Abstract
Background
Although the impact of obstructive sleep apnea (OSA) on cardiovascular risk is reasonably well established in middle-aged patients, debate persists whether OSA also increases this risk in the elderly. Arterial stiffness has been used as an early independent predictor of cardiovascular risk.
Study Objectives
We sought to determine whether OSA had significant effects on the arterial stiffness in the elderly population and evaluate the impacts of comorbidities on the association between arterial stiffness and OSA.
Methods
We performed a cross-sectional study in a university hospital. Elderly participants (≥60 yr) were invited to participate in our study between November 2010 and January 2013. OSA was diagnosed using gold standard polysomnography and arterial stiffness was assessed by brachial-ankle pulse wave velocity (baPWV) and cardio-ankle vascular index (CAVI) as well as by central systolic and diastolic blood pressure (cSBP and cDBP). High-sensitivity C-reactive protein (hs-CRP) level was also measured.
Results
We found no significant association between the severity of OSA and arterial stiffness-related parameters such as cSBP, cDBP, baPWV, CAVI, and hs-CRP. However, in patients without comorbid medical conditions or use of medications (n=101), we showed a modest association between OSA and arterial stiffness-related parameters and hs-CRP.
Conclusion
We conclude that OSA is associated with increased arterial stiffness in otherwise healthy elderly population, although the association was obviated by comorbidities and medications perhaps due to ceiling effects.
Keywords: obstructive sleep apnea (OSA), arterial stiffness, elderly population, pulse wave velocity, lung
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repetitive pauses in breathing during sleep resulting in an intermittent reduction in blood oxygen saturation. The prevalence of OSA is about 6–13% in the middle-aged population with higher values up to 19–37% in older individuals [1–3]. In the era of population aging, OSA in older people might be a serious health threat, but the consequences of OSA in the elderly have been debated. OSA is known to increase the risk of cardiovascular diseases, such as coronary artery disease, heart failure, and stroke, and mortality [4–7]. In older people, some authors have shown that OSA was also associated with increased blood pressure (BP) [8], high cardiovascular risk [4, 5] and high mortality [9]. On the other hand, others have shown that OSA in the elderly is not associated with either systolic/diastolic hypertension or mortality [10, 11].
Arterial stiffness has been implicated as an early independent predictor of cardiovascular risk in OSA patients [12, 13]. There are several indices for measuring arterial stiffness such as brachial-ankle pulse wave velocity (baPWV) and cardio-ankle vascular index (CAVI). A substantial body of evidence shows that these measurements of arterial stiffness are increased in middle-aged patients with OSA [12], and our group has also shown that nocturnal hypoxemia is associated with arterial stiffness in the middle-aged male patients with OSA [14]. However, there is a paucity of research addressing the association between arterial stiffness, an early marker of cardiovascular risk, and OSA in the elderly population. Considering the controversies regarding OSA in the elderly, we sought to test the hypothesis that elderly OSA patients would have increased vascular stiffness as compared to matched individuals without OSA, independent of known comorbidities. Since the elderly frequently present with comorbid medical conditions, excluding those with comorbidities might only result in an artificially healthy group of the elderly. Conversely, the impact of comorbidities on vascular stiffness might lead to ceiling effects which would limit the ability to assess the impact of OSA. Moreover, certain comorbidities such as hypertension and diabetes may be causally linked to OSA which further complicates decisions to include or exclude these patients in rigorous analyses. Thus, we planned to assess elderly OSA patients with and without known comorbidities to allow determination of the OSA on vascular stiffness and potential interactive effects with comorbidities.
METHODS
Study subjects
We conducted a cross-sectional observational study of 471 elderly individuals aged at least 60 years who were recruited by convenience sampling from the sleep laboratory of Seoul National University Bundang Hospital (SNUBH) in Seongnam-si, South Korea and adjacent communities by advertisement between November 2010 and January 2013. We excluded patients with a history of prior therapy for snoring or OSA, heart failure, cerebrovascular diseases with neurologic deficits, inflammatory diseases, chronic obstructive pulmonary disease, nocturnal asthma, Cheyne-Stoke respiration, and other sleep disorders such as parasomnias and circadian rhythm disorders. All of the participants underwent nocturnal polysomnography and clinical evaluation. Our Institutional Review Board approved the study (B-1006/103-010), and a signed informed consent was obtained from all of the subjects or from their legal guardians.
Clinical evaluation
Clinical data were collected using a standardized protocol, including questions about current medical conditions and medication use, Pittsburgh sleep quality index (PSQI), and Epworth sleepiness scale (ESS). Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or current use of antihypertensive medications. Diabetes mellitus (DM) was defined as fasting blood glucose level ≥ 126 mg/dL, or current use of anti-diabetic medications. Metabolic dyslipidemia was considered to be present if subjects were taking lipid-lowering medications at recruitment or had high serum triglyceride level (≥ 150 mg/dL) or low high-density lipoprotein cholesterol level (≤ 50mg/dl for men and ≤ 40mg/dL for women) [15, 16]. Laboratory tests were carried out for measuring the high-sensitivity C-reactive protein (hs-CRP) level, lipid profile, fasting blood sugar level, and insulin level.
Nocturnal polysomnography
We used Embla N7000 (Embla, Reykjavik, Iceland) with standard electrodes and sensors. Electroencephalography electrodes were applied at C4/A1, C3/A2, O1/A2, and O2/A1, and two electrooculography electrodes were applied on the sides of both eyes to record horizontal and vertical eye movements. Electromyography electrodes were applied on the submentalis muscles and both anterior tibialis muscles. Strain gauges were used for recording chest and abdominal respiratory movements, and nasal pressure cannulae were used to record airflow. Oxygen saturation was measured using a pulse oximeter applied on the index finger. Based on the criteria of Rechtschaffen and Kales [17], sleep was scored at every 30-second epoch of the nocturnal polysomnography. Apnea was defined as complete cessation of airflow for at least 10 seconds. Hypopnea was defined as a discernible reduction in airflow for at least 10 seconds associated with electroencephalographic arousal or oxygen desaturation (≥ 4%) [18]. The apnea-hypopnea index (AHI) was defined as the total number of apneas and hypopneas per hour of sleep, and the oxygen desaturation index (ODI) was calculated as the number of oxygen desaturations (≥ 4%) per hour of sleep. OSA was diagnosed if AHI was equal to or more than 15/hr, and was divided into two groups, mild-to-moderate (AHI between 15 and 29/hr) and severe (AHI ≥ 30/hr) OSA [9].
Assessment of arterial stiffness
Arterial stiffness was assessed by brachial-ankle pulse wave velocity (baPWV), cardio-ankle vascular index (CAVI), and central blood pressures, both systolic and diastolic pressures (cSBP and cDBP). To evaluate baPWV, we measured electrocardiogram, phonocardiogram and oscillometric signals from 4 extremities and ankles, as well as tonometric signals from the right common carotid and right femoral arteries by using a noninvasive vascular screening device system (VP-2000; Omron-Colin, Kyoto, Japan) [19]. The measurement of baPWV and CAVI was performed based on the literature [13, 20]. The time interval between the wave front of the brachial waveform and that of the ankle waveform was defined as the time interval between the brachium and ankle (ΔTba). The path length from the suprasternal notch to the brachium (Lb) and ankle (La) was obtained from superficial measurements and was calculated using the following equations: Lb = 0.2195 x height (in cm) - 2.0734; La = 0.8129 x height (in cm) + 12.328. Finally, baPWV was calculated by the following equation: baPWV = (La - Lb) / ΔTba [20]. We measured baPWV after at least 5 min of rest, and subsequently CAVI was calculated using the following equation: CAVI = 2ρ × 1 / (Ps - Pd) × ln (Ps/Pd) × baPWV2 (ρ, density of blood; Ps and Pd, systolic and diastolic blood pressure, respectively) [13]. Central blood pressures were assessed non-invasively using an algorithm that derives the pressure wave at the ascending aorta from an external measurement taken at the radial artery (SphygmoCor; AtCor Medical, IL, USA).
Statistical Analysis
Subjects were divided into three subgroups based on the AHI, i.e., normal (AHI < 15 /hr), mild-to-moderate (15 ≤ AHI < 30 /hr), and severe OSA (AHI ≥ 30 /hr). We used an AHI cutoff value of 15 for making the diagnosis of OSA (control group) in the elderly [1]. Comparison between subgroups was done with analysis of covariance after adjusting for body mass index and gender. Multiple linear regression analysis was used to determine the relationship between cardiovascular variables as dependent variables and polysomnographic and clinical variables as independent variables. All significance tests were two sided, and P value < 0.05 was considered statistically significant. Statistical analysis was done with SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Clinical characteristics of subjects
Overall, 71.0% of male and 34.7% of female subjects were diagnosed as OSA. Table 1 shows the clinical characteristics of the study population. Age was not different among the three subgroups, i.e., normal (n = 226, 68.0 ± 5.6 yr), mild-to-moderate OSA (n = 127, 67.7 ± 5.4 yr), and severe OSA (n = 118, 68.2 ± 5.7 yr). We found that the proportion of male subjects and body mass index (BMI) were different among subgroups (χ2 = 67.34, df = 2, P < 0.001; F = 26.21, df = 2, P < 0.001, respectively). Hypertension, diabetes, and dyslipidemia were equally distributed among the AHI subgroups (P = 0.498; P = 0.802; P = 0.930, respectively). The proportion of medicated subjects with hypertension, diabetes, and dyslipidemia in the comorbidity group was 80.4%, 70.8%, and 43.3%, respectively.
Table 1.
Clinical characteristics of the study population.
| Variable | Normal (AHI<15/hr) | Mild to moderate (15≤AHI<30/hr) | Severe (AHI≥30/hr) | P |
|---|---|---|---|---|
| Number of subjects | 226 | 127 | 118 | |
| Male | 66, (29.2) | 77 (60.6) | 85 (72.0) | <0.001† |
| $ Age, yr | 67.9 (5.60) | 67.7 (5.43) | 68.2 (5.73) | 0.743 |
| $ BMI, kg/m2 | 23.3 (2.47)a | 24.4 (2.79)a | 25.5 (3.17)a | <0.001* |
| Current smokers | 12 (5.4) | 12 (9.5) | 13 (12.4) | 0.078 |
| Hypertension | 123 (54.4) | 71 (55.9) | 72 (61.0) | 0.498 |
| Diabetes | 44 (19.5) | 24 (18.9) | 26 (22.0) | 0.802 |
| Dyslipidemia | 98 (43.6) | 53 (42.1) | 52 (44.4) | 0.930 |
Data are presented as number of subjects (% within each AHI group).
Data are presented as means (s.d.);
P<0.05 by chi square test;
P<0.05 by ANOVA;
P<0.05 by pairwise comparison between the means with same superscript with Boferroni’s correction; AHI, apnea-hypopnea index; BMI, body mass index
2. Polysomnographic findings and arterial stiffness-related variables
We compared scales for sleep quality and sleepiness, polysomnographic findings and arterial stiffness-related variables among three groups defined by AHI (Table 2). As expected, subjective sleepiness measured by ESS was higher in the severe OSA group (P = 0.001 by ANOVA; P = 0.001 between the normal and severe OSA group by post-hoc test with Bonferroni’s method). Polysomnographic data showed significant group differences in AHI, lowest O2 (% of sleep time), oxygen desaturation below 90% (% of sleep time), and oxygen desaturation index (P<0.001, P<0.001, P<0.001, and P<0.001, respectively). However, we could not identify any significant difference in cardiovascular variables such as cSBP, cDBP, baPWV, CAVI and hs-CRP (P=0.118, P=0.159, P=0.202, P=0.062, and P=0.154, respectively).
Table 2.
Polysomnographic and cardiovascular variables of all subjects.
| All subjects (n=471)
|
P | |||
|---|---|---|---|---|
| Normal (n=226) | Mild-to-moderate (n=127) | Severe (n=118) | ||
| PSQI | 6.49 (0.27) | 7.18 (0.34) | 7.55 (0.39) | 0.077 |
| ESS | 6.71 (0.32)a | 7.31 (0.40) | 8.16 (0.46)a | 0.048* |
| AHI (events/h) | 6.79 (0.51)a | 21.62 (0.65)a | 46.56 (0.71)a | <0.001* |
| Lowest O2 (%) | 87.78 (0.39)a | 84.24 (0.49)a | 78.59 (0.53)a | <0.001* |
| Desat. < 90% (%) | 1.20 (0.52)a | 1.58 (0.66) | 8.83 (0.72)a | <0.001* |
| ODI | 5.02 (0.66)a | 14.83 (0.84)a | 36.01 (0.91)a | <0.001* |
| cSBP (mmHg) | 115.4 (1.05) | 118.6 (1.32) | 118.5 (1.44) | 0.118 |
| cDBP (mmHg) | 74.6 (0.63) | 75.9 (0.80) | 76.8 (0.87) | 0.159 |
| baPWV (cm/s) | 1632.9 (19.6) | 1633.9 (248.0) | 1675.3 (282.7) | 0.202 |
| CAVI | 5.64 (0.14) | 5.79 (0.18) | 6.25 (0.20) | 0.062 |
| hs-CRP (mg/L) | 1.04 (0.11) | 1.31 (0.14) | 1.39 (0.15) | 0.154 |
Data are presented as means (s.e.m.).
P<0.05, gender- and BMI-adjusted ANCOVA;
P<0.05 by pairwise comparison between the means with same superscript with Boferroni’s correction
PSQI, Pittsburgh sleep quality index; ESS, Epworth sleepiness scale; AHI, apnea-hypopnea index; Lowest O2, lowest O2 saturation during sleep study; Desat. < 90%, percentage of sleep with desaturation of oxygen below 90%; ODI, oxygen desaturation index; cSBP, central systolic blood pressure; cDBP, central diastolic blood pressure; baPWV, brachial-ankle pulse wave velocity; CAVI, cardio-ankle vascular index; hs-CRP, high-sensitivity C-reactive protein
3. Subgroup analysis: comorbidity and healthy groups
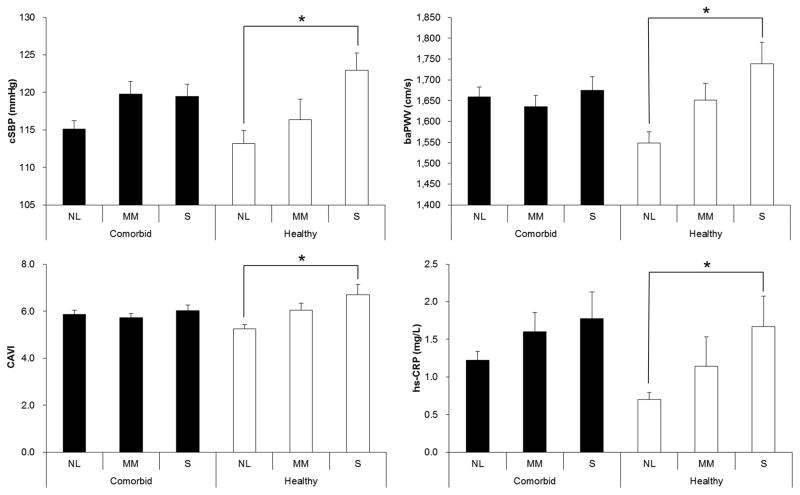
We stratified the subjects into two groups for subgroup analysis by design, which were comorbidity and no comorbidity groups (n = 370; n = 101, respectively). The AHI subgroups of the healthy group did not show any differences in the proportion of males and BMI, whereas the AHI subgroups of the comorbidity group showed the differences (χ2 = 68.1, df = 2, P < 0.001; F = 25.3, df = 2, P <0.001, respectively). Therefore, adjustment for gender and BMI was performed only for the comorbid group. The healthy group revealed a significant subgroup difference in cSBP, cDBP, baPWV, CAVI and hs-CRP (F = 4.460, P = 0.014; F = 3.408, P = 0.037; F = 8.703, P < 0.001; F = 9.554, P < 0.001; F = 3.953, P = 0.022, respectively; Table 3 and Figure 1). In Figure 1, cSBP is representative of central BPs, since cDBP showed similar results to cSBP. In contrast, the comorbid group did not show subgroup differences in any of cSBP, cDBP, baPWV, CAVI or hs-CRP (F = 1.320, P = 0.268; F = 0.808, P = 0.447; F = 0.341, P = 0.711; F = 0.623, P = 0.537; F = 0.443, 0.642, respectively). Within comorbid group, further subgroup analysis for the patients with or without medication (n=233 and 137, respectively), such as antihypertensive or antidiabetics, showed no difference of cSBP, cDBP, baPWV, and CAVI among AHI subgroups after adjusting sex and BMI. To investigate the relationship between OSA and arterial stiffness in the healthy group, we first performed univariate regression analysis and it showed a significant correlation between AHI and arterial stiffness-related variables (R = 0.308, P = 0.002 for cSBP; R = 0.251, P = 0.012 for cDBP; R = 0.355, P < 0.001 for baPWV; R = 0.354, P < 0.001 for CAVI; R = 0.322, P = 0.001 for hs-CRP). We also performed multiple linear regression analysis for the arterial stiffness-related variables. These 5 variables were used as dependent variables, and AHI, sex, and BMI as independent variables. Central BPs, baPWV, CAVI, and hs-CRP showed a significant association with AHI (β = 0.286, P = 0.007; β = 0.232, P = 0.024; β = 0.326, P = 0.002; β = 0.326, P = 0.002; β = 0.333, P = 0.007, respectively) (Table 4).
Table 3.
Polysomnographic and cardiovascular variables of subjects in the comorbid and healthy group
| Comorbid group (n=370)
|
P | Healthy group (n=101)
|
P | |||||
|---|---|---|---|---|---|---|---|---|
| Normal (n=171) | Mild-to-moderate (n=103) | Severe (n=96) | Normal (n=55) | Mild-to-moderate (n=24) | Severe (n=22) | |||
| Age (yr) | 68.1 (5.65) | 67.67 (5.46) | 67.95 (5.95) | 0.832 | 67.45 (5.49) | 67.58 (5.44) | 69.32 (4.62) | 0.363 |
| $ Male (%) | 48 (28.1) | 67 (65.0) | 73 (76.0) | <0.001 * | 18 (34.5) | 10 (41.7) | 12 (54.5) | 0.204 |
| BMI (kg/m2) | 23.39 (2.42) | 24.51 (2.78) | 25.84 (3.13) | <0.001 * | 22.85 (2.6) | 24.08 (2.85) | 23.85 (2.89) | 0.124 |
| PSQI | 6.68 (3.82) | 7.08 (3.71) | 6.99 (3.82) | 0.044 * | 7.29 (3.82) | 6.42 (3.96) | 7.52 (4.25) | 0.583 |
| ESS | 6.35 (3.87) | 7.57 (4.13) | 8.32 (5.63) | 0.144 | 6.98 (4.7) | 6.79 (4.78) | 9.05 (4.51) | 0.187 |
| AHI (events/h) | 6.06 (4.14) | 22.13 (3.87) | 47.16 (13.68) | <0.001 * | 6.77 (4.27) | 20.73 (3.63) | 42.89 (7.89) | <0.001 * |
| Lowest O2 (%) | 88.26 (3.88) | 83.81 (4.89) | 78.44 (7.66) | <0.001 * | 86.94 (5.12) | 85.92 (4.09) | 78.00 (9.21) | <0.001 * |
| Desat.<90% (%) | 0.48 (2.17) | 1.56 (2.03) | 10.33 (14.66) | <0.001 * | 1.41 (7.41) | 2.53 (6.3) | 6.41 (8.0) | 0.013 * |
| ODI | 3.89 (3.39) | 15.54 (4.74) | 38.02 (16.21) | <0.001 * | 5.83 (13.41) | 13.2 (5.59) | 32.89 (11.88) | <0.001 * |
| cSBP (mmHg) | 114.99 (14.48) | 119.64 (17.13) | 119.24 (15.17) | 0.268 | 112.93 (12.32) | 116.38 (13.32) | 122.09 (10.92) | 0.014 * |
| cDBP (mmHg) | 73.02 (9.24) | 76.99 (10.16) | 78.43 (9.16) | 0.447 | 73.8 (8.97) | 75.25 (7.94) | 79.5 (8.53) | 0.037 * |
| baPWV (cm/s) | 1660.95 (305.45) | 1629.87 (259.07) | 1656.99 (288.97) | 0.711 | 1546.04 (189.67) | 1651.29 (197.18) | 1755.23 (243.78) | <0.001 * |
| CAVI | 5.89 (2.27) | 5.68 (1.75) | 5.96 (2.29) | 0.537 | 5.23 (1.26) | 6.05 (1.49) | 6.9 (2.15) | <0.001 * |
| hs-CRP (mg/dL) | 1.15 (1.3) | 1.34 (1.89) | 1.37 (1.82) | 0.642 | 0.69 (0.67) | 1.14 (1.9) | 1.62 (1.83) | 0.022 * |
Data are presented as means (s.d.).
Data are presented as number of subjects (% within each AHI group)
P<0.05, adjustment for gender and BMI for comorbid group
BMI, body mass index; PSQI, Pittsburgh sleep quality index; ESS, Epworth sleepiness scale; AHI, apnea-hypopnea index; Lowest O2, lowest O2 saturation during sleep study; Desat. < 90%, percentage of sleep with desaturation of oxygen below 90%; ODI, oxygen desaturation index; cSBP, central systolic blood pressure; cDBP, central diastolic blood pressure; baPWV, brachial-ankle pulse wave velocity; CAVI, cardio-ankle vascular index; hs-CRP, high-sensitivity C-reactive protein
Figure 1.
The normal AHI and severe OSA subgroups of the healthy group showed differences in cSBP, baPWV, CAVI and hs-CRP. Black and white bars indicate the mean of comorbidity and healthy groups, respectively, and the error bars show SEM. *P<0.05, post hoc test by Bonferroni method following ANOVA. NL, normal group; MM, mild-to-moderate OSA group; S, severe OSA group; cSBP, central systolic blood pressure; baPWV, brachial-ankle pulse wave velocity; CAVI, cardio-ankle vascular index; hs-CRP, high sensitivity C-reactive protein; AHI, apnea-hypopnea index; OSA, obstructive sleep apnea; SEM, standard error of means
Table 4.
Multiple linear regression analysis of arterial stiffness variables in the healthy group.
| Independent variables | Dependent variables
|
||||
|---|---|---|---|---|---|
| cSBP | cDBP | baPWV | CAVI | hs-CRP | |
| AHI | 0.286 (0.007)* | 0.232 (0.024)* | 0.326 (0.002)* | 0.326 (0.002)* | 0.333 (0.002)* |
| Sex | −0.103 (0.478) | −0.271 (0.061) | −0.119 (0.415) | −0.102 (0.486) | 0.209 (0.152) |
| BMI | 0.113 (0.271) | 0.080 (0.428) | −0.015 (0.882) | −0.029 (0.782) | 0.045 (0.657) |
| Current smoking | −0.007 (0.960) | −0.010 (0.945) | −0.128 (0.381) | −0.154 (0.293) | 0.092 (0.523) |
Data are presented as standardized β coefficient (P value).
P<0.05, multiple linear regression; AHI, apnea hypopnea index; BMI, body mass index; cSBP, central systolic blood pressure; baPWV, brachial-ankle pulse wave velocity; CAVI, cardio-ankle vascular index; hs-CRP, high-sensitivity C-reactive protein
DISCUSSION
We studied the elderly population for assessing the relationship between obstructive sleep apnea and arterial stiffness. The elderly patients with OSA did not show an association between AHI and arterial stiffness-related variables, but a subgroup without comorbidities revealed a modest association between AHI and the given variables, such as cSBP, cDBP, baPWV and CAVI, and a systemic inflammatory marker hs-CRP.
Arterial stiffness is one of the important biological markers for arterial health at various sites in the arterial system and is caused by structural changes in the vascular wall, including fibrosis, medial smooth muscle cell necrosis, breaks in elastin fibers, calcifications and diffusion of macromolecules into the arterial wall [21]. Higher arterial stiffness is associated with atherosclerosis [22], which increases the risk of cardiovascular diseases and events. PWV is the most thoroughly and clinically investigated measure of arterial stiffness, which is defined as the wave speed within a material (distance travelled/transit time) [12]. The association between OSA and arterial stiffness has been studied by many researchers and a review, conducted by Philips et al., summarized that 20 out of 28 cross-sectional studies in the middle-aged patients found a significant difference in arterial stiffness between controls and patients with OSA [12]. baPWV has been suggested as a predictor of the presence of coronary artery disease, however, it can be affected by changes in BP during measurement in patients with OSA [22]. Thus, CAVI was an improved measurement obtained by adjusting peripheral BPs so that the effect of BP at the time of the measurement might be reduced [23]. Recently good reproducibility of CAVI was validated in OSA [13]. It is also noteworthy that central BPs are closely related to arterial stiffness and are more relevant than peripheral BP in the pathogenesis of cardiovascular diseases [24].
After we have demonstrated that nocturnal hypoxemia is associated with arterial stiffness and endothelial dysfunction in the middle-aged [14, 25], we questioned whether OSA in the elderly could also have an impact on the arterial stiffness. Previous studies about the association between OSA and arterial stiffness were performed in the middle-aged patients [12]. To the best of our knowledge, our study is the first cross-sectional study investigating the association between OSA and arterial stiffness exclusively in the elderly population. Unlike the middle-aged population, the elderly with OSA did not show an association between OSA and arterial stiffness-related variables. Evaluation of endothelial dysfunction could yield positive results [26], but it is reported that in older adults the measurements for both arterial stiffness and endothelial dysfunction are directly associated, providing similar information [27, 28]. On the other hand, as the elderly are prone to various medical diseases and consequently they take more medications, the subgroup without comorbidities was analyzed and then, the relationship between OSA and arterial stiffness was found in this healthy group. Our subgroup analysis based on medication status within comorbid group showed no worsening of arterial stiffness according to OSA in both medication and non-medication subgroups. For the ‘non-medication’ subgroup, medical conditions per se, such as hypertension, diabetes, and dyslipidemia, might aggravate arterial stiffness regardless of the presence of OSA [29, 30], whereas, for the ‘medication’ subgroup, antihypertensive and antidiabetic medications might improve arterial stiffness in patients with OSA [31, 32]. Thus, it is plausible that the presence of these comorbidities and the use of medication may negate the possible relationship between OSA and arterial stiffness in the elderly via the mixture of ceiling effect of increased stiffness and medication effect of decreasing arterial stiffness. Although we found a modest but significant association between AHI and arterial stiffness after eliminating potential confounding effects of medical conditions and medication use, we should be cautious about the interpretation of these results. The healthy OSA-subgroup consisted of only one fourths of all subjects, making representativeness debatable. Still, we believe that our analyses provide value by removing the confounding effects of comorbidities/ medications to explore the isolated impact of OSA on cardiovascular risk in the elderly.
CRP is an important serum biomarker of inflammation that can predict cardiovascular risk in apparently healthy subjects [33] and heart failure patients with central sleep apnea [34]. However, the association between CRP and OSA is controversial [15, 35–37]. Previously we have shown that CRP was associated with obesity rather than AHI [25]. It should be noted that the subjects of the previous study consisted of only middle-aged men (42.1 ± 8.7 years, n = 90), but not the elderly population. In this context, the study of Roche et al. in 2009 is relevant to our current study, because their study subjects included the elderly who had OSA without cardiovascular morbidity [15]. Their study population was similar to that of our study, especially the healthy group, and they also showed a significant association between CRP and OSA. Therefore, the discovery of the association between CRP and OSA might indicate a characteristic of the elderly population without comorbidity.
The issue of potential ceiling effects deserves further comment. Because the patients with OSA and comorbidities did not have worse vascular stiffness than the patients with comorbidities alone, the possibility exists that a ‘maximum’ stiffness in elderly humans occurs above which further worsening is unlikely. Both biological as well as methodological explanations could be at play. For example, OSA and DM could be working through autonomic, inflammatory or oxidative stress mechanisms such that the combination of diseases may not worsen risk at least as assessed through vascular stiffness [38, 39]. The instrumentation required to measure pulse wave velocity may also have reduced sensitivity at maximal values such that measurement of increased vascular stiffness may be problematic in some patients. Because our severe OSA patients with comorbidities did not have velocities above those of other groups, we suggest that our findings are more likely to be driven by biological rather than methodological issues. However, further work is clearly needed in this area.
The main strengths of this study are a large number of subjects with laboratory polysomnographic data as well as detailed medical evaluation, which enabled us to stratify the subjects based on the presence or absence of medical illnesses. However, we acknowledge some limitations of our study. First, the elderly without comorbidities were only one fourth of our subjects, which limited our statistical power. Due to this limitation, we could parsimoniously suggest that the comorbidities and their medications attenuate the impact of OSA on arterial stiffness. Second, we found modest sizes of correlation between AHI and cardiovascular variables, indicating other factors might also influence cardiovascular variables. Third, the arterial stiffness is an early marker of atherosclerosis. It is a good cardiovascular marker for the middle-aged population, but may not be ideal for the elderly, because the ceiling effect of the arterial stiffness might be present in this group, especially those with comorbidities. Despite these limitations, we could conclude that OSA was associated with increased arterial stiffness in otherwise healthy elderly population, although the association was obviated by comorbidities and medications perhaps due to ceiling effects.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (to I.Y.Y.) [grant number 2010-0008886]. Dr. Malhotra is supported by the National Institutes of Health.
Footnotes
Conflict of interest statement: All the authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
Tae Kim performed statistical analyses and data interpretation, and wrote the manuscript. In-Young Yoon conceived and designed the study, contributed to data acquisition and interpretation, supervised the study, critically revised the manuscript, and approved the final version. Chung Suk Lee contributed to data acquisition and interpretation, and approved the final version. Sang Don Lee contributed to designing the study and data acquisition, and approved the final version. Suk-Hoon Kang contributed to designing the study and data acquisition, and approved the final version. Ji Won Han contributed to data acquisition, and approved the final version. Atul Malhotra critically revised the manuscript and approved the final version. Ki Woong Kim contributed to data acquisition, and approved the final version to be published. The corresponding author (In-Young Yoon) confirms that all the data in the study were fully accessible and he had final responsibility for the decision to submit this manuscript for publication.
FINANCIAL DISCLOSURE AND CONFLICT OF INTEREST
All the authors have no conflict of interest. Dr. Malhotra relinquished all outside personal sources of income in 2012.
References
- 1.Ancoli-Israel S, Kripke DF, Klauber MR, Fell R, Stepnowsky C, Estline E, Khazeni N, Chinn A. Morbidity, mortality and sleep-disordered breathing in community dwelling elderly. Sleep. 1996;19:277–282. doi: 10.1093/sleep/19.4.277. [DOI] [PubMed] [Google Scholar]
- 2.Lee SD, Kang SH, Ju G, Han JW, Kim TH, Lee CS, Kim T, Kim KW, Yoon IY. The prevalence of and risk factors for sleep-disordered breathing in an elderly korean population. Respiration. 2014;87:372–378. doi: 10.1159/000358442. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: Eighteen-year follow-up of the wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 7.Barcelo A, Esquinas C, Pierola J, De la Pena M, Sanchez-de-la-Torre M, Montserrat JM, Marin JM, Duran J, Arque M, Bauca JM, Barbe F. Vitamin d status and parathyroid hormone levels in patients with obstructive sleep apnea. Respiration. 2013;86:295–301. doi: 10.1159/000342748. [DOI] [PubMed] [Google Scholar]
- 8.Endeshaw YW, White WB, Kutner M, Ouslander JG, Bliwise DL. Sleep-disordered breathing and 24-hour blood pressure pattern among older adults. J Gerontol A Biol Sci Med Sci. 2009;64:280–285. doi: 10.1093/gerona/gln011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, Soler-Cataluna JJ, Almeida-Gonzalez C, De la Cruz Moron I, Duran-Cantolla J, Montserrat JM. Cardiovascular mortality in obstructive sleep apnea in the elderly: Role of long-term continuous positive airway pressure treatment: A prospective observational study. Am J Respir Crit Care Med. 2012;186:909–916. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 10.Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, Young T, Pickering TG. Age-dependent associations between sleep-disordered breathing and hypertension: Importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the sleep heart health study. Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 11.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips CL, Butlin M, Wong KK, Avolio AP. Is obstructive sleep apnoea causally related to arterial stiffness? A critical review of the experimental evidence. Sleep Med Rev. 2013;17:7–18. doi: 10.1016/j.smrv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Kumagai T, Kasai T, Kato M, Naito R, Maeno K, Kasagi S, Kawana F, Ishiwata S, Narui K. Establishment of the cardio-ankle vascular index in patients with obstructive sleep apnea. Chest. 2009;136:779–786. doi: 10.1378/chest.09-0178. [DOI] [PubMed] [Google Scholar]
- 14.Chung S, Yoon IY, Lee CH, Kim JW. The association of nocturnal hypoxemia with arterial stiffness and endothelial dysfunction in male patients with obstructive sleep apnea syndrome. Respiration. 2010;79:363–369. doi: 10.1159/000228905. [DOI] [PubMed] [Google Scholar]
- 15.Roche F, Gaspoz JM, Pichot V, Picard-Kossovsky M, Maudoux D, Garcin A, Celle S, Sforza E, Barthelemy JC Proof Groups SS. Association between c-reactive protein and unrecognised sleep-disordered breathing in the elderly. Eur Respir J. 2009;33:797–803. doi: 10.1183/09031936.00023208. [DOI] [PubMed] [Google Scholar]
- 16.Trzepizur W, Le Vaillant M, Meslier N, Pigeanne T, Masson P, Humeau MP, Bizieux-Thaminy A, Goupil F, Chollet S, Ducluzeau PH, Gagnadoux F Institut de Recherche en Sante Respiratoire des Pays de la Loire Sleep Cohort G. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest. 2013;143:1584–1589. doi: 10.1378/chest.12-1652. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. A manual of standardized terminology, technique, and scoring system for sleep stages of human subjects. Los Angeles, California: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 18.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The report of an american academy of sleep medicine task force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 19.Yu WC, Chuang SY, Lin YP, Chen CH. Brachial-ankle vs carotid-femoral pulse wave velocity as a determinant of cardiovascular structure and function. J Hum Hypertens. 2008;22:24–31. doi: 10.1038/sj.jhh.1002259. [DOI] [PubMed] [Google Scholar]
- 20.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 21.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Rio F, Pino JM, Alonso A, Arias MA, Martinez I, Alvaro D, Villamor J. White coat hypertension in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2004;125:817–822. doi: 10.1378/chest.125.3.817. [DOI] [PubMed] [Google Scholar]
- 23.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (cavi) J Atheroscler Thromb. 2006;13:101–107. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 24.Agabiti-Rosei E, Mancia G, O’Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: A consensus document. Hypertension. 2007;50:154–160. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]
- 25.Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Lee T, Choi DJ, Ahn HJ. Endothelial dysfunction and c-reactive protein in relation with the severity of obstructive sleep apnea syndrome. Sleep. 2007;30:997–1001. doi: 10.1093/sleep/30.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayers L, Stoewhas AC, Ferry B, Stradling J, Kohler M. Elevated levels of endothelial cell-derived microparticles following short-term withdrawal of continuous positive airway pressure in patients with obstructive sleep apnea: Data from a randomized controlled trial. Respiration. 2013;85:478–485. doi: 10.1159/000342877. [DOI] [PubMed] [Google Scholar]
- 27.Koivistoinen T, Virtanen M, Hutri-Kahonen N, Lehtimaki T, Jula A, Juonala M, Moilanen L, Aatola H, Hyttinen J, Viikari JS, Raitakari OT, Kahonen M. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: The cardiovascular risk in young finns study and the health 2000 survey. Atherosclerosis. 2012;220:387–393. doi: 10.1016/j.atherosclerosis.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Buchner NJ, Quack I, Woznowski M, Stahle C, Wenzel U, Rump LC. Microvascular endothelial dysfunction in obstructive sleep apnea is caused by oxidative stress and improved by continuous positive airway pressure therapy. Respiration. 2011;82:409–417. doi: 10.1159/000323266. [DOI] [PubMed] [Google Scholar]
- 29.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–1698. doi: 10.1097/HJH.0b013e32833a6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safar ME, Balkau B, Lange C, Protogerou AD, Czernichow S, Blacher J, Levy BI, Smulyan H. Hypertension and vascular dynamics in men and women with metabolic syndrome. J Am Coll Cardiol. 2013;61:12–19. doi: 10.1016/j.jacc.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 31.Takami T, Saito Y. Azelnidipine plus olmesartan versus amlodipine plus olmesartan on arterial stiffness and cardiac function in hypertensive patients: A randomized trial. Drug Des Devel Ther. 2013;7:175–183. doi: 10.2147/DDDT.S42338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutouyrie P, Lacolley P, Briet M, Regnault V, Stanton A, Laurent S, Mahmud A. Pharmacological modulation of arterial stiffness. Drugs. 2011;71:1689–1701. doi: 10.2165/11593790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM. High-sensitivity c-reactive protein: Potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 34.Schmalgemeier H, Bitter T, Fischbach T, Horstkotte D, Oldenburg O. C-reactive protein is elevated in heart failure patients with central sleep apnea and cheyne-stokes respiration. Respiration. 2014;87:113–120. doi: 10.1159/000351115. [DOI] [PubMed] [Google Scholar]
- 35.Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated c-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 36.Guven SF, Turkkani MH, Ciftci B, Ciftci TU, Erdogan Y. The relationship between high-sensitivity c-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012;16:217–221. doi: 10.1007/s11325-011-0492-2. [DOI] [PubMed] [Google Scholar]
- 37.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, Foster GD, Maislin G, Saif H, Broderick P, Chittams J, Hanlon AL, Pack AI. Cpap, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doupis J, Rahangdale S, Gnardellis C, Pena SE, Malhotra A, Veves A. Effects of diabetes and obesity on vascular reactivity, inflammatory cytokines, and growth factors. Obesity (Silver Spring) 2011;19:729–735. doi: 10.1038/oby.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yim-Yeh S, Rahangdale S, Nguyen AT, Jordan AS, Novack V, Veves A, Malhotra A. Obstructive sleep apnea and aging effects on macrovascular and microcirculatory function. Sleep. 2010;33:1177–1183. doi: 10.1093/sleep/33.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]