Abstract
Serial femtosecond crystallography (SFX) has enabled the damage-free structural determination of metalloenzymes and filled the gaps of our knowledge between crystallographic and spectroscopic data. Crystallographers, however, scarcely know whether the rising technique provides truly new structural insights into mechanisms of metalloenzymes partly because of limited resolutions. Copper nitrite reductase (CuNiR), which converts nitrite to nitric oxide in denitrification, has been extensively studied by synchrotron radiation crystallography (SRX). Although catalytic Cu (Type 2 copper (T2Cu)) of CuNiR had been suspected to tolerate X-ray photoreduction, we here showed that T2Cu in the form free of nitrite is reduced and changes its coordination structure in SRX. Moreover, we determined the completely oxidized CuNiR structure at 1.43 Å resolution with SFX. Comparison between the high-resolution SFX and SRX data revealed the subtle structural change of a catalytic His residue by X-ray photoreduction. This finding, which SRX has failed to uncover, provides new insight into the reaction mechanism of CuNiR.
Keywords: copper, electron transfer, enzyme, serial femtosecond crystallography, X-ray free-electron laser
Since the invention of the Haber–Bosch process, the amount of nitrogen oxides fixed in soils and waters has been increasing and the global nitrogen cycle has gradually changed (1, 2). In the cycle, nitrogen fixed in the form of ammonium salts are converted to nitrogen oxides and then reduced to a dinitrogen gas in a stepwise manner (NO3− → NO2− → NO → N2O → N2) (3). This reduction process, denitrification, is the main path for fixed nitrogen to be removed and hence has major agronomic and environmental impacts. Chemical reactions in denitrification are performed by microorganisms and coupled with their anaerobic respiratory systems in which metalloenzymes are utilized (3, 4). Nitrite reduction to nitric oxide (NO2− + e− + 2H+ → NO + H2O) is an important step in denitrification where the ion is changed to the toxic and highly reactive gas. Two types of dissimilatory nitrite reductase (NiR) have been identified to date (3, 4). One of them is cd1-type heme nitrite reductase (cd1NiR), which functions as a homodimer (5). The other one is copper nitrite reductase (CuNiR): a homotrimeric copper-containing enzyme. CuNiR can also reduce dioxygen to hydrogen peroxide (6, 7) and catalyse the dismutation of superoxide (8). Each monomer of typical CuNiR contains two copper sites: Type 1 Cu (T1Cu) with a Cys–Met–His2 ligand set and Type 2 Cu (T2Cu) with a His3 ligand set (9–13). The T1Cu site accepts an electron from c-type cytochromes (14, 15) or blue copper proteins (6, 16, 17), when CuNiR and the donor protein form a transient electron transfer (ET) complex. The received electrons are transferred to the T2Cu site, the catalytic centre, through a Cys–His pathway. The Asp–His pair (Aspcat and Hiscat), which is conserved above the T2Cu site and connected via a water molecule (bridging water), is essential to the enzymatic activity (18, 19), though the exact role has been ambiguous.
In conventional synchrotron radiation crystallography (SRX), strong X-ray beams induce photoreduction of metal centres and destroy their natural structures (20–23). Spectroscopic analysis revealed that T1Cu in CuNiR is rapidly reduced by synchrotron X-ray (24). T2Cu is more resistant, than T1Cu, to X-ray damage in the absence of NO2−. When the substrate binds to T2Cu, X-ray photoreduction of T1Cu is followed by ET from T1Cu to T2Cu, which results in reduction of NO2− in crystallo (24). This gated ET is explained by the concept of proton-coupled ET (25, 26), although the detailed mechanism remains to be elucidated. Because the presence of NO2− accelerates intramolecular ET also in solution (25–27), it is obvious that ET from T1Cu to T2Cu is gated to some extent. However, kinetic studies demonstrated the random sequential mechanism of nitrite reduction; i.e. intramolecular ET can occur both with and without the binding of the substrate (28, 29). Especially, intramolecular ET before substrate binding is dominant at low pH (<6.5) (28). Moreover, we have recently shown that substrate-free T2Cu in a CuNiR crystal crystallized at pH 4.5 may be reduced by synchrotron X-rays and that an unknown chemical reaction occurs on T2Cu during data collection (30).
To investigate the nitrite reduction mechanism in CuNiR, detailed structural comparison between its oxidized and reduced state is necessary. Here, we closely examined X-ray-induced structural changes and chemical reactions at the T2Cu site using a helical scan method combined with microfocus X-ray beams (31). Furthermore, we determined the first completely oxidized CuNiR structure using serial femtosecond crystallography (SFX) with X-ray free-electron laser (XFEL) (32), which has enabled damage-free structural determination of metalloenzymes even at room temperature (RT) (33–37). Because the Bragg spacings of previously determined SFX structures of metalloenzymes were longer than typical covalent bond lengths found in macromolecules (∼1.5 Å), it has been difficult to obtain, at the chemical level, new structural insights into the reaction mechanisms of metalloenzymes. CuNiR crystals used in this study have been known to diffract X-rays well; therefore, we could determine its high-resolution SFX structure. We here would like to report our results in detail.
Materials and Methods
Sample preparation of CuNiR from Geobacillus thermodenitrificans (GtNiR)
Geobacillus thermodenitrificans copper nitrite reductase (GtNiR) was expressed and purified as described previously (30). We used chloride-free buffers for all the steps of purification and crystallization. Microcrystals for SFX were obtained by a rotational crystallization technique using nanoseeds of the protein as follows. Macrocrystals were transferred to a 1.5 ml tube (Eppendorf, Hamburg, Germany) containing 1 ml of solution composed of 100 mM sodium acetate buffer (pH 4.5), 5.5% (w/v) polyethylene glycol 4,000 and 75 mM CuSO4. After sonicating the crystals on ice with a UD-211 ultrasonicator (Tomy Seiko Co., Tokyo, Japan), the solution was centrifuged and the supernatant was collected as a nanoseed solution. In a 15 ml centrifuge tube (AS ONE Co., Osaka, Japan), 4 ml of the 20 mg/ml protein solution was mixed with 4 ml of the precipitant solution, which was composed of 100 mM sodium acetate buffer (pH 4.5), 15% (w/v) polyethylene glycol 4,000, 75 mM CuSO4 and then 160 μl of the nanoseed solution was added. The centrifuge tube had been rotated on a RT-50 culture rotator (TITEC, Saitama, Japan) at ∼30 rpm for 1 week at RT. The microcrystal solution was filtered through a 30 μm CellTrics filter (Chiyoda Sci. Co., Tokyo, Japan) and adjusted to a number density of ∼4.4 × 108 crystals/ml by adding 4 ml of the precipitant solution.
Synchrotron data collection
Cryogenic SRX datasets were collected using microfocus beamline BL32XU at SPring-8 (38). A large single crystal of GtNiR (915 × 620 × 230 µm3) was flash-cooled by immersion in liquid nitrogen and mounted on a conventional goniometer with the longest axis roughly directed towards the horizontal rotation axis. Along the longest edge of the crystal, 120 irradiation points were chosen. The regular intervals between the irradiation points were 7.6 µm, which is sufficient to separate the radiation damage at each irradiation point. The beam at a wavelength of 0.7500 Å was focused to 15 μm (height, H) × 1.0 μm (width, W) with a photon flux of 8 × 1011 photons/s. Using the helical scan method, a total of seven datasets (SR1–SR7) were repeatedly collected in order from the same points of the same crystal except for absorbed X-ray dose per frame. The exposure time for each image was 1 s. For datasets SR1, SR3, SR5 and SR7, each image was collected with an absorbed dose of 0.064 MGy/frame with a 92.3% attenuated beam, while for datasets SR2, SR4 and SR6, the dose corresponded to 8.252 MGy/frame without attenuation. The X-ray doses were calculated with RADDOSE (39). The parameters and statistics are summarized in Table I.
Table I.
Data collection and refinement statistics for the cryogenic SRX structures
| Name/PDB ID | SR1/4YSO | SR2/4YSP | SR3/4YSQ | SR4/4YSR | SR5/4YSS | SR6/4YST | SR7/4YSU |
|---|---|---|---|---|---|---|---|
| Data collection at BL32XU of SPring-8 (Wavelength 0.7500 Å) | |||||||
| X-ray dose (MGy) | 0.064 | 8.316 | 8.380 | 16.632 | 16.696 | 24.948 | 25.012 |
| Space group | R3 | R3 | R3 | R3 | R3 | R3 | R3 |
| Unit cell a = b, c (Å) | 114.8, 84.10 | 114.9, 84.23 | 115.0, 84.23 | 115.0, 84.34 | 115.1, 84.31 | 115.2, 84.38 | 115.2, 84.39 |
| Resolution (Å) | 50.0–1.50 (1.55–1.50) | 50.0–1.34 (1.39–1.34) | 50.0–1.50 (1.55–1.50) | 50.0–1.34 (1.39–1.34) | 50.0–1.50 (1.55–1.50) | 50.0–1.34 (1.39–1.34) | 50.0–1.50 (1.55–1.50) |
| Rsym (%) | 10.9 (53.3) | 9.8 (31.2) | 10.7 (55.0) | 10.1 (30.5) | 11.7 (64.4) | 12.2 (36.9) | 13.9 (78.7) |
| Rpim (%) | 7.4 (35.1) | 6.7 (17.5) | 7.4 (36.7) | 7.1 (21.0) | 8.0 (43.4) | 8.3 (25.4) | 9.5 (55.0) |
| CC1/2 | (0.530) | (0.895) | (0.576) | (0.871) | (0.465) | (0.810) | (0.277) |
| Completeness (%) | 96.9 (98.5) | 93.6 (98.2) | 96.6 (98.6) | 92.8 (98.0) | 96.4 (98.6) | 93.1 (98.1) | 96.1 (97.4) |
| Unique reflections | 63,495 (6,470) | 87,908 (9,204) | 64,868 (6,624) | 87,653 (9,255) | 64,668 (6,623) | 88,348 (9,311) | 64,638 (6,537) |
| <I/σ (I)> | 12.2 (2.77) | 11.8 (6.03) | 11.2 (2.01) | 11.3 (4.66) | 10.0 (1.60) | 8.77 (3.53) | 8.05 (1.34) |
| Redundancy | 3.0 (3.0) | 3.1 (3.0) | 2.9 (3.0) | 3.0 (3.0) | 2.9 (3.0) | 3.0 (2.9) | 2.8 (2.8) |
| Refinement | |||||||
| Resolution (Å) | 38.73–1.50 (1.54–1.50) | 20.08–1.34 (1.37–1.34) | 34.35–1.50 (1.53–1.50) | 50.00–1.34 (1.37–1.34) | 33.23–1.50 (1.54–1.50) | 20.12–1.34 (1.37–1.34) | 42.94–1.50 (1.54–1.50) |
| Rwork/Rfree (%) | 12.9/17.7 | 12.6/15.0 | 14.0/18.7 | 14.3/17.4 | 14.8/19.1 | 15.5/18.5 | 16.3/20.8 |
| No. of | |||||||
| protein atoms | 2,375 | 2,383 | 2,344 | 2,352 | 2,377 | 2,355 | 2,359 |
| ligand/ions | 20 | 20 | 28 | 20 | 28 | 19 | 18 |
| water | 371 | 294 | 331 | 302 | 311 | 294 | 273 |
| Average B (Å2) | |||||||
| All | 13.9 | 13.8 | 19.0 | 14.7 | 16.3 | 17.1 | 16.2 |
| Protein atoms | 11.7 | 12.3 | 17.1 | 12.8 | 14.7 | 15.5 | 14.7 |
| Water atoms | 26.8 | 24.5 | 31.5 | 28.2 | 27.5 | 29.2 | 26.1 |
| T1Cu, T2Cu | 8.6, 8.7 | 9.6, 8.7 | 14.0, 13.3 | 9.83, 9.13 | 11.4, 10.7 | 12.2, 11.7 | 10.8, 10.6 |
| Other atoms | 29.6 | 31.2 | 32.8 | 32.6 | 33.5 | 35.9 | 38.6 |
| Ramachandran | |||||||
| Favoured (%) | 98.0 | 97.7 | 98.3 | 98.0 | 98.0 | 97.3 | 97.3 |
| Allowed (%) | 2.0 | 2.3 | 1.7 | 2.0 | 2.0 | 2.7 | 2.7 |
| Outliers (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
RT data collection was performed at BL38B1 of SPring-8 (40), as described previously (41). The dataset was collected from one position of a single crystal using an ADSC Quantum 315 charge-coupled device (CCD) detector (Area Detector Systems Co., CA, USA). The beam size was 50 μm (H) × 88 μm (W). The oscillation angle and exposure time per image were set to 1° and 1.5 s, respectively. A total of 120 diffraction images were collected from the single crystal. The parameters and statistics are summarized in Table II.
Table II.
Data collection and refinement statistics for the RT SRX structure
| Data collection at BL38B1 of SPring-8 (Wavelength 0.9000 Å) | |
| Space group | R3 |
| Unit cell a = b, c (Å) | 116.2, 85.55 |
| Resolution range (Å) | 50.0–1.35 (1.55–1.35) |
| Rsym (%) | 9.6 (36.2) |
| Completeness (%) | 99.8 (100) |
| Unique reflections | 94,321 (4,709) |
| <I/σ (I)> | 24.4 (2.4) |
| Redundancy | 4.2 (3.6) |
| Refinement | |
| Resolution (Å) | 26.5–1.35 (1.39–1.35) |
| Rwork (%)/Rfree (%) | 9.7/12.0 |
| No. of protein atoms | 2,574 |
| No. of ligand atoms and ions | 17 |
| No. of water molecules | 219 |
| Average B (Å2) | |
| All | 18.9 |
| Protein atoms | 17.6 |
| Water | 34.1 |
| T1Cu atom | 11.6 |
| T2Cu atom | 10.5 |
| Other atoms | 26.8 |
| Ramachandran plot (%) | |
| Favoured | 97.3 |
| Allowed | 2.7 |
| Outliers | 0 |
| Coordinate error (Å) | 0.020 |
| PDB code | 4YSD |
Structure determination of the SRX structures
All of the datasets were indexed and integrated using HKL2000 (42). The phases were determined by the molecular replacement method using MOLREP (43) with a GtNiR monomer (Protein Data Bank (PDB) code 4ZK8) as a search model. Manual model building was performed using WinCoot 0.7 (44). The program REFMAC5 (45) from the CCP4 suite (ver. 6.5.0) (46) was used for structural refinement. Anisotropic displacement parameters were introduced after water molecules were built in the models. The final models were checked for stereochemical quality using MolProbity (47).
Single-shot XFEL data collection
The experiment was performed at BL3 of SPring-8 Angstrom Compact Free-Electron Laser (SACLA) in Hyogo, Japan (48). Using 2–10 fs XFEL pulses, we collected diffraction patterns from greenish-blue (aerobically oxidized) GtNiR microcrystals (Supplementary Fig. S1a). The pulse duration shorter than 10 fs is quite important to obtain intact metalloprotein structures, because ultrabright XFEL beams damage electronic structures of heavy atoms in a few tens of femtoseconds (49) and can destroy the natural structures of metal centres (50). A liquid injector (nozzle aperture diameter: 200 μm) with a sample circulation system was used (51). Microcrystal sample (5.5 ml) was placed in a reservoir. The flow rate was set to 5.3 ml/min (70 cm/s). The injector was installed in a helium ambiance, diffraction chamber enclosure: Diverse Application Platform for Hard X-ray Diffraction in SACLA (DAPHNIS) (52). The liquid–stream width was nearly the same as the aperture size. The sample chamber was maintained at a temperature of 300 K with a humidity of 85–99%. The diffraction patterns were collected using a short-working-distance octal multiport CCD detector (53) with XFEL radiation. The microcrystals were exposed to single X-ray pulses at a photon energy of 11.0 keV. The pulses consisted of 5 × 1010 photons/pulse were focused to 2.5 μm (H) × 2.0 μm (W) at the interaction point using Kirkpatrick–Baez mirrors (54). The repetition rate was 30 Hz, and the typical pulse energy at the sample was 90 μJ/pulse. The parameters and statistics are summarized in Table III.
Table III.
Data collection and refinement statistics for the SFX structure
| Data collection | |
| Beamline | SACLA BL3 |
| Wavelength (Å) | 1.129 |
| Space group | R3 |
| Unit cell a = b, c (Å) | 116.2, 85.55 |
| Resolution range (Å) | 34.8–1.43 (1.47–1.43) |
| Rsplit (%) | 17.70 (113.8) |
| Completeness (%) | 100 (99.97) |
| Unique reflections | 79,590 (7,859) |
| CC1/2 | 0.970 (0.0417) |
| <I/σ (I)> | 3.38 (0.99) |
| Redundancy | 245.3 (207.0) |
| Refinement | |
| Resolution range (Å) | 34.80–1.43 (1.47–1.43) |
| Rwork (%)/Rfree (%) | 13.7/14.9 |
| No. of protein atoms | 2,414 |
| No. of heterogen atoms | 9 |
| No. of water molecules | 134 |
| Average B (Å2) | |
| All | 24.0 |
| Protein atoms | 23.3 |
| Water | 36.6 |
| T1Cu | 20.4 |
| T2Cu | 17.9 |
| Other atoms | 31.7 |
| Ramachandran plot (%) | |
| Favoured | 96.4 |
| Allowed | 3.6 |
| Outliers | 0 |
| PDB code | 4YSA |
Structural determination of the SFX structure
A total of 180,942 images were collected, of which 139,391 diffraction images were identified and 37,186 images were indexed and merged using CrystFEL (55). The data was indexed as space group R3 and processed as space group H3. Indexing ambiguity in SFX was solved by an algorithm that clusters snapshots (56). The phase was determined by molecular replacement using MOLREP with the GtNiR monomeric unit (PDB code 4ZK8) as a search model. Manual model building was performed using WinCoot 0.7. The program REFMAC5 from the CCP4 suite (ver. 6.5.0) was used for structure refinement. The microcrystals diffracted X-rays beyond 1.4 Å resolution (Supplementary Fig. S1b) but the statistics of high-resolution shells, such as CC1/2 and Rsplit, were poor (Supplementary Fig. S2). We therefore performed paired refinement to determine the ‘nominal resolution’, based on the idea that proper use of weaker and noisier but higher-resolution data can provide better models (57, 58). Actually, inclusion of noisy high-resolution data enables the better analysis of SFX data (59). The result of paired refinement showed that including higher-resolution data provided a better model than rejecting it (Supplementary Fig. S3a). The student’s t-test P value calculated from CC1/2 in the highest resolution shell cut at 1.43 Å resolution (0.0417, n = 7,859) was 0.000214, which is less than the significance level α = 0.001 (Supplementary Fig. S3b). Conversely, the P value calculated from CC1/2 in the highest resolution shell cut at 1.42 Å resolution (0.0246, n = 8,164) was 0.0261, which is greater than α (Supplementary Fig. S3b). Based on these things, we chose to use data up to 1.43 Å resolution, and performed further refinement. The final model was checked for stereochemical quality using MolProbity.
Results
Radiation damages to GtNiR in SRX
The structures for SR1, SR3, SR5 and SR7 data were refined against data to 1.50 Å resolution, whereas those for SR2, SR4 and SR6 data were refined to 1.34 Å resolution (Table I). Root-mean-square deviations (RMSDs) for Cα atoms between seven structures were at most 0.1 Å.
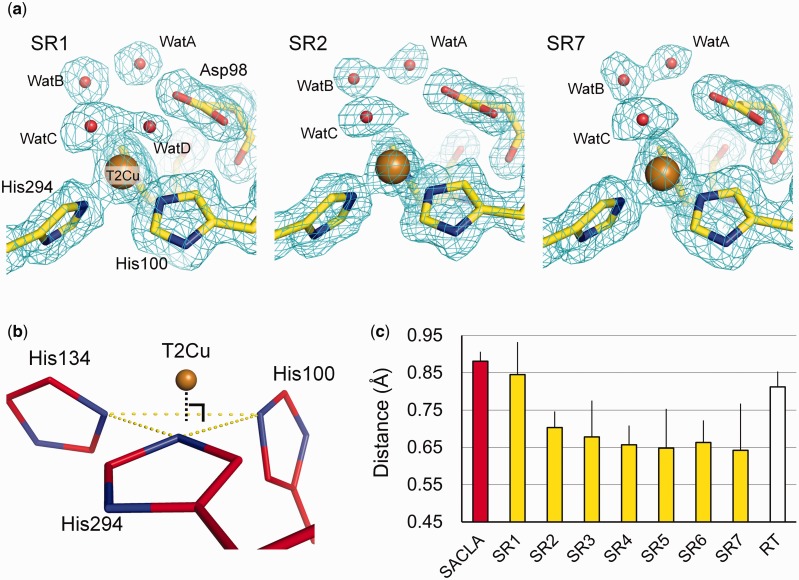
Changes in ligand–T1Cu distance fell within the range of coordinate errors (Table IV), indicating that the T1Cu site did not show significant structural changes that can be detected at resolutions of our present structures. Conversely, the T2Cu site showed obvious structural changes induced by X-ray irradiation: two water ligands (WatC and WatD) were present on T2Cu in the SR1 structure, while the electron density of WatD was not observed in other SR data (Fig. 1a). Furthermore, with increasing X-ray dose, the T2Cu atom gradually sank towards a ‘ligand plane’ composed of three Nε2 atoms of His ligands (Fig. 1b and c).
Table IV.
Coordination geometries of the copper sites
| SFX | SR1 | SR2 | SR3 | SR4 | SR5 | SR6 | SR7 | |
|---|---|---|---|---|---|---|---|---|
| Coordinate error (Å) | 0.012 | 0.042 | 0.021 | 0.048 | 0.025 | 0.052 | 0.029 | 0.062 |
| I. T1Cu–Ligand Distances (Å) | ||||||||
| Cu-H95Nδ1 | 2.03 | 2.02 | 2.04 | 2.01 | 2.03 | 2.03 | 2.06 | 2.05 |
| Cu-C135Sγ | 2.27 | 2.21 | 2.23 | 2.25 | 2.25 | 2.25 | 2.22 | 2.22 |
| Cu-H143Nδ1 | 1.91 | 1.97 | 2.05 | 2.02 | 2.02 | 2.04 | 2.03 | 2.03 |
| Cu-M148Sδ | 2.66 | 2.70 | 2.64 | 2.67 | 2.64 | 2.67 | 2.61 | 2.67 |
| II. T1Cu–Ligand Angles (°) | ||||||||
| H95-Cu-C135 | 135.3 | 136.6 | 134.3 | 133.4 | 133.1 | 132.9 | 133.2 | 134.2 |
| H95-Cu-H143 | 104.5 | 102.6 | 104.2 | 106.0 | 105.2 | 105.1 | 105.4 | 104.0 |
| His95-Cu-M148 | 81.1 | 82.1 | 81.5 | 82.1 | 82.1 | 84.1 | 80.8 | 82.1 |
| C135-Cu-H143 | 105.8 | 105.1 | 106.8 | 106.9 | 106.7 | 105.7 | 105.5 | 106.1 |
| C135-Cu-M148 | 110.2 | 109.8 | 112.1 | 111.8 | 111.6 | 112.0 | 112.9 | 113.6 |
| H143-Cu-M148 | 120.2 | 121.8 | 116.9 | 115.2 | 117.3 | 116.4 | 118.7 | 115.9 |
| III. T2Cu–Ligand Distances (Å) | ||||||||
| Cu-H100Nε2 | 2.07 | 2.07 | 2.04 | 2.09 | 2.07 | 2.02 | 2.04 | 2.03 |
| Cu-H134Nε2 | 2.12 | 1.99 | 2.01 | 2.03 | 1.98 | 2.01 | 1.97 | 2.01 |
| Cu-H294Nε2 | 2.02 | 2.06 | 1.99 | 2.01 | 1.99 | 2.04 | 2.01 | 2.01 |
| IV. T2Cu–Ligand Angles (°) | ||||||||
| H100-Cu-H134 | 106.0 | 106.0 | 113.6 | 115.2 | 116.1 | 116.1 | 115.3 | 114.2 |
| H100-Cu-H294 | 97.24 | 97.72 | 99.9 | 102.7 | 100.7 | 102.6 | 101.3 | 104.3 |
| H134-Cu-H294 | 106.3 | 108.2 | 111.0 | 110.5 | 112.4 | 111.7 | 111.9 | 111.9 |
| V. Distances from T2Cu to the ligand planes (Å) | ||||||||
| 0.881 | 0.845 | 0.703 | 0.678 | 0.657 | 0.648 | 0.663 | 0.642 | |
Fig. 1.
Structural changes in SRX. (a) Changes in the hydration structures at the T2Cu site. The 2Fo–Fc maps contoured at 1.0 σ are shown as cyan meshes. Carbon, oxygen, nitrogen and copper atoms are yellow, red, blue and brown, respectively. (b) The ligand plane composed of three Nε2 atoms of His residues at the T2Cu site (yellow-dashed lines). Carbon, nitrogen and copper atoms are coloured in bright red, blue and brown, respectively. (c) Distances from T2Cu to the ligand plane. The error bars represent twice the values of the coordinate errors estimated by the maximum likelihood method. (A colour version of this figure is available online at: http://jb.oxfordjournals.org)
Completely oxidized GtNiR structure determined by SFX
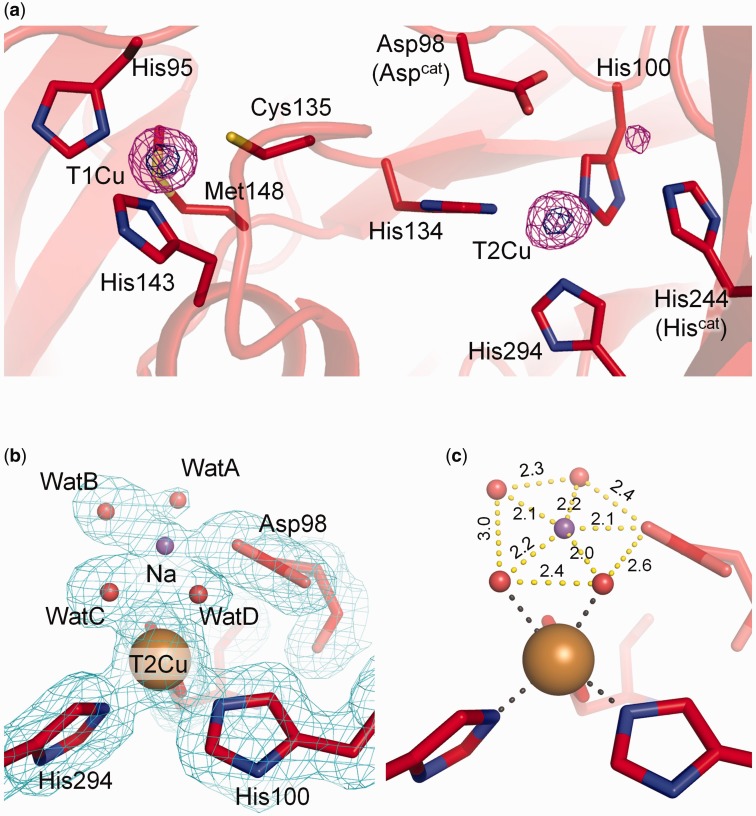
The SFX structure was refined to 1.43 Å resolution (Table III, see Materials and Methods). The final Rwork and Rfree values were 13.7 and 14.9%, respectively, showing that the model has a good agreement with the experimental data. RMSDs of Cα atoms between the SFX structure and cryogenic SRX structures were <0.2 Å. Anomalous peaks of T1Cu and T2Cu were clearly observed (Fig. 2a). The ligand–T1Cu distances in the SFX structure were the same as those in the SRX structures within the range of coordinate errors (Table IV). T2Cu in the SFX structure was coordinated by three histidine residues, WatC and WatD (Fig. 2b). The distance from T2Cu to the ligand plane was longer than those of the SRX structures (Fig. 1c), although it is almost the same as that in the SR1 structure within the range of coordinate errors.
Fig. 2.
SFX structure of GtNiR. Carbon, oxygen, nitrogen, sulfur and copper atoms are bright red, red, blue, yellow and brown, respectively. (a) Copper binding sites in the SFX structure. The anomalous Fourier maps are contoured at 4.0 (magenta) and 12 σ (dark blue). (b) Hydration structure of the T2Cu site in the SFX structure. The 2Fo–Fc maps contoured at 1.0 σ are shown as cyan meshes. Na+ and water molecules are shown as a purple sphere. (c) Penta-coordinated Na+ above the T2Cu site. Distances are shown in Å. (A colour version of this figure is available online at: http://jb.oxfordjournals.org)
An unidentified electron density was observed in the vicinity of T2Cu–ligand water molecules in the SFX data (Fig. 2b). We tentatively assigned this to a 40% occupancy sodium ion (Na+) from the crystallization buffer for the following reasons. The peak assigned to Na+ was close to WatB, WatC, WatD and Asp98 (Aspcat) as shown in Fig. 2c. The distances from the assigned Na+ to water molecules or Asp98 were shorter than typical hydrogen bonds but longer than typical covalent bonds in protein crystal structures. We could rule out a disordered water model because all water molecules in Fig. 2c showed full occupancy. It is, therefore, reasonable to speculate that the atom at the peak is a metal ion and forms coordination bonds with water and Aspcat. The peak did not show an anomalous peak, meaning that it is not Cu. Na+ was the only metal ion, other than Cu, in the crystallization solution. Furthermore, Aspcat can easily form a coordination bond with a cation because it is deprotonated in the resting state (60).
Presumably because of the high concentration of CuSO4 in the crystallization buffer, there existed an anomalous peak of 20% occupancy Cu bound to His244 (Hiscat) in the SFX data (Fig. 2a, Supplementary Fig. S5). Because the Cu atom was very close to bridging water (1.44 Å, Supplementary Fig. S5), we regarded the Cu atom and bridging water were alternative structures. The occupancy of bridging water was 80%. This anomalous peak of Cu was also observed in a previously determined cryogenic SRX structure (PDB code 3X1E, Supplementary Fig. S6). CuNiR has evolutionary and structural relationship with some multicopper oxidases (MCOs) (61–64), and Hiscat in CuNiR is superimposed to one of the His ligands to a trinuclear copper centre in the MCOs (63, 64). Therefore, the binding of extra Cu to Hiscat is not a surprising phenomenon.
RT SRX structure of GtNiR
Cryo-manipulations of protein crystals in SRX can change the population of amino acid side chains (65, 66). Due to its thermostability, GtNiR macrocrystals can be used in SRX without cryo-cooling (41); therefore, we determined an RT SRX structure of GtNiR to judge whether the observed structural differences between cryogenic SRX and SFX structures were derived from X-ray photoreduction and not from the difference of measurement temperatures. The RT SRX structure was refined to 1.35 Å resolution (Table II). The final Rwork and Rfree values were 9.7 and 12.0%, respectively. RMSDs of Cα atoms between the RT SRX and cryogenic SRX structures were <0.2 Å. The RMSD of Cα atoms between the RT SRX and SFX structure was 0.08 Å.
The T2Cu in the RT SRX structure was coordinated by three His ligands and two water molecules (Supplementary Fig. S7). The distance from T2Cu to the ligand plane was shorter than that of the SR1 structure and longer than those of other SRX structures (Fig. 1c), albeit within the range of errors. However, it is obvious that the distance was shorter than that in the SFX structure. The electron density map of the RT SRX data has a strong positive peak around WatC with full occupancy (Supplementary Fig. S4c). We also observed an unidentified positive electron density peak (Supplementary Fig. S4c) at the corresponding position of the putative Na+ site in the SFX structure (Supplementary Fig. S4b), though the signal was too weak to place any atomic model there. The observation that both RT SRX and SFX data show this electron density means, at least, that it is not the result of photoreduction.
The electron density map of the RT SRX data showed a strong electron density peak near Hiscat. When water is assigned there, the modelled molecule was too close to Hiscat (<2.0 Å). Therefore, it was likely to be a Cu ion coordinated by Hiscat (Supplementary Fig. S7) as was observed in the SFX structure and the 3X1E structure.
Redox-coupled conformational change in Hiscat
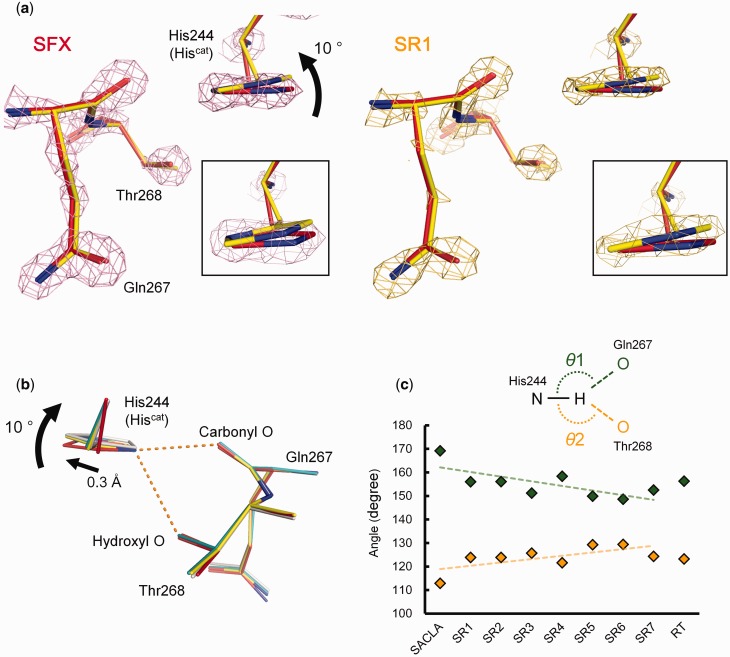
Compared with the SFX structure, cryogenic and RT SRX structures revealed that the imidazole ring and the Cβ atom of Hiscat rotated ∼10° and moved 0.3 Å, respectively (Fig. 3a and b, Supplementary Fig. S8). To confirm that it is not the result of cutting the SFX data at 1.43 Å resolution, we refined the SFX structure against the data to 1.65 Å resolution, where CC1/2 ∼ 0.5 and Rsplit < 100 (Supplementary Fig. S2), and the conformation of Hiscat did not change (Supplementary Fig. S9). The Nδ1 atom of Hiscat forms a bifurcated hydrogen bond with the hydroxyl oxygen atom of Thr268 and the backbone carbonyl oxygen atom of Gln267 (Fig. 3b). The Hiscat rotation changes the states of the bifurcated hydrogen bond. To qualitatively evaluate this subtle change, we modelled hydrogen atoms of Hiscat at the ideal positions without refinement. Figure 3c shows that with increasing of X-ray dose, the θ1 (Nδ1–H–OGln) angle and the θ2 (Nδ1–H–OThr) angle decreased and increased, respectively.
Fig. 3.
Redox-coupled rotation of Hiscat. (a) Electron density maps (contoured at 4.0 σ) around Hiscat in the SFX (red) and SR1 (yellow) structures. (b) Comparison of Hiscat of the SFX (red), SR1 (yellow), SR7 (teal blue) and RT SRX (white) structures. (c) Changes in the angle of the bifurcated hydrogen bond. The upper and lower series show θ1 and θ2, respectively. Trends are shown by dashed lines. (A colour version of this figure is available online at: http://jb.oxfordjournals.org)
Discussion
Geometrical changes at Cu sites
We recently demonstrated that T1Cu in a GtNiR crystal is reduced by at least an X-ray dose of 0.041 Mgy (30). Because even the X-ray dose for SR1 was higher than this value (Table I), T1Cu in the present SRX structures must be, more or less, reduced. However, the geometries around T1Cu did not show significant differences between the SRX structures and the completely oxidized SFX structure (Table IV). It is not surprising because spectroscopic studies have revealed that the typical changes in ligand–T1Cu distance in reduction are <0.1 Å so as to minimize the reorganization energy in ET (67).
We revealed that X-ray irradiation made the T2Cu atom gradually move towards to the ligand plane (Fig. 1c). Sunken T2Cu atoms have been observed in the reduced forms of other CuNiRs (68–70). Therefore, contrary to the previous report (24), our result indicates that T2Cu free of NO2− can be reduced in SRX. This conclusion is reasonable because our crystal was crystallized at a pH low enough (pH 4.5) for intramolecular ET without NO2− binding to occur dominantly (28). Although the T2Cu sites in CuNiR crystals lose ligand water when it is harshly reduced by artificial reductants (68–70), it is just one of the reduced states of T2Cu, called inactive reduced state, and a normal reduced state retains water ligand (29) as was observed in our structures (Fig. 1a).
Conformational change of Hiscat
The structural change of Hiscat cannot be caused by coordination of Cu or the presence of Na+ above the T2Cu site, because the former was found also in the cryogenic and RT SR structures (Supplementary Fig. S6 and S7) and the latter was found also in the RT SR structure (Supplementary Fig. S4c). The difference of measurement temperature is not a major cause of the structural change (Fig. 3b). Thus, the conformational difference in Hiscat between the SFX and SRX structures is most likely to result from photoreduction of the Cu sites. Indeed, the observed conformational change was small and is probably due to the rigid catalytic site of thermostable GtNiR (71).
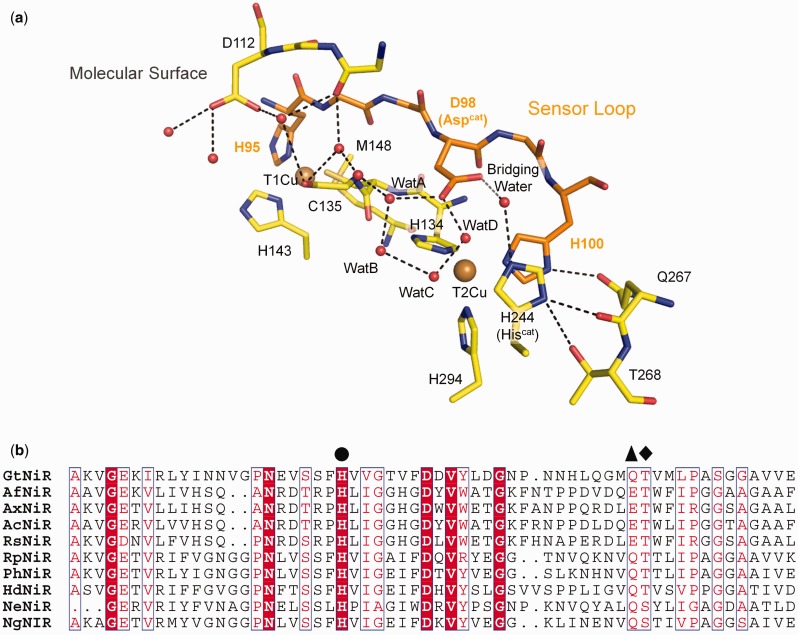
Interestingly, there is a hydrogen bond between T2Cu ligand His100 and the side chain of Gln267, and His100 is located at the end of a sensor loop (Fig. 4a), which is thought to transmit information about T1Cu’s redox state to T2Cu (8, 72). Although the Thr–Gln pair (or Thr–Glu in some CuNiRs) composing the bifurcated hydrogen bond has been ignored for long, they are conserved in CuNiRs except for a few CuNiRs, which have Ser at the position of Thr (Fig. 4b). The nitrite reduction activity of Ser-containing CuNiR is lower than the activity of Thr-containing CuNiR (70, 73). The crystal structures of Ser-containing CuNiRs show that the Ser residue does not always form a hydrogen bond with Hiscat (70, 73). These facts imply the enzymatic importance of the hydrogen bond between Hiscat and Thr.
Fig. 4.
Conserved hydrogen bond network. (a) Redox sensor loop in GtNiR. The hydrogen bond network is shown by dotted lines. (b) Amino acid sequences of CuNiRs. Hiscat, Gln/Glu and Thr/Ser are indicated by filled circle, triangle and diamond, respectively. Ax, Ac, Rs, Rp, Ph and Hd means Achromobacter xylosoxidans, Achromobacter cycloclastes, Rhodobacter sphaeroides, Ralstonia pickettii, Pseudoalteromonas haloplanktis and Hyphomicrobium denitrificans, respectively. (A colour version of this figure is available online at: http://jb.oxfordjournals.org)
The rotation of the imidazole ring changes the θ angles of the bifurcated hydrogen bond (Fig. 3c). Generally, the θ angle is used as an indicator of hydrogen bond strength. Strong, moderate and weak hydrogen bonds tend to show 170–180°, 150–180°, 90–150° θ angles, respectively (74). Therefore, the Hiscat rotation strengthens the hydrogen bond between Hiscat and Thr and weakens the hydrogen bond between Hiscat and Gln/Glu. Because the hydroxyl oxygen atom is less negatively polarized than the carbonyl oxygen atom, this structural change may destabilize the positive charge on Hiscat, which may facilitate transfer of a proton to the bridging water between Aspcat and Hiscat (Fig. 4a). Hiscat has been suggested to directly provide a proton to NO2− (19, 75); however, our results indicate another possibility. Hiscat may function as a switch for proton relay.
Supplementary Data
Supplementary Data are available at JB Online.
Acknowledgements
The SFX experiment was carried out at BL3 of the SPring-8 Angstrom Compact Free-Electron Laser (SACLA) with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (proposal no. 2013B8045). We are grateful for support from the SACLA High Performance Computing (HPC) system and the Mini-K super computer system. The microfocus SRX experiment at BL32XU of SPring-8 was supported by the Platform for Drug Discovery, Informatics and Structural Life Science. The authors thank M.E.P. Murphy for discussion; K. Baba and N. Mizuno for their help in the experiment at BL38B1 (proposal no. 2013A1592); E. Yamashita and A. Higashiura for their support at BL44XU of the SPring-8 and all staff at the SACLA for technical assistance.
Funding
This work was supported by the X-ray Free-Electron Laser Priority Strategy Program of the Ministry of Education, Culture, Sports, Science and Technology in Japan (MEXT), the Grant-in-Aid for Scientific Research on Innovative Areas from MEXT, the Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Research Fellows (Grant no. 254626), and the JSPS KAKENHI (Grant no. 15K18487).
Conflict of Interest
None declared.
Glossary
Abbreviations
- CuNiR
copper nitrite reductase
- ET
electron transfer
- GtNiR
Geobacillus thermodenitrificans copper nitrite reductase
- PCET
proton-coupled electron transfer
- RT
room temperature
- SFX
serial femtosecond crystallography
- SRX
synchrotron radiation crystallography
- T1Cu
Type 1 copper
- T2Cu
Type 2 copper
- XFEL
X-ray free-electron laser
References
- 1.Gruber N., Galloway J.N. (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296 [DOI] [PubMed] [Google Scholar]
- 2.Galloway J.N., Townsend A.R., Erisman J.W., Bekunda M., Cai Z., Freney J.R., Martinelli L.A., Seitzinger S.P., Sutton M.A. (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 [DOI] [PubMed] [Google Scholar]
- 3.Zumft W.G. (1997) Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61, 533–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavares P., Pereira A.S., Moura J.J., Moura I. (2006) Metalloenzymes of the denitrification pathway. J. Inorg. Biochem. 100, 2087–2100 [DOI] [PubMed] [Google Scholar]
- 5.Fülöp V., Moir J.W.B., Ferguson S.J., Hajdu J. (1995) The anatomy of a bifunctional enzyme: structural basis for reduction of oxygen to water and synthesis of nitric oxide by cytochrome cd1. Cell 81, 369–377 [DOI] [PubMed] [Google Scholar]
- 6.Kakutani T., Watanabe H., Arima K., Beppu T. (1981) A blue protein as inactivating factor for nitrite reductase from Alcaligenes faecalis Strain S-6. J. Biochem. 89, 463–472 [DOI] [PubMed] [Google Scholar]
- 7.MacPherson I.S., Rosell F.I., Scofield M., Mauk A.G., Murphy M.E. (2010) Directed evolution of copper nitrite reductase to a chromogenic reductant. Protein Eng. Des. Sel. 23, 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strange R.W., Murphy L.M., Dodd F.E., Abraham Z.H.L., Eady R.R., Smith B.E., Hasnain S.S. (1999) Structural and kinetic evidence for an ordered mechanism of copper nitrite reductase. J. Mol. Biol. 287, 1001–1009 [DOI] [PubMed] [Google Scholar]
- 9.Godden J., Turley S., Teller D., Adman E., Liu M., Payne W., LeGall J. (1991) The 2.3 angstrom X-ray structure of nitrite reductase from Achromobacter cycloclastes. Science 253, 438–442 [DOI] [PubMed] [Google Scholar]
- 10.Kukimoto M., Nishiyama M., Murphy M.E.P., Turley S., Adman E.T., Horinouchi S., Beppu T. (1994) X-ray structure and site-directed mutagenesis of a nitrite reductase from Alcaligenes Faecalis S-6: roles of two copper atoms in nitrite reduction. Biochemistry 33, 5246–5252 [DOI] [PubMed] [Google Scholar]
- 11.Dodd F.E., Beeumen J.V., Eady R.R., Hasnain S.S. (1998) X-ray structure of a blue-copper nitrite reductase in two crystal forms. The nature of the copper sites, mode of substrate binding and recognition by redox partner. J. Mol. Biol. 282, 369–382 [DOI] [PubMed] [Google Scholar]
- 12.Inoue T., Gotowda M., Deligeer, Kataoka K., Yamaguchi K., Suzuki S., Watanabe H., Gohow M., Kai Y. (1998) Type 1 Cu structure of blue nitrite reductase from Alcaligenes xylosoxidans GIFU 1051 at 2.05 Å resolution: comparison of blue and green nitrite reductases. J. Biochem. 124, 876–879 [DOI] [PubMed] [Google Scholar]
- 13.Merkle A.C., Lehnert N. (2012) Binding and activation of nitrite and nitric oxide by copper nitrite reductase and corresponding model complexes. Dalton Trans. 41, 3355–3368 [DOI] [PubMed] [Google Scholar]
- 14.Koteishi H., Nojiri M., Nakagami T., Yamaguchi K., Suzuki S. (2009) Cytochrome c551 is a mediator of electron transfer between copper-containing nitrite reductase and azurin in a denitrifying bacterium, Achromobacter xylosoxidans. Bull. Chem. Soc. Jpn. 82, 1003–1005 [Google Scholar]
- 15.Nojiri M., Koteishi H., Nakagami T., Kobayashi K., Inoue T., Yamaguchi K., Suzuki S. (2009) Structural basis of inter-protein electron transfer for nitrite reduction in denitrification. Nature 462, 117–120 [DOI] [PubMed] [Google Scholar]
- 16.Vlasie M.D., Fernandez-Busnadiego R., Prudencio M., Ubbink M. (2008) Conformation of pseudoazurin in the 152 kDa electron transfer complex with nitrite reductase determined by paramagnetic NMR. J. Mol. Biol. 375, 1405–1415 [DOI] [PubMed] [Google Scholar]
- 17.Impagliazzo A., Blok A.J., Cliff M.J., Ladbury J.E., Ubbink M. (2007) Redox-state-dependent complex formation between pseudoazurin and nitrite reductase. J. Am. Chem. Soc. 129, 226–233 [DOI] [PubMed] [Google Scholar]
- 18.Boulanger M.J., Kukimoto M., Nishiyama M., Horinouchi S., Murphy M.E. (2000) Catalytic roles for two water bridged residues (Asp-98 and His-255) in the active site of copper-containing nitrite reductase. J. Biol. Chem. 275, 23957–23964 [DOI] [PubMed] [Google Scholar]
- 19.Kataoka K., Furusawa H., Takagi K., Yamaguchi K., Suzuki S. (2000) Functional analysis of conserved aspartate and histidine residues located around the type 2 copper site of copper-containing nitrite reductase. J. Biochem. 127, 345–350 [DOI] [PubMed] [Google Scholar]
- 20.Yano J., Kern J., Irrgang K.D., Latimer M.J., Bergmann U., Glatzel P., Pushkar Y., Biesiadka J., Loll B., Sauer K., Messinger J., Zouni A., Yachandra V.K. (2005) X-ray damage to the Mn4Ca complex in single crystals of photosystem II: a case study for metalloprotein crystallography. Proc. Natl. Acad. Sci. USA 102, 12047–12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlichting I., Berendzen J., Chu K., Stock A.M., Maves S.A., Benson D.E., Sweet R.M., Ringe D., Petsko G.A., Sligar S.G. (2000) The catalytic pathway of cytochrome P450cam at atomic resolution. Science 287, 1615–1622 [DOI] [PubMed] [Google Scholar]
- 22.Berglund G.I., Carlsson G.H., Smith A.T., Szöke H., Henriksen A., Hajdu J. (2002) The catalytic pathway of horseradish peroxidase at high resolution. Nature 417, 463–468 [DOI] [PubMed] [Google Scholar]
- 23.Beitlich T., Kuhnel K., Schulze-Briese C., Shoeman R.L., Schlichting I. (2007) Cryoradiolytic reduction of crystalline heme proteins: analysis by UV-Vis spectroscopy and X-ray crystallography. J. Synch. Rad. 14, 11–23 [DOI] [PubMed] [Google Scholar]
- 24.Hough M.A., Antonyuk S.V., Strange R.W., Eady R.R., Hasnain S.S. (2008) Crystallography with online optical and X-ray absorption spectroscopies demonstrates an ordered mechanism in copper nitrite reductase. J. Mol. Biol. 378, 353–361 [DOI] [PubMed] [Google Scholar]
- 25.Brenner S., Heyes D.J., Hay S., Hough M.A., Eady R.R., Hasnain S.S., Scrutton N.S. (2009) Demonstration of proton-coupled electron transfer in the copper-containing nitrite reductases. J. Biol. Chem. 284, 25973–25983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leferink N.G., Han C., Antonyuk S.V., Heyes D.J., Rigby S.E., Hough M.A., Eady R.R., Scrutton N.S., Hasnain S.S. (2011) Proton-coupled electron transfer in the catalytic cycle of Alcaligenes xylosoxidans copper-dependent nitrite reductase. Biochemistry 50, 4121–4131 [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi K., Tagawa S., Deligeer, Suzuki S. (1999) The pH-dependent changes of intramolecular electron transfer on copper-containing nitrite reductase. J. Biochem. 126, 408–412 [DOI] [PubMed] [Google Scholar]
- 28.Wijma H.J., Jeuken L.J., Verbeet M.P., Armstrong F.A., Canters G.W. (2006) A random-sequential mechanism for nitrite binding and active site reduction in copper-containing nitrite reductase. J. Biol. Chem. 281, 16340–16346 [DOI] [PubMed] [Google Scholar]
- 29.Wijma H.J., Jeuken L.J.C., Verbeet M.P., Armstrong F.A., Canters G.W. (2007) Protein film voltammetry of copper-containing nitrite reductase reveals reversible inactivation. J. Am. Chem. Soc. 129, 8557–8565 [DOI] [PubMed] [Google Scholar]
- 30.Fukuda Y., Tse K.M., Kado Y., Mizohata E., Matsumura H., Inoue T. (2015) Insights into unknown foreign ligand in copper nitrite reductase. Biochem. Biophys. Res. Commun. 464, 622–628 [DOI] [PubMed] [Google Scholar]
- 31.Flot D., Mairs T., Giraud T., Guijarro M., Lesourd M., Rey V., van Brussel D., Morawe C., Borel C., Hignette O., Chavanne J., Nurizzo D., McSweeney S., Mitchell E. (2010) The ID23-2 structural biology microfocus beamline at the ESRF. J. Synch. Rad. 17, 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman H.N., Fromme P., Barty A., White T.A., Kirian R.A., Aquila A., Hunter M.S., Schulz J., DePonte D.P., Weierstall U., Doak R.B., Maia F.R., Martin A.V., Schlichting I., Lomb L., Coppola N., Shoeman R.L., Epp S.W., Hartmann R., Rolles D., Rudenko A., Foucar L., Kimmel N., Weidenspointner G., Holl P., Liang M., Barthelmess M., Caleman C., Boutet S., Bogan M.J., Krzywinski J., Bostedt C., Bajt S., Gumprecht L., Rudek B., Erk B., Schmidt C., Homke A., Reich C., Pietschner D., Struder L., Hauser G., Gorke H., Ullrich J., Herrmann S., Schaller G., Schopper F., Soltau H., Kuhnel K.U., Messerschmidt M., Bozek J.D., Hau-Riege S.P., Frank M., Hampton C.Y., Sierra R.G., Starodub D., Williams G.J., Hajdu J., Timneanu N., Seibert M.M., Andreasson J., Rocker A., Jonsson O., Svenda M., Stern S., Nass K., Andritschke R., Schroter C.D., Krasniqi F., Bott M., Schmidt K.E., Wang X., Grotjohann I., Holton J.M., Barends T.R., Neutze R., Marchesini S., Fromme R., Schorb S., Rupp D., Adolph M., Gorkhover T., Andersson I., Hirsemann H., Potdevin G., Graafsma H., Nilsson B., Spence J.C. (2011) Femtosecond X-ray protein nanocrystallography. Nature 470, 73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kern J., Alonso-Mori R., Hellmich J., Tran R., Hattne J., Laksmono H., Glockner C., Echols N., Sierra R.G., Sellberg J., Lassalle-Kaiser B., Gildea R.J., Glatzel P., Grosse-Kunstleve R.W., Latimer M.J., McQueen T.A., DiFiore D., Fry A.R., Messerschmidt M., Miahnahri A., Schafer D.W., Seibert M.M., Sokaras D., Weng T.C., Zwart P.H., White W.E., Adams P.D., Bogan M.J., Boutet S., Williams G.J., Messinger J., Sauter N.K., Zouni A., Bergmann U., Yano J., Yachandra V.K. (2012) Room temperature femtosecond X-ray diffraction of photosystem II microcrystals. Proc. Natl. Acad. Sci. USA 109, 9721–9726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson L.C., Arnlund D., Katona G., White T.A., Barty A., DePonte D.P., Shoeman R.L., Wickstrand C., Sharma A., Williams G.J., Aquila A., Bogan M.J., Caleman C., Davidsson J., Doak R.B., Frank M., Fromme R., Galli L., Grotjohann I., Hunter M.S., Kassemeyer S., Kirian R.A., Kupitz C., Liang M., Lomb L., Malmerberg E., Martin A.V., Messerschmidt M., Nass K., Redecke L., Seibert M.M., Sjohamn J., Steinbrener J., Stellato F., Wang D., Wahlgren W.Y., Weierstall U., Westenhoff S., Zatsepin N.A., Boutet S., Spence J.C., Schlichting I., Chapman H.N., Fromme P., Neutze R. (2013) Structure of a photosynthetic reaction centre determined by serial femtosecond crystallography. Nat. Commun. 4, 2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kupitz C., Basu S., Grotjohann I., Fromme R., Zatsepin N.A., Rendek K.N., Hunter M.S., Shoeman R.L., White T.A., Wang D., James D., Yang J.H., Cobb D.E., Reeder B., Sierra R.G., Liu H., Barty A., Aquila A.L., Deponte D., Kirian R.A., Bari S., Bergkamp J.J., Beyerlein K.R., Bogan M.J., Caleman C., Chao T.C., Conrad C.E., Davis K.M., Fleckenstein H., Galli L., Hau-Riege S.P., Kassemeyer S., Laksmono H., Liang M., Lomb L., Marchesini S., Martin A.V., Messerschmidt M., Milathianaki D., Nass K., Ros A., Roy-Chowdhury S., Schmidt K., Seibert M., Steinbrener J., Stellato F., Yan L., Yoon C., Moore T.A., Moore A.L., Pushkar Y., Williams G.J., Boutet S., Doak R.B., Weierstall U., Frank M., Chapman H.N., Spence J.C., Fromme P. (2014) Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser. Nature 513, 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kern J., Alonso-Mori R., Tran R., Hattne J., Gildea R.J., Echols N., Glöckner C., Hellmich J., Laksmono H., Sierra R.G., Lassalle-Kaiser B., Koroidov S., Lampe A., Han G., Gul S., DiFiore D., Milathianaki D., Fry A.R., Miahnahri A., Schafer D.W., Messerschmidt M., Seibert M.M., Koglin J.E., Sokaras D., Weng T.-C., Sellberg J., Latimer M.J., Grosse-Kunstleve R.W., Zwart P.H., White W.E., Glatzel P., Adams P.D., Bogan M.J., Williams G.J., Boutet S., Messinger J., Zouni A., Sauter N.K., Yachandra V.K., Bergmann U., Yano J. (2013) Simultaneous femtosecond X-ray spectroscopy and diffraction of photosystem II at room temperature. Science 340, 491–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern J., Tran R., Alonso-Mori R., Koroidov S., Echols N., Hattne J., Ibrahim M., Gul S., Laksmono H., Sierra R.G., Gildea R.J., Han G., Hellmich J., Lassalle-Kaiser B., Chatterjee R., Brewster A.S., Stan C.A., Glockner C., Lampe A., DiFiore D., Milathianaki D., Fry A.R., Seibert M.M., Koglin J.E., Gallo E., Uhlig J., Sokaras D., Weng T.C., Zwart P.H., Skinner D.E., Bogan M.J., Messerschmidt M., Glatzel P., Williams G.J., Boutet S., Adams P.D., Zouni A., Messinger J., Sauter N.K., Bergmann U., Yano J., Yachandra V.K. (2014) Taking snapshots of photosynthetic water oxidation using femtosecond X-ray diffraction and spectroscopy. Nat. Commun. 5, 4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirata K., Kawano Y., Ueno G., Hashimoto K., Murakami H., Hasegawa K., Hikima T., Kumasaka T., Yamamoto M. (2013) Achievement of protein micro-crystallography at SPring-8 beamline BL32XU. J. Phys. Conf. Ser. 425, 012002 [Google Scholar]
- 39.Paithankar K.S., Garman E.F. (2010) Know your dose: RADDOSE. Acta Cryst. D 66, 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanida H., Kikuchi A., Miura K., Takeshita K., Goto S., Shiro Y., Ishikawa T. (2004) XAFS and protein crystallography beamline BL38B1 at SPring-8. AIP Conf. Proc. 705, 486–489 [Google Scholar]
- 41.Fukuda Y., Inoue T. (2015) High-temperature and high-resolution crystallography of thermostable copper nitrite reductase. Chem. Commun. 51, 6532–6535 [DOI] [PubMed] [Google Scholar]
- 42.Otwinowski Z., Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 43.Vagin A., Teplyakov A. (2010) Molecular replacement with MOLREP. Acta Cryst. D 66, 22–25 [DOI] [PubMed] [Google Scholar]
- 44.Emsley P., Lohkamp B., Scott W.G., Cowtan K. (2010) Features and development of Coot. Acta Cryst. D 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murshudov G.N., Skubak P., Lebedev A.A., Pannu N.S., Steiner R.A., Nicholls R.A., Winn M.D., Long F., Vagin A.A. (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Cryst. D 67, 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winn M.D., Ballard C.C., Cowtan K.D., Dodson E.J., Emsley P., Evans P.R., Keegan R.M., Krissinel E.B., Leslie A.G., McCoy A., McNicholas S.J., Murshudov G.N., Pannu N.S., Potterton E.A., Powell H.R., Read R.J., Vagin A., Wilson K.S. (2011) Overview of the CCP4 suite and current developments. Acta Cryst. D 67, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen V.B., Arendall W.B., III, Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tono K., Togashi T., Inubushi Y., Sato T., Katayama T., Ogawa K., Ohashi H., Kimura H., Takahashi S., Takeshita K., Tomizawa H., Goto S., Ishikawa T., Yabashi M. (2013) Beamline, experimental stations and photon beam diagnostics for the hard x-ray free electron laser of SACLA. New J. Phys. 15, 083035 [Google Scholar]
- 49.Son S.-K., Chapman H.N., Santra R. (2011) Multiwavelength anomalous diffraction at high X-ray intensity. Phys. Rev. Lett. 107, 218102. [DOI] [PubMed] [Google Scholar]
- 50.Nass K., Foucar L., Barends T.R., Hartmann E., Botha S., Shoeman R.L., Doak R.B., Alonso-Mori R., Aquila A., Bajt S., Barty A., Bean R., Beyerlein K.R., Bublitz M., Drachmann N., Gregersen J., Jonsson H.O., Kabsch W., Kassemeyer S., Koglin J.E., Krumrey M., Mattle D., Messerschmidt M., Nissen P., Reinhard L., Sitsel O., Sokaras D., Williams G.J., Hau-Riege S., Timneanu N., Caleman C., Chapman H.N., Boutet S., Schlichting I. (2015) Indications of radiation damage in ferredoxin microcrystals using high-intensity X-FEL beams. J. Synch. Rad. 22, 225–238 [DOI] [PubMed] [Google Scholar]
- 51.Sugahara M., Mizohata E., Nango E., Suzuki M., Tanaka T., Masuda T., Tanaka R., Shimamura T., Tanaka Y., Suno C., Ihara K., Pan D., Kakinouchi K., Sugiyama S., Murata M., Inoue T., Tono K., Song C., Park J., Kameshima T., Hatsui T., Joti Y., Yabashi M., Iwata S. (2014) Grease matrix as a versatile carrier of proteins for serial crystallography. Nat. Methods 12, 61–63 [DOI] [PubMed] [Google Scholar]
- 52.Tono K., Nango E., Sugahara M., Song C., Park J., Tanaka T., Tanaka R., Joti Y., Kameshima T., Ono S., Hatsui T., Mizohata E., Suzuki M., Shimamura T., Tanaka Y., Iwata S., Yabashi M. (2015) Diverse application platform for hard X-ray diffraction in SACLA (DAPHNIS): application to serial protein crystallography using an X-ray free-electron laser. J. Synch. Rad. 22, 532–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joti Y., Kameshima T., Yamaga M., Sugimoto T., Okada K., Abe T., Furukawa Y., Ohata T., Tanaka R., Hatsui T., Yabashi M. (2015) Data acquisition system for X-ray free-electron laser experiments at SACLA. J. Synch. Rad. 22, 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yumoto H., Mimura H., Koyama T., Matsuyama S., Tono K., Togashi T., Inubushi Y., Sato T., Tanaka T., Kimura T., Yokoyama H., Kim J., Sano Y., Hachisu Y., Yabashi M., Ohashi H., Ohmori H., Ishikawa T., Yamauchi K. (2012) Focusing of X-ray free-electron laser pulses with reflective optics. Nat. Photonics 7, 43–47 [Google Scholar]
- 55.White T.A., Kirian R.A., Martin A.V., Aquila A., Nass K., Barty A., Chapman H.N. (2012) CrystFEL: a software suite for snapshot serial crystallography. J. Appl. Cryst. 45, 335–341 [Google Scholar]
- 56.Brehm W., Diederichs K. (2014) Breaking the indexing ambiguity in serial crystallography. Acta Cryst. D 70, 101–109 [DOI] [PubMed] [Google Scholar]
- 57.Karplus P.A., Diederichs K. (2012) Linking crystallographic model and data quality. Science 336, 1030–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evans P.R., Murshudov G.N. (2013) How good are my data and what is the resolution? Acta Cryst. D 69, 1204–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bublitz M., Nass K., Drachmann N.D., Markvardsen A.J., Gutmann M.J., Barends T.R.M., Mattle D., Shoeman R.L., Doak R.B., Boutet S., Messerschmidt M., Seibert M.M., Williams G.J., Foucar L., Reinhard L., Sitsel O., Gregersen J.L., Clausen J.D., Boesen T., Gotfryd K., Wang K.-T., Olesen C., Møller J.V., Nissen P., Schlichting I. (2015) Structural studies of P-type ATPase–ligand complexes using an X-ray free-electron laser. IUCrJ 2, 409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H., Boulanger M.J., Mauk A.G., Murphy M.E.P. (2000) Carbon monoxide binding to copper-containing nitrite reductase from Alcaligenes faecalis. J. Phys. Chem. B. 104, 10738–10742 [Google Scholar]
- 61.Nakamura K., Go N. (2005) Function and molecular evolution of multicopper blue proteins. Cell. Mol. Life Sci. 62, 2050–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lawton T.J., Sayavedra-Soto L.A., Arp D.J., Rosenzweig A.C. (2009) Crystal structure of a two-domain multicopper oxidase: implications for the evolution of multicopper blue proteins. J. Biol. Chem. 284, 10174–10180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komori H., Miyazaki K., Higuchi Y. (2009) X-ray structure of a two-domain type laccase: a missing link in the evolution of multi-copper proteins. FEBS Lett. 583, 1189–1195 [DOI] [PubMed] [Google Scholar]
- 64.MacPherson I.S., Murphy M.E. (2007) Type-2 copper-containing enzymes. Cell. Mol. Life Sci. 64, 2887–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fraser J.S., Clarkson M.W., Degnan S.C., Erion R., Kern D., Alber T. (2009) Hidden alternative structures of proline isomerase essential for catalysis. Nature 462, 669–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fraser J.S., van den Bedem H., Samelson A.J., Lang P.T., Holton J.M., Echols N., Alber T. (2011) Accessing protein conformational ensembles using room-temperature X-ray crystallography. Proc. Natl. Acad. Sci. USA 108, 16247–16252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solomon E.I., Szilagyi R.K., DeBeer George S., Basumallick L. (2004) Electronic structures of metal sites in proteins and models: contributions to function in blue copper proteins. Chem. Rev. 104, 419–458 [DOI] [PubMed] [Google Scholar]
- 68.Murphy M.E.P., Turley S., Adman E.T. (1997) Structure of nitrite bound to copper-containing nitrite reductase from Alcaligenes faecalis. J. Biol. Chem. 272, 28455–28460 [DOI] [PubMed] [Google Scholar]
- 69.Jacobson F., Guo H., Olesen K., Okvist M., Neutze R., Sjolin L. (2005) Structures of the oxidized and reduced forms of nitrite reductase from Rhodobacter sphaeroides 2.4.3 at high pH: changes in the interactions of the type 2 copper. Acta Cryst. D 61, 1190–1198 [DOI] [PubMed] [Google Scholar]
- 70.Lawton T.J., Bowen K.E., Sayavedra-Soto L.A., Arp D.J., Rosenzweig A.C. (2013) Characterization of a nitrite reductase involved in nitrifier denitrification. J. Biol. Chem. 288, 25575–25583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukuda Y., Tse K.M., Lintuluoto M., Fukunishi Y., Mizohata E., Matsumura H., Takami H., Nojiri M., Inoue T. (2014) Structural insights into the function of a thermostable copper-containing nitrite reductase. J. Biochem. 155, 123–135 [DOI] [PubMed] [Google Scholar]
- 72.Hough M.A., Ellis M.J., Antonyuk S., Strange R.W., Sawers G., Eady R.R., Samar Hasnain S. (2005) High resolution structural studies of mutants provide insights into catalysis and electron transfer processes in copper nitrite reductase. J. Mol. Biol. 350, 300–309 [DOI] [PubMed] [Google Scholar]
- 73.Boulanger M.J., Murphy M.E.P. (2002) Crystal structure of the soluble domain of the major anaerobically induced outer membrane protein (AniA) from pathogenic Neisseria: a new class of copper-containing nitrite reductases. J. Mol. Biol. 315, 1111–1127 [DOI] [PubMed] [Google Scholar]
- 74.Desiraju G.R., Steiner T. (1999) The Weak Hydrogen Bonds in Structural Chemistry and Biology Oxford University Press, Oxford [Google Scholar]
- 75.Antonyuk S.V., Strange R.W., Sawers G., Eady R.R., Hasnain S.S. (2005) Atomic resolution structures of resting-state, substrate- and product-complexed Cu-nitrite reductase provide insight into catalytic mechanism. Proc. Natl. Acad. Sci.USA 102, 12041–12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.