Abstract
Despite the prevalence of migraine, the pathophysiology of the disease remains unclear. Current understanding of migraine has alluded to the possibility of a hyperexcitable brain. The aim of the current study is to investigate human brain metabolite differences in the anterior cingulate cortex (ACC) during the interictal phase in migraine patients. We hypothesized that there may be differences in levels of excitatory neurotransmitters and/or their derivatives in the migraine cohort in support of the theory of hyperexcitability in migraine. 2D J-resolved proton magnetic resonance spectroscopy (1H-MRS) data were acquired on a 3 Tesla (3 T) MRI from a voxel placed over the ACC of 32 migraine patients (MP; 23 females, 9 males, age 33 ± 9.6 years) and 33 healthy controls (HC; 25 females, 8 males, age 32 ± 9.6 years). Amplitude correlation matrices were constructed for each subject to evaluate metabolite discriminability. ProFit-estimated metabolite peak areas were normalized to a water reference signal to assess subject differences. The initial analysis of variance (ANOVA) was performed to test for group differences for all metabolites/creatine (Cre) ratios between healthy controls and migraineurs but showed no statistically significant differences. In addition, we used a multivariate approach to distinguish migraineurs from healthy subjects based on the metabolite/Cre ratio. A quadratic discriminant analysis (QDA) model was used to identify 3 metabolite ratios sufficient to minimize minimum classification error (MCE). The 3 selected metabolite ratios were aspartate (Asp)/Cre, N-acetyl aspartate (NAA)/Cre, and glutamine (Gln)/Cre. These findings are in support of a ‘complex’ of metabolite alterations, which may underlie changes in neuronal chemistry in the migraine brain. Furthermore, the parallel changes in the three-metabolite ‘complex’ may confer more subtle but biological processes that are ongoing. The data also support the current theory that the migraine brain is hyperexcitable even in the interictal state.
Keywords: Magnetic resonance spectroscopy (MRS), Excitatory neurotransmitters, Interictal migraine, Anterior cingulate cortex (ACC), 2D J-resolved, Central sensitization
Highlights
-
•
3 T MRI was used to acquire 2D J-resolved proton magnetic resonance spectroscopy.
-
•
Metabolite alterations are reported in the anterior cingulate cortex of episodic migraineurs.
-
•
The complex of metabolites may reflect multiple chemical changes in migraineurs.
-
•
The observed chemical changes support the theory that the brain of migraineurs is hyperexcitable.
1. Introduction
Although the cause of migraine attacks remains unknown, a number of studies have indicated that migraine alters brain structure and function, including behavioral studies (Borsook et al., 2014, Brigo et al., 2013, Hoffken et al., 2009) and neuroimaging studies (Sprenger and Borsook, 2012). An increasing number of studies have evaluated measures of brain chemistry in migraine patients (see below). Altered chemistry in migraine may provide a basis for the purported cortical hyperexcitability (Coppola et al., 2007, Demarquay et al., 2013), and could be a target for evaluating treatment efficacy in responders and non-responders, as has been reported in other chronic pain conditions such as fibromyalgia (Harris et al., 2013), or evaluation of the effects of treatments (e.g., topiramate) on brain metabolites (Moore and Wardrop, 2006).
MRS studies of migraine have recently been reviewed elsewhere (Reyngoudt et al., 2012). These studies have consistently suggested altered energy metabolism (Reyngoudt et al., 2011) in migraine patients but few studies have reported alterations in glutamate (Glu) or gamma aminobutyric acid (GABA) in migraine patients, the latter two components providing a potential basis for hyperexcitability (either increased Glu or decreased GABA or some combination that contributes to potential neuronal hyperexcitability). A recent paper reported significantly increased GABA in migraine using a Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) sequence (Aguila et al., 2015) and higher glutamine (Gln)/glycine (Gly) ratios in women than in men during their interictal phase of migraine (Gonzalez de la Aleja et al., 2013), offering some support for the hyperexcitability thesis. The hyperexcitability thesis relates to the sensitization that results from cortical spreading depression (CSD). CSD has been implicated in the pathogenesis of migraine but is still not fully understood. Studies have indicated that the wave of activation and subsequent inhibition caused by CSD results in the sensitization of the trigeminal vascular system and its central projections (Cutrer and Charles, 2008). Others have suggested that hyperexcitability of the trigeminovascular neurons may stem from altered descending modulation of the brain stem which may cause a loss of inhibition or enhanced facilitation (Moulton et al., 2008). MRS studies have found evidence of this with increases in both excitatory (Gonzalez de la Aleja et al,. 2013) and inhibitory (Aguila et al., 2015) neurotransmitters suggesting a potentially more complex changes in brain neurochemistry that contributes to a hyperexcitable state.
Here we have used an approach to optimize the measurements of both GABA and Glu with a 2D J-resolved approach, focusing on the anterior cingulate cortex (ACC). We have chosen the ACC because of its putative role in multiple processing in migraine including involvement in salience (Borsook et al., 2013), descending modulation (Mainero et al., 2011), and altered resting state connectivity (Russo et al., 2012, Tessitore et al., 2015, Xue et al., 2013). Conventional proton MRS (1H-MRS) is often hampered by low spectral resolution and metabolite peak overlap, especially to measure GABA and Gln/Glu concentrations. Reliable detection and quantization of metabolite concentrations is thus problematic. Two-dimensional (2D) 1H-MRS methodologies effectively increase spectral resolution by spreading metabolite resonances over a 2D surface and accounting for J-coupling resonances. We hypothesized that we would be able to determine measures of GABA and/or Glu in migraine and healthy subjects and that differences between two populations can be differentiated based on the chemical signature as has been done in other pain states (Foerster et al., 2012, Niddam et al., 2011) as well as acute pain (Cleve et al., 2015). We have previously shown that metabolites can differentiate healthy subjects from migraine patients using a linear discriminate analysis (LDA) approach (Prescot et al., 2009). Here in a larger patient population along with a QDA analysis we hope to gain a more thorough understanding of how metabolite concentrations (specifically GABA and Glu) differ and if the differences are in support of the current theory of a hyperexcitable migraine brain (Welch et al., 1990).
2. Methods
2.1. Subject selection
The local Institutional Review Board (IRB) of Partners Health Care approved the present study protocol, which met the requirements for investigations in human subjects. A total of 33 healthy control (HC; 25 females, 8 males, mean age = 32 ± 9.6 years) subjects and 32 migraine patients (MP; 23 females, 9 males, mean age = 33 + 9.6 years) were included in this study. All subjects were between the ages of 18–50, right-handed, non-smokers, and had no significant history of other chronic pain, psychiatric, neurological or any other major disorders. At the beginning of each study visit, subjects were screened for depression (Beck Depression Inventory II (Beck et al., 1996), exclusionary score > 25 [moderate to severe depression]) as well as a urine test for barbiturates, benzodiazepines, amphetamine, cocaine, tetrahydrocannabinol, phencyclidine and opioids (excluding prescription pain medications). Healthy controls were also excluded if they were on any prescription medications or had any history of migraine. All migraine subjects fit the criteria for episodic migraine in accordance with the International Classification for Headache II and confirmed by a neurologist. The migraine subjects also had to have had a history of migraine for at least 3 years and were excluded if they were on any daily preventative medications for their migraine. In order to confirm the migraine subjects were in the interictal phase, we asked if they had an attack within 24 h leading to their study visit and then followed up with them 72 h after the study visit. If subjects experienced an attack within that window of time, they were disqualified.
2.2. 2D MRS data acquisition
All MR imaging and 2D J-resolved 1H MRS measurements were performed using a 3 Tesla Siemens (Erlangen, Germany) TIM Trio™ whole-body MRI system housed at McLean Hospital (Belmont, MA). Radiofrequency (RF) excitation and signal reception was achieved using a manufacturer-supplied circularly polarized body RF coil and a 12-channel phased array receive-only RF coil (operated in the four-cluster mode, 3 elements per cluster), respectively. Subjects were positioned supine and foam pads were utilized to fixate the head within the RF receive coil. Following the acquisition of low-resolution localizer MR images, high-resolution 3D magnetization-prepared rapid gradient echo (MP-RAGE; TR/TE/TI = 2000/3.53/1100 ms; FOV = 256 × 256 × 224 mm; isotropic 1 mm in-plane resolution) MRI data then were acquired to facilitate accurate MRS voxel positioning and for post hoc within-voxel tissue-type segmentation. All spectroscopy data were acquired from a voxel (30 × 22 × 25 mm3) positioned within the anterior cingulate cortex (ACC), which was obliqued along the sagittal plane and positioned to cover predominantly gray matter. B0 shimming was performed over the MRS voxel using the manufacturer-supplied phase map method as well as additional interactive manual shimming until a full-width at half-maximum (FWHM) of ≤ 12 Hz was observed for the real component of the unsuppressed water signal line width. 2D J-resolved 1H MRS data were recorded using a modified PRESS sequence as described in an earlier report (Prescot and Renshaw, 2013). Briefly, 2D MRS acquisition parameters were as follows: TR/TE = 2000/31–229 ms; ΔTE = 2 ms; 4 signal averages per TE step; 2D spectral width = 2000 × 500 Hz; 2D matrix size = 2048 × 100 Hz; maximum echo sampling based on the echo center of the first echo (i.e. TE = 31 ms); total acquisition time = 28 min 18 s. Outer-volume suppression and solvent water suppression were achieved using manufacturer-supplied techniques as described elsewhere (Prescot and Renshaw, 2013). Water unsuppressed 2D 1H MRS data also were recorded from the ACC voxel using 2 signal averages per TE step. The RF transmitter frequency was set relative to total ACC (3.0 ppm) and tissue water (4.7 ppm) resonances for the water suppressed and unsuppressed measurements, respectively.
2.3. Tissue segmentation
The BET (Smith, 2002) and FAST (Zhang et al., 2001) tools provided with the freely-available FMRIB Software Library (FSL) (Smith et al., 2004) were used to perform skull stripping and whole brain tissue segmentation, respectively. For each subject dataset, home-written MATLAB (version 2010b, The MathWorks, Natick, MA) functions then were used to extract the obliqued ACC MRS voxel to obtain within-voxel gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) tissue content for each subject. The GM percentage was calculated as the ration to total brain matter (i.e. 100 × GM / [WM + GM]).
2.4. 2D MRS data processing
2D 1H MRS data were stored as Siemens TWIX files and transferred to a personal computer system for pre-processing using MATLAB scripts. Recombination of the four receiver channels was performed using a scaled square-root of sum-of-squares conjugate reconstruction method for both water suppressed and unsuppressed data. For the metabolite water suppressed data, eddy current distortions initially were accounted for using an established time-domain method,(Klose, 1990) where each TE step from a given 2D 1H MRS dataset was corrected using the corresponding water unsuppressed data recorded at the same TE. Following coil cluster combination and eddy current correction, the residual water signal was removed on a TE-by-TE basis using a Hankel singular value decomposition (HSVD) routine. Finally, the corrected and water filtered metabolite data were reconstructed to yield a 2D matrix characterized by 100 TE steps and 2048 complex points, and reformatted to produce individual file types recorded for analysis by the ProFit software. The ProFit algorithm was applied identically to all ACC 2D 1H MRS data as detailed in previous reports, (Prescot and Renshaw, 2013, Schulte and Boesiger, 2006) where following zero-filling of the 2D matrix to 200 points along the indirectly detected dimension, 2D fast Fourier Transformation, and phase correction and frequency realignment, the software fitted the a basis set of nineteen metabolites in fully automated fashion. The basis set consisted of: total creatine (Cre), N-acetyl aspartate (NAA), glycerophosphorylcholine (GPC), phosphorylcholine (PCh), alanine (Ala), aspartate (Asp), γ-amino butyric acid (GABA), glucose (Glc), Gln, Glu, glycine (Gly), glutathione (GSH), lactate (Lac), myoinositol (Ins), N-acetyl aspartylglutamate (NAAG), phosphoethanolamine (PE), taurine (Tau), scyllo-inositol (sI), and ascorbic acid (Asc). The Cre methylene (CH2) and methyl (CH3) protons were fitted separately whereas the separate GPC and PCh peaks ultimately were considered as a composite resonance (Cho). The Cramer-Rao lower bound (CRLB) values reported by ProFit were used for assessing final metabolite fit precision. Metabolites with CRLB outputs of < 20% are considered sufficient. Any CRLB values > 20% were excluded from the analysis.
2.5. 2D MRS data statistical analysis
Analysis of variance (ANOVA) initially was performed using OriginLab (OriginLab Corporation, Northampton, MA) to test for group differences for all metabolite/Cre ratios between healthy controls and migraineurs. In addition, we used a multivariate approach to classify migraineurs from healthy subjects based on the ratio of all 19 metabolites expressed as the ratio to Cre (features). A quadratic discriminant analysis (QDA) model was used in a forward feature selection wrapper method (Kohavi and John, 1997) as implemented in MATLAB, with the dataset (33 healthy controls, 32 migraineurs) being randomly divided into training (N = 49) and testing (N = 16) groups. The feature selection procedure uses the minimum classification error (MCE) of the learning algorithm QDA on each feature subset as the performance indicator for each subset. The training set is used to select the features and fit the QDA model while the test set is used to evaluate the performance of the selected feature. Each candidate feature subset is evaluated and compared with the application of a stratified 10-fold cross-validation of the training set. These steps were implemented in MATLAB using the functions ‘cvpartition’, ‘sequentialfs’, and ‘crossval’, which are all provided with the Statistics Toolbox™.
3. Results
3.1. Subjects
Clinical characteristics (including medication usage) for individual migraine subjects are given in Table 1 and all migraine subjects were matched with age-gender to controls.
Table 1.
Clinical characteristics (including medication usage) for individual migraine subjects.
| Subject ID | Age | Gender | Disease duration years |
Avg. migraine days/month | Visual aura | Medications for migraine |
|---|---|---|---|---|---|---|
| M01 | 34 | M | 20 | 12 | No | Excedrin migraine |
| M02 | 32 | M | 15 | 2 | No | Tylenol, ibuprofen |
| M03 | 49 | F | 39 | 13 | Yes | Imitrex |
| M04 | 36 | F | 20 | 12 | No | Aspirin, tylenol, ibuprofen |
| M05 | 34 | F | 14 | 14 | Yes | Excedrin |
| M06 | 24 | F | 8 | 6 | Yes | Tylenol, aleve, rizatriptan |
| M07 | 22 | F | 7 | 10 | No | Tylenol, ibuprofen |
| M08 | 25 | F | 7 | 10 | Yes | Ibuprofen |
| M09 | 22 | F | 3 | 2 | Yes | + Acetaminophen |
| M10 | 32 | F | 21 | 2 | Yes | Ibuprofen |
| M11 | 48 | F | 23 | 2 | No | None |
| M12 | 31 | F | 3 | 13 | No | Aspirin, ibuprofen |
| M13 | 37 | M | 33 | 2 | No | None |
| M14 | 24 | F | 4 | 1 | No | Tylenol |
| M15 | 26 | M | 3 | 10 | No | None |
| M16 | 30 | F | 9 | 4 | No | Naratriptan |
| M17 | 50 | F | 30 | 3 | Yes | Sumatriptan, ibuprofen |
| M18 | 50 | F | 15 | 4 | Yes | Imitrex, ibuprofen |
| M19 | 38 | F | 27 | 12 | Yes | Sumatriptan, ibuprofen, aspirin |
| M20 | 38 | F | 12 | 2 | No | Imitrex, naproxen |
| M21 | 32 | M | 26 | 7 | Yes | Tylenol |
| M22 | 32 | F | 14 | 6 | No | None |
| M23 | 41 | F | 23 | 3 | No | Ibuprofen |
| M24 | 46 | M | 6 | 6 | Yes | Ibuprofen, tylenol |
| M25 | 19 | F | 5 | 1 | Yes | None |
| M26 | 18 | M | 10 | 6 | No | None |
| M27 | 21 | M | 15 | 3 | No | Ibuprofen |
| M28 | 45 | F | 30 | 4 | Yes | Ibuprofen, excedrin |
| M29 | 29 | M | 12 | 3 | No | None |
| M30 | 23 | F | 10 | 5 | No | Acetaminophen |
| M31 | 22 | F | 3 | 14 | Yes | None |
| M32 | 39 | F | 6 | 6 | Yes | Excedrin |
3.2. MRI/MRS
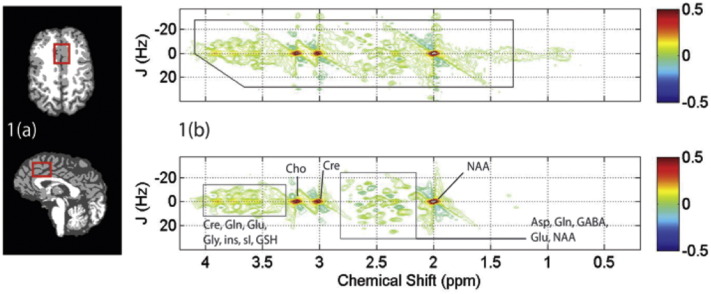
Fig. 1(a) shows tissue segmented axial and sagittal T1-weighted MRI data obtained from a single MP subject, displaying the typical ACC MRS voxel location used for the present study. Group analysis for within-voxel tissue composition revealed no significant differences between GM expressed as the percentage of total brain matter (HC 62 ± 4%; MP 60 ± 8%, p = 0.1), or CSF (HC 13 ± 6%; MP 13 ± 5%, p = 0.6). A representative 2D J-resolved 1H MRS dataset recorded from the same subject is presented in Fig. 1(b), displaying the raw 2D spectral data and its estimated spectral fit as determined using ProFit (see Figure legend for more details). Statistical group mean analysis failed to reveal significant differences for the Cre-normalized metabolite levels.
Fig. 1.
(a) Skull stripped T1-weighted MRI data recorded from a single MP subject demonstrating typical ACC MRS voxel positioning (red box) on axial (top panel) and sagittal (bottom panel) image slices. (b) 2D J-resolved 1H MRS data recorded from the MRS voxel shown in (a). The raw 2D MRS data (real component) is shown in the top panel with the black box depicting the region used for spectral fitting. The resulting 2D fit as calculated using ProFit is shown in the bottom panel and tentative signal assignments are provided. The color bars to the right show contouring amplitudes and signal phase. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Statistical analysis
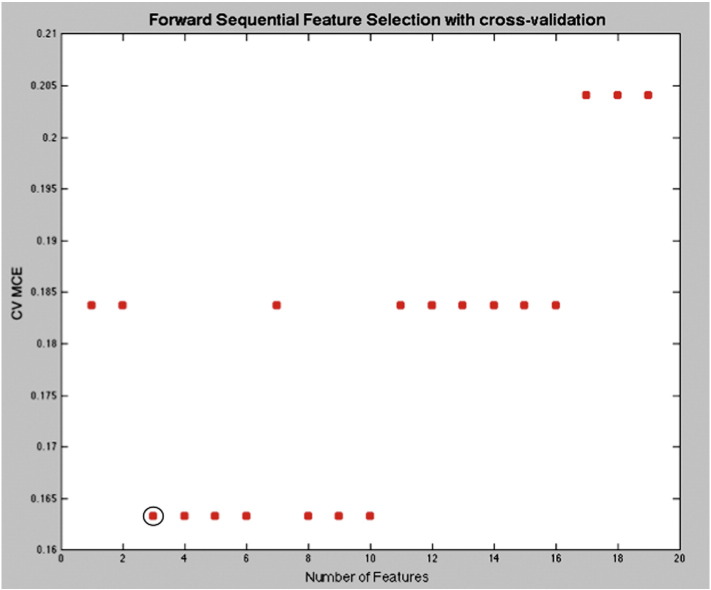
Standard statistical analysis using ANOVA yielded no significant metabolite differences between the migraine and the healthy control groups. The results of feature extraction with cross-validation, however, indicated that 3 metabolite ratios were sufficient to minimize MCE. The resulting MCE was 0.0625, which corresponds to 1 subject in the test group and the 3 selected metabolite ratios were Asp/Cre, NAA/Cre, and Gln/Cre. Fig. 2 displays where the classification error is first minimized by increasing the number of features used for a cross-validation test of the discrimination. For these metabolites, the mean CRLB values were 13.8 ± 8.2% (HC) and 11.5 ± 4.3% (MP) for Asp, 0.8 ± 0.4% (HC) and 0.7 ± 0.3% (MP) for NAA, and 10.7 ± 3.6% (HC) and 9.4 ± 3.2% (MP) for Gln. No significant group differences existed for any of the metabolite CRLB values. Neither the raw Cre amplitude (HC 6.26 × 10− 5 ± 6.13 × 10− 6; MP 6.31 × 10− 5 ± 7.33 × 10− 6; p = 0.7) nor the Cre CRLB values (HC 0.84 ± 0.36%; MP 0.74 ± 0.17%; p = 0.14) were found to significantly differ between the two groups. In addition, no significant symptom correlations were found after running a correlation coefficient analysis across multiple co-factors (age, sex, frequency, and aura).
Fig. 2.
Number of features selection: The graph displays the cross-validation minimum classification error (CV MCE) as the number of selected features is increased. The CV MCE acquires the first true minimum with 3 selected features.
4. Discussion
We report results on interictal measures of metabolites, reproduced and expanded from our intial findings previously reported (Prescot et al., 2009) albeit using a different statistical technique and with a larger cohort. Our findings indicated that three metabolite ratios were sufficient to minimize the minimum classification error (MCE) which is a measure of misclassification of erroneous group allocation. The approach used thus differentiated the patient group from the healthy controls at both a group and at a matched level. The analysis allows for the determination of discrimination of two or more groups (e.g., migraine vs. healthy) based on CRE-normalized levels of specific metabolites. The major finding relates to Gln (involved in both Glu and GABA metabolism) and the excitatory amino acid neurotransmitter Asp (see below). Changes in NAA may relate to a measure of ongoing physiological processes including neuronal osmoregulation and myelination (see below). The metabolites Asp, NAA, and Gln as a group differentiate patients from controls but is not possible to state (with statistical significance) that they are either elevated or reduced compared to patients. We can state that the average of these metabolites in patients were smaller than in controls but did not achieve statistical significance. In addition, we report the metabolite ratios as a ‘complex’ in an effort to not only understand what changes have occurred but also to attempt an explanation of why, based on metabolite interaction.
4.1. The cingulate cortex, chemistry and migraine
As noted in the introduction we chose the ACC as a target to measure metabolic changes. We, and others, have observed functional and morphometric changes in the cingulate in episodic migraine (Maleki et al., 2012). As noted previously, the cingulate is involved in numerous processes in migraine behaviors (see Devinsky for a review (Devinsky et al., 1995)) such as interoception (Critchley et al., 2004), executive function (Carter et al., 2000), salience (Goldstein et al., 2009), pain modulation (Zhang et al., 2005), amongst others. Studies have noted changes in many of these functions in migraineurs (Mainero et al., 2011, Niddam et al., 2015, Tessitore et al., 2015). As Shyu and Vogt have noted in their evaluation of cingulate sub-territory or region functions (Vogt, 2005), these differences may relate to “modifications of cingulate synapses (from repeated migraine attacks) appear to regulate afferent signals that may be important to the transition from acute to chronic pain conditions associated with persistent peripheral noxious stimulation” (Shyu and Vogt, 2009).
Receptor architecture of the ACC has been reviewed (Palomero-Gallagher et al., 2009). Furthermore in vivo receptor binding studies in humans, using positron emission tomography, have reported a number of receptors in the region including dopamine D2 or D1 (Olver et al., 2010, Suhara et al., 2002), GABA and benzodiazepine (Oblak et al., 2009) as well as Glu receptors (Zavitsanou et al., 2002). Evaluation of metabolic changes, that include molecules that may act on some of these receptors (e.g., GABA or Glu) in the cingulate have been reported across a number of disease states, including generalized anxiety disorders (Strawn et al., 2013), obsessive compulsive disorders (Brennan et al., 2015), autism (Baruth et al., 2013), alcoholism (Cohen-Gilbert et al., 2015) and healthy subjects (Kuhn et al., 2015).
4.2. 2D resolved measures define metabolites that allow for segregation of patient vs. controls
In our prior report we found that in interictal migraineurs there may be alterations in excitatory amino acids in the cingulate cortex of migraineurs compared with healthy controls (Prescot et al., 2009). This was a small study of 10 patients and 10 controls. In this study 33 patients and 32 healthy matched controls were evaluated. The results reported here show that there were 3 metabolite ratio's - NAA/Cre, Asp/Cre and Gln/Cre - that could provide a segregation of the disease vs. heathy state:
NAA is widely distributed in the CNS in both neurons and glia (Baslow, 2003). It is syntehsized from Asp and acetyl-coenzyme A in neurons (Moffett et al., 2007). It serves in a number of putative roles including being a potential marker for neuronal health as measured using MRS techniques (Luyten and den Hollander, 1986). It may play a role in CNS metabolism, nitrogen balance, neuronal osmoregulation and axon-glial signaling (Moffett et al., 2007). In the healthy brain, NAA is one of the largest peaks in the acquired MRS spectrum. The ratio between NAA versus total creatine (NAA/Cre), have been found to be clinically useful, since the latter usually remains constant (Miller, 1991). Most clinical studies using MRS have reported decreases in NAA levels (e.g., ischemia (Berthet et al., 2014), stroke (Igarashi et al., 2015), multiple sclerosis (Aboul-Enein et al., 2010), traumatic brain injury (Moffett et al., 2013). Impairments of energy metabolism decrease NAA levels in the brain. Given NAA's main role in energy metabolism and a source of acetate for fatty acid and steroid synthesis in oligodendrocytes (Moffett et al., 2007), what could its putative role in migraine be? The migraine brain has been proposed to be (1) hyperexcitable or have alterations in migraine energetics due to potential mitochondrial dysfunction. Consistent with these findings, 1H-MRS studies have evaluated metabolite ratios in cluster headache in the hypothalamus and show that the NAA/Cre ratio is lower in patients with cluster headache vs. chronic migraine or controls (Wang et al., 2006). As in the case of other conditions, the decrease in NAA may correlate with progressive changes in migraine, worsened with increasing frequency (Mohamed et al., 2013) or chronicity (Lai et al., 2015). As reported for TBI, for example, decreased NAA may be associated with an impairment of acetyl coenzyme A dependent functions (viz., energy derivation, lipid synthesis, and protein acetylation) (Vagnozzi et al., 2007).
Gln is a major metabolite of Glu, a principle excitatory neurotransmitter in the brain (Albrecht et al., 2010). Gln on the other hand is synthesized exclusively in glial cells from Glu and ammonia by the enzyme glutamine synthetase as a means for the brain to protect itself against excitotoxicity from excess Glu and ammonia (Suarez et al., 2002). Subsequently, Gln is released back into the extracellular space, shuttled back into neurons and converted to Glu by glutaminase. The Glu that is regenerated may then go on to play a direct role in excitatory neurotransmission (Prescot et al., 2009). Cerebrospinal fluid (CSF) Gln, Gly and taurine (Tau) concentrations are elevated in migraineurs (Rothrock et al., 1995) suggesting glurgic systems are likely to be altered in the migraine brain. It has been postulated that increased brain Glu leads to cortical hyperexcitability typical of migraine (Goadsby, 2005).
Asp is a major excitatory amino acid in the nervous system. It acts on n-methyl-d-asparatate (NMDA) receptors. Measures of Asp are not normally reported in MRS studies. Our method has allowed for a more specific determination of metabolites rendering signals from metabolites that could not previously be parsed out such as Asp. The amino acid is also part of a metabolic pathway involved in making NAA (Bates et al., 1996).
The ‘complex’ of metabolites is described as such to not only understand individual changes in metabolite ratios but to also discuss the interactions since our analysis detected a significant difference as a group of metabolites. All three of the above mentioned metabolites have been suggested as eventual precursers of glutimate (Albrecht et al., 2010, Clark et al., 2006).
4.3. Consistent results across studies
While alterations in metabolites were reported in our prior study in a small sample of migraine patients, the method used here is more sensitive: Gln and NAAG (a molecule of NAA and covalently bonded Glu) were the linear discriminants. Here we observed that Gln is again noted as a differentiator and NAA (previously NAAG), and Asp, all related to each other metabolically. Thus, although a slightly different method was employed, we find somewhat consistent results. The confirmation of Gln is of particular interest since in this static evaluation of metabolites, the changes may represent abnormalities in a pathway related to both Glu and GABA synthesis.
4.4. Lack of GABA and glutamate changes
Although we had surmised that we should be able to evalute Glu and GABA, such changes have been hard to define in migraine (Bridge et al., 2015) and other chronic pain conditions (Foerster et al., 2015). Evaluation of changes during a migraine attack (ictally) compared with interictal timepoints may be one issue. During a migraine, while the release of excitatory amino acids including Glu and Asp may be ongoing, as noted above, the changes are at micro-molar levels in the synaptic clefts. Such changes cannot be measured using MRS. However, what we observed were changes in Gln – the storage system of Glu. Interictally the alterations in this metabolite, synthesized in glial cells, may represent a major abnormality in migraine. For example, there may be a malfunction with Gln transport into neurons. The method however, does not evaluate dynamic changes in Glu and indeed could not measure micro-molar levels in the synaptic clefts. More novel methods such as 13C spectroscopy are needed (Gruetter et al., 2003, Yen et al., 2011). 13C spectroscopy is a method using an infusion of 13C labelled glucose that then gets incorporated into cell cycle (i.e., CREB). This method may allow for understanding how metabolic forces may be skewed in conditions such as migraine by observing alterations in Gln shuffling (de Graaf et al., 2003).
Unlike Glu and Glx, GABA does not have a major role in metabolism, and as such, 1H-MRS-derived GABA levels may be more indicative of neural activity (Muthukumaraswamy et al., 2009). If the brain were functioning normally, then an increase in Glu (or Glu precursors) would indicate an increase in GABA (Eichler and Meier 2008). Our results suggest the possibility of excess excitation from excess Glu but no excess inhibition by excess GABA. The Glu to GABA balance is imperative for normal neuronal activity and without it may ultimately lead to a hyperexcitable brain.
5. Caveats
5.1. Sensitivity
The method reported here allows for an improved spectral analysis. However, there are issues with spectroscopy including acquisition (e.g., movement, shimming standards) or voxel location in the brain. In this sample there was < 1 mm movement and the voxel placement was always performed in a routine manner by the same individual. Variances of concentration measures may also be present (van de Bank et al., 2015).
5.2. Metabolite-to-creatine ratios
Although metabolite ratios are commonly used to report changes (Lirng et al., 2015), determining absolute concentrations is also becoming a usual approach. Both methods have advantages and disadvantages including factors affecting water quantification (Hernandez-Tamames et al., 2016) or scanning parameters and models (Yamamoto et al., 2015). Furthermore, pathological changes may affect metabolic ratios (Cheong et al., 2006). Potential utilization of absolute quantification in the clinical setting might require additional resources and expertise not easily available and metabolite ratios might be a solution to assess changes in brain chemistry.
5.3. Patient phenotype
In this cohort patients were recruited primarily for the frequency of migraine. Thus we have a group of patients with high and low frequency attacks (i.e., episodic migraine). Some patients also had auras. Our analysis did not show any differences when these and other variables (including sex or age) were evaluated.
5.4. Medications
As noted in our patient inlcusion criteria (see Methods), patients recruited were taking only either NSAIDS, triptans or both. Drugs including NSAIDS may alter chemisty directly and thus may confound or contribute to the observed changes.
6. Conclusion
Our results indicate changes in certain metabolites in migraineurs compared to controls. Our results also replicate and further validate a previous, smaller study (Prescot et al., 2009). They also extend metabolites to differentiate patients from controls including now NAA, Gln and aspartate. Understanding the biochemical alterations in the brains of migraine patients may allow for targeted new therapeutic approaches of treatment as well as an approach to better understanding of the effects of medications on the brain.
Acknowledgements
The authors have no conflicts of interest to declare.
Research reported in this publication was supported by the National Institutes of Health (NIH) award number RO1 NS073977 and K24 NS064050.
References
- Aboul-Enein F., Krssak M., Hoftberger R., Prayer D., Kristoferitsch W. Reduced NAA-levels in the NAWM of patients with MS is a feature of progression. A study with quantitative magnetic resonance spectroscopy at 3 Tesla. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguila M.E., Lagopoulos J., Leaver A.M., Rebbeck T., Hubscher M., Brennan P.C., Refshauge K.M. Elevated levels of GABA + in migraine detected using (1) H-MRS. NMR Biomed. 2015;28:890–897. doi: 10.1002/nbm.3321. [DOI] [PubMed] [Google Scholar]
- Albrecht J., Sidoryk-Wegrzynowicz M., Zielinska M., Aschner M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010;6:263–276. doi: 10.1017/S1740925X11000093. [DOI] [PubMed] [Google Scholar]
- Baruth J.M., Wall C.A., Patterson M.C., Port J.D. Proton magnetic resonance spectroscopy as a probe into the pathophysiology of autism spectrum disorders (ASD): a review. Autism Res. 2013;6:119–133. doi: 10.1002/aur.1273. [DOI] [PubMed] [Google Scholar]
- Baslow M.H. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem. Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Bates T.E., Strangward M., Keelan J., Davey G.P., Munro P.M., Clark J.B. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berthet C., Xin L., Buscemi L., Benakis C., Gruetter R., Hirt L., Lei H. Non-invasive diagnostic biomarkers for estimating the onset time of permanent cerebral ischemia. J. Cereb. Blood Flow Metab. 2014;34:1848–1855. doi: 10.1038/jcbfm.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D., Edwards R., Elman I., Becerra L., Levine J. Pain and analgesia: the value of salience circuits. Prog. Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D., Erpelding N., Lebel A., Linnman C., Veggeberg R., Grant P.E., Buettner C., Becerra L., Burstein R. Sex and the migraine brain. Neurobiol. Dis. 2014;68:200–214. doi: 10.1016/j.nbd.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan B.P., Tkachenko O., Schwab Z.J., Juelich R.J., Ryan E.M., Athey A.J., Pope H.G., Jenike M.A., Baker J.T., Killgore W.D., Hudson J.I., Jensen J.E., Rauch S.L. An examination of rostral anterior cingulate cortex function and neurochemistry in obsessive-compulsive disorder. Neuropsychopharmacology. 2015;40:1866–1876. doi: 10.1038/npp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H., Stagg C.J., Near J., Lau C.I., Zisner A., Cader M.Z. Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia. 2015;35:1025–1030. doi: 10.1177/0333102414566860. [DOI] [PubMed] [Google Scholar]
- Brigo F., Storti M., Tezzon F., Manganotti P., Nardone R. Primary visual cortex excitability in migraine: a systematic review with meta-analysis. Neurol. Sci. 2013;34:819–830. doi: 10.1007/s10072-012-1274-8. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Macdonald A.M., Botvinick M., Ross L.L., Stenger V.A., Noll D., Cohen J.D. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J.L., Cady E.B., Penrice J., Wyatt J.S., Cox I.J., Robertson N.J. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. AJNR Am. J. Neuroradiol. 2006;27:1546–1554. [PMC free article] [PubMed] [Google Scholar]
- Clark J.F., Doepke A., Filosa J.A., Wardle R.L., Lu A., Meeker T.J., Pyne-Geithman G.J. N-acetylaspartate as a reservoir for glutamate. Med. Hypotheses. 2006;67:506–512. doi: 10.1016/j.mehy.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Cleve M., Gussew A., Reichenbach J.R. In vivo detection of acute pain-induced changes of GABA + and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. NeuroImage. 2015;105:67–75. doi: 10.1016/j.neuroimage.2014.10.042. [DOI] [PubMed] [Google Scholar]
- Cohen-Gilbert J.E., Sneider J.T., Crowley D.J., Rosso I.M., Jensen J.E., Silveri M.M. Impact of family history of alcoholism on glutamine/glutamate ratio in anterior cingulate cortex in substance-naive adolescents. Dev Cogn Neurosci. 2015 doi: 10.1016/j.dcn.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G., Pierelli F., Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia. 2007;27:1427–1439. doi: 10.1111/j.1468-2982.2007.01500.x. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Cutrer F.M., Charles A. The neurogenic basis of migraine. Headache. 2008;48:1411–1414. doi: 10.1111/j.1526-4610.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- de Graaf R.A., Mason G.F., Patel A.B., Behar K.L., Rothman D.L. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–357. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- Demarquay G., Andre-Obadia N., Caclin A., Morlet D., Mauguiere F. Neurophysiological evaluation of cortical excitability in migraine: a review of the literature. Rev. Neurol. (Paris) 2013;169:427–435. doi: 10.1016/j.neurol.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Eichler S.A., Meier J.C. E–I balance and human diseases — from molecules to networking. Front. Mol. Neurosci. 2008;1:2. doi: 10.3389/neuro.02.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster B.R., Petrou M., Edden R.A., Sundgren P.C., Schmidt-Wilcke T., Lowe S.E., Harte S.E., Clauw D.J., Harris R.E. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579–583. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster B.R., Nascimento T.D., DeBoer M., Bender M.A., Rice I.C., Truong D.Q., Bikson M., Clauw D.J., Zubieta J.K., Harris R.E., DaSilva A.F. Excitatory and inhibitory brain metabolites as targets of motor cortex transcranial direct current stimulation therapy and predictors of its efficacy in fibromyalgia. Arthritis Rheum. 2015;67:576–581. doi: 10.1002/art.38945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby P.J. Migraine, allodynia, sensitisation and all of that. Eur. Neurol. 2005;53(Suppl. 1):10–16. doi: 10.1159/000085060. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Alia-Klein N., Tomasi D., Carrillo J.H., Maloney T., Woicik P.A., Wang R., Telang F., Volkow N.D. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez de la Aleja J., Ramos A., Mato-Abad V., Martinez-Salio A., Hernandez-Tamames J.A., Molina J.A., Hernandez-Gallego J., Alvarez-Linera J. Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache. 2013;53:365–375. doi: 10.1111/head.12030. [DOI] [PubMed] [Google Scholar]
- Gruetter R., Adriany G., Choi I.Y., Henry P.G., Lei H., Oz G. Localized in vivo 13C NMR spectroscopy of the brain. NMR Biomed. 2003;16:313–338. doi: 10.1002/nbm.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.E., Napadow V., Huggins J.P., Pauer L., Kim J., Hampson J., Sundgren P.C., Foerster B., Petrou M., Schmidt-Wilcke T., Clauw D.J. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119:1453–1464. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- Hernandez-Tamames J.A., Mato Abad V., Garcia-Alvarez R., Gonzalez-Zabaleta J., Pereira Loureiro J., Alvarez-Linera J. Effect of water T2 shortening in the quantification of in-vitro proton MR spectroscopy. J. Neuroimaging. 2016;26:58–61. doi: 10.1111/jon.12258. [DOI] [PubMed] [Google Scholar]
- Hoffken O., Stude P., Lenz M., Bach M., Dinse H.R., Tegenthoff M. Visual paired-pulse stimulation reveals enhanced visual cortex excitability in migraineurs. Eur. J. Neurosci. 2009;30:714–720. doi: 10.1111/j.1460-9568.2009.06859.x. [DOI] [PubMed] [Google Scholar]
- Igarashi H., Suzuki Y., Huber V.J., Ida M., Nakada T. N-acetylaspartate decrease in acute stage of ischemic stroke: a perspective from experimental and clinical studies. Magn. Reson. Med. Sci. 2015;14:13–24. doi: 10.2463/mrms.2014-0039. [DOI] [PubMed] [Google Scholar]
- Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn. Reson. Med. 1990;14:26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- Kohavi R., John G.H. Wrappers for feature subset selection. Artif. Intell. 1997;97:273–324. [Google Scholar]
- Kuhn S., Schubert F., Mekle R., Wenger E., Ittermann B., Lindenberger U., Gallinat J. 2015. Neurotransmitter changes during interference task in anterior cingulate cortex: evidence from fMRI-guided functional MRS at 3 T. Brain Struct. Funct. [DOI] [PubMed] [Google Scholar]
- Lai T.H., Protsenko E., Cheng Y.C., Loggia M.L., Coppola G., Chen W.T. Neural plasticity in common forms of chronic headaches. Neural Plast. 2015;2015:205985. doi: 10.1155/2015/205985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirng J.F., Chen H.C., Fuh J.L., Tsai C.F., Liang J.F., Wang S.J. Increased myo-inositol level in dorsolateral prefrontal cortex in migraine patients with major depression. Cephalalgia. 2015;35:702–709. doi: 10.1177/0333102414557048. [DOI] [PubMed] [Google Scholar]
- Luyten P.R., den Hollander J.A. Observation of metabolites in the human brain by MR spectroscopy. Radiology. 1986;161:795–798. doi: 10.1148/radiology.161.3.3786735. [DOI] [PubMed] [Google Scholar]
- Mainero C., Boshyan J., Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann. Neurol. 2011;70:838–845. doi: 10.1002/ana.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N., Becerra L., Brawn J., Bigal M., Burstein R., Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012;32:607–620. doi: 10.1177/0333102412445622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.L. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-l-aspartate, creatine and choline. NMR Biomed. 1991;4:47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Moffett J.R., Ross B., Arun P., Madhavarao C.N., Namboodiri A.M. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett J.R., Arun P., Ariyannur P.S., Namboodiri A.M. N-Acetylaspartate reductions in brain injury: impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation. Front. Neuroenerg. 2013;5:11. doi: 10.3389/fnene.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed R.E., Aboelsafa A.A., Al-Malt A.M. Interictal alterations of thalamic metabolic concentration ratios in migraine without aura detected by proton magnetic resonance spectroscopy. Egypt. J. Radiol. Nucl. Med. 2013;44:859–870. [Google Scholar]
- Moore C.M., Wardrop M., Frederick B.deB., Renshaw P.F. Topiramate raises anterior cingulate cortex glutamine levels in healthy men; a 4.0 T magnetic resonance spectroscopy study. Psychopharmacology. 2006;188:236–243. doi: 10.1007/s00213-006-0451-y. [DOI] [PubMed] [Google Scholar]
- Moulton E.A., Burstein R., Tully S., Hargreaves R., Becerra L., Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Edden R.A., Jones D.K., Swettenham J.B., Singh K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niddam D.M., Tsai S.Y., Lu C.L., Ko C.W., Hsieh J.C. Reduced hippocampal glutamate-glutamine levels in irritable bowel syndrome: preliminary findings using magnetic resonance spectroscopy. Am. J. Gastroenterol. 2011;106:1503–1511. doi: 10.1038/ajg.2011.120. [DOI] [PubMed] [Google Scholar]
- Niddam D.M., Lai K.L., Fuh J.L., Chuang C.Y., Chen W.T., Wang S.J. Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia. 2015 doi: 10.1177/0333102415583144. [DOI] [PubMed] [Google Scholar]
- Oblak A., Gibbs T.T., Blatt G.J. Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Res. 2009;2:205–219. doi: 10.1002/aur.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver J.S., O'Keefe G., Jones G.R., Burrows G.D., Tochon-Danguy H.J., Ackermann U., Scott A.M., Norman T.R. Dopamine D(1) receptor binding in the anterior cingulate cortex of patients with obsessive-compulsive disorder. Psychiatry Res. 2010;183:85–88. doi: 10.1016/j.pscychresns.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N., Vogt B.A., Schleicher A., Mayberg H.S., Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum. Brain Mapp. 2009;30:2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot A.P., Renshaw P.F. Two-dimensional J-resolved proton MR spectroscopy and prior knowledge fitting (ProFit) in the frontal and parietal lobes of healthy volunteers: assessment of metabolite discrimination and general reproducibility. J. Magn. Reson. Imaging. 2013;37:642–651. doi: 10.1002/jmri.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot A., Becerra L., Pendse G., Tully S., Jensen E., Hargreaves R., Renshaw P., Burstein R., Borsook D. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol. Pain. 2009;5:34. doi: 10.1186/1744-8069-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyngoudt H., Paemeleire K., Descamps B., De Deene Y., Achten E. 31P-MRS demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia. 2011;31:1243–1253. doi: 10.1177/0333102410394675. [DOI] [PubMed] [Google Scholar]
- Reyngoudt H., Achten E., Paemeleire K. Magnetic resonance spectroscopy in migraine: what have we learned so far? Cephalalgia. 2012;32:845–859. doi: 10.1177/0333102412452048. [DOI] [PubMed] [Google Scholar]
- Rothrock J.F., Mar K.R., Yaksh T.L., Golbeck A., Moore A.C. Cerebrospinal fluid analyses in migraine patients and controls. Cephalalgia. 1995;15:489–493. doi: 10.1046/j.1468-2982.1995.1506489.x. [DOI] [PubMed] [Google Scholar]
- Russo A., Tessitore A., Giordano A., Corbo D., Marcuccio L., De Stefano M., Salemi F., Conforti R., Esposito F., Tedeschi G. Executive resting-state network connectivity in migraine without aura. Cephalalgia. 2012;32:1041–1048. doi: 10.1177/0333102412457089. [DOI] [PubMed] [Google Scholar]
- Schulte R.F., Boesiger P. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed. 2006;19:255–263. doi: 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- Shyu B.C., Vogt B.A. Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway. Mol. Pain. 2009;5:51. doi: 10.1186/1744-8069-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sprenger T., Borsook D. Migraine changes the brain: neuroimaging makes its mark. Curr. Opin. Neurol. 2012;25:252–262. doi: 10.1097/WCO.0b013e3283532ca3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn J.R., Chu W.J., Whitsel R.M., Weber W.A., Norris M.M., Adler C.M., Eliassen J.C., Phan K.L., Strakowski S.M., DelBello M.P. A pilot study of anterior cingulate cortex neurochemistry in adolescents with generalized anxiety disorder. Neuropsychobiology. 2013;67:224–229. doi: 10.1159/000347090. [DOI] [PubMed] [Google Scholar]
- Suarez I., Bodega G., Fernandez B. Glutamine synthetase in brain: effect of ammonia. Neurochem. Int. 2002;41:123–142. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- Suhara T., Okubo Y., Yasuno F., Sudo Y., Inoue M., Ichimiya T., Nakashima Y., Nakayama K., Tanada S., Suzuki K., Halldin C., Farde L. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch. Gen. Psychiatry. 2002;59:25–30. doi: 10.1001/archpsyc.59.1.25. [DOI] [PubMed] [Google Scholar]
- Tessitore A., Russo A., Conte F., Giordano A., De Stefano M., Lavorgna L., Corbo D., Caiazzo G., Esposito F., Tedeschi G. Abnormal connectivity within executive resting-state network in migraine with Aura. Headache. 2015;55:794–805. doi: 10.1111/head.12587. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R., Tavazzi B., Signoretti S., Amorini A.M., Belli A., Cimatti M., Delfini R., Di Pietro V., Finocchiaro A., Lazzarino G. Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment–part I. Neurosurgery. 2007;61:379–388. doi: 10.1227/01.NEU.0000280002.41696.D8. discussion 388-379. [DOI] [PubMed] [Google Scholar]
- van de Bank B.L., Emir U.E., Boer V.O., van Asten J.J., Maas M.C., Wijnen J.P., Kan H.E., Oz G., Klomp D.W., Scheenen T.W. Multi-center reproducibility of neurochemical profiles in the human brain at 7 T. NMR Biomed. 2015;28:306–316. doi: 10.1002/nbm.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.J., Lirng J.F., Fuh J.L., Chen J.J. Reduction in hypothalamic 1H-MRS metabolite ratios in patients with cluster headache. J. Neurol. Neurosurg. Psychiatry. 2006;77:622–625. doi: 10.1136/jnnp.2005.081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K.M., D'Andrea G., Tepley N., Barkley G., Ramadan N.M. The concept of migraine as a state of central neuronal hyperexcitability. Neurol. Clin. 1990;8:817–828. [PubMed] [Google Scholar]
- Xue T., Yuan K., Cheng P., Zhao L., Zhao L., Yu D., Dong T., von Deneen K.M., Gong Q., Qin W., Tian J. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed. 2013;26:1051–1058. doi: 10.1002/nbm.2917. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Isobe T., Akutsu H., Masumoto T., Ando H., Sato E., Takada K., Anno I., Matsumura A. Influence of echo time in quantitative proton MR spectroscopy using LCModel. Magn. Reson. Imaging. 2015;33:644–648. doi: 10.1016/j.mri.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Yen Y.F., Nagasawa K., Nakada T. Promising application of dynamic nuclear polarization for in vivo (13)C MR imaging. Magn. Reson. Med. Sci. 2011;10:211–217. doi: 10.2463/mrms.10.211. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K., Ward P.B., Huang X.F. Selective alterations in ionotropic glutamate receptors in the anterior cingulate cortex in schizophrenia. Neuropsychopharmacology. 2002;27:826–833. doi: 10.1016/S0893-133X(02)00347-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang Y., Zhao Z.Q. Anterior cingulate cortex contributes to the descending facilitatory modulation of pain via dorsal reticular nucleus. Eur. J. Neurosci. 2005;22:1141–1148. doi: 10.1111/j.1460-9568.2005.04302.x. [DOI] [PubMed] [Google Scholar]