inducible enzyme, which is up-regulated by lesion of nerves, abnormal mechanical forces, and other various factors (Amin, et al., 1995; Dinerman, Lowenstein, Snyder, 1990; Ma, et al., 2000; Sessa, et al., 1993). nNOS expression in the gracile nucleus is markedly increased in rats with sciatic axotomy and accompanied by an increased number of cells showing expression of NADPH diaphorase (NADPHd) reactivity, a marker of nNOS (Ma, et al., 2000).
The gracile nucleus receives ascending input from the sciatic nerve, which has the longest axons in the body (Leem, et al., 1994; Ueyama, et al., 1994). Recent experiments have suggested that the gracile nucleus is an integration center for visceral and somatic information flowing into the thalamus, which possesses functions for sensory and pain processing in the dorsal column pathway (Al-Chaer, et al., 1996; Al-Chaer, et al., 1997). The afferent sensory fibers in the sciatic nerve originate from the skin or muscle, and synapse directly on dorsal horn neurons, or on dorsal horn interneurons in the spinal cord, which ascends to the gracile nucleus (Leem, et al., 1994; Ueyama, et al., 1994). Previous studies have demonstrated that neuropeptide Y and substance P immunoreactivities increase in the gracile nucleus after sciatic nerve damage or transection (Noguchi, K., et al., 1995; Ohara, et al., 1994; Zhang, et al., 1993).
The purpose of the present study was to determine the influence of 2,5-HD intoxication, which induces axonal damage and impaired axonal transport, on nNOS expression in the brainstem and on functional neuropathic changes in ZDF rats compared to normal lean control (LC) rats. Baseline and 2,5-HD intoxication-induced nNOS expressions in the brainstem nuclei were examined by using nNOS immunohistochemistry and NADPHd histochemistry, a marker of nNOS activity. Functional neuropathic changes were examined by measuring the mechanical tolerance threshold of the foot using Von Frey Filaments and by testing the withdrawal latencies of the foot in response to heat and cold stimuli.
2. Results
2.1. Functional neuropathic changes
The blood glucose levels, body weights, mechanical tolerance threshold of the foot, and withdrawal latencies to application of heat or cold stimuli were measured in seven ZDF rats compared to seven age matched LC rats. Blood glucose level was markedly elevated (P< 0.001, n=7 /group) in ZDF rats (459.6±35.2 mg/dl, M±SE) vs. LC rats (105.7±12.7). However, the body weights were of minimal variation, 468.0±8.0 g in ZDF rats vs. 422.7±11.1 in LC rats.
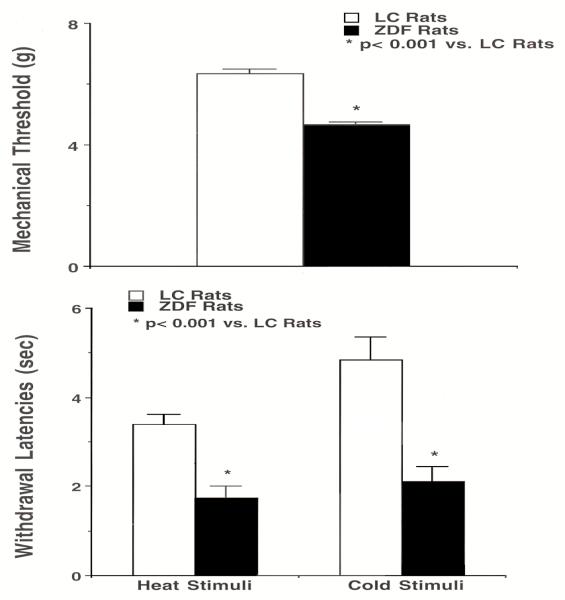
The withdrawal latencies to heat and cold stimuli and mechanical tolerance threshold of the foot in ZDF and LC rats were continually observed for three days, and the averaged values served as baseline control (Fig. 1). The mechanical tolerance thresholds of foot were significantly decreased (P< 0.01, n=7) in ZDF vs. LC rats (Fig. 1, top). The withdrawal latencies to heat stimuli were markedly reduced in ZDF rats compared to LC rats, as shown in figure 1, bottom (P<0.001, n=7). The withdrawal latencies to cold stimuli of ZDF rats were considerably reduced (P<0.001, n=7). ZDF rats were almost two times as sensitive to heat and cold temperatures compared to LC rats. There were no detectable differences in mechanical tolerance threshold and withdrawal latencies between the left and right foot in both ZDF and LC rats at baseline levels.
Fig. 1.
Foot-mechanical thresholds and withdrawal latencies to heat and cold stimuli in ZDF rats vs. Lean control (LC) rats. Mechanical thresholds in the ZDF rat are significantly lower than that in the LC rat (top panels). Bottom panels show that ZDF rats have a markedly faster foot withdrawal to heat (left) and cold (right) stimuli compared to LC rats. Values are mean ± SEM (n = 7/group). *: P < 0.001, compared with LC rats.
2.2. Responses of functional neuropathic changes to 2,5-HD intoxication
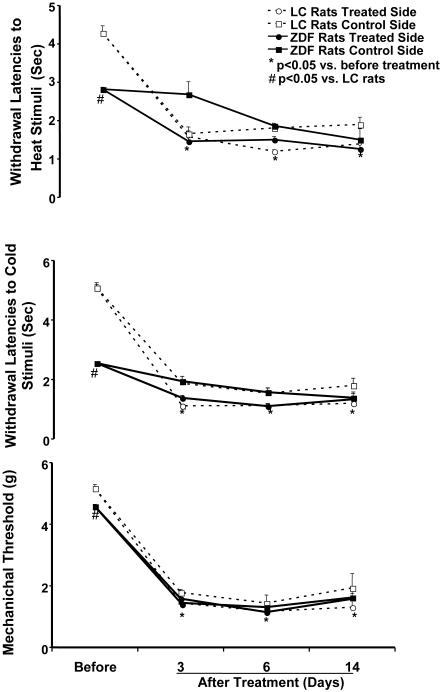
Changes in mechanical tolerance threshold and withdrawal latencies of the foot in response to 2,5-HD treatments were examined in ZDF and LC rats (n = 5-6). Figure 2 shows the time intervals of the changes in mechanical tolerance threshold and withdrawal latencies to heat and cold stimuli in ZDF compared to LC rats following 2,5-HD intoxication. The withdrawal latencies to heat and cold stimuli were consistently reduced in both the left and right foot of ZDF and LC rats after 2,5-HD intoxication compared to their control values (P < 0.05, Fig. 2, top and middle panels). The mechanical tolerance thresholds were significantly decreased in both feet following treatment with 2,5-HD (P < 0.01, Fig. 2, bottom). There were no differences between LC and ZDF rats on the withdrawal latencies to mechanical, heat, and cold stimuli after 2,5-HD intoxication. Administration of 2,5-HD on unilateral sciatic nerve plus systemic intoxication tended to further decrease the withdrawal latencies to heat and cold stimuli on the ipsilateral side of the foot, although the differences failed short of statistical significance, as shown in figure 2, top and middle. There was no detectable difference of the mechanical tolerance thresholds between control and treated sides of the foot following unilateral treatment with 2,5-HD, as shown in figure 2, bottom.
Fig. 2.
The time intervals of changes in withdrawal latency responses to heat and cold stimuli (top and middle panels) and mechanical threshold (bottom panels) on the control and treated sides of foot induced by 2,5-hexanedione (2,5-HD) intoxication in ZDF rats compared to lean control (LC) rats. Withdrawal latency responses to heat and cold stimuli and mechanical threshold showed significant decreases in the control value of ZDF rats compared to LC rats. Mechanical tolerance thresholds and withdrawal latencies to heat and cold stimuli were significantly decreased on both sides of the foot at 3, 6, and 14 days after 2,5-HD treatments in ZDF and LC rats. Values are mean ± SEM (n = 5-6/group). *: P < 0.05, compared with before treatment; #: P < 0.05, compared with LC rats.
ANOVA analysis revealed significant differences in withdrawal latencies to heat and cold stimuli on both feet at 3, 6, and 14 days after 2,5-HD treatments in ZDF and LC rats compared to control values (P < 0.05), as well as mechanical tolerance thresholds over these days (P < 0.01). The significant decrease of the withdrawal latencies and mechanical tolerance threshold of the foot occurred at 3 days after injection (P < 0.05). The maximum responses to 2,5-HD were at 6 days and maintained at 14 days after treatments (Fig. 2).
2.3. NADPHd staining
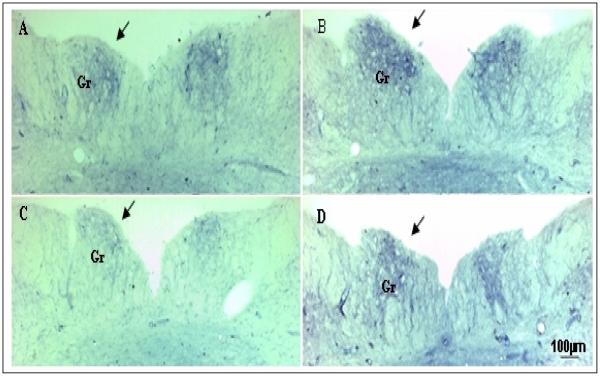
LC and ZDF rats at baseline level were stained and compared with groups of ZDF rats and six LC rats with 2,5-HD intoxication (n = 6/group). Figures 3 shows the rostral part of medulla sections containing variable NADPHd reactivity as evidenced by the color density of the cells from a baseline LC rat and a ZDF rat at baseline level compared to a LC rat and a ZDF rat treated with 2,5-HD. The gracile nucleus of LC rats exhibited a moderately dense NADPHd staining of medium-sized neurons and nerve fiber network (Fig. 3 A), which were high than the staining in a ZDF rat at baseline level (Fig. 3C vs. 3A). Fourteen days after 2,5-HD intoxication, an increase in NADPHd-stained neurons and axons were recognized on the rostral regions of the gracile nucleus in a LC rat (Fig 3 B vs. A). The increase was pronounced on the ipsilateral side of the gracile nucleus compared to the contralateral side (Fig. 3B, pointed by an arrow). In a 2,5-HD treated ZDF rat, moderate increases in NADPHd-positive cells existed in the gracile nucleus (Fig. 3D). Such changes were not apparent in the gracile nucleus of non-treated control rats nor in other nuclei of the medulla.
Fig. 3.
The low magnification micrographs from the rostral part of medulla sections show the structures and distributions of NADPH diaphorase staining in ZDF rat compared to LC rat and their response to 2,5-hexanedione (2,5-HD) treatments. The micrographs (A and C, the left side) show the rostral part of medulla sections from non-treated LC (A) and ZDF rat (C) vs. the micrographs (B and D, the right side) are the sections from LC (B) and ZDF rat (D) with 2,5-HD treatments. NADPH diaphorase staining in the gracile nucleus (Gr) was reduced in a control ZDF rat (C) compared to a LC rat (A). Unilateral 2,5-HD intoxication over the sciatic nerve caused an increase in NADPH diaphorase staining on the ipsilateral gracile nucleus (indicated by arrows) in a LC rat (B) and a ZDF rat (D) compared to non-treated controls (A and C).
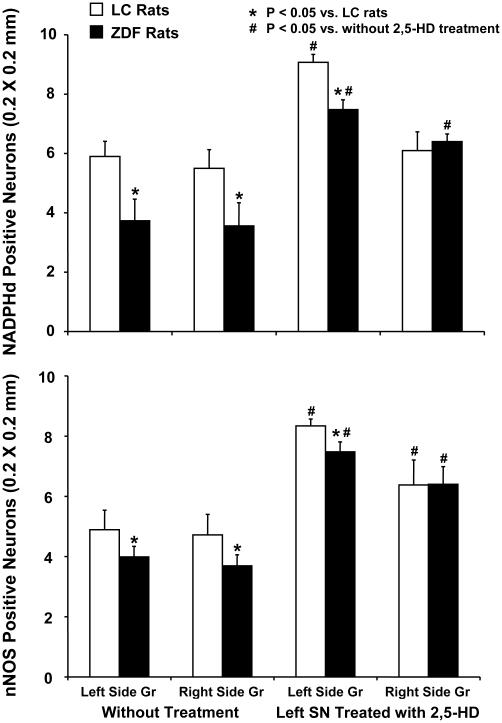
The average number of NADPHd positive neurons (200 × 200 μm) were significantly reduced in both sides of the gracile nucleus in ZDF rats compared to LC rats at baseline level (Fig. 4, top). Following 2,5-HD intoxication in LC rats, the number of NADPHd positive neurons were markedly increased in the rostral region of the ipsilateral side of the gracile nucleus. The number of neurons stained with NADPHd tended to increase in the contralateral sides of the gracile nucleus, although the change fell short of statistical significance. Following 2,5-HD intoxication in ZDF rats, the number of NADPHd positive neurons were moderately increased in the ipsilateral side of the gracile nucleus. The number of neurons stained with NADPHd in the ipsilateral gracile nucleus was significantly higher in LC rats when compared to those in ZDF rats induced by 2,5-HD intoxication, as shown in figure 4, top panel. The number of NADPHd positive cells in the ipsilateral gracile nucleus was significantly higher in both LC and ZDF rats than those induced in the contralateral sides (P<0.05).
Fig. 4.
Quantitation of NADPH diaphorase reactivity (top panels) and nNOS immunoreactivity (bottom panels) in the ipsilateral and contralateral sides of gracile nucleus in LC and ZDF rats with and without 2,5-HD treatments. Left panels show baseline NADPH diaphorase staining cells and nNOS immunostaining cells in the left and right sides of the gracile nucleus of ZDF rats compared to LC rats. Right panels show that NADPH diaphorase reactivity and nNOS immunostaining neurons in ipsilateral and contralateral sides of gracile nucleus were altered by 2,5-HD intoxication in ZDF rats compared to LC rats. Each bar represents the mean values and vertical bars represent ± SEM (n=5-6). *: P < 0.05, compared to LC rats; #: compared to control without treatment.
The baseline levels of NADPHd positive neurons were less (about 50-60 %) in the caudal region of the gracile nucleus than those in the rostral gracile nucleus in both ZDF and LC rats. In the caudal region of the gracile nucleus following 2,5-HD intoxication in LC rats (n = 5), the number of NADPHd positive cells (200 × 200 μm) in the ipsilateral side was 7.3 ± 0.7 S.E.M., which was significantly increased compared with baseline value in the same side (3.7 ± 0.5, P < 0.01) and contralateral side (4.7 ± 1.0, P < 0.05). In ZDF rats with 2,5-HD intoxication (n = 5), the number of NADPHd positive neurons on the ipsilateral side gracile nucleus was 5.7 ± 0.5, which failed to show statistical different changes compared to baseline value in the same side (4.1 ± 0.6) and contralateral side (4.7 ± 0.9). 2,5-HD intoxication induced a marginal high level of NADPHd positive neurons in the caudal region of ipsilateral gracile nucleus in LC rats compared to ZDF rats (P = 0.09).
2.4. nNOS immunohistochemistry
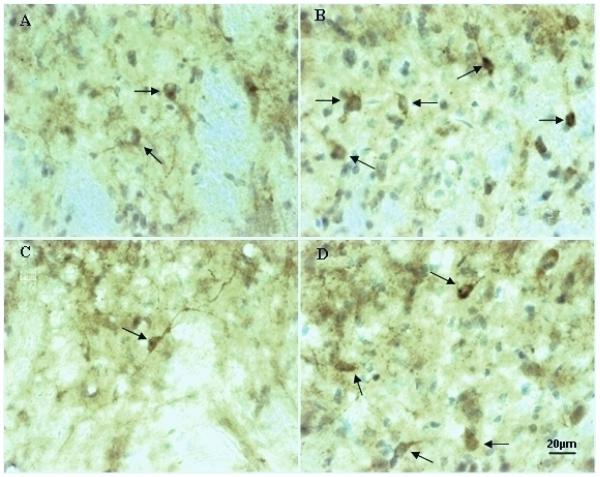
The medulla sections of ZDF and LC rats at baseline level were stained with nNOS immunoreactivity and compared with the rats with 2,5-HD intoxication (n = 5-6). Figure 5 shows that neurons in the gracile nucleus contain variable nNOS immunoreactivity evidenced by the color density of the cells from a LC rat and a ZDF rat (A and C) compared to the rats treated with 2,5-HD (B and D). As shown in figure 5A, nNOS immunostained cells were recognized in the gracile nucleus in a LC rat, which were higher than those stained cells of the same area in a ZDF rat (Fig. 5C). 2,5-HD intoxication in a LC rat caused higher increases in nNOS immunostained neurons in the ipsilateral gracile nucleus compared to the moderate increases in a ZDF rat, as shown in figure 5 B and D, pointed by arrows).
Fig. 5.
The high magnification micrographs show nNOS immunoreactivity in the gracile nucleus of a LC rat (A) and a ZDF rat (C) compared to a LC rat (B) and a ZDF rat (D) with 2,5-HD intoxication. nNOS immunostained cells (indicated by arrows) were increased in the ipsilateral side of the gracile nucleus in a LC rat and a ZDF rat (B and D) with 2,5-HD treatment (B) compared to a LC rat and a ZDF rat without treatment (A and C). nNOS immunoreactivity was also reduced in the gracile nucleus of a control ZDF rat (C) compared to a LC rat (A).
The number of nNOS positive neurons (200 × 200 μm) in the rostral regions of the gracile nucleus was significantly less in ZDF rats than those in LC rats, as shown in figure 4, bottom panel. After 2,5-HD intoxication in LC rats (n = 5), the number of nNOS immunoreactive cells was markedly increased in the rostral region of the ipsilateral gracile nucleus compared to baseline value (Fig. 4, bottom). In LC rats with 2,5-HD intoxication, the number of nNOS-positive cells was significantly higher in the rostral region of ipsilateral gracile nucleus than those in baseline rats. Following 2,5-HD intoxication in ZDF rats, the nNOS positive neurons in the contralateral sides of the gracile nucleus suggested a significant elevation compared to control value (P < 0.05). However, the numbers of nNOS positive neurons in the ipsilateral gracile nucleus were marginally less in ZDF rats than those in the same side of LC rats with 2,5-HD treatment (P < 0.07). The number of nNOS positive neurons in the ipsilateral gracile nucleus was marginally less than those in the contralateral gracile nucleus in both LC rats (P < 0.09) and ZDF rats (P < 0.06).
In the caudal region of the gracile nucleus of LC rats with 2,5-HD intoxication (n = 5), the number of nNOS positive cells was significantly increased in the ipsilateral side (5.8 ± 0.2) and contralateral gracile nucleus (4.9 ± 0.5) compared to baseline value (3.5 ± 0.3), which was less (about 50%) than those in the rostral gracile nucleus. The 2,5-HD intoxication failed to show detectable changes of nNOS reactivity between the ipsilateral side and contralateral region in LC rats. 2,5-HD intoxication in ZDF rats caused an increase in nNOS immunoreactive neurons in the caudal region of the ipsilateral gracile nucleus (5.9 ± 0.8) compared to control rats (3.7 ± 0.2). In the contralateral side of the gracile nucleus, the number of nNOS-positive cells in control ZDF rats (4.3 ± 0.3) was similar to the rats treated with 2,5-HD (4.4 ± 0.6).
3. Discussion
We examined the expression of nNOS and NADPHd in the brainstem nuclei in ZDF compared to LC rats at baseline level, and determined the responses of nNOS expression and NADPHd reactivity in the nuclei to 2,5-HD intoxication in the rats. The functional neuropathic changes were tested by measurements of mechanical tolerance thresholds and withdrawal latencies in the rats. The major new findings of this study are: 1) nNOS and NADPHd positive cells in the gracile nucleus were decreased in ZDF rats associated with a reduction of mechanical thresholds and withdrawal latencies; 2) NADPHd reactivity and nNOS positive neurons were significantly increased in the ipsilateral side and moderately elevated in the contralateral side of the gracile nucleus by 2,5-HD intoxication in LC rats; 3) 2,5-HD intoxication induced expressions of nNOS and NADPHd reactivity in the ipsilateral gracile nucleus were impaired in ZDF rats; and 4) Withdrawal latencies and mechanical threshold were consistently decreased by 2,5-HD intoxication in both LC and ZDF rats. The present study is the first evidence showing that ZDF rats possesses a consistent impairment of nNOS expression and NADPHd reactivity in the gracile nucleus at both baseline level and with 2,5-HD intoxication. 2,5-HD intoxication induces a considerable upregulation of nNOS and NAPDHd expression in the gracile nucleus in LC rats; however, the levels of 2,5-HD intoxication-induced elevation of nNOS expression and NADPHd reactivity are attenuated in ZDF rats. The differences of nNOS expression and NADPHd reactivity in ZDF and LC rats were evident in the gracile nucleus, a central site receiving ascending input from primary sciatic sensory afferent fibers in the brainstem, but not evident in other brainstem regions. The mechanical tolerance thresholds and withdrawal latencies were consistently reduced in ZDF rats and in the rats with 2,5-HD intoxication. The results suggest that the gracile nucleus in the medulla is a selective site, in which nNOS expression and NADPHd reactivity are upregulated by 2,5-HD-induced sciatic neuropathy and downregulated in ZDF neuropathic rats. The levels of nNOS expression in the gracile nucleus, associated with hyperalgesia and somatosensive hypersensitivity in both diabetic and chemical neuropathies, demonstrate that NO levels in the nucleus participate in the pathophysiological processes of neuropathies. Impaired nNOS-NO generation in the gracile nucleus plays a role in the pathophysiological processes of type II diabetic neuropathy and chemical neuropathy in ZDF rats.
With regard to the potential role of 2,5-HD-induced transganglionic upregulation of nNOS expression in the gracile nucleus, it has been demonstrated that 2,5-HD is an axonal neurotoxin that prevents the normal proximal-to-distal transport of neurofilaments, resulting in the formation of neurofilamentous axonal swellings (Anthony, et al., 1983; LoPachin, et al., 1994; Spencer, et al., 1979). The gracile nucleus receives ascending input from primary afferent fibers of the sciatic nerve, which has the longest axons in the body (Leem, et al., 1994; Ueyama, et al., 1994). Electrophysiological mapping studies and anterograde axonal tracing techniques have provided evidence for the somatotopic organization of the gracile nucleus in various mammals including the cat, rat, raccoon, sheep, and opossum (Cliffer, Hasegawa, Willis, 1992; Leem, et al., 1994; McComas, 1963; Ueyama, et al., 1994). Earlier investigators have demonstrated that transection injury of the sciatic nerve causes dystrophic central terminals in the ipsilateral gracile nucleus (Wessels, Feirabend, Marani, 1991). Neuropeptide Y has been shown to be upregulated in the ipsilateral gracile nucleus following unilateral transection injury of the sciatic nerve in rats (Zhang, et al., 1993). Our previous studies have demonstrated that nNOS immunoreactivity and NADPHd reactivity are increased in the gracile nucleus by sciatic axotomy in rats, suggesting that sciatic injury induces transganglionic or transsynaptic nNOS expression in the gracile nucleus (Ma, et al., 2000). Present studies show that nNOS positive neurons in the ipsilateral gracile nucleus were increased by unilateral administration of 2,5-HD on the sciatic nerve in both ZDF and LC rats. These results are consistent with our previous studies, and other investigators reported that nNOS expression is induced in the parent neurons following damage of the peripheral nerve (Ma, et al., 2000; Wu, et al., 1994). The results of the present study suggest that impairment of proximal-to-distal transport of neurofilaments and other substances, which results from axonal damage/swellings on peripheral nerve, causes upregulation of nNOS expression in the central sensory nucleus. The data also agree with the previous studies, which suggest that lesion–induced transganglionic and/or transsynaptic expression of an endogenous substance or pathological changes is predominately in the ipsilateral gracile nucleus.
Previous studies have shown that nociceptive pressure thresholds were significantly decreased in diabetic animal models vs. normal control groups (Dyck, et al., 1993; Vinik, et al., 1992; Wuarin-Bierman, et al., 1987). The ZDF rat, a type II diabetic rat model, begins to develop hyperglycemia at approximately 7 weeks of age (Kava, Greenwood, Johnson, 1990; Peterson, 1995). Changes in nerve conduction velocity and some morphological changes of the ZDF rat resemble human diabetic neuropathy (Otto, et al., 2011). The results of the present study suggest that mechanical thresholds and withdrawal latencies to heat or cold stimuli are consistently decreased in both ZDF rats and chemical neuropathies induced by 2,5-HD intoxication. The data also demonstrates that hyperalgesia and hypersensitivity to temperature and pressure presented in chemical neuropathies in LC rats are similar with neuropathies developed in ZDF rats. However, baseline levels of nNOS expression in the gracile nucleus and the withdrawal latencies were reduced in ZDF compared to LC rats. It appears that the mechanism for an upregulation of nNOS expression in the gracile nucleus in response to damage of sciatic nerve is impaired in ZDF rats at baseline level. Consistently, the level of upregulation of nNOS expression in the ipsilateral gracile nucleus following sciatic nerve intoxication with 2,5-HD is also attenuated in ZDF rats compared to LC rats. Our results agree with these previous studies reporting that the gracile nucleus is an important site in somatosensory regulation through interaction of peripheral somatosensory information with central pathways, and further suggest that upregulation of nNOS expression in the gracile nucleus is impaired in both chemical intoxication and diabetic neuropathy in ZDF rats. A decrease in nNOS-NO production in the gracile nucleus is a probable pathophysiological processes of diabetic and chemical neuropathies involved in change of sensory functions in ZDF rats.
An increasing body of evidence shows that activation of the NO-cGMP pathway is the common denominator for the mode of analgesics which block hyperalgesia. L-arginine NO activates the non-opioid analgesic system in the brain (Kumar, et al., 1993) and also causes peripheral analgesics which inhibit hyperalgesia directly (Duarte, Lorenzetti, Ferreira, 1990; Kumar, et al., 1993). Sasaki et al., 1998, have reported that nNOS expression is decreased in DRG neurons, and is closely associated with decreased paw withdrawal threshold in both L-NAME-treated and diabetic rats. The results of the present study show that nNOS in the gracile nucleus is reduced in ZDF neuropathic rats at baseline level. A chemical neuropathy-induced up-regulation of nNOS in the nucleus is also impaired in ZDF rats. A number of recent studies have suggested that the gracile nucleus plays an important role in somatic and visceral pain processing (Al-Chaer, et al., 1996; Al-Chaer, et al., 1997). Our results show that there are no differences between LC and ZDF rats on withdrawal latencies to mechanical, heat, and cold stimuli after 2,5-HD intoxication. One reason is that the chemical neuropathic model is too strong to reflect the difference between LC and ZDF rats since the withdrawal latencies to mechanical, heat, and cold stimuli were markedly decreased by 2,5-HD treatments compared to control values in both LC and ZDF rats. In addition, NO may also contribute to neuroprotection or cell damage (Gross, Wolin, 1995; Lipton, et al., 1993), and NO promotes the survival of DRG neurons (Thippeswamy, Morris, 1997). These studies cannot exclude the neuroprotective mechanism of NO in the gracile nucleus during 2,5-HD intoxication. A more sophisticated approach would be required to address these issues. Despite these limitations, our results from chemical and diabetic neuropathies, consistently suggest that damage of the sciatic nerve leads to an increase in nNOS expression in the gracile nucleus, and its upregulation is attenuated in ZDF rats. The results suggest that modified nNOS-NO generation in the gracile nucleus is the common pathophysiological processes of type II diabetic neuropathy and chemical neuropathy.
In conclusion, expression of nNOS and NADPHd reactivity in the gracile nucleus was decreased in ZDF rats, accompanied by a reduction of mechanical thresholds and withdrawal latencies on the hind foot. 2,5-HD intoxication induced an upregulation of nNOS expression and NADPHd reactivity in the gracile nucleus in LC rats, associated with hyperalgesia and hypersensitivity to temperature and pressure on foot. The 2,5-HD intoxication-induced upregulation of nNOS expression and NADPHd reactivity in the gracile nucleus was attenuated in ZDF rats, and functional neuropathic changes was further worsened on the treated side. The results suggest that impaired axonal transport, resulting from axonal damage on peripheral nerve, causes upregulation of nNOS expression in the gracile nucleus; and axonal damage-induced nNOS expression in the gracile nucleus is consistently attenuated in ZDF rats both at baseline level and in chemical intoxication. Reduction of nNOS-NO generation in the gracile nucleus is involved in the pathophysiological changes of neuropathies in Type II diabetes and in 2,5-HD intoxication in ZDF rats.
4. Methods and materials
4.1. Animal preparation
These experiments were performed using inbred male Zucker diabetic fatty (ZDF Gmi™fa/fa) rats (4 months) and age-matched lean, non-diabetic (ZDF Gmi™ +/+ or +/fa) control rats (Genetic Models, Inc., Indianapolis) (Peterson, 1995; Rong, Ma, 2011). The protocol was approved by the Animal Care and Use Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, and was in accord with AAALAC and NIH guidelines. The animals were maintained on a 12-h light-dark cycle in temperature and humidity controlled rooms. Food (Purina 5008 rat chow) and water were available ad libitum.
4.2. Measurements of Mechanical, Thermal, and Cold Hyperalgesia
Experiments were conducted to determine the foot withdrawal responses to mechanical, heat, and cold stimuli of ZDF diabetic rats compared to LC rats. Mechanical stimuli tests were performed by the application of Von Frey filaments from a Semmes-Weinstein Von Frey Anesthesiometer Kit as previously described (Hargreaves, et al., 1988; Rong, Ma, 2011; Zhuang, et al., 1997). Different diameters, ranging from 1.65g to 6.65g, of the Von Frey filaments were applied, starting from the weakest, in ascending order to the dorsal surface of the rat’s hind paw. A single gram of stimulus consisted of 6-8 applications of a von Frey filament within a 2-3 sec period on each hind paw. The mechanical threshold was expressed in grams, which was the amount of stimuli applied to the rat’s hind paw to induce a 50 % response of foot withdrawal. Withdrawal latencies of the hind foot were tested using a brass tool maintained at 4°C and 52°C to determine the withdrawal latencies to cold and heat stimuli, respectively. The brass tool was applied to the dorsal surface of the hind foot once in each trial, for five trials (Hargreaves, et al., 1988; Kim, Chung, 1992; Rong, Ma, 2011). The withdrawal latencies were expressed in seconds, which was the length of time for the rat to withdraw its hind foot in reaction to the stimulation.
4.3. Intoxication with 2,5-HD and Experimental Protocol
The rats were anesthetized by an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (13 mg/kg). The neurotoxicity of 2,5-HD in rats were performed by systemic injection plus local administration of the compound to induce intoxication on the sciatic nerve and conducted the same as previously described (Al-Chaer, et al., 1997; LoPachin, et al., 1994). Briefly, animals were treated by a single dose injection of 2,5-HD (400 mg/kg, i.p.) to produce systemic intoxication. Then, the region of the left gluteus was exposed and the sciatic nerve was identified under an operation microscope. The nerve was carefully isolated and placed under a plastic paper, and 10 μl of 2,5-HD (diluted 1:1 with Ringer’s solution) was injected and spread over the left side of the sciatic nerve about 2.0 cm length by using a 50 μl microsyringe three times at 10 minutes each during 30 minutes. The incision was closed, and the rats were allowed to recover for 24-48 h. The withdrawal latencies and mechanical tolerance thresholds of the foot were measured before and 3, 6, and 14 days after the treatment. At the end of the experiment, rats were perfused with 4% paraformaldehyde under anesthesia with sodium pentobarbital (50 mg/kg, i.p.). Sections of rat medulla tissue were examined by immunolabeling with polyclonal antibodies directed against nNOS and by NADPH diaphorase (NADPHd) histochemistry.
4.4. Histological Method
Fourteen days after intoxication, the rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). The chest cavity was opened, and a cannula was implanted into the ascending aorta via the left ventricle, and the right atrium was cut. Perfusion was performed using 100-150 ml of 0.9% NaCl, then 4% paraformaldehyde in sodium phosphate buffer for 45 minutes (Ma, et al., 1997; Ma, et al., 2000). The brain was rapidly removed and postfixed with 4% paraformaldehyde in 0.1 M phosphate buffer at 4 °C overnight and then placed in 30% sucrose for 24 hours. The lower brainstem was cut coronally at 30 μm thickness on a cryostat (−18 °C). According to the topography of the important nuclei located along the rostrocaudal axis, the medulla oblongata was conventionally divided and cut into quadrants as previously described (Kuo, et al., 1994; Ma, et al., 1997; Ma, et al., 2000). The 1st and 2nd quadrant belong to the caudal region of the gracile nucleus, while the 3rd and 4th quadrant belong to the rostral region of the gracile nucleus. The cross sections containing the four quadrants were compared in immunohistochemistry and NADPHd histochemistry.
4.5. nNOS immunohistochemistry
The tissue sections were incubated in 0.1M phosphate-buffered saline (PBS) for 15 min, 3% normal goat serum for 1 hour, and 0.05% Triton X-100, 0.1M PBS in heparin containing 1:800 dilution of a rabbit polyclonal nNOS (Transduction Laboratories, Lexington, KY) overnight at 4° C (Ma, et al., 2000). The antigen-antibody complex was visualized using the DAB method as described by the manufacturer (Zymed Laboratories Inc., South San Francisco, CA) using Streptavidin-Biotin Amplification. The sections were then counterstained with hematoxylin and mounted on poly-L-lysine coated slides for examination using a microscope.
4.6. NADPH-diaphorase histochemistry
The cross sections of the medulla were also examined by NADPHd histochemistry. NADPHd staining was performed by incubating slide-mounted tissue sections in 0.1 M Tris HCl (pH 8.0), 0.3% Triton X-100 containing 1.0 mM reduced nicotinamide adenine dinucleotide phosphate and 0.2 mMnitro blue tetrazolium, at 37 °C for 90-120 min (Ma, et al., 1997; Ma, et al., 2000). Washing the sections with 0.1M PBS stopped the reaction. The sections were mounted on poly-L-lysine coated slides, mounted and then photographed and examined under a light microscope.
4.7. Data presentation and statistical analysis
The nNOS immunoreactivity and NADPHd reactivity in the brainstem nuclei were expressed as the number of positive cells in a microscopic area (200 × 200 μm) as described (Kuo, et al., 1994; Ma, et al., 1997; Ma, et al., 2000). The micrographs were quantified using a microscope with reticule grid to measure the number of positive cells containing color staining in 8-10 non-overlapping tissue sections in each nucleus. An averaged number of positive neurons in each nucleus, per ipsilateral and contralateral sides for each animal were obtained. The quantitation for all subjects was determined in a blinded fashion before comparison of different groups. The orders of measurements between control and intervention animals were randomized.
Results were expressed as mean ± SEM. Five to seven rats were used for each defined group. Analysis of variances and Student's t-test were used to analyze significant differences. P values less than 0.05 were considered significant.
Highlights.
We report selective impairment of nNOS expression in the gracile nucleus in ZDF rats.
nNOS expression is up-regulated in the gracile nucleus by 2,5-HD intoxication in rats.
2,5-HD-induced nNOS expression in the gracile nucleus is attenuated in ZDF rats.
We examine functional neuropathic changes in the rats with and without intoxication.
Impaired gracile nNOS levels is involved in neuropathic pathophysiology in ZDF rats.
Acknowledgments
This research was supported by ADA RA0056 and NIH Grants AT004620 and AT004504 to SXM. These studies were conducted at the biomedical research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Abbreviations Used
- 2,5-HD
2,5-hexanedione
- LC
lean control
- NO
nitric oxide
- NADPHd
NADPH diaphorase
- nNOS
neuronal nitric oxide synthase
- ZDF
Zucker Diabetic Fatty
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J. Neurophysiol. 1996;76:2661–2674. doi: 10.1152/jn.1996.76.4.2661. [DOI] [PubMed] [Google Scholar]
- [2].Al-Chaer ED, Westlund KN, Willis WD. Nucleus gracilis: an integrator for visceral and somatic information. J. Neurophysiol. 1997;78:521–527. doi: 10.1152/jn.1997.78.1.521. [DOI] [PubMed] [Google Scholar]
- [3].Amin AR, Cesare PE, Vyas P, Attur M, Tzeng E, Billiar TR, Stuchin SA, Abramson SB. The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: Evidence for up-regulated neuronal nitric oxide synthase. J. Exp. Med. 1995;182:2097–2102. doi: 10.1084/jem.182.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anthony DC, Giangaspero F, Graham DG. The spatio-temporal pattern of the axonopathy associated with the neurotoxicity of 3,4-Dimethyl-2,5-Hexanedione in the rat. J. Neuropathol. Exp. Neurol. 1983;42:548–560. doi: 10.1097/00005072-198309000-00007. [DOI] [PubMed] [Google Scholar]
- [6].Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;18:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- [7].Cliffer KD, Hasegawa T, Willis WD. Responses of neurons in the gracile nucleus of cats to innocuous and noxious stimuli: basic characterization and antidromic activation from the thalamus. J. Neurophysiol. 1992;68:818–832. doi: 10.1152/jn.1992.68.3.818. [DOI] [PubMed] [Google Scholar]
- [8].Dinerman JL, Lowenstein CJ, Snyder SH. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circ. Res. 1990;73:217–222. doi: 10.1161/01.res.73.2.217. [DOI] [PubMed] [Google Scholar]
- [9].Duarte IDG, Lorenzetti BB, Ferreira SH. Peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Euro. J. Pharmacol. 1990;186:289–293. doi: 10.1016/0014-2999(90)90446-d. [DOI] [PubMed] [Google Scholar]
- [10].Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O’Brien PC, Melton LJ. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort. Neurology. 1993;43:817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- [11].Gross SS, Wolin MS. Nitric oxide: Pathophysiological mechanisms. Annu. Rev. Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- [12].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- [13].Kava R, Greenwood MRC, Johnson PR. Zucker (fa/fa) rat. ILAR. News. 1990;32:4–8. [Google Scholar]
- [14].Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- [15].Kumar A, Raghubir R, Srimal RC, Dhawan BN. Evidence for involvement of nitric oxide in pretectal analgesia in rat. Neuroreport. 1993;4:706–708. doi: 10.1097/00001756-199306000-00026. [DOI] [PubMed] [Google Scholar]
- [16].Kuo H, Grant S, Muth N, Hengemihle J, Ingram DK. The correlation between neuron counts and optical density of NADPH-diaphorase histochemistry in the rat striatum: a quantitative study. Brain Res. 1994;660:57–65. doi: 10.1016/0006-8993(94)90838-9. [DOI] [PubMed] [Google Scholar]
- [17].Leem JW, Lee BH, Willis WD, Chung JM. Grouping of somatosensory neurons in the spinal cord and the gracile nucleus of the rat by cluster analysis. J. Neurophysiol. 1994;72:2590–2597. doi: 10.1152/jn.1994.72.6.2590. [DOI] [PubMed] [Google Scholar]
- [18].Kilo S, Berghoff M, Hilz M, Freeman R. Neural and endothelial control of the microcirculation in diabetic peripheral neuropathy. Neurology. 2000;54:1246–1252. doi: 10.1212/wnl.54.6.1246. [DOI] [PubMed] [Google Scholar]
- [19].Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HSV, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–631. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- [20].LoPachin RM, Lehning EJ, Stack EC, Hussein SJ, Saubermann AJ. 2,5-Hexanedione alters elemental composition and water content of rat peripheral nerve myelinated axons. J Neurochem. 1994;63:2266–2278. doi: 10.1046/j.1471-4159.1994.63062266.x. [DOI] [PubMed] [Google Scholar]
- [21].Ma SX, Holley AT, Sandra A, Cassell MD, Abboud FM. Increased expression of nitric oxide synthase in the gracile nucleus of aged rats. Neuroscience. 1997;76:659–663. [PubMed] [Google Scholar]
- [22].Ma SX, Wei X, Cornford ME, Vahabnezhad I, Li XY. Responses of Nitric oxide synthase in the gracile nucleus to sciatic nerve injury in young and aged rats, Brain. Research. 2000;855:124–131. doi: 10.1016/s0006-8993(99)02379-3. [DOI] [PubMed] [Google Scholar]
- [23].McComas AJ. Responses of the rat dorsal column system to mechanical stimulation of the hindpaw. J. Physiol. 1963;166:435–448. doi: 10.1113/jphysiol.1963.sp007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Europ. J. Clin. Invest. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- [25].Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K. Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J. Neurosci. 1995;15:7633–7643. doi: 10.1523/JNEUROSCI.15-11-07633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ohara S, Roth KA, Beaudet LN, Schmidt RE. Transganglionic neuropeptide Y response to sciatic nerve injury in young and aged rats. J. Neuropathol. Exp. Neurol. 1994;53:646–662. doi: 10.1097/00005072-199411000-00012. [DOI] [PubMed] [Google Scholar]
- [27].Otto KJ, Wyse BD, Cabot PJ, Smith MT. Longitudinal study of painful diabetic neuropathy in the Zucker diabetic fatty rat model of type 2 diabetes: impaired basal G-protein activity appears to underpin marked morphine hyposensitivity at 6 months. Pain. Med. 2011;12:437–450. doi: 10.1111/j.1526-4637.2011.01067.x. [DOI] [PubMed] [Google Scholar]
- [28].Peterson RG. The Zucker diabetic fatty (ZDF) rat. In: Shafrir E, editor. Lessons from Animal Diabetes V. Smith-Gordon; Great Britain: 1995. pp. 225–230. [Google Scholar]
- [29].Rong PJ, Ma SX. Electroacupuncture Zusanli (ST36) on release of nitric oxide in the gracile nucleus and improvement of sensory neuropathies in Zucker Diabetic Fatty rats. Evid. Based Complement. Alternat. 20112011:134545. doi: 10.1093/ecam/nep103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Russell JW, Berent-Spillson A, Vincent AM, Freimann CL, Sullivan KA, Feldman EL. Oxidative injury and neuropathy in diabetes and impaired glucose tolerance. Neurobiol. Dis. 2008;30:420–429. doi: 10.1016/j.nbd.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sasaki T, Yasuda H, Maeda K, Kikkawa R. Hyperalgesia and decreased neuronal nitric oxide synthase in diabetic rats. Neuro. Report. 1998;9:243–247. doi: 10.1097/00001756-199801260-00013. [DOI] [PubMed] [Google Scholar]
- [32].Sessa WC, Harrison JK, Luthin DR, Pollock JS, Lynch KR. Genomic analysis and expression patterns reveal distinct genes for endothelial and brain nitric oxide synthase. Hypertension. 1993;21:934–938. doi: 10.1161/01.hyp.21.6.934. [DOI] [PubMed] [Google Scholar]
- [33].Spencer PS, Sabri MI, Schaumburg HH, Moore CL. Does a defect of energy metabolism in the nerve fiber underlie axonal degeneration in polyneuropathies? Ann Neurol. 1979;5:501–507. doi: 10.1002/ana.410050602. [DOI] [PubMed] [Google Scholar]
- [34].Thippeswamy T, Morris R. Cyclic guanosine 3’,5’-monophosphate-mediated neuroprotection by nitric oxide in dissociated cultures of rat dorsal root ganglion neurons. Brain Res. 1997;774:116–122. doi: 10.1016/s0006-8993(97)81694-0. [DOI] [PubMed] [Google Scholar]
- [35].Ueyama T, Houtani T, Ikeda M, Sato K, Sugimoto T, Mizuno N. Distribution of primary afferent fibers projecting from hindlimb cutaneous nerves to the medulla oblongata in the cat and rat, J. Comp. Neurol. 1994;341:145–158. doi: 10.1002/cne.903410202. [DOI] [PubMed] [Google Scholar]
- [36].Vinik AI, Holland MT, Le Beau JM, Liuzzi FJ, Stansberry KB, Colen LB. Diabetic neuropathies, Diabetes. Care. 1992;15:1926–1975. doi: 10.2337/diacare.15.12.1926. [DOI] [PubMed] [Google Scholar]
- [37].Wessels WJT, Feirabend HKP, Marani E. Development of projections of primary afferent fibers from the hindlimb to the gracile nucleus: a WGA-HRP study in the rat. Dev. Brain Res. 1991;63:265–279. doi: 10.1016/0165-3806(91)90086-x. [DOI] [PubMed] [Google Scholar]
- [38].Wu W, Liuzzi FJ, Schinco FP, Depto AS, Li Y, Mong JA, Dawson TM, Snyder SH. Neuronal nitric oxide synthase is induced in spinal neurona by traumatic injury. Neuroscience. 1994;61:719–726. doi: 10.1016/0306-4522(94)90394-8. [DOI] [PubMed] [Google Scholar]
- [39].Wuarin-Bierman L, Zahnd GR, Kaufmann F, Burcklen L, Alder J. Hyperalgesia in spontaneous and experimental animal models of diabetic neuropathy. Diabetolgia. 1987;30:653–658. doi: 10.1007/BF00277324. [DOI] [PubMed] [Google Scholar]
- [40].Zhang X, Meister B, Elde R, Verge VMK, Hokfelt T. Large calibre primary afferent neurons projecting of the gracile nucleus express neuropeptide Y after sciatic nerve lesions: an immunohistochemical and in situ hybridization study in rats, Europ. J. Neurosci. 1993;5:1510–1519. doi: 10.1111/j.1460-9568.1993.tb00219.x. [DOI] [PubMed] [Google Scholar]
- [41].Zhuang H, Wuarin L, Fei Z, Ishii DN. Insulin-like growth factor (IGF) gene expression is reduced in nueronal tissues and liver from rats with non-insulin dependent diabetes mellitis, and IGF treatment Ameliorates diabetic neuropathy. J. Pharm. Exp. Therapeutics. 1997;283:366–374. [PubMed] [Google Scholar]