Abstract
Apoptosis is the main driver of cell death in bioreactor suspension cell cultures during the production of biopharmaceuticals from animal cell lines. It is known that apoptosis also has an effect on the quality and quantity of the expressed recombinant protein. This has raised the importance of studying apoptosis for implementing culture optimization strategies. The work here describes a novel approach to obtain near real time data on proportion of viable, early apoptotic, late apoptotic and necrotic cell populations in a suspension CHO culture using automated sample preparation in conjunction with flow cytometry. The resultant online flow cytometry data can track the progression of apoptotic events in culture, aligning with analogous manual methodologies and giving similar results. The obtained near-real time apoptosis data are a significant improvement in monitoring capabilities and can lead to improved control strategies and research data on complex biological systems in bioreactor cultures in both academic and industrial settings focused on process analytical technology applications.
Keywords: Flow cytometry, Online, At-line, Automation, CHO cells, PAT, Monitoring, Apoptosis
Introduction
Bioprocess engineering requires an understanding of a process in order to ensure the goal of final product quality with optimum yield. A variety of analytical tools have been developed to help monitor and control bioreactor operations and optimize biopharmaceutical productions in mammalian cell culture bioprocesses. The study and use of real time or near real time online or at-line monitoring tools has gained steady momentum since the inception of the process analytical technology initiative (Rathore et al. 2010) set out by the US Food and Drug Administration in September 2004 in their published guideline document: “PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance” (Food and Drug Administration 2004). When it comes to analytical tools for mammalian cell culture bioreactors, biopharmaceutical manufacturers have traditionally used in situ sensors to monitor mammalian culture parameters for feedback control of the process. These parameters have traditionally been restricted, due to technical challenges, to only a few, such as pH, dissolved oxygen, optical density, capacitance, and temperature (Carvell and Dowd 2006; Hanson et al. 2007; Konstantinov et al. 1992; Naciri et al. 2008; Zeiser et al. 1999). The limitation of these common measured parameters is that no direct information is given on the state of the cell such as viability, cell cycle, cell size, or apoptotic state, all of which are metrics that can help improve recombinant protein production and reproducibility of a mammalian culture bioprocess (Al-Rubeai et al. 1991a; Ibarra et al. 2003; Plasier et al. 1999). Parameters that directly give cell and product quality information can improve upon current cell culture processes together with traditional parameters to provide dynamic modelling and prediction on product quantity and quality in real time and validate the bioprocess to the best extent (Streefland et al. 2013). It has been demonstrated that it is possible to obtain online data representing cell cycle states and viable cell number (Kuystermans et al. 2012). The aim of this study is to demonstrate, for the first time, using a near real-time and automated technique specifically designed for apoptosis measurements that we can obtain both early/late apoptosis, viable and necrotic cell data from a mammalian cell culture using an online system.
Apoptosis is a form of programmed cell death first described in a 1972 publication by Kerr, Wyllie and Currie et al. (1972). Apoptosis was defined in terms of several morphological changes occurring in the cell such as the appearance of blebbing, cell shrinkage, nuclear fragmentation and chromatin condensation. The biochemical events that accompany these morphological changes include the fragmentation of chromosomal DNA, externalization of phosphatidylserine (PS; Martin et al. 1995) and in some cases, the translocation of calreticulin to the cell surface (Obeid et al. 2007) and the proteolytic cleavage of intracellular substrates (Martin and Green 1995). The net cumulative effect results in the apoptotic cells undergoing phagocytosis.
Cell culture bioprocessing has emphasized the importance of apoptosis ever since it was observed in the context of mammalian cell cultures for bioprocessing (Cotter and Al-Rubeai 1995). Although apoptosis plays a role, necrosis may also be caused by extreme conditions. But often in bioreactors an environmental factor contributing to cell death steadily builds up, thus, termination of bioreactor cell cultures is often due to apoptosis rather than necrosis as a result of external factors such as excessive shear forces (Chisti 1993; Emery et al. 1995; Kuystermans and Al-Rubeai 2011; Petersen et al. 1988), metabolic nutrient depletion (Bibila et al. 1994; Heath et al. 1990; Leelavatcharamas et al. 1994; Singh and Al-Rubeai 1998), metabolic by-product accumulation (Martinelle et al. 1996; Xie and Wang 1994), hypoxia (Jan et al. 1997; Ogawa et al. 1992), sub-optimal pH (Naciri et al. 2008; Singh 1994) and hyper-osmolality (deZengotita et al. 2002). Since apoptosis is regulated through a cascade of molecular events in response to external factors (Arden and Betenbaugh 2004) it is possible to monitor the progress of apoptosis within a cell population. One such method is with the Annexin-V affinity assay, where the externalization of PS can be used as a target for a labeled and cell membrane impermeable Annexin-V molecule to detect apoptosis. In combination with a cell membrane impermeable dye, such as propidium iodide (PI), the technique can be used to detect early and late apoptosis (Plasier et al. 1999) by fluorescence microscopy or flow cytometry.
Flow cytometry is a versatile and valuable tool in research on mammalian cell cultures (Al-Rubeai et al. 1991b; Borth et al. 2000; Carroll and Al-Rubeai 2004; Lee and Lee 2012). The execution of flow cytometry experiments traditionally involves several manual sample preparation steps, depending on the protocol. As an example, the typical apoptosis protocol involves several manual centrifugation, staining and incubations before the actual flow cytometric analysis takes place. These manual operations mean that it would be laborious to gather apoptotic data on a bioreactor culture every 2–6 h, not to mention that the manual procedures may not be consistent due to the possible introduction of human error. In this article we will take a novel approach, using automated online flow cytometry, to quantify the levels of early and late apoptosis and necrotic percentage cell formation in suspension Chinese Hamster Ovary (CHO) cell culture for the first time.
Materials and methods
Cell line culture and maintenance
The CHO cell line CHOK1SV expressing monoclonal antibody B72.3-IgG4 was kindly provided by Lonza Biologics Plc. The CHO cells were cultured in 250 ml GL45 shaker flasks (Bellco Glass, Vineland, NJ, USA) in a working volume of 50 ml CD-CHO Medium (Invitrogen, Paisley, UK) supplemented with 50 µM l-methionine sulfoximine (MSX) (Sigma, Gillingham, UK). All cell culture work was carried out aseptically in a laminar airflow chamber. Cells were maintained in suspension culture growing in an orbital shaker incubator at 37 °C. The seeding density was 3 × 105 cells/ml, with a new subculture initiated every 3–4 days when the cells reached mid-exponential phase, an approximate density of 3 × 106 cells/ml.
Stirred suspension batch culture and sampling
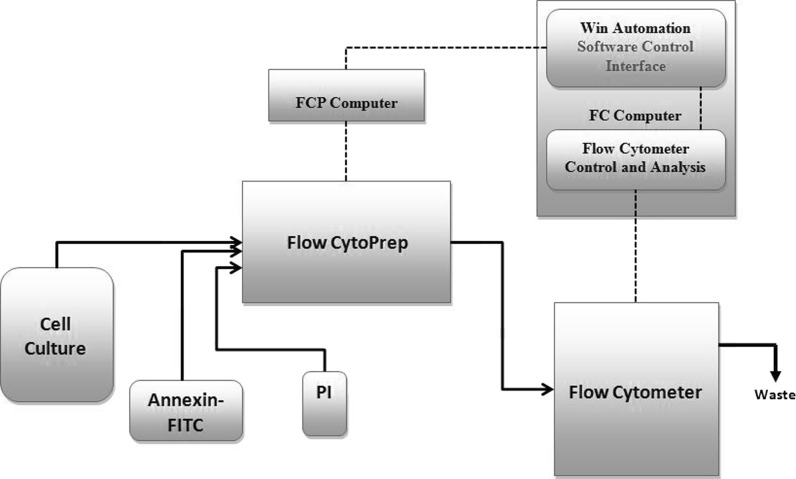
The cells were grown in 500 ml spinner flasks vented through a 0.2 µm filter (Bellco Glass) connected via silicon tubing to the Flow CytoPrep (FCP) 5000 (MSP Corporation, Minneapolis, MN, USA). The CHOK1SV cells were cultivated in the spinner flasks in CD-CHO medium (Invitrogen), supplemented with 50 µM MSX to give a total working volume of 300 ml. The initial seeding density was 3 × 105 cells/ml. The spinner flask was placed in a water bath (Julabo, Peterborough, UK) on top of a magnetic stirrer plate (Bellco Glass) set at 110 rpm to agitate the cell culture in the spinner flask. The water bath temperature was maintained at 37 °C. Manual samples were taken; under aseptic conditions in a laminar flow hood by disconnecting and reconnecting the quick connect fittings from the sample transfer line (described in the automated sample preparation section) by submerging in a beaker of 70 % alcohol. Samples were for the manual Annexin-V FITC apoptosis assay and to determine cell number and viability via TB dye exclusion. A basic mechanical setup and diagram for the automated sampling and preparation of single stained samples is described in a previous publication (Kuystermans et al. 2012) but this work demonstrates that with few modifications and a newly designed control protocol the system can be utilized for dual staining intended for apoptosis analysis. Briefly, the spinner flask was connected to a modified FCP 5000 (MSP Corporation) which conducted the preprogrammed automated sample preparation and delivered the sample to a sample cup of the flow cytometer (FC) (Beckman Coulter, High Wycombe, UK). The FCP and FC setup was controlled by the WinAutomation (Softomotive Solutions Ltd, Athens, Greece) supervisory control running on the FC computer and networked to the FCP via a transmission control protocol (see Fig. 1).
Fig. 1.
General illustration of the automated cell culture analysis setup with the CHO cell culture processing steps required to obtain apoptosis data. The solid lines represent process flow paths and the dashed lines represent the process control interface between the FCP and FC
Manual sample preparation for apoptosis analysis
CHO-K1SV cells were removed at 24 h intervals during batch culture so as not to interfere with the automated analysis and were analyzed for the percentages of viable, apoptotic and necrotic cells. Dual staining for both Annexin-V binding to PS by Annexin-V-Fluorescein isothiocyanate (FITC) (BD Biosciences, Oxford, UK) and cellular DNA using PI was performed as follows (Ishaque and Al-Rubeai 1999): 1 × 106 cells/ml were washed in ice cold PBS (0.01 M phosphate buffer, 0.0027 M potassium chloride and 0.137 M sodium chloride, pH 7.4, at 25 °C) and centrifuged at 300g for 5 min at 4 °C before resuspending in 1× binding buffer [(10 mM HEPES solution) pH 7.4, 140 mM NaCl, and 2.5 mM CaCl2]. One microliter of Annexin-V FITC was added to 100 μl of cell suspension and incubated for 10 min at 20–25 °C before adding 400 µl of 1× binding buffer and 10 μl of PI (50 µg/ml). The mix was then incubated for 1 min before analysis. The samples were analysed with the same FC filter and laser setup used in the automated protocol.
Automated sample preparation program and apoptosis analysis
Sample preparation steps for flow cytometric analysis were programmed into the FCP using the designed protocol steps shown in Table 1. A modification was made to the FCP so that both injection port 1 and 2 can use the injection sample loop for stain incubation and mixing. The new dual staining setup consisted of the stirred cell culture vessel connected by 43.18 mm length (1.6 mm ID) autoclaved silicon tubing to FCP sample port 1, which was pre-rinsed with 70 % alcohol before being primed. A sample transfer line connected between FCP injection port 2 and the FC sample receptacle. 50 μl of Annexin-V FITC was mixed with 10 ml of PBS (0.01 M phosphate buffer, 0.0027 M potassium chloride and 0.137 M sodium chloride, pH 7.4, at 25 °C) and used as a port 6 reservoir. A 50 ml solution of PI at 1 mg/ml in PBS was connected to port 7 as a reservoir. A WinAutomation job designer script was used to control both the FCP and FC. Flow cytometer compensation was carried out using the first samples of the culture before full automation was initiated. The sample was analysed with a Beckman FC equipped with a 488 nm diode laser. A 525 band pass detection filter was used to obtain FITC fluorescence and a 670 nm long pass filter was used to collect the maximal emission of PI fluorescence. For the analysis, an electronic volume (EV) versus side scatter (SS) signal plot was used to discriminate between cells and debris and give accurate selection of CHO cells with the exact cell size range of 6–15 µm for CHO cells (Song et al. 2006). A second plot with FITC integral log versus PI signal integral log was generated for 20,000 cells defined by the gate on the first EV versus SS plot. Gating was set out empirically on the second plot to divide it into four quadrants defined as viable cells (“low” FITC and “low” PI signal), early apoptotic cells (“high” FITC and “low” PI signal), late apoptotic cells (“high” FITC and “high” PI signal), and necrotic cells (“low” FITC and “high” PI signal). The percentage of total cells in the population analysed in the total of the four quadrants was used to express the results, and fluorescence compensation was carried out before taking experimental readings.
Table 1.
Programmed command sequence for apoptosis staining procedure in the FCP system, SL = sample loop as described in Kuystermans et al. (2012)
| Steps | Command | Stream/port | Flow (ml/min) | Volume (μl/ml) | Time (s) | |
|---|---|---|---|---|---|---|
| 1 | Draw sample | 1 | 4 | 1,000 | – | These steps are for removing the dead volume from the tubings |
| 2 | Incubate | – | 30 | |||
| 3 | Test trigger | – | – | – | ||
| 4 | Inject SL | – | 3 | 1,000 | – | |
| 5 | Draw sample | 1 | 10 | 1,000 | – | |
| 6 | Incubate | – | – | – | 10 | |
| 7 | Inject SL | – | 5 | 1,000 | – | |
| 8 | Test trigger | – | – | – | – | |
| 9 | Wash | – | – | |||
| 10 | Draw sample | 1 | 4 | 900 | 0 | |
| 11 | Incubate | – | – | – | 30 | |
| 12 | Test trigger | – | – | – | – | |
| 13 | Incubate | – | 50 | Apoptosis sample preparations steps | ||
| 14 | Draw sample | 1 | 5 | 800 | – | |
| 15 | Draw stain (Annexin V) | 6 | 5 | 200 | – | |
| 16 | Draw sample (air) | 2 | 5 | 500 | – | |
| 17 | Inject SL | – | 4 | 500 | – | |
| 18 | Incubate | – | – | – | 600 | |
| 19 | Draw stain (PI) | 7 | 5 | 10 | – | |
| 20 | Draw sample (air) | 2 | 5 | 500 | – | |
| 21 | Inject SL | – | 4 | 500 | – | |
| 22 | Incubate | – | – | – | 30 | |
| 23 | Default | – | – | – | – | |
| 24 | Incubate | – | – | – | 250 | |
| 25 | Test trigger | – | – | – | – | Washing and incubation steps |
| 26 | Incubate | – | – | – | 50 | |
| 27 | Test trigger | – | – | – | ||
| 28 | Incubate | – | – | – | 50 | |
| 29 | Wash | – | 10 | 2,000 | – | |
| 30 | Incubate | – | – | – | 3,600 | |
| 31 | Incubate | – | – | – | 3,600 | |
| 32 | Incubate | – | – | – | 3,600 | |
| 33 | Incubate | – | – | – | 3,600 | |
| 34 | Test trigger | – | – | – | – | |
| 35 | Incubate | – | – | – | 3,600 |
Results and discussion
This study was initiated to determine the applicability of acquiring near real-time automated apoptosis data on mammalian cell suspension cultures. This would prove invaluable in various research and industrial PAT scenarios, as it would give further insight on the given conditions for the onset of apoptosis leading to tighter control and cell culture processes.
Stirred suspension cell culture
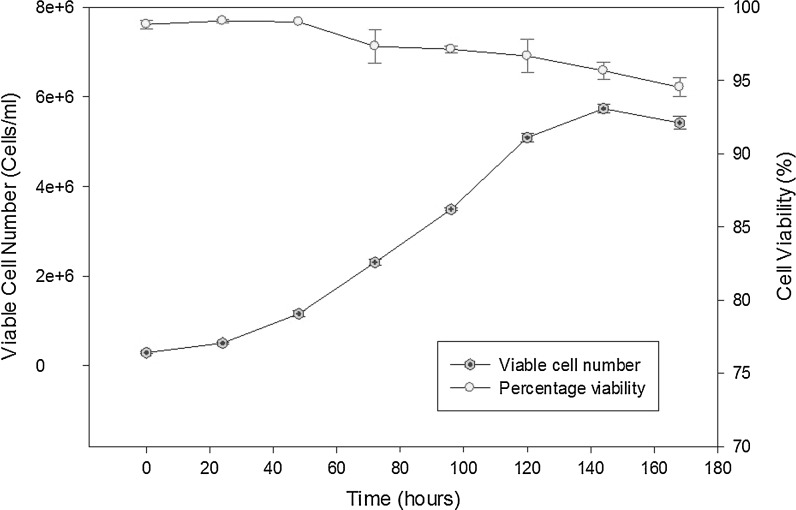
Cell samples were taken in triplicate every 24 h from the 500 ml suspension culture and analysed by TB exclusion hemocytometer counts (see Fig. 2). From the growth profile, the specific growth rate of the CHOK1SV cells was found to be 0.027 h−1, a typical value for suspension CHOK1SV cells according to the literature (Davies et al. 2012). The TB exclusion assay is commonly used for determining cell viability but cannot determine the percentage of early apoptotic cells and give a deeper insight into whether optimal bioreactor conditions are being met. Apoptosis can increase significantly from the late exponential phase onwards in mammalian cell culture. With apoptosis the major contributor to cell death in suspension CHO culture (Singh 1994), the importance of detection for biopharmaceutical processes can be significant.
Fig. 2.
A 500 ml spinner batch culture of CHOK1SV cells. Samples were taken in triplicate every 24 h over a period of 168 h and viable cells as well as percentage viability were determined by hemocytometer counts in conjunction with the TB exclusion assay. The cells reached a maximum cell density of 5.74 × 106 cells/ml with a viability of 95.67 ± 0.58 % at 144 h
One concern that would be critical in industrial bioprocess monitoring is the risk of contamination of bioreactor cell cultures from the sampling mechanism. Although there are several systems on the market that address this issue, none have been fully demonstrated to have full acceptance in a cGMP environment. With the push for PAT in biologics manufacturing environments it should not be long until an appropriate sampling system should be accepted and give rise to the possibility of online flow cytometry for bioprocess monitoring and control. Several sampling systems designed for both industrial and laboratory research settings are already on the market which allow aseptic sample withdrawal from suspension based bioreactor systems (BBI-Biotech 2012; Flownamics 2012) and a future study will be to incorporate a sampling system appropriate for industrial bioprocesses PAT applications.
Automated versus manual apoptosis measurements
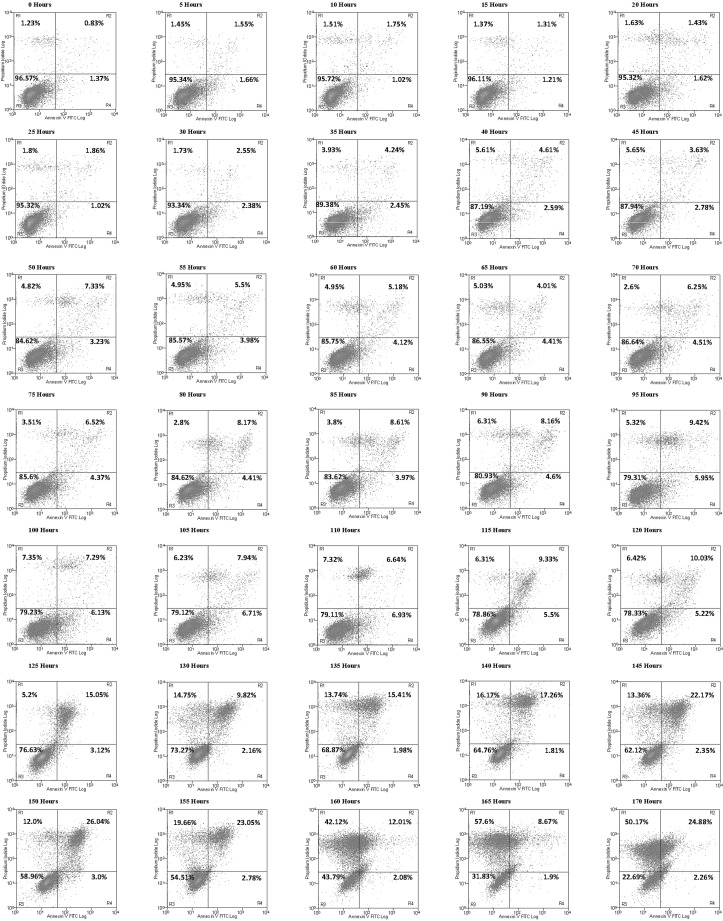
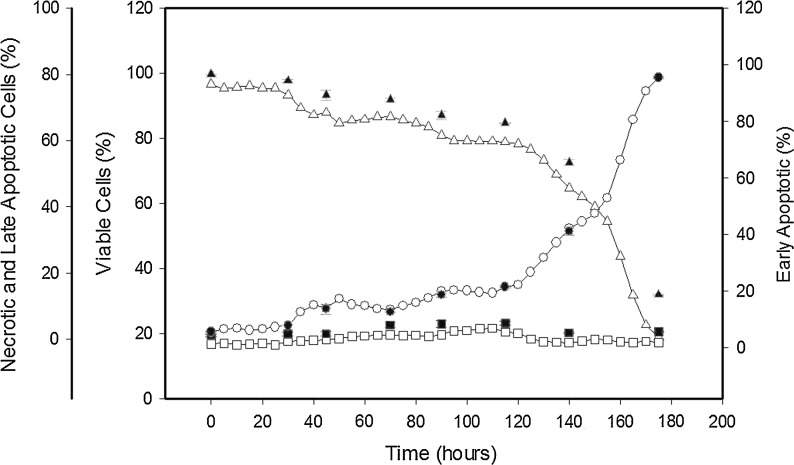
In order to determine if online flow cytometry can be performed to obtain apoptosis data and hence observe the transition to apoptosis in a 500 ml stirred suspension cell culture, a manual apoptosis assay was carried out on the culture alongside the automated assay at timed intervals without interfering with the automated analysis. The automated online analysis has the capability to increase the sampling interval for this experiment, which was set for every 5 h. The data obtained via the online flow cytometry run is shown as quadrant dot plots in Fig. 3, where the data indicate that the highest population of early apoptotic cells by percentage is achieved at 110 h, reaching 6.93 %, after which a significant increase in the amount of late apoptotic and necrotic cells occurs as the culture progresses to the late exponential and stationary phase (see Fig. 4). At the point where the culture has reached the stationary phase (140 h), apoptosis has clearly set in with 65.44 ± 1.15 % viable cells in the culture detected using the FITC/PI assay. This compared to the manual TB exclusion assay which still gives a cell viability of 95.67 ± 0.58 % at 144 h in the stationary phase. The automated apoptosis data correlates well with the manual flow cytometric analysis (see Fig. 4), and in comparison to the traditional TB exclusion assay it shows that the TB membrane integrity assay does not give a true measure of CHO culture viability. The same would hold true for most membrane integrity assays as they would not include early apoptosis data where the membrane is still intact but the cell has initiated the biochemical pathways leading to cell death. Apoptosis can have an impact on protein production (Browne and Al-Rubeai 2011; Gregory et al. 2009) with the benefit of early detection and monitoring of apoptotic events helping to determine optimal harvest time in addition to presenting the opportunity to employ further apoptosis prevention control strategies during the cell culture process.
Fig. 3.
Automated online flow cytometric analyses of CHOK1SV cells. Quadrant plots were generated every 5 h between 0 and 170 h. The x-axis represents Annexin-V-FITC fluorescence and the y-axis PI fluorescence with the plot divided into four representative regions labeled R1–R4. R1 = necrotic cells, R2 = late-apoptotic cells, R3 = viable cells, R4 = early-apoptotic cells. The data show that these CHO cells enter early apoptosis and progress to late apoptosis in the plots as the CHO culture progresses, with time, until reaching a point where late apoptosis and eventually necrosis dominates. In this study, the importance of entering early apoptosis is key, as this gives an early indicator of negative environmental factors an indication of when to harvest the cells depending on the tolerance of the product to the percentage of early and late apoptosis within the culture or serve as an early indicator for process adjustments (nutrient feeds or the activation of apoptosis suppressors) to compensate for the negative environmental stimuli
Fig. 4.
Online flow cytometry to measure apoptosis versus manual flow cytometry measurements via dual Annexin-V FITC and PI staining on CHOK1SV cultured cells grown in a 500 ml stirred vessel. The plots show online readings for the percentages of viable (blank triangles), early apoptotic (blank squares), and late apoptotic plus necrotic cells (blank circles) compared to the manual readings for the percentages of viable (black filled triangles), early apoptotic (black filled squares), and late apoptotic plus necrotic cells (black filled circles)
Typically, apoptosis detection and monitoring is limited to the manual sampling intervals that are humanly possible over the course of the culture due to the time constraints of sample preparation and flow cytometry operations. Sitton et al. (2006), did show the possibility of using forward scatter (FSC) and side scatter (SS) to discriminate between apoptotic and non-apoptotic cells using an online FC setup but this does not give any definitive information about early apoptotic cells within the population. In biologics manufacturing it is important to closely monitor culture conditions and it is beneficial to carefully track the progress of apoptosis; it can have an effect on final product quality and quantity, as the proteases activated by apoptosis may degrade the desired protein products. The ability to detect in near real time the start of an increase in early apoptotic cells in bioreactor culture can be advantageous to the overall control strategy and process design as the early detection can enable an early response in order to reduce apoptosis. Previous research with antibody producing hybridoma cell cultures has also indicated that there is a significant inverse correlation between the percentage of apoptotic cells and antibody productivity at the initiation of a culture (Gregory et al. 2009). Thus, it could be an important determinant of the culture’s productivity at different stages and indicate the optimal harvest time for maximal quality and quantity of protein products. Fed-batch systems can be finely tuned with the addition of apoptosis monitoring and control where adding a particular substrate to drive an alternative metabolic pathway such as introducing additives at particular culture stages that influence apoptosis and hence the final product quality and quantity (Whitford 2006). Apoptosis monitoring provides an additional parameter for the development of a bioprocess that improves upon the information that traditional parameters currently achieve improving dynamic modelling and prediction of cell culture. In addition, fragmented DNA (Janicke et al. 1998) can complicate downstream processing operations as fragmentation increases with apoptosis (Shukla et al. 2007). Detection can lead to easier downstream processing with less product loss.
Conclusion
We present here a novel approach and successful implementation to obtain near real-time apoptosis data from suspension cell culture using automated sample preparation and flow cytometry. This is of importance to the research and industrial communities, especially in biologics manufacturing in the light of the development of the PAT initiative to improve end product quality and quantity through improved bioprocess monitoring and control in operations such as cell culture. In addition, the ability to closely follow the development of apoptosis overtime can improve cell death research, offering greater understanding of the mechanisms involved and the temporal aspect of its onset.
Acknowledgments
We thank Christian Lavarreda of MSP Corporation for the use of the Flow CytoPrep 5000 unit. This work was supported by funds from Enterprise Ireland for the apPAT project.
References
- Al-Rubeai M, Chalder S, Bird R, Emery AN. Cell cycle, cell size and mitochondrial activity of hybridoma cells during batch cultivation. Cytotechnology. 1991;7:179–186. doi: 10.1007/BF00365929. [DOI] [PubMed] [Google Scholar]
- Al-Rubeai M, Emery AN, Chalder S. Flow cytometric study of cultured mammalian cells. J Biotechnol. 1991;19:67–81. doi: 10.1016/0168-1656(91)90075-7. [DOI] [PubMed] [Google Scholar]
- Arden N, Betenbaugh MJ. Life and death in mammalian cell culture: strategies for apoptosis inhibition. Trends Biotechnol. 2004;22:174–180. doi: 10.1016/j.tibtech.2004.02.004. [DOI] [PubMed] [Google Scholar]
- BBI-Biotech (2012) BioPROBE. http://bbi-biotech.com/en/produkte/probenahme-bioprobe/. Accessed 8 Jan 2014
- Bibila T, Ranucci C, Glazomitsky K, Buckland B, Aunins J. Investigation of NS0 cell metabolic behavior in monoclonal antibody producing clones. Ann NY Acad Sci. 1994;745:277–284. doi: 10.1111/j.1749-6632.1994.tb44382.x. [DOI] [PubMed] [Google Scholar]
- Borth N, Zeyda M, Kunert R, Katinger H. Efficient selection of high-producing subclones during gene amplification of recombinant Chinese hamster ovary cells by flow cytometry and cell sorting. Biotechnol Bioeng. 2000;71:266–273. doi: 10.1002/1097-0290(2000)71:4<266::AID-BIT1016>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Browne SM, Al-Rubeai M. Defining viability in mammalian cell cultures. Biotechnol Lett. 2011;33:1745–1749. doi: 10.1007/s10529-011-0644-2. [DOI] [PubMed] [Google Scholar]
- Carroll S, Al-Rubeai M. The selection of high-producing cell lines using flow cytometry and cell sorting. Expert Opin Biol Ther. 2004;4:1821–1829. doi: 10.1517/14712598.4.11.1821. [DOI] [PubMed] [Google Scholar]
- Carvell JP, Dowd JE. On-line measurements and control of viable cell density in cell culture manufacturing processes using radio-frequency impedance. Cytotechnology. 2006;50:35–48. doi: 10.1007/s10616-005-3974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisti Y. Animal cell culture in stirred bioreactors: observations on scale-up. Bioprocess Biosyst Eng. 1993;9:191–196. doi: 10.1007/BF00369402. [DOI] [Google Scholar]
- Cotter TG, Al-Rubeai M. Cell death (apoptosis) in cell culture systems. Trends Biotechnol. 1995;13:150–155. doi: 10.1016/S0167-7799(00)88926-X. [DOI] [PubMed] [Google Scholar]
- Davies SL, Lovelady CS, Grainger RK, Racher AJ, Young RJ, James DC. Functional heterogeneity and heritability in CHO cell populations. Biotechnol Bioeng. 2012 doi: 10.1002/bit.24621. [DOI] [PubMed] [Google Scholar]
- deZengotita VM, Abston LR, Schmelzer AE, Shaw S, Miller WM. Selected amino acids protect hybridoma and CHO cells from elevated carbon dioxide and osmolality. Biotechnol Bioeng. 2002;78:741–752. doi: 10.1002/bit.10255. [DOI] [PubMed] [Google Scholar]
- Emery AN, Jan DC, Al-Rubeai M. Oxygenation of intensive cell-culture system. Appl Microbiol Biotechnol. 1995;43:1028–1033. doi: 10.1007/BF00166920. [DOI] [PubMed] [Google Scholar]
- Flownamics (2012) Seg-Flow. http://www.flownamics.com/products_segflow.php. Accessed 8 Jan 2014
- Food and Drug Administration (2004) Guidance for Industry: PAT- A framework for innovative pharmaceutical development, manufacturing, and quality assurance. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070305.pdf. Accessed 12 Dec 2013
- Gregory CD, Pound JD, Devitt A, Wilson-Jones M, Ray P, Murray RJ. Inhibitory effects of persistent apoptotic cells on monoclonal antibody production in vitro: simple removal of non-viable cells improves antibody productivity by hybridoma cells in culture. mAbs. 2009;1:370–376. doi: 10.4161/mabs.1.4.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Ge X, Kostov Y, Brorson KA, Moreira AR, Rao G. Comparisons of optical pH and dissolved oxygen sensors with traditional electrochemical probes during mammalian cell culture. Biotechnol Bioeng. 2007;97:833–841. doi: 10.1002/bit.21320. [DOI] [PubMed] [Google Scholar]
- Heath C, Dilwith R, Belfort G. Methods for increasing monoclonal antibody production in suspension and entrapped cell cultures: biochemical and flow cytometric analysis as a function of medium serum content. J Biotechnol. 1990;15:71–89. doi: 10.1016/0168-1656(90)90052-D. [DOI] [PubMed] [Google Scholar]
- Ibarra N, Watanabe S, Bi JX, Shuttleworth J, Al-Rubeai M. Modulation of cell cycle for enhancement of antibody productivity in perfusion culture of NS0 cells. Biotechnol Prog. 2003;19:224–228. doi: 10.1021/bp025589f. [DOI] [PubMed] [Google Scholar]
- Ishaque A, Al-Rubeai M. Role of Ca2+, Mg2+ and K+ ions in determining apoptosis and extent of suppression afforded by bcl-2 during hybridoma cell culture. Apoptosis. 1999;4:335–355. doi: 10.1023/A:1009643204200. [DOI] [PubMed] [Google Scholar]
- Jan DCH, Petch DA, Huzel N, Butler M. The effect of dissolved oxygen on the metabolic profile of a murine hybridoma grown in serum-free medium in continuous culture. Biotechnol Bioeng. 1997;54:153–164. doi: 10.1002/(SICI)1097-0290(19970420)54:2<153::AID-BIT7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinov KB, Pambayun R, Matanguihan R, Yoshida T, Perusicn CM, Hu WS. On-line monitoring of hybridoma cell growth using a laser turbidity sensor. Biotechnol Bioeng. 1992;40:1337–1342. doi: 10.1002/bit.260401107. [DOI] [PubMed] [Google Scholar]
- Kuystermans D, Al-Rubeai M. Bioreactor systems for producing antibody from mammalian cells. In: Al-Rubeai M, editor. Antibody expression and production. cell engineering. 7. Netherlands: Springer; 2011. pp. 25–52. [Google Scholar]
- Kuystermans D, Mohd A, Al-Rubeai M. Automated flow cytometry for monitoring CHO cell cultures. Methods. 2012;56:358–365. doi: 10.1016/j.ymeth.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lee GM (2012) Monitoring of autophagy in Chinese hamster ovary cells using flow cytometry. Methods 56:375–382. doi:10.1016/j.ymeth.2011.11.006 [DOI] [PubMed]
- Leelavatcharamas V, Emery AN, Al-Rubeai M. Growth and interferon-gamma production in batch culture of CHO cells. Cytotechnology. 1994;15:65–71. doi: 10.1007/BF00762380. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelle K, Westlund A, Haggstrom L. Ammonium ion transport-a cause of cell death. Cytotechnology. 1996;22:251–254. doi: 10.1007/BF00353945. [DOI] [PubMed] [Google Scholar]
- Naciri M, Kuystermans D, Al-Rubeai M. Monitoring pH and dissolved oxygen in mammalian cell culture using optical sensors. Cytotechnology. 2008;57:245–250. doi: 10.1007/s10616-008-9160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Kamihira M, Yoshida H, Iijima S, Kobayashi T. Effect of dissolved oxygen concentration on monoclonal antibody production in hybridoma cell cultures. J Ferment Bioeng. 1992;74:372–378. doi: 10.1016/0922-338X(92)90034-R. [DOI] [Google Scholar]
- Petersen JF, Mcintire LV, Papoutsakis ET. Shear sensitivity of cultured hybridoma cells (Crl-8018) depends on mode of growth, culture age and metabolite concentration. J Biotechnol. 1988;7:229–246. doi: 10.1016/0168-1656(88)90054-5. [DOI] [Google Scholar]
- Plasier B, Lloyd DR, Paul GC, Thomas CR, Al-Rubeai M. Automatic image analysis for quantification of apoptosis in animal cell culture by annexin-V affinity assay. J Immunol Methods. 1999;229:81–95. doi: 10.1016/S0022-1759(99)00107-6. [DOI] [PubMed] [Google Scholar]
- Rathore AS, Bhambure R, Ghare V. Process analytical technology (PAT) for biopharmaceutical products. Anal Bioanal Chem. 2010;398:137–154. doi: 10.1007/s00216-010-3781-x. [DOI] [PubMed] [Google Scholar]
- Shukla AA, Hubbard B, Tressel T, Guhan S, Low D. Downstream processing of monoclonal antibodies–application of platform approaches. J Chromatogr B Anal Technol Biomed Life Sci. 2007;848:28–39. doi: 10.1016/j.jchromb.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Singh MA. Cell death in bioreactors: a role for apoptosis. Biotechnol Bioeng. 1994;44:720–726. doi: 10.1002/bit.260440608. [DOI] [PubMed] [Google Scholar]
- Singh RP, Al-Rubeai M. Apoptosis and bioprocess technology. Adv Biochem Eng Biotechnol. 1998;62:167–184. doi: 10.1271/bbb.62.167. [DOI] [PubMed] [Google Scholar]
- Sitton G, Hansgate A, Srienc F. Transient gene expression in CHO cells monitored with automated flow cytometry. Cytotechnology. 2006;52:13–24. doi: 10.1007/s10616-006-9020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Suen Y, Yee BS (2006) Apoptosis and cell cycle analysis with the QuantaTM SC Flow System. AACR Meeting Abstracts 2006, p 1166
- Streefland M, Martens DE, Beuvery EC, Wijffels RH. Process analytical technology (PAT) tools for the cultivation step in biopharmaceutical production. Eng Life Sci. 2013;13:212–223. doi: 10.1002/elsc.201200025. [DOI] [Google Scholar]
- Whitford WG. Fed-batch mammlian cell culture in bioproduction. Bioprocess Int. 2006;4:30–40. [Google Scholar]
- Xie L, Wang DI. Fed-batch cultivation of animal cells using different medium design concepts and feeding strategies. Biotechnol Bioeng. 1994;43:1175–1189. doi: 10.1002/bit.260431123. [DOI] [PubMed] [Google Scholar]
- Zeiser A, Bedard C, Voyer R, Jardin B, Tom R, Kamen AA. On-line monitoring of the progress of infection in Sf-9 insect cell cultures using relative permittivity measurements. Biotechnol Bioeng. 1999;63:122–126. doi: 10.1002/(SICI)1097-0290(19990405)63:1<122::AID-BIT13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]