Abstract
Objectives
Inflammatory myopathies are a group of idiopathic, heterogeneous systemic diseases affecting predominantly skeletal muscles, though they can also involve the skin and internal organs. The association between cancer and idiopathic inflammatory myopathies, particularly dermatomyositis, which is termed cancer-associated myositis (CAM), has been reported in the medical literature. A newly described autoantibody to a 155-kDa nuclear protein, identified as transcription intermediary factor 1-gamma (TIF1-γ), has proven useful for cancer screening in patients with dermatomyositis.
Material and methods
Based on our database of laboratory results, between November 2014 and January 2016, we found 80 patients with a positive autoimmune inflammatory myopathy immunoblot profile.
Results
Eleven of 80 patients revealed the presence of anti-TIF1-γ antibodies: 8 women and 3 men with average age 54.2 years. Dermatomyositis (DM) was diagnosed in 6 cases, polymyositis in 1 case, myositis limited to ocular muscles and rhabdomyolysis in 1 case each, and undifferentiated connective tissue disease in 2 cases. Neoplasm was found in 4 cases. All of those patients had DM. The average time between DM and diagnosis of neoplasm was 7.5 months (from 1 to 18 months).
Conclusions
The association between cancer and idiopathic inflammatory myopathies, particularly DM, is well known, and cancer screening should be obligatory in such patients. So far there is no consensus as to the method or frequency with which patients with an idiopathic inflammatory myopathy should be tested to rule out neoplasm. Detection of anti-TIF1-γ antibodies in patients with DM gives the clinicians the very important suggestion of CAM. It seems reasonable that these patients should have more detailed and often repeated differential diagnostics.
Keywords: neoplasm, anti-TIF1-γ, clinical characteristic
Introduction
Inflammatory myopathies are a group of idiopathic, heterogeneous systemic diseases affecting predominantly skeletal muscles, though they can also involve the skin and internal organs. In adults they include mainly polymyositis (PM), dermatomyositis (DM) and inclusion body myositis (IBM) [1, 2]. The association between cancer and idiopathic inflammatory myopathies, particularly dermatomyositis, which is termed cancer-associated myositis (CAM), has been reported in the medical literature. A newly described autoantibody to a 155-kDa nuclear protein, identified as transcription intermediary factor 1-γ (TIF1-γ), has proven useful for cancer screening in patients with dermatomyositis.
The aim of this study was to evaluate the clinical picture of patients with the presence of TIF1-γ antibodies in one center.
Material and methods
We searched our database of laboratory results with the aim of finding patients with a positive autoimmune inflammatory myopathy profile (EUROLINE, EUROIMMUN). Between November 2014 and January 2016, we found 80 such patients.
Results
In a group of 80 Caucasian patients, 11 revealed the presence of anti-TIF1-γ antibodies, but in 3 cases anti-TIF-γ were only low positive. There were 8 women and 3 men with average age 54.2 years (from 27 to 84 years). Dermatomyositis was diagnosed in 6 cases, polymyositis in 1 case, myositis limited to ocular muscles in 1 case and rhabdomyolysis in 1 case. In 2 patients with low positive antibodies undifferentiated connective tissue disease was recognized. In this group of patients with anti-TIF-γ antibodies neoplasm was found in 5 cases. All of those patients had DM. The average time between DM and diagnosis of neoplasm was 7.5 months (from 1 to 18 months). In 2 cases neoplasm was recognized concurrently with myositis. In 2 other cases, the neoplasm was detected 8 and 18 months after diagnosis of myositis. None of the patients had lung involvement, but 3 of them had severe skin changes and 2 of them dysphagia. Clinical characteristics of patients with TIF1-γ positivity are presented in Table I.
Table I.
Clinical manifestations in our patients who exhibited anti-TIF1-γ positivity
| N | Gender | Age at disease onset | Diagnosis | Observation (months) | Cancer | Death | Smoking | Muscle symptoms | Skin lesions | Skeletal symptoms | Lung involvement | GI involve-ment | CK [U/l] | LDH [U/l] | AST [U/l] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 33 | NDCTD | 9 | no | no | no | mild | no | arthralgia | no | no | 65 | 170 | 27 |
| 2 | F | 51 | limited ocular myositis | 5 | no | no | no | localized | no | no | no | no | 34 | 122 | 19 |
| 3 | M | 55 | DM | 1 | stomach | no data | yes | no | swelling of the eyelids | no | no | no | 2337 | NP | 149 |
| 4 | F | 27 | no | 4 | no | no | no | mild | no | no | no | no | 1414 | 263 | 87 |
| 5 | F | 59 | PM | 102 | no | no | no | severe | no | severe | no | dysphagia | 25630 | 365 | 720 |
| 6 | F | 57 | DM | 18 | pulmonis | no | yes | severe | heliotrope rash, V-sign, erythematous and oedematous rashes on face, rashes on the trunk | no | no | no | 305 | 258 | 31 |
| 7 | M | 74 | rhabdomyolysis | 1 | no | no | yes | severe | no | no | no | no | 145924 | 4221 | 1767 |
| 8 | M | 84 | DM | 1 | no | yes | no | severe | erythematous rashes Gottron's signs V-sign, scarf-sign periungual erythema | no | fibrotic changes | dysphagia | 307 | 349 | 65 |
| 9 | F | 41 | DM | 9 | no | no | no | no | erythematous and oedematous rashes on face, rashes on the trunk; Raynaud | arthralgia | no | no | 101 | 328 | 46 |
| 10 | F | 53 | DM | 8 | ovarian | yes | no | severe | erythematous rashes on face, heliotrope rash, V-sign, scarf-sign; arm and leg oedema | no | no | dysphagia | 17288 | 871 | 504 |
| 11 | F | 62 | DM | 3 | colon | yes | no | severe | swelling of the eyelids, heliotrope rash, V-sign, scarf-sign | no | no | dysphagia | 1280 | 333 | 75 |
DM – dermatomyositis; NDCTD – undifferentiated connective tissue disease; NP – not performed
In some patients the coexistence of anti-TIF-γ and other antibodies was found: in 2 cases anti-Ro52, anti-MDA5 in another 2 cases, and anti-OJ and anti-Ku in individual cases. In patients with low positive TIF-γ, anti-Mi2 and anti-DFS70 were found. It is worth pointing out that in patients with neoplasm, anti-TIF-γ were highly positive and were the only positive antibodies. The immune profile of patients with the presence of anti-TIF1-γ antibodies is presented in Table II.
Table II.
Immune profile of patients with the presence of anti-TIF1-γ antibodies
| No. | Sex | Antinuclear antibodies | TIF1-γ | Mi-2 | Ku | PM100 | PM75 | Jo1 | SRP | PL7 | PL12 | EJ | OJ | MDA5 | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 1: 2560 | low positive | + + + | + | – | – | – | – | – | – | – | – | – | – |
| 2 | F | NP | + | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 | M | NP | + + + | – | – | – | – | – | – | – | – | – | – | – | – |
| 4 | F | 1: 2560 | low positive | – | – | – | – | – | – | – | – | – | – | + | DFS70 |
| 5 | F | 1: 320 | low positive | – | – | – | – | – | – | – | – | – | – | – | – |
| 6 | F | 1: 2560 | + + + | – | – | – | – | – | – | – | – | – | – | – | Ro52 |
| 7 | M | 1280 | + | – | – | – | – | – | – | – | – | – | + | – | |
| 8 | M | 2560 | + + + | – | – | – | – | – | – | – | – | – | + + + | – | Ro52 |
| 9 | F | 1280 | + | – | – | – | – | – | – | – | – | – | – | – | – |
| 10 | F | 2560 | ++ | – | – | – | – | – | – | – | – | – | – | – | – |
| 11 | F | negative | + + + | – | – | – | – | – | – | – | – | – | – | – | – |
NP – not performed; Mi-2 – anti-helicase protein antibodies; PM100 – anti-human exosome complex PM100; PM75 – anti-human exosome complex PM75; SRP – anti-signal recognition particle antibodies; PL7 – anti-threonyl tRNA; Pl12 – anti-alanyl tRNA; EJ – anti-glycyl tRNA; OJ – anti-isoleucyl tRNA; MDA5 – melanoma; differentiation-associated gene 5 antibodies; DFS70 – autoantibodies to dense fine speckles; Anti-Jo1 – anti-histidyl tRNA
Discussion
The association between cancer and idiopathic inflammatory myopathies is well described in the literature [3, 4]. The incidence of cancer in published series of patients with idiopathic inflammatory myopathy ranges from 9% to 42%. A great variety of cancer types may occur: the most frequent are ovarian, breast, lung, gastric, colorectal tumors and lymphomas in dermatomyositis, and lung, urinary bladder cancers and lymphomas in polymyositis [5]. Cancer can develop before, concurrently, or subsequent to the onset of idiopathic inflammatory myopathy, but is usually recognized within 3 years of myositis diagnosis, with most diagnoses within 12 months [5]. The mean period in our group of patients was 7.5 months (from 1 to 18 months). In 2 cases neoplasm was recognized concurrently with myositis.
Malignant disease is also one of the main causes of mortality in patients with inflammatory myopathies. Thus cancer screening is obligatory in such patients, but there is no consensus as to the method or frequency with which patients with an idiopathic inflammatory myopathy should be tested to rule out neoplasm during the follow-up. Clinical research studies have shown that older age, male sex, dysphagia, and skin manifestations, such as skin necrosis, periungual erythema, and the ‘V’ or ‘shawl’ sign, are associated with occult malignancy in patients with myositis. Refractory or recurrent disease has also been related to cancer-associated myositis. In contrast, the presence of interstitial lung disease seems to be protective for the development of cancer. None of our patients with cancer had lung involvement. An absence of autoantibodies seems to be predictive of a high risk of occult malignancy, whereas the presence of antisynthetase antibodies seems to have a protective value against cancer.
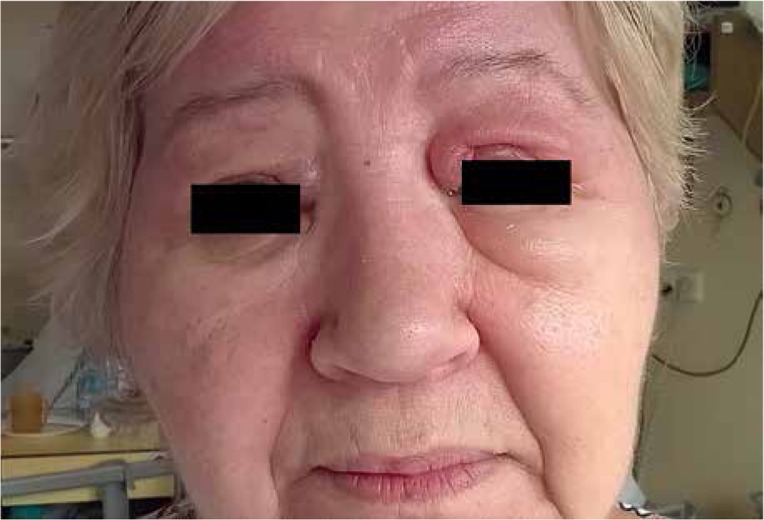
In 2006, Targoff et al. [6] described a novel autoantibody against a 155-kDa protein in a large series of patients with myositis. This antibody was then identified as human TIF1-γ [7, 8]. It seemed to be a myositis-specific autoantibody, as it was not detected in other autoimmune diseases or in other noninflammatory myopathies [7]. Among the 85 patients with idiopathic inflammatory myopathy, Trallero-Araguás et al. [9] found anti-p155 (TIF1-γ) autoantibody in 10 of the 16 patients classified as having CAM. They found that in DM, the negative and positive predictive value of presence of the TIF1-γ autoantibody for a diagnosis of CAM was 92% and 66.7%, respectively. No myositis-specific autoantibodies were detected in patients positive for anti-p155 (TIF1-γ) autoantibody. In contrast, myositis-associated autoantibodies were identified in some anti-TIF1-γ-positive patients [9]. None of the patients with CAM in our group had myositis-specific and only one had myositis-associated autoantibodies. Chinoy et al. [10] found the coexistence of TIF1-γ antibodies and other myositis-specific antibodies in only 3 patients, 2 of whom had anti-Mi-2 autoantibodies. Some authors have suggested an association between anti-TIF1-γ autoantibody and a specific clinical phenotype in adult DM, characterized by severe skin disease [11, 12]. Three of our patients had such a clinical presentation (Fig. 1).
Fig. 1.
Clinical presentation of DM.
Vincent et al. [13] in their study proposed that TIF1-γ is involved in the transforming growth factor β (TGF-β) signaling pathway, which is inactivated in some malignancies [13]. TIF1-γ acts on TGF-β and is essential in chromatin-mediated transcriptional regulation, functioning as a positive (agonist) or negative (antagonist) regulator of this pathway, which seems to be related to carcinogenesis.
Saleva-O'Callaghan et al. in their meta-analysis concluded that testing positive for TIF1-γ has an 18-fold higher association with cancer than TIF1-γ-negative status, that is, 93 of every 100 negative dermatomyositis patients will not develop cancer. They also proposed a reasonable approach for detecting occult malignancy in myositis [14]. Testing for the anti-TIF1-γ antibody alone had 50% sensitivity and 96% specificity for detection of CAM. The risk of developing cancer-associated DM in anti-TIF-γ-positive patients has also been assessed by a systematic review and meta-analysis on six cohort studies including a total of 312 adult patients with DM [15]. It was concluded that when the test for anti-TIF1-γ determination is negative, it reasonably rules out the presence of associated cancer. It was suggested, however, that it would be desirable to perform a single PET/CT study.
Conclusions
The association between cancer and idiopathic inflammatory myopathies, particularly dermatomyositis, is well known, and cancer screening should be obligatory in such patients. As yet there is no consensus as to the method or frequency with which patients with an idiopathic inflammatory myopathy should be tested to rule out neoplasm. Detection of anti-TIF1-γ antibodies in patient with DM gives the clinicians the very important suggestion of CAM. It seems reasonable that these patients should have more detailed and often repeated differential diagnostics.
The authors declare no conflict of interest.
References
- 1.Dobloug C, Garen T, Bitter H, et al. Prevalence and clinical characteristics of adult polymyositis and dermatomyositis; data from a large and unselected Norwegian cohort. Ann Rheum Dis. 2015;74:1551–1556. doi: 10.1136/annrheumdis-2013-205127. [DOI] [PubMed] [Google Scholar]
- 2.Váncsa A, Gergely L, Ponyi A, et al. Myositis-specific and myositis-associated antibodies in overlap myositis in comparison to primary dermatopolymyositis: Relevance for clinical classification: Retrospective study of 169 patients. Joint Bone Spine. 2010;77:125–130. doi: 10.1016/j.jbspin.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Huang YL, Chen YJ, Lin MW, et al. Malignancies associated with dermatomyositis and polymyositis in Taiwan: a nationwide population-based study. Br J Dermatol. 2009;161:854–860. doi: 10.1111/j.1365-2133.2009.09274.x. [DOI] [PubMed] [Google Scholar]
- 4.Antiochos BB, Brown LA, Li Z, et al. Malignancy is associated with dermatomyositis but not polymyositis in Northern New England, USA. J Rheumatol. 2009;36:2704–2710. doi: 10.3899/jrheum.090549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dankó K, Ponyi A, Molnar AP, et al. Paraneoplastic myopathy. Curr Opin Rheumatol. 2009;21:594–598. doi: 10.1097/BOR.0b013e3283317fa5. [DOI] [PubMed] [Google Scholar]
- 6.Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682–3689. doi: 10.1002/art.22164. [DOI] [PubMed] [Google Scholar]
- 7.Targoff IN, Trieu EP, Levy-Neto M, et al. Autoantibodies to transcriptional intermediary factor-1 gamma (TIF-1gamma) in dermatomyositis [abstract] Arthritis Rheum. 2006;54:S518. [Google Scholar]
- 8.Fujimoto M, Hamaguchi Y, Kaji K, et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 2012;64:513–522. doi: 10.1002/art.33403. [DOI] [PubMed] [Google Scholar]
- 9.Trallero-Araguás E, Labrador-Horrillo M, Selva-O'Callaghan A, et al. Cancer-associated myositis and anti-p155 autoantibody in a series of 85 patients with idiopathic inflammatory myopathy. Medicine. 2010;89:47–52. doi: 10.1097/MD.0b013e3181ca14ff. [DOI] [PubMed] [Google Scholar]
- 10.Chinoy H, Fertig N, Oddis CV, et al. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis. 2007;66:1345–1349. doi: 10.1136/ard.2006.068502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaji K, Fujimoto M, Hasegawa M, et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology (Oxford) 2007;46:25–28. doi: 10.1093/rheumatology/kel161. [DOI] [PubMed] [Google Scholar]
- 12.Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology (Oxford) 2008;47:324–328. doi: 10.1093/rheumatology/kem359. [DOI] [PubMed] [Google Scholar]
- 13.Vincent DF, Yan KP, Treilleux I, et al. Inactivation of TIF1gamma cooperates with Kras to induce cystic tumors of the pancreas. PLoS Genet. 2009;5:e1000575. doi: 10.1371/journal.pgen.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selva-O'Callaghan A, Trallero-Araguás E, Grau-Junyent JM, Labrador-Horrillo M. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol. 2010;22:627–632. doi: 10.1097/BOR.0b013e32833f1075. [DOI] [PubMed] [Google Scholar]
- 15.Trallero-Araguás E, Rodrigo-Pendás JÁ, Selva-O'Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis. A systematic review and meta-analysis. Arthritis Rheum. 2012;64:523–532. doi: 10.1002/art.33379. [DOI] [PubMed] [Google Scholar]