Abstract
The aim of this study was to determine the skeletal effect of total ethanolic extract from the stem-bark of Ulmus davidiana (UDE) in a rat model of postmenopausal bone loss. Effective dose of UDE was determined in adult female Sprague-Dawley (SD) rats by measuring bone regeneration at fracture site. UDE (250 mg/kg p.o.) was administered to ovariectomized (OVX) osteopenic SD rats for 12 weeks. OVX rats treated with vehicle or 17β-estradiol, and sham-operated rats treated with vehicle served as various controls. Bone mineral density (BMD), microarchitecture, biomechanical strength, turnover markers, and uterotrophic effect were studied. Bioactive markers in UDE were analyzed by HPLC. Human osteoblasts was used to study the effect of compounds on differentiation by alkaline phosphase assay. One-way ANOVA was used to test significance of effects. OVX+UDE group showed BMD, microarchitectural parameters and compressive strength at lumbar vertebra (L5) comparable to sham. At proximal femur, OVX+UDE group exhibited significantly higher BMD, better microarchitecture and compressive strength compared with OVX+vehicle. OVX-induced decrease in Ca/P ratio was completely restored at both skeletal sites by UDE treatment. Serum procollagen N-terminal propeptide and carboxy-terminal collagen crosslinks were respectively higher and lower in OVX+UDE group compared with OVX+vehicle group. Osteogenic genes were upregulated in L5 and anti-resorptive genes were suppressed in proximal femur of OVX+UDE group compared with OVX+vehicle. UDE had no uterine estrogenicity. Analysis of markers yielded two osteogenic isoforms of catechin. In conclusion, UDE completely restored vertebral trabecular bones and strength in osteopenic rats by an osteogenic mechanism and prevented bone loss at proximal femur.
Keywords: Lumbar vertebra, proximal femur, gene expression, bone mineral density, biomechanical strength
Introduction
Osteoporosis is a metabolic disease of bone that presents with low bone mineral density (BMD) and poor bone micro-architecture, thus increasing the risk of fracture [1]. Rapid falls in estrogen levels after menopause lead to accelerated bone loss and enhanced fracture risk. Accelerated bone loss occurs in the trabecular bones including lumbar vertebra and femur neck (hip bone), which are the major weight-bearing sites of the body. Vertebral and hip fractures are the major causes of morbidity in postmenopausal osteoporosis. The estrogen deficient condition in postmenopausal women can be mitigated with exogenous estrogen supplementation and has been used to improve the symptoms of postmenopausal bone loss [2]. The Women’s Health Initiative (WHI), a randomized, placebo-controlled trial on healthy women aged 50-79 years (BMD T-score <-2.5) concluded that with estrogen replacement therapy (ERT) there was a significant reduction (34%) in hip and vertebral fractures and in the prevalence of osteoporotic fractures (24% decrease) [3]. However, the use of ERT in osteoporotic women to mitigate fracture risk has raised serious safety concerns due to their potential side effects which include increased risk of breast cancer, stroke and myocardial infarction [4].
Several pharmacological interventions are currently available to reduce the fracture risk including, bisphosphonates, selective estrogen receptor modulators (SERMs) [5] and neutralizing antibody against receptor activator of nuclear factor kappaB (denosumab) [6], which act by suppressing increased rate of bone resorption in postmenopausal women. These therapies prevent additional bone loss but have no effect on bone formation and thus are ineffective in building new bone. Intermittent injection of 1-34 peptide fragment of human parathyroid hormone (PTH) is the only clinically used osteogenic therapy that helps in gaining lost bone in postmenopausal osteoporosis [7]. However, this PTH therapy carries the black box warning from the U.S. FDA due to oncological concerns, as the compound has been shown to cause osteogenic sarcoma in rats. Worldwide, due to the adverse effects of synthetic drugs and their higher cost, many postmenopausal women prefer to use medicinal plants to cut the risk of fracture. Phytochemicals derived from the medicinal plants have preventive or therapeutic pharmacological potential against many metabolic disorders [8].
Ulmus davidiana Planch (family Ulmaceae) (UD) is a deciduous tree, which is widely distributed in Korean peninsula. Stem-bark of this plant has been used in Chinese and Korean traditional medicine for the treatment of inflammation, edema and stomach cancer [9-11]. UD has been reported to prevent cartilage degeneration and promote regeneration of damaged tissue in mouse model of rheumatoid arthritis [12]. It has been shown to stimulate proliferation and differentiation of murine osteoblastic cells [13,14]. Furthermore, UD has been shown to inhibit bone resorption in vitro by inhibiting the processing of cathepsin K, the collagenolytic protein secreted by osteoclasts [15]. The plant contains flavonoids including (+)-catechin and (-)-catechin and several glucosides of both catechin isomers [16,17]. Among the catechin family of compounds, epigallocatechin (EGCG) present in green tea has been shown to have bone conserving as well as osteogenic effect [18], although effect of (+)-catechin and (-)-catechin has not been studied in bone cells. Moreover, there is no preclinical study assessing the effect of UD in osteopenic bone.
We prepared an ethanolic extract made from stem-bark of UD (UDE). We first determined whether UDE was effective in promoting bone regeneration in a femur drill-hole fracture model of rat, which enabled us to determine an effective dose. At the effective dose determined thereof, the skeletal effect of UDE at the trabecular sites (lumbar vertebra and proximal femur) was assessed in osteopenic rats wherein osteopenia was induced by prolonged OVX. Orally doses of 17β-estradiol (E2) were used as a reference treatment for evaluating skeletal effect of UD in therapeutic protocol. Estrogenicity of UD was assessed at the uterine level. Finally, the osteogenic marker compounds present in UD were identified.
Materials and methods
Reagents and chemicals
Cell culture medium and all fine chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) and cell culture supplements such as FBS and diaspase were purchased from Invitrogen (Carlsbad, CA, USA). Procollagen type 1 amino-terminal propeptide (P1NP) ELISA kit was purchased from MyBioSource (USA) and C-terminal crosslinked telopeptide of type I collagen (CTX-1) ELISA kit was purchased from Immunodiagnostic Systems Ltd. (Tyne & Wear, UK).
Preparation of ethanolic extract
Powdered stem bark of U. davidiana (5.0 kg) was put in glass percolator with ethanol (20 L) for 18 h at room temperature, after which the percolate was collected. This process of extraction was repeated 5 times and the extracts were pooled. The pooled extract was filtered and concentrated at 45°C, which yielded the final extract, UDE. The yield of UDE (w/w, calculated from the weight of initial powder and the final powder obtained after evaporation of solvent) was 610.0 g or 12.2% of the dry weight. UDE was then triturated with hexane (500 ml × 5) to remove non-polar impurities.
In vivo studies
Animals and experimental procedure
All animal experimental procedures were prior approved by the local Institutional Animal Care and Use Committee and conducted as per the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China. All animals used for the study were obtained from Experimental Animal Center of Jilin University and were subjected to 12 h dark-light cycle under controlled temperature (23-25°C) and humidity (50-60%).
Drill-hole injury in the femur
Thirty two adult female rats (250-270 g) were anesthetized by i.p. injection of a cocktail of ketamine (80 mg/kg) and xylazine (10 mg/kg). Drill-hole injury was created by inserting a drill bit having a diameter of 0.8 mm in the anterior diaphysis of femurs, ~2 cm above the knee joint [19,20]. One day after surgery, rats were divided into five groups (n = 8/group). One group received methylcellulose (vehicle) and other groups received daily UDE treatment at 50 mg/kg, 100 mg/kg, 250 mg/kg or 500 mg/kg dose for 14 days by oral gavage. On the 14th day, rats were killed and femurs were collected and kept in 70% isopropanol until further use. Determination of bone volume per tissue volume in the drill-hole region of excised femurs was carried out using a high resolution micro-computed tomography (μCT) as described subsequently.
Metabolic assessment of UDE by Oxymax calorimetry
Effect of UDE on overall metabolism of rats were studied using Oxymax Laboratory Animal Monitoring System (CLAMS) (Columbus Instuments, Columbus, OH, USA). Twelve adult female SD rats (250-270 g) were used in the experiment: half were administered vehicle (methylcellulose) and the other half UDE (250 mg/kg p.o.) for 12 weeks. After the treatments, rats were housed in CLAMS cages for 3 days with access to food and water ad libitum with the continuation of treatment to continuously record food intake, water intake, energy expenditure parameters (VO2, VCO2, respiratory exchange ratio and heat output), metabolic parameters (basal- and resting metabolic rates) and behavioral parameters (ambulatory activity and sleep cycle).
Assessment of UDE in osteopenic rats
Sixty four adult female SD rats (250-270 g) were used in the experiment. Out of these, 48 were bilaterally ovariectomized (OVX) and 16 were sham-operated (ovary intact). Rats were then left untreated for 12 weeks for osteopenia to develop in OVX rats [21]. Rats were then divided into four groups (16 rats/group) as follows: sham operated (ovary intact) + methylcellulose (vehicle), OVX+vehicle, OVX+UDE (250 mg/kg p.o.) and OVX+ 10 µg/kg (E2) [22]. After 12 weeks of various treatments described above, all groups were first acclimatized and then kept in metabolic cages with no food but water ad libitum for 24 h. Urine samples were collected. Rats were then returned to the cages with food and water ad libitum with various treatments as before for an additional 24 h. Rats were then killed and autopsied to collect blood, bones (femur and lumber vertebrae) and uteri.
Uteri were dissected out, blotted, weighed and fixed in 4% paraformaldehyde for histology, as described before [23]. About 5.0 mm pieces from the middle segment of each uterus were dehydrated in ascending grades of ethanol, cleared in xylene, and embedded in paraffin wax using standard procedures. Representative transverse sections (5.0 μm) were stained with H&E. Photomicrographs of sections were obtained using a Nikon microscope (Eclipse Ni-E/Ni-U) fitted with NIS Elements BR Image Acquisition software fitted to the microscope. For determination of progesterone receptor (PR) mRNA levels, uteri were snap frozen in liquid nitrogen and stored at -70°C until further use.
Determination of bone mineral density (BMD) and trabecular microarchitecture
CT on excised bones (including bones with drill-hole) was performed by Scanco Medical μCT 40 (Scanco Medical AG, Brüttisellen, Switzerland), having a cone-beam x-ray source of 30-70 kVp and 3-72 µm nominal isotropic (pixel size) resolution as described before [24]. Briefly, trabecular bone volume fraction and microarchitecture in excised L5 and proximal femur was assessed by 12-m isotropic voxels. For L5 trabecular region, we measured ~300 transverse CT slices between the cranial and caudal endplates, and excluded 100 μm near each endplate. Following a standard convolution-backprojection method, CT images were reconstructed in 1024 × 1024 pixel matrices. The resultant gray-scale images were segmented using a constrained 3D Gaussian filter (σ = 0.8, support = 1.0) to minimize noise, and a constant thresholding (22% of maximal gray scale value) was used to extract the structure of mineralized tissue. Using this system, we measured bone volume per tissue volume percent (BV/TV, %), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), connection density (Conn.D), structure model index (S.M.I.) and trabecular pattern factor (Tb.pf).
Using μCT scans, trabecular BMD of femurs was determined from the volume of interest (VOI) made for selected trabecular regions, i.e. lumbar vertebra 5 (L5) and proximal femur. For calibration, hydroxyapatite phantom rods of 4 mm of diameter with known BMD (0.25 g/cm3 and 0.75 g/cm3) were employed [25]. The measurements yielded volumetric BMD (vBMD).
Bone miomechanical strength
L5 was isolated and its spinous and transverse processes were removed. Next, the articular surfaces were removed to make two flat and parallel faces for compression testing. Individual L5 was mounted between the faces of a compression jig and a constant force (2 mm/min) was applied in the craniocaudal direction using Universal Testing Machine (Huntington Beach, CA, USA) [26]. Individual femur head was similarly subjected to compression testing.
Scanning electron microscopy
L5 and proximal femurs were embedded in an acrylic material and cross sections (50 μm) were made and coated with gold sputter coater (108 Auto Sputter Coater, Ted Pella Inc. Redding, CA, USA). Energy-dispersive X-ray spectroscopy (EDX) analysis was performed with an X-ray detector system attached to field emission Philips Scanning Electron Microscope 515 equipped with a LINK QX 2000 EDX-spectrometer. Ca and P weight (%) and Ca/P molar ratio were determined with reference standard (hydroxyapatite) and synthetic pure samples having similar Ca/P ratio (tricalcium phosphate) [27]. The standard EDX graph output was used to correct all observed ratio in various groups. Correction of the data was done by the ZAF (Z = atomic number, A = absorption and F = fluorescent excitation) method [28-30].
Ex vivo mineralization of bone marrow stromal/osteoprogenitor cells (BMSCs)
Ex vivo mineralization was performed according to a previously published protocol. Briefly, at the end of various treatments, BMSCs were squeezed out of L5 and proximal femurs in osteoblast differentiation medium (α-MEM containing 10% FBS, 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate and 100 nM dexamethasone). BMSCs were seeded onto 12-well plates (2 × 106 cells/well) and cultured for 18 days with changing of medium every third day. After 18 days, cells were stained with alizarin red-S. Extraction of the stain was performed by 10% cetylpyridinium chloride (CPC) for colorimetric quantification at 595 nm [31].
Measurement of bone turnover markers
Serum CTX-1 and P1NP were measured by ELISA following the manufacturer’s protocols.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qPCR) was performed to determine the relative expressions of osteoblast and osteoclast specific genes in L5 and proximal femurs of various groups. Bone segments were quickly dissected on ice, cleaned of muscle and then snap frozen in liquid nitrogen. Bones were then stored at -70°C until RNA extraction. After total RNA extraction, cDNA was synthesized with RevertAid cDNA synthesis kit (Fermentas, Austin, USA) using 2 μg total RNA. SYBR green chemistry was employed to perform quantitative detection of relative expression of mRNA levels of these genes using a Light Cycler 480 (Roche Molecular Biochemicals, Indianapolis, USA). GAPDH was used as the internal control. Primers were designed by the Universal Probe Library (Roche Applied Science) for the following genes: type 1 collagen (Col-1), runt-related transcription factor (Runx2), receptor activator of nuclear factor kappa-B (RANK) and tartrate-resistant acid phosphatase (TRAP). PR mRNA levels were determined using snap frozen uteri harvested from various groups. Primer sequences used for all genes are given in Table 1.
Table 1.
Primer sequences of various genes used in qPCR
| Gene name | Primer sequence | Accession number |
|---|---|---|
| Col-1 | F-CATGTTCAGCTTTGTGGACCT | NM_053304 |
| R-GCAGCTGACTTCAGGGATGT | ||
| Runx-2 | F-CCACAGAGCTATTAAAGTGACAGTG | NM_053470 |
| R-AACAAACTAGGTTTAGAGTCATCAAGC | ||
| RANK | F-TGAGGTTTCCAGAGGACCAC | NM_012870.2 |
| R-GGAAAGGTTTCCTGGGTTGT | ||
| TRAP | F-GCCTCTTGCGTCCTCTATGA | NM_019144.1 |
| R-AGCACCATCCACGTATCCA | ||
| PR | F-GGCAGCTGCTTTCAGTAGTCA | NM_022847.1 |
| R-CCTTGAATGTGTAGCTACTGGT | ||
| GAPDH | F-TTTGATGTTAGTGGGGTCTCG | NM_017008 |
| R-AGCTTGTCATCAACGGGAAG |
Measurement of active biomarkers in the extract
HPLC system (Shimadzu Prominence Series, Japan) equipped with binary gradient pump (10 ATVP), Rheodyne (Cotati, CA, USA) model 7125 injector with a 50 ml loop and diode array detector (10 ATVP) was used. Processed samples were injected (50 μl) to a C18 column (100mm × 2.1 mm, 5 μm). The system was run in isocratic mode with the mobile phase consisting of methanol and 0.01% formic acid in the ratio of 90:10 (v/v) at the flow rate of 1.5 ml/min. Mobile phase was subsequently filtered through 0.22 µm Millipore filter (Billerica, USA) and degassed ultrasonically for 20 min prior to use. Chromatographic separations were performed at room temperature.
Culturing of human osteoblastic cells
A human osteoblast cell line (hOB 1.19) was purchased from ATCC, Manassas, USA and cultured in DMEM-F-12 with glutamax containing 10% FBS (v/v) and G-418 (0.3 mg/ml).
Alkaline phosphatase (ALP) assay
For determination of ALP activity, hOB (2 × 103 cells/well) were seeded in 96-well plates. Cells were treated with increasing concentrations of (-)-catechin, (+)-catechin or EGCG. Control cells were treated with vehicle (0.1% DMSO). All treatments were given for 48 h in α-MEM supplemented with 5% charcoal-treated FBS, 10 mM β-glycerophosphate, 50 mg/ml ascorbic acid and 1% penicillin/streptomycin. At the end of the incubation period, total ALP activity was measured using p-nitrophenylphosphate (PNPP) as a substrate and quantitated colorimetrically at 405 nm.
Statistics
Data are expressed as mean ± SEM unless otherwise indicated. The data obtained in experiments with multiple treatments were subjected to one-way ANOVA followed by post hoc Newman-Kuels multiple comparison test of significance using GraphPad Prism 5.
Results
Effect of UD extract on fracture healing and determination of its effective dose
Effect of increasing doses of UDE (100-, 250- and 500 mg/kg p.o.) on the regeneration of bone was quantified by μCT scans performed at the drill-hole site (femur diaphysis). Quantification of data is shown in Figure 1. In comparison to the control, UDE increased BV/TV (p<0.01) at 250 mg/kg dose. UDE at 100 mg/kg dose had no significant effect on BV/TV. There was no difference in BV/TV between 250- and 500 mg/kg dose, which suggested that the maximum effective dose was 250 mg/kg. Thus, in subsequent studies, 250 mg/kg dose was used.
Figure 1.
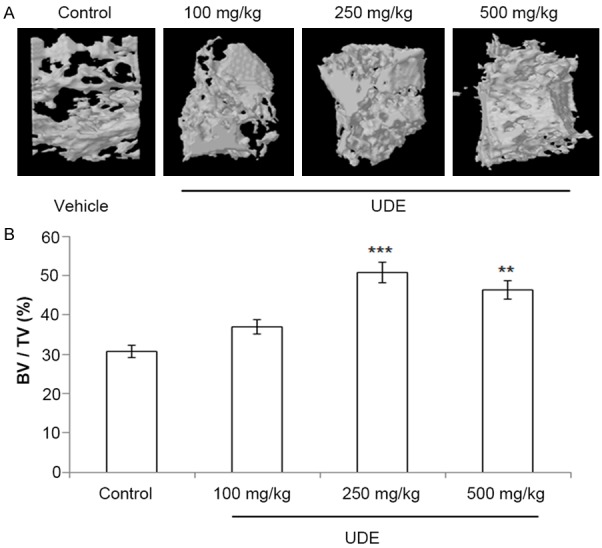
UDE enhanced bone regeneration in drill-hole. A. Representative μCT images from the center of the drill-hole in various groups. B. μCT analysis showing BV/TV%. All values are expressed as mean ± SEM (n = 8 rats/group); **p<0.01, ***p<0.001 compared with control (vehicle treated).
Long-term metabolic assessment of UDE
Because postmenopausal osteoporosis is a metabolic bone disease, we assessed the effect of UDE (250 mg/kg dose) given for 12 weeks on various metabolic parameters of skeletally mature female rats (270 ± 20 g) using Oxymax Calorimetry. At the end of 12 weeks, food intake, water intake, energy expenditure parameters (VO2, VCO2, RER and heat output), metabolic parameters (BMR and RMR) and behavioral parameters (ambulatory activity and sleep cycle) were not different between the control and UDE groups (Supplementary Figure 1).
Effect of UDE on uterine estrogenecity and changes in body weight
Skeletally mature rats were made osteopenic as described in Materials and Methods, after which various treatments were dispensed for 12 weeks. OVX resulted in increased body weight compared to the sham group. Body weight was not different between the E2 and sham groups. Body weight was not different between the OVX+vehicle and OVX+UDE groups, and between the sham and OVX+E2 groups (Supplementary Table 1).
Efficacy of OVX was confirmed by studying uterus. OVX caused significant decrease in uterine weight compared with sham group. Uterine weight was not different between the sham and OVX+E2 groups, and between the OVX+vehicle and OVX+UDE groups (Table 2). Analysis of uterine histomorphometry showed that OVX resulted in marked decrease in total uterine area, luminal area and luminal epithelial cell height compared with sham group. OVX+E2 group exhibited comparable uterine and luminal areas to the sham, but the luminal epithelial cell height was increased. All these parameters were not different between the OVX and OVX+UDE groups (Table 2).
Table 2.
Uterine parameters
| Sham+V | OVX+V | OVX+UDE | OVX+E2 | |
|---|---|---|---|---|
| Uterine wt (g) | 0.92 ± 0.08 | 0.17 ± 0.03*** | 0.14 ± 0.06*** | 0.82 ± 0.1 |
| Uterine area (mm2) | 312.4 ± 8.4 | 173.7 ± 7.1*** | 184.2 ± 3.7*** | 318.3 ± 12.4* |
| Luminal area (mm2) | 6.9 ± 0.4 | 3.2 ± 0.17** | 3.4 ± 0.08*** | 6.88 ± 0.16 |
| Luminal epithelial cell height (μm) | 0.32 ± 0.03 | 0.15 ± 0.02*** | 0.17 ± 0.05*** | 0.38 ± 0.02* |
| PR mRNA (fold change over sham+V) | 1.0 | 0.47 ± 0.03*** | 0.52 ± 0.08*** | 2.3 ± 0.04*** |
Data are mean ± SEM;
p<0.05 compared with sham (vehicle treated);
p<0.01 compared with sham (vehicle treated);
p<0.001 compared with sham (vehicle treated).
To exclude a possibility that UDE may have very low estrogenic potency, the expression of PR mRNA in the uterus was studied in the various groups. OVX group had a drastic reduction in PR transcript compared with sham, and E2 supplementation to OVX rats robustly increased the transcript level. PR mRNA level was not different between the OVX and OVX+UDE groups (Table 2).
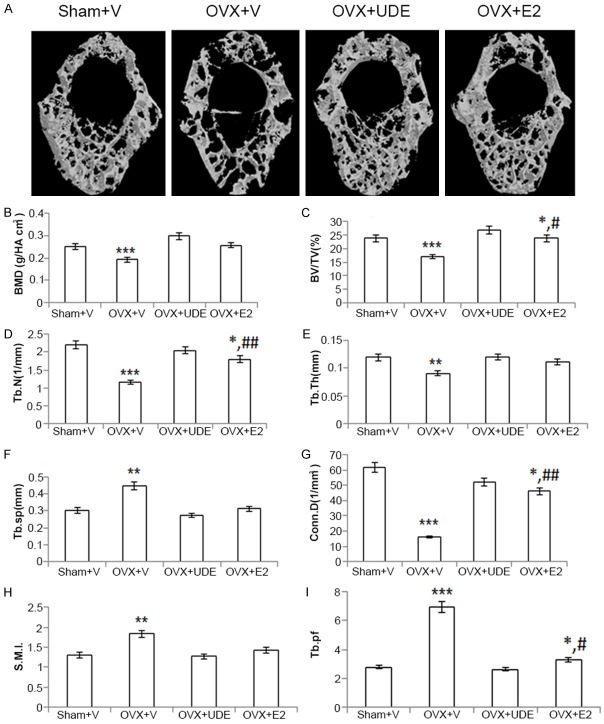
Effect of UDE in osteopenic skeleton
Images of 3D-µCT revealed deterioration of the trabecular architecture due to destruction of trabecular bones of L5 in OVX+vehicle group compared with intact trabecular bones in sham+vehicle group (representative photographs presented in Figure 2A). Trabecular response to UDE (250 mg/kg) was quantified and data presented in Figure 2B-I. In comparison to the sham group, the OVX+vehicle group had reduced vBMD, BV/TV, Tb.Th, Tb.N and conn.D (p<0.001 in all cases), and increased Tb.sp and SMI (p<0.001 in both cases). In OVX, trabeculae with unfavorable rod-like structure over plate and less concave surface resulted in higher S.M.I. and Tb.pf values respectively over the sham.
Figure 2.
UDE completely reversed vertebral osteopenia in OVX rats. (A) Representative μCT images of excised lumbar vertebra (L5) of various groups. μCT parameters are shown from panels (B-I). (D-F) are trabecular connectivity and (G-I) are trabecular geometry parameters. All values are expressed as mean ± SEM (n = 8 rats/group); *p<0.05, **p<0.01 and ***p<0.001 compared with control (vehicle treated); #p<0.05 and ##p<0.01 compared with OVX+vehicle.
UDE treatment to OVX rats resulted in complete restoration of L5 as all the parameters in OVX+UDE were comparable to the sham group. Although OVX+E2 group had higher BV/TV, Tb.N and Conn.D compared with OVX+vehicle, these parameters were significantly lower than in the sham group. In addition, Tb.pf was significantly lower in the OVX+E2 group compared with OVX+vehicle but significantly higher as compared to the sham grop. These data suggested that E2 had partial restorative effect on L5 whereas UDE had complete restorative effect.
Femoral data show that compared with the sham group, the OVX+vehicle group had reduced vBMD, BV/TV, Tb.N and conn.D (p<0.001 in all cases); and increased Tb.sp and SMI (p<0.001 in both cases) (Figure 3). UDE treatment reversed the OVX-induced decreases in vBMD, BV/TV, Tb.N and conn.D and increases in SMI and Tb.sp. Although these parameters were significantly altered in the OVX+UDE group compared with OVX+vehicle group, they failed to reach the levels observed in sham, suggesting that UDE failed to completely restore trabecular bones in femur. In this regard, UDE had effects similar to E2 (Figure 3).
Figure 3.
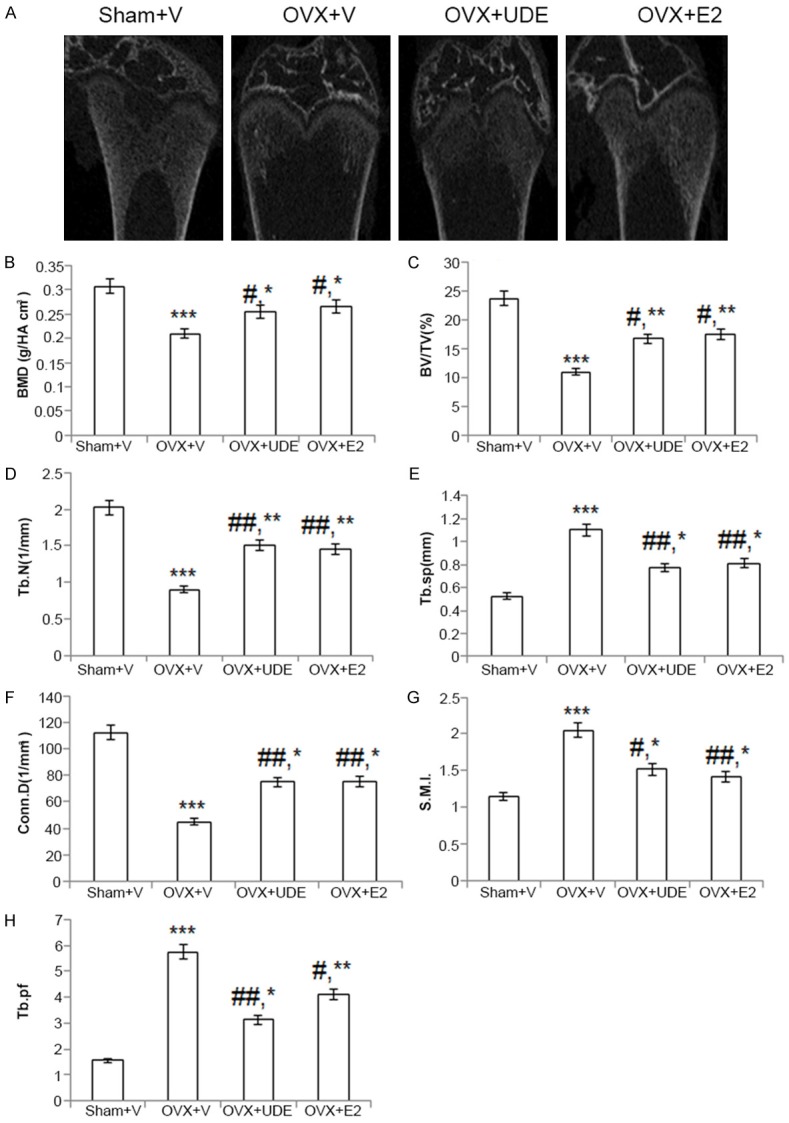
UDE improves trabecular osteopenia at proximal femur in OVX rats. (A) Representative μCT images from the proximal region of excised femurs of various groups. (B-H) All parameters quantified and presented. All values are expressed as mean ± SEM (n = 12 rats/group); *p<0.05, **p<0.01 and ***p<0.001 compared with sham (vehicle treated); #p<0.05 and ##p<0.01 compared with OVX+vehicle.
Effect of UDE on biomechanical strength of trabecular bones
We next assessed biomechanical strength of L5 and femur head. Data in Table 3 showed that the compressive strength parameters including load, energy and stiffness were significantly decreased in the OVX group compared with the sham group at both sites (p<0.001 in all cases). These strength parameters were not different between the OVX+UDE and sham groups, suggesting complete restoration of vertebral bone strength by UDE. However, at proximal femur, OVX+UDE group showed significant increase in these parameters compared with OVX+vehicle but still lower than in the sham group, thus suggesting incomplete restoration by UDE. The strength parameters of L5 in OVX+E2 group was comparable to the sham but that of proximal femur comparable to OVX+UDE.
Table 3.
Bone biomechanical strength parameters
| Sham+V | OVX+V | OVX+UDE | OVX+E2 | |
|---|---|---|---|---|
| L5 | ||||
| Ultimate Load (N) | 190.08 ± 18.5 | 104.63 ± 11.47*** | 196.81 ± 6.77 | 168.17 ± 8.05 |
| Energy (mJ) | 248.62 ± 3.24 | 142.34 ± 7.06*** | 258.52 ± 4.57 | 231.68 ± 9.39 |
| Stiffness (N/mm) | 143.28 ± 8.43 | 64.56 ± 5.23*** | 139.75 ± 4.47 | 145.63 ± 7.83 |
| Proximal femur | ||||
| Ultimate Load (N) | 387.4 ± 18.7 | 280.8 ± 13.3*** | 323.2 ± 17.4#,** | 335.8 ± 8.1##,* |
| Energy (mJ) | 490.6 ± 15.6 | 356.0 ± 21.2*** | 400.1 ± 16.5#,** | 406.7 ± 9.3#,* |
| Stiffness (N/mm) | 158.6 ± 3.8 | 78.8 ± 4.9*** | 98.2 ± 3.1#,** | 104.6 ± 5.6#,** |
Data are mean ± SEM (for L5, n = 6 rats/group and for proximal femur, n = 8 rats/group);
p<0.05 compared with sham (vehicle treated);
p<0.01 compared with sham (vehicle treated);
p<0.001 compared with sham (vehicle treated);
p<0.05 compared with OVX+vehicle;
p<0.01 compared with OVX+vehicle.
Effect of UDE on bone Ca/P ratio
Higher Ca/P ratio is considered a consistent indicator of bone quality. The ratio was shown to be lower in postmenopausal osteoporosis and in the rabbits with osteopenia when compared with E2-sufficient controls [30,32]. EDX-SEM microscopy of L5 and proximal femur showed a decreased Ca/P ratio in the OVX group compared to sham group, and the decrease was due to reduced total Ca in the OVX group relative to sham (Table 4). The Ca/P ratio in proximal femur was not different between the sham, OVX+UDE and OVX+E2 groups in L5. In proximal femur, the ratio was higher in OVX+UDE and OVX+E2 group compared with OVX+vehicle but lower than in the sham group.
Table 4.
Bone calcium and phosphorus content, and Ca/P ratio
| Sham+V | OVX+V | OVX+UDE | OVX+E2 | |
|---|---|---|---|---|
| L5 | ||||
| Calcium (%) | 63.8 ± 0.3 | 57.8 ± 0.7** | 64.7 ± 0.4 | 62.4 ± 0.5 |
| Phosphorus (%) | 33.08 ± 0.08 | 33.73 ± 0.09 | 33.26 ± 0.2 | 32.25 ± 0.6 |
| Ca/P | 1.93 ± 0.06 | 1.71 ± 0.02** | 1.92 ± 0.2 | 1.91 ± 0.5 |
| Proximal femur | ||||
| Calcium (%) | 68.6 ± 0.1 | 62.7 ± 0.4*** | 65.6 ± 0.08#,* | 66.3 ± 0.4##,* |
| Phosphorus (%) | 32.9 ± 0.2 | 33.53 ± 0.2 | 32.9 ± 0.6 | 33.5 ± 0.3 |
| Ca/P | 2.08 ± 0.03 | 1.86 ± 0.04** | 1.98 ± 0.03#,* | 1.97 ± 0.07#,* |
Data are mean ± SEM (n = 6 rats/group);
p<0.05 compared with sham (vehicle treated);
p<0.01 compared with sham (vehicle treated);
p<0.001 compared with sham (vehicle treated);
p<0.05 compared with OVX+vehicle;
p<0.01 compared with OVX+vehicle.
Effect of UDE on skeletal gene expression
Using the total RNA prepared from proximal femur and L5, the expression levels of osteogenic genes (Col-1 and Runx-2) and osteoclastogenic genes (RANK and TRAP) were measured in various groups of rats. In OVX+vehicle rats, osteogenic genes expression was reduced and osteoclastogenic genes expression was increased compared with the sham (Figure 4). In L5, the expression of both osteogenic genes was comparable between the sham and OVX+UDE groups. E2 failed to increase OVX-induced decrease in Runx2 mRNA but significantly increased Col-1 (Figure 4A). Both RANK and TRAP mRNA levels were comparable between the sham and OVX+UDE groups, but their levels in OVX+E2 group were significantly lower than in the sham (Figure 4A).
Figure 4.
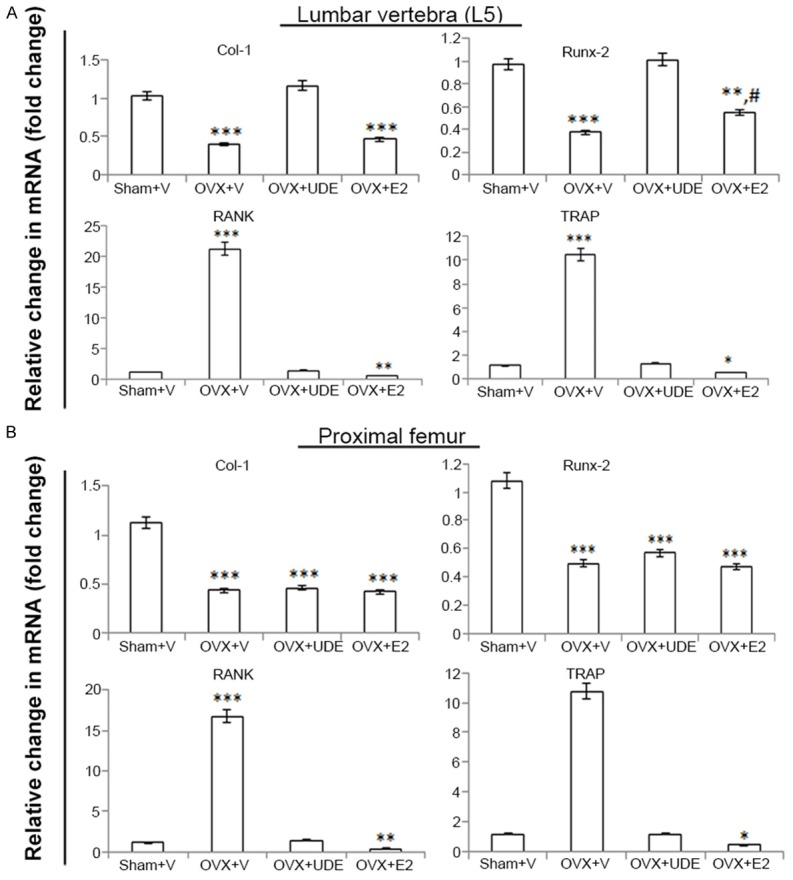
UDE differentially alters skeletal gene expression in OVX rats. qPCR determination of relative mRNA levels of osteogenic (Col1 and Runx2) and osteoclastogenic (RANK and TRAP) genes in L5 (A) and proximal femur (B) in various groups are shown. All values are expressed as mean ± SEM (n = 6 rats/group); *p<0.05, **p<0.01 and ***p<0.001 compared with sham (vehicle treated); #p<0.05 compared with OVX+vehicle.
In femur, OVX group showed significant increase in the mRNA levels of osteoclastogenic genes and decrease in osteogenic genes (Figure 4B). Osteogenic gene mRNAs were not different between the OVX, OVX+UDE and OVX+E2 groups. Osteoclastogenic gene mRNA levels were not different between the sham and OVX+UDE, but in OVX+E2 these two mRNA levels were lower than in the sham group (Figure 4B).
Effect of UDE on ex vivo mineralized nodule formation
Ex vivo nodule formation from BMSC derived from L5 over proximal femur was assessed. BMSC derived from L5 of OVX+UDE group showed nodule formation comparable to sham (Figure 5A) but that derived from proximal femur was comparable to OVX (Figure 5B). Taken together, these data suggest that UDE had osteogenic effect at L5 but not proximal femur.
Figure 5.
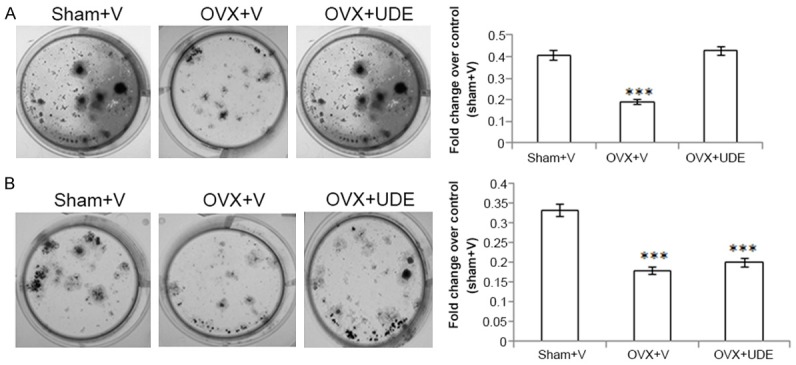
UDE increased ex vivo nodule formation in BMSC derived from L5 of OVX rats. BMSC harvested from L5 (A) and proximal femur (B) from the groups indicated, and cultured in osteogenic medium to induce mineralization (see Materials and Methods). Left panels represent photomicrographs of mineralized nodules stained with alizarin red-S. Right panels, stain was extracted and quantified colorimetrically. All values are expressed as mean ± SEM (n = 6 rats/group); ***p<0.001 compared with sham (vehicle treated).
Effect of UDE on serum biochemical markers of bone
Serum CTX-1 is one of the most widely used biochemical markers of bone loss due to estrogen deficiency, and in our experiments it was found to be robustly increased in the OVX group compared with the sham (Figure 5A). OVX-induced rise in serum CTX-1 was significantly suppressed in the OVX+UDE group and the level was comparable to the sham group. Serum CTX-1 in the OVX+E2 group was significantly lower than the sham group (Figure 6A).
Figure 6.
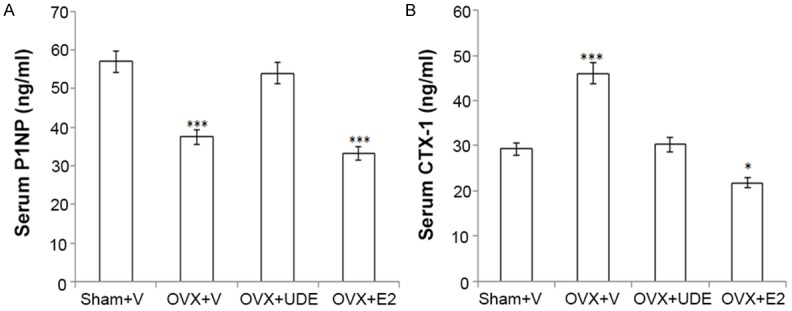
UDE favourably alters bone biochemical markers in serum of OVX rats. (A) P1NP, a serum osteogenic marker and (B) CTX-1, a serum resorptive marker. All values are expressed as mean ± SEM (n = 12 rats/group); *p<0.05 and ***p<0.001 compared with sham (vehicle treated).
Serum P1NP is a reliable marker of bone formation [33]. We observed decreased serum P1NP in the OVX+vehicle group compared with the sham group (Figure 5B). P1NP level in the OVX+UDE group was significantly higher than in the OVX+vehicle group but lower than in the sham groups. E2 treatment failed to increase OVX-induced reduction in serum P1NP (Figure 6B).
Determination of marker compounds in UDE and assessment of their osteogenic effect
Both (+)-catechin and (-)-catechin have been reported to be present in UDE [16,17]. The principal component analysis of both catechin isomers was performed by HPLC. (-)-catechin was found to account for 7.46 ± 0.82% of the extract, and (+)-catechin for 3.74 ± 0.38% of the extract.
We next assessed the in vitro osteogenic effects of these two catechins and compared them with the effect of (-)-epigallocatechin gallate (EGCG), the green tea catechin that is known to have osteogenic effect. In human osteoblasts (hOB.1.19), (-)-Catechin was more potent in inducing the osteoblast differentiation (increased ALP production) than other catechins (Figure 7).
Figure 7.
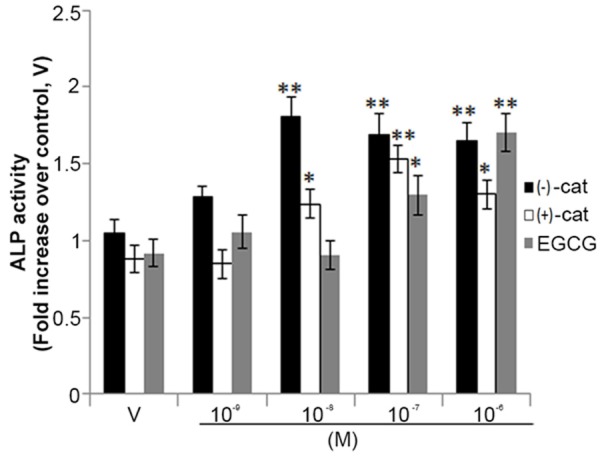
(-)-Catechin is more potent than other naturally occurring catechins in promoting osteoblast differentiation. Human osteoblasts (hOB.1.19, 2 × 103 cells/well) were seeded in 96-well plates. Cells were treated with increasing concentrations of (-)-catechin, (+)-catechin and EGCG as indicated 48 h in osteoblast differentiation medium. ALP production was measured as described in Materials and Methods. Assay was performed in triplicate and data represented as mean ± SEM; *p<0.05 and **p<0.01 versus corresponding vehicle treated cells (V).
Discussion
The findings reported here demonstrate that UDE accelerated fracture healing and had significant anti-osteoporotic effect as it completely restored trabecular bones in lumbar vertebra lost due to OVX. Although UDE failed to attend the trabecular restoration at proximal femur to the level seen in the sham group, the bone volume was significantly higher than in the OVX rats. Mechanistically, the beneficial outcome of UDE treatment on osteopenic skeleton was due to its both osteogenic and anti-osteoclastogenic effects. At the effective dose required for these skeletal actions, UDE neither displayed uterine estrogenicity nor altered metabolism. Two catechin isomers were present in the UDE which showed osteogenic effect in vitro.
Extract of UD has been shown to promote differentiation of mouse osteoblastic cells [13,14], thus raising the possibility that it might have osteogenic effect. Healing of fracture involves the formation of cartilaginous soft callus which is subsequently resorbed and gradually replaced with woven bone. Ultimately, this bone becomes mineralized and undergoes remodeling event to restore the original cortex of the lamellar bone [34,35]. Fracture healing model allows testing the osteogenic effect of a given agent by measuring the bone volume at the callus. We used this model and found that UDE at 250 mg/kg dose significantly enhanced bone volume at the callus over control, suggesting osteogenic potential of UDE. As fracture healing involves endochondral ossification, wherein cartilaginous scaffold is first deposited by chondrocytes, it is possible that UDE promotes chondrogenesis.
The pathogenesis of postmenopausal osteoporosis is not only increases the bone resorption due to increased number of osteoclasts and their hyper-active states but also reduces the ability of osteoblasts to form new bone [36]. Most of the therapies are aimed at inhibiting the resoprtion without addressing the impaired formation aspect of the bone remodeling cycle in postemenopausal skeleton. Although PTH is clinically used in the osteogenic therapy, it is a sole agent and has several limitations pertaining to safety, cost and mode of administration (to be injected daily for two years). Therefore, an oral osteogenic therapy for postmenopausal osteoporosis remains an unmet medical need. We tested UDE in the therapeutic mode, i.e. first allowed the bone loss to occur at the trabecular sites. Substantial loss of trabecular bone 3 months after OVX has been extensively reported [37,38]. One striking observation of our study was the site-selective effect of UDE, i.e. the extract had complete restorative effect in vertebra but only partial effect in proximal femur. It should be noted that PTH also showed stronger bone restorative effect in vertebra than in other trabecular sites [39].
The preservation of trabecular microarchitecture significantly contributes to the bone strength and may reduce fracture risk beyond BMD [40]. We observed that the bone strength data correlated with microarchitectural data, as UDE completely restored vertebral compressive strength to the sham group level whereas the compressive strength of proximal femur was significantly better than in OVX group but failed to achieve the level of sham group. Further, reduced Ca/P ratio in OVX group at both trabecular sites was restored to the sham group level only at the vertebra in UDE group, thus suggesting that this ratio also correlated with the BMD and strength.
Gene expression profile showed that at L5, UDE increased osteogenic genes expression and decreased the expression of osteoclastogenic genes compared with OVX group. On the other hand, in proximal femur, UDE suppressed only osteoclastogenic genes compared with OVX but had no effect on the osteogenic genes expression. Attesting to the gene expression data showing osteogenic effect of UDE in vertebra, ex vivo nodule formation was increased in BMSC derived from L5 of UDE-treated rats compared to the OVX rats. These cellular and molecular data explained why UDE completely restored L5 trabecular bones but achieved only partial restoration in case of proximal femur. It appears that the complete restoration of the lost bones in L5 was due to the dual action of UDE (osteogenic as well as anti-resorptive) whereas its partial (or bone conserving) effect in femur was due to resorption-suppressive effect similar to that of E2.
Increased serum CTX-1 as a result of OVX was significantly suppressed by UDE, confirming an anti-resorptive effect of this extract. Phytoestrogens (flavonoids and stilbenes) are common secondary plant metabolites that are abundantly present in various plant extracts. UDE has been reported to have various flavonoids, the most abundant being catechins and their glycosylated forms [16,17]. It is conceivable that these phytoestrogens contributed to the observed anti-resorptive effect of UDE. However, the serum P1NP level declined due to OVX was remarkably increased upon UDE treatment. Since serum P1NP is an established osteogenic marker [33,41], it is reasonable to conclude that UDE also had a significant osteogenic impact. A human equivalent dose (H.E.D.) of 250 mg/kg of UDE in rats translates to ~40 mg/kg based on the available dose conversion table between human and other species [42]. For a 60 kg person, daily requirement of BF is calculated at ~2400 mg.
The increases in serum P1NP and osteogenic genes in the L5 of OVX+UDE group over OVX+vehicle group was likely caused by catechins (- and + isomers) as both isomers increased osteoblast differentiation, although (-)-catechin was relatively more potent than (+)-catechin. It should be noted that (-)-catechin was more effective in osteogenic differentiation compared to EGCG, a form of catechin present in green tea. The major forms of catechin in green tea are epi-catechins. Green tea has been reported to have bone conserving effect in preclinical models of bone loss as well as in human studies [43-45]. As (-)-catechin is abundant in UDE and has osteogenic potential, it would be interesting to compare osteogenic efficacy of UDE and green tea in preclinical model of osteopenia to test whether UDE possesses better efficacy.
The stem-bark of another species, Ulmus wallichiana, from the same genus Ulmus has been reported to have anti-osteporotic effect in OVX rats [46]. The bioactive compounds in U. wallichiana are C-glucoside flavonones whereas in case of U. davidiana the bioactive compounds are flavanols. So far, no catechin C-glucoside has been reported in U. davidiana but a large number of O-glucosides have been identified. It would be interesting to study whether O-glucosides of catechins have osteogenic effect.
A secondary concern with the use of plant extracts rich in phytoestrogens for the treatment of postmenopausal osteoporosis is their potential for endometrial hyperplasia, as those cells may at times become pre-cancerous. In rats, assessment of wet weight, luminal area and luminal epithelial cell height of uterus revealed absence of estrogen-‘like’ action of UDE, suggesting that UDE is safe (devoid of undesirable uterine effects) for the use in postmenopausal osteoporosis.
Conclusion
Our study demonstrates that daily treatment of a phytopreparation made from the stem-bark of U. davidiana completely restores bone mass and strength in lumbar vertebra as well as prevents bone loss at proximal femur in osteopenic OVX rats. In addition, UDE contains two osteogenic compounds and is devoid of uterine estrogenicity. Based on these preclinical data, an alternative therapeutic application of UDE could be suggested in the setting of postmenopausal bone loss.
Acknowledgements
This study was supported by national natural science foundation of China (NSFC: 31300778).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell RG, Espina B, Hulley P. Bone biology and the pathogenesis of osteoporosis. Curr Opin Rheumatol. 2006;18(Suppl 1):S3–10. doi: 10.1097/01.bor.0000229521.95384.7d. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger B, Genant HK, Cann CE. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med. 1985;102:319–324. doi: 10.7326/0003-4819-102-3-319. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 6.Diedhiou D, Cuny T, Sarr A, Norou Diop S, Klein M, Weryha G. Efficacy and safety of denosumab for the treatment of osteoporosis: A systematic review. Ann Endocrinol (Paris) 2015;76:650–657. doi: 10.1016/j.ando.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi R, Goswami R, Chattopadhyay N. Investigational anabolic therapies for osteoporosis. Expert Opin Investig Drugs. 2010;19:995–1005. doi: 10.1517/13543784.2010.501077. [DOI] [PubMed] [Google Scholar]
- 8.Sharan K, Siddiqui JA, Swarnkar G, Maurya R, Chattopadhyay N. Role of phytochemicals in the prevention of menopausal bone loss: evidence from in vitro and in vivo, human interventional and pharma-cokinetic studies. Curr Med Chem. 2009;16:1138–1157. doi: 10.2174/092986709787581806. [DOI] [PubMed] [Google Scholar]
- 9.Ahn J, Lee JS, Yang KM. Ultrafine particles of Ulmus davidiana var. japonica induce apoptosis of gastric cancer cells via activation of caspase and endoplasmic reticulum stress. Arch Pharm Res. 2014;37:783–792. doi: 10.1007/s12272-013-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyu J, Kim BJ, Kim H. Anti-Allergic Effect of Ulmus davidiana Cortex on Contact Dermatitis Induced by Dinitrofluoro- Benzene in Mice. J Pharmacopuncture. 2013;16:41–45. doi: 10.3831/KPI.2013.16.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun CD, Pae HO, Kim YC, Jeong SJ, Yoo JC, Lee EJ, Choi BM, Chae SW, Park RK, Chung HT. Inhibition of nitric oxide synthesis by butanol fraction of the methanol extract of Ulmus davidiana in murine macrophages. J Ethnopharmacol. 1998;62:129–135. doi: 10.1016/s0378-8741(98)00063-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim KS, Lee SD, Kim KH, Kil SY, Chung KH, Kim CH. Suppressive effects of a water extract of Ulmus davidiana Planch (Ulmaceae) on collagen-induced arthritis in mice. J Ethnopharmacol. 2005;97:65–71. doi: 10.1016/j.jep.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Suh SJ, Yun WS, Kim KS, Jin UH, Kim JK, Kim MS, Kwon DY, Kim CH. Stimulative effects of Ulmus davidiana Planch (Ulmaceae) on osteoblastic MC3T3-E1 cells. J Ethnopharmacol. 2007;109:480–485. doi: 10.1016/j.jep.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Kang SK, Kim KS, Byun YS, Suh SJ, Jim UH, Kim KH, Lee IS, Kim CH. Effects of Ulmus davidiana planch on mineralization, bone morphogenetic protein-2, alkaline phosphatase, type I collagen, and collagenase-1 in bone cells. In Vitro Cell Dev Biol Anim. 2006;42:225–229. doi: 10.1290/0510068.1. [DOI] [PubMed] [Google Scholar]
- 15.Kim KW, Park JS, Kim KS, Jin UH, Kim JK, Suh SJ, Kim CH. Inhibition of Ulmus davidiana Planch (Ulmaceae) on bone resorption mediated by processing of cathepsin K in cultured mouse osteoclasts. Phytother Res. 2008;22:511–517. doi: 10.1002/ptr.2366. [DOI] [PubMed] [Google Scholar]
- 16.Hosny M, Zheng MS, Zhang H, Chang HW, Woo MH, Son JK, Lee SK. (--)-Catechin glycosides from Ulmus davidiana. Arch Pharm Res. 2014;37:698–705. doi: 10.1007/s12272-013-0264-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee GY, Jang DS, Kim J, Kim CS, Kim YS, Kim JH, Kim JS. Flavan-3-ols from Ulmus davidiana var. japonica with inhibitory activity on protein glycation. Planta Med. 2008;74:1800–1802. doi: 10.1055/s-0028-1088324. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Kang L, Lin RW, Fu YC, Lin YS, Chang JK, Chen HT, Chen CH, Lin SY, Wang GJ, Ho ML. (-)-Epigallocatechin-3-gallate improves bone microarchitecture in ovariectomized rats. Menopause. 2013;20:687–694. doi: 10.1097/GME.0b013e31828244f0. [DOI] [PubMed] [Google Scholar]
- 19.Ngueguim FT, Khan MP, Donfack JH, Tewari D, Dimo T, Kamtchouing P, Maurya R, Chattopadhyay N. Ethanol extract of Peperomia pellucida (Piperaceae) promotes fracture healing by an anabolic effect on osteoblasts. J Ethnopharmacol. 2013;148:62–68. doi: 10.1016/j.jep.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 20.Ngueguim FT, Khan MP, Donfack JH, Siddiqui JA, Tewari D, Nagar GK, Tiwari SC, Theophile D, Maurya R, Chattopadhyay N. Evaluation of Cameroonian plants towards experimental bone regeneration. J Ethnopharmacol. 2012;141:331–337. doi: 10.1016/j.jep.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 21.Trivedi R, Kumar A, Gupta V, Kumar S, Nagar GK, Romero JR, Dwivedi AK, Chattopadhyay N. Effects of Egb 761 on bone mineral density, bone microstructure, and osteoblast function: Possible roles of quercetin and kaempferol. Mol Cell Endocrinol. 2009;302:86–91. doi: 10.1016/j.mce.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Bain SD, Jensen E, Celino DL, Bailey MC, Lantry MM, Edwards MW. High-dose gestagens modulate bone resorption and formation and enhance estrogen-induced endosteal bone formation in the ovariectomized mouse. J Bone Miner Res. 1993;8:219–230. doi: 10.1002/jbmr.5650080213. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui JA, Sharan K, Swarnkar G, Rawat P, Kumar M, Manickavasagam L, Maurya R, Pierroz D, Chattopadhyay N. Quercetin-6-Cbeta-D-glucopyranoside isolated from Ulmus wallichiana planchon is more potent than quercetin in inhibiting osteoclastogenesis and mitigating ovariectomy-induced bone loss in rats. Menopause. 2011;18:198–207. doi: 10.1097/gme.0b013e3181e84e67. [DOI] [PubMed] [Google Scholar]
- 24.Pierroz DD, Bonnet N, Bianchi EN, Bouxsein ML, Baldock PA, Rizzoli R, Ferrari SL. Deletion of beta-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res. 2012;27:1252–1262. doi: 10.1002/jbmr.1594. [DOI] [PubMed] [Google Scholar]
- 25.Sharan K, Mishra JS, Swarnkar G, Siddiqui JA, Khan K, Kumari R, Rawat P, Maurya R, Sanyal S, Chattopadhyay N. A novel quercetin analogue from a medicinal plant promotes peak bone mass achievement and bone healing after injury and exerts an anabolic effect on osteoporotic bone: the role of aryl hydrocarbon receptor as a mediator of osteogenic action. J Bone Miner Res. 2011;26:2096–2111. doi: 10.1002/jbmr.434. [DOI] [PubMed] [Google Scholar]
- 26.Mosekilde L, Danielsen CC, Knudsen UB. The effect of aging and ovariectomy on the vertebral bone mass and biomechanical properties of mature rats. Bone. 1993;14:1–6. doi: 10.1016/8756-3282(93)90248-9. [DOI] [PubMed] [Google Scholar]
- 27.Cassella JP, Garrington N, Stamp TC, Ali SY. An electron probe X-ray microanalytical study of bone mineral in osteogenesis imperfecta. Calcif Tissue Int. 1995;56:118–122. doi: 10.1007/BF00296342. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki T, Debari K, Garant PR. Ameloblast modulation and changes in the Ca, P, and S content of developing enamel matrix as revealed by SEM-EDX. J Dent Res. 1987;66:778–783. doi: 10.1177/00220345870660031501. [DOI] [PubMed] [Google Scholar]
- 29.Akesson K, Grynpas MD, Hancock RG, Odselius R, Obrant KJ. Energy-dispersive X-ray microanalysis of the bone mineral content in human trabecular bone: a comparison with ICPES and neutron activation analysis. Calcif Tissue Int. 1994;55:236–239. doi: 10.1007/BF00425881. [DOI] [PubMed] [Google Scholar]
- 30.Kourkoumelis N, Balatsoukas I, Tzaphlidou M. Ca/P concentration ratio at different sites of normal and osteoporotic rabbit bones evaluated by Auger and energy dispersive X-ray spectroscopy. J Biol Phys. 2012;38:279–291. doi: 10.1007/s10867-011-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schecroun N, Delloye C. Bone-like nodules formed by human bone marrow stromal cells: comparative study and characterization. Bone. 2003;32:252–260. doi: 10.1016/s8756-3282(02)00970-5. [DOI] [PubMed] [Google Scholar]
- 32.Fountos G, Tzaphlidou M, Kounadi E, Glaros D. In vivo measurement of radius calcium/phosphorus ratio by X-ray absorptiometry. Appl Radiat Isot. 1999;51:273–278. doi: 10.1016/s0969-8043(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 33.Hale LV, Galvin RJ, Risteli J, Ma YL, Harvey AK, Yang X, Cain RL, Zeng Q, Frolik CA, Sato M, Schmidt AL, Geiser AG. PINP: a serum biomarker of bone formation in the rat. Bone. 2007;40:1103–1109. doi: 10.1016/j.bone.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(Suppl 1):S11–25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trivedi R, Mithal A, Chattopadhyay N. Anabolics in osteoporosis: the emerging therapeutic tool. Curr Mol Med. 2010;10:14–28. doi: 10.2174/156652410791065372. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava K, Khan K, Tyagi AM, Khan MP, Yadav DK, Trivedi R, Maurya R, Singh D, Chattopadhyay N. Greater Skeletal Gains in Ovary Intact Rats at Maturity Are Achieved by Supplementing a Standardized Extract of Butea monosperma Stem Bark that Confers Better Bone Conserving Effect following Ovariectomy and Concurrent Treatment Withdrawal. Evid Based Complement Alternat Med. 2013;2013:519387. doi: 10.1155/2013/519387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan K, Sharan K, Swarnkar G, Chakravarti B, Mittal M, Barbhuyan TK, China SP, Khan MP, Nagar GK, Yadav D, Dixit P, Maurya R, Chattopadhyay N. Positive skeletal effects of cladrin, a naturally occurring dimethoxydaidzein, in osteopenic rats that were maintained after treatment discontinuation. Osteoporos Int. 2013;24:1455–1470. doi: 10.1007/s00198-012-2121-8. [DOI] [PubMed] [Google Scholar]
- 39.Nakano T, Shiraki M, Sugimoto T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Kuroda T, Nakamura T. Once-weekly teriparatide reduces the risk of vertebral fracture in patients with various fracture risks: subgroup analysis of the Teriparatide Once-Weekly Efficacy Research (TOWER) trial. J Bone Miner Metab. 2014;32:441–446. doi: 10.1007/s00774-013-0505-2. [DOI] [PubMed] [Google Scholar]
- 40.Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, Basle MF, Audran M. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res. 2000;15:13–19. doi: 10.1359/jbmr.2000.15.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Lund AM, Hansen M, Kollerup G, Juul A, Teisner B, Skovby F. Collagen-derived markers of bone metabolism in osteogenesis imperfecta. Acta Paediatr. 1998;87:1131–1137. doi: 10.1080/080352598750031112. [DOI] [PubMed] [Google Scholar]
- 42.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 43.Shen CL, Wang P, Guerrieri J, Yeh JK, Wang JS. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos Int. 2008;19:979–990. doi: 10.1007/s00198-007-0527-5. [DOI] [PubMed] [Google Scholar]
- 44.Shen CL, Chyu MC, Yeh JK, Zhang Y, Pence BC, Felton CK, Brismee JM, Arjmandi BH, Doctolero S, Wang JS. Effect of green tea and Tai Chi on bone health in postmenopausal osteopenic women: a 6-month randomized placebo-controlled trial. Osteoporos Int. 2012;23:1541–1552. doi: 10.1007/s00198-011-1731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian G, Xue K, Tang L, Wang F, Song X, Chyu MC, Pence BC, Shen CL, Wang JS. Mitigation of oxidative damage by green tea polyphenols and Tai Chi exercise in postmenopausal women with osteopenia. PLoS One. 2012;7:e48090. doi: 10.1371/journal.pone.0048090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharan K, Siddiqui JA, Swarnkar G, Tyagi AM, Kumar A, Rawat P, Kumar M, Nagar GK, Arya KR, Manickavasagam L, Jain GK, Maurya R, Chattopadhyay N. Extract and fraction from Ulmus wallichiana Planchon promote peak bone achievement and have a nonestrogenic osteoprotective effect. Menopause. 2010;17:393–402. doi: 10.1097/gme.0b013e3181bfae38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.