Abstract
Background
Obesity promotes a state of low-grade inflammation that exacerbates chronic inflammatory diseases such as asthma and inflammatory bowel disease. In transplantation, the survival of organs transplanted into obese patients is reduced compared to allografts in lean recipients. However, whether this is due to increased alloimmunity remains to be addressed conclusively.
Methods
We used a mouse model of high fat diet (HFD)-induced obesity and assessed immune responses to allogeneic stimulation in vitro, allogeneic splenocyte immunization in vivo, and allogeneic heart transplantation.
Results
Our results indicate that HFD altered the composition and phenotype of splenic antigen-presenting cells (APCs) that led to their enhanced capacity to stimulate T cells. Immunization with allogeneic splenocytes in vivo resulted in increased alloreactivity, as determined by IFNγ production. Moreover, cardiac allograft rejection in HFD mice was modestly accelerated compared to aged-matched control animals fed a low fat diet (LFD), correlating with enhanced alloreactive T cell function.
Conclusions
Our results highlight the increased alloresponse triggered by HFD-induced obesity and its negative impact on transplant outcome.
INTRODUCTION
In the last 30 years, significant changes in diet and physical activity in the American people have greatly contributed to the current obesity epidemic1. Obesity has a stark impact on public health, as it is associated with an increase in cardiovascular diseases, severity of asthma and gastrointestinal inflammatory diseases2–4. The estimated cost for direct obesity-related medical treatment in the US is over $100 billion5 and this amount is increasing.
The location and duration of fat deposition impacts the severity of the disease, with visceral obesity being linked to inflammation of white adipose tissue6. At the cellular level, adipocytes produce inflammatory cytokines that enhance the prevalence of local pro-inflammatory M1 macrophages at the expense of anti-inflammatory and tissue-repairing M2 macrophages7. The change in the local cytokine milieu also impacts the local adaptive immune system, by recruiting CD4+ Th1 and CD8+ cytotoxic T cells while depleting Th2 and regulatory T (Tregs) cells involved in tissue homeostasis8. This local adipose inflammation may exacerbate chronic inflammatory diseases. Indeed, preclinical mouse models indicate that diet-induced inflammation increases the severity of T cell-mediated diseases in models of inflammatory bowel disease and multiple sclerosis9–11.
The role for obesity in transplant alloreactivity remains controversial. When kidneys from a single donor were transplanted into different recipients, the organs grafted into obese patients displayed reduced long-term survival compared to the paired kidneys transplanted into lean recipients12. However, whether this is due to increased alloimmunity or to non-immune factors leading to increased graft damage remains unclear as obesity is a risk factor for non-immune mediated comorbidities and surgical complications, which might affect graft function and fate3,13. While some studies have reported and association between increased body mass index (BMI) with delayed graft function14,15 (itself a risk for acute rejection), and risk of acute rejection within the first 6 months post-transplantation15,16, other studies have failed to reveal such associations17,18. The discrepancy in the literature may be due to the fact that not all types of obesity seem to drive inflammation, as visceral obesity is more closely associated with inflammation19, and it may therefore be the type of obesity that impacts alloimmunity. Thus, despite elegant studies linking obesity to enhanced autoimmunity, there is a lack of consensus regarding whether obesity affects alloimmunity. In this study, we used a mouse model of HFD-induced obesity that has been reported to mimic inflammatory obesity linked with metabolic syndrome20, to investigate the consequences of this type of obesity on alloimmune responses.
The composition of the immune system, as well as T cell responses were assessed in male C57BL/6 mice fed a HFD for at least 12 weeks, using age-matched control male mice fed a nutrient-equivalent LFD. APCs from mice fed a HFD elicited more robust proliferation and cytokine production by CD8+ and CD4+ T cells than APCs from LFD controls. HFD-fed mice displayed enhanced alloreactivity following immunization with fully allogeneic splenocytes. In a minor mismatched heart transplantation model, HFD mice rejected cardiac allografts with faster kinetics compared to their LFD counterparts, correlating with increased effector function of alloreactive T cells. Overall, our results indicate that HFD-induced obesity exacerbates the alloimmune response, and suggest that obese patients could be at higher risk for T cell-mediated immune rejection of solid organ transplants.
MATERIALS AND METHODS
Mice and diets
Six-week old male C57BL/6 mice (Jackson Laboratories) were fed for at least 12 weeks a HFD or a LFD in which 60% or 10% kcal, respectively, came from fat (D12492i or D12450Bi, Research Diets Inc.). For most experiments, HFD diet weighed over 45 grams. Eight week-old female BALB/c and 129/Sv mice were purchased from Charles River and Taconic, respectively. Marilyn anti-male TCR transgenic female mice were obtained from NCI Frederickson and have been described previously21.
Weight and glucose assessment
Body weight was measured using a laboratory scale. Blood glucose was measured after 6h of fasting using glucose strips.
Purification of APCs and T cells
HFD and LFD mice were sacrificed, spleens removed, prepared into a single cell suspension and red blood cells lysed using ammonium chloride potassium hypotonic buffer. T cells were purified using the ‘negative selection T cell purification’ kit (StemCell) with magnetic beads. T cells were verified to have a purity of > 90% by flow cytometry and were labeled with 5 µM CFSE. For preparation of splenic APCs, spleens were incubated for 10 min with biotin-labeled antibodies against CD4, CD8, Thy1.2 and NK1.1 (GK4.5, 53–6.7, Thy1.2 and NK1.1 from eBioscience), further incubated for 5 min with streptavidin-coupled magnetic beads (ThermoFisher) and finally negatively selected using a magnet (StemCell). The presence of T cells and NK cells was less than 1%. APCs were irradiated with 2000 rads using a Cesium-137 source. Cell quantification was performed using Countess (Invitrogen). 5×104 T cells were cultured in complete (c)DMEM (10% Fetal Calf Serum, 50 µM β-mercaptoethanol, 1× non-essential aminoacids, 1× L-glutamine, 1× penicillin/strepmtomycin and 10 mM HEPES) with 2×105 APCs in the presence of 1 µg/ml of anti-CD3 monoclonal antibody (145-2C11 mAb) for 3 days at 37°C and 8% CO2.
Flow cytometry
To assess the composition and phenotype of APCs, splenocytes (1–2×106 cells/tube) resuspended in FACS buffer (1% BSA/PBS/ 0.09% sodium azide) were stained with fluorochrome-labeled antibodies against CD11b, CD11c, Gr-1, B220, Kb, IAb, CD80, CD86, CD40 and flurochrome-matched isotype controls. T cell preparations were stained with fluorochrome-labeled anti-CD4, -CD8, -CD44, -CD62L antibodies in FACS buffer, fixed and permeabilized in ‘Foxp3 staining buffer’ (eBioscience) and stained with anti-Foxp3 fluorochrome-labeled antibody. Antibodies were purchased from eBioscience and flow cytometry acquisition was performed on an LSR Fortessa (BD Biosciences). The analysis was performed using FlowJo software.
IFNγ ELISpot
IFNγ ELISpots were performed using 106 cervical lymph node or splenic cells from C57BL/6 mice, cultured for 18h in the presence of 2000 rads γ-irradiated T-depleted allogeneic splenocytes, using an IFNγ ELISpot kit and following the manufacturer’s instructions (BD Biosciences). Spot quantification was performed using a C.T.L. quantification device.
Donor-specific transfusion (DST) immunization
C57BL/6 mice fed a LFD or HFD for >12 weeks were immunized subcutaneously with BALB/c splenocytes (106) at the base of the neck. Cervical lymph nodes draining this site were harvested 2 weeks later and their leukocytes were restimulated with syngeneic or donor irradiated splenocytes for analysis of IFNγ production by ELISpots, as described above.
Heart transplantation
Abdominal heterotopic cardiac transplantation was performed using a technique adapted from that originally described by Corry et al22. Briefly, cardiac allografts were transplanted in the abdominal cavity by anastomosing the aorta and pulmonary artery of the graft end-to-side to the recipient’s aorta and vena cava, respectively. The day of rejection was defined as the last day of a detectable heartbeat.
Statistical analysis
Cardiac graft mean survival time, standard deviation and p-values were calculated using Kaplan–Meier/Log-rank test methods. Comparisons of means were performed using the Student’s t-test, the Tukey test for multiple comparisons, or the Mann-Whitney test when appropriate.
RESULTS
HFD-induced obesity enhances the stimulatory capacity of APCs in vitro
As a first step to test the impact of HFD-induced obesity on alloimmunity, aged-matched male C57BL/6 mice were fed for at least 12 weeks a HFD (60% kcal from fat) or a control LFD (10% kcal from fat). As expected, the body weight and fasting blood glucose levels were increased in HFD-fed when compared to LFD-fed mice (Fig. 1).
Figure 1. Mice fed a HFD are obese and hyperglycemic.
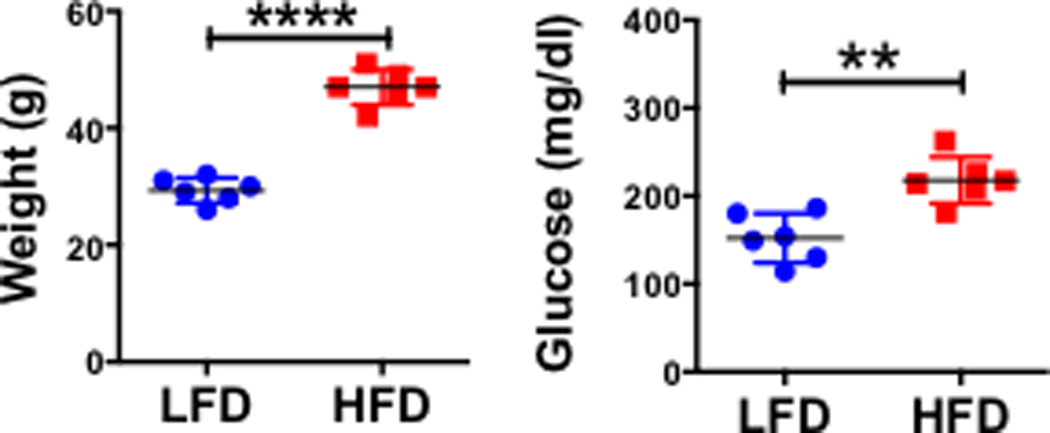
Weight (A) and fasting glucose levels (B) of C57BL/6 male mice (mean +/− SEM) fed for >12 weeks a HFD (n=6) or a LFD (n=6). Comparisons were performed using a two-tailed unpaired t test. **: p<0.01, ****: p<0.0001.
To determine if HFD-induced obesity affected the systemic T cell compartment, the composition of T cells was assessed by flow cytometry in splenocytes from HFD and LFD mice. No differences were observed in the frequencies (Fig. 2A) or total numbers (not shown) of CD4+, CD8+, CD4+Foxp3+ Tregs, or CD44loCD62Lhi naïve and CD44hiCD62Llo memory subsets. To assess whether HFD modulates T cell responses, CFSE-labeled T cells from HFD or LFD mice were stimulated for 3 days with equal numbers of APCs (T- and NK-depleted splenocytes) from HFD or LFD mice in the presence of activating soluble anti-CD3 mAb. Strikingly, anti-CD3 mAb induced greater proliferation of CD4+ and CD8+ T cells when in the presence of HFD compared to LFD APCs, regardless of whether T cells originated from HFD or LFD mice (Fig. 2B). Similarly, IL-17 expression by CD4+ and IFNγ production by CD8+ T cells were increased when T cells were stimulated with HFD, as compared with LFD, APCs, irrespective of the T cell provenance (Fig. 2C).
Figure 2. HFD results in greater T cell activation.
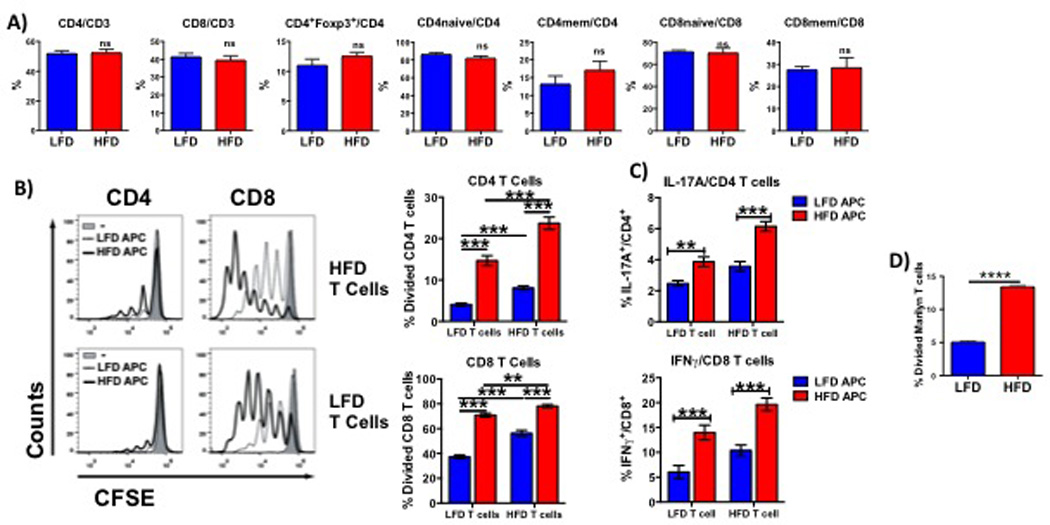
Percentages of CD4+, CD8+, Tregs, memory and naïve T cells (A) in splenocytes from HFD and LFD mice were analyzed by flow cytometry. Proliferation (B), IFNγ production by CD8+ cells (C) and IL-17 expression by CD4+ cells were assessed in HFD and LFD T cells stimulated with HFD or LFD APCs. (D) Proliferation of CFSE-labeled CD4+ Marilyn T cells stimulated for 3 days with APCs isolated from mice maintained on HFD or LFD for >12 weeks. Experiments were performed in groups of at least 3 mice and repeated at least twice. Results are displayed as mean +/− SEM. Comparisons were performed using a two-tailed unpaired t test. **: p<0.01, ***: p<0.001, ****: p<0.0001, ns: not significant.
To investigate if these effects held true during cognate alloreactive T cell interactions and to exclude the possibility that differences were due to a different TCR repertoire in HFD versus LFD mice, we investigated the proliferative capacity of TCR-transgenic T cells from lean Marilyn female mice, in which monoclonal CD4+ T cells recognize an H-Y peptide presented by I-Ab. CFSE-labeled Marilyn T cells were stimulated with APCs from HFD or LFD male mice, and similarly to the polyclonal T cell response, the proliferation of Marilyn T cells was greater when cultured with HFD than LFD APCs (Fig. 2D). These results indicate that APCs from HFD mice have an increased T cell stimulatory capacity when compared to APCs from LFD mice, suggesting that the APC compartment in HFD mice was altered, either in composition or in phenotype.
HFD-induced obesity alters the composition and phenotype of splenic APCs
To investigate whether the APC composition in the spleen was altered by the HFD, the percentages and total numbers of macrophages (CD11b+CD11c−/intGr1−), conventional dendritic cells (cDC, CD11c+CD11b+), plasmacytoid DCs (pDCs, CD11cintB220+CD11b−), CD8α DCs (CD11c+CD8α+CD11b−), B cells (B220+CD11c−CD11b−) and granulocytes (FSChiSSChi Gr1+) were analyzed by flow cytometry. The percentages and total numbers of cDCs, macrophages and granulocytes were increased in splenocytes from HFD compared to LFD mice (Fig. 3A, B). Importantly, cellularity of the spleen from HFD mice was greater than that from LFD mice, such that the total numbers of B cells and pDCs were also significantly greater in splenocytes from HFD than LFD mice (Fig. 3B). In addition, macrophages and cDCs from HFD mice expressed higher levels of the costimulatory molecule CD80 than their counterparts from LFD mice (Fig 3C), providing a possible explanation for the greater activation ability of HFD APCs.
Figure 3. HFD alters the composition and stimulatory capacity of splenic APCs.
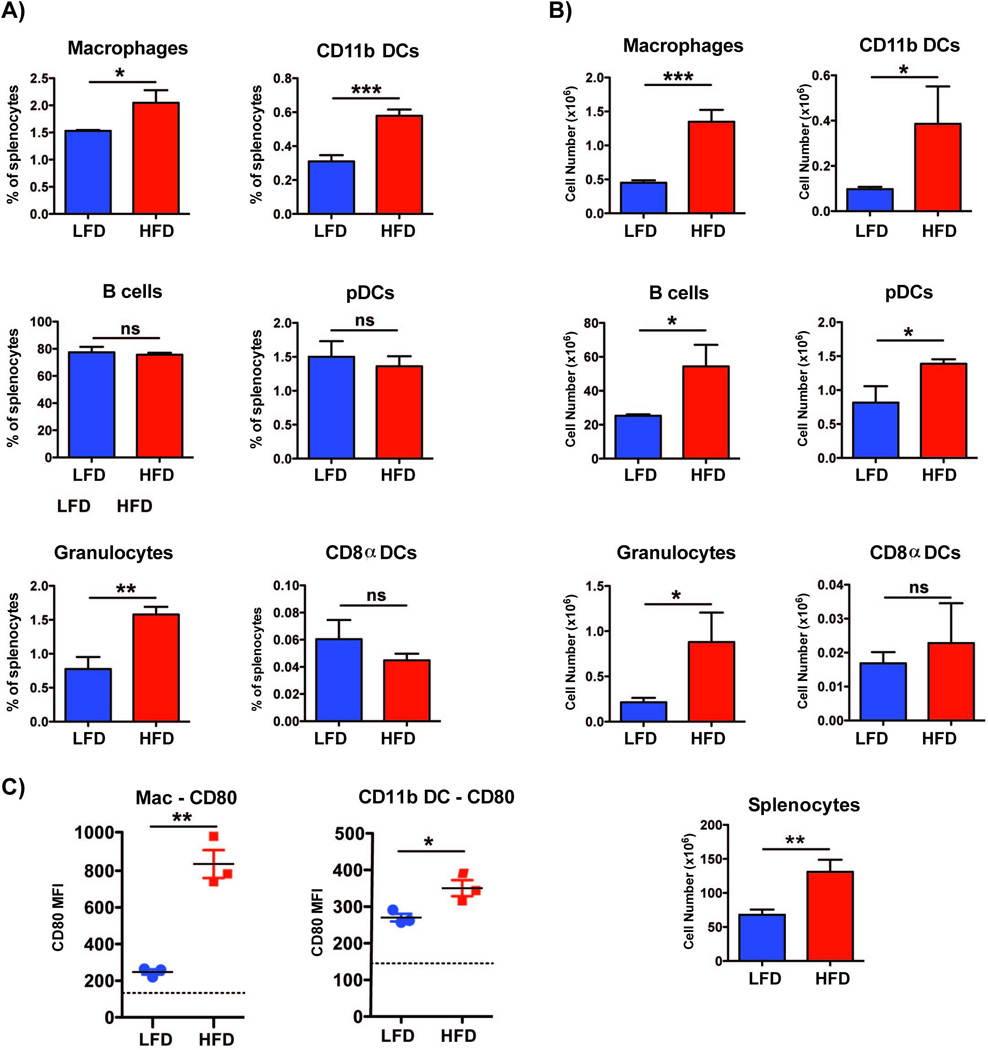
APCs were isolated from the spleens of mice maintained on HFD or LFD for >12 weeks. Percentages (A) and total numbers (B) of splenic macrophages (CD11b+CD11c−/intGr1−), conventional DCs (cDC, CD11c+CD11b+), plasmacytoid DCs (pDC, CD11cintB220+CD11b−), CD8α DCs (CD8-like DC, CD11c+CD8α+CD11b−), B cells (B220+CD11c−CD11b−) and granulocytes (FSChiSSChi Gr1+) were analyzed by flow cytometry. (C) MFI analysis of CD80 expression on macrophages and cDCs, as assessed by flow cytometry. Dotted lines represent MFI for isotype control. Three animals were analyzed in each group and the experiment was repeated twice. Results are displayed as mean +/− SEM. Comparisons were performed using two-tailed unpaired t test. *: p <0.05; **: p<0.01, ***: p<0.001, ns: not significant.
HFD-induced obesity results in greater alloresponse to allogeneic DST immunization
Our in vitro results support the hypothesis that HFD mice may develop stronger T cell immune responses in vivo. To test this possibility, LFD or HFD C57BL/6 mice (H-2b) were injected subcutaneously in the base of the neck with splenocytes from BALB/c mice (H-2d). Cells from the draining lymph nodes were harvested 2 weeks later and restimulated with syngeneic (C57BL/6) or donor (BALB/c) irradiated splenocytes to determine the frequency of IFNγ-producing cells. Unimmunized naïve C57BL/6 mice fed normal chow were used as controls. As shown in Figure 4, the production of IFNγ by cells from BALB/c-immunized HFD mice was significantly greater than that from BALB/c-immunized LFD mice. Thus, HFD-induced obese mice displayed increased alloreactivity to DST in vivo.
Figure 4. Mice in HFD develop increased alloreactivity to in vivo DST immunization.
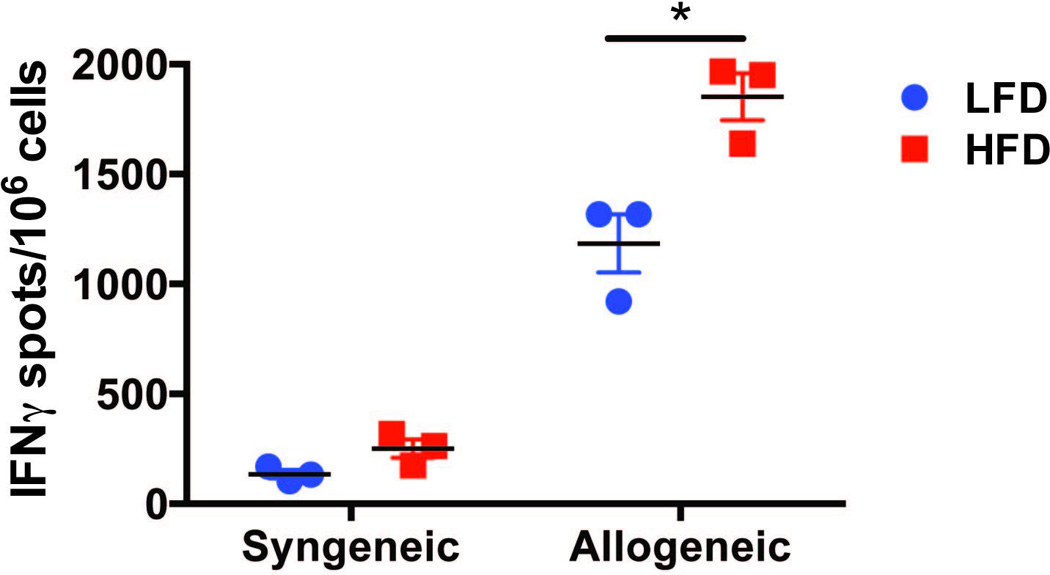
C57BL/6 mice (H-2b) in LFD and HFD were immunized with allogeneic BALB/c splenocytes (106 cells) subcutaneously under the skin of the neck and cells isolated from cervical draining lymph nodes were analyzed 14 days later by ELISpot for their IFNγ production upon in vitro restimulation with irradiated syngeneic or donor splenocytes. The data (mean +/− SEM of 3 mice/group) are representative of 2 independent experiments with at least 3 mice/group. Comparisons were performed by one-way ANOVA. *: p <0.05.
HFD-induced obesity results in faster cardiac allograft rejection kinetics
To determine the effect of HFD on the alloimmune response towards solid organs, hearts from BALB/c females were transplanted into C57BL/6 HFD or LFD male mice. In this fully mismatched strain combination, both LFD and HFD mice rejected their grafts within 7–8 days (data not shown). Because the tempo of rejection of fully mismatched cardiac allografts may already be maximal in LFD mice, we investigated the effect of HFD-induced obesity on the kinetics of rejection of minor mismatched allografts. To this end, hearts from 129/Sv (H-2b) females were transplanted into C57BL/6 (H-2b) HFD and LFD male mice. In this strain combination, cardiac allografts transplanted into HFD recipients underwent an accelerated rejection compared to LFD recipients, whereas obesity did not affect the survival of syngeneic heart transplants (Fig. 5A). Allospecific T cell responses, measured 2–3 weeks post-rejection, were significantly increased in transplanted HFD mice, as demonstrated by higher 129/Sv-specific IFNγ ELISpots (Fig. 5B). This difference was not solely driven by the one high producer, as elimination the highest IFNγ-producer in each group still resulted in statistically significant differences (data not shown). Taken together, our data support the conclusion that HFD-induced obesity moderately exacerbates alloimmune responses and can accelerate rejection of solid organs.
Figure 5. Mice in HFD have faster kinetics of cardiac allograft rejection.
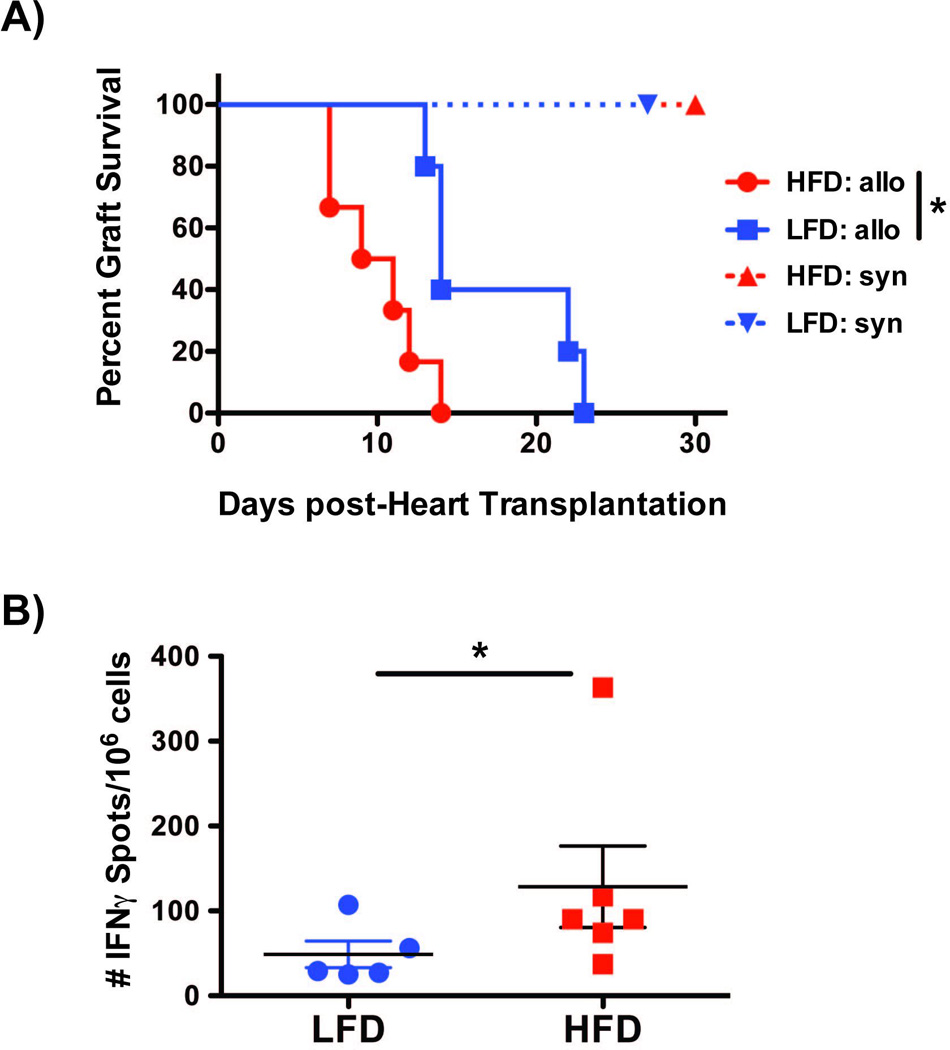
(A) Rejection kinetics of syngeneic (C57BL/6, LFD n=5, HFD n=5; both survived >d30 post-transplantation), and minor mismatched 129/Sv (H2) cardiac allografts (LFD n=5, mean survival time +/− SD = 17 +/− 5; HFD n=6, mean survival time +/− SD = 10 +/− 3) C57BL/6 mice. Comparisons were performed using Kaplan–Meier/Log-rank test. (B) Splenocytes were harvested from all the mice in A, 2–3 weeks following rejection of 129/Sv cardiac allografts by LFD and HFD C57BL/6 recipients. An IFNγ ELISpot assay was performed upon overnight restimulation with γ-irradiated T cell-depleted 129/Sv splenocytes. Data are displayed as mean +/− SEM. Comparisons were performed using a one-tailed Mann-Whitney test.
DISCUSSION
Most studies suggest that obesity has a negative impact on graft outcome23–28. However, because obesity is associated with surgical complications, cardiovascular comorbidities, and other factors that may negatively impact transplant function and half-life, whether obesity directly affects alloimmunity is unclear. Using an experimental setting that has been reported to model inflammatory obesity, we tested directly whether HFD-induced obesity impacts T cell responses and alloimmunity. Our results indicate that HFD-induced obesity modestly enhances T cell alloimmunity and accelerates allograft rejection.
Several animal models of obesity have been used in various experimental settings, including genetic- and diet-induced models. The genetic models comprise mice that are deficient in the leptin/leptin receptor axis and become obese from hyperphagia29, whereas the HFD-mediated obese mice have increased leptin levels (data not shown) and do not display hyperphagia but rather have calorie-rich diet-induced weight gain. We used male C57BL/6 mice subjected to a HFD because they have been reported to develop obesity, hyperinsulinemia, hyperglycemia and hypertension that mimic the metabolic syndrome observed in patients with inflammatory obesity, whereas other mouse strains are more resistant to these effects20. In addition, we used a LFD as a control diet, as opposed to normal chow, to ensure that only the kilocalories from fat differed between the 2 diets such that possible differences in graft outcome could not be ascribed to different dietary antigens between the 2 feeds. Our results using HFD-fed obese male C57BL/6 mice demonstrate that HFD leads to increased T cell proliferation, or IFNγ production in response to alloantigen in 3 independent models: in vitro using Marilyn T cells stimulated with minor antigen-expressing APCs, in vivo following DST immunization with fully allogeneic splenocytes, and in vivo in a model of minor mismatched heart transplantation.
The rejection kinetics of heart allografts in HFD obese mice were only modestly accelerated compared to LFD mice, even when all parameters were carefully controlled: LFD and HFD hosts genetically identical and aged-matched, matched composition of the LFD and HFD that differed only in kilocalories from fat, controlled environment. Moreover, the effects of HFD-induced obesity were only detectable in mice transplanted with minor rather than major mismatched cardiac allografts and when HFD had been administered for over 12 weeks but not 8–10 weeks (data not shown). These data suggest that it takes time for the immune consequences of obesity to take hold. Similarly, frequency of IFNγ-producing cells following ex vivo donor restimulation was only slightly increased in HFD versus LFD mice. This may be due to the fact IFNγ production was measured 2–3 weeks post-rejection. At this time point, the differentiation of alloreactive T cells into effector/memory cells in LFD mice may have caught up with that in HFD mice. Future experiments investigating the kinetics of effector alloreactive T cell differentiation following transplantation should be able to determine if differences between LFD and HFD mice become more apparent when compared at a time point just before rejection starts occurring in the HFD mice. The modest effects of HFD-induced obesity may explain the difficulty in detecting the consequences of obesity on alloimmunity in obese patients with different degrees of genetic disparities between donors and recipients, different duration of obesity, and when all types of obesity are considered together. In patients, it has been suggested that waist circumference or waist-to-hip-ratio may be a better prognostic factor for transplant outcomes than BMI19,30,31, as abdominal fat rather than fat accumulation in other parts of the body has been linked with increased inflammation32 and therefore may be the type of obesity that results in enhanced alloimmunity.
The fact that not all types of obesity may increase alloimmunity is exemplified by results in leptin-deficient obese mice that were transplanted with allogeneic skin grafts and displayed prolongation of graft survival rather than accelerated graft rejection33. In this case, leptin deficiency was associated with increased Tregs and a Th2 profile, prompting the authors to speculate that the adverse outcome of transplanted organs in obese patients may be due to increased leptin levels, which are pro-inflammatory. Because HFD-induced obesity in male C57BL/6 mice but not leptin-deficiency is associated with visceral inflammation and because HFD C57BL/6 mice display elevated levels of serum leptin (data not shown), our results are consistent with this interpretation and we favor the hypothesis that only inflammatory obesity leads to enhanced alloimmunity. However, serum levels of TNFα, IL-1β and IL-6 were similar in our lean and HFD mice (data not shown), suggesting that visceral inflammation may be sufficient to promote alloreactivity in obesity without requiring detectable levels of systemic inflammation in the circulation. Alternatively, visceral inflammation and increased alloreactivity may be 2 independent consequences of obesity.
Our results suggest that the basis for increased alloreactivity may be due to APCs from HFD mice having intrinsically enhanced capacity to stimulate T cells, correlating with increased expression of the costimulatory molecule CD80. These results are consistent with a recent report showing that both aging mice that acquire increased visceral adiposity as they get older, and HFD-fed obese mice respond with higher cytokine production than lean mice to immune stimulation in vivo with anti-CD40 and IL-234. Our results also show increased production of IL-17 by CD4+ T cells stimulated with APCs from HFD mice, consistent with data showing that obesity leads to a Th17 bias and increased susceptibility to autoimmunity35. The mechanisms that link obesity to increased baseline APC function and increased Th17 frequency as well as subsequent adaptive alloreactivity remain to be elucidated.
Overfeeding has been linked with increased intestinal permeability to endotoxin36, which may cause innate inflammation and be able to lead to secondary augmentation of adaptive alloimmunity. Moreover, diet is a strong determinant of the microbial composition of the intestine37,38. Because there is a known cross-talk between the microbiota and the immune system39,40, one possibility is that obesity-induced dysbyosis affects systemic alloimmunity41. Another possibility is that obesity can be also linked to hyperlipidemia, and hyperlipidemia has recently been shown to promote accelerated rejection of cardiac allografts in mice, by inducing anti-donor Th17 reactivity42. Similarly, obesity is often associated with hyperglycemia and our HFD animals displayed higher serum levels of fasting glucose. However, whether hyperglycemia is another variable that affects alloimmunity remains to be determined. Obesity is also linked to an increased ratio of the pro-inflammatory adipokine leptin to the anti-inflammatory adipokine adiponectin, which may skew immune responses towards increased alloreactivity and accelerated acute rejection. Indeed, as previously mentioned, mice deficient in leptin had prolonged skin allograft survival and enhanced Treg and Th2 responses33. In contrast, mice deficient in adiponectin displayed more rapid cardiac allograft rejection43. Additional factors have linked obesity to inflammation, including inflammatory lipids44. Interestingly, atorvastatin, an inhibitor of HMG-CoA reductase that reduces levels of cholesterol, was described to have immunomodulatory capacity in models of cardiac transplantation45 and a retrospective analysis in the same study revealed that kidney transplant patients taking statins had an increased overall survival compared to the non-statin treated group. In addition to pharmacological treatment, obesity can be reduced with lifestyle changes or bariatric surgery. It would be of interest that future studies test whether lifestyle and surgical treatment of obesity restore a lower T cell activation capacity by APCs and normalize kinetics of transplant rejection.
Acknowledgments
We would like to thank Michelle Miller for critical reading and comments.
Funding: NIH/NIAID 1R01AI115716 (MLA) and NIH/NIAID 5P01AI097113 (MLA and ASC).
Abbreviations
- APC
antigen-presenting cell
- BMI
body mass index
- DST
donor-specific transfusion
- HFD
high fat diet
- LFD
low fat diet
- MST
mean survival time
- Treg
Regulatory T cell
Footnotes
Disclosure: The authors declare no conflicts of interest.
LLM: No conflict of interest.
DY: No conflict of interest.
KL: No conflict of interest.
LC: No conflict of interest.
YW: No conflict of interest.
ASC: No conflict of interest.
MLA: No conflict of interest.
Author participation:
participated in research design, performance of the research, data analysis and writing of the manuscript. molinero.luciana@gene.com
performed experiments and data analysis. dyin@surgery.bsd.uchicago.edu
performed experiments and data analysis. leik@uchicago.edu
performed experiments and data analysis. lchen@medicine.bsd.uchicago.edu
performed experiments. yingwang@medicine.bsd.uchicago.edu
participated in research design and data analysis. achong@surgery.bsd.uchicago.edu
participated in research design, data analysis and writing of the manuscript. malegre@medicine.bsd.uchicago.edu
REFERENCES
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 2.Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007;62:1205. doi: 10.1111/j.1398-9995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 3.Lovren F, Teoh H, Verma S. Obesity and Atherosclerosis: Mechanistic Insights. Can J Cardiol. 2015;31:177. doi: 10.1016/j.cjca.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol. 2014;5:462. doi: 10.3389/fimmu.2014.00462. 10.3389/fimmu.2014.00462. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamosky L. The obesity epidemic. While America swallows $147 billion in obesity-related healthcare costs, physicians called on to confront the crisis. Med Econ. 2013;90:14. [PubMed] [Google Scholar]
- 6.Tian YF, Chang WC, Loh CH, Hsieh PS. Leptin-mediated inflammatory signaling crucially links visceral fat inflammation to obesity-associated beta-cell dysfunction. Life Sci. 2014;116:51. doi: 10.1016/j.lfs.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 7.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262:134. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Priceman SJ, Kujawski M, Shen S, et al. Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2013;110:13079. doi: 10.1073/pnas.1311557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccio L, Cantoni C, Henderson JG, et al. Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur J Immunol. 2013;43:2089. doi: 10.1002/eji.201242836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paik J, Fierce Y, Treuting PM, Brabb T, Maggio-Price L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a−/− male mice. J Nutr. 2013;143:1240. doi: 10.3945/jn.113.174615. [DOI] [PubMed] [Google Scholar]
- 11.Gruber L, Kisling S, Lichti P, et al. High fat diet accelerates pathogenesis of murine Crohn's disease-like ileitis independently of obesity. PLoS One. 2013;8:e71661. doi: 10.1371/journal.pone.0071661. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto S, Hanley E, Hahn AB, et al. The impact of obesity in renal transplantation: an analysis of paired cadaver kidneys. Clin Transplant. 2002;16:252. doi: 10.1034/j.1399-0012.2002.01080.x. [DOI] [PubMed] [Google Scholar]
- 13.Flancbaum L, Choban PS. Surgical implications of obesity. Annu Rev Med. 1998;49:215. doi: 10.1146/annurev.med.49.1.215. [DOI] [PubMed] [Google Scholar]
- 14.Weissenbacher A, Jara M, Ulmer H, et al. Recipient and donor body mass index as important risk factors for delayed kidney graft function. Transplantation. 2012;93:524. doi: 10.1097/TP.0b013e318243c6e4. [DOI] [PubMed] [Google Scholar]
- 15.Gore JL, Pham PT, Danovitch GM, et al. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6:357. doi: 10.1111/j.1600-6143.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang SH, Coates PT, McDonald SP. Effects of body mass index at transplant on outcomes of kidney transplantation. Transplantation. 2007;84:981. doi: 10.1097/01.tp.0000285290.77406.7b. [DOI] [PubMed] [Google Scholar]
- 17.Massarweh NN, Clayton JL, Mangum CA, Florman SS, Slakey DP. High body mass index and short- and long-term renal allograft survival in adults. Transplantation. 2005;80:1430. doi: 10.1097/01.tp.0000181094.68259.88. [DOI] [PubMed] [Google Scholar]
- 18.LaMattina JC, Foley DP, Fernandez LA, et al. Complications associated with liver transplantation in the obese recipient. Clin Transplant. 2012;26:910. doi: 10.1111/j.1399-0012.2012.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinbokel T, Floerchinger B, Schmiderer A, et al. Obesity and its impact on transplantation and alloimmunity. Transplantation. 2013;96:10. doi: 10.1097/TP.0b013e3182869d2f. [DOI] [PubMed] [Google Scholar]
- 20.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 22.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Papalia T, Greco R, Lofaro D, Maestripieri S, Mancuso D, Bonofiglio R. Impact of body mass index on graft loss in normal and overweight patients: retrospective analysis of 206 renal transplants. Clin Transplant. 2010;24:E241. doi: 10.1111/j.1399-0012.2010.01258.x. [DOI] [PubMed] [Google Scholar]
- 24.Sampaio MS, Reddy PN, Kuo HT, et al. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation. 2010;89:1117. doi: 10.1097/TP.0b013e3181d2bfb2. [DOI] [PubMed] [Google Scholar]
- 25.Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002;73:70. doi: 10.1097/00007890-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 26.Meier-Kriesche HU, Vaghela M, Thambuganipalle R, Friedman G, Jacobs M, Kaplan B. The effect of body mass index on long-term renal allograft survival. Transplantation. 1999;68:1294. doi: 10.1097/00007890-199911150-00013. [DOI] [PubMed] [Google Scholar]
- 27.Grady KL, White-Williams C, Naftel D, et al. Are preoperative obesity and cachexia risk factors for post heart transplant morbidity and mortality: a multi-institutional study of preoperative weight-height indices. Cardiac Transplant Research Database (CTRD) Group. J Heart Lung Transplant. 1999;18:750. doi: 10.1016/s1053-2498(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 28.Grady KL, Costanzo MR, Fisher S, Koch D. Preoperative obesity is associated with decreased survival after heart transplantation. J Heart Lung Transplant. 1996;15:863. [PubMed] [Google Scholar]
- 29.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10:131. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham PT, Danovitch GM, Pham PC. Obesity and its impact on transplantation and alloimmunity. Transplantation. 2013;96:e71. doi: 10.1097/01.TP.0000436929.53768.93. [DOI] [PubMed] [Google Scholar]
- 31.Kovesdy CP, Czira ME, Rudas A, et al. Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant. 2010;10:2644. doi: 10.1111/j.1600-6143.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- 32.van Ree RM, de Vries AP, Oterdoom LH, et al. Abdominal obesity and smoking are important determinants of C-reactive protein in renal transplant recipients. Nephrol Dial Transplant. 2005;20:2524. doi: 10.1093/ndt/gfi052. [DOI] [PubMed] [Google Scholar]
- 33.Moraes-Vieira PM, Bassi EJ, Larocca RA, et al. Leptin deficiency modulates allograft survival by favoring a Th2 and a regulatory immune profile. Am J Transplant. 2013;13:36. doi: 10.1111/j.1600-6143.2012.04283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirsoian A, Bouchlaka MN, Sckisel GD, et al. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med. 2014;211:2373. doi: 10.1084/jem.20140116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winer S, Paltser G, Chan Y, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 36.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 37.Brandsma E, Houben T, Fu J, Shiri-Sverdlov R, Hofker MH. The immunity-diet-microbiota axis in the development of metabolic syndrome. Curr Opin Lipidol. 2015;16:16. doi: 10.1097/MOL.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 38.Zhao L. The gut microbiota and obesity: from correlation to causality. Nature reviews. Microbiology. 2013;11:639. doi: 10.1038/nrmicro3089. [DOI] [PubMed] [Google Scholar]
- 39.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 40.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alegre ML, Mannon RB, Mannon PJ. The microbiota, the immune system and the allograft. Am J Transplant. 2014;14:1236. doi: 10.1111/ajt.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan J, Bagley J, Iacomini J. Hyperlipidemia Promotes Anti-Donor Th17 Responses That Accelerate Allograft Rejection. Am J Transplant. 2015;15:2336. doi: 10.1111/ajt.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto Y, Christen T, Shimizu K, et al. Adiponectin inhibits allograft rejection in murine cardiac transplantation. Transplantation. 2009;88:879. doi: 10.1097/TP.0b013e3181b6efbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khatri P, Roedder S, Kimura N, et al. A common rejection module (CRM) for acute rejection across multiple organs identifies novel therapeutics for organ transplantation. J Exp Med. 2013;210:2205. doi: 10.1084/jem.20122709. [DOI] [PMC free article] [PubMed] [Google Scholar]


