Abstract
Disorders of copper metabolism are associated with neurological dysfunction including Wilson’s disease (WD). WD is a autosomal recessive disorder caused by mutations in the ATP7B gene resulting in the inability of the hepatocytes to remove excess copper. Gradual copper accumulation causes damage to liver, brain and other organs manifesting in liver disease, neurological and psychiatric symptoms. Also scond copper-neurometaboic disorder: Menkes disease charaterized with mutated ATP7A gene, is ralated with abnormally neuroal transmission and synaptogenesis. Parkinson’s disease and Alzheimer’s disease both are refered to some degree of copper/iron metabolism changes. The precise mechanisms by which excess copper causes neurological damage remain to be elucidated. In this study, we aimed to investigate the influence of excessive amounts of Cu2+ on the oxidative damage response and survival of primary astrocytes from newborn rats. Primary cultured rat astrocytes were divided into three groups: 30 μmol/L CuCl2, 100 μmol/L CuCl2 and control. At 12, 24, 48, 96 and 120 hours of CuCl2 intervention, cell viability, intracellular reduced glutathione level and glutathion reductase activity, and nitric oxide secretion were determined. It was found that 30 μmol/L CuCl2 might stimulate the exaltation and the compensatory proliferation of astrocytes. The survival rate of astrocytes in the 100 μmol/L CuCl2 group was significantly decreased relative to the 30 μmol/L CuCl2 group. At 24 hours of CuCl2 intervention, intracellular reduced glutathione level and glutathion reductase activity were significantly decreased in the 100 μmol/L CuCl2 group compared to the control group. At 120 hours of CuCl2 intervention, nitric oxide secretion in the 100 μmol/L CuCl2 group was significantly greater than in the control group. Under pathological conditions, excessive amounts of Cu2+ greatly damaged the growth and proliferation of astrocytes, reduced the anti-oxidative capacity of astrocytes by reducing intracellular glutathione level and glutathion reductase activity, worsened oxidative stress, and activated inflammation pathway by increasing nitric oxide secretion. By the way, all these findings might provide potential molecular therapeutic targets for the neurodegenerative diseases related Cu2+ Metabolic Disorders, e.g., Wilson’s disease, Parkinson’s disease and Alzheimer’s disease.
Keywords: Copper, Wilson’s disease, menkes disease, Parkinson’s disease, astrocytes, oxidative stress, glutathione
Introduction
Copper is a metal that possesses oxidative-reductive property and exists in vivo in two forms Cu2+ and Cu+ [1]. It is one of the essential trace metals for living organisms. Copper can match with some ligands in the living organism and participate in the interaction between cations through oxidation-reduction reaction. Copper is essential for brain cells as a cofactor and for structural components of various enzymes that are involved in redox reactions and participate in important biochemical pathways such as the respiratory chain, the antioxidative defense and iron metabolism [2]. It is associated with oxidase activity electron transfer and mitochondrial reactive oxygen species production. Therefore, copper homeostasis is important for cell survival. However, excess of copper is harmful, since copper in redox-active form in cells can catalyze the production of hydroxyl radicals in a Fenton-like reaction, thereby inducing oxidative stress and cell damage. There is strong evidence that copper plays a key role in many diseases, including disorders of copper metabolism. An example of a disease that leads to copper deficiency in brain is Menkes disease, while copper overload in brain is observed in Wilson’s disease (WD). Alterations of copper homeostasis in brain have also been connected with neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) and epilepsy [3-5].
Wilson’s disease is an autosomal recessive disease that is caused by mutations in the ATP7B gene, resulting in abnormal biliary excretion of copper and finally excessive amounts of copper in some tissues and organs, like hepatocytes, brain, kidney, and cornea. The copper absorbed by the brain mainly accumulates in the basal ganglia, discus lentiformis and cerebral cortex, causing the degeneration and loss of neurons and astrocytes with clinical psychological and extrapyramidal symptoms [6,7].
Astrocyte proliferation in the brain of patients with Wilson’s disease has been well documented. Astrocytes being in close contact to both neurons and to endothelial cells of brain capillaries are considered the first brain parenchymal cells that encounter metal ions that cross the blood-brain barrier and play an important role in metal metabolism in the brain. Astrocytes are considered as key regulators of the homeostasis of the redox-active metals iron and copper in the brain [8]. Astrocytes absorb more copper than the neurons cultured in vitro [9]. It is presumed that astrocytes protect neurons against copper toxicity by reducing excessive amounts of copper through rapid absorption. In addition, astrocytes contain high levels of metallothionein and glutathione (GSH) [10], indicating that astrocytes exhibit stronger capacity against metal toxicity and metal-induced oxidative stress damage.
The ability of astrocytes to efficiently take up, store and export copper suggests that these cells have an important function as regulators of the copper homeostasis in brain. However, the fate of astrocytes once exposed to copper remains unclear. To elucidate the effects of copper on astrocytes, we treated primary cultured rat astrocytes using CuCl2 at micromolar concentrations, observed cell growth and survival, determined intracellular GSH level, glutathione reductase (GR) activity and nitric oxide (NO) level.
Materials and methods
Reagents and instruments
Fetal bovine serum and high glucose Dulbecco’s modified Eagle’s medium (DMEM) were from Gibco (New York, NY, USA); rabbit anti-rat glial fibrillary acidic protein (GFAP) antibody, goat anti-rabbit IgG antibody, Cy3 dye, and NO detection kit from the Sigma (St. Louis, MO, USA); GSH level and GR activity detection kits from Beyotime Institute of Biotechnology, Beijing, China; CO2 incubator (NAPCO model 5410 incubator) from Precision Scientific (Chicago, IL, USA); thermostatic water bath machine from Beijing Medical Equipment Co., Ltd., (Beijing, China); BX-40 inverted microscope from Olympus (Tokyo, Japan); low speed centrifuge from Beckman Coulter Inc., (Brea, CA, USA); double distilled water machine from Millipore (Nepean, Ontario, Canada); LS-B50L high-pressure steam sterilizer from Shanghai Huaxian Medical Nuclear Instrument Co., Ltd., (Shanghai, China); thermostatic oscillator from Hualida Experimental Equipment Co., Ltd., (Shanghai, China).
Primary culture, purification and identification of astrocytes
Sprague-Dawley rats at 2-3 days of age were sacrificed by dislocation. Under sterile conditions, cerebral cortex was harvested. Under the microscope, the cerebral pia mater and blood vessels were removed and then cerebral cortex was chopped into small pieces, triturated into a cell suspension, and then centrifuged at 250 × g for 10 minutes. The resulting precipitates were re-suspended with high glucose DMEM containing 10% FBS, filtered with a 200-mesh screen, seeded onto a poly-L-lysine plastic culture flask and stained with trypan blue. Cells at a density of 1 × 106/mL were incubated at 37°C in a 5% CO2 incubator. The culture medium was refreshed once every 3 days. After primary culture for 10 days, the cells were dissociated into two layers using a thermostatic oscillator at 37°C. Cells in the lower chamber were the purified astrocytes. The cells were identified by GFAP immunohistochemical staining. Cells at a density of 1 × 104/mL were seeded in a 6-well plate. After 30 minutes, when cells completely adhered to the flask wall, 1 mL of culture medium was added to each well, cells were fixed with 4% paraformaldehyde, treated with Trition at room temperature for 30 minutes, blocked with 7% goat serum, treated with rabbit anti-rat GFAP antibody (primary antibody) at 4°C overnight, incubated with Cy3 dye-labeled goat anti-rabbit IgG (secondary antibody) at room temperature for 2 hours, and stained with 4’,6-diamidino-2-phenylindole (DAPI) for 20 minutes. There were three PBS washes between each above step. After glycerol mounting, cells were observed under the fluorescence microscope. GFAP-positive cells were counted. Cells from 10 visual fields per coverslip, a total of 10 coverslips, were included in the later experiments.
Determination of cell survival rate by cell counting Kit-8 (CCK-8) assay
The purified primary cultured cells were seeded in a 96-well plate, with 1 ×104 cells/100 μL per well. Then the 96-well plate was incubated at 37°C in a 5% CO2 incubator for 2 hours. When the cells adhered to the wall completely, CuCl2 solution was added to a final concentration of 0, 30, and 100 μmol/L. Seven parallel wells were designated for each group. At 24, 48, 72, 96 and 120 hours of CuCl2 intervention, cells were collected and treated with DMEM (10 μL per well) and CCK-8 solution (100 μL per well) for additional 2 hours. Optical density at 450 nm was determined using an ELISA reader. Survival rate of astrocytes (%) = optical density experimental group/optical density control group × 100%.
Detection of intracellular GSH level
According to the method initially proposed by Tietze [11], intracellular GSH level was determined as follows. Astrocytes were cultured with 100 μmol/L CuCl2 solution for 12, 24, 48, 72, and 96 hours, fully lysed with cell lysis solution, centrifuged at 14,000 × g for 10 minutes. The supernatant was used for experiments.
Using 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) reaction, oxidized glutathione (GSSG) and total GSH level were determined using optical density at 412 nm. The concentration of total protein in cell lysates was determined using the BCA assay. Intracellular GSH level (nmol/mg protein) = total GSH level-GSSG × 2.
Detection of GR activity
GR utilizes the H+ provided by NADPH to convert GSSG to reduced GSH, thus NADPH reduces. According to method of determining GSH level, cells were lysed and the supernatant was collected. Following a modification of the method provided by Gutterer et al. [12], optical density of NADPH at 340 nm was measured, and GR activity was calculated. Approximately 100 μL of supernatant was collected from the 96-well plate and then 80 μL of NADPH, followed by 180 μL of GSSG, was added. After reaction at room temperature for 20 minutes, the final concentration in the resultant mixture was 1 mmol/L EDTA, 1 mmol/L GSSG, 0.2 mmol/L NADPH, and 100 mmol/L KPi (pH = 7.0). The optical density of the mixture at 340 nm was reduced. The concentration of total protein in cell lysates was determined using the BCA assay and expressed in nmol of reduced NADPH [nmol NADPH·min-1· (mg protein)-1].
Determination of NO level
NO in vivo exists primarily in two forms as nitrite and nitrate and its concentration can be indirectly determined. According to Griess Reaction method [13], astrocytes were cultured with 100 μmol/L CuCl2 solution for 12, 24, 48, 72 and 96 hours. After removal of cell culture medium, 50 μL of Griess Reagent R1 and Griess Reagent R2 were added to each well. After reaction at room temperature for 10 minutes, optical density of NO at 540 nm was read. NO level was indirectly detected by measuring the NO2 level in the supernatant.
Statistical analysis
All measurement data were expressed as the mean ± SD. One-way analysis of variance was used for comparison between different time points in the same group and t-test for comparison between groups. A level of P < 0.05 was considered statistically significant.
Results
Primary culture of newborn rat astrocytes and its purity identification by GFAP
Immunohistochemical staining (Figure 1A Cy3 (red)-labeled GFAP-immunoreactive cells; Figure 1B DAPI (blue)-stained nuclei; Figure 1C double labeling of the same field): Newborn rat astrocytes were immunohistochemically stained for GFAP with red cytoplasm and blue nuclei. GFAP-immunoreactive cells accounted for 97% of all astrocytes.
Figure 1.
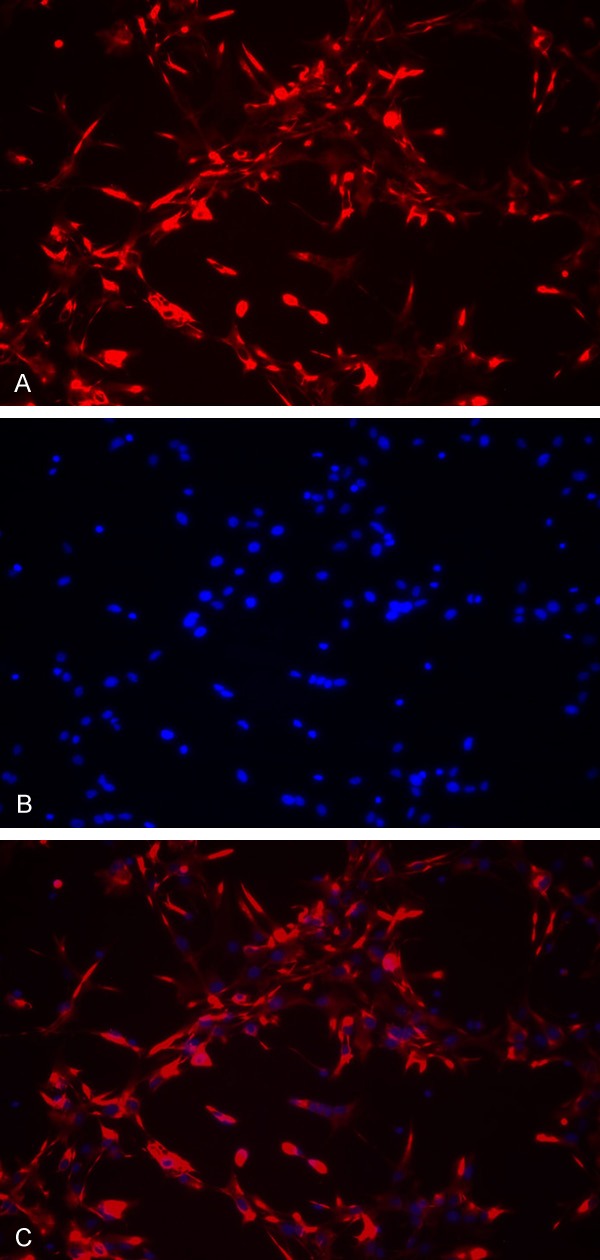
Immunofluorescence images of primary cultured newborn rat astrocytes (× 200). Astrocytes cover the whole coverslip and present with a large spherical appearance with many neurites and connections between neurites. A. Cy3 (red)-labeled GFAP-immunoreactive cells; B. DAPI (blue)-stained nuclei; C. Double labeling of the same field.
Effects of copper on survival rate of astrocytes
Astrocytes were treated with two different concentrations of CuCl2 to investigate the survival rate of astrocytes using the CCK-8 assay. As shown in Figure 2A, there was no significant difference in cell survival rate between 30 μmol/L CuCl2 and 100 μmol/L CuCl2 groups at 24 or 48 hours. At 72 hours, cells in the 30 μmol/L CuCl2 and control groups tended to grow. The cell survival rate in the 100 μmol/L CuCl2 group was 93%, 90% and 87% of that in the control group at 72, 96 and 120 hours, respectively. After 96 and 120 hours of treatment, cell growth in the control group was slightly decreased, while cells in the 30 μmol/L CuCl2 group still grew and tended to be stable. After 72 hours, cell survival rate in the 100 μmol/L CuCl2 group was significantly lower than that in the control and 30 μmol/L CuCl2 groups. According to the survival rate-time curve (Figure 2B), the survival rate of cells in the 30 μmol/L CuCl2 group at 72 hours was significantly increased compared to that at 24 hours, indicating that low concentration of CuCl2 can promote the proliferation of astrocytes.
Figure 2.
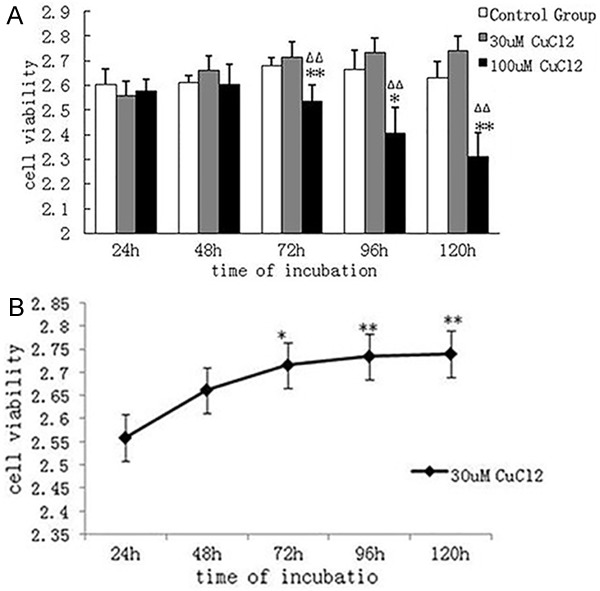
A. Changes in survival rate of astrocytes in each group. Compared with the control group, survival rate of astrocytes in the 30 μmol/L CuCl2 group did not change significantly, while that in the 100 μmol/L CuCl2 group was significantly decreased. *P < 0.05, **P < 0.01, vs. control group. ΔΔP < 0.01, vs. 30 μmol/L CuCl2 group. B. Change in survival rate of astrocytes after 30 μmol/L CuCl2 intervention for different time periods. Compared with treatment for 24 hours, cell survival rate was significantly increased after treatment for 72, 96 and 120 hours. *P < 0.05, **P < 0.01, vs. treatment for 24 hours.
Copper attenuated the anti-oxidative capacity of astrocytes
Intracellular GR activity and GSH level were reduced. To investigate whether copper-caused reduction in survival rate of astrocytes and cell function damage are related to oxidative stress, we incubated astrocytes using 100 μmol/L CuCl2 and determined intracellular GSH level and GR activity. At 24 hours, GR activity in the 100 μmol/L CuCl2 group was 76% of that in the control group (P < 0.01) (Figure 3A). After 24 hours, intracellular GSH level in the 100 μmol/L CuCl2 group was significantly lower than that in the control group (P < 0.05) (Figure 3B). Prior to decrease in survival rate of astrocytes, GR activity and GSH level had begun to decrease significantly, indicating that damage to the anti-oxidative capacity of astrocytes occurred earlier than cell destruction.
Figure 3.
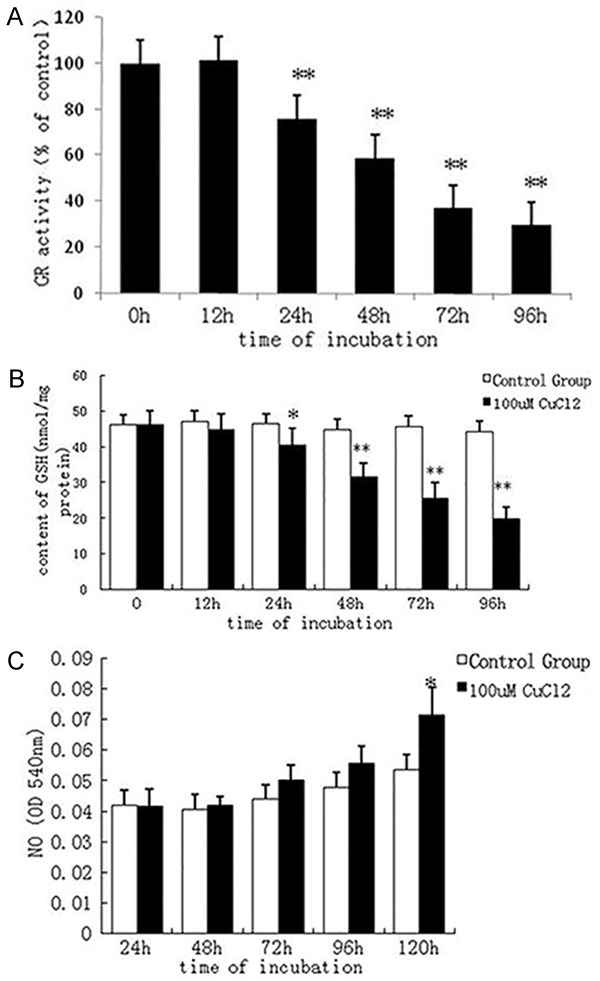
A. Effect of 100 μmol/L CuCl2 on glutathione reductase (GR) activity. After intervention with 100 μmol/L CuCl2 for 24 hours, GR activity was obviously decreased. **P < 0.01, vs. control group. B. Effect of 100 μmol/L CuCl2 on glutathione (GSH) level in the astrocyte. After intervention with 100 μmol/L CuCl2 for 24 hours, GSH level in the astrocyte was significantly decreased compared with the control group. *P < 0.05, **P < 0.01, vs. control group. C. Effect of 100 μmol/L CuCl2 on nitric oxide (NO) level in the astrocyte. After intervention with 100 μmol/L CuCl2 for 120 hours, NO level was significantly increased. *P < 0.05, vs. control group.
Copper promoted NO secretion by astrocytes
With the prolongation of time taken for 100 μmol/L CuCl2 intervention, no obvious change in NO level was found. But after 120 hours, NO level was significantly increased (P < 0.05) (Figure 3C). This suggests that long-time intervention by excessive amounts of copper increased intracellular NO level.
Discussion
Normal brain copper concentration in the extracellular fluid ranges between 0.2-1.7 μmol/L, but in the synaptic cleft, the peak concentration can attain at 200 μmol/L [14-15]. It is reported that the copper concentration in the brain of patients with Wilson’s disease is 10-15 times that in the normal individuals. Studies regarding neurodegenerative diseases, such as Wilson’s disease and Alzheimer’s disease, have demonstrated that excessive amounts of copper are closely related to the pathological mechanisms underlying these diseases and cause the degeneration and loss of neurons in the brain [15].
In the present study, we treated primary cultured rat astrocytes using 30 and 100 μmol/L CuCl2 and investigated whether CuCl2 influenced the survival rate of astrocytes at different time periods. CCK-8, used in this study, is a water-soluble, highly sensitive reagent used for determining the number of viable cells. Results from this study showed that in the initial 48 hours, both 30 and 100 μmol/L CuCl2 did not influence the growth of astrocytes. After 72 hours, cell survival rate in the 100 μmol/L CuCl2 group was decreased, and over time, cell injury significantly worsened. These results suggest that long-term 100 μmol/L CuCl2 intervention influences the survival of astrocytes, while no obvious astrocyte injury was observed during the 12 hours of 30 μmol/L CuCl2 intervention period. While the astrocytes in the control group showed a decreased growth tendency, the growth of astrocytes in the 30 μmol/L CuCl2 group was active, indicating that 30 μmol/L CuCl2 might stimulate the exaltation and the compensatory proliferation of astrocytes. Proliferation of reactive astrocytes leads to strong molecular motion, which plays an important role in the response to central nervous system injury. Astrocytes are a group of glial cells in the brain and are found throughout the interspace between neurons and their neuritis. They support and maintain the normal function of the central nervous system, including regulating blood flow, providing energy for neuronal metabolism, maintaining extracellular ion homeostasis and body fluid balance, modulating synaptic transmission and plasticity, and serving as transporters [16]. Also, astrocytes produce a response to cellular injury in the brain, and defend the brain against oxidative stress and toxins. Activated astrocytes encounter abnormal products, such as excessive amounts of reactive oxygen species or inflammatory cytokines, which play harmful effects in injuries or diseases [16,17]. Studies have demonstrated that astrocyte dysfunction plays an important role in the occurrence and development of neurodegenerative diseases [18,19]. Our results show that prolonged exposure to excessive copper leads to the death of these cells suggesting one mechanisms underlying neurological dysfunction in WD [20].
Results from this study showed that after astrocytes were cultured with 100 μmol/L CuCl2 for 24 hours, intracellular GR activity was decreased and simultaneously GSH level was decreased. Reduced GSH level attenuates the anti-oxidative capacity of astrocytes, which further worsens mitochondrial injury. ATP is necessary for GSH synthesis, so deficiency of mitochondrial energy supply possibly influences GSH circulation [28]. Therefore, intracellular GR activity and GSH level are closely related to mitochondrial function and cell ability against oxidative stress injury. In addition, astrocytes also release compounds to prevent against copper-mediated deactivation/degradation of extracellular GSH [29]. There is evidence that neurons resist hydrogen peroxide-induced injury via the GSH system [30]. Neurons maintain intracellular GSH level based on the extracellular GSH secreted by astrocytes. Pope et al. [29] reported that astrocytes protect against copper-catalyzed loss of extracellular GSH via two mechanisms: (1) cleaning and (or) chelating extracellular copper; (2) secreting anti-oxidative molecules. It has been confirmed that astrocyte can intake extracellular copper. When astrocytes are deficient, free copper ions that added in the conditioned culture medium are reduced, indicating that astrocyte conditioned culture medium contains some molecules that competitively bind to copper ions, such as amino acids, prion protein [31], amyloid precursor protein [32] and ceruloplasmin [33]. Wang et al. [34] found that reaction of astrocyte-released pyruvate with hydrogen peroxide prevents the formation of hydroxyl radicals and protects neurons against the neurotoxicity produced by copper-catalyzed oxidative stress. Astrocytes protect neurons against reactive oxygen species injury by interacting with neurons via many pathways. The anti-oxidative capacity of astrocytes is attenuated once intracellular GSH concentration is decreased, leading to apoptosis or necrosis, thus astrocytes are not able to protect neurons and maintain neuronal function.
In spite of poorly understood mechanisms underlying copper toxicity, oxidative stress has been proven to be a key factor of central nervous system injury. Oxidative stress is mediated by free radicals (mainly hydroxy radicals) produced during electron transfer which occurs when copper alters its potency. Copper can directly inhibit protein function, for example, lactate dehydrogenase, because it can catalyze the generation of hydroxyl radicals [22]. Copper can also cause oxidative damage of astrocytes, which can be protected against by high density lipoprotein, and 250 μmol/L copper ions can deactivate cells [23,24].
Mammalian central nervous system is susceptible to reactive oxygen species injury. Compared with neurons, astrocytes contain a larger number of anti-oxidative systems, for example GSH-GSSG system [25], which plays an important anti-oxidative stress role in the central nervous system. Intracellular GSH exists in its reduced form. Copper is considered the most efficient trace metal that catalyzes GSH oxidation [26], which is related to neurodegenerative disease with GSH loss. Approximately 90% of intracellular GSH locates in the cytoplasm, and the remaining part in the mitochondrion. GSH loss may cause mitochondrial injury [27]. It has been proven that excessive amounts of copper can cause oxidative stress, leading to mitochondrial dysfunctions, including mitochondrial membrane permeability transition, decrease in membrane potential, reduced enzyme activity, and ATP exhaust [15].
Decreased GSH level in the neurons is highly vulnerable to NO injury [35]. It has been confirmed that NO can induce mitochondrial membrane permeability transition, cause mitochondrial function damage [36] and then lead to the occurrence of neuroinflammation. Copper can increase NO level [37] through up-regulating inducible nitric oxide synthase expression and then influences mitochondrial membrane permeability transition. NO binds to excessive reactive oxygen species in the brain to form peroxynitrite anions, and the later decomposes and produces highly toxic hydroxy radicals that cause injury to the adjacent cells. These were similar to our results that, after in vitro cultured astrocytes were treated with 100 μmol/L CuCl2 for 120 hours, NO level was increased to 133% of that in the control group (P < 0.01). NO level increase occurred after reductions of GSH level and GR activity in the astrocytes and was accompanied by decrease in survival rate of astrocytes. These indicate that the mechanism underlying astrocyte anti-oxidation is unbalanced, and oxidative stress damage to astrocytes is further worsened.
A better understanding of the mechanism underlying Cu2+ overload-caused astrocyte injury and prevention will provide implications for the potentially adjunct treatment of neurodegenerative diseases, such as Wilson’s disease, Parkinson’s disease and Alzheimer’s disease [38-40].
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81071065) and Shanghai Jiao Tong University School of Medicne Brain Innovative plan 2015 & Translational medicine 2015.
Disclosure of conflict of interest
None.
References
- 1.Festa RA, Thiele DJ. Copper: An essential metal in biology. Curr Biol. 2011;21:R877–883. doi: 10.1016/j.cub.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheiber IF, Dringen R. Astrocyte functions in the copper homeostasis of the brain. Neurochem Int. 2013;62:556–65. doi: 10.1016/j.neuint.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Scheiber I, Dringen R, Mercer JF. Copper: effects of deficiency and overload. Met Ions Life Sci. 2013;13:359–87. doi: 10.1007/978-94-007-7500-8_11. [DOI] [PubMed] [Google Scholar]
- 4.Hung YH, Bush AI, Fontaine SL. Links between copper and cholesterol in Alzheimer’s disease. Front Physiol. 2013;4:111. doi: 10.3389/fphys.2013.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowinska-Zyrek M, Salerno M, Kozlowski H. Neurodegenerative diseases - Understanding their molecular bases and progress in the development of potential treatments. Coord Chem Rev. 2015;284:298–312. [Google Scholar]
- 6.Ala A, Walker A, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 7.Bertrand E, Lechowicz W, Szpak GM, Lewandowska E, Członkowska A, Dymecki J. Quantitative study of pathological forms of astroglia in Wilson’s disease. Folia Neuropathol. 1997;35:227–232. [PubMed] [Google Scholar]
- 8.Tiffany-Castiglioni E, Hong S, Qian Y. Copper handling by astrocyte: insights into neurodegenerative diseases. Int J Dev Neurosci. 2011;29:811–818. doi: 10.1016/j.ijdevneu.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Dineley KE, Scanlon JM, Kress GJ, Stout AK, Reynolds IJ. Astrocytes are more resistant than neurons to the cytotoxic effects of increased [Zn (2+)] (i) Neurobiol Dis. 2000;7:310–320. doi: 10.1006/nbdi.2000.0303. [DOI] [PubMed] [Google Scholar]
- 10.Dringen R, Hamprecht B. Glutathione restoration as indicator for cellular metabolism of astroglial cells. Dev Neurosci. 1998;20:401–407. doi: 10.1159/000017337. [DOI] [PubMed] [Google Scholar]
- 11.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 12.Gutterer JM, Dringen R, Hirrlinger J, Hamprecht B. Purification of glutathione reductase from bovine brain, generation of an antiserum, and immunocytochemical localization of the enzyme in neural cells. J Neurochem. 1999;73:1422–1430. doi: 10.1046/j.1471-4159.1999.0731422.x. [DOI] [PubMed] [Google Scholar]
- 13.Suk K, Lee J, Hur J, Kim YS, Lee M, Cha S, Yeou Kim S, Kim H. Activation-induced cell death of rat astrocytes. Brain Res. 2001;900:342–347. doi: 10.1016/s0006-8993(01)02326-5. [DOI] [PubMed] [Google Scholar]
- 14.Mathie A, Sutton GL, Clarke CE, Veale EL. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol Ther. 2006;111:567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Castro PA, Ramirez A, Sepúlveda FJ, Peters C, Fierro H, Waldron J, Luza S, Fuentealba J, Muñoz FJ, De Ferrari GV, Bush AI, Aguayo LG, Opazo CM. Copper-uptake is critical for the down regulation of synapsin and dynamin induced by neocuproine: modulation of synaptic activity in hippocampal neurons. Front Aging Neurosci. 2014;6:319. doi: 10.3389/fnagi.2014.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 19.Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 20.Haywood S, Paris J, Ryvar R, Botteron C. Brain copper elevation and neurological changes in north ronaldsay sheep: a model for neurodegenerative disease? J Comp Pathol. 2008;139:252–255. doi: 10.1016/j.jcpa.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Scheiber IF, Mercer JF, Dringen R. Copper accumulation by cultured astrocytes. Neurochem Int. 2010;56:451–460. doi: 10.1016/j.neuint.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Pamp K, Bramey T, Kirsch M, De Groot H, Petrat F. NAD(H) enhances the Cu(II)-mediated inactivation of lactate dehydrogenase by increasing the accessibility of sulfhydryl groups. Free Radic Res. 2005;39:31–40. doi: 10.1080/10715760400023671. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti G, Bacchetti T, Moroni C, Vignini A, Curatola G. Copper-induced oxidative damage on astrocytes: protective effect exerted by human high density lipoproteins. Biochim Biophys Acta. 2003;1635:48–54. doi: 10.1016/j.bbalip.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Merker K, Hapke D, Reckzeh K, Schmidt H, Lochs H, Grune T. Copper related toxic effects on cellular protein metabolism in human astrocytes. Biofactors. 2005;24:255–261. doi: 10.1002/biof.5520240130. [DOI] [PubMed] [Google Scholar]
- 25.Steele ML, Robinson SR. Reactive astrocytes give neurons less support: implications for Alzheimer’s disease. Neurobiol Aging. 2012;33:423, e1–13. doi: 10.1016/j.neurobiolaging.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 26.White AR, Cappai R. Neurotoxicity from glutathione depletion is dependent on extracellular trace copper. J Neurosci Res. 2003;71:889–897. doi: 10.1002/jnr.10537. [DOI] [PubMed] [Google Scholar]
- 27.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 28.Mazzetti AP, Fiorile MC, Primavera A, Lo Bello M. Glutathione transferases and neurodegenerative diseases. Neurochem Int. 2015;82:10–18. doi: 10.1016/j.neuint.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Pope SA, Milton R, Heales SJ. Astrocytes protect against copper-catalysed loss of extracellular glutathione. Neurochem Res. 2008;33:1410–1418. doi: 10.1007/s11064-008-9602-3. [DOI] [PubMed] [Google Scholar]
- 30.Aoyama K, Nakaki T. Impaired Glutathione Synthesis in Neurodegeneration. Int J Mol Sci. 2013;14:21021–21044. doi: 10.3390/ijms141021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown DR. Role of the prion protein in copper turnover in astrocytes. Neurobiol Dis. 2004;15:534–543. doi: 10.1016/j.nbd.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Brown DR, Kozlowski H. Biological inorganic and bioinorganic chemistry of neurodegeneration based on prion and Alzheimer diseases. Dalton Trans. 2004;13:1907–1917. doi: 10.1039/b401985g. [DOI] [PubMed] [Google Scholar]
- 33.Lafon-Cazal M, Adjali O, Galéotti N, Poncet J, Jouin P, Homburger V, Bockaert J, Marin P. Proteomic analysis of astrocytic secretion in the mouse. Comparison with the cerebrospinal fluid proteome. J Biol Chem. 2003;278:24438–24448. doi: 10.1074/jbc.M211980200. [DOI] [PubMed] [Google Scholar]
- 34.Wang XF, Cynader MS. Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J Neurosci. 2001;21:3322–3331. doi: 10.1523/JNEUROSCI.21-10-03322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell KF. Insight into a neuron’s preferential susceptibility to oxidative stress. Biochem Soc Trans. 2013;41:1541–1545. doi: 10.1042/BST20130245. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, Li Q, Jin A, Cui M, Liu X. E3 ubiquitin ligase Siah-1 downregulates synaptophysin expression under high glucose and hypoxia. Am J Transl Res. 2015;7:15–27. [PMC free article] [PubMed] [Google Scholar]
- 37.Cuzzocrea S, Persichini T, Dugo L, Colasanti M, Musci G. Copper induces type II nitric oxide synthase in vivo. Free Radic Biol Med. 2003;34:1253–1262. doi: 10.1016/s0891-5849(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 38.Xu P, Lu ZL, Wang X, Dosher B, Zhou J, Zhang D, Zhou Y. Category and perceptual learning in subjects with treated Wilson’s disease. PLoS One. 2010;5:e9635. doi: 10.1371/journal.pone.0009635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao JJ, Yin M, Wang ZJ, Wang XP. Transplanted Neural Stem Cells: Playing a Neuroprotective Role by Ceruloplasmin in the Substantia Nigra of PD Model Rats? Oxid Med Cell Longev. 2015;2015:618631. doi: 10.1155/2015/618631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei X, He S, Wang Z, Wu J, Zhang J, Cheng Y, Yang J, Xu X, Chen Z, Ye J, Chen L, Lin L, Xiao J. ibroblast growth factor 1attenuates 6-hydroxydopamine-induced neurotoxicity: an in vitro and in vivo investigation in experimental models of parkinson’s disease. Am J Transl Res. 2014;6:664–677. [PMC free article] [PubMed] [Google Scholar]


