Abstract
Nucleotide sequences from the plastome are currently the main source for assessing taxonomic and phylogenetic relationships in flowering plants and their historical biogeography at all hierarchical levels. One major exception is the large and economically important genus Quercus (oaks). Whereas differentiation patterns of the nuclear genome are in agreement with morphology and the fossil record, diversity patterns in the plastome are at odds with established taxonomic and phylogenetic relationships. However, the extent and evolutionary implications of this incongruence has yet to be fully uncovered. The DNA sequence divergence of four Euro-Mediterranean Group Ilex oak species (Quercus ilex L., Q. coccifera L., Q. aucheri Jaub. & Spach., Q. alnifolia Poech.) was explored at three chloroplast markers (rbcL, trnK/matK, trnH-psbA). Phylogenetic relationships were reconstructed including worldwide members of additional 55 species representing all Quercus subgeneric groups. Family and order sequence data were harvested from gene banks to better frame the observed divergence in larger taxonomic contexts. We found a strong geographic sorting in the focal group and the genus in general that is entirely decoupled from species boundaries. High plastid divergence in members of Quercus Group Ilex, including haplotypes shared with related, but long isolated oak lineages, point towards multiple geographic origins of this group of oaks. The results suggest that incomplete lineage sorting and repeated phases of asymmetrical introgression among ancestral lineages of Group Ilex and two other main Groups of Eurasian oaks (Cyclobalanopsis and Cerris) caused this complex pattern. Comparison with the current phylogenetic synthesis also suggests an initial high- versus mid-latitude biogeographic split within Quercus. High plastome plasticity of Group Ilex reflects geographic area disruptions, possibly linked with high tectonic activity of past and modern distribution ranges, that did not leave imprints in the nuclear genome of modern species and infrageneric lineages.
Keywords: Fagaceae, Mediterranean, Ancient introgression, Incomplete lineage sorting, Decoupled phylogenies
Introduction
Quercus L. (oaks) is among the most ecologically diverse and economically important extratropical tree genera in the northern hemisphere (Kubitzki, 1993). Quercus is the largest genus in the order Fagales, comprising ca. 400–500 species (Govaerts & Frodin, 1998). Species diversity is highest in the Americas (Groups Quercus, Lobatae and Protobalanus; Flora of North America Editorial Committee, 1997) and Southeast Asia and southern China (Group Cyclobalanopsis; Flora of China Editorial Committee, 1999). In contrast, a relatively lower number of species occurs in western Eurasia and the Mediterranean (Groups Ilex and Cerris; Kubitzki, 1993; Menitsky, 2005). The six major infrageneric lineages of Quercus occur from the tropics to the high mountains of the temperate zone and to the boreal continental, cold temperate regions (Denk & Grimm, 2010). The northern limit of oaks in North America and Eurasia coincides with the border of Dfb to Dfc and Dwb to Dwc climates, snow climates with warm versus cool summers (Köppen, 1936; Kottek et al., 2006; Peel, Finlayson & McMahon, 2007).
Recent molecular phylogenetic studies at and below the genus level focussed on the nucleome of oaks (Oh & Manos, 2008; Denk & Grimm, 2010; Hipp et al., 2014; Hubert et al., 2014). These studies consistently recovered two main lineages, the ‘New World Clade’ comprising the white oaks (Group Quercus), red oaks (Group Lobatae) and golden-cup oaks (Group Protobalanus), and the ‘Old World Clade’ consisting of the cycle-cup oaks (Group Cyclobalanopsis), the Ilex oaks (Group Ilex) and the Cerris oaks (Group Cerris). Evidence from nuclear markers and the fossil record suggests that the initial split in the ‘New World Clade’ was pre-Oligocene between the lineages leading to Group Lobatae and Group Protobalanus/Quercus (Bouchal et al., 2014; Hubert et al., 2014; Grímsson et al., 2015). This early radiation of the Quercus/Protobalanus lineage left its imprints in the molecular signatures of the few modern species of Group Protobalanus and two narrow endemic white oak species, Quercus pontica (north-eastern Turkey, south-western Georgia; Denk & Grimm, 2010) and Q. sadleriana (California; Hubert et al., 2014). Within the ‘Old World Clade’, the major split was established between the evergreen Groups Cyclobalanopsis and Ilex during the Eocene/Oligocene, whereas the chiefly temperate Group Cerris is suggested to have evolved (‘budded’) from a Group Ilex stock, possibly in Europe, not before the earliest Miocene (Denk & Grimm, 2009; Kmenta, 2011; Hubert et al., 2014; Velitzelos, Bouchal & Denk, 2014).
Nuclear amplicon data sets have also contributed to resolve the circumscription of these six groups and to delineate some intergroup and interspecies relationships (López de Heredia et al., 2007; Pearse & Hipp, 2009; Denk & Grimm, 2010; Hubert et al., 2014); well-resolved within-lineage relationships were recently obtained from phylogenomic data in the genetically least-diverged, but species-rich Group Quercus (Hipp et al., 2014). Nucleome-based studies, therefore, clearly indicate a strong correlation between morphology/speciation and nuclear differentiation in oaks. In contrast, oak plastid haplotypes are extensively shared between groups of related species (Whittemore & Schaal, 1991; Belahbib et al., 2001; Petit et al., 2002; Kanno et al., 2004; López de Heredia et al., 2007; Okaura et al., 2007; Neophytou et al., 2010; Gugger & Cavender-Bares, 2013). Notably, this was also observed in other genera of Fagaceae such as Fagus (Fujii et al., 2002; Lei et al., 2012; Zhang et al., 2013b) and Lithocarpus (Cannon & Manos, 2003), and other Fagales such as the northern hemispheric Carya (Juglandaceae; Zhang et al., 2013a) and the South American Nothofagus (Nothofagaceae; Acosta & Premoli, 2010; Premoli et al., 2012). Plastomes of this large group of long-lived woody plants appear to retain molecular signatures of evolutionary events that cannot be investigated when considering the nuclear DNA alone (e.g., Cavender-Bares et al., 2011; Premoli et al., 2012). As such, they can provide additional information to complement hypotheses on diversification and speciation processes. However, the extent and evolutionary implications of nuclear-plastome incongruence in Quercus have yet to be fully uncovered.
Testing the potential of DNA barcoding in western Eurasian oaks, Simeone et al. (2013) recently found puzzling diversity in the plastid haplotypes of samples belonging to Group Ilex. Several members of this oak group appeared paraphyletic with Groups Cerris and Quercus, and an underlying geographic partitioning was suggested in addition to the detected interspecific haplotype sharing. In the present study, we increased the geographic coverage and taxon sampling to explore the complex patterns of plastome evolution in Quercus Group Ilex. This species group is today confined to extra-tropical regions of Eurasia, spanning from arid Mediterranean maquis to high mountain and sub-alpine Himalayan forests and thickets, and to subtropical forests of SE Asia. Group Ilex includes some 35 evergreen, mostly sclerophyllous taxa, whose taxonomy is still controversial (see Table 1) and biogeographic history is not yet well understood (Menitsky, 2005; Denk & Grimm, 2010). In this work, we compiled plastid sequence data for 81 accessions of 20 oak taxa of Group Ilex. The main sampling effort was put into the four species currently occurring in the Mediterranean and adjacent regions in North Africa (Atlas Mountains) and northern Turkey (Black Sea region): the widespread Quercus ilex L. and Q. coccifera L., and the two East Mediterranean narrow endemics Q. aucheri Jaub. & Spach. and Q. alnifolia Poech. Data for additional 56 individuals of ca. 40 species were also produced to integrate all subgeneric Quercus groups and their worldwide geographic distribution. Additionally, Fagales data sets were harvested from gene banks to allow interpretation of the observed divergence in the plastid markers within a larger taxonomic frame. Our objectives were: (1) to assess the extent of plastome diversity in the Euro-Mediterranean focal group; (2) to outline key phylogeographic patterns within Quercus Group Ilex; (3) to establish major evolutionary steps for the differentiation of the ‘Old World Clade’.
Table 1. Species list.
Species included in Quercus Group Ilex according to Denk & Grimm, (2010); nomenclature followed Govaerts & Frodin (1998); species investigated in the present study are bolded. Taxonomic remarks and species distributions according to Govaerts & Frodin (1998).
| Species | Taxonomic remarks | Distribution |
|---|---|---|
| Q. acrodonta Seemen | Includes Q. handeliana A. Camusa/b | C, E and S China |
| Q. alnifolia Poech | Cyprus | |
| Q. aquifolioides Rehder & E.H.Wilson | Includes Q. semecarpifolia subsp. glabraa | Tibet, C and SW China to Myanmar |
| Q. aucheri Jaub. & Spach | SW Anatolia | |
| Q. baloot Griff. | Pakistan, Afghanistan | |
| Q. baronii Skan | Numerous morpho-ecological traits in common with members of Group Cerrisa | NC and SW China |
| Q. bawanglingensis C.C. Huang, Ze X. Li & F.W. Xing | Poorly known; uncertain status, related to Q. phillyreoidesb | SE China |
| Q. coccifera L. | Includes Q. calliprinos Webba | Mediterranean |
| Q. cocciferoides Hand.-Mazz. | Includes Q. taliensis A. Camusb | CS China |
| Q. floribunda Lindl. ex A. Camus | Basionym: Q. dilatata Lindl. ex A.DC. nom. illegit. | Pakistan, Afghanistan, Nepal |
| Q. dolicholepis A. Camus | Includes Q. fimbriatab | CW to SW China |
| Q. engleriana Seemen | Tibet to E China, Myanmar | |
| Q. fimbriata Y.C. Hsu & H. Wei Jen | Included in Q. semecarpifoliaa or Q. dolicholepisb | C China |
| Q. franchetii Skan | C China to N Vietnam | |
| Q. gilliana Rehder & E.H. Wilson | Included in Q. spinosab | Tibet, C and S China |
| Q. guyavifolia H. Lév. | Included in Q. semecarpifolia subsp. glabraa; Q. pannosa or Q. aquifolioides var. rufescensb | C and S China |
| Q. ilex L. | Mediterranean | |
| Q. kingiana Craib | C China to N Thailand | |
| Q. lanata Sm. | Included in Q. leucotrichophorab | Buthan to Vietnam |
| Q. leucotrichophora A. Camus | Basionym: Q. incana Roxb. nom. illegit. | N Pakistan, N India, Nepal to N Vietnam |
| Q. lodicosa O.E. Warb. & E.F. Warb. | SE Tibet to Myanmar | |
| Q. longispica (Hand.-Mazz.) A. Camus | Includes Q. semecarpifolia subsp. glabraa; Q. rehderianab | C and S China |
| Q. marlipoensis Hu & Cheng | Poorly knowna; very close to Q. englerianab | C China |
| Q. monimotricha Hand.-Mazz. | C and S China | |
| Q. oxyphylla Hand.-Mazz. | Includes Q. spathulata Seemen; included in Q. dolicholepisb | C and S China |
| Q. pannosa Hand.-Mazz. | Possibly conspecific with Q. semecarpifoliaa | C and S China |
| Q. phillyreoides A. Gray | Includes Q. utilisa | C China to Japan |
| Q. pseudosemecarpifolia A. Camus | Includes Q. semecarpifolia subsp. glabraa, Q. rehderianab | Tibet to CS China |
| Q. rehderiana Hand.-Mazz. | Included in Q. semecarpifolia subsp. glabraa; includes Q. longispica and Q. pseudosemecarpifoliab | Tibet to C and S China |
| Q. semecarpifolia Sm. | Includes: Q. fimbriata, Q. gujavifolia, Q. aquifolioides, Q. rehderiana, Q. longispica, Q. pseudosemecarpifoliaa | Afghanistan to Myanmar |
| Q. senescens Hand.-Mazz. | E Himalaya, Tibet, C and S China | |
| Q. setulosa Hickel & A. Camus | C China to Vietnam | |
| Q. spinosa David | NC and SW China to Taiwan | |
| Q. tarokoensis Hayata | E Taiwan | |
| Q. utilis Hu & Cheng | Included in Q. phillyreoides subsp. fokiensisa | C China |
Material and Methods
Plant material, DNA amplification and sequencing
Our analysis included 59 individuals of the four Mediterranean Quercus Group Ilex species (File S1) covering their entire range in North Africa and western Eurasia. Additionally, 22 individuals of 16 Asian species of Group Ilex were analysed. The final dataset also included all species of the western North American Group Protobalanus (five species, 10 individuals), 16 species of Group Quercus (20 individuals, from North America and Eurasia), five species of the East Asian Group Cyclobalanopsis (11 individuals), seven species of the American Group Lobatae (eight individuals), and six species of Group Cerris (seven individuals). The outgroup set was represented by one sample each of the monotypic genera Notholithocarpus and Chrysolepis (western North America) and one species each of Castanea and Castanopsis (NCBI GenBank accessions HQ336406 (complete plastid genome of C. mollissima), JN044213, JF941179, FJ185053). Based on their genetic (plastid) signatures these genera are the closest relatives of Quercus within the Fagaceae (Manos, Cannon & Oh, 2008). For voucher information and accession numbers see File S1. The molecular analyses included three plastid DNA regions: a part of the rbcL gene, the trnH-psbA intergenic spacer and a portion of the trnK/matK region (3′ intron and partial gene). These markers were chosen based on the variability displayed in previous analyses (e.g., Manos, Zhou & Cannon, 2001; Okaura et al., 2007; Simeone et al., 2013) and the high number of their sequences available on GenBank. Primer sequences for the three regions were obtained from Kress & Erickson (2007), Shaw et al. (2005) and Piredda et al. (2011), respectively. DNA extractions and PCR protocols were the same as in Piredda et al. (2011). Sequencing of both DNA strands was performedat Macrogen (http://www.macrogen.com), using the forward and reverse PCR primers,; electropherograms were edited with CHROMAS 2.3 (http://www.technelysium.com.au) and checked visually.
Assessment of overall diversity in Quercus and Fagaceae
The diversity of the investigated regions was evaluated with Mega 5.2 (Tamura et al., 2011) and DnaSP5.1 (Librado & Rozas, 2009). For comparisons of divergence patterns across all Fagales, available data in gene banks were processed using gbk2fas (Göker et al., 2009); multiple sequence alignments were done with mafft v.7 (Katoh & Standley, 2013) using default settings and checked by eye to remove inconsistencies and erroneous sequences (taxa and sequence numbers are given in the Online Supporting Archive; www.palaeogrimm.org/data/Smn15_OSA.zip). To minimise the effect of alignment gaps, and since we were primarily interested in assessing intra- and intergeneric divergence, alignments included only subsets of the Fagales: (1) Nothofagaceae (data covering all four genera); (2) Fagaceae (10 genera including Quercus); (3) Betulaceae-Ticodendron-Casuarinaceae (11 genera); (4) Juglandaceae (9 genera); (5) Myricaceae (4 genera). Pairwise distance matrices (uncorrected p-distance, K2P, HKY, GTR+Γ) for each marker were calculated with PAUP* 4.0 (Swofford, 2002). Minimum intra-specific and minimum/maximum inter-specific distances (calculated with g2cef; Göker & Grimm, 2008) within and between genera, subgenera in the case of Fagus, and infrageneric groups in case of Quercus, are listed in File S2.
Phylogenetic analyses
Multiple sequence alignments for the focal group were obtained with ClustalW 1.81 (Thompson, Higgins & Gibson, 1994) and checked by eye. The matrices were concatenated with the Python programme combinex2_0.py (Python v. 2.6.4; Biopython 1.57).
Maximum likelihood trees were inferred with GARLI (Zwickl, 2006; run on the CIPRES portal, http://www.phylo.org/sub_sections/portal/) using four data partitions (rbcL and matK codons, trnK intron and trnH-psbA spacer). MrModeltest 2.0 (Nylander, 2004) and the Akaike Information Criterion (AIC; Akaike, 1974) were used to decide on the best-fitting substitution model for each partition.
MrModeltest 2.0 results were also used for setting up Bayesian inference, performed with MrBayes 3.4b4 (Ronquist & Huelsenbeck, 2003; Ronquist et al., 2012). RAxML v. 7.0.4 (Stamatakis, Hoover & Rougemont, 2008) was used for calculating maximum likelihood bootstrap support (1,000 replicates). Trees were edited with Figtree 1.3.1 (Rambaut, 2014) and Mesquite v. 2.75 (Maddison & Maddison, 2011). Median-joining (MJ) haplotype networks were inferred with Network 4.6.1.1 (http://www.fluxus-engineering.com/) for each gene region (rbcL, trnK/matK, trnH-psbA), treating gaps either as missing or 5th state. The MJ algorithm was invoked with default parameters (equal weight of transversion/transition), in order to handle large datasets and multistate characters.
Primary data, analyses, results and Files S1–S3 are provided as online supporting archives at www.palaeogrimm.org/data/Smn15_OSA.zip.
Results
Levels of intra- and interspecies plastome divergence in Quercus
The entire dataset included 423 plastid DNA sequences (141 samples, three markers each). The sequence quality was high for all the marker regions: 100% of unambiguous full-length electropherograms with 100% overlap between complementary sequences were recovered across all taxa. Table 2 shows that trnH-psbA was the most variable marker region (a 34-bp inversion occurring in approximately 50% of the samples was replaced with its reverse-complementary sequence and a binary character was inserted to keep record of it). As expected, the least variable region was rbcL. No indels were found in the rbcL and matK coding regions. The combined cpDNA dataset (trnH-psbA, trnK/matK, rbcL) resulted in an alignment of 2082 characters (sites), of which 122 were variable (thereof 72 parsimony-informative; gaps not considered). The combined regions had a nucleotide diversity of 0.006 and included 74 different haplotypes of which 50 were unique (restricted to a single accession). As a result, the overall haplotype diversity was high (Hd = 0.978 ± 0.005). With gaps considered, the number of haplotypes increased to 110, of which 89 were unique (Hd = 0.994).
Table 2. Diversity values and models of DNA evolution of the fragments used for the analyses in 59 Quercus species (137 individuals) and 4 outgroup taxa.
| Markers | AL | P | Nhap | Hd | S | PICs | θw | π | ME |
|---|---|---|---|---|---|---|---|---|---|
| rbcL | 743 | 0.00–0.008 | 28 | 0.846 ± 0.027 | 26 | 18 | 0.0063 | 0.0027 | HKY+ I |
| trnH-psbA | 634 | 0.00–0.035 | 37 (84) | 0.944 ± 0.008 | 38 | 23 | 0.0159 | 0.009 | GTR+ G |
| trnK/matK | 705 | 0.00–0.022 | 49 (51) | 0.952 ± 0.008 | 59 | 31 | 0.0156 | 0.0064 | n.d. |
| trnK (intron) | 401 | n.d. | 32 (34) | 0.821 ± 0.028 | 36 | 16 | 0.0169 | 0.0048 | GTR+ G |
| matK (codons) | 304 | n.d. | 25 | 0.925 ± 0.008 | 23 | 15 | 0.0146 | 0.0086 | HKY |
| rbcL+trnK/matK+trnH-psbA | 2,082 | 0.00–0.014 | 74 (110) | 0.978 ± 0.005 | 122 | 72 | 0.0119 | 0.0056 | Combined |
| rbcL +matK | 1,047 | n.d. | 49 | 0.965 ± 0.006 | 49 | 33 | 0.0085 | 0.0044 | n.d. |
| trnH-psbA +trnK | 1,035 | n.d. | 57 (103) | 0.954 ± 0.008 | 69 | 34 | 0.0161 | 0.0067 | n.d. |
| trnH-psbA +trnK-matK | 1339 | n.d. | 65 (110) | 0.970 ± 0.006 | 92 | 49 | 0.0155 | 0.0072 | n.d. |
Notes.
- AL
- Aligned length (bp)
- P
- uncorrected p-distance (min.–max.)
- N hap
- Number of identified haplotypes, brackets: with gaps considered
- Hd
- Haplotype diversity
- S
- Number of polymorphic sites
- θ
- Nucleotide polymorphism
- π
- Nucleotide diversity
- PIC
- Number of Parsimony Informative Characters
- ME
- Model of evolution
In general, the infrageneric divergence calculated in Quercus is comparable to that found in other genera of the Fagaceae and Betulaceae, and higher than in Juglandaceae (Table 3). All three gene regions allow distinguishing the generic affinity of an oak individual; the same haplotype may be shared by several or many oak species (usually within the same infrageneric group; Table 3), but not with other genera of the Fagaceae.
Table 3. Divergence patterns in other Fagaceae and Fagales.
Divergence patterns in Quercus compared to other Fagaceae and Fagales based inter-species pair wise uncorrected p-distances of sequences retrieved from GenBank and produced in this study.
| Intrageneric divergence | Mean intergeneric divergence at family levelc | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rbcL | matK | trnH-psbA | rbcL | matK | trnH-psbA | |||||||||
| Taxon | Nt | Ns | Min. | Max. | Min. | Max. | Min. | Max. | Min. | Max. | Min. | Max. | Min. | Max. |
| Quercus | 87/87/86 | 219/255/382 | 0.000 | 0.010 | 0.000 | 0.023 | 0.000 | 0.042 | 0.003 | 0.011 | 0.006 | 0.015 | 0.006 | 0.042 |
| Fagus | 8/9/6 | 33/30/19 | 0.000 | 0.014 | 0.000 | 0.007 | 0.000 | 0.042 | 0.024 | 0.036 | 0.091 | 0.098 | 0.120 | 0.147 |
| Other Fagaceae | 37/21/28 | 102/32/86 | 0.000 | 0.020 | 0.000 | 0.021 | 0.000 | 0.020 | 0.003 | 0.013 | 0.000 | 0.019 | 0.011 | 0.032 |
| Nothofagaceaea | 23/b /14 | 35/b /53 | 0.000 | 0.027 | –b | –b | 0.000 | 0.017 | 0.012 | 0.023 | –b | –b | 0.017 | 0.041 |
| Betulaceae | 55/19/77 | 131/34/247 | 0.000 | 0.011 | 0.000 | 0.006 | 0.000 | 0.069 | 0.006 | 0.024 | 0.014 | 0.033 | 0.011 | 0.079 |
| Juglandaceae | 18/b /21 | 23/b/28 | 0.000 | 0.005 | –b | –b | 0.000 | 0.007 | 0.000 | 0.021 | –b | –b | 0.006 | 0.034 |
Notes.
- Nt
- number of taxa
- Ns
- number of sequences
Values for rbcL may be over-estimated (data usually older than 15 years; sequences show features characteristic for sequencing and editing artifacts).
Insufficient data.
Values for Quercus and other Fagaceae not including Fagus (see Fagus for max. inter-generic divergence in Fagaceae).
At the infrageneric level in Quercus, minimal inter-species distances can be zero for all three markers and within all infrageneric groups. Notably, maximal inter-species distances within infrageneric groups of Quercus can reach or even exceed the level of inter-generic differentiation in Fagaceae (e.g., between Notholithocarpus, Lithocarpus, Castanopsis, Castanea, Chrysolepis), Juglandaceae and Myricaceae. The maximum intra-specific distance found in Mediterranean individuals of Quercus Group Ilex equals the maximum inter-specific divergence found within this group (Table 3; for additional data see File S2).
Phylogenetic placement of Mediterranean Quercus Group Ilex plastid haplotypes
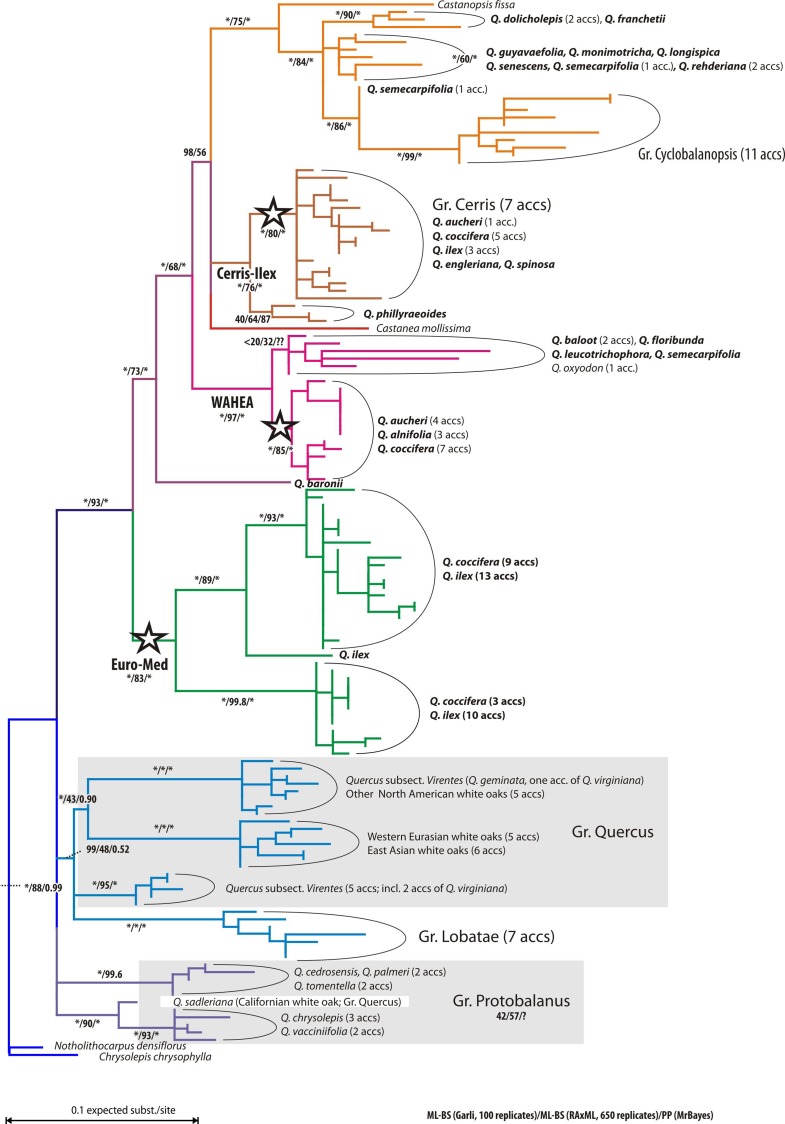
Individuals of the Mediterranean species of Quercus Group Ilex cluster in three well supported clades (Fig. 1). The first clade (‘Euro-Med’) accommodates most accessions of Q. ilex and Q. coccifera. In the second clade (‘Cerris-Ilex’), accessions of Q. ilex, Q. coccifera, and one of the five samples of Q. aucheri group together with all representatives of Quercus Group Cerris and two Himalayan-East Asian species of Group Ilex. Sister to this clade are the three representatives of the single Japanese species of Group Ilex (Q. phillyraeoides). In the third clade (West Asia-Himalaya-East Asia; ‘WAHEA’) the remaining specimens of Q. aucheri form a subclade along with the Cypriote endemic Q. alnifolia, and several Eastern Mediterranean Q. coccifera. The second, more divergent and poorly supported subclade comprises two western Himalayan species (Q. baloot, Q. floribunda), two individuals of Himalayan-East Asian species of Quercus Group Ilex, and one Central China accession of a Cyclobalanopsis member (Q. oxyodon) sympatric with many group Ilex oaks, including Q. semecarpifolia, Q. leucotrichophora and Q. floribunda (Menitsky, 2005). In contrast to Group Ilex, all other infrageneric groups show relatively high chlorotypic coherence, usually forming clades or grouping within the same subtree. The actual root of the tree is obscured; representatives of Castanea, Castanopsis, and Notholithocarpus/Chrysolepis that could be used as putative outgroups are placed in different subtrees.
Figure 1. ML tree of the investigated oak accessions.
ML tree of plastid accessions; tentatively rooted with the Notholithocarpus-Chrysolepis subtree. Stars indicate subtrees comprising accessions of Mediterranean members of Quercus Group Ilex. Colouration refers to the taxonomic affiliations and main clades of specimens. Number at branches indicate non-parametric bootstrap support under maximum likelihood using two different implementations and posterior probabilities calculated using Bayesian inference.
Evolutionary significance of plastid haplotypes in western Mediterranean oaks of Quercus Group Ilex
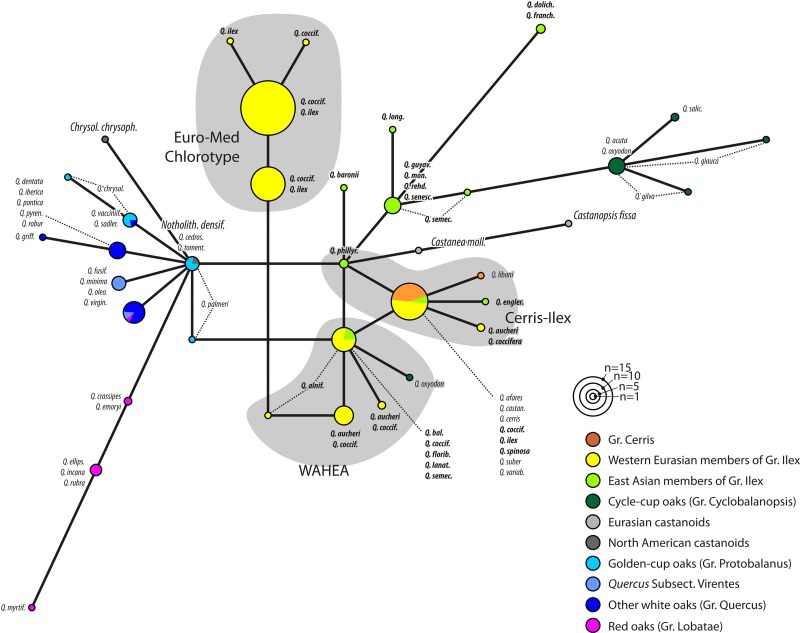
The MJ network for the plastid region with the highest overall variability (trnH-psbA, only length-homogenous parts considered; Fig. 2; MJ networks for the other gene regions can be found in File S3 and in the online archive www.palaeogrimm.org/data/Smn15_OSA.zip) highlights the evolutionary significance of the three main haplotypes, ‘Euro-Med’, ‘Cerris-Ilex’, and ‘WAHEA’. Three main clusters differ by a minimum of two conserved mutations: (1) Group Quercus, Protobalanus and Lobatae (‘New World Oaks’); (2) individuals with ‘Euro-Med’ haplotypes; (3) individuals with ‘Cerris-Ilex’ and ‘WAHEA’ haplotypes, representatives of Group Cerris and East Asian species of Group Ilex and Group Cyclobalanopsis (‘Old World Oaks’). In general, haplotypes (File S3 includes MJ-networks for the other three regions, rbcL gene, matK gene, 3’ trnK intron) found in the western Eurasian members of Group Ilex represent unique or ancestral variants. Unique haplotypes of Group Cerris are directly derived from the Group Ilex or shared ‘Cerris-Ilex’ haplotypes. Haplotypes of Group Cyclobalanopsis are identical to or can be derived from East Asian members of Group Ilex. The graphs further highlight a close relationship of haplotypes of Chrysolepis and Notholithocarpus with those of the ‘New World’ oaks; the haplotypes of Castanea and Castanopsis can be derived from the ‘Old World’ oaks basic type.
Figure 2. Haplotype network based on the trnH-psbA spacer.
Haplotype network based on length-conserved portions of the trnH-psbA spacer. Colouration refers to the taxonomic affiliation of specimens.
Figures 1 and 2 clearly illustrate that differentiation in the plastid sequences of Quercus (and related Fagaceae) is independent from the formation of the modern clades or, at least, from their genetic homogenization (lineage sorting).
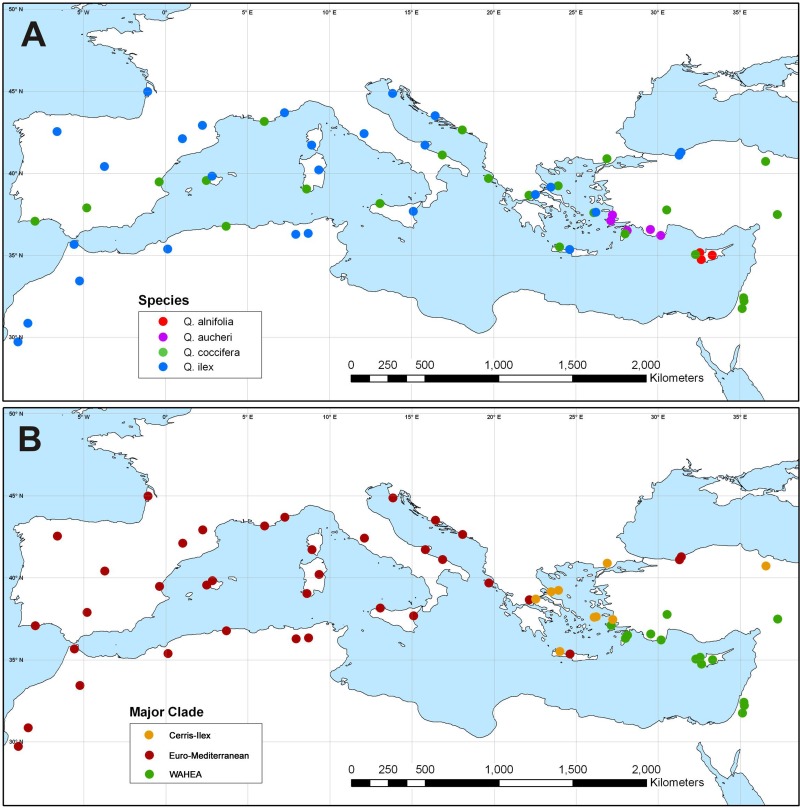
Phylogeographic structure in Quercus Group Ilex
Haplotypes forming the ‘Euro-Med’, ‘Cerris-Ilex’ and ‘WAHEA’ lineages are geographically sorted but shared among species. The phylogenetically isolated ‘Euro-Med’ haplotypes are encountered in the western Mediterranean populations of Q. ilex and Q. coccifera (North Africa, Iberia, Southern France, Italy), along the Adriatic coast and into Central Greece (Fig. 3). Also included here are isolated populations of Q. ilex from Crete and the southern Black Sea coast. ‘Cerris-Ilex’ and ‘WAHEA’ haplotypes are confined to the eastern Mediterranean region. ‘Cerris-Ilex’ haplotypes are found in the Aegean region (Q. ilex, Q. coccifera and Q. aucheri individuals) and replaced by ‘WAHEA’ haplotypes (Q. coccifera, Q. aucheri, Q. alnifolia) in south-western Turkey and extending to the east (Levante region; Fig. 3). The ‘Cerris-Ilex’ type is also found in the Q. coccifera individual from northern Turkey, representing the north-easternmost population of this species.
Figure 3. Plastid haplotype variation in Mediterranean members of Quercus Group Ilex.
Geographic pattern of plastid haplotype variation in Mediterranean members of Quercus Group Ilex. (A) Map showing the taxonomic identity of sampled specimens. (B) Map showing the plastid haplotypes of sampled specimens.
Discussion
All currently available molecular data on Fagaceae show a deep incongruence between nuclear and plastid data. Nuclear phylogenies unambiguously point towards an inclusive common origin of all oaks, i.e., a monophyletic (s. str.) genus Quercus (Oh & Manos, 2008; Denk & Grimm, 2010; Hubert et al., 2014). At the same time plastid data repeatedly failed to resolve all oaks as one clade (Manos, Cannon & Oh, 2008; this study). Instead, a split emerges (with varying support) between the North American Notholithocarpus and a North American/northern temperate clade of oaks, the ‘New World Oaks’, and the Eurasian Castanea, Castanopsis and oak lineages, the ‘Old World oak’ clade. If we accept the monophyly of the genus Quercus, which is further supported by morphology and evidence from the fossil record, haplotypes of Castanea/Castanopsis and Notholithocarpus that group with the ‘New World’ and ‘Old World’ oaks, respectively, can only be the result of incomplete lineage sorting during the formation of the modern genera and/or ancestral gene flow between early diverged lineages. In addition, the plastid gene pool of the earliest oaks must have shown a genetic gradient that reflected to some extent a biogeographic pattern. Although it is impossible to pinpoint the place of origin of oaks, it is clear that paleoclimatic and paleogeographic conditions during the Eocene facilitated a rapid spread over the Holarctic region, allowing them to pick up and propagate geographic signatures inherited from their common ancestors with Notholithocarpus, Castanea and Castanopsis.
Major trends of plastome differentiation
Overall low genetic intra- and intertaxonomic (intrageneric lineages, genera) distances suggest low evolutionary rates for the chloroplast genomes of Fagales at the examined loci. However, the data coverage is far from sufficient for most genera and families to precisely assess the potential variation within the plastome of this plant group. In Fagaceae, a comparison with the (genetically) more diverse Nothofagaceae and Betulaceae families shows that haplotype variation at the trnH-psbA locus can be sufficiently high to allow phylogeographic and systematic inferences (see Premoli et al., 2012; Grimm & Renner, 2013). We observed similar levels of variation for haplotypes of intrageneric lineages of Quercus at this marker. Furthermore, a geographic pattern is evident for the most widely sampled groups. Groups Ilex, Lobatae and Quercus were the most variable groups, whereas Group Cerris exhibited the lowest variation rates among its species. Interclade differentiation among all Quercus groups equalled or exceeded that found in the four outgroup genera (Castanea, Castanopsis, Notholithocarpus and Chrysolepis). As a consequence, the outgroup taxa appear scattered across the tree, rather than being culled in a distinct subtree, rendering the plastome of Quercus ‘non-monophyletic’. Outgroup selection as a potential source of topological ambiguity has previously been pointed out by Hubert et al. (2014) who analyzed 108 oak taxa at eight nuclear markers. Ambiguous relationships within Fagales independently of the strength of the obtained phylogenetic signal were also suggested in a recent study based on plastid DNA, fossil and reproductive syndromes which resolved the majority of inter-generic relationships in each family except for in the Quercoideae group making Castanopsis and Quercus “non-monophyletic” (Xiang et al., 2014).
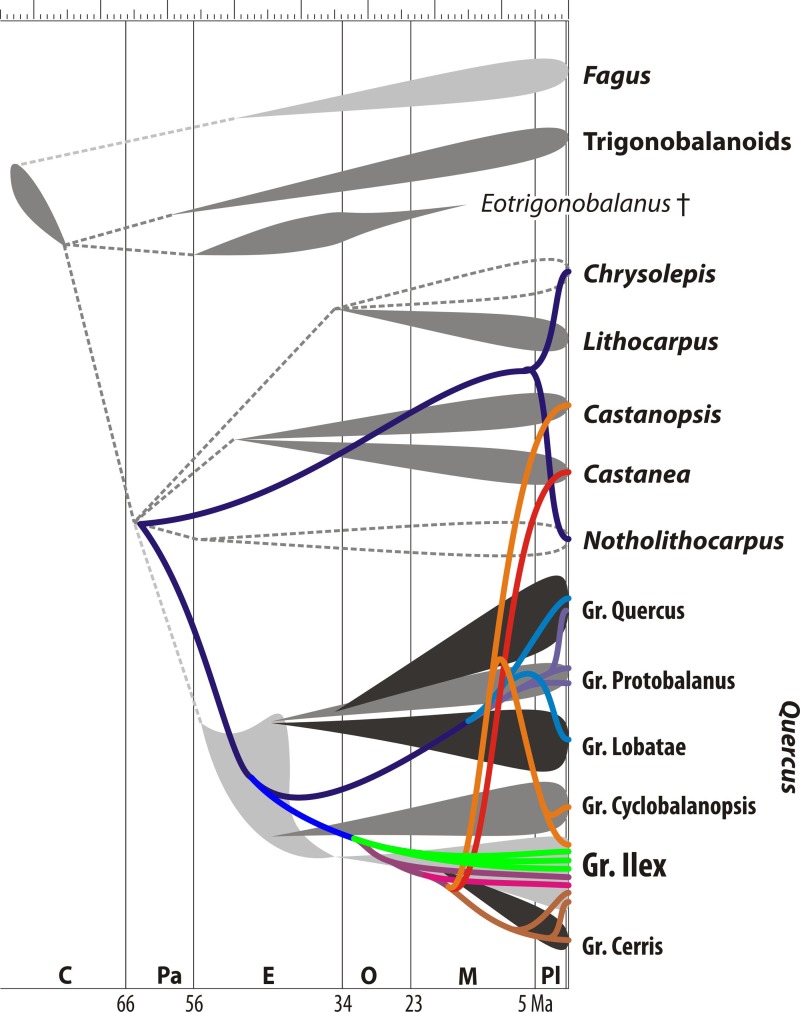
How polyphyletic is Quercus Group Ilex?
Figure 4 highlights the incongruence of the plastid genealogy tree with the current understanding of the evolution of Fagaceae and oaks based on molecular sequence data from non-coding nuclear gene regions (Manos, Zhou & Cannon, 2001; Denk & Grimm, 2010), a recent time-calibrated nuclear phylogeny of oaks (Hubert et al., 2014), and the fossil record of modern lineages as documented by pollen investigated under the scanning-electron microscope (Grímsson et al., 2015; see also Denk & Grimm, 2009). Extensive sampling is more likely to reveal polyphyly (Wiens & Servedio, 2000), but caution is needed when trees are interpreted regarding single (monophyly, paraphyly) or multiple (polyphyly) origins of species. Indeed, polyphyly as expressed in phylogenetic trees, ‘non-monophyly’, is more likely due to homoplasy between distant branches in a tree. Additional reasons for topological polyphyly is that we work with gene trees, and not species trees: in this case, topological polyphyly (‘non-monophyly’) may be due to ancestral polymorphisms or introgression causing incongruences between the trees. However, distinguishing the effects of these mechanisms may be very difficult in the absence of nuclear markers and (palaeo-)geography as complementary information (Funk & Omland, 2003). As a general rule, deep putative polyphyly or paraphyly (lineages resolved as a “basal” grade) hints at retained ancestral polymorphism, i.e., a lineage sorting phenomenon, while recently introgressed haplotypes may assume a highly derived position in a gene tree. At the same time, incomplete lineage sorting is not predicted to promote the geographic proximity of interspecifically shared haplotypes that may be seen under local introgression (Hare & Avise, 1998; Masta et al., 2002).
Figure 4. Map of chloroplast evolution in oaks.
Mapping of chloroplast evolution in oaks (using the same rooting scenario as in Fig. 1) on current evolutionary synopsis (based on nuclear sequence data, morphology, and the fossil record; modified after Grímsson et al. (2015, Fig. 16). Colouring of the plastid lineages refers to branches/subclades in Fig. 1: bluish, common (ancestral) and ‘New World’ oak/castanoids plastid haplotype lineages; green, lineages of the unique ‘Euro-Med’ plastid haplotype found only in Mediterranean members of Group Ilex; reddish, lineages of ‘Old World’ oaks and Eurasian castanoids. Note that members of Group Ilex keep plastid haplotypes of five different evolutionary sources/systematic affinities. Abbreviations: C, Cretaceous; Pa, Paleocene; E, Eocene; O, Oligocene; M, Miocene; Pl, Plio-/Pleistocene.
A strictly polyphyletic origin of Quercus Group Ilex or its Mediterranean members is unlikely. Nuclear data covering the entire range of Q. ilex and Q. coccifera in the Mediterranean region unambiguously resolved the two species as close, but mutually monophyletic sister taxa (Denk & Grimm, 2010). The ‘non-monophyly’ of Group Ilex plastomes seen in the tree (Fig. 1), including haplotypes shared with Group Cerris or closely related to Group Cyclobalanopsis therefore reflects either incomplete lineage sorting or introgression or both. In the absence of nucleome data for all here included individuals, it is impossible to infer to which degree introgression and incomplete lineage sorting contributed to the plastid gene pool of the Mediterranean species of Group Ilex. Nevertheless, the most straightforward explanation for the observed scenario would be a combined effect: asymmetrical introgression of ancestral haplotypes resulting in local genetic clusters decoupled from taxonomic boundaries, in which plastome accessions of species or species complexes may form grades or multiple clades in phylogenetic trees, thus appearing para- or polyphyletic (e.g., Rieseberg & Soltis, 1991; Whittemore & Schaal, 1991).
As modelled by Excoffier, Foll & Petit (2009), interspecific interactions during historical range fluctuations can profoundly affect the observed phylogeographic patterns, and manifest as paraphyly or reticulation (polyphyly in a broad sense). In fact, most range expansions do not occur in completely uninhabited areas, and interbreeding between local and an expanding (invasive) species with subsequent asymmetrical introgression can develop also in absence of selection, e.g., when one species is dominant and most abundant (Lepais et al., 2009). Plastid haplotypes referring to the original (‘lost’) species are indeed likely to persist over long evolutionary periods, and may still be found in the invading species. Noteworthy, environmental changes and disturbance of local communities have been shown to increase hybridisation rates (Lagache et al., 2013), hence, the potential for widespread, imbalanced introgression. In Group Ilex oaks, the interspecific capture of plastids among sexually incompletely isolated species likely occurred on the geological timescale, concealing the species relationships at various stages in the history of the genus. In a comprehensive study of the genus Ilex (Manen et al., 2010), the high incongruence between a taxonomically compatible nuclear gene tree and a geographically structured plastid tree was explained with extensive extinctions between the Cretaceous and Miocene and multiple hybridization and introgression events between distantly related lineages. This has been documented also for Platanus (Grimm & Denk, 2010) and more recently suggested for the evergreen white oaks of Quercus subsection Virentes (Eaton et al., 2015). Similar ancient lateral transfers have been also inferred to explain the paraphyly of the maternally inherited mtDNA of Picea species (Bouillé, Senneville & Bousquet, 2011) and Pinus species (Tsutsui et al., 2009).
Decoupling of plastid signatures and taxonomy in oaks
Speciation processes in Quercus do not immediately leave imprints in the plastome (e.g., Neophytou et al., 2010; Cavender-Bares et al., 2011) as also well documented for Nothofagus (Acosta & Premoli, 2010; Premoli et al., 2012). Low mutation rates and long generation times can contribute to slow evolutionary rates and incomplete lineage sorting of organellar genomes (Cavender-Bares et al., in press; Besnard, Rubio de Casas & Vargars, 2007). In addition, reiterated extinction and re-colonisation involving bottlenecks, genetic drift, and founder effects may cause random fixation of haplotypes, increasing the probability for retaining ancestral traits. Oaks in general, and especially the Mediterranean taxa, are also characterised by a marked resprouting ability in response to environmental disturbances (Barbero et al., 1990). This could have contributed to clonally preserve and transmit ancestral plastid lineages (maternally inherited) during multiple unfavourable conditions since the origin of the Mediterranean region (Blondel & Aronson, 1999). At the same time, large population sizes and long distance pollen dispersal might have contributed to homogenise the nuclear genomes in local populations of a species but not their organelle genomes. Topographic barriers hindering seed dispersal and balancing selection on the organelle genomes could have preserved ancestral polymorphisms within the species’ gene pools, also (Funk & Omland, 2003). However, although there is some evidence for balancing selection on the mtDNA in plants (Städler & Delph, 2002) it has not yet been suggested as an explanation of intraspecific plastid heterogeneity.
Additionally, Fagaceae lineages are susceptible to hybridisation and introgression (Arnold, 2006). This may lead to the formation of morphologically unambiguous individuals of a species with plastid signatures of another (Whittemore & Schaal, 1991; Petit et al., 2004). There is strong evidence for local introgression in oak communities with morphologically distinct species in the case of European white oaks (Group Quercus; Q. robur, Q. petraea, Q. pyrenaica, Q. pubescens, Q. frainetto; Curtu, Gailing & Finkeldey, 2007; Valbuena-Carabaña et al., 2007; Lepais et al., 2009), as well as in members of Quercus subsection Virentes, a subgroup of Group Quercus, in North America (Cavender-Bares et al., in press), and across a wide range of Group Lobatae (Dodd & Afzal-Rafii, 2004; Peñaloza-Ramírez et al., 2010; Moran, Willis & Clark, 2012; Valencia-Cuevas et al., 2015). In our focal group, hybrids and different levels of genetic introgression among morphologically pure individuals were molecularly documented via genetic assignment analysis in Q. ilex/Q. coccifera (Ortego & Bonal, 2010) and, to a lesser extent, in Q. coccifera/Q. alnifolia (Neophytou et al., 2011). Also, the potential for inter-group hybridisation was experimentally demonstrated for Q. ilex and Q. robur (Group Quercus; Schnitzler et al., 2004), and natural introgression in Q. ilex and Q. suber (Group Cerris) was identified in southern France (Mir et al., 2009) and the Iberian Peninsula (Burgarella et al., 2009). Therefore, it is possible that ancient hybridization and introgression, favoured by the well-known sexual promiscuity between closely related taxa and their ability to disperse pollen over long distances, obscure the interpretation of the evolutionary origin of an oak species or entire lineage.
In the Mediterranean, the profound geological and ecological changes during the Neogene (Blondel & Aronson, 1999) likely caused extinction, re-colonisation, range fragmentation and hybridisation linked to secondary contact, especially when species were still young and reproductive barriers likely weaker than today. Taken together, incomplete sorting of ancestral traits and introgression of haplotypes thus appear highly likely mechanisms to decrease inter-species plastid differentiation while at the same time increasing intra-species variation.
Temporal and spatial framework of plastome evolution
The three distinct plastid haplotypes observed in modern Mediterranean members of Quercus Group Ilex may reflect three radiation phases (range extensions), followed by range disruptions and isolation of plastome lineages within the ‘Old World Clade’ of Quercus. Considering the high diversity of haplotypes in Group Ilex as compared to other major oak lineages (or other genera in the Fagales; see Table 3; File S2) it can be hypothesized that the geographical disruptions in the plastome of the ancestors of Group Ilex and interacting lineages predate the manifestation of modern taxa (species and infrageneric groups; Fig. 1). Haplotypes shared between members of Group Ilex and its sister lineages Group Cerris and Group Cyclobalanopsis may indicate the same geographic origin or may be the result of secondary contact and asymmetrical introgression.
Evolutionary hypotheses concerning the unique ‘Euro-Med’ haplotype
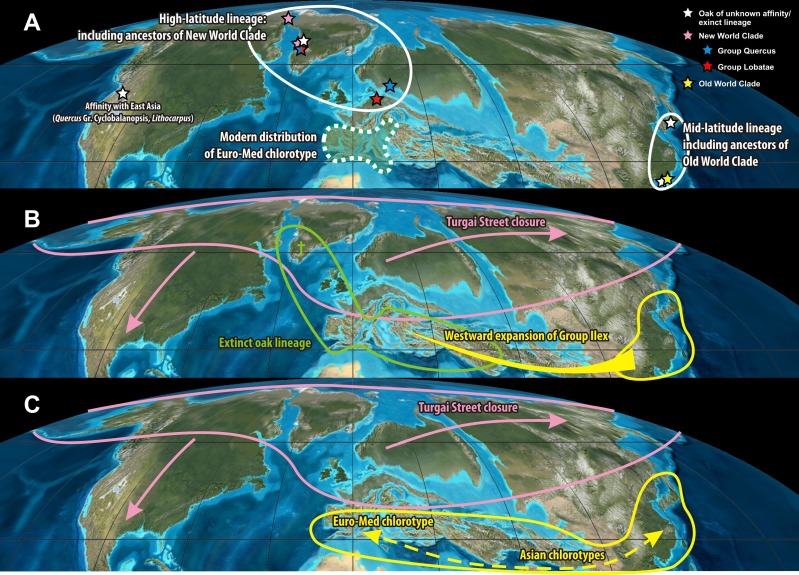
Independent from the actual position of the plastid root (note the scattered placement of putative outgroups in Fig. 1), the divergence of the ‘Euro-Med’ haplotype must have coincided with the initial differentiation in Quercus (Fig. 1). Oaks had achieved a wide northern hemispheric range by the Eocene. Unequivocal fossils are known from high latitudes (North America, Greenland, North Europe; Crepet & Nixon, 1989; Manchester, 1994; Grímsson et al., 2015) and mid-latitudes (Central Europe, South East Asia; Kvaček & Walther, 1989; Hofmann, 2010). All major lineages of oaks were established by the end of the Eocene, ca. 35 Ma, as evidenced by the fossil record and molecular dating using eight nuclear gene regions (Bouchal et al., 2014: Fig. 14; Hubert et al., 2014; Grímsson et al., 2015). During this time, one fraction of oaks, represented by the ‘Euro-Med’ plastids, must have been geographically and reproductively isolated which would have caused a major split in the plastid gene pool (Fig. 1). Today, the ‘Euro-Med’ haplotype within Quercus Group Ilex is the only one exclusively shared by just two, but widespread Mediterranean species of Group Ilex, Q. ilex and Q. coccifera. This haplotype is markedly distinct from haplotypes in other oaks or Fagaceae (Fig. 1). Two evolutionary hypotheses could explain the establishment of this unique haplotype in Q. ilex-Q. coccifera (Fig. 5): (i) The ‘Euro-Med’ haplotype is the remnant of an extinct oak lineage that was intrograded (invaded) and consumed by members of Group Ilex (Fig. 5B); under this scenario Group Ilex would have migrated into Europe at some point prior to the Miocene where it came into contact with this extinct oak lineage. (ii) The ‘Euro-Med’ haplotype represents an original plastome of Group Ilex; under this scenario, the first split within the modern ‘Old World clade’ would have been between western members of Group Ilex and an eastern cluster comprising precursors, members of Group Ilex and Group Cyclobalanopsis (Fig. 5C). At the moment we can only speculate which hypothesis applies.
Figure 5. Origin of the ‘Euro-Med’ haplotype.
Eocene set-up and the origin of the ‘Euro-Med’ haplotype. (A) Unequivocal fossil record of oaks in the Eocene mapped on a palaeotopographic map highlighting a primary split into a high-latitude and mid-latitude lineage that likely correspond to the deep phylogenetic split seen in nuclear and plastid sequence data of modern oaks between the ‘New World Clade’ (Groups Protobalanus, Quercus and Lobatae) and the ‘Old World Clade’ (Groups Cyclobalanopsis, Ilex, Cerris). (B–C) Scenarios that can explain the occurrence of the unique ‘Euro-Med’ haplotype in westernmost members of Quercus Group Ilex. (B) The ‘Euro-Med’ haplotype belonged to an extinct oak lineage geographically/biologically separated from both the ancestors of the New World and Old World Clade. Westward expansion of Himalayan members of Group Ilex and subsequent large-scale introgression/hybridisation homogenised the western members of Group Ilex and the extinct oak lineage, retaining and evolving the original haplotype in the Mediterranean region. (C) The ‘Euro-Med’ haplotype reflects geographic fragmentation within the Paleogene range of the Old World Clade that was overprinted to some degree after later radiation phases of Group Ilex. Palaeotopographic map base used with permission from Ron Blakey, © Colorado Plateau Geosystems.
During the Eocene and Oligocene a number of extinct genera of Fagaceae (e.g., Eotrigonobalanus, Trigonobalanopsis) and extinct lineages of Quercus were present in the northern hemisphere (Kohlman-Adamska & Ziembínska-Tworzydło, 2000; Kohlman-Adamska & Ziembínska-Tworzydło, 2001; Stuchlik, Ziembínska-Tworzydło & Kohlman-Adamska, 2007; Denk, Grímsson & Zetter, 2012; Grímsson et al., 2015). It is possible that the plastid of one of these lineages was captured by members of Quercus Group Ilex when expanding westwards into the modern area of the Euro-Med haplotype. This would fit with the first hypothesis.
Scenario (ii) would be in agreement with geographic fragmentation and subsequent incomplete lineage sorting across the former Paratethyan and current Himalayan arcs. During its evolution, Group Ilex must have been continuously affected by range disruptions caused by tectonic activity south of the Paratethys linked to the collision of Africa and the Indian subcontinent with Eurasia (Fig. 6); progressive rarefaction of the original haplotypes and the occurrence of (repeated) invasion and introgression events that left imprints in the plastome even within the same species is highly likely in such a topographic setting. Nevertheless, under scenario (ii) the diversity and distinctness of the ‘Euro-Med’ haplotype would require an initial geographic separation between the westernmost (European) and other members of Group Ilex, predating the formation of the modern lineages and surviving later (Miocene) migration waves. Therefore, scenario (ii) appears less probable than scenario (i).
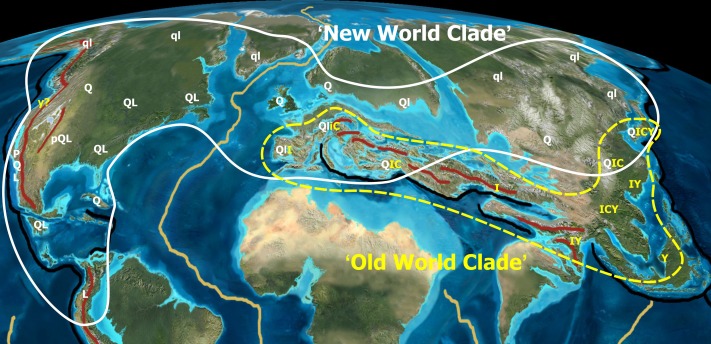
Figure 6. Tectonic activity during the Eocene.
Tectonic activity during the Eocene and past and modern distribution of the New World (white) and Old World (yellow) groups within Quercus. Black lines indicate major subduction zones, red lines major orogenies. Note that the high latitude lineage of oaks (Quercus Group Lobatae, Group Quercus, Group Protobalanus) evolved in tectonically stable regions, whereas the low latitude lineage (Quercus Group Ilex, Group Cyclobalanopsis, Group Cerris) evolved in tectonically unstable regions. Uppercase and lowercase letters refer to extant and extinct distribution areas of major oak lineages: P,p, Group Protobalanus; Q,q, Group Quercus; L,l, Group Lobatae; I,i, Group Ilex; C,c, Group Cerris; Y,y, Group Cyclobalanopsis. Palaeotopographic map base used with permission from Ron Blakey, © Colorado Plateau Geosystems.
Evolutionary significance of the ‘Cerris-Ilex’ haplotype and the origin of Group Cerris
The ‘Cerris-Ilex’ haplotype is shared between all species of Quercus Group Cerris (western Eurasian and East Asian), East Mediterranean (Aegean) individuals and two East Asian species of Group Ilex. This is in agreement with Denk & Grimm (2010) who suggested that Quercus Group Cerris evolved from Group Ilex by budding (a hypothesis further confirmed by the 8-nuclear gene data set used by Hubert et al. (2014)), and the low support for a Group Ilex clade in an all-Fagaceae (excluding Fagus) tree based on over 1000 nuclear ITS sequences (Denk & Grimm, 2010). Hubert et al. (2014) inferred a Miocene age for this budding event, which corresponds to the earliest unequivocal fossil of Quercus Group Cerris (Kmenta, 2011) and is younger than the earliest definite fossil record of Quercus Group Ilex in Europe (early Oligocene, Cospuden; (Denk, Grímsson & Zetter, 2012)). Also, dispersed pollen from the Paleogene Changchang Formation, Hainan (Hofmann, 2010), resembles both Quercus Group Ilex and Group Cyclobalanopsis; the age of this formation is considered late early to early late Eocene (Lei et al., 1992). The haplotype most closely related to the ‘Cerris-Ilex’ haplotype is encountered in the widespread East Asian Q. phillyraeoides, the only species of Group Ilex extending to Japan (in contrast, the East Asian members of Group Cerris have a much wider range in north-eastern Asia; Menitsky, 2005). Regarding its phylogenetic position, the emergence of the ‘Cerris-Ilex’ haplotype appears to have been linked with a major taxonomic sorting event in Eurasian Fagaceae, resulting in distinct haplotypes restricted to genera and intrageneric groups of Quercus (Fig. 1). Based on the palaeobotanical record, these lineages (Castanopsis, Castanea, Quercus Group Ilex, Quercus Group Cyclobalanopsis) were well established at least by the Eocene (Table 4 and Fig. 5; Grímsson et al., 2015); a deep divergence is reflected by their distinctly different nuclear genomes (Oh & Manos, 2008; Denk & Grimm, 2010; Hubert et al., 2014). Again, two evolutionary scenarios could explain the occurrence of the ‘Cerris-Ilex’ haplotype in Aegean individuals of Q. ilex and Q. coccifera and the westernmost Q. aucheri: (i) Group Cerris evolved in western Eurasia/Himalaya from an (extinct) subtropical to temperate sublineage of Group Ilex, which left its imprint in the Aegean members of Group Ilex, and Q. spinosa, Q. engleriana and Q. phillyraeoides. (ii) Group Cerris shares a common ancestry with the north-east Asian Q. phillyraeoides of Group Ilex. Under the latter scenario, the budding event of the group would have taken place in north-eastern Asia, from where it migrated into western Eurasia and the Aegean region. In relatively recent times, Group Cerris came into contact with the Mediterranean members of Group Ilex and was locally introgressed (e.g., Burgarella et al., 2009; Mir et al., 2009). However, it is difficult to explain why Q. ilex-coccifera should only intrograde into populations of Cerris oaks at a large scale in the Aegean region. Therefore, scenario (i) appears to be more plausible. The fossil record of Group Cerris, in particular the Central and East Asian one, could potentially shed further light on this hypothesis. Unfortunately, it is currently not very well understood.
Table 4. Eocene fossil record of Quercus.
| Locality, site, age | Reference | Taxon, organ | Affinity |
|---|---|---|---|
| Clarno Fm., Oregon, western U.S.A.; ∼48 Ma | Manchester, 1994 | “Quercus” paleocarpa Manchester; cupules and acorns | Group Cyclobalanopsis (? Lithocarpus) |
| Axel-Heiberg Island, Canadian Arctic; ∼45 Ma | McIntyre, 1991; McIver & Basinger, 1999 | Pollen and leaves | New World Clade; Quercus Group Quercus/Lobatae |
| Hareøn, western Greenland; ∼42 Ma | Grímsson et al., 2015 | Quercus sp. 4–5; pollen | Extinct/ancestral type |
| Quercus sp. 6–7; pollen | New World Clade (aff. Group Protobalanus) | ||
| Quercus sp. 1–3; pollen | Quercus Group Quercus and/or Lobatae | ||
| Baltic amber, northern Europe; ∼45 Ma | Crepet & Nixon, 1989; Weitschat & Wichard, 2003 | Flower and in situ pollen | Quercus Group Quercus |
| Königsaue, near Aschersleben, Germany; middle Eocene (48–38 Ma) | Kvaček & Walther, 1989 | Quercus subhercynica H Walther & Kvaček; leaf | Quercus Group Lobatae |
| Ube coal-field, southwestern Honshu, Japan; middle Eocene (48–38 Ma) | Huzioka & Takahasi, 1970 | Cyclobalanopsis nathoi Huzioka & Takahashi; leaf, acorn (?), cupule (?) | Quercus, affinity unclear |
| Changchang, Hainan; middle (?) Eocene (50–35 Ma) | Hofmann, 2010 | Quercus pollen types 2–8, 10; pollen | Quercus, affinity unclear (extinct, Group Quercus/Lobatae?, Group Protobalanus?) |
| Quercus pollen type 1; pollen | Quercus Group Ilex | ||
| Quercus pollen type 9; pollen | Quercus Group Cyclobalanopsis |
Origin and evolutionary significance of the ‘WAHEA’ haplotype of Group Ilex
The West Asian–Himalayan-East Asian (WAHEA) haplotype represents Eastern Mediterranean members ofQuercus Group Ilex and is sister to a clade comprising several Asian species of Group Ilex (Himalayas to the mountains of Southeast Asia). Based on its position in the plastid tree, this haplotype seems to reflect a second radiation within the Old World Clade and allies after the isolation of the ‘Euro-Med’ lineage and prior to the radiation and subsequent sorting within the clade comprising the ‘Cerris-Ilex’ haplotypes (Fig. 1). The modern distribution of species with the WAHEA haplotype follows the Himalayan corridor (Kitamura, 1955; Güner & Denk, 2012). The Himalayan corridor is a narrow band along the southern flanks of the Himalaya with a subtropical to temperate climate (Cwa, Cwb; Peel, Finlayson & McMahon, 2007) providing a pathway for plants originating from humid temperate Cenozoic laurel and mixed broad-leaved deciduous and evergreen forests. In addition to Quercus Group Ilex (Zhou, 1992; Velitzelos, Bouchal & Denk, 2014), prominent relic taxa include species of Acer, Aesculus, Cedrus, Cotinus, Juglans, Platanus, and Rhododendron among others. The ‘WAHEA’ haplotype represents the western counterpart to the haplotype lineage found in East Asian species of Group Ilex and Cyclobalanopsis (Fig. 1). The relic Q. alnifolia, today only found in the mid-montane region of Cyprus (Mt. Troodos), would be a witness of this expansion (Menitsky, 2005).
Conclusion
Plant biogeographic studies at the genus level have commonly relied on few to many chloroplast markers and a single or very few accessions per taxon. In the case of woody angiosperms with a subtropical to temperate distribution such as e.g., Nothofagaceae (Svenson et al., 2001; Knapp et al., 2005), Rhus (Yi, Miller & Wen, 2004), Cornus (Xiang et al., 2005), Carpinus (Yoo & Wen, 2007), Castanea (Lang et al., 2007), Juglans (Aradhya et al., 2007), and Carya (Zhang et al., 2013a), this approach runs the risk of capturing but a limited aspect of the evolutionary history of the focal group. Mere combination with e.g., nuclear ITS data can be problematic, too (compare data shown here with data provided by Denk & Grimm (2010), on western Eurasian members of Group Ilex). The decoupled evolutionary signals in plastomes and the nucleome/morphology as documented for Nothofagus (Acosta & Premoli, 2010; Premoli et al., 2012) and Quercus Group Ilex (this study) suggest that the traditional placeholder sampling strategy is not ideal. Signals from few-marker/many-samples data sets are likely to be complex or even puzzling (Figs. 1–4), but at the same time provide entirely new perspectives on plant evolution worth exploring. For Quercus Group Ilex, our pilot study focussing on Mediterranean species reveals a crucial aspect of oak evolution not seen in the combined nuclear, morphological, and fossil data: large-scale introgression and/or incomplete lineage sorting among ancestral lineages of modern major groups and species. The new data corroborate hypotheses that Group Cerris evolved (‘budded’) relatively recent from Group Ilex as discussed by Denk & Grimm (2010), using over 600 ITS and over 900 5S-IGS accessions covering all western Eurasian oak species and inferred by Hubert et al. (2014), using dated phylogenies based on seven single-copy nuclear regions and the ITS region. Our preferred hypothesis is that Quercus Group Cerris evolved in western Eurasia and the Himalayas when the then chiefly subtropical low latitude Group Ilex radiated into temperate niches. Accordingly, within modern members of Group Cerris, a wide spectrum of leaf traits is found from pseudo-evergreen in Q. suber, to semi-evergreen in Q. brantii, Q. ithaburensis, Q. trojana (partly) and fully deciduous in Q. acutissima, Q. castaneifolia, Q. cerris, Q. libani and Q. variabilis. The conspicuous plastid diversity in the Mediterranean species of Group Ilex and the lineage in general (Figs. 1 and 2; Table 2) can only be interpreted as reflecting the highly complex geographical history of this group, due to growing geographic isolation between clades from the Oligocene onwards. The ‘Euro-Med’ haplotype evidences an initial phase of geographic differentiation predating the formation of modern lineages, but its origin and evolutionary significance remain enigmatic (Fig. 5).
Although decoupled from taxonomy, the plastid phylogeny provides important, independent information on the geographic differentiation of Quercus prior to the formation of modern species/species groups. So far, the major split within oaks has been between ‘New World’ and ‘Old World’ oaks (following Manos, Zhou & Cannon, 2001) because of the current distribution of the major, molecular-defined lineages of oaks. This view replaced traditional concepts (reviewed in Denk & Grimm, 2010) recognising two subgenera/genera, one in subtropical to tropical East Asia (subgenus/genus Cyclobalanopsis = Group Cyclobalanopsis) and the other ubiquitous on the northern hemisphere (subgenus/genus Quercus, including all other infrageneric groups). The plastid data presented here suggest that the early evolution of oaks instead was geographically bound to high latitude Arctic regions and to low latitude subtropical regions (Fig. 5). The high latitude lineages remained genetically homogeneous in the nucleome, but also in the plastome to some degree. Continuous circum-polar distribution could have prevented pronounced genetic drift in the high latitude lineage, which became the ‘New World Clade’, and explains low genetic differentiation in deciduous high and mid latitude white oaks until today (Denk & Grimm, 2010). At the same time, the Atlantic, the proto-Mediterranean, the Paratethys and the rise of the Himalaya isolated the Eurasian low latitude lineage, and may explain the differentiation between plastome clades, known to be mirror of geographic isolation rather than species taxonomy in case of introgression. The high diversity of southern plastomes, the low diversity of northern plastomes, and the propensity of oaks to introgress, altogether built a consistent framework for deciphering the historical biogeography of this group.
Our data should only be viewed as a first step towards a more complete understanding of the biogeography and evolution of oaks. The next step would be to map the plastid variation of Quercus Group Ilex across its entire range by sampling multiple stands of the Himalayan and East Asian species to characterise the geographic and taxonomic ranges of the various plastid lineages. Finally, because of similar strong correlation between plastome differentiation and geographic distribution at the population level and the species/genus level, similar or identical plastid haplotypes typically shared between co-occurring and often distantly related taxa, polyphyletic signals and reproductive biology, the same processes could have likely played a key role in the evolutionary history of other Fagaceae (e.g., Fagus, Castanea, Castanopsis). As well, broadening the sampling efforts of phylogenetic analyses of the plastome could help decipher the speciation history of these genera. At the same time, extended nucleome investigations will be necessary to definitely assess a clear molecular phylogeny of Fagaceae.
Supplemental Information
Intra- and intertaxonomic minimum and maximum pairwise genetic distances in Fagales based on the used plastid markers.
haplotype networks of the investigated dataset based on rbcL and trnK-matK markers.
Acknowledgments
We thank all the Directors and curators of the Botanic Gardens and Arboreta who provided the investigated material: Anthony S. Aiello, Wolfgang Bopp, Anne Boscawen, Peter Brownless, Béatrice Chassé, Laszlo Csiba, Dirk De Meyere, Holly Forbes, Anett Krämer, Isabel Larridon, Gitte Petersen, David Scherberich, Patrick Thompson, Jef Van Meulder, Michael Wall, David Zuckerman, and all the colleagues and friends who contributed to the sampling design: Jeannine Cavender-Bares, Hanno Schaefer, Charalambos Neophytou, Martina Temunovic, Gianni Bedini, Laura Genco, Enara Otaegi Veslin.
Funding Statement
This project was funded by a Swedish Research Council (VR) grant to TD. GWG is financed by the Austrian Science Fund (FWF), grant M-1751-B16. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Marco Cosimo Simeone and Guido W. Grimm conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Alessio Papini analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Federico Vessella analyzed the data, reviewed drafts of the paper.
Simone Cardoni and Enrico Tordoni performed the experiments, analyzed the data.
Roberta Piredda analyzed the data, contributed reagents/materials/analysis tools.
Alain Franc and Thomas Denk analyzed the data, wrote the paper, reviewed drafts of the paper.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
All sequence data generated as part of this study are available on GenBank (http://www.ncbi.nlm.nih.gov/genbank/) under accession numbers LM652969– LM653098.
Data Availability
The following information was supplied regarding data availability:
Primary data and analyses are provided for download in the Online Supplementary Archive (OSA) at the journal’s homepage and at www.palaeogrimm.org/data/Smn15_OSA.zip. See GuideToFile.txt included in the OSA for further information. Other relevant data are within the paper and Supplemental Information.
References
- Acosta & Premoli (2010).Acosta MC, Premoli AC. Evidence of chloroplast capture in South American Nothofagus (subgenus Nothofagus, Nothofagaceae) Molecular Phylogenetics and Evolution. 2010;54:235–242. doi: 10.1016/j.ympev.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Akaike (1974).Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- Aradhya et al. (2007).Aradhya MK, Potter D, Gao F, Simon CJ. Molecular phylogeny of Juglans (Juglandaceae): a biogeographic perspective. Tree Genetics and Genomes. 2007;3:363–378. doi: 10.1007/s11295-006-0078-5. [DOI] [Google Scholar]
- Arnold (2006).Arnold ML. Evolution through genetic exchange. Vol. 3. Oxford: Oxford University Press; 2006. [Google Scholar]
- Barbero et al. (1990).Barbero M, Bonin G, Loisel R, Quezel P. Changes and disturbances of forest ecosystems caused by human activities in the western part of the Mediterranean Basin. Vegetatio. 1990;87:151–173. doi: 10.1007/BF00042952. [DOI] [Google Scholar]
- Belahbib et al. (2001).Belahbib N, Pemonge MH, Ouassou A, Sbay H, Kremer A, Petit R. Frequent cytoplasmic exchanges between oak species that are not closely related: Quercus suber and Q. ilex in Morocco. Molecular Ecology. 2001;10:2003–2012. doi: 10.1046/j.0962-1083.2001.01330.x. [DOI] [PubMed] [Google Scholar]
- Besnard, Rubio de Casas & Vargars (2007).Besnard G, Rubio de Casas R, Vargars P. Plastid and nuclear DNA polymorphism reveals historical processes of isolation and reticulation in the olive tree complex (Olea europaea) Journal of Biogeography. 2007;34:736–752. doi: 10.1111/j.1365-2699.2006.01653.x. [DOI] [Google Scholar]
- Blondel & Aronson (1999).Blondel J, Aronson J. Biology and wildlife of the Mediterranean region. Oxford: Oxford University Press; 1999. [Google Scholar]
- Bouchal et al. (2014).Bouchal J, Zetter R, Grímsson F, Denk T. Evolutionary trends and ecological differentiation in early Cenozoic Fagaceae of western North America. Americal Journal of Botany. 2014;101:1–18. doi: 10.3732/ajb.1300450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillé, Senneville & Bousquet (2011).Bouillé M, Senneville S, Bousquet J. Discordant mtDNA and cpDNA phylogenies indicate geographic speciation and reticulation as driving factors for the diversification of the genus Picea. Tree Genetics and Genomes. 2011;7:469–484. doi: 10.1007/s11295-010-0349-z. [DOI] [Google Scholar]
- Burgarella et al. (2009).Burgarella C, Lorenzo Z, Jabbour-Zahab R, Lumaret R, Guichoux E, Petit R, Soto A, Gil L. Detection of hybrids in nature: application to oaks (Quercus suber and Q. ilex) Heredity. 2009;102:442–452. doi: 10.1038/hdy.2009.8. [DOI] [PubMed] [Google Scholar]
- Cannon & Manos (2003).Cannon CH, Manos PS. Phylogeography of the Southeast Asian stone oaks (Lithocarpus) Journal of Biogeography. 2003;30:211–226. doi: 10.1046/j.1365-2699.2003.00829.x. [DOI] [Google Scholar]
- Cavender-Bares et al. (in press).Cavender-Bares J, Gonzalez-Rodriguez A, Eaton DAR, Hipp AAL, Beulke A, Manos PS. Phylogeny and biogeography of the American live oaks (Quercus subsection Virentes): a genomic and population genetics approach. Molecular Ecology. 2015 doi: 10.1111/mec.13269. In Press. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares et al. (2011).Cavender-Bares J, Gonzalez-Rodriguez A, Pahlich A, Koehler K, Deacon N. Phylogeography and climatic niche evolution in live oaks (Quercus series Virentes) from the tropics to the temperate zone. Journal of Biogeography. 2011;38:962–981. doi: 10.1111/j.1365-2699.2010.02451.x. [DOI] [Google Scholar]
- Crepet & Nixon (1989).Crepet WL, Nixon KC. Earliest megafossil evidence of Fagaceae: phylogenetic and biogeographic implications. American Journal of Botany. 1989;76:842–855. doi: 10.2307/2444540. [DOI] [Google Scholar]
- Curtu, Gailing & Finkeldey (2007).Curtu AL, Gailing O, Finkeldey R. Evidence for hybridization and introgression within a species-rich oak (Quercus spp.) community. BMC Evolutionary Biology. 2007;7:218. doi: 10.1186/1471-2148-7-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk & Grimm (2009).Denk T, Grimm GW. Significance of pollen characteristics for infrageneric classification and phylogeny in Quercus (Fagaceae) International Journal of Plant Sciences. 2009;170:926–940. doi: 10.1086/600134. [DOI] [Google Scholar]
- Denk & Grimm (2010).Denk T, Grimm GW. The oaks of western Eurasia: traditional classifications and evidence from two nuclear markers. Taxon. 2010;59:351–366. [Google Scholar]
- Denk, Grímsson & Zetter (2012).Denk T, Grímsson F, Zetter R. Fagaceae from the early Oligocene of Central Europe: persisting New World and emerging Old World biogeographic links. Review of Palaeobotany and Palynology. 2012;169:7–20. doi: 10.1016/j.revpalbo.2011.09.010. [DOI] [Google Scholar]
- Dodd & Afzal-Rafii (2004).Dodd RS, Afzal-Rafii Z. Selection and dispersal in a multispecies oak hybrid zone. Evolution. 2004;58:261–269. doi: 10.1111/j.0014-3820.2004.tb01643.x. [DOI] [PubMed] [Google Scholar]
- Eaton et al. (2015).Eaton DAR, Hipp AL, Gonzalez-Rodriguez A, Cavender-Bares J. Historical introgression among the American live oaks and the comparative nature of tests for introgression. Evolution. 2015;69:2587–2601. doi: 10.1101/016238. [DOI] [PubMed] [Google Scholar]
- Excoffier, Foll & Petit (2009).Excoffier L, Foll M, Petit RJ. Genetic consequences of range expansions. Annual Review of Ecology, Evolution and Systematics. 2009;40:481–501. doi: 10.1146/annurev.ecolsys.39.110707.173414. [DOI] [Google Scholar]
- Flora of China Editorial Committee (1999).Flora of China Editorial Committee . Flora of China, Cycadaceae through Fagaceae. Vol. 4. St. Louis: Missouri Botanical Garden; 1999. [Google Scholar]
- Flora of North America Editorial Committee (1997).Flora of North America Editorial Committee . Flora of North America north of Mexico. Vol. 3. New York: Oxford University Press; 1997. [Google Scholar]
- Fujii et al. (2002).Fujii N, Tomaru N, Okuyama K, Koike T, Mikami T, Ueda K. Chloroplast DNA phylogeography of Fagus crenata (Fagaceae) in Japan. Plant Systematics and Evolution. 2002;232:21–33. doi: 10.1007/s006060200024. [DOI] [Google Scholar]
- Funk & Omland (2003).Funk D, Omland K. Species-level paraphyly and polyphyly: frequency, causes and consequences, with insights from animal mitochondrial DNA. Annual Reviews of Ecology, Evolution and Systematics. 2003;34:397–423. doi: 10.1146/annurev.ecolsys.34.011802.132421. [DOI] [Google Scholar]
- Göker et al. (2009).Göker M, García-Blázquez G, Voglmayr H, Tellería MT, Martín MP. Molecular taxonomy of phytopathogenic fungi: a case study in Peronospora. PLoS ONE. 2009;4:e1897. doi: 10.1371/journal.pone.0006319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göker & Grimm (2008).Göker M, Grimm GW. General functions to transform associate data to host data, and their use in phylogenetic inference from sequences with intra-individual variability. Bmc Evolutionay Biology. 2008;8:86. doi: 10.1186/1471-2148-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govaerts & Frodin (1998).Govaerts R, Frodin DG. World checklist and bibliography of Fagales (Betulaceae, Corylaceae, Fagaceae and Ticodendraceae) Kew: Royal Botanic Gardens; 1998. [Google Scholar]
- Grimm & Denk (2010).Grimm GW, Denk T. The reticulate origin of modern plane trees (Platanus, Platanaceae)—a nuclear marker puzzle. Taxon. 2010;59:134–147. [Google Scholar]
- Grimm & Renner (2013).Grimm GW, Renner SS. Harvesting Betulaceae sequences from GenBank to generate a new chronogram for the family. Botanical Journal of the Linnean Society. 2013;172:465–477. doi: 10.1111/boj.12065. [DOI] [Google Scholar]
- Grímsson et al. (2015).Grímsson F, Zetter R, Grimm GW, Krarup Pedersen G, Pedersen AK, Denk T. Fagaceae pollen from the early Cenozoic of West Greenland: revisiting Engler’s and Chaney’s Arcto-Tertiary hypotheses. Plant Systematics and Evolution. 2015;301:809–832. doi: 10.1007/s00606-014-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugger & Cavender-Bares (2013).Gugger PF, Cavender-Bares J. Molecular and morphological support for a Florida origin of the Cuban oak. Journal of Biogeography. 2013;40:632–645. doi: 10.1111/j.1365-2699.2011.02610.x. [DOI] [Google Scholar]
- Güner & Denk (2012).Güner TH, Denk T. The genus Mahonia in the Miocene of Turkey: taxonomy and biogeographic implications. Review of Palaeobotany and Palynology. 2012;175:32–46. doi: 10.1016/j.revpalbo.2012.02.005. [DOI] [Google Scholar]
- Hare & Avise (1998).Hare MP, Avise JC. Population structure in the American oyster as inferred by nuclear gene genealogies. Molecular Biology and Evolution. 1998;15:119–128. doi: 10.1093/oxfordjournals.molbev.a025908. [DOI] [PubMed] [Google Scholar]
- Hipp et al. (2014).Hipp AL, Eaton DAR, Cavender-Bares J, Fitzek E, Nipper R, Manos PS. A framework phylogeny of the American oak clade based on sequenced RAD data. PLoS ONE. 2014;9:e1897. doi: 10.1371/journal.pone.0093975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann (2010).Hofmann C-C. Microstructure of Fagaceae pollen from Austria (Paleocene/Eocene boundary) and Hainan Island (?middle Eocene). Paper presented at the 8th European Palaeobotany-Palynology Conference, 6-19 July; Budapest, Hungary. 2010. [Google Scholar]
- Hubert et al. (2014).Hubert F, Grimm GW, Jousselin E, Berry V, Franc A, Kremer A. Multiple nuclear genes stabilize the phylogenetic backbone of the genus Quercus. Systematics and Biodiversity. 2014;12:405–423. doi: 10.1080/14772000.2014.941037. [DOI] [Google Scholar]
- Huzioka & Takahasi (1970).Huzioka K, Takahasi E. The Eocene flora of the Ube coal-field, southwest Honshu, Japan. Japan: Akita University, Mining College; 1970. [Google Scholar]
- Kanno et al. (2004).Kanno M, Yokoyama J, Suyama Y, Ohyama M, Itoh T, Suzuki M. Geographical distribution of two haplotypes of chloroplast DNA in four oak species (Quercus) in Japan. Journal of Plant Research. 2004;117:311–317. doi: 10.1007/s10265-004-0160-8. [DOI] [PubMed] [Google Scholar]
- Katoh & Standley (2013).Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura (1955).Kitamura S. Flowering plants and ferns. In: Kihara H, editor. Fauna and Flora of Nepal Himalaya. Kyoto: Fauna and Flora Research Society; 1955. pp. 73–290. [Google Scholar]
- Kmenta (2011).Kmenta M. Die Mikroflora der untermiozänen Fundstelle Altmittweida, Deutschland. Wien: University of Wien; 2011. [Google Scholar]
- Knapp et al. (2005).Knapp M, Stöckler K, Havell D, Delsuc F, Sebastiani F, Lockhart PJ. Relaxed molecular clock provides evidence for long-distance dispersal of Nothofagus (Southern Beech) PLoS Biology. 2005;3:e1897. doi: 10.1371/journal.pbio.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlman-Adamska & Ziembínska-Tworzydło (2000).Kohlman-Adamska A, Ziembínska-Tworzydło M. Morphological variability and botanical affinity of some species of the genus Tricolporopollenites Pf. et Thoms. from the Middle Miocene Lignite association at Lubstów (Konin region—Central Poland) Acta Palaeobotanica. 2000;40:49–71. [Google Scholar]
- Kohlman-Adamska & Ziembínska-Tworzydło (2001).Kohlman-Adamska A, Ziembínska-Tworzydło M. Morphological variability and botanical affinity of Fususpollenites Kedves 1978 (LM and SEM investigations) Acta Palaeobotanica. 2001;41:147–159. [Google Scholar]
- Köppen (1936).Köppen W. Das geographische System der Klimate. In: Köppen W, Geiger R, editors. Handbuch der Klimatologie, Band 1. Berlin: Teil C. Gebr. Borntraeger; 1936. pp. 1–44. [Google Scholar]
- Kottek et al. (2006).Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift. 2006;15:259–263. doi: 10.1127/0941-2948/2006/0130. [DOI] [Google Scholar]
- Kress & Erickson (2007).Kress WJ, Erickson DL. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnHpsbA spacer region. PLoS ONE. 2007;2(6):e1897. doi: 10.1371/journal.pone.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitzki (1993).Kubitzki K. Fagaceae. In: Kubitzki K, Rohwer JG, Bittrich V, editors. The families and genera of vascular plants. Vol 2. Berlin: Spinger; 1993. pp. 301–309. [Google Scholar]
- Kvaček & Walther (1989).Kvaček Z, Walther H. Palaeobotanical studies in Fagaceae of the European Tertiary. Plant Systematics and Evolution. 1989;162:213–229. doi: 10.1007/BF00936918. [DOI] [Google Scholar]
- Lagache et al. (2013).Lagache L, Klein EK, Guichoux E, Petit RJ. Fine-scale environmental control of hybridization in oaks. Molecular Ecology. 2013;22:423–436. doi: 10.1111/mec.12121. [DOI] [PubMed] [Google Scholar]
- Lang et al. (2007).Lang P, Dane F, Kubisiak TL, Huang H. Molecular evidence for an Asian origin and a unique westward migration of species in the genus Castanea via Europe to North America. Molecular Phylogenetics and Evolution. 2007;43:49–59. doi: 10.1016/j.ympev.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Lei et al. (2012).Lei M, Wang Q, Wu ZJ, Lopez-Pujol J, Li DZ, Zhang ZY. Molecular phylogeography of Fagus engleriana (Fagaceae) in subtropical China: limited admixture among multiple refugia. Tree Genetics and Genomes. 2012;8:1203–1212. doi: 10.1007/s11295-012-0507-6. [DOI] [Google Scholar]
- Lei et al. (1992).Lei YZ, Zhang QR, He W, Cao XP. Tertiary. In: Wang XF, editor. Geology of Hainan Island. I. Stratigraphy and palaeontology. Beijing: Geological Publishing House; 1992. pp. 218–266. (in Chinese) [Google Scholar]
- Lepais et al. (2009).Lepais O, Petit R, Guichoux E, Lavabre J, Alberto F, Kremer A, Gerber S. Species relative abundance and direction of introgression in oaks. Molecular Ecology. 2009;18:2228–2242. doi: 10.1111/j.1365-294X.2009.04137.x. [DOI] [PubMed] [Google Scholar]
- Librado & Rozas (2009).Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- López de Heredia et al. (2007).López de Heredia U, Jiménez P, Collada C, Simeone MC, Bellarosa R, Schirone B, Cervera MT, Gil L. Multi-marker phylogeny of three evergreen oaks reveals vicariant patterns in the Western Mediterranean. Taxon. 2007;56:1209–1209. doi: 10.2307/25065912. [DOI] [Google Scholar]
- Maddison & Maddison (2011).Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. (Version 2.75) 2011 Available at http://mesquiteproject.org .
- Manchester (1994).Manchester SR. Fruits and seeds of the Middle Eocene nut beds flora, Clarno Formation, Oregon. Palaeontographica Americana. 1994;58:1–205. [Google Scholar]
- Manen et al. (2010).Manen J-F, Barriera G, Loizeau P-A, Naciri Y. The history of extant Ilex species (Aquifoliaceae): evidence of hybridization within a Miocene radiation. Molecular Phylogenetics and Evolution. 2010;57:961–977. doi: 10.1016/j.ympev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Manos, Cannon & Oh (2008).Manos PS, Cannon CH, Oh S-H. Phylogenetic relationships and taxonomic status of the paleoendemic Fagaceae of Western North America: recognition of a new genus. Notholithocarpus Madroño. 2008;55:181–190. [Google Scholar]
- Manos, Zhou & Cannon (2001).Manos PS, Zhou ZK, Cannon CH. Systematics of Fagaceae: Phylogenetic tests of reproductive trait evolution. International Journal of Plant Sciences. 2001;162:1361–1379. doi: 10.1086/322949. [DOI] [Google Scholar]
- Masta et al. (2002).Masta SE, Sullivan B, Lamb T, Routman EJ. Phylogeography, species boundaries, and hybridization among toads of the Bufo americanus group. Molecular Phylogenetics and Evolution. 2002;24:302–314. doi: 10.1016/S1055-7903(02)00216-6. [DOI] [PubMed] [Google Scholar]
- McIntyre (1991).McIntyre DJ. Pollen and spore flora of an Eocene forest, eastern Axel Heiberg Island, N.W.T. In: Christie RL, McMillan NJ, editors. Tertiary fossil forest of the Geodetic Hills, Axel Heiberg Island, Arctic Archipelago. vol. 403. Ottawa, Ontario: Geological Survey of Canada; 1991. pp. 83–97. (Geological Survey of Canada, Bulletin). [Google Scholar]
- McIver & Basinger (1999).McIver EE, Basinger JF. Early tertiary floral evolution in the Canadian high arctic. Annals of the Missouri Botanical Garden. 1999;86:523–545. doi: 10.2307/2666184. [DOI] [Google Scholar]
- Menitsky (2005).Menitsky YL. Oaks of Asia. Enflied, New Hampshire: Science Publishers; 2005. [Google Scholar]
- Mir et al. (2009).Mir C, Jarne P, Sarda V, Bonin A, Lumaret R. Contrasting nuclear and cytoplasmic exchanges between phylogenetically distant oak species (Quercus suber L. and Q. ilex L.) in Southern France: inferring crosses and dynamics. Plant Biology. 2009;11:213–226. doi: 10.1111/j.1438-8677.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Moran, Willis & Clark (2012).Moran EV, Willis J, Clark JS. Genetic evidence for hybridization in red oaks (Quercus sect. Lobatae, Fagaceae) American Journal of Botany. 2012;99:92–100. doi: 10.3732/ajb.1100023. [DOI] [PubMed] [Google Scholar]
- Neophytou et al. (2011).Neophytou C, Aravanopoulos FA, Fink S, Dounavi A. Interfertile oaks in an island environment: II. Limited hybridization Quercus alnifolia Poech and Q. coccifera L. in a mixed stand. European Journal of Forest Research. 2011;130:623–635. doi: 10.1007/s10342-010-0454-4. [DOI] [Google Scholar]
- Neophytou et al. (2010).Neophytou C, Dounavi A, Fink S, Aravanopoulos FA. Interfertile oaks in an island environment: I. High nuclear genetic differentiation and high degree of chloroplast DNA sharing between Q. alnifolia and Q. coccifera in Cyprus. A multipopulation study. European Journal of Forest Research. 2010;130:543–555. doi: 10.1007/s10342-010-0442-8. [DOI] [Google Scholar]
- Nylander (2004).Nylander JAA. MrModeltest. 2.1. Uppsala: Evolutionary Biology Centre, Uppsala University; 2004. Program distributed by the author. [Google Scholar]
- Oh & Manos (2008).Oh S-H, Manos PS. Molecular phylogenetics and cupule evolution in Fagaceae as inferred from nuclear CRABS CLAW sequences. Taxon. 2008;57:434–451. [Google Scholar]
- Okaura et al. (2007).Okaura T, Quang ND, Ubukata M, Harada K. Phylogeographic structure and late Quaternary population history of the Japanese oak Quercus mongolica var. crispula and related species revealed by chloroplast DNA variation. Genes and Genetics Systems. 2007;82:465–477. doi: 10.1266/ggs.82.465. [DOI] [PubMed] [Google Scholar]
- Ortego & Bonal (2010).Ortego J, Bonal R. Natural hybridisation between kermes (Quercus coccifera L.) and holm oaks (Q. ilex L.) revealed by microsatellite markers. Plant Biology. 2010;12:234–238. doi: 10.1111/j.1438-8677.2009.00244.x. [DOI] [PubMed] [Google Scholar]
- Pearse & Hipp (2009).Pearse IS, Hipp AL. Phylogenetic and trait similarity to a native species predict herbivory on non-native oaks. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18097–18102. doi: 10.1073/pnas.0904867106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel, Finlayson & McMahon (2007).Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences. 2007;11:1633–1644. doi: 10.5194/hess-11-1633-2007. [DOI] [Google Scholar]
- Peñaloza-Ramírez et al. (2010).Peñaloza-Ramírez JM, González-Rodríguez A, Mendoza-Cuenca L, Caron H, Kremer A, Oyama K. Interspecific gene flow in a multispecies oak hybrid zone in the Sierra Tarahumara of Mexico. Annals of Botany. 2010;105:389–399. doi: 10.1093/aob/mcp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit et al. (2004).Petit RJ, Bodénès C, Ducousso A, Roussel G, Kremer A. Hybridization as a mechanism of invasion in oaks. New Phytologist. 2004;161:151–164. [Google Scholar]
- Petit et al. (2002).Petit RJ, Csaikl UM, Bordács S, Burg K, Coart E, Cottrell J, Van Dam B, Deans JD, Dumolin-Lapègue S, Fineschi S. Chloroplast DNA variation in European white oaks: phylogeography and patterns of diversity based on data from over 2600 populations. Forest Ecology and Management. 2002;156:5–26. doi: 10.1016/S0378-1127(01)00645-4. [DOI] [Google Scholar]
- Piredda et al. (2011).Piredda R, Simeone MC, Attimonelli M, Bellarosa R, Schirone B. Prospects of barcoding the Italian wild dendroflora: oaks reveal severe limitations to tracking species identity. Molecular Ecology Resources. 2011;11:72–83. doi: 10.1111/j.1755-0998.2010.02900.x. [DOI] [PubMed] [Google Scholar]
- Premoli et al. (2012).Premoli AC, Mathiasen P, Acosta MC, Ramos VA. Phylogeographically concordant chloroplast DNA divergence in sympatric Nothofagus s.s. How deep can it be? New Phytologist. 2012;193:261–275. doi: 10.1111/j.1469-8137.2011.03861.x. [DOI] [PubMed] [Google Scholar]
- Rambaut (2014).Rambaut A. FigTree, a graphical viewer of phylogenetic trees, v1.4.2. Institute of Evolutionary Biology University of Edinburgh; 2014. Available at http://tree.bio.ed.ac.uk/software/figtree/ [Google Scholar]
- Rieseberg & Soltis (1991).Rieseberg LH, Soltis DE. Phylogenetic consequences of cytoplasmic gene flow in plants. Evolutionary Trends in Plants. 1991;5:65–84. [Google Scholar]
- Ronquist & Huelsenbeck (2003).Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Klopfstein S, Vilhelmsen L, Schulmeister S, Murray DL, Rasnitsyn AP. A total-evidence approach to dating with fossils, applied to the early radiation of the hymenoptera. Systematics Biology. 2012;61:973–999. doi: 10.1093/sysbio/sys058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler et al. (2004).Schnitzler JP, Steinbrecher R, Zimmer I, Steigner D, Fladung M. Hybridization of European oaks (Quercus ilex × Q. robur) results in a mixed isoprenoid emitter type. Plant Cell and Environment. 2004;27:585–593. doi: 10.1111/j.1365-3040.2003.01169.x. [DOI] [Google Scholar]
- Shaw et al. (2005).Shaw J, Lickey EB, Beck JT, Farmer SB, Liu W, Miller J, Siripun KC, Winder CT, Schilling EE, Small RL. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany. 2005;92:142–166. doi: 10.3732/ajb.92.1.142. [DOI] [PubMed] [Google Scholar]
- Simeone et al. (2013).Simeone MC, Piredda R, Papini A, Vessella F, Schirone B. Application of plastid and nuclear markers to DNA barcoding of Euro–Mediterranean oaks (Quercus, Fagaceae): problems, prospects and phylogenetic implications. Botanical Journal of the Linnean Society. 2013;172:478–499. doi: 10.1111/boj.12059. [DOI] [Google Scholar]
- Städler & Delph (2002).Städler T, Delph LF. Ancient mitochondrial haplotypes and evidence for intragenic recombination in a gynodioecious plant. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11730–11735. doi: 10.1073/pnas.182267799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis, Hoover & Rougemont (2008).Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Systematics Biology. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Stuchlik, Ziembínska-Tworzydło & Kohlman-Adamska (2007).Stuchlik L, Ziembínska-Tworzydło M, Kohlman-Adamska A. Botanical affinity of some Neogene sporomorphs and nomenclatural problems. Acta Palaeobotany. 2007;47:291–311. [Google Scholar]
- Svenson et al. (2001).Svenson U, Backlund A, McLoughlin S, Hill RS. Nothofagus biogeography revisited with special emphasis on the enigmatic distribution of subgenus Brassospora in New Caledonia. Cladistics. 2001;17:28–47. doi: 10.1111/j.1096-0031.2001.tb00109.x. [DOI] [Google Scholar]
- Swofford (2002).Swofford DL. PAUP*: Phylogenetic analysis using parsimony (and other methods) 4.0 Beta. Sunderland: Sinauer Associates; 2002. [Google Scholar]
- Tamura et al. (2011).Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, Higgins & Gibson (1994).Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui et al. (2009).Tsutsui K, Suwa A, Sawada K, Kato T, Ohsawa TA, Watano Y. Incongruence among mitochondrial, chloroplast and nuclear gene trees in Pinus subgenus Strobus (Pinaceae) Journal of Plant Research. 2009;122:509–521. doi: 10.1007/s10265-009-0246-4. [DOI] [PubMed] [Google Scholar]
- Valbuena-Carabaña et al. (2007).Valbuena-Carabaña M, González-Martínez SC, Hardy OJ, Gil L. Fine-scale spatial genetic structure in mixed oak stands with different levels of hybridization. Molecular Ecology. 2007;16:1207–1219. doi: 10.1111/j.1365-294X.2007.03231.x. [DOI] [PubMed] [Google Scholar]
- Valencia-Cuevas et al. (2015).Valencia-Cuevas L, Mussali-Galante P, Piñero D, Castillo-Mendoza E, Rangel-Altamirano G, Tovar-Sánchez E. Hybridization of Quercus castanea (Fagaceae) across a red oak species gradient in Mexico. Plant Systematics and Evolution. 2015;301:1085–1097. doi: 10.1007/s00606-014-1151-4. [DOI] [Google Scholar]
- Velitzelos, Bouchal & Denk (2014).Velitzelos D, Bouchal JM, Denk T. Review of the Cenozoic floras and vegetation of Greece. Review of Palaeobotany and Palynology. 2014;204:56–117. doi: 10.1016/j.revpalbo.2014.02.006. [DOI] [Google Scholar]
- Weitschat & Wichard (2003).Weitschat W, Wichard W. Atlas of Plants and Animals in Baltic Amber. Munchen: Pfeil Verlag; 2003. [Google Scholar]
- Whittemore & Schaal (1991).Whittemore AT, Schaal BA. Interspecific gene flow in sympatric oaks. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2540–2544. doi: 10.1073/pnas.88.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens & Servedio (2000).Wiens JJ, Servedio MR. Species delimitation in systematics: inferring diagnostic differences between species. Proceedings of the Royal Society of London Series B. 2000;267:631–36. doi: 10.1098/rspb.2000.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang et al. (2005).Xiang Q-Y, Manchester SR, Thomas DT, Zhang W, Fan C. Phylogeny, biogeography, and molecular dating of Cornelian cherries (Cornus, Cornaceae): Tracking Tertiary plant migration. Evolution. 2005;59:1685–1700. doi: 10.1111/j.0014-3820.2005.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Xiang et al. (2014).Xiang X-G, Wang W, Li R-Q, Lin L, Liu Y, Zhou Z-K, Li Z-Y, Chen Z-D. Large-scale phylogenetic analyses reveal fagalean diversification promoted by the interplay of diaspores and environments in the Paleogene. Perspectives in Plant Ecology, Evolution and Systematics. 2014;16:101–110. doi: 10.1016/j.ppees.2014.03.001. [DOI] [Google Scholar]
- Yi, Miller & Wen (2004).Yi T, Miller AJ, Wen J. Phylogenetic and biogeographic diversification of Rhus (Anacardiaceae) in the Northern Hemisphere. Molecular Phylogenetics and Evolution. 2004;33:861–879. doi: 10.1016/j.ympev.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Yoo & Wen (2007).Yoo K-O, Wen J. Phylogeny of Carpinus and subfamily Coryloideae (Betulaceae) based on chloroplast and nuclear ribosomal sequence data. Plant Systematics and Evolution. 2007;267:25–35. doi: 10.1007/s00606-007-0533-2. [DOI] [Google Scholar]
- Zhang et al. (2013a).Zhang J-B, Li R-Q, Xiang X-G, Manchester SR, Lin L, Wang W, Wen J, Chen Z-D. Integrated fossil and molecular data reveal the biogeographic diversification of the eastern Asian-eastern North American disjunct hickory genus (Carya Nutt.) Plos One. 2013a;8:e1897. doi: 10.1371/journal.pone.0070449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2013b).Zhang ZY, Wu R, Wang Q, Zhang ZR, Lopez-Pujol J, Fan DM, Li DZ. Comparative phylogeography of two sympatric beeches in subtropical China: species-specific geographic mosaic of lineages. Ecology and Evolution. 2013b;3:4461–4472. doi: 10.1002/ece3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou (1992).Zhou Z. The fossil history of Quercus. Acta Botanica Yunnanica. 1992;15:21–33. [Google Scholar]
- Zwickl (2006).Zwickl DJ. PhD thesis. 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the Maximum Likelihood criterion. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intra- and intertaxonomic minimum and maximum pairwise genetic distances in Fagales based on the used plastid markers.
haplotype networks of the investigated dataset based on rbcL and trnK-matK markers.
Data Availability Statement
The following information was supplied regarding data availability:
Primary data and analyses are provided for download in the Online Supplementary Archive (OSA) at the journal’s homepage and at www.palaeogrimm.org/data/Smn15_OSA.zip. See GuideToFile.txt included in the OSA for further information. Other relevant data are within the paper and Supplemental Information.